Abstract
To date, much of the work in rodents implicating vasopressin (Avp) in the regulation of social behavior has focused on its action via the Avp 1a receptor (Avpr1a). However, there is mounting evidence that the Avp 1b receptor (Avpr1b) also plays a significant role in Avp's modulation of social behavior. The Avpr1b is heavily expressed on the anterior pituitary cortiocotrophs where it acts as an important modulator of the endocrine stress response. In the brain, the Avpr1b is prominent in the CA2 region of the hippocampus, but can also be found in areas such as the paraventicular nucleus of the hypothalamus and the olfactory bulb. Studies that have employed genetic knockouts or pharmacological manipulation of the Avpr1b point to the importance of central Avpr1b in the modulation of social behavior. However, there continues to be a knowledge gap in our understanding of where in the brain this is occurring, as well as how and if the central actions of Avp acting via the Avpr1b interact with the stress axis. In this review we focus on the genetic and pharmacological studies that have implicated the Avpr1b in the neural regulation of social behaviors, including social forms of aggressive behavior, social memory, and social motivation.
Keywords: Avpr1b, aggressive behavior, social recognition memory, social motivation, stress
Introduction
Arginine vasopressin (Avp) is a cyclic nonapeptide produced primarily within the paraventricular nucleus (PVN) and the supraoptic nucleus (SON) of the hypothalamus. Three specific receptor subtypes mediate the actions of Avp: the Avp 1a receptor (Avpr1a), the Avp 1b receptor (Avpr1b), and the Avp 2 receptor (Avpr2). All three receptor subtypes can be found in the periphery (Arsenijevic et al., 1994; Jard et al., 1987; Knepper, 1997; Koshimizu et al., 2006; Thibonnier et al., 2002), but only the centrally expressed Avpr1a and Avpr1b are known to mediate the effects of Avp on social behavior (Foletta et al., 2002; Lolait et al., 1995; Young et al., 2006). While the role of the Avpr1a in the neural regulation of social behavior has been studied extensively, pharmacological studies as well as data from Avpr1b knockout (Avpr1b −/−) mice suggest a significant role for the Avpr1b as well.
The Avpr1b is expressed in a variety of tissues, including the pancreas, where it has been linked to insulin secretion, and the adrenal gland, where it has been linked to catecholamine release. It is also heavily expressed in the corticotrophes of the anterior pituitary gland (Antoni, 1984; Jard et al., 1986), but is also found in the brain. In rat brain, Avpr1b transcripts and immunoreactive cell bodies are localized to the cerebellum, cerebral cortex, hippocampus, olfactory bulb, PVN, piriform cortical layer II, red nucleus, septum, and suprachiasmatic nucleus (Barberis and Tribollet, 1996; Hernando et al., 2001; Lolait et al., 1995; Saito et al., 1995; Stemmelin et al., 2005; Vaccari et al., 1998). However, a more recent in situ hybridization study, in which more specific riboprobes and more stringent wash conditions were utilized, found that the Avpr1b of mice, rats, and humans is more discretely localized than previous studies suggested, with prominence in the dorsal one-third of pyramidal cells of the CA2 region of the hippocampus (Figure 1), and in a few cells within the anterior amygdala and the PVN (Young et al., 2006).
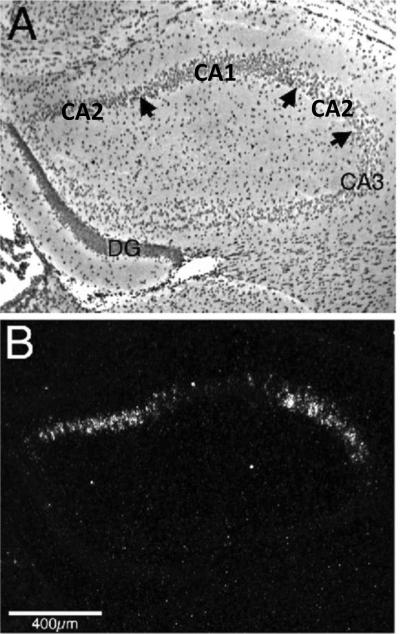
Figure 1.
Vasopressin 1b receptor (Avpr1b) in situ hybridization in a coronal section of mouse hippocampus, approximately 1.1 mm posterior to bregma. A) A brightfield photomicrograph with the two left arrows indicating the CA1–CA2 pyramidal cell borders and the far right arrow the CA2–CA3 pyramidal cell border. B) A darkfield photomicrograph, which highlights the presence of Avpr1b transcripts within the CA2 region of hippocampus. The arrangement of the CA2 region of the hippocampus is unusual in this rostral portion of hippocampus as the CA1 region is between portions of the CA2 region (Lein et al., 2005). DG=dentate gyrus. Adapted from Young, Li, Wersinger, and Palkovits, Neuroscience, 2006; 143(3): 1031–1039, ©2006 with permission from Elsevier.
The apparent discrepancy between the Hernando et al. (2001) study and the Young et al. (2006) study probably reflect methodological differences. The original riboprobe had stretches of sequence that had fairly high identity (> 80%) with the Avpr1a and the oxytocin receptor (Oxtr), likely resulting in cross-hybridization (Hernando et al., 2001). On the other hand, when Young and colleagues (2006) used RT-PCR to quantify Avpr1b mRNA, the distribution was found to be more extensive than that seen with in situ hybridization; which suggests that some areas of the brain have so few Avpr1b transcripts that in situ hybridization is not sensitive enough to detect them. The issue of where exactly in the brain the Avpr1b is located is further complicated by the lack of antibodies in species such as mice and humans, as well as the lack of specific radiolabeled ligands. To date there are no published studies using receptor autoradiography to map the central distribution of the Avpr1b; thus, in humans and mice the presence of Avpr1b protein is inferred from the in situ hybridization studies. While we may not know where in the brain Avp acting via the Avpr1b is affecting behavior, it is clear that the central Avpr1b is important to aspects of social behavior, such as aggression and social memory (DeVito et al., 2009; Wersinger et al., 2007; Wersinger et al., 2002; Wersinger et al., 2004; Wersinger et al., 2008). This review will focus on the behavioral evidence implicating the Avpr1b in the neural regulation of social behavior (summarized in Table 1).
Table 1.
| Behavior | Behavioral Test | Avpr1b −/− | SSR149415 | References |
|---|---|---|---|---|
| Aggression | Social Dominance (mounting behavior) | ↑ | N/A | Caldwell etal., 2010 |
| Competitive (food deprivation/competition) | ↓ | N/A | Wersinger et al., 2007 | |
| Defensive (attack avoidance) | ← → | N/A | Wersinger et al., 2007 | |
| Defensive (reverse resident-intruder) | ↓ | ↓ | Wersinger et al., 2007; Griebel etal., 2002 | |
| Maternal (pup defense) | ↓ | N/A | Wersinger et al., 2007 | |
| Predatory (attack cricket) | ← → | N/A | Wersinger et al., 2007 | |
| Offensive (neutral arena) | ↓ | N/A | Wersinger et al., 2002 | |
| Offensive (resident intruder) | ↓ | ↓ | Wersinger et al., 2002; Caldwell and Young, 2009; Blanchard et al., 2005 (hamster); Griebel et al., 2002(mouse) | |
| Social memory/Memory | Littermate vs. novel animal recognition | ↓ | N/A | DeVitoetal., 2009 |
| Temporal order memory | ↓ | N/A | DeVitoetal., 2009 | |
| Bruce Effect | ↓ | N/A | Wersinger et al., 2008 | |
| Novel vs. familiar female | ↓ | N/A | Wersinger et al., 2002 | |
| Social motivation/Preference | Sociability (familiar littermate interaction) | ↓ | N/A | DeVitoetal., 2009 |
| Social preference (novel animal vs. novel object) | ← → | N/A | Yang et al., 2007 | |
| Bedding preference | ↓ | N/A | Wersinger et al., 2004 | |
| Social Anxiety | Sociability test following chronic social defeat | N/A | ↓ | Litvin etal., 2011 |
Aggressive Behavior
Displays of aggressive behavior are important to the survival and reproductive success of many species. Based on work in Avpr1b −/− mice, there is compelling evidence that the Avpr1b is essential for displays of aggressive behavior that are directed towards a conspecific (for review see Caldwell et al., 2008a; Caldwell et al., 2008b). In resident-intruder and neutral-cage aggression tests Avpr1b −/− mice display fewer attacks and have longer attack latencies than Avpr1b wildtype (+/+) controls (Wersinger et al., 2007; Wersinger et al., 2002). Further, in a reversal of a resident-intruder test, where the experimental mice are intruders rather than residents, Avpr1b −/− mice display normal defensive avoidance behaviors (i.e., boxing stance and protection of their flanks) when attacked by a stimulus animal, but are less likely to initiate retaliatory attacks (Figure 2) (Wersinger et al., 2007). Pharmacological studies using the Avpr1b antagonist SSR149415 are consistent with the work in Avpr1b −/− mice. Syrian hamsters orally administered SSR149415 have reductions in the frequency and duration of offensive attacks, chase behaviors, flank marking, and in the olfactory investigation that often precedes and accompanies an offensive attack (Blanchard et al., 2005). In addition, mice that are orally administered SSR149415, display fewer defensive bites when forced to encounter a threatening predator and reductions in the duration of offensive aggression in a resident-intruder test (Griebel et al., 2002).
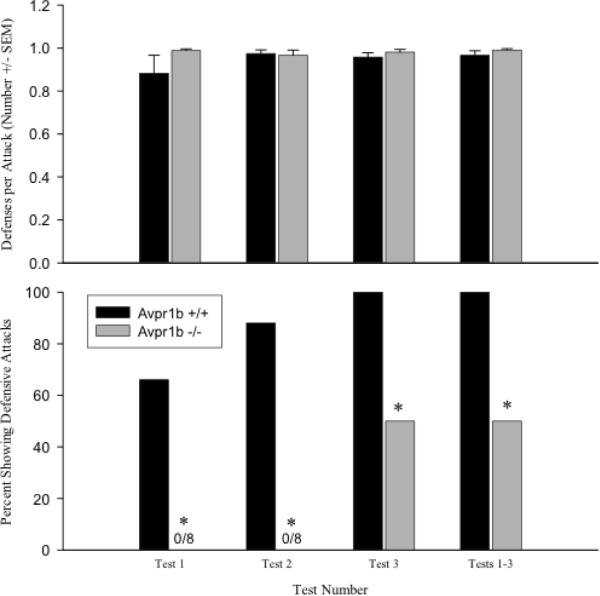
Figure 2.
Defensive behaviors in a reversal of a resident-intruder test in vasopressin 1b knockout (Avpr1b −/−) and wildtype (Avpr1b +/+) mice. Top panel: the mean number of defensive avoidance behaviors per an attack by a stimulus animal (+SEM) observed in each test and in tests 1–3 combined. Defensive avoidance behaviors included flight and boxing. There are no differences between the genotypes. Bottom panel: the percentage of defensive attacks by Avpr1b wildtype and knockout mice. A defensive attack is defined as an attack by the experimental animal that directly follows an attack by a stimulus animal. For tests 1 and 2, the percentage of Avpr1b −/− mice that display defensive attacks is significantly lower than controls. *p < 0.05. Adapted from Wersinger, Caldwell, Christiansen, and Young, Genes, Brain and Behavior, 2007; 6(7): 653–660.
While repeated aggression testing or food competition can increase aggressive behaviors in Avpr1b −/− mice, they never reach the levels observed in control animals (Wersinger et al., 2007); which may reflect the importance of the Avpr1b for normal displays of aggressive behavior under social conditions or could be an artifact resulting from developmental compensation in Avpr1b −/− mice. In tests of social dominance, male Avpr1b −/− mice are able to form dominance hierarchies, but they do so by employing alternative strategies and fewer displays of aggressive behaviors. Specifically, in early hierarchy formation, socially dominant Avpr1b −/− mice display more mounting behavior than Avpr1b +/+ mice, and non-socially dominant Avpr1b −/− mice engage in fewer attacks and have shorter attack durations compared to controls (Caldwell et al., 2010). The reduced aggression phenotype observed in Avpr1b −/− mice does not appear to be strain specific, as Avpr1b −/− mice crossed with the more “wild” outbred strain, Mus musculus castaneus, have reduced aggressive behaviors relative to controls (Caldwell and Young, 2009).
The deficits in aggressive behaviors observed in Avpr1b −/− mice are not limited to males. Following parturition, female Avpr1b −/− mice have reductions in maternal aggressive behaviors, compared to control mice, as measured by longer attack latencies and fewer attacks, directed towards a male intruder (Wersinger et al., 2007). Interestingly, the disruption of the Avpr1b does not affect all forms of aggressive behavior. In a non-social context, such as the predation of a cricket, Avpr1b −/− and Avpr1b +/+ mice have similar attack latencies (Wersinger et al., 2007). These data are important because they demonstrate that Avpr1b −/− mice are capable of detecting and attacking a stimulus. Interestingly, in tests of juvenile play behaviors, which include play soliciting behaviors such as “push/crawl” and investigative behaviors such as nose-to-nose sniff, Avpr1b −/− mice and controls spend similar amounts of time engaged in sniffing, pushing, crawling over and following of littermates. The only measureable differences in behavior at this time in development are that Avpr1b −/− mice spend less time huddling with littermates when compared to controls (Yang et al., 2007). While juvenile play behaviors do not include aggressive behaviors per se, there are aspects of agonistic behaviors that, at least in some rodents, appear to morph into adult aggressive behaviors (Delville, 2005; Wommack et al., 2003). Thus, the lack of genotypic differences in predatory aggression and juvenile play behaviors as well as the other impairments in social behavior (described below) suggest that disruption of the Avpr1b does not result in a global disruption of aggressive behavior, but rather affects aggressive behavior only in specific social contexts.
Social Recognition Memory, Social Motivation, and Social Preference
In addition to reductions in aggressive behavior, Avpr1b −/− mice also have mild impairments in social recognition memory and reduced social motivation (for review see Caldwell et al., 2008a; Caldwell et al., 2008b). In both an 11-trial habituation/dishabituation social recognition test and a 2-trial social recognition test, Avpr1b −/− mice display deficits in social memory. Specifically, Avpr1b −/− males spend more trials of an 11-trial habituation/dishabituation task (with 5-minute intertrial intervals) investigating a familiar mouse than their Avpr1b +/+ counterpart. The impairment is considered mild as Avpr1b −/− males do eventually decrease the amount of time spent investigating a familiar mouse over the 11 trials, which indicates recognition of the familiar animal. However, following either a 30-minute or 24-hour delay, Avpr1b −/− males are unable to discriminate between a novel female and a familiar female or a littermate and a novel animal, respectively (DeVito et al., 2009; Wersinger et al., 2002) (Figure 3). Avpr1b −/− mice also have difficulty associating objects with odors in a specific sequence when the training sessions and testing sessions are separated by 24 hours during an object-trace-odor test, suggesting that Avpr1b −/− mice have impaired temporal memory in which they cannot integrate and/or recall information after a substantial time delay (DeVito et al., 2009). This may explain why Avpr1b −/− males are able to recognize a novel animal when there is a short time delay between presentations, such as in the 11-trial habitation/dishabituation test where there is only a 5-minute interval between trials, but not in a test with a longer time delay, such as 30 minutes (Wersinger et al., 2002).
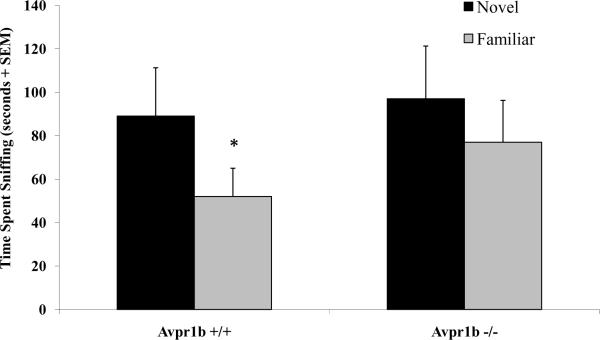
Figure 3.
In a social recognition test with a 30-minute delay between trials, vasopressin 1b knockout mice (Avpr1b −/−) mice show impairments in their ability to recognize a familiar female. *p<0.05 compared to the initial exposure. Adapted from Wersinger, Ginns, O'Carroll, Lolait, and Young, Molecular Psychiatry, 2002; 7(9): 975–984, ©2002 with permission from Nature Publishing Group.
Since the standard tests used to measure social recognition memory are generally less robust in females, Wersinger and colleagues (2008) measured the Bruce effect in Avpr1b −/− females. While social recognition and the Bruce effect are likely mediated by different neural pathways, both require the detection and processing of olfactory cues. The Bruce effect is the termination of a pregnancy by a recently mated female when exposed to the pheromones of an unfamiliar male (Bruce, 1959; Bruce, 1960; Bruce and Parrott, 1960). The removal of the vomeronasal organ, but not the main olfactory epithelium, eliminates pregnancy block, indicating that the Bruce effect is mediated by the accessory olfactory system (Bellringer et al., 1980). Unlike controls, female Avpr1b −/− mice fail to terminate their pregnancies when presented with an unfamiliar male (Wersinger et al., 2008). These data suggest that, if the time interval is long enough, female Avpr1b −/− mice, like males, cannot discriminate between novel and familiar conspecifics.
For an animal to display normal social behaviors it must be motivated to interact socially with other animals. In an olfactory-based social motivation test in which Avpr1b −/− and control mice investigate the following pairings of bedding materials: 1) female-soiled bedding vs. male-soiled bedding, 2) female-soiled bedding vs. clean bedding, and 3) male-soiled bedding vs. clean bedding (with socially motivated individuals expected to spend more time investigating female-soiled bedding over male-soiled bedding and either soiled bedding over clean bedding), Avpr1b −/− mice fail to exhibit a preference for any bedding. Rather, Avpr1b −/− mice spend equal amounts of time interacting with either of the choices and less time overall interacting with the bedding (Wersinger et al., 2004). In a social preference test, Avpr1b −/− prefer a novel mouse over an inanimate object, suggesting that in the presence of myriad sensory cues Avpr1b −/− mice are still interested in social stimuli (Yang et al., 2007). Though, the amount of time spent interacting with another mouse tends to be much lower in Avpr1b −/− mice compared to controls (DeVito et al., 2009). Taken together, these data suggest that Avpr1b −/− mice have deficits in their motivation to interact with social stimuli.
As a functional olfactory system is critical for normal displays of social behaviors in rodents, many of the behavioral deficits observed in Avpr1b −/− mice could be explained if they were to have an impaired olfactory system. However, in a simple test of olfactory ability Avpr1b −/− mice are as adept at finding a hidden cookie as Avpr1b +/+ mice (Wersinger et al., 2002). In a more refined olfactory discrimination test using an olfactometer, Avpr1b −/− mice are able to discriminate between male and female urine (Wersinger et al., 2004). Further, when compared to Avpr1b +/+ mice, Avpr1b −/− mice display a similar elevation in the number of c-fos containing neurons in the main and accessory olfactory bulbs after a social encounter with another male (Wersinger et al., 2002). These data suggest that the chemosensory neural circuit is not compromised in Avpr1b −/− mice.
Stress, Mood, and Social Behavior
The Avpr1b has also been identified as an important regulator of the hypothalamic-pituitary-adrenal-axis (HPA) (Antoni, 1993; Jard et al., 1987; Volpi et al., 2004). Pituitary Avpr1b located on the corticotrophes mediate the synergistic effects of PVN-derived Avp and corticotropin releasing hormone (CRH) on the release of adrenocorticotropic hormone (ACTH). Studies utilizing Avpr1b −/− mice have shown that while they appear to have normal resting ACTH levels, there are abnormalities in their endocrine response to stress. When faced with different types of acute and repeated stressors, genetic disruption of the Avpr1b results in a blunted ACTH release compared to controls (Lolait et al., 2007a; Lolait et al., 2007b; Roper et al., 2010; Stewart et al., 2008a; Tanoue et al., 2004). Interestingly, the blunting of the ACTH response does not always result in a corresponding reduction in corticosterone (CORT) (Lolait et al., 2007a). The coupling of the ACTH response with the CORT response (or lack there of) is stressor specific. For example, in male Avpr1b −/− mice, acute stressors such as mild restraint, forced swim, and shaker stress all result in a decrease in ACTH but no change in CORT (Steward et al., 2008a; Roper et al., 2011). Conversely, novel environment, ethanol (♀s also), insulin-induced hypoglycemia, lipopolysaccharide treatment (♀s only), and antidepressant treatment (♀s also), result in decreases in ACTH and CORT (Lolait et al., 2007b; Stewart et al., 2008b). Why there is an observed disconnect between ACTH and CORT in Avpr1b −/− mice under the aforementioned conditions is not understood. Roper and colleagues (2011) propose that it could be a methodological issue, as only one time point was measured, or that the release of Avp and CRH in Avpr1b −/− mice is not the same as it is in control mice. There is also the possibility that other pathways, such as direct innervation of the adrenal medulla or cortex may affect CORT in sex- and stress-specific ways (Bornstein et al., 2008; Ehrhart-Bornstein et al., 1998).
Studies that have utilized SSR149415 (as reviewed by Serradeil-Le Gal et al., 2005) have focused primarily its potential use as a treatment for affective disorders that are associated with chronic stress, such as anxiety and depression. There is pharmacological evidence that both peripheral (intraperitoneal (i.p.) and oral) and central (intracranial) administration of SSR149415 can reduce anxiety-like and depressive-like behaviors in rats and mice (Griebel et al., 2002; Hodgson et al., 2007; Iijima and Chaki, 2007; Overstreet and Griebel, 2005; Salome et al., 2006; Shimazaki et al., 2006; Stemmelin et al., 2005); suggestive that the effects of SSR149415 on mood may be due primarily to its central actions. However, as SSR149415 is known to also bind to the Oxtr (with a selectivity ratio of 3.2 Avpr1b/Oxtr), which can also be found at low levels in the anterior pituitary of rats, it is not known whether these effects are solely do to its action on the Avpr1b (Antoni, 1986; Chadio and Antoni, 1989; Samson and Schell, 1995; Griffante, 2005). Interestingly, disruption of the Avpr1b in Avpr1b −/− mice has not been found to affect normal displays of anxiety-like and depression-like behavior (Caldwell et al., 2010; Caldwell et al., 2006; Wersinger et al., 2002). The observed differences between genetic and pharmacological models on the role of the Avpr1b mood may be due to developmental compensation in Avpr1b −/− mice or the promiscuity of SSR149415.
While the studies that have explored the role of the Avpr1b in stress and mood have not examined social behavior per se, dysregulation of the HPA-axis is linked to changes in anxiety-like and depression-like behavior and aggression (de Kloet et al., 2005; Mello et al., 2003; Plotsky et al., 1998). A recent study by Litvin and colleagues (2011) is the first to begin to explore the complex interactions between the HPA-axis, mood, and social behavior. Specifically, the authors examined whether i.p. adminstration of SSR149415 modulates anxiety-like behavior in a sociability test following chronic social defeat. They found that SSR149415 attenuates the increases in social anxiety that occur following chronic social defeat. Further, while chronic social defeat increases the expression of the immediate early gene c-fos and Avp/c-fos double labeling within the PVN, peripheral administration of SSR149415 attenuates this effect. The authors also measured the expression of Avpr1a, Avpr1b, and Oxtr using quantitative PCR across the different conditions. They found a decrease in Avpr1b and Oxtr expression within the medial amygdala of defeated mice compared to undefeated controls, but no change in the olfactory bulb, hippocampus, or lateral septum. The authors suggest that chronic stress affects the central Avp system by altering expression of Avpr1b within the medial amygdala and may be important to stress coping (Litvin et al., 2011). Whether the Avpr1b within the medial amygdala is important to the changes in social behavior observed in Avpr1b −/− mice is unknown. It may be that the Avpr1b within the limbic system is important to stress-related responses and that the Avpr1b elsewhere, such as within the hippocampus, is important to the coordination of the correct behavioral output under certain social conditions. Given the potential complexities of these interactions it will be important for future work to consider how the HPA-axis may be interacting with the central nervous system to affect mood and how these alterations in mood may impact social behavior.
Central Avpr1b in the Regulation of Social Behavior
While it is clear that the Avpr1b is important to the neural regulation of social behavior, the specific molecular mechanisms and brain regions involved are unknown. Though, its prominent expression in the CA2 region of the hippocampus in mouse, rat, and human is intriguing and suggests conservation across species. A possible role for the CA2 region in the regulation of social behaviors is revealed in hippocampal lesion studies in rodents that include the CA2 region. When the CA2 region is a part of the lesion, rodents show a phenotype similar to that found in Avpr1b −/− mice. Specifically, reduced aggression and impaired social recognition (Ely et al., 1977; Maaswinkel et al., 1996; Uekita and Okanoya, 2011). Based on these data and the function of the hippocampus, it has been hypothesized that the Avpr1b within the CA2 field may aid in the formation and/or recall of memories related to social encounters and in particular those that are accessory olfactory system-based (Caldwell et al., 2008b; Young et al., 2006). The CA2 region is quite distinct from the CA2 and the CA3 regions, receiving input from the posterior hypothalamus, particularly the supramammilary nucleus (Bartesaghi et al., 2006; Borhegyi and Leranth, 1997) and the perforant pathway, which connects the entorhinal cortex to the hippocampal formation, bypassing the granule cell layer (Bartesaghi and Gessi, 2004). While Avp is often found in the dorsal hippocampus (Landgraf et al., 1991), there is no known direct Avp innervation to the CA2 region. Possible sources of Avp to this part of hippocampus, and ultimately to the Avpr1b, may be from extracellular and cerebral spinal fluid via distant axonal release and long distance diffusion (Herkenham, 1987; Landgraf and Neumann, 2004), nearby axonal release from the entorhinal cortices, or from the pyramidal cells of the CA1 and CA3 fields of the hippocampus (Hallbeck et al., 1999). It is also possible that the prominence of the Avpr1b in the CA2 region does not reflect its importance in the neural regulation of social behavior, and that one, or several, of the other brain areas that express the Avpr1b are also important.
Summary
One of the major challenges of this field continues to be the determination of where in the brain Avp is acting via the Avpr1b to affect social behavior and whether there is a link between the actions of Avp on the HPA-axis and social behavior. To complicate things further, the Avpr1b −/− mice that are currently available are traditional knockout mice and thus are missing the gene throughout development and throughout their entire body. Likewise, the stability and selectivity of the pharmacological agents currently used have been called into question and are primarily administered peripherally rather than centrally (Oost et al., 2011). To make significant advances in the field, better pharmacological and genetic techniques, such as RNAi and conditional knockouts, will need to be employed to help narrow in on the neuroanatomical substrates where Avp acts via the Avpr1b to affect social behavior. Cross-species work will also be important to help determine if the role of the Avpr1b in the neural regulation of social behavior is conserved across species. We are hopeful that future work in our laboratory, as well as others, will help provide some insight into where in the brain Avp is acting through the Avpr1b to affect social behavior.
Highlights
A review of the contributions of the Avpr1b to the neural regulation of behavior.
Focus on the Avpr1b, aggressive behavior, social memory, and social motivation.
A discussion of where in the brain Avp acts via the Avpr1b to exert its effects.
Acknowledgements
The authors would like to express their gratitude to Drs. Elliott Albers, Colleen Novak, and W. Scott Young, III for taking the time to make suggestions for the improvement of this manuscript. This work was supported in part by NIH MH083963 awarded to HKC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES CITED
- Antoni FA. Novel ligand specificity of pituitary vasopressin receptors in the rat. Neuroendocrinology. 1984;39:186–8. doi: 10.1159/000123976. [DOI] [PubMed] [Google Scholar]
- Antoni FA. Oxytocin receptors in rat adenohypophysis: evidence from radioligand binding studies. Endocrinology. 1986;119:2393–5. doi: 10.1210/endo-119-5-2393. [DOI] [PubMed] [Google Scholar]
- Antoni FA. Vasopressinergic control of pituitary adrenocorticotropin secretion comes of age. Front Neuroendocrinol. 1993;14:76–122. doi: 10.1006/frne.1993.1004. [DOI] [PubMed] [Google Scholar]
- Arsenijevic Y, et al. Vasopressin-binding sites in the pig pituitary gland: competition by novel vasopressin antagonists suggests the existence of an unusual receptor subtype in the anterior lobe. J Endocrinol. 1994;141:383–91. doi: 10.1677/joe.0.1410383. [DOI] [PubMed] [Google Scholar]
- Barberis C, Tribollet E. Vasopressin and oxytocin receptors in the central nervous system. Crit Rev Neurobiol. 1996;10:119–54. doi: 10.1615/critrevneurobiol.v10.i1.60. [DOI] [PubMed] [Google Scholar]
- Bartesaghi R, Gessi T. Parallel activation of field CA2 and dentate gyrus by synaptically elicited perforant path volleys. Hippocampus. 2004;14:948–63. doi: 10.1002/hipo.20011. [DOI] [PubMed] [Google Scholar]
- Bartesaghi R, et al. Input-output relations in the entorhinal cortex-dentate-hippocampal system: evidence for a non-linear transfer of signals. Neuroscience. 2006;142:247–65. doi: 10.1016/j.neuroscience.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Bellringer JF, et al. Involvement of the vomeronasal organ and prolactin in pheromonal induction of delayed implantation in mice. J Reprod Fertil. 1980;59:223–8. doi: 10.1530/jrf.0.0590223. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, et al. AVP V1b selective antagonist SSR149415 blocks aggressive behaviors in hamsters. Pharmacol Biochem Behav. 2005;80:189–94. doi: 10.1016/j.pbb.2004.10.024. [DOI] [PubMed] [Google Scholar]
- Borhegyi Z, Leranth C. Distinct substance P- and calretinin-containing projections from the supramammillary area to the hippocampus in rats; a species difference between rats and monkeys. Exp Brain Res. 1997;115:369–74. doi: 10.1007/pl00005706. [DOI] [PubMed] [Google Scholar]
- Bornstein SR, et al. Dissociation of ACTH and glucocorticoids. Trends Endocrinol Metab. 2008;19:175–80. doi: 10.1016/j.tem.2008.01.009. [DOI] [PubMed] [Google Scholar]
- Bruce HM. An exteroceptive block to pregnancy in the mouse. Nature. 1959;184:105. doi: 10.1038/184105a0. [DOI] [PubMed] [Google Scholar]
- Bruce HM. A block to pregnancy in the mouse caused by proximity of strange males. J Reprod Fertil. 1960;1:96–103. doi: 10.1530/jrf.0.0010096. [DOI] [PubMed] [Google Scholar]
- Bruce HM, Parrott DM. Role of olfactory sense in pregnancy block by strange males. Science. 1960;131:1526. doi: 10.1126/science.131.3412.1526. [DOI] [PubMed] [Google Scholar]
- Caldwell HK, et al. Social dominance in male vasopressin 1b receptor knockout mice. Horm Behav. 2010;58:257–63. doi: 10.1016/j.yhbeh.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell HK, et al. Vasopressin: behavioral roles of an “original” neuropeptide. Prog Neurobiol. 2008a;84:1–24. doi: 10.1016/j.pneurobio.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell HK, et al. The acute intoxicating effects of ethanol are not dependent on the vasopressin 1a or 1b receptors. Neuropeptides. 2006;40:325–37. doi: 10.1016/j.npep.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Caldwell HK, et al. The role of the vasopressin 1b receptor in aggression and other social behaviours. Prog Brain Res. 2008b;170:65–72. doi: 10.1016/S0079-6123(08)00406-8. [DOI] [PubMed] [Google Scholar]
- Caldwell HK, Young WS., 3rd Persistence of reduced aggression in vasopressin 1b receptor knockout mice on a more “wild” background. Physiol Behav. 2009;97:131–4. doi: 10.1016/j.physbeh.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadio SE, Antoni FA. Characterization of oxytocin receptors in rat adenohypophysis using a radioiodinated receptor antagonist peptide. J Endocrinol. 1989;122:465–70. doi: 10.1677/joe.0.1220465. [DOI] [PubMed] [Google Scholar]
- de Kloet ER, et al. Stress and the brain: from adaptation to disease. Nat Rev Neurosci. 2005;6:463–75. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- Delville Y, Newman ML, Wommack JC, Taravost-Lahn K, Cervantes MC. Development of aggression. (Ed.) 2005 Oxford University Press; New York: 2005. [Google Scholar]
- DeVito LM, et al. Vasopressin 1b receptor knock-out impairs memory for temporal order. J Neurosci. 2009;29:2676–83. doi: 10.1523/JNEUROSCI.5488-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrhart-Bornstein M, et al. Intraadrenal interactions in the regulation of adrenocortical steroidogenesis. Endocr Rev. 1998;19:101–43. doi: 10.1210/edrv.19.2.0326. [DOI] [PubMed] [Google Scholar]
- Ely DL, et al. Effects of hippocampal lesion on cardiovascular, adrenocortical and behavioral response patterns in mice. Physiol Behav. 1977;18:1075–83. doi: 10.1016/0031-9384(77)90014-2. [DOI] [PubMed] [Google Scholar]
- Foletta VC, et al. Cloning of rat ARHGAP4/C1, a RhoGAP family member expressed in the nervous system that colocalizes with the Golgi complex and microtubules. Brain Res Mol Brain Res. 2002;107:65–79. doi: 10.1016/s0169-328x(02)00448-5. [DOI] [PubMed] [Google Scholar]
- Griebel G, et al. Anxiolytic- and antidepressant-like effects of the non-peptide vasopressin V1b receptor antagonist, SSR149415, suggest an innovative approach for the treatment of stress-related disorders. Proc Natl Acad Sci U S A. 2002;99:6370–5. doi: 10.1073/pnas.092012099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffante C, et al. Selectivity of d[Cha4]AVP and SSR149415 at human vasopressin and oxytocin receptors: evidence that SSR149415 is a mixed vasopressin V1b/oxytocin receptor antagonist. Br J Pharmacol. 2005;146:744–51. doi: 10.1038/sj.bjp.0706383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallbeck M, et al. Distribution of preprovasopressin mRNA in the rat central nervous system. J Comp Neurol. 1999;411:181–200. [PubMed] [Google Scholar]
- Herkenham M. Mismatches between neurotransmitter and receptor localizations in brain: observations and implications. Neuroscience. 1987;23:1–38. doi: 10.1016/0306-4522(87)90268-5. [DOI] [PubMed] [Google Scholar]
- Hernando F, et al. Immunohistochemical localization of the vasopressin V1b receptor in the rat brain and pituitary gland: anatomical support for its involvement in the central effects of vasopressin. Endocrinology. 2001;142:1659–68. doi: 10.1210/endo.142.4.8067. [DOI] [PubMed] [Google Scholar]
- Hodgson RA, et al. Comparison of the V1b antagonist, SSR149415, and the CRF1 antagonist, CP-154,526, in rodent models of anxiety and depression. Pharmacol Biochem Behav. 2007;86:431–40. doi: 10.1016/j.pbb.2006.12.021. [DOI] [PubMed] [Google Scholar]
- Iijima M, Chaki S. An arginine vasopressin V1b antagonist, SSR149415 elicits antidepressant-like effects in an olfactory bulbectomy model. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:622–7. doi: 10.1016/j.pnpbp.2006.12.008. [DOI] [PubMed] [Google Scholar]
- Jard S, et al. Neurohypophyseal hormone receptor systems in brain and periphery. Prog Brain Res. 1987;72:173–87. doi: 10.1016/s0079-6123(08)60206-x. [DOI] [PubMed] [Google Scholar]
- Jard S, et al. Vasopressin antagonists allow demonstration of a novel type of vasopressin receptor in the rat adenohypophysis. Mol Pharmacol. 1986;30:171–7. [PubMed] [Google Scholar]
- Knepper MA. Molecular physiology of urinary concentrating mechanism: regulation of aquaporin water channels by vasopressin. Am J Physiol. 1997;272:F3–12. doi: 10.1152/ajprenal.1997.272.1.F3. [DOI] [PubMed] [Google Scholar]
- Koshimizu TA, et al. V1a vasopressin receptors maintain normal blood pressure by regulating circulating blood volume and baroreflex sensitivity. Proc Natl Acad Sci U S A. 2006;103:7807–12. doi: 10.1073/pnas.0600875103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landgraf R, et al. Septal and hippocampal release of vasopressin and oxytocin during late pregnancy and parturition in the rat. Neuroendocrinology. 1991;54:378–83. doi: 10.1159/000125917. [DOI] [PubMed] [Google Scholar]
- Landgraf R, Neumann ID. Vasopressin and oxytocin release within the brain: a dynamic concept of multiple and variable modes of neuropeptide communication. Front Neuroendocrinol. 2004;25:150–76. doi: 10.1016/j.yfrne.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Lein ES, et al. Redefining the boundaries of the hippocampal CA2 subfield in the mouse using gene expression and 3-dimensional reconstruction. J Comp Neurol. 2005;485:1–10. doi: 10.1002/cne.20426. [DOI] [PubMed] [Google Scholar]
- Litvin Y, et al. Effects of chronic social defeat on behavioral and neural correlates of sociality: Vasopressin, oxytocin and the vasopressinergic V1b receptor. Physiol Behav. 2011;103:393–403. doi: 10.1016/j.physbeh.2011.03.007. [DOI] [PubMed] [Google Scholar]
- Lolait SJ, et al. Extrapituitary expression of the rat V1b vasopressin receptor gene. Proc Natl Acad Sci U S A. 1995;92:6783–7. doi: 10.1073/pnas.92.15.6783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lolait SJ, et al. The hypothalamic-pituitary-adrenal axis response to stress in mice lacking functional vasopressin V1b receptors. Endocrinology. 2007a;148:849–56. doi: 10.1210/en.2006-1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lolait SJ, et al. Attenuated stress response to acute lipopolysaccharide challenge and ethanol administration in vasopressin V1b receptor knockout mice. J Neuroendocrinol. 2007b;19:543–51. doi: 10.1111/j.1365-2826.2007.01560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maaswinkel H, et al. Roles of the basolateral amygdala and hippocampus in social recognition in rats. Physiol Behav. 1996;60:55–63. doi: 10.1016/0031-9384(95)02233-3. [DOI] [PubMed] [Google Scholar]
- Mello AF, et al. Update on stress and depression: the role of the hypothalamic-pituitary-adrenal (HPA) axis. Rev Bras Psiquiatr. 2003;25:231–8. doi: 10.1590/s1516-44462003000400010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oost T, et al. Potent and selective oxindole-based vasopressin 1b receptor antagonists with improved pharmacokinetic properties. Bioorg Med Chem Lett. 2011;21:3828–31. doi: 10.1016/j.bmcl.2011.03.012. [DOI] [PubMed] [Google Scholar]
- Overstreet DH, Griebel G. Antidepressant-like effects of the vasopressin V1b receptor antagonist SSR149415 in the Flinders Sensitive Line rat. Pharmacol Biochem Behav. 2005;82:223–7. doi: 10.1016/j.pbb.2005.07.021. [DOI] [PubMed] [Google Scholar]
- Plotsky PM, et al. Psychoneuroendocrinology of depression. Hypothalamic-pituitary-adrenal axis. Psychiatr Clin North Am. 1998;21:293–307. doi: 10.1016/s0193-953x(05)70006-x. [DOI] [PubMed] [Google Scholar]
- Roper JA, et al. Attenuated stress response to acute restraint and forced swimming stress in arginine vasopressin 1b receptor subtype (Avpr1b) receptor knockout mice and wild-type mice treated with a novel Avpr1b receptor antagonist. J Neuroendocrinol. 2010;22:1173–80. doi: 10.1111/j.1365-2826.2010.02070.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito M, et al. Molecular cloning and characterization of rat V1b vasopressin receptor: evidence for its expression in extra-pituitary tissues. Biochem Biophys Res Commun. 1995;212:751–7. doi: 10.1006/bbrc.1995.2033. [DOI] [PubMed] [Google Scholar]
- Salome N, et al. Differential roles of amygdaloid nuclei in the anxiolytic- and antidepressant-like effects of the V1b receptor antagonist, SSR149415, in rats. Psychopharmacology (Berl) 2006;187:237–44. doi: 10.1007/s00213-006-0424-1. [DOI] [PubMed] [Google Scholar]
- Samson WK, Schell DA. Oxytocin and the anterior pituitary gland. Adv Exp Med Biol. 1995;395:355–64. [PubMed] [Google Scholar]
- Serradeil-Le Gal C, et al. An overview of SSR149415, a selective nonpeptide vasopressin V(1b) receptor antagonist for the treatment of stress-related disorders. CNS Drug Rev. 2005;11:53–68. doi: 10.1111/j.1527-3458.2005.tb00035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimazaki T, et al. The pituitary mediates the anxiolytic-like effects of the vasopressin V1B receptor antagonist, SSR149415, in a social interaction test in rats. Eur J Pharmacol. 2006;543:63–7. doi: 10.1016/j.ejphar.2006.06.032. [DOI] [PubMed] [Google Scholar]
- Stemmelin J, et al. Evidence that the lateral septum is involved in the antidepressant-like effects of the vasopressin V1b receptor antagonist, SSR149415. Neuropsychopharmacology. 2005;30:35–42. doi: 10.1038/sj.npp.1300562. [DOI] [PubMed] [Google Scholar]
- Stewart LQ, et al. Pituitary-adrenal response to acute and repeated mild restraint, forced swim and change in environment stress in arginine vasopressin receptor 1b knockout mice. J Neuroendocrinol. 2008a;20:597–605. doi: 10.1111/j.1365-2826.2008.01704.x. [DOI] [PubMed] [Google Scholar]
- Stewart LQ, et al. The role of the arginine vasopressin Avp1b receptor in the acute neuroendocrine action of antidepressants. Psychoneuroendocrinology. 2008b;33:405–15. doi: 10.1016/j.psyneuen.2007.12.009. [DOI] [PubMed] [Google Scholar]
- Tanoue A, et al. The vasopressin V1b receptor critically regulates hypothalamic-pituitary-adrenal axis activity under both stress and resting conditions. J Clin Invest. 2004;113:302–9. doi: 10.1172/JCI19656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibonnier M, et al. Molecular pharmacology and modeling of vasopressin receptors. Prog Brain Res. 2002;139:179–96. doi: 10.1016/s0079-6123(02)39016-2. [DOI] [PubMed] [Google Scholar]
- Uekita T, Okanoya K. Hippocampus lesions induced deficits in social and spatial recognition in Octodon degus. Behav Brain Res. 2011;219:302–9. doi: 10.1016/j.bbr.2011.01.042. [DOI] [PubMed] [Google Scholar]
- Vaccari C, et al. Comparative distribution of vasopressin V1b and oxytocin receptor messenger ribonucleic acids in brain. Endocrinology. 1998;139:5015–33. doi: 10.1210/endo.139.12.6382. [DOI] [PubMed] [Google Scholar]
- Volpi S, et al. Regulation of vasopressin V1b receptors and stress adaptation. Ann N Y Acad Sci. 2004;1018:293–301. doi: 10.1196/annals.1296.035. [DOI] [PubMed] [Google Scholar]
- Wersinger SR, et al. Disruption of the vasopressin 1b receptor gene impairs the attack component of aggressive behavior in mice. Genes Brain Behav. 2007;6:653–60. doi: 10.1111/j.1601-183X.2006.00294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wersinger SR, et al. Vasopressin V1b receptor knockout reduces aggressive behavior in male mice. Mol Psychiatry. 2002;7:975–84. doi: 10.1038/sj.mp.4001195. [DOI] [PubMed] [Google Scholar]
- Wersinger SR, et al. Social motivation is reduced in vasopressin 1b receptor null mice despite normal performance in an olfactory discrimination task. Horm Behav. 2004;46:638–45. doi: 10.1016/j.yhbeh.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Wersinger SR, et al. Inactivation of the oxytocin and the vasopressin (Avp) 1b receptor genes, but not the Avp 1a receptor gene, differentially impairs the Bruce effect in laboratory mice (Mus musculus) Endocrinology. 2008;149:116–21. doi: 10.1210/en.2007-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wommack JC, et al. Repeated exposure to social stress alters the development of agonistic behavior in male golden hamsters. Horm Behav. 2003;43:229–36. doi: 10.1016/s0018-506x(02)00029-6. [DOI] [PubMed] [Google Scholar]
- Yang M, et al. Social approach behaviors are similar on conventional versus reverse lighting cycles, and in replications across cohorts, in BTBR T+ tf/J, C57BL/6J, and vasopressin receptor 1B mutant mice. Front Behav Neurosci. 2007;1:1. doi: 10.3389/neuro.08/001.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young WS, et al. The vasopressin 1b receptor is prominent in the hippocampal area CA2 where it is unaffected by restraint stress or adrenalectomy. Neuroscience. 2006;143:1031–9. doi: 10.1016/j.neuroscience.2006.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]