Abstract
The 1-[4-(2-aminoethoxy)phenylcarbonyl]-3,5-bis-(benzylidene)-4-oxopiperidines 5–8 are a novel cluster of highly potent P-glycoprotein dependent multidrug resistance (MDR) revertants. Using a concentration of 4 μg/mL, these compounds possess 11–43 times the potency of verapamil in reversing MDR in murine L-5178 lymphoma cells transfected with the human MDR1 gene. Structure–activity relationships reveal that the attachment of the N-aroyl group to various 3,5-bis(benzylidene)-4-piperidones is essential for MDR reversal to occur. In terms of potencies, the 1-piperidinyl group is the preferred terminal amine while the 4-methyl and 4-chloro substituents are the optimal groups for placement in the benzylidene aryl rings.
Keywords: Multidrug resistance; Structure1–activity relationships; Physicochemical constants; N-Aroyl-3,5-bis(benzylidene)-4-piperidones
The principal objective of our laboratory is finding chemical approaches to counteract the ravages caused by cancer. A recent emphasis has been directed to finding compounds which reverse the vexatious problem of multidrug resistance (MDR).1,2 The study described herein reveals the remarkable potencies of a novel series of P-glycoprotein (P-gp) associated MDR revertants namely the 1-[4-(2-aminoethoxy)phenylcarbonyl]-3,5-benzylidene-4-oxo-piperidines.
The problem of drug resistance occurs in many tumours and leads to an increased drug efflux from the neoplasms causing decreased intracellular drug concentrations thereby reducing the effectiveness of anticancer drugs. The mechanism of drug resistance is multifactorial but principally it is due to the overexpres-sion of P-gp, which is a member of the ABC (ATP-binding cassette) family of transporters.3 P-gp is a 170 kDa membrane protein which behaves as a drug efflux pump with a very broad spec-ificity, and appears to act from the intracellular leaflet.4 In humans, this protein is encoded by the mdr1 and mdr3 genes. While the MDR3 glycoprotein can bind to some of the substrates and inhibitors of MDR,5 the low rate of this transport process usually makes it undetectable.
A number of studies revealed the increased potencies of anti-cancer drugs when administered with MDR revertants.6,7 These chemosensitizers act in different ways, namely by binding to P-gp, inhibiting the efflux of the anticancer drugs from the tumours and reducing the binding of cytotoxins to P-gp.8,9 In addition these compounds may reduce P-gp synthesis and/or inhibit MDR gene expression. A number of MDR modulators have pronounced bioac-tivities of their own such as verapamil and cyclosporine A,10 which limit their clinical usefulness while most inhibitors of MDR are transporter substrates thus requiring high concentrations to overcome MDR.11,12 To the best knowledge of the authors, these severe limitations have resulted in there being no clinically available MDR reversal agents to date and hence such medication is urgently required.
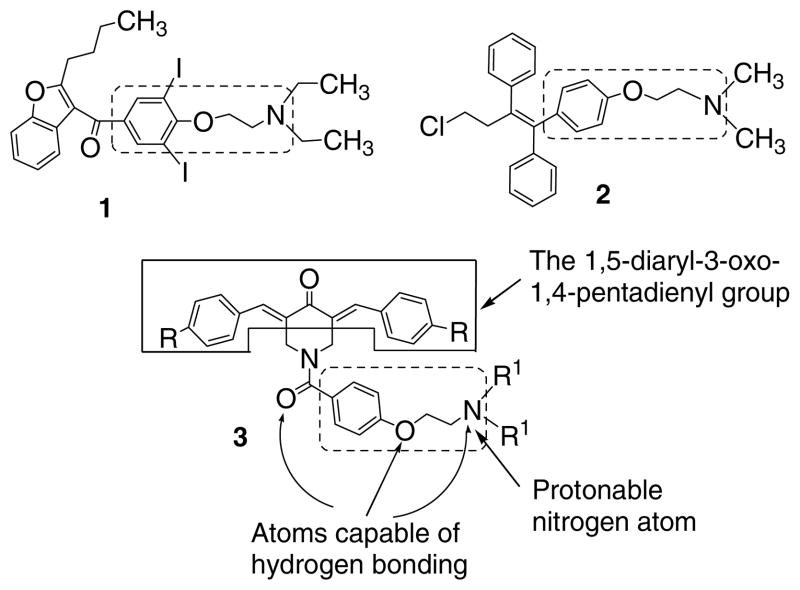
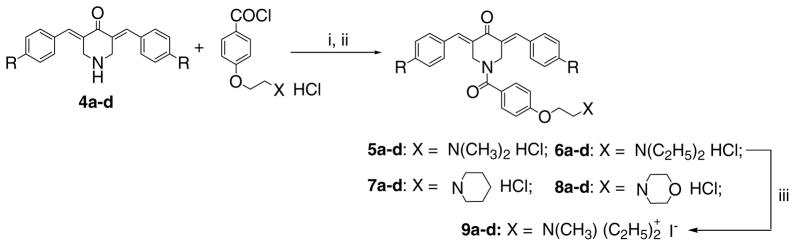
The design of a novel cluster of MDR revertants was made as follows. First, the common molecular features of a number of MDR modulators are their hydrophobicity, having two aryl rings and atoms capable of hydrogen bonding as well as bearing a positive charge at neutral pH.13,14 Second, in terms of specific groups to be incorporated into the candidate MDR revertants, previous investigations from this laboratory revealed that various compounds which contain the 1,5-diaryl-3-oxo-1,4-pentadienyl moiety reverse MDR.1,2 In addition, the 4-(2-aminoethoxy)phenyl substituent is present in the established MDR modulators amiodarone 115 and toremifene 2.16,17 These general and specific molecular features were incorporated into the design of the candidate MDR revertants, whose general structure 3 is presented in Figure 1. The decision was made to evaluate the effect of placing different R groups in the arylidene aryl rings as well as varying the basic centre X. The compounds in series 5–9 were prepared by a previously reported methodology18 and the synthetic chemical route is summarized in Figure 2. Since the 4-piperidones 4a–d possess some of the molecular features found in series 5–9, their assessment for MDR reversal properties was undertaken.
Figure 1.
The structures of amidarone 1, toremiphene 2 and the general formula 3 of the candidate MDR revertants prepared in this study. The groups within the dotted lines in structures 1–3 are the common molecular features of some MDR revertants.
Figure 2.
Synthesis of the compounds 5–9. The reaction conditions are as follows: i—CH2Cl2/N(C2H5)3, ii—HCl/ (CH3)2CHOH; iii—a, K2CO3; b, CH3I/CH3COCH3. The nature of the R groups in series 4–9 are as follows, namely a: R = H; b: R = CH3; c: R = Cl; d: R = NO2.
The compounds were assessed for MDR revertant properties using a literature method.19 This assay employed murine L-5178 lymphoma cells transfected with the human MDR1 gene and in these cells the levels of P-gp are substantially higher than in the parental cells.20 The concentrations of rhodamine 123 were measured in treated and untreated transfected and parental cells and the ratios of the fluorescence intensities are referred to as fluorescence activity ratio (FAR) values. Since MDR is due, inter alia, to an increase in the efflux of a compound from cells, a FAR value of greater than 1 indicates that reversal of MDR has taken place. All of the compounds were assessed using concentrations of 4 and 40 μg/mL and the data are presented in Table 1.
Table 1.
MDR-reversing properties in murine L-5178Y/MDR1 lymphoma cells and logP values of the compounds in series 4–9 and verapamil
| Compound | FAR valuea
|
logPb | |
|---|---|---|---|
| 4 μg/ml | 40 μg/ml | ||
| 4a | 1.58 | 2.00 | 3.38 |
| 4b | 3.26 | 4.03 | 4.25 |
| 4c | 1.25 | 0.91 | 4.71 |
| 4d | 1.04 | 0.80 | 3.27 |
| 5a | 45.5 | 51.6 | 4.60 |
| 5b | 83.7 | —c | 5.50 |
| 5c | 98.0 | 98.9 | 5.95 |
| 5d | 63.0 | 3.23 | 4.52 |
| 6a | 49.5 | 23.8 | 5.35 |
| 6b | 106 | 66.6 | 6.25 |
| 6c | 118 | 99.7 | 6.71 |
| 6d | 77.5 | 14.1 | 5.27 |
| 7a | 123 | 136 | 5.51 |
| 7b | 179 | 146 | 6.40 |
| 7c | 157 | 133 | 6.86 |
| 7d | 145 | 51.5 | 5.42 |
| 8a | 48.5 | 63.3 | 4.64 |
| 8b | 95.8 | 124 | 5.34 |
| 8c | 76.0 | 6.88 | 5.80 |
| 8d | 63.3 | 10.9 | 4.36 |
| 9a | 1.37 | 1.05 | 1.37 |
| 9b | 1.93 | 114 | 2.27 |
| 9c | 1.21 | 1.85 | 2.73 |
| 9d | 1.03 | 1.22 | 1.30 |
| Verapamil | 4.2 | —d | 4.55 |
The fluorescence activity ratio (FAR) values are the ratios of the fluorescent intensities of rhodamine 123 in treated and untreated murine L-5178Y cells transfected with human MDR1 gene.
The logP values are of the unprotonated molecules and were determined using a commercial software package.30
A concentration of 40 μg/ml of 5b was toxic to the cells.
Limitations of solubility precluded an assessment of verapamil at 40 μg/mL. The FAR value of this compound at 10 μg/mL is 5.61.
On a few occasions, the FAR values are lower at 40 μg/ml than when the lower concentration is used. This observation has been observed previously.1 A possible explanation for this phenomenon is that when the concentration of a compound is elevated, although binding to P-gp continues, other behavioural mechanisms are activated which expedite the extrusion of cellular contents. However the FAR values using 4 μg/mL will be considered since, with the exception of 8b and 9b, the higher concentration of compounds did not lead to substantial increases in the FAR data. The following observations were made from the MDR reversal experiments. First, MDR-reversal was displayed by all of the compounds in series 5–8 and the FAR values of these compounds ranged from 11 (5a) to 43 (7b) times that of the established MDR-reversing agent verapamil. These data clearly indicate that the incorporation of the 4-(2-aminoethoxy)phenyl carbonyl group into series 4 leading to series 5–8 generated a novel class of potent MDR-reversal agents. In general, the quaternary ammonium compounds 9 were inactive in this bioassay. Second, the optimal basic centre in series 5–8 was considered and the data presented in Table 1 reveals that the presence of a 1-piperidino group in series 7 is greatly preferred. Thus the average FAR values for the compounds in series 5–8 are 72.6, 87.8, 151 and 70.9, respectively. The pKa values of dimethylamine, diethylamine, piperidine and morpholine are 10.73, 10.84, 11.12 and 8.50, respectively,21 revealing that under the conditions of the bioassay, all of these compounds exist principally as ions.22 The solvent-accessible surface area (SASA) figures for the protonated dimethylamino, diethylamino, piperidino and morpholino species are 183.8, 233.0, 234.6 and 224.1, respectively.23 Thus apart from the smaller SASA figure for the dimethylamino group, neither the basicity nor the size of the amino group appears to govern the magnitude of the FAR values. However the biodata generated suggest that development of analogs of series 7 should take place in which the terminal basic group is a heterocycle containing one nitrogen atom such as hexamethyleneimine or heptamethyle-neimine. Third, the substituents in the arylidene aryl rings were examined in terms of MDR-reversing potencies. In each of the series 5–8, greater potencies were noted with the compounds bearing 4-methyl and 4-chloro substitutents. This observation may have been due to the π and molecular refractivity (MR) values of these groups. The π constants for hydrogen, methyl, chloro and nitro group are 0.00, 0.56, 0.71 and −0.28, respectively,24 indicating the greater hydrophobicity of the methyl and chloro substituents. The MR figures of hydrogen, methyl, chloro and nitro groups are 1.03, 5.65, 6.03 and 7.36, respectively,24 suggesting that an optimal MR value may have been reached while smaller and larger MR figures detract from MDR-reversing properties. Thus groups which are lipophilic and have MR values of approximately 5–6 should be placed in the arylidene aryl rings, for example, the trifluoro-methyl substituent has π and MR values of 0.88 and 5.02, respectively.24
A number of studies revealed the high lipophilicity of various MDR-reversal agents25,26; for example, the calculated logP values of a number of 1,4-dihydropyridines which reverse MDR were in the region of 5.1–7.5.27 The calculated logP values of the compounds in series 4–9 and verapamil are presented in Table 1. The average logP figures for the compounds in series 4–9 are 3.90, 5.14, 5.90, 6.05, 4.99 and 1.92, respectively. Thus the compounds displaying significant capabilities in reversing MDR, namely series 5–8, have logP values in the region of 5–6. On the other hand, the compounds in series 4 and 9, which are virtually bereft of anti-MDR properties, have lower logP values. However, linear and logarithmic plots between the FAR values and the logP figures of the compounds in series 5–8 did not reveal any correlation (p > 0.05).
The potential of these compounds as MDR revertants will be enhanced if bioactivity is displayed at lower concentrations than 4 μg/ml and reversal of MDR occurs in a different species and another neoplastic disease. Consequently, the most potent compound identified in this study, namely 7b along with its analog which is bereft of a N-acyl group viz. 4b was evaluated further. These results are presented in Table 2. A concentration of 0.4 μg/mL of 7b but not 4b demonstrated MDR-revertant properties using L5178/MDR1 cells. In addition, 7b reverses MDR in Colo 320/MDR1 cells while 4b displays marginal potencies. These data confirm the relative potencies of 4b and 7b and in particular emphasize the potential of 7b as a MDR revertant.
Table 2.
Evaluation of 4b and 7b for MDR-revertant properties using murine L5178Y/MDR1 and human colo320/MDR1 cells
| Compound | FAR value
|
||
|---|---|---|---|
| L5178Y/MDR1 cells | colo320/MDR1 cells
|
||
| 0.4 μg/mLa | 0.4 μg/mLa | 4 μg/mLb | |
| 4b | 1.00 | 1.24 | 2.42 |
| 7b | 7.35 | 2.49 | 10.8 |
Verapamil is inactive using this concentration.
The FAR value of verapamil is 3.84 using a concentration of 10 μg/mL.
A further issue to be resolved is whether the N-acylpiperidones described in this report are cytocidal to neoplasms which are multidrug-resistant. Consequently, several representative compounds were assayed for cytocidal activity towards L5178Y/MDR1 cells.28 The ID50 figures for 6a–c are 3.27, 1.56 and 1.53 μg/mL, respectively, while for 7a–d, the relevant values are 3.28, 2.83, 4.17 and 2.15 μg/mL, respectively. Verapamil has an ID50 figure of 42 μg/ mL. This observation emphasizes further the importance of developing these cytotoxins, which possess MDR-revertant properties.
The MDR modulators are believed to bind to the transmembrane domains of P-gp which leads to inhibition of ABC transporters due to the induced conformational changes.25 The functionally active conformation of P-gp depends on the integrity of the membrane bilayer in which P-gp is embedded. Due to the existence of 12 transmembrane domains of P-gp, the co-crystallization of MDR inhibitors and P-gp is not possible thereby precluding direct evidence of ligand binding.
A final consideration which demonstrates the importance of this seminal work is the very recent disclosure of the excellent tolerability of the compounds in series 4–8.29 Thus doses of up to and including 300 mg/kg of each of these compounds did not induce lethalities in a short-term toxicity study in mice. A few compounds were examined at lower doses in rats which also did not induce mortalities. However, most of the compounds in series 9 causing deaths in some of the mice and bearing in mind the lack of MDR revertant properties in general, suggest that formation of further quaternary ammonium analogs of 9 is not pursued.
In conclusion, this study has identified a novel class of P-gp associated MDR-reversal agents 5–8. These compounds demonstrate high potencies which far exceed that of a reference drug verapamil. Optimal structural fragments in terms of potencies are the 1-piperidinyl group as the terminal base and 4-methyl and 4-chloro substituents are present in the arylidene aryl rings. Series 4 has the general molecular features of various MDR revertants, as well as possessing the 1,5-diaryl-3-oxo-1,4-pentadienyl group. However, the absence of the 4-(2-aminoethoxy)- phenylcar-bonyl group attached to the piperidyl nitrogen atom resulted in compounds displaying a lack of MDR reversal. Development of one or more of these molecules may produce a single drug candidate to treat P-gp mediated MDR cancers and structural modifications can be undertaken in the future in order to increase potencies still further. In this regard, various guidelines for amplifying the project have been obtained based on the physicochemical properties of these molecules.
Acknowledgments
The authors thank the Canadian Institutes of Health Research for an operating Grant to J.R. Dimmock. Support was also provided by the Szeged Foundation of Cancer Research, Hungary to J. Molnár.
References and notes
- 1.Dimmock JR, Das U, Gul HI, Kawase M, Sakagami H, Baráth Z, Oscovsky I, Molnár J. Bioorg Med Chem Lett. 2005;15:1633. doi: 10.1016/j.bmcl.2005.01.054. [DOI] [PubMed] [Google Scholar]
- 2.Das U, Kawase M, Sakagami H, Ideo A, Shimada J, Molnár J, Baráth Z, Bata Z, Dimmock JR. Bioorg Med Chem. 2007;15:3373. doi: 10.1016/j.bmc.2007.03.022. [DOI] [PubMed] [Google Scholar]
- 3.Leonard GD, Polgar O, Bates SE. Curr Opin Invest Drugs. 2002;3:1652. [PubMed] [Google Scholar]
- 4.Chen Y, Pant AC, Simon SM. Cancer Res. 2001;61:7763. [PubMed] [Google Scholar]
- 5.Smith AJ, Van Helvoort A, Van Meer G, Szabo K, Welker E, Szakacs G, Varadi A, Sarckadi B, Borst P. J Biol Chem. 2000;275:23530. doi: 10.1074/jbc.M909002199. [DOI] [PubMed] [Google Scholar]
- 6.Limtrakul P, Anuchapreeda S, Buddhasukh D. BMC Cancer. 2004;4:13. doi: 10.1186/1471-2407-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fu L, Liang Y, Deng L, Ding Y, Chen L, Ye Y, Yang X, Pan Q. Cancer Chemother Pharmacol. 2004;53:349. doi: 10.1007/s00280-003-0742-5. [DOI] [PubMed] [Google Scholar]
- 8.Beck WT, Qian XD. Biochem Pharmacol. 1992;43:89. doi: 10.1016/0006-2952(92)90665-6. [DOI] [PubMed] [Google Scholar]
- 9.Safa AR. Cancer Inv. 1993;11:46. doi: 10.3109/07357909309020260. [DOI] [PubMed] [Google Scholar]
- 10.Ford JM, Hait WN. Pharmacol Rev. 1990;42:155. [PubMed] [Google Scholar]
- 11.Pauli-Magnus CH, von Richter O, Burk O, Ziegler A, Mettang T, Eichelbaum M, Fromm MF. J Pharmacol Exp Ther. 2000;293:376. [PubMed] [Google Scholar]
- 12.Wang EJ, Casciano CN, Clement RP, Johnson WW. Cancer Res. 2001;61:4805. [PubMed] [Google Scholar]
- 13.Roberts J, Jarry C. J Med Chem. 2003;46:4805. doi: 10.1021/jm030183a. [DOI] [PubMed] [Google Scholar]
- 14.Shen X, Chen G, Zhu G, Fong WF. Bioorg Med Chem. 2006;14:7138. doi: 10.1016/j.bmc.2006.06.066. [DOI] [PubMed] [Google Scholar]
- 15.Chaufferet B, Martin M, Hammann A, Michael MF, Martin F. Cancer Res. 1986;46:825. [PubMed] [Google Scholar]
- 16.DeGregoria MW, Ford JM, Benz CC, Wiebe VJ. J Clin Oncol. 1989;7:1359. doi: 10.1200/JCO.1989.7.9.1359. [DOI] [PubMed] [Google Scholar]
- 17.Braybrooke JP, Vallis KA, Houlbrook S, Rockett H, Ellmen J, Anttila M, Ganesan TS, Harris AL, Talbot DC. Cancer Chemother Pharmacol. 2000;46:27. doi: 10.1007/s002809900085. [DOI] [PubMed] [Google Scholar]
- 18.Das U, Alcorn J, Shrivastav A, Sharma RK, De Clercq E, Balzarini J, Dimmock JR. Eur J Med Chem. 2007;42:71. doi: 10.1016/j.ejmech.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 19.Kawase M, Sakagami H, Motohashi N, Hauer H, Chatterjee SS, Spengler G, Vigyikanne AV, Molnár A, Molnár J. In Vivo. 2005;19:705. In brief, both the L-5178 MDR and parental cells were grown in McCoy’s 5A medium containing heat-inactivated horse serum (10%), L-glutamine and antibiotics. A solution of the test compound (2 mg/ml, 10 μl) in dimethylsulfoxide was added to aliquots of the cell suspension and incubated at room temperature for 10 min. Then 10 μl of rhodamine 123 in dimethylsulfoxide was added so that its final concentration was 5.2 μM and the cells were incubated for a further 20 min at 37 °C. The cells were washed twice and resuspended in PBS (pH 7.4) after which the fluorescence was measured using a Beckton Dickinson FACScan instrument. The fluorescence of the cells was measured in treated MDR cells (F1), untreated MDR cells (F2), treated parental cells (F3) and untreated parental cells (F4) and the FAR values were obtained from the following equation, viz. F1/ F2 over F3/ F4. The colo320/MDR1 cells were obtained from the ATCC (ATCC #CCL-220.1). The colo 320 assay was undertaken in a similar manner except that prior to flow cytometry, the cells which form a monolayer were treated with 0.25% trypsin solution for 2–3 min in order to produce a suspension of individual cells. [PubMed] [Google Scholar]
- 20.Gyémánt N, Tanaka M, Antus S, Hohmann J, Csuka O, Mandoky L, Molnár J. In Vivo. 2005;19:367. [PubMed] [Google Scholar]
- 21.Albert A, Serjeant EP. The Determination of Ionization Constants. 3. Chapman and Hall; London: 1984. pp. 151–152. [Google Scholar]
- 22.Albert A. Selective Toxicity. 7. Chapman and Hall; London: 1985. p. 643. [Google Scholar]
- 23.MacroModel 7.1. Department of Chemistry, Columbia University; New York: 2000. [Google Scholar]
- 24.Hansch C, Leo AJ. Substituent Constants for Correlation Analysis in Chemistry and Biology. Wiley; New York: 1979. p. 49. [DOI] [PubMed] [Google Scholar]
- 25.Molnár J, Gyémánt N, Tanaka M, Hohmann J, Bergmann-Leitner E, Molnár P, Deli J, Didiziapetris R, Ferreira MJU. Curr Pharm Des. 2006;12:287. doi: 10.2174/138161206775201893. [DOI] [PubMed] [Google Scholar]
- 26.Zamora JM, Pearce HL, Beck WT. Mol Pharmacol. 1988;33:454. [PubMed] [Google Scholar]
- 27.Kawase M, Shah A, Gaveriya H, Motohashi N, Sakagami H, Varga A, Molnár J. Bioorg Med Chem. 2002;10:1051. doi: 10.1016/s0968-0896(01)00363-7. [DOI] [PubMed] [Google Scholar]
- 28.Richter M, Molnár J, Hilgeroth A. J Med Chem. 2006;49:2838. doi: 10.1021/jm058046w. The cytocidal activity of 3a–c and 4a–d towards L5178Y/MDR1 was accomplished by a literature methodology.27 All values were expressed as means of duplicate experiments. In brief, cells were incubated with varying concentrations of the compounds at 37 °C for 48 h and the ID50 values were determined by the MTT method. [DOI] [PubMed] [Google Scholar]
- 29.Das U, Das S, Bandy B, Stables JP, Dimmock JR. Bioorg Med Chem. 2008;16:3602. doi: 10.1016/j.bmc.2008.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Molinspiration Cheminformatics. http://www.molinspiration.com.