Abstract
The primary objective of this study was to discover one or more clusters of compounds which are not equitoxic but display cytoselectivity toward different malignant cells. Furthermore a most important consideration is that such molecules should also display greater cytotoxic potencies to tumors than normal tissues. Two series of compounds are described which meet these criteria, namely the 1-aryl-2-dimethylaminomethyl-2-propen-1-one hydrochlorides 1a–e and 1-aryl-3-dimethylamino-2-hydroxymethyl-1-propanone hydrochlorides 2a–e. A number of these compounds possess marked cytotoxic potencies (IC50 and CC50 values within the 10−6 and 10−7 molar range) which are greater than these of the reference drug melphalan. Statistical analyses demonstrated that cytotoxic potencies are influenced by the size of the aryl substituents in series 1 and to some extent by the electronic properties of the aryl groups in series 2. The mode of action of a representative compound 1e in HL-60 cells included inducing apoptosis and activation of caspases –3, –8, and –9.
Keywords: 1-Aryl-3-dimethylamino-1-propanones, Cytotoxicity, Apoptosis, Caspases
1. Introduction
One of the major problems involved in treating different cancers with drugs is the lack of their demonstrating appreciably greater toxicity to malignancies than normal cells. Hence novel groups of molecules are required which are not general biocidal agents displaying equi-toxicity but vary considerably in their potencies toward different cells. In particular, these compounds should provide unequivocal evidence of being more cytotoxic to neoplastic cells rather than normal tissues. The objective of this report is to disclose that the 1-aryl-2-dimeth-ylaminomethyl-2-propen-1-one hydrochlorides 1 and the related adducts 2 are novel clusters of compounds with selective toxicity to tumors.
The reasons for preparing series 1 and the related adducts 2 and 3 included the following general considerations. First, conjugated styryl ketones are designed as thiol alkylators having little or no capacity to interact with the amino or hydroxy groups of cellular constituents.1–3 Consequently these molecules may be devoid of the genotoxic properties of a number of currently available anticancer drugs4 since thiol groups are absent in nucleic acids. This concept of thiol-specificity represents a markedly different approach in the design of putative anticancer drugs than is often followed. Second, this novel design may lead to cytotoxics which are not cross-resistant to contemporary anticancer medication. Support for this contention comes from the observation that several drug resistant cell lines were free from cross-resistance to a series of Mannich bases of conjugated styryl ketones.5 Third, since enones may undergo indiscriminate thiol alkylation prior to reaching a target site, a pro-drug approach was incorporated into the project.
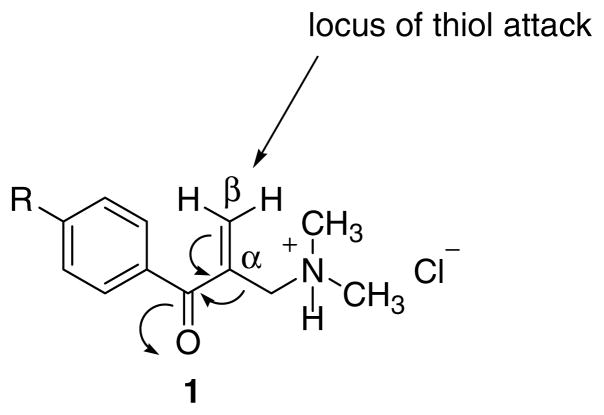
The specific reasons for the design of the compounds in series 1 were to create a series of molecules having the following structural features, namely (1) a conjugated enone group attached to an aryl ring, (2) a sterically unhindered β carbon atom where interactions with thiols take place, and (3) an amino group located close to the α,β-unsaturated keto pharmacophore. The reasons for the last molecular component are as follows. First, protonation of the basic center leading to a quadrivalent nitrogen atom will increase the electrophilicity to thiols as illustrated in Figure 1. Second, on occasions the extracellular pH around tumor cells may be low6,7 and the pH of neoplasms may be lower than in normal tissues.8 Since the ratio of ions to free base is dependent on the pH of the medium,9 the percentage of ions can be greater, close to or in the malignant cells which may lead to preferential toxicity to neoplasms. Third, the amine hydrochloride portion of 1–3 confers drug-likeness and increases water solubility.
Figure 1.
Design of the thiol alkylator 1.
In the light of these considerations, a twofold strategy was adopted, namely Phase I (an initial examination to ascertain whether some preliminary data warranted proceeding to Phase II) and Phase II (a detailed investigation of whether the compounds display cytoselectivity and a probing of the mode of action of these novel cytotoxins). The Phase I study has been completed.10 The compounds were prepared as follows. Condensation of the appropriate arylethanone with dimethylamine hydrochloride and excess of formaldehyde led to the formation of 1a–e. In the presence of water, the Mannich bases 1a–e were converted into the corresponding aminoalcohols 2a–e while reaction of 1c with 2-mercap-toethanol gave 3. The IC50 values of the compounds in the series 1 and 2 toward human WiDr colon cancer cells were in the low micromolar range. On the other hand, 3 has very low potency (IC50 = 311 μM) and further analogs in series 3 were not prepared. A stability study revealed that 2c but not 3 reverted to 1c in solution. The purpose of the current report is to reveal the results of the Phase II investigation in which the selective toxicity of various compounds to neoplastic cells is clearly demonstrated as well as an indication of the means whereby bioactivity is mediated (Fig. 2).
Figure 2.
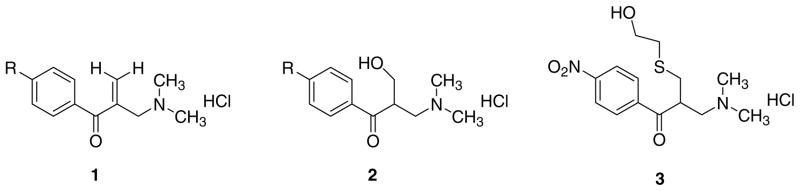
Structures of series 1–3. The aryl substituents in series 1 and 2 are as follows, namely (a) R = H; (b) R = Cl; (c) R = NO2; (d) R = CH3: (e) R = OCH3.
2. Bioevaluations
All of the compounds in series 1–3 were evaluated against transformed human CEM and Molt 4/C8 T-lymphocytes. In addition, these compounds were assessed against murine L1210 leukemic cells. These data are presented in Table 1. Six representative compounds 1a, b, e, 2b, e, and 3 were evaluated against approximately 54 human tumor cell lines representing different types of cancers. The cytotoxicity against these neoplasms, and in particular toward leukemic cell lines, is summarized in Table 2. The cytotoxic potencies of 1a–e, 2a–e, and 3 were also assessed using the following human neoplastic cells lines, namely HL-60 promyelocytic leukemic cells and HSC-2 and HSC-4 squamous cell carcinomas. In addition, evaluation toward non-malignant human HGF gingival fibroblasts, HPLF periodontal ligament fibroblasts and HPC pulp cells was undertaken. These results are portrayed in Table 3. A representative compound 1e caused apoptosis in HL-60 cells as indicated in Figure 3. In addition, 1e activated caspases-3, –8 and –9 in HL-60 cells and caspase-3 in HSC-2 carcinomas which are presented in Figure 3.
Table 1.
Evaluation of series 1–3 against human CEM and Molt 4/C8 T-lymphocytes and murine L1210 leukemic cells
| Compound | IC50 (μM)a
|
SIb | IC50 (μM)a | |
|---|---|---|---|---|
| CEM | Molt 4/C8 | L1210 | ||
| 1a | 65.6 ± 33.8 | 146 ± 91 | 2.2 | 52.2 ± 15.8 |
| 1b | 32.6 ± 7.0 | 30.2 ± 19.0 | 1.1 | 26.5 ± 21.7 |
| 1c | 7.91 ± 0.53 | 8.95 ± 0.20 | 1.1 | 8.61 ± 1.85 |
| 1d | 11.4 ± 1.9 | 35.5 ± 7.4 | 3.1 | 11.1 ± 0.9 |
| 1e | 10.6 ± 8.5 | 21.3 ± 14.0 | 2.0 | 5.08 ± 4.52 |
| 2a | 1.42 ± 0.07 | 5.84 ± 1.89 | 4.1 | 1.65 ± 0.10 |
| 2b | 33.1 ± 19.5 | 30.9 ± 18.6 | 1.1 | 22.0 ± 18.5 |
| 2c | 12.2 ± 0.0 | 36.8 ± 2.6 | 3.0 | 42.6 ± 1.7 |
| 2d | 8.22 ± 2.28 | 21.0 ± 13.0 | 2.6 | 6.55 ± 4.29 |
| 2e | 1.15 ± 0.03 | 4.90 ± 2.19 | 4.3 | 1.57 ± 0.05 |
| 3 | >500 | >500 | ~1.0 | >500 |
| Melphalanc | 2.47 ± 0.03 | 3.24 ± 0.79 | 1.3 | 2.13 ± 0.03 |
The IC50 value is the concentration of compound required to inhibit the growth of the cells by 50%.
The letters SI refer to the selectivity index of each compound which is the ratio of the highest to lowest IC50 values of each compound toward the two T-lymphocytes.
The data for melphalan is taken from Ref. 23 copyright (2006) with permission of Elsevier.
Table 2.
Evaluation of 1a, b, e, 2b, e, 3, and melphalan against a panel of human tumor cell linesa
| Compound | All cell lines
|
Leukemic cell lines, IC50 (μM)b
|
SId | |||||
|---|---|---|---|---|---|---|---|---|
| Average GI50 (μM)b | SIc | CCRF-CEM | K562 | RPMI-8226 | SR | Average | ||
| 1a | 7.94 | 67.6 | 0.40 | 2.19 | 1.91 | 0.55 | 1.26 | 6.3 |
| 1b | >20.4 | >30.2 | 3.31 | 3.47 | — | 3.98 | 3.59 | >5.7 |
| 1e | >10.2 | >269 | 0.37 | 1.05 | 21.4 | 14.5 | 9.33 | >1.1 |
| 2b | 4.68 | 251 | 0.32 | 0.33 | — | 0.63 | 0.43 | 10.9 |
| 2e | 4.57 | 2512 | — | 0.49 | 20.0 | — | 10.3 | 0.4 |
| 3 | >97.7 | >2.63 | >100 | >100 | 38.0 | >100 | >84.5 | ~1.2 |
| Melphalan | 26.9 | 118 | 6.17 | 43.7 | 66.1 | 1.86 | 29.5 | 0.9 |
The compounds were evaluated against several groups of human tumors including leukemia.
The GI50 and IC50 values refer to the quantities of compounds required to inhibit 50% of the growth of the cells as explained in the text.
The selectivity index (SI) figures are the differences between the highest and lowest GI50 values.
The selectivity index (SI) figures refer to the quotients of the average GI50 value of all the cell lines and the average IC50 figure for leukemic cells.
Table 3.
Evaluation of 1a–e, 2a–e, 3, and melphalan against normal and malignant cells
| Compound | Normal cells, CC50 (μM)a
|
Tumor cells, CC50 (μM)a
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HGF | HPC | HPLF | ave | HL-60 | SIb | HSC-2 | SIb | HSC-4 | SIb | |
| 1a | 26 | 31 | 52 | 36 | 12 | 3.0 | 29 | 1.2 | 33 | 1.1 |
| 1b | 6.1 | 6.0 | 9.2 | 7.1 | <3.1 | >2.3 | 5.1 | 1.4 | 6.4 | 1.1 |
| 1c | 19.3 | 30 | 26 | 25 | 1.6 | 16 | 8.2 | 3.1 | 19 | 1.3 |
| 1d | 6.1 | 6.9 | 8.1 | 7.0 | 0.45 | 16 | 3.4 | 2.1 | 7.6 | 0.9 |
| 1e | 6.8 | 5.1 | 9.2 | 7.0 | <3.4 | >2.1 | 5.9 | 1.2 | 6.1 | 1.2 |
| 2a | 4.4 | 5.5 | 5.2 | 5.0 | 0.41 | 12 | 5.2 | 1.0 | 6.5 | 0.8 |
| 2b | 5.8 | 5.4 | 12 | 7.7 | <3.1 | >2.5 | 6.0 | 1.3 | 8.0 | 1.0 |
| 2c | 271 | 227 | 176 | 225 | 35 | 6.4 | 73 | 3.1 | 99 | 2.3 |
| 2d | 4.9 | 5.7 | 9.8 | 6.8 | <3.1 | >2.2 | 6.3 | 1.1 | 5.8 | 1.2 |
| 2e | 4.0 | 5.3 | 4.9 | 4.7 | 0.46 | 10 | 2.5 | 1.9 | 5.2 | 0.9 |
| 3 | 390 | 400 | 400 | 397 | 353 | 1.1 | 165 | 2.4 | 264 | 1.5 |
| Melphalanc | >200 | >200 | >200 | >200 | 6.0 | >33 | 35 | >5.7 | 81 | >2.5 |
The CC50 value is the concentration of the compound required to kill 50% of the cells. The figures quoted are the average of two independent determinations which differed by less than 5%. The highest concentrations employed were 400 μM except solubility considerations precluded the use of more than 200 μM of melphalan.
The letters SI refer to the selectivity index which was obtained by dividing the average CC50 value for the three normal cells by the CC50 figure of each malignant cell line.
The data for melphalan was taken from Ref. 21 copyright (2007) with permission of Elsevier.
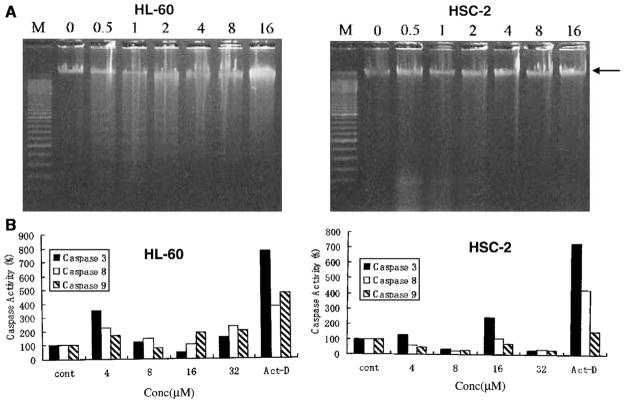
Figure 3.
(A) Evaluation of the effects of 1e in HL-60 and HSC-2 cells (5 × 105 cells) on DNA fragmentation after incubation for 6 h with the concentration indicated (in μM). M is the molecular weight marker of DNA. The arrow indicates a large DNA fragment. Reproducible results were obtained when repeating the experiment. (B) The effect of 1e on the activation of caspases in neoplastic cells (4 × 104 cells) after incubation for 4 h. Each value is the mean of two or three experiments.
3. Discussion
All of the compounds 1–3 were evaluated toward CEM and Molt 4/C8 T-lymphocytes in order to discern the ability of these compounds to inhibit the growth of transformed cells of human origin. In addition, many anticancer drugs are effective toward murine L1210 cells11 and therefore this bioassay was employed with a view to detecting promising lead molecules. The data are presented in Table 1. In particular, the potencies of 2a and 2e having IC50 values of <2 μM in two-thirds of the screens as well as 1c and 2d with IC50 figures below 10 μM in most of the assays are noteworthy.
The different cytotoxic potencies of 1a–e may have been caused by the variation in the electronic, hydrophobic and steric properties of the aryl substituents. Accordingly linear and semilogarithmic plots were made between the IC50 values of 1a–e in each screen and the Hammett sigma, Hansch pi, and molar refractivity (MR) constants of the aryl group. Negative correlations were noted between the MR values of the aryl substituents and the IC50 figures in the CEM, Molt4/C8 and L1210 bioassays (p < 0.05).Thus in developing series 1, groups with greater size than the aryl substituents employed in 1a–e should be used. A similar analysis was undertaken with 2a–e which revealed a positive correlation between the σ constants and the IC50 values in the L1210 screen. This observation reveals that in the future the placement of electron releasing groups in the aryl ring in analogs of 2a–e should lead to compounds with greater cytotoxic properties. No other correlations were noted (p > 0.05).
In view of the important contribution of steric factors to cytotoxic potencies in series 1, consideration was given to the possibility that the topography of the aryl ring may also affect the magnitude of the IC50 values. Accordingly the torsion angles (θ) between the aryl rings and the adjacent carbonyl group were calculated. In the case of 1a–e, the θ figures are –73.1, –69.5, –59.5, –64.4, and –67.4, respectively. Linear and semilogarithmic plots between these figures and the IC50 values revealed a negative correlation in the CEM test (p < 0.05). Hence, the insertion of bulky substituents in the ortho and meta positions of the aryl rings when creating further analogs of series 1 may lead to compounds with increased potencies. The θ values of 2a–e are 17.5, 24.3, 26.8, 30.7, and 15.7, respectively, but no correlations between these figures and cytotoxic potencies were noted (p > 0.05) confirming that no evidence was generated that steric factors contribute significantly to bioactivity in series 2.
The question of whether masking the enone structure of 1a–e by hydration yielding 2a–e led to greater cytotoxic potencies was addressed by comparing the IC50 values of compounds bearing the same aryl substituents in each screen. Thus 1a was compared with 2a in the CEM assay, then in the Molt 4/C8 test and finally in the L1210 screen and so forth. Standard derivations were taken into account. The results indicated that 2a > 1a and 1c > 2c in all three bioassays while 2e > 1e when considering CEM and Molt 4/C8 T-lymphocytes. In the remaining cases equal potencies were observed. Hence both the nature of the aryl ring and the general structure of the series of the compounds need to be considered when developing these compounds.
A comparison was made between the IC50 values of the most potent compounds with the alkylating agent melphalan which is used in cancer chemotherapy. In the CEM, Molt 4/C8 and L1210 assays, 2a possesses 1.7, 0.6, and 1.3 times the potencies of melphalan, respectively, while the relevant figures for 2e are 2.2, 0.7, and 1.4, respectively. Clearly both of these aminoalcohols are lead molecules which should be developed further. Other compounds with 25% or more of the potencies of melphalan are 1c (in all three screens), 1e (L1210 assay), and 2d (CEM and L1210 tests).
The biodata in Table 1, and also Tables 2 and 3 vide in-fra, confirm the lack of cytotoxic potency of 3. However, the concept of activated soft compounds has been proposed which refers to the combination of a bioactive molecule to an inert carrier moiety.12 Thus the attachment of a pharmacophore to 3 such as esterification of a cytotoxic acid via the hydroxy group of 3 may be a worthwhile venture in the future.
An important feature of antineoplastic agents is their ability to display preferential cytotoxicity toward malignant cells rather than normal tissues. Such a property would be displayed by compounds which are not general biocidal agents and therefore exert different potencies in bioassays. Thus a comparison between the cytotoxicity of the compounds toward transformed human CEM and Molt 4/C8 cells was undertaken. The selectivity index (SI) values of each compound were calculated using the ratio of the higher and lower IC50 figures and these data are presented in Table 1. Compounds in which the difference in IC50 values were more than doubled (SI > 2) were noted, namely 1a, d, and all members of series 2 except 2b. This result compares favourably with melphalan with a SI figure of 1.3.
Compounds 1a, b, e, 2b, e, and 3 were evaluated against approximately 54 human tumor cell lines which were derived from the following groups of neoplasms namely leukemia, melanoma and non-small cell lung, colon, central nervous system, ovarian, renal and prostate cancers; with the exception of 1a, all compounds were also evaluated using various malignant breast cells.13 In this determination the quantity of compound required to inhibit the growth of the cells by 50% is computed. However in those cases where 50% inhibition of growth was not achieved at the highest concentration, namely 100 μM, the figure of 100 μM was used in determining the average inhibitory concentration. Hence, the term GI50 rather than IC50 is utilized.
The biodata are presented in Table 2. The average IC50 figures reveal that the compounds in series 1 and 2 are potent cytotoxins in general. Of particular interest are the two compounds in series 2 with GI50 figures of less than 5 μM. Examination of the mean graphs14 revealed that most of the compounds displayed a greater toxicity to leukemic cells than other cell lines. With the exception of 3, the potencies toward various leukemic cells are noteworthy; specifically 2b has submicromolar IC50 values and 1a, b possess IC50 figures of less than 5 μM. All of the compounds in series 1 and 2 demonstrated greater antileukemic properties than melphalan. These evaluations confirm the cytotoxic properties of series 1 and 2. In regard to selective toxicity for leukemic cells, impressive SI values were obtained for 1a, 1b and in particular 2b.
This preferential toxicity for certain neoplastic cells which have been observed may translate into a display for selective toxicity to malignant rather than normal cells. This possibility was addressed in the third bioassay involving both normal and malignant cells. All of the compounds in series 1–3 were evaluated against HGF, HPC, and HPLF normal cell lines as well as toward HL-60, HSC-2, and HSC-4 neoplasms. These data are presented in Table 3. The results confirm that the compounds in series 1 and 2 are potent cytotoxins toward malignant cells whereby 77% of the CC50 values are less than 10 μM. The HL-60 cells are the most sensitive to 1a–e and 2a–e and in the case of 1d, 2a, and 2e, the CC50 figures are submicromolar toward this cell line. The CC50 values of most of the compounds against HL-60, HSC-2, and HSC-4 cells are lower than melphalan. Linear and semilogarithmic plots were undertaken between the CC50 figures generated in the HL-60, HSC-2 and HSC-4 assays with the σ, π, and MR constants of the aryl substituents and the θ values of the compounds in series 1 and 2. Cytotoxic potencies were negatively correlated with the MR constants in the HSC-2 screen while in the case of series 2, a positive correlation was noted between the σ values in all three cell lines. These results are similar to the observations made in reviewing the biodata in Table 1.
The SI values are clearly dependent on the cell line under consideration. Thus the percentage of compounds which possess SI figures greater than 2 is 100, 30, and 10, respectively, when considering HL-60, HSC-2, and HSC-4 cells, respectively. Of particular note are the SI values of 10 or greater displayed by 1c, d, and 2a, e when considering the greater toxicity toward HL-60 neoplasms than normal cells. The CC50 values against the malignant cells vary considerably and in general the relative sensitivities to each chemical are HL-60 > HSC-2 > HSC-4. This observation demonstrates further the selective toxicity of these compounds which may reveal a disparity in the lethal effects to normal and neoplastic cells.
The final segment of this study was directed to obtaining some understanding of the way in which cytotoxicity was caused and why potencies to various malignant cells differed. Many cytotoxic agents cause apoptosis,15 which can be due to activation of caspases. Treatment of HL-60 cells by a representative compound 1e revealed that internucleosomal DNA was induced using 0.5, 1, and 2 μM of this Mannich base (Fig. 3A). At higher concentrations of 4, 8, and 16 μM, this phenomenon did not occur and only a smear pattern of DNA fragmentation was observed. On the other hand, there was no unequivocal evidence of DNA fragmentation using the same concentrations of 1e with HSC-2 cells. Figure 3B indicates that activation of caspases –3, –8, and –9 occurred in HL-60 cells by 1e but this process was virtually absent in HSC-2 cells. Thus 1e caused apoptotic cell death in HL-60 cells but the death of HSC-2 cells is by alternative mechanisms. These results suggest that some of the compounds in series 1 and 2 cause apoptosis. In addition, a compound may cause toxicity by different mechanisms depending on the cell line. This phenomenon may be a contributing factor to the different SI values observed in this project which further enhances the potential of these compounds for further development.
4. Conclusions
The biodata presented in Table 1 reveal that the amino-alcohols 2a and 2e are clearly lead molecules in terms of both cytotoxic potencies and SI values. When evaluations of representative compounds against a number of human tumor cell lines took place, 2b emerged as a noteworthy candidate for future development in terms of potency and selective toxicity especially to leukemic cells (Table 2). An important feature of most of these compounds in series 1 and 2 is their lethal effects toward promyelocytic leukemic HL-60 cells as the figures in Table 3 reveal. In particular 1d, 2a, and 2e have submicromolar CC50 values. Of considerable importance is the observation of the selective toxicity for HL-60 cells displayed by various molecules, especially 1c, d and 2a, e with SI figures of 10 or more. A representative compound 1e caused apoptosis in HL-60 but not HSC-2 cells. In addition, while 1e activated caspases –3, –8, and –9 in HL-60 cells, this effect was virtually absent in HSC-2 cells. This observation of different mechanisms of action is mandatory when compounds are to be developed which are tumor-specific and spare normal tissues. This study has demonstrated the need to develop these prototypic molecules in series 1 and 2. In particular the very favourable properties of 2a and 2e both in terms of cytotoxic potencies and selective toxicity to different cells should be noted.
5. Experimental protocols
5.1. Chemistry
5.1.1. Synthesis of compounds
The compounds in series 1–3 were prepared by the methodologies described previously.10
5.1.2. Molecular modeling
Molecular modeling used a BioMedCache programme.16
5.1.3. Statistical analyses
The Hammett sigma, Hansch pi, and molar refractivity constants were obtained from the literature.17 The linear and semilogarithmic plots were made using a commercial statistics package.18 The significant p values (<0.05) that are generated from the data presented in Table 1 when the IC50 values of the compounds in series 1 were plotted against various physicochemical parameters are as follows [bioassay, physical constant, linear(l) or semilogarithmic(sl) plot in parentheses]: 0.023 (CEM, MR, l), 0.005 (Molt4/C8, MR, l), 0.025 (Molt4/C8, MR, sl), 0.015 (L1210, MR, l), 0.046 (L1210, MR, sl), 0.040 (CEM, θ, sl). In the case of series 2, the relevant figure is as follows: 0.009 (L1210, σ, l).
The related p value (<0.05) obtained from the CC50 figures of the compounds in series 1 evaluated against HL-60, HSC-2, and HSC-4 cells is as follows: 0.045 (HSC-2, MR, l). In the case of series 2, the relevant figures are as follows: 0.035 (HL-60, σ, l), 0.035 (HSC-2, σ, l), 0.019 (HSC-2, σ, sl), 0.036 (HSC-4, σ, l), 0.017 (HSC-4, σ, sl).
5.2. Bioassays
5.2.1. Evaluation of 1a–e, 2a–e, and 3 against transformed CEM and Molt 4/C8 T-lymphocytes and murine L1210 leukemic cells
The methodology for these bioassays using CEM, Molt 4/C8 and L1210 cells has been described previously.19 In brief, different concentrations of each compound were incubated at 37 °C for 72 h (CEM and Molt 4/C8 T-lymphocytes) or 48 h (L1210 cells).
5.2.2. Examination of 1a, b, e, 2b, e, and 3 against approximately 54 human tumor cell lines
A literature procedure was utilized when assaying various compounds against a number of human tumor cell lines.13 The concentrations of the compounds were 10−8–10−4 M except in the case of melphalan quantities of 10−7.6–10−3.6 M were employed. The number of cell lines whose growth was not inhibited by 50% at the maximum concentration of 10−4 M was not achieved/ total number of cell lines examined were 5/53 (1b), 4/49 (1e), and 54/55 (3).
5.2.3. Evaluation of the compounds in series 1–3 against normal and malignant human tumor cell lines
The methodology used in assessing the cytotoxicity of various compounds to the normal HGF, HPC, and normal HPLF cell lines as well as neoplastic HL-60, HSC-2, and HSC-4 cells has been described previously20 and recently summarized.21
5.2.4. Evaluation of the ability of 1e to cause apoptosis and activate caspases in HSC-2 and HL-60 cells
The methodology of evaluating whether apoptosis and activation of caspases –3, –8, and –9 occurs have been previously described22 and summarized.21
Acknowledgments
The authors thank the Canadian Institutes of Health Research for an operating grant to J.R. Dimmock and the Ministry of Education, Science, Sports and Culture of Japan for a Grant-in-Aid (No. 14370607) to H. Sakagami. The Flemish Fonds voor Wetenschappelijk Onderzoek provided funds to J. Balzarini and E. De Clercq who thank Mrs. L. van Berckelaer for undertaking the CEM, Molt 4/C8, and L1210 assays. The U.S. National Cancer Institute kindly generated the biodata which are presented in Table 2.
References and notes
- 1.Pati HN, Das U, Sharma RK, Dimmock JR. Mini-Rev Med Chem. 2007;7:131–139. doi: 10.2174/138955707779802642. [DOI] [PubMed] [Google Scholar]
- 2.Mutus B, Wagner JD, Talpas CJ, Dimmock JR, Phillips OA, Reid RS. Anal Biochem. 1989;177:237–243. doi: 10.1016/0003-2697(89)90045-6. [DOI] [PubMed] [Google Scholar]
- 3.Dimmock JR, Raghavan SK, Logan BM, Bigam GE. Eur J Med Chem. 1983;18:248–254. [Google Scholar]
- 4.Okey AB, Harper PA. In: Principles of Medical Pharmacology. 7. Kalant H, Grant DM, Mitchell J, editors. Elsevier; Canada, Toronto: 2007. p. 902. [Google Scholar]
- 5.Dimmock JR, Kumar P, Quail JW, Pugazhenthi U, Yang J, Chen M, Reid RS, Allen TM, Kao GY, Cole SPC, Batist G, Balzarini J, De Clercq E. Eur J Med Chem. 1995;30:209–217. [Google Scholar]
- 6.Ojugo ASE, McSheehy PMJ, McIntyre DJO, McCoy C, Stubbs M, Leach MO, Judson IR, Griffiths JR. NMR Biomed. 1999;12:495–504. doi: 10.1002/(sici)1099-1492(199912)12:8<495::aid-nbm594>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 7.Stubbs M, McSheey PMJ, Griffiths JR, Bashford CL. Mol Med Today. 2000;6:15–19. doi: 10.1016/s1357-4310(99)01615-9. [DOI] [PubMed] [Google Scholar]
- 8.Van den Berg AP, Wike-Hooley JL, Van den Berg-Blok AE, Van der Zee J, Reinhold HS. Eur J Cancer Clin Oncol. 1982;18:457–462. doi: 10.1016/0277-5379(82)90114-6. [DOI] [PubMed] [Google Scholar]
- 9.Albert A. Selective Toxicity. 7. Chapman and Hall; London: 1985. pp. 642–643. [Google Scholar]
- 10.Pati HN, Das U, Ramirez-Erosa IJ, Dunlop DM, Hickie RA, Dimmock JR. Chem Pharm Bull. 2007;55:511–515. doi: 10.1248/cpb.55.511. [DOI] [PubMed] [Google Scholar]
- 11.Suffness M, Douros J. In: Methods in Cancer Research. Part A. De Vita VT Jr, Busch H, editors. XVI. Academic Press; New York: 1979. p. 84. [Google Scholar]
- 12.Bodor N. Adv Drug Res. 1984;13:255–331. [Google Scholar]
- 13.Boyd MR, Paull KD. Drug Dev Res. 1995;34:91–109. [Google Scholar]
- 14.Grever MR, Schepartz SA, Chabner BA. Semin Oncol. 1992;19:622–638. [PubMed] [Google Scholar]
- 15.Tsurusawa M, Saeki K, Fujimoto T. Int J Hematol. 1997;66:79–88. doi: 10.1016/s0925-5710(97)00583-5. [DOI] [PubMed] [Google Scholar]
- 16.BioMedCache 6.1 Windows, BioMedCache. Fujitsu America, Inc; 2003. [Google Scholar]
- 17.Hansch C, Leo AJ. Substituent Constants for Correlation Analysis in Chemistry and Biology. John Wiley and Sons; New York: 1979. p. 49. [Google Scholar]
- 18.Statistical Package for Social Sciences. SPSS for Windows, Standard Version, release 13.0. Chicago: SPSS Inc; 2004. [Google Scholar]
- 19.Baraldi PB, Nunez M, Del C, Tabrizo MA, De Clercq E, Balzarini J, Bermejo J, Estévez F, Romagnoli R. J Med Chem. 2004;47:2877–2886. doi: 10.1021/jm031104y. [DOI] [PubMed] [Google Scholar]
- 20.Motohashi N, Wakabayashi H, Kurihara T, Fukushima H, Yamada T, Kawase M, Sohara Y, Tani S, Shirataki Y, Sakagami H, Satoh K, Nakashima H, Molnár A, Spengler G, Gyémánt N, Ugocsai K, Molnár J. Phytother Res. 2004;18:212–223. doi: 10.1002/ptr.1426. [DOI] [PubMed] [Google Scholar]
- 21.Das U, Kawase M, Sakagami H, Ideo A, Shimada J, Molnár J, Baráth Z, Bata Z, Dimmock JR. Bioorg Med Chem. 2007;15:3373–3380. doi: 10.1016/j.bmc.2007.03.022. [DOI] [PubMed] [Google Scholar]
- 22.Takeuchi R, Hoshijima H, Onuki N, Nagasaka H, Chowdbury SA, Kawase M, Sakagami H. Anticancer Res. 2005;25:4037–4042. [PubMed] [Google Scholar]
- 23.Das U, Gul HI, Alcorn J, Shrivastav A, George T, Sharma RK, Nienaber RK, De Clercq E, Balzarini J, Kawase M, Kan N, Tanaka T, Tani S, Werbovetz KA, Yakovich AJ, Manavathu EK, Stables JP, Dimmock JR. Eur J Med Chem. 2006;41:577–585. doi: 10.1016/j.ejmech.2005.12.014. [DOI] [PubMed] [Google Scholar]