Abstract
Purpose of the review
Children with severe asthma have a high degree of respiratory morbidity despite treatment with high doses of inhaled corticosteroids and are therefore very difficult to treat. This review will discuss phenotypic and pathogenic aspects of severe asthma in childhood, as well as remaining knowledge gaps.
Recent findings
As a group, children with severe asthma have a number of distinct phenotypic features compared to children with mild-to-moderate asthma. Clinically, children with severe asthma are differentiated by greater allergic sensitization, increased exhaled nitric oxide, and significant airflow limitation and air trapping that worsens as a function of age. These findings are accompanied by structural airway changes and increased and dysregulated airway inflammation and oxidant stress which may explain the differential nature of corticosteroid responsiveness in this population. Because children with severe asthma themselves are a heterogeneous group, current efforts are focused on improved definition and sub-phenotyping of the disorder. While the clinical relevance of phenotyping approaches in severe asthma is not yet clear, they may provide important insight into the mechanisms underlying the disorder.
Summary
Improved classification of severe asthma through unified definitions, careful phenotypic analyses, and mechanism-focused endotyping approaches may ultimately advance knowledge and personalized treatment.
Keywords: Severe asthma, difficult asthma, children, phenotype, endotype
Introduction
While the implementation of asthma clinical practice guidelines has led to important reductions in overall asthma morbidity and mortality over the past two decades [1], there remains a relatively small yet significant group of patients with severe asthma who suffer from ongoing respiratory symptoms despite appropriate treatment with inhaled corticosteroids (ICS) [2, 3]. Although there is some symptom heterogeneity within this subpopulation, affected patients share the risk for adverse outcomes, including life-threatening exacerbations and ultimately, asthma-related death [4, 5]. Patients with severe asthma therefore account for up to 50% of all asthma-related costs given the disproportionate medication requirements and healthcare utilization associated with the disorder [6, 7].
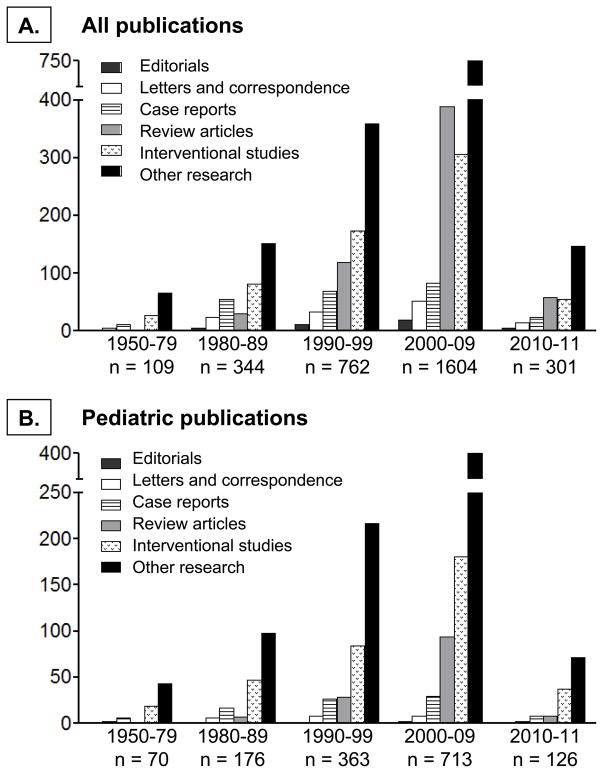
Interest in the underlying biological mechanisms of severe asthma and specialized diagnostic and management strategies for the disorder has increased substantially in recent years. This has resulted in the formation of several international severe asthma research initiatives, from which a number of pivotal publications have ensued (Table 1) [8–12]. These efforts have ultimately resulted in a rapid increase in the number of indexed (PubMed) publications on severe asthma over the past two decades (Figure 1). However, while the body of available literature on severe asthma has grown, there remain few publications on children. This knowledge gap has important public health consequences given the high underlying prevalence of asthma in children and the significant burden of asthma disease in this age group [13–15]. This review will therefore discuss recent findings related to severe asthma in children that shed new light on the burden and peculiarities of the disorder. Because clinical management approaches to the child with severe asthma have been the subject of several recent reviews [16–19], this review will focus instead on phenotypic and pathogenic aspects of severe asthma in childhood, as well as remaining knowledge gaps.
Table 1.
| Name | Clinical centers | Patients enrolled | Original reference |
|---|---|---|---|
| European Network for Understanding Mechanisms of Severe Asthma (ENFUMOSA) | Twelve clinical specialty centers in nine European countries | Aged 17–65 years with asthma diagnosed by a specialist receiving ICS treatment for at least 1 year | [8] |
| Epidemiology and Natural History of Asthma: Outcomes and Treatment Regimens (TENOR) | Two hundred eighty-three office-based private practice, hospital, and academic sites across the US | Aged 6 years and older with severe or difficult-to-treat asthma treated by an asthma specialist | [9] |
| Global Asthma and Allergy European Network (GA2LEN) | Twenty-seven research centers and 60 collaborating centers in 16 European countries in 35 countries | Project-specific, includes birth cohorts and patients with mild-to-severe asthma across the age spectrum | [10] |
| National Heart, Lung and Blood Institute Severe Asthma Research Program (SARP) | Eight academic clinical specialty centers in the US and UK | Project-specific, aged 6 years and older with physician-diagnosed mild to severe asthma and healthy nonsmoking adults without asthma | [11] |
| Unbiased Biomarkers for the Prediction of Respiratory Disease Outcome (U-BIOPRED) | Twenty academic centers across Europe | Adults and children with severe asthma taking high-dose ICS, adults with mild-to-moderate asthma, and healthy nonsmoking adults without asthma | [12] |
ICS, inhaled corticosteroids.
Figure 1.
Publications on (A) severe asthma and (B) severe asthma in children from the 1950’s to present. Publications were limited to those indexed on PubMed and written in English. Searches were made for “severe asthma,” “difficult asthma,” and “difficult-to-treat asthma,” excluding “acute asthma” and animal studies.
What is severe asthma? The struggle for a common definition
Undoubtedly, one of the greatest challenges in the field is the definition of severe asthma as a unique disease entity. Some controversy stems from poor understanding of asthma itself, which is clearly a heterogeneous syndrome associated with a constellation of clinical features [20]. Indeed, in a recent review of publications involving relatively large (n > 100) cohorts of children with asthma diagnosed between 6 and 18 years, 122 publications yielded 60 different definitions of asthma that had a large impact on prevalence estimates and asthma predictive probabilities [21]. Nonetheless, several concerted efforts have been made to standardize the definition of severe asthma in order to advance the field.
An important advance came from the recognition that asthma severity and control are related but not interchangeable concepts. Whereas asthma control refers to the extent to which asthma symptoms or associated features are alleviated by treatment, asthma severity refers to the difficulty in controlling asthma with treatment (i.e., the activity of the underlying disease state) [22, 23]. These distinctions were incorporated into a uniform definition of severe asthma by the World Health Organization (WHO) during a workshop in 2009. According to the WHO definition, severe asthma is defined as “uncontrolled asthma which can result in risk of frequent severe exacerbations (or death) and/or adverse reactions to medications and/or chronic morbidity (including impaired lung function or reduced lung growth in children)” [24]. Severe asthma therefore includes three groups: 1) untreated severe asthma due to failure of diagnosis, lack of access to medical care, or non-adherence to therapy, 2) difficult-to-treat severe asthma from co-morbid conditions or adverse environmental circumstances, and 3) treatment-resistant severe asthma, which encompasses asthma for which control is not achieved despite intensive therapy and asthma for which control can only be maintained with intensive therapy (Figure 2) [24, 25]. This definition has a number of global advantages. In low- or middle-income countries where asthma is highly prevalent, asthma may be undiagnosed due to limited training and accessibility of medical professionals or may be undertreated due to limited availability of asthma controller medications. This definition will capture these individuals and will therefore permit more meaningful estimates of the prevalence of severe asthma worldwide. This will ultimately promote health care planning and policy for public health purposes and will facilitate epidemiologic comparisons across different populations. The prevalence of severe asthma is also expected to increase as a result.
Figure 2.
The WHO definition of severe asthma.
Because the WHO definition of severe asthma was drafted for global application, the American Thoracic Society (ATS) and the European Respiratory Society (ERS) convened a similar workshop in 2011 to develop a definition of severe asthma for countries in which there is reasonable access to asthma medications (publication expected in 2012). This workshop built upon the ATS definition of severe asthma proposed 10 years earlier [26] and distinguishes only between patients with treatment-resistant severe asthma and patients in whom the asthma is difficult to treat. According to the new ATS-ERS definition, once the asthma diagnosis is confirmed and differentiated from difficult-to-treat asthma, severe asthma is defined as the requirement for treatment with high-dose ICS plus a second controller medication. This definition applies to two groups: 1) patients who require intensive treatment to maintain asthma control, and 2) others who fail to achieve control with this treatment regimen (personal communication, Sally Wenzel, ATS-ERS workshop chair). This approach is in keeping with that proposed by the WHO and attempts to identify individuals at high risk from the disease and for medication-related side effects. While research on the application and utility of these emerging definitions of severe asthma is clearly needed, these attempts to improve classification of the disorder are expected to lead to refinement in clinical and mechanistic explorations that are essential for the development of novel therapeutics.
Phenotyping severe asthma: what is it and why does it matter?
For the purpose of this review, the term “phenotype” refers to observable characteristics, often with no direct relationship to disease process, such as airway physiology, triggers, and inflammatory parameters [27]. However, there is no consensus definition of what a “phenotype” is and therefore the term is used in different ways by different groups. For instance, geneticists frequently refer to a “phenotype” as any observable trait of an organism. In the clinical realm, a “phenotype” may be viewed along the spectrum of a “useful but entirely artificial construct” to “an underlying disease entity that, like biological species, awaits discovery” [28]. These controversies have formed the subject of two recent reviews on childhood asthma [28, 29]. While the clinical benefit of phenotyping severe asthma is not yet clear, it may be the key to major advances in the field, which is presently plagued by a lack of clear genetic markers for disease onset and severity [30, 31] and highly heterogeneous disease presentation in terms of symptoms, exacerbations, and pharmacologic responses [32–34]. However, until convincing data are available, the decision whether or not to phenotype severe asthma ultimately rests with the theoretical underpinnings of the investigative team.
Severe asthma as a unique phenotype: clinical studies
Severe asthma presents in childhood as a heterogeneous disorder determined by unique socio-cultural, environmental and biological factors, including suboptimal adherence to prescribed asthma therapies [35–37]. Although early investigations in childhood severe asthma identified a highly atopic group of children with persistent airflow obstruction, airway eosinophilia and reticular basement membrane thickening despite corticosteroid treatment [38–40], the presence of unique severe asthma “phenotype” in children has been questioned since asthma per se in children tends to be more episodic with a high exacerbation frequency despite relatively normal lung function [41]. Therefore, over the past decade, the Epidemiology and Natural History of Asthma: Outcomes and Treatment Regimens (TENOR) study group and the National Heart, Lung and Blood Institute’s Severe Asthma Research Program (SARP) led concerted efforts to identify the unique features of children with severe versus mild-to-moderate asthma. Both groups found that children with severe asthma had a high burden of asthma symptoms and increased frequency of asthma exacerbations despite high-dose inhaled and oral corticosteroid treatment [2, 42, 43]. Furthermore, children with severe asthma were also more likely to experience a subsequent asthma exacerbation requiring systemic corticosteroids, an emergency visit, or hospitalization when followed prospectively after enrollment [2, 4], a finding that was strongest in children with baseline airflow limitation [2].
Other interesting findings from SARP and TENOR have centered on comparisons of children and adults with severe asthma (Table II). Although children and adults with severe asthma share a similar burden of symptoms, emergency department visits and hospitalizations are more frequent in children [2, 42] and are greatest in children with daily symptoms [4]. Furthermore, unlike adults with severe asthma wherein markers of atopy are relatively less prevalent [3], children with severe asthma are highly atopic with increased peripheral blood eosinophilia, aeroallergen sensitivity, and elevated serum IgE concentrations [2, 42, 44]. Whereas exhaled nitric oxide concentrations are not consistently elevated in adults with severe asthma [3], by contrast, children with severe asthma have sustained increases in exhaled nitric oxide [2] in keeping with the reactive, at-risk, adult severe asthma phenotype [45]. Airflow limitation and air trapping are also present in children with severe asthma [2, 42], albeit to a lesser degree than what is commonly seen in adults [46]. Interestingly, whereas pre-pubertal girls with severe asthma had no residual air trapping after maximal bronchodilation, boys with severe asthma had incomplete reversal of air trapping with persistent elevations in the ratio of residual volume to total lung capacity (RV/TLC) [47]. This finding suggests that the adult physiological patterns of severe asthma are already present in school-age boys but may not yet be fully developed in girls. Thus the years surrounding puberty may represent a critical window where the phenotype of severe asthma in children intensifies and worsens.
Table 2.
| Feature | Adults (18 years+) with severe asthma
|
Children (6–17 years) with severe asthma
|
||
|---|---|---|---|---|
| Observation {versus nonsevere asthma) | Reference | Observation (versus nonsevere asthma) | Reference | |
| Exacerbation severity | Frequency emergency department visits and hospitalizations with ~30% hospitalized in the previous year; 20–25% with lifetime history of intubation | [3] | Frequent emergency department visits and hospitalizations with ~55% hospitalization in the previous year; 10–15% with lifetime history of intubation | [2,42] |
|
| ||||
| Allergic sensitization | Varying degrees of atopy according to age of asthma onset and phenotype cluster | [3,20▪▪] | Highly atopic with increased peripheral blood eosinophilia, aeroallergen sensitivity, and elevated serum IgE concentrations | [2,42.44▪▪] |
|
| ||||
| Exhaled nitric oxide | Not distinguishing overall but associated with exacerbations in a selected phenotype | [3,45▪] | Sustained elevations | [2] |
|
| ||||
| Airflow limitation | Moderate-to-severe airflow limitation, often with incomplete reversal after bronchodilation | [3,20▪▪,46] | Some (mild) airflow limitation with near-complete reversal after bronchodilation; significant acceleration of airflow limitation in some adolescents after puberty | [2,42,48▪▪] |
|
| ||||
| Air trapping | Increased air trapping (increased RV/TLC) at the same threshold of airflow limitation (FEV1/FVC) | [46] | Increased air trapping (increased RV/TLC) at baseline; reversible in girls but persistent in boys | [2,47▪▪] |
FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; RV/TLC, ratio of residual volume to total lung capacity.
Given the limited number of longitudinal studies in children with severe asthma, the natural history of severe asthma is poorly understood. Thus an important unanswered question is the stability of the severe asthma phenotype in children over time. In the TENOR study, 38% of children with poorly controlled severe asthma had improvement in their asthma symptoms after 2 years of follow-up [4]. However, 62% of children remained persistently very poorly controlled and were six times more likely to have severe exacerbations [4]. A similar subset of pre-pubertal children with severe asthma enrolled in SARP also demonstrated declines in the forced expiratory volume in one second (FEV1), forced vital capacity (FVC), and post-bronchodilator FEV1 percent predicted values during the early adolescent years [48]. Children with the most significant declines were those who had ongoing daily symptoms and a greater magnitude of allergic sensitization [48]. These findings are similar to those of the Childhood Asthma Management Research Program [49, 50] and the Dunedin Multidisciplinary Health and Development Study [51, 52] which demonstrated increased and persistent airflow limitation in children with persistent versus remitting asthma that was associated with greater aeroallergen sensitization and increased airway hyperresponsiveness during early childhood. The Melbourne Asthma study further noted early and irreversible loss of lung function by age 14 in children with severe asthma [53, 54], suggesting that the changes in lung function in severe asthma occur early during the course of the disease. Whether these observations represent a reduction in lung growth or progression of structural airway remodeling is not clear [55].
Severe asthma as a unique phenotype: translational studies
Considerably fewer studies have focused on the mechanistic underpinnings of severe asthma in children, in part due to research limitations in children. One example involves bronchoscopy, which typically can only be done for clinical purposes in children and therefore is associated with significant challenges stemming from selection bias. Yet despite these challenges, critical findings have been made. Importantly, structural airway alterations are apparent in children with severe asthma, like adults, and include greater airway smooth muscle mass [56], increased reticular basement membrane (RBM) thickening [39, 57] and epithelial damage and angiogenesis [58, 59]. While airway mucosal and tissue eosinophilia are often accompanying findings [38, 60], these structural airway changes may also be present in the absence of a prominent eosinophilic infiltrate [59] and may instead develop as a function of age [61, 62] and asthma duration [60, 63, 64]. Interestingly, airway eosinophilia and RBM thickening are not readily identifiable in 12–24 month infants with wheezing disorders [61] but are present in some preschool children after 24 months of age [60]. Although other preschool children have more neutrophilic-predominant patterns of inflammation with increased IL-8 expression [65–67], these findings suggest that there is a classical and perhaps severe form of asthma that is identifiable very early in life and is distinct from other wheezing disorders. However, contrary to what is seen in fibrotic conditions, the ratio of fibril to matrix in the RBM of children and adults with asthma is normal and is not associated with increased interstitial collagen [68]. These findings challenge the notion that asthma is a fibrotic disorder and therefore additional studies are needed to understand how these airway structural changes evolve across the early lifespan.
The airway structural changes that accompany severe asthma do not occur in isolation but rather are accompanied by a number of other inflammatory features. In contrast to children with mild-to-moderate asthma, children with severe asthma have altered regulation of the antioxidant, glutathione, in the epithelial lining fluid that corresponds to the magnitude of airflow obstruction [69]. These disturbances of airway glutathione are further associated with increased lipid peroxidation and DNA nucleoside oxidation byproducts [69, 70], increased nitric oxide oxidation products [71], increased catabolism of endogenous S-nitrosothiols [72], and impaired airway macrophage function, including altered innate immune defense [70, 73]. These findings may account for the increased severity of respiratory infections as well as the relative corticosteroid resistance of this population. Despite best attempts at corticosteroid therapy, children with severe asthma have decreased histone deacetylase activity in airway macrophages [70] as well as sustained expression of pro-inflammatory cytokines and chemokines resulting in a unique molecular phenotype that is neither Th1- nor Th2- predominant [74]. These observations are likely mediated by post-translational modification of key proteins and transcription factors such as nuclear factor (erythroid-derived 2)-like 2 [75] and suggest that that interventions to restore airway redox status may be warranted in children with severe asthma.
Sub-phenotypes within severe asthma
These prior efforts suggest that there is indeed a unique group of children with severe asthma who, as a group, differ considerably from children with mild-to-moderate asthma with regard to a number of clinical, pathophysiologic and molecular features. However, within the group of children severe asthma, clearly there are a number of sub-phenotypes given the observed heterogeneity in the disorder. These questions have resulted in the application of non-biased cluster analyses to diverse samples of highly characterized patients to determine “clusters” or groups of patients with shared phenotypic characteristics. This approach was recently applied to large samples of adults with asthma and revealed unique subgroups with differences in lung function, age of asthma onset, inflammatory features and clinical treatment responses [20, 76]. The SARP investigators also applied the same approach to a highly characterized sample of children and identified four unique cluster phenotypes of asthma defined by different degrees of lung function, asthma duration, and asthma controller medication use (Figure 3) [77]. Children with ATS-defined severe asthma [26] were present in all four clusters, thus confirming its marked heterogeneity even in childhood [77]. Furthermore, no cluster corresponded to the asthma severity classifications proposed in asthma treatment guidelines [78, 79]. Additional studies are now needed to understand the clinical relevance of these clusters, including their stability over time and their predictive validity with regard to a number of important disease outcomes.
Figure 3.
Unbiased hierarchical cluster analysis reveals four clusters of asthma with shared phenotypic features. Although clusters 3 and 4 tend to be more “severe,” children with ATS-defined severe asthma are present in all clusters, thus highlighting the heterogeneity of the disorder [77].
Conclusion - From phenotype to endotype: Next steps
Although considerable advances have been made in the understanding of severe asthma in children over the past decade, the next decade will inevitably be focused on personalized medicine for the disorder [80]. In contrast to the “one-size-fits-all” approach to asthma treatment provided by current asthma treatment guidelines [78, 79], the next decade will likely see major revisions to these guidelines based on emerging phenotypic findings [81], including the highly differential nature of the corticosteroid response in children with severe asthma [34]. A number of asthma clinical trials have already included analyses of pre-selected phenotypic predictors [82, 83] and differential responses within treatment groups [84]. However, the biology linking phenotypic characteristics to clinical responses remains unknown. While the quest for biomarkers of severe asthma in children has yielded interesting developments with regard to urinary leukotriene E4 [85, 86] and vitamin D [87] analyses, more attention on the “endotype” of asthma (i.e., the distinct disease entity present in a phenotype that is defined by a specific biological mechanism) is clearly needed [27]. Whereas current treatment guidelines assume that: 1) asthma is a unified disorder with a common inflammatory mechanism, 2) there is concordance between inflammation and symptoms, and 3) the nature of the inflammation is corticosteroid responsive [78, 79], developments in asthma endotyping are likely to challenge this paradigm in the next decade. This would represent a huge advance for both practitioners and patients alike and may be an important next step toward mitigating the morbidity and mortality that accompanies severe asthma in children, which remains a heterogeneous condition that is extremely difficult to treat.
Key Points.
Children with severe asthma are a heterogeneous group that includes children with severe, therapy-resistant asthma and children in whom the asthma is difficult-to-treat.
Although there is no consensus definition of “phenotype,” it typically refers to observable characteristics that may or may not be related to disease, such as airway physiology, triggers, and inflammatory parameters.
While the phenotype of severe asthma in children as a group is unique from that of children with mild-to-moderate asthma, there are also sub-phenotypes within severe asthma.
The clinical relevance of asthma phenotypes ultimately rests with endotyping, which focuses on the biological mechanisms that underlie a distinct disease entity present within a phenotype.
Acknowledgments
Supported in part by NIH RO1 NR012021 and U10 HL109164
Footnotes
Conflicts of interest
Leonard Bacharier is on the Speaker’s bureau and/or has received honoraria from Merch and GlaxoSmithKline. Leonard Bacharier and Anne Fitzpatrick receive research funding from the National Institutes of Health. Carlos E. Baena-Cagnani has no disclosures relevant to this work.
References and Recommended Reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
* Of special interest
** Of outstanding interest
- 1*.Szefler SJ. Advancing asthma care: The glass is only half full! J Allergy Clin Immunol. 2011;128(3):485–94. doi: 10.1016/j.jaci.2011.07.010. This article reviews trends in asthma morbidity and morality over the past 20 years as well as remaining gaps in asthma knowledge and treatment. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fitzpatrick AM, Gaston BM, Erzurum SC, Teague WG. Features of severe asthma in school-age children: Atopy and increased exhaled nitric oxide. J Allergy Clin Immunol. 2006;118(6):1218–25. doi: 10.1016/j.jaci.2006.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moore WC, Bleecker ER, Curran-Everett D, et al. Characterization of the severe asthma phenotype by the National Heart, Lung, and Blood Institute’s Severe Asthma Research Program. J Allergy Clin Immunol. 2007;119(2):405–13. doi: 10.1016/j.jaci.2006.11.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haselkorn T, Fish JE, Zeiger RS, et al. Consistently very poorly controlled asthma, as defined by the impairment domain of the Expert Panel Report 3 guidelines, increases risk for future severe asthma exacerbations in The Epidemiology and Natural History of Asthma: Outcomes and Treatment Regimens (TENOR) study. J Allergy Clin Immunol. 2009;124(5):895–902. e1–4. doi: 10.1016/j.jaci.2009.07.035. [DOI] [PubMed] [Google Scholar]
- 5.Sullivan SD, Wenzel SE, Bresnahan BW, et al. Association of control and risk of severe asthma-related events in severe or difficult-to-treat asthma patients. Allergy. 2007;62(6):655–60. doi: 10.1111/j.1398-9995.2007.01383.x. [DOI] [PubMed] [Google Scholar]
- 6*.Barnett SB, Nurmagambetov TA. Costs of asthma in the United States: 2002–2007. J Allergy Clin Immunol. 2011;127(1):145–52. doi: 10.1016/j.jaci.2010.10.020. This article presents current estimates of the incremental direct costs of asthma and productivity losses due to asthma as determined by the Medical Expenditure Panel Survey. [DOI] [PubMed] [Google Scholar]
- 7**.Szefler SJ, Zeiger RS, Haselkorn T, et al. Economic burden of impairment in children with severe or difficult-to-treat asthma. Ann Allergy Asthma Immunol. 2011;107(2):110–9. e1. doi: 10.1016/j.anai.2011.04.008. This article presents mean total annual asthma costs for children with severe or difficult-to-treat asthma enrolled in The Epidemiology and Natural History of Asthma: Outcomes and Treatment Regimens study according to the level of asthma control. [DOI] [PubMed] [Google Scholar]
- 8.The ENFUMOSA cross-sectional European multicentre study of the clinical phenotype of chronic severe asthma. European Network for Understanding Mechanisms of Severe Asthma. Eur Respir J. 2003;22(3):470–7. doi: 10.1183/09031936.03.00261903. [DOI] [PubMed] [Google Scholar]
- 9.Dolan CM, Fraher KE, Bleecker ER, et al. Design and baseline characteristics of the epidemiology and natural history of asthma: Outcomes and Treatment Regimens (TENOR) study: a large cohort of patients with severe or difficult-to-treat asthma. Ann Allergy Asthma Immunol. 2004;92(1):32–9. doi: 10.1016/S1081-1206(10)61707-3. [DOI] [PubMed] [Google Scholar]
- 10.Bousquet J, Burney PG, Zuberbier T, et al. GA2LEN (Global Allergy and Asthma European Network) addresses the allergy and asthma ‘epidemic’. Allergy. 2009;64(7):969–77. doi: 10.1111/j.1398-9995.2009.02059.x. [DOI] [PubMed] [Google Scholar]
- 11.Wenzel SE, Busse WW. Severe asthma: lessons from the Severe Asthma Research Program. J Allergy Clin Immunol. 2007;119(1):14–21. doi: 10.1016/j.jaci.2006.10.025. quiz 2–3. [DOI] [PubMed] [Google Scholar]
- 12.Unbiased Biomarkers for the Prediction of Respiratory Disease Outcomes (U-BIOPRED) 2010 [updated 2010; cited]; Available from: http://www.upiopred.edu.
- 13.Akinbami LJ, Moorman JE, Garbe PL, Sondik EJ. Status of childhood asthma in the United States, 1980–2007. Pediatrics. 2009;123 (Suppl 3):S131–45. doi: 10.1542/peds.2008-2233C. [DOI] [PubMed] [Google Scholar]
- 14*.Akinbami LJ, Moorman JE, Liu X. Asthma prevalence, health care use, and mortality: United States, 2005–2009. Natl Health Stat Report. 2011;(32):1–14. This article presents data from several national surveys on recent trends in asthma prevalence healthcare use, school and work absences, and asthma mangement practices. [PubMed] [Google Scholar]
- 15.Bousquet J, Khaltaev N. Global Alliance Against Chronic Respiratory Diseases. Geneva: World Health Organization; 2007. Global surveillance, prevention and control of chronic respiratory diseases: a comprehensive approach. [Google Scholar]
- 16**.Bush A, Saglani S. Management of severe asthma in children. Lancet. 2010;376(9743):814–25. doi: 10.1016/S0140-6736(10)61054-9. This article reviews clinical diagnostic approaches and therapeutic management strategies for children with severe and difficult-to-treat asthma. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17**.Lodrup Carlsen KC, Hedlin G, Bush A, et al. Assessment of problematic severe asthma in children. Eur Respir J. 2011;37(2):432–40. doi: 10.1183/09031936.00091410. This article presents recommendations for clinical diagnostic approaches for children with severe and difficult-to-treat asthma and reviews relevant practice gaps. [DOI] [PubMed] [Google Scholar]
- 18**.Hedlin G, Bush A, Lodrup Carlsen K, et al. Problematic severe asthma in children, not one problem but many: a GA2LEN initiative. Eur Respir J. 2010;36(1):196–201. doi: 10.1183/09031936.00104809. This consensus statement from the Problematic Severe Asthma in Childhood Initiative Group discusses recommended nomenclature and clinical approaches to the child with problematic severe asthma. [DOI] [PubMed] [Google Scholar]
- 19**.Bush A, Pedersen S, Hedlin G, et al. Pharmacological treatment of severe, therapy-resistant asthma in children: what can we learn from where? Eur Respir J. 2011;38(4):947–58. doi: 10.1183/09031936.00030711. This article reviews pharmacological treatment options for children with severe, therapy-resistant asthma. [DOI] [PubMed] [Google Scholar]
- 20**.Moore WC, Meyers DA, Wenzel SE, et al. Identification of asthma phenotypes using cluster analysis in the Severe Asthma Research Program. Am J Respir Crit Care Med. 2010;181(4):315–23. doi: 10.1164/rccm.200906-0896OC. This article presents findings from an unbiased cluster analysis of adolescents and adults enrolled in the Severe Asthma Research Program, which identified five distinct clusters or “phenotypes” of asthma with shared clinical characteristics. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21*.Van Wonderen KE, Van Der Mark LB, Mohrs J, et al. Different definitions in childhood asthma: how dependable is the dependent variable? Eur Respir J. 2010;36(1):48–56. doi: 10.1183/09031936.00154409. This article describes differences in the epidemiological definition of asthma in children and how this may affect prediction of asthma at age 6 years. [DOI] [PubMed] [Google Scholar]
- 22.Reddel HK, Taylor DR, Bateman ED, et al. An official American Thoracic Society/European Respiratory Society statement: asthma control and exacerbations: standardizing endpoints for clinical asthma trials and clinical practice. Am J Respir Crit Care Med. 2009;180(1):59–99. doi: 10.1164/rccm.200801-060ST. [DOI] [PubMed] [Google Scholar]
- 23.Taylor DR, Bateman ED, Boulet LP, et al. A new perspective on concepts of asthma severity and control. Eur Respir J. 2008;32(3):545–54. doi: 10.1183/09031936.00155307. [DOI] [PubMed] [Google Scholar]
- 24**.Bousquet J, Mantzouranis E, Cruz AA, et al. Uniform definition of asthma severity, control, and exacerbations: document presented for the World Health Organization Consultation on Severe Asthma. J Allergy Clin Immunol. 2010;126(5):926–38. doi: 10.1016/j.jaci.2010.07.019. This position statement from the World Health Organization provides a uniform definition for asthma severity, control and exacerbations with important implications for global health. [DOI] [PubMed] [Google Scholar]
- 25**.Bush A, Zar HJ. WHO universal definition of severe asthma. Curr Opin Allergy Clin Immunol. 2011;11(2):115–21. doi: 10.1097/ACI.0b013e32834487ae. This article reviews the World Health Organization’s definition of asthma severity and control, with emphasis on the relevance to children. [DOI] [PubMed] [Google Scholar]
- 26.Proceedings of the ATS workshop on refractory asthma: current understanding, recommendations, and unanswered questions. American Thoracic Society. Am J Respir Crit Care Med. 2000;162(6):2341–51. doi: 10.1164/ajrccm.162.6.ats9-00. [DOI] [PubMed] [Google Scholar]
- 27**.Lotvall J, Akdis CA, Bacharier LB, et al. Asthma endotypes: a new approach to classification of disease entities within the asthma syndrome. J Allergy Clin Immunol. 2011;127(2):355–60. doi: 10.1016/j.jaci.2010.11.037. This PRACTALL (PRACtical ALLergy) consensus report proposes definitions for asthma phenotypes versus endotypes and discusses how these terms can be used in the context of clinical research and drug development. [DOI] [PubMed] [Google Scholar]
- 28**.Spycher BD, Silverman M, Kuehni CE. Phenotypes of childhood asthma: are they real? Clin Exp Allergy. 2010;40(8):1130–41. doi: 10.1111/j.1365-2222.2010.03541.x. This article reviews definitions and approaches to asthma phenotyping with an emphasis on applicability and relevance to clinical practice. [DOI] [PubMed] [Google Scholar]
- 29**.Bush A, Fleming L. Phenotypes of refractory/severe asthma. Paediatr Respir Rev. 2011;12(3):177–81. doi: 10.1016/j.prrv.2011.01.003. This article reviews current understanding of asthma phenotypes and provides suggestions for the approach to asthma “phenotypes” in the clinical setting. [DOI] [PubMed] [Google Scholar]
- 30*.Barnes KC. Genetic studies of the etiology of asthma. Proc Am Thorac Soc. 2011;8(2):143–8. doi: 10.1513/pats.201103-030MS. This article reviews progress on the study of genes as they relate to asthma etiology, including genome-wide association studies. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31*.Meyers DA. Genetics of asthma and allergy: what have we learned? J Allergy Clin Immunol. 2010;126(3):439–46. doi: 10.1016/j.jaci.2010.07.012. quiz 47–8. This article reviews progress on understanding of the genetic basis of disease severity with a focus on asthma and also discusses future approaches to personalized medicine. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32**.Fleming L, Wilson N, Regamey N, Bush A. Use of sputum eosinophil counts to guide management in children with severe asthma. Thorax. 2011 Aug 8; doi: 10.1136/thx.2010.156836. [Epub ahead of print]. This study found that incorporating sputum eosinophil counts into the management of children with severe asthma was not useful in the clinical setting. [DOI] [PubMed] [Google Scholar]
- 33*.Wu AC, Tantisira K, Li L, et al. Predictors of Symptoms are Different from Predictors of Severe Exacerbations from Asthma in Children. Chest. 2011;140(1):100–7. doi: 10.1378/chest.10-2794. This study found that children with persistent symptoms were more likely to have severe exacerbations, although the predictors of symptoms and exacerbations differed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bossley CJ, Saglani S, Kavanagh C, et al. Corticosteroid responsiveness and clinical characteristics in childhood difficult asthma. Eur Respir J. 2009;34(5):1052–9. doi: 10.1183/09031936.00186508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McNally KA, Rohan J, Schluchter M, et al. Adherence to combined montelukast and fluticasone treatment in economically disadvantaged african american youth with asthma. J Asthma. 2009;46(9):921–7. doi: 10.3109/02770900903229651. [DOI] [PubMed] [Google Scholar]
- 36**.Edgecombe K, Latter S, Peters S, Roberts G. Health experiences of adolescents with uncontrolled severe asthma. Arch Dis Child. 2010;95(12):985–91. doi: 10.1136/adc.2009.171579. This study found that adolescents with uncontrolled severe asthma were often non-adherent with their asthma therapy and also had a poor understanding of their medication. [DOI] [PubMed] [Google Scholar]
- 37.Fitzpatrick AM, Kir T, Naeher LP, et al. Tablet and inhaled controller medication refill frequencies in children with asthma. J Pediatr Nurs. 2009;24(2):81–9. doi: 10.1016/j.pedn.2008.02.027. [DOI] [PubMed] [Google Scholar]
- 38.Payne DN, Adcock IM, Wilson NM, et al. Relationship between exhaled nitric oxide and mucosal eosinophilic inflammation in children with difficult asthma, after treatment with oral prednisolone. Am J Respir Crit Care Med. 2001;164(8 Pt 1):1376–81. doi: 10.1164/ajrccm.164.8.2101145. [DOI] [PubMed] [Google Scholar]
- 39.Payne DN, Rogers AV, Adelroth E, et al. Early thickening of the reticular basement membrane in children with difficult asthma. Am J Respir Crit Care Med. 2003;167(1):78–82. doi: 10.1164/rccm.200205-414OC. [DOI] [PubMed] [Google Scholar]
- 40.Payne DN, Wilson NM, James A, et al. Evidence for different subgroups of difficult asthma in children. Thorax. 2001;56(5):345–50. doi: 10.1136/thorax.56.5.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bacharier LB, Strunk RC, Mauger D, et al. Classifying asthma severity in children: mismatch between symptoms, medication use, and lung function. Am J Respir Crit Care Med. 2004;170(4):426–32. doi: 10.1164/rccm.200308-1178OC. [DOI] [PubMed] [Google Scholar]
- 42.Chipps BE, Szefler SJ, Simons FE, et al. Demographic and clinical characteristics of children and adolescents with severe or difficult-to-treat asthma. J Allergy Clin Immunol. 2007;119(5):1156–63. doi: 10.1016/j.jaci.2006.12.668. [DOI] [PubMed] [Google Scholar]
- 43**.Fitzpatrick AM, Teague WG. Severe Asthma in Children: Insights from the National Heart, Lung, and Blood Institute’s Severe Asthma Research Program. Pediatr Allergy Immunol Pulmonol. 2010;23(2):131–8. doi: 10.1089/ped.2010.0021. This article reviews findings on severe asthma in children from the Severe Asthma Research Program. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44**.Haselkorn T, Szefler SJ, Simons FE, et al. Allergy, total serum immunoglobulin E, and airflow in children and adolescents in TENOR. Pediatr Allergy Immunol. 2010;21(8):1157–65. doi: 10.1111/j.1399-3038.2010.01065.x. This article presents findings on the relationship between allergic sensitization and airflow limitation un children with severe or difficult-to-treat asthma enrolled in The Epidemiology and Natural History of Asthma: Outcomes and Treatment Regimens study. [DOI] [PubMed] [Google Scholar]
- 45*.Dweik RA, Sorkness RL, Wenzel S, et al. Use of exhaled nitric oxide measurement to identify a reactive, at-risk phenotype among patients with asthma. Am J Respir Crit Care Med. 2010;181(10):1033–41. doi: 10.1164/rccm.200905-0695OC. This study fron the Severe Asthma Research Program found that although exhaled nitric oxide did not differentiate subjects with severe versus non-severe asthma, it did identify a group of subjects with severe asthma with significant airflow limitation and exacerbations. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sorkness RL, Bleecker ER, Busse WW, et al. Lung function in adults with stable but severe asthma: air trapping and incomplete reversal of obstruction with bronchodilation. J Appl Physiol. 2008;104(2):394–403. doi: 10.1152/japplphysiol.00329.2007. [DOI] [PubMed] [Google Scholar]
- 47**.Sorkness RL, Teague WG, Penugonda M, Fitzpatrick AM. Sex dependence of airflow limitation and air trapping in children with severe asthma. J Allergy Clin Immunol. 2011;127(4):1073–1074. doi: 10.1016/j.jaci.2010.12.1079. This study from the Severe Asthma Research Program describes differences in pre- and post-bronchodilator airflow limitation and air trapping in asthmatic children according to sex. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48**.Fitzpatrick AM, Teague WG. Progressive airflow limitation is a feature of children with severe asthma. J Allergy Clin Immunol. 2011;127(1):282–4. doi: 10.1016/j.jaci.2010.10.036. This small cohort study from the Severe Asthma Research Program descibes accelerated loss of lung function in children with severe asthma associated with daily symptoms and allergic sensitization. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Covar RA, Spahn JD, Murphy JR, Szefler SJ. Progression of asthma measured by lung function in the childhood asthma management program. Am J Respir Crit Care Med. 2004;170(3):234–41. doi: 10.1164/rccm.200308-1174OC. [DOI] [PubMed] [Google Scholar]
- 50**.Covar RA, Strunk R, Zeiger RS, et al. Predictors of remitting, periodic, and persistent childhood asthma. J Allergy Clin Immunol. 2010;125(2):359–66. e3. doi: 10.1016/j.jaci.2009.10.037. This article from the Childhood Asthma Management Program found that allergic sensitization and exposure, low lung function, and airway hyperresponsiveness are associated with asthma persistence in childhood. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rasmussen F, Taylor DR, Flannery EM, et al. Risk factors for airway remodeling in asthma manifested by a low postbronchodilator FEV1/vital capacity ratio: a longitudinal population study from childhood to adulthood. Am J Respir Crit Care Med. 2002;165(11):1480–8. doi: 10.1164/rccm.2108009. [DOI] [PubMed] [Google Scholar]
- 52.Sears MR, Greene JM, Willan AR, et al. A longitudinal, population-based, cohort study of childhood asthma followed to adulthood. N Engl J Med. 2003;349(15):1414–22. doi: 10.1056/NEJMoa022363. [DOI] [PubMed] [Google Scholar]
- 53.Horak E, Lanigan A, Roberts M, et al. Longitudinal study of childhood wheezy bronchitis and asthma: outcome at age 42. BMJ. 2003;326(7386):422–3. doi: 10.1136/bmj.326.7386.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Phelan PD, Robertson CF, Olinsky A. The Melbourne Asthma Study: 1964–1999. J Allergy Clin Immunol. 2002;109(2):189–94. doi: 10.1067/mai.2002.120951. [DOI] [PubMed] [Google Scholar]
- 55.Strunk RC, Weiss ST, Yates KP, et al. Mild to moderate asthma affects lung growth in children and adolescents. J Allergy Clin Immunol. 2006;118(5):1040–7. doi: 10.1016/j.jaci.2006.07.053. [DOI] [PubMed] [Google Scholar]
- 56.Regamey N, Ochs M, Hilliard TN, et al. Increased airway smooth muscle mass in children with asthma, cystic fibrosis, and non-cystic fibrosis bronchiectasis. Am J Respir Crit Care Med. 2008;177(8):837–43. doi: 10.1164/rccm.200707-977OC. [DOI] [PubMed] [Google Scholar]
- 57.Payne DN, Qiu Y, Zhu J, et al. Airway inflammation in children with difficult asthma: relationships with airflow limitation and persistent symptoms. Thorax. 2004;59(10):862–9. doi: 10.1136/thx.2003.017244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Barbato A, Turato G, Baraldo S, et al. Epithelial damage and angiogenesis in the airways of children with asthma. Am J Respir Crit Care Med. 2006;174(9):975–81. doi: 10.1164/rccm.200602-189OC. [DOI] [PubMed] [Google Scholar]
- 59**.Baraldo S, Turato G, Bazzan E, et al. Noneosinophilic asthma in children: relation with airway remodelling. Eur Respir J. 2011;38(3):575–83. doi: 10.1183/09031936.00168210. This study found evidence of airway remodeling in children with non-eosinophilic asthma that was not significantly different from that observed in children with eosinophilic asthma. [DOI] [PubMed] [Google Scholar]
- 60.Saglani S, Payne DN, Zhu J, et al. Early detection of airway wall remodeling and eosinophilic inflammation in preschool wheezers. Am J Respir Crit Care Med. 2007;176(9):858–64. doi: 10.1164/rccm.200702-212OC. [DOI] [PubMed] [Google Scholar]
- 61.Saglani S, Malmstrom K, Pelkonen AS, et al. Airway remodeling and inflammation in symptomatic infants with reversible airflow obstruction. Am J Respir Crit Care Med. 2005;171(7):722–7. doi: 10.1164/rccm.200410-1404OC. [DOI] [PubMed] [Google Scholar]
- 62**.Tsartsali L, Hislop AA, McKay K, et al. Development of the bronchial epithelial reticular basement membrane: relationship to epithelial height and age. Thorax. 2011;66(4):280–5. doi: 10.1136/thx.2010.149799. This study found that the reticular basement membrane was microscopically identifiable by 30 weeks gestation and thickened further in early childhood and adolescence. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Barbato A, Turato G, Baraldo S, et al. Airway inflammation in childhood asthma. Am J Respir Crit Care Med. 2003;168(7):798–803. doi: 10.1164/rccm.200305-650OC. [DOI] [PubMed] [Google Scholar]
- 64**.Malmstrom K, Pelkonen AS, Malmberg LP, et al. Lung function, airway remodelling and inflammation in symptomatic infants: outcome at 3 years. Thorax. 2011;66(2):157–62. doi: 10.1136/thx.2010.139246. This study found that infants with decreased pulmonary function, increased reticular basement membrane thickening, and increaed mucosal mast cells had more asthma symptoms and greater inhaled asthma medication requirements at 3 years of age. [DOI] [PubMed] [Google Scholar]
- 65.Hauk PJ, Krawiec M, Murphy J, et al. Neutrophilic airway inflammation and association with bacterial lipopolysaccharide in children with asthma and wheezing. Pediatr Pulmonol. 2008;43(9):916–23. doi: 10.1002/ppul.20880. [DOI] [PubMed] [Google Scholar]
- 66.Marguet C, Bocquel N, Benichou J, et al. Neutrophil but not eosinophil inflammation is related to the severity of a first acute epidemic bronchiolitis in young infants. Pediatr Allergy Immunol. 2008;19(2):157–65. doi: 10.1111/j.1399-3038.2007.00600.x. [DOI] [PubMed] [Google Scholar]
- 67.Marguet C, Jouen-Boedes F, Dean TP, Warner JO. Bronchoalveolar cell profiles in children with asthma, infantile wheeze, chronic cough, or cystic fibrosis. Am J Respir Crit Care Med. 1999;159(5 Pt 1):1533–40. doi: 10.1164/ajrccm.159.5.9805028. [DOI] [PubMed] [Google Scholar]
- 68.Saglani S, Molyneux C, Gong H, et al. Ultrastructure of the reticular basement membrane in asthmatic adults, children and infants. Eur Respir J. 2006;28(3):505–12. doi: 10.1183/09031936.06.00056405. [DOI] [PubMed] [Google Scholar]
- 69.Fitzpatrick AM, Teague WG, Holguin F, et al. Airway glutathione homeostasis is altered in children with severe asthma: evidence for oxidant stress. J Allergy Clin Immunol. 2009;123(1):146–52. e8. doi: 10.1016/j.jaci.2008.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70**.Fitzpatrick AM, Teague WG, Burwell L, et al. Glutathione oxidation is associated with airway macrophage functional impairment in children with severe asthma. Pediatr Res. 2011;69(2):154–9. doi: 10.1203/PDR.0b013e3182026370. This substudy from the Severe Asthma Research Program found that children with severe asthma have oxidation of the antioxidant, glutathione, which is associated with impaired airway macrophage function including apoptosis and phagocytosis of infectious particles. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fitzpatrick AM, Brown LA, Holguin F, Teague WG. Levels of nitric oxide oxidation products are increased in the epithelial lining fluid of children with persistent asthma. J Allergy Clin Immunol. 2009;124(5):990–6. e1–9. doi: 10.1016/j.jaci.2009.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72**.Greenwald R, Fitzpatrick AM, Gaston B, et al. Breath formate is a marker of airway S-nitrosothiol depletion in severe asthma. PLoS One. 2010;5(7):e11919. doi: 10.1371/journal.pone.0011919. This study found that exhaled breath condensate concentrations of formate were higher in children with severe versus non-severe asthma, suggestive of increased catabolism of S-nitrosothiols. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fitzpatrick AM, Holguin F, Teague WG, Brown LA. Alveolar macrophage phagocytosis is impaired in children with poorly controlled asthma. J Allergy Clin Immunol. 2008;121(6):1372–8. 8 e1–3. doi: 10.1016/j.jaci.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74**.Fitzpatrick AM, Higgins M, Holguin F, et al. The molecular phenotype of severe asthma in children. J Allergy Clin Immunol. 2010;125(4):851–7. e18. doi: 10.1016/j.jaci.2010.01.048. This substudy from the Severe Asthma Research Program found that children with severe asthma have a unique airway molecular phenotype that is not clearly associated with a Th1 or Th2 phenotype. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75**.Fitzpatrick AM, Stephenson ST, Hadley GR, et al. Thiol redox disturbances in children with severe asthma are associated with posttranslational modification of the transcription factor nuclear factor (erythroid-derived 2)-like 2. J Allergy Clin Immunol. 2011;127(6):1604–11. doi: 10.1016/j.jaci.2011.03.031. This study found that children with severe asthma have impaired activation of the transcription factor Nrf2 associated with glutathione depletion and increased oxidant stress. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Haldar P, Pavord ID, Shaw DE, et al. Cluster analysis and clinical asthma phenotypes. Am J Respir Crit Care Med. 2008;178(3):218–24. doi: 10.1164/rccm.200711-1754OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77**.Fitzpatrick AM, Teague WG, Meyers DA, et al. Heterogeneity of severe asthma in childhood: Confirmation by cluster analysis of children in the National Institutes of Health/National Heart, Lung, and Blood Institute Severe Asthma Research Program. J Allergy Clin Immunol. 2011;127(2):382–9. e13. doi: 10.1016/j.jaci.2010.11.015. This article describes the results of an unbiased cluster analysis of children enrolled in the Severe Asthma Research Program and identified four distinct phenotypic clusters differentiated by lung function, the age of asthma onset, and controller medication use. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention. 2009 [updated 2009; cited]; Available from: www.ginasthma.org.
- 79.Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007. J Allergy Clin Immunol NHLBI. 2007;120(5 Suppl):S94–138. doi: 10.1016/j.jaci.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 80*.Hamburg MA, Collins FS. The path to personalized medicine. N Engl J Med. 2010;363(4):301–4. doi: 10.1056/NEJMp1006304. This article reviews obstacles to personalized medicine and strategies that have been put into place to help promote its use in the clinical setting. [DOI] [PubMed] [Google Scholar]
- 81*.Szefler SJ. Is it time to revise the asthma guidelines? J Allergy Clin Immunol. 2011 Sept 22; doi: 10.1016/j.jaci.2011.09.004. [Epub ahead of print] This article reviews the development of the National Asthma Education and Prevention Program’s asthma treatment guidelines and current knowledge gaps remaining related to the care of patients with asthma. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82*.Corren J, Lemanske RF, Hanania NA, et al. Lebrikizumab Treatment in Adults with Asthma. N Engl J Med. 2011;365(12):1088–98. doi: 10.1056/NEJMoa1106469. This clinical trial found that lebrikizumab, an anti-IL-13 monoclonal antibody, improved lung function in adult patients with poorly controlled asthma despite high-dose ICS therapy. [DOI] [PubMed] [Google Scholar]
- 83*.Lemanske RF, Jr, Mauger DT, Sorkness CA, et al. Step-up therapy for children with uncontrolled asthma receiving inhaled corticosteroids. N Engl J Med. 2010;362(11):975–85. doi: 10.1056/NEJMoa1001278. This clinical trial found that for children with asthma that was poorly controlled despite low-dose ICS, LABA step up was more likely to provide the best response compared to higher-dose ICS or LTRA, although overall responses were highly differential. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84*.Peters SP, Kunselman SJ, Icitovic N, et al. Tiotropium bromide step-up therapy for adults with uncontrolled asthma. N Engl J Med. 2010;363(18):1715–26. doi: 10.1056/NEJMoa1008770. This clinical trial found that triotrium added to ICS improved symptoms and lung function in adult patients with asthma poorly controlled on low doses of ICS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85*.Rabinovitch N, Reisdorph N, Silveira L, Gelfand EW. Urinary leukotriene E4 levels identify children with tobacco smoke exposure at risk for asthma exacerbation. J Allergy Clin Immunol. 2011;128(2):323–7. doi: 10.1016/j.jaci.2011.05.035. This study found that children with second-hand tobacco smoke exposure with severe asthma exacerbations were differentiated by high urinary leukotriene levels. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86*.Rabinovitch N, Graber NJ, Chinchilli VM, et al. Urinary leukotriene E4/exhaled nitric oxide ratio and montelukast response in childhood asthma. J Allergy Clin Immunol. 2010;126(3):545–51. e1–4. doi: 10.1016/j.jaci.2010.07.008. This study found that the ratio of urinary leukotriene E4 to exhaled nitric oxide predicted asthmatic children with a better response to montelukast than fluticasone. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87**.Gupta A, Sjoukes A, Richards D, et al. Relationship Between Serum Vitamin D, Disease Severity and Airway Remodeling in Children with Asthma. Am J Respir Crit Care Med. 2011 Sept 15; doi: 10.1164/rccm.201107-1239OC. [Epub ahead of print] This study found that low serum 25-hydroxyvitamin D (25[OH]D3) was associated with increased symptoms, lower pulmonary function, more exacerbations and asthma medication requirements, and increased airway smooth muscle mass. [DOI] [PMC free article] [PubMed] [Google Scholar]