Abstract
GABAB receptors (GABABRs) have been linked to a wide range of physiological and cognitive processes and are of interest for treating a number of neurodegenerative and psychiatric disorders. As many of these diseases are associated with advanced age, it is important to understand how the normal aging process impacts GABABR expression and signaling. Thus, we investigated GABABR expression and function in the prefrontal cortex (PFC) and hippocampus of young and aged rats characterized in a spatial learning task. Baclofen-stimulated GTP-binding and GABABR1 and GABABR2 proteins were reduced in the PFC of aged rats but these reductions were not associated with spatial learning abilities. In contrast, hippocampal GTP-binding was comparable between young and aged rats but reduced hippocampal GABABR1 expression was observed in aged rats with spatial learning impairment. These data demonstrate marked regional differences in GABABR complexes in the adult and aged brain and could have implications for both understanding the role of GABAergic processes in normal brain function and the development of putative interventions that target this system.
Keywords: Aging, Baclofen, Spatial learning, GABABR, PFC, Memory
1. Introduction
GABAB receptors (GABABRs) are G-protein coupled receptors (GPCRs) and modulation of these receptors shows potential for treating a number of neurological and psychiatric disorders. In post-synaptic neurons, GABABRs bind γ-aminobutyric acid (GABA) and are coupled to the Gi/o class of Gα-proteins that inhibit adenylyl cyclase to decrease intracellular levels of cyclic AMP (cAMP; Odagaki et al., 2000; Odagaki and Koyama, 2001). Additionally, the Gβγ-subunit activates the inward rectifying postassium current that modulates the late, or slow, phase of the inhibitory post-synaptic potential (Luscher et al., 1997). GABABRs are also located on axon terminals where their activation decreases Ca2+ influx (Takahashi et al., 1998) and inhibits neurotransmitter release (Waldmeier et al., 2008). GABABRs are unique among GPCRs as they are obligate heterodimers comprised of at least one GABABR1 subunit with one GABABR2 subunit (Jones et al., 1998; Kaupmann et al., 1998; White et al., 1998), although more complex arrangements are speculated (Pin et al., 2009). The R1 subunit contains the orthosteric binding site and is expressed as one of two isoforms, GABABR1a or GABABR1b (Kaupmann et al., 1997). While no ligands can distinguish between the two (Kaupmann et al., 1997, 1998), molecular and biochemical evidence has identified distinct cellular distributions and functions for each isoform. GABABR1a contains a pair of short consensus repeats at the N-terminal that act as an axonal targeting factor that trafficks GABABR complexes containing this isoform to presynaptic terminals where they modulate neurotransmitter release (Biermann et al., 2010). Conversely, GABABR1b lacks this N-terminal extension and is preferentially trafficked to dendrites where it controls postsynaptic inhibition (Vigot et al., 2006). However, functionality of the receptor is not observed until this R1 subunit associates with an R2 subunit; the R2 subunit mediates interactions with the G-protein (Robbins et al., 2001) as well as facilitates expression of the receptor complex at the plasma membrane (Margeta-Mitrovic et al., 2000).
Despite the significant functional implications, surprisingly little is known regarding the normal composition of GABABR complexes across distinct brain regions and the extent to which such complexes and their activity change with age. Such information is vital given the diversity of signaling offered by unique receptor configurations and emerging evidence that GABAergic indices change with age. For example, in aged rats, prefrontal and hippocampal interneurons degenerate or cease to express glutamic acid decarboxylase (GAD-67), the GABA-synthesizing enzyme (Shetty and Turner, 1998; Stanley and Shetty, 2004; Stranahan et al., 2011). Moreover, evoked GABA release is decreased in the CA1 subregion of the aged rat hippocampus (Stanley et al., 2011).
Among the functional consequences that could stem from age-related alterations in GABAergic signaling is a loss of cognitive abilities. Indeed, GABAergic signaling has been implicated in cognitive processes supported by both medial temporal lobe and frontal cortical systems, and these brain regions are particularly vulnerable to changes associated with advancing age. Age-related frontal cortical dysfunction, reflected in a loss of behavioral flexibility, has been detected in aged rodents using tasks such as attentional set-shifting (Barense et al., 2002; Schoenbaum et al., 2002; Rodefer and Nguyen, 2008). Loss of declarative/spatial memory supported by hippocampus is also a prominent feature of advanced age and such deficits can be modeled in aged rats using spatial learning tasks such as the Morris water maze. A unique feature of aged rat models characterized on spatial learning tasks is that reliable individual differences in performance can be detected, such that aged rats can be subgrouped into those that perform within the range of young rats and those that perform outside this range, demonstrating hippocampal-dependent learning impairment. Such behavioral models have been used to implicate a number of neurobiological alterations in age-related cognitive deficits, including marked alterations of signaling downstream of muscarinic acetylcholine (mAChR) or Group I metabotropic glutamate receptors (mGluRs; Chouinard et al., 1995; Nicolle et al., 1999; Zhang et al., 2007). In contrast, the relationship between cognitive abilities in aged animals and GABABR signaling has not been thoroughly investigated, although GABABR antagonists are known to restore memory function in various animal models of aging (Froestl et al., 2004; Lasarge et al., 2009) while GABABR agonists impair spatial learning in young rats (McNamara and Skelton, 1996). Consequently, we hypothesized that aging may modulate GABABR expression or function in close association with cognition in a brain region-dependent manner. To test this hypothesis, we performed parallel pharmacological and biochemical analyses of hippocampal and PFC GABABRs in tissues obtained from young and aged rats previously tested for spatial learning ability.
2. Materials and methods
2.1. Animals
Young adult (6 mo, n=8) and aged (22 mo, n=16) male Fischer 344 (F344) rats were obtained from the National Institute on Aging colony (Harlan, IN, USA) and individually housed in the AALAC-accredited Psychology Department vivarium at Texas A&M University for 2 weeks prior to the onset of behavioral testing. The vivarium was maintained at a constant 25°C with a regular 12:12h light/dark cycle (lights on at 08:00), and rats had free access to food and water at all times. All rats were screened daily for health problems including but not limited to cataracts, jaundice, food and water intake, and the appearance of tumors. Sentinel rats housed in the same room were routinely screened and found to be negative for a range of pathogens. All animal procedures were conducted in accordance with approved institutional animal care procedures and NIH guidelines.
2.3. Behavioral testing
Animals were tested for spatial learning ability according to the methods developed by Gallagher and colleagues (Gallagher et al., 1993) with specific modifications for training F344 rats (Bizon et al., 2009). Rats were trained in a Morris water maze apparatus consisting of a circular tank (1.8 m diameter) filled with water (27°C) made opaque by the addition of nontoxic tempera paint. A retractable escape platform (12 cm diameter) was submerged 2 cm below the surface in the southwest quadrant of the maze. Each rat received 3 trials a day for 8 consecutive days to learn to swim to the submerged platform using white, geometric spatial cues affixed to a black curtain surrounding the maze. Rats were placed into the water facing the wall of the maze at one of four equally spaced start positions (north, south, east or west) in a pseudo randomized order such that all rats started from each of the locations the same number of times. Rats were allowed to swim for up to 90 s in order to locate the platform before they were guided to it by the experimenter. Rats remained on the platform for 30 s, and subsequently transferred a holding cage for a 30 s inter trial interval (ITI). Every sixth trial (i.e. the third trial on days 2, 4, 6 and 8) was a probe trial during which the platform was lowered to the bottom of the tank and made unavailable for escape for the first 30 s of the trial, after which the platform was raised and subjects were allowed to escape. Data were acquired via a video camera mounted above the maze that was connected to a DVD recorder and computer with a video tracking system (HVS Image, Buckingham, UK).
After the last day of spatial training, rats received one session with six trials of cue training to assess sensorimotor function and motivation. Here, rats were trained to escape to a visible black platform extending 2 cm above the water surface, the position of which varied from trial to trial. On each trial, rats were given 90 s to reach the platform followed by a 30 s ITI.
2.4. Membrane preparation
Approximately 2 weeks following the completion of behavioral testing, animals were decapitated, brains were removed from the skull and PFC and hippocampus were dissected on an ice-cold plate and stored at −80°C until use. As cognitive task performance will elicit changes in protein expression, this interval was selected to evaluate baseline, rather than behaviorally-stimulated, protein levels and functions. Frozen tissue was weighed, thawed and homogenized in 10 volumes of an ice-cold buffer (50 mM HEPES, pH 7.4, 1 mM EDTA and 1 mM EGTA and protease inhibitors; Roche, Mannheim, Germany) using a glass-teflon dounce homogenizer. Homogenates were centrifuged at 14,000 RPM for 20 minutes at 4°C. The supernatant was discarded and the pellet resuspended in 20 ml of the same buffer without protease inhibitors and incubated on ice for 30 minutes followed by centrifugation at 16,500 RPM for 15 minutes at 4°C. This pellet was resuspended in 10 volumes of 50 mM HEPES, pH 7.4, and aliquots were stored at −80°C until used for GTP-binding or Western blotting assays. Protein concentration was determined using the Pierce BCA Kit according to the manufacturer’s protocol (Rockford, IL).
2.5. GTP-binding assay
All reactions were run in triplicate at room temperature. GTP-binding reactions were run in a buffer of 50 mM HEPES (pH 7.4), 150 mM NaCl, 10 mM MgCl2 and 50 μM GDP with the appropriate concentration of baclofen (100 nM – 1 mM; Tocris, Ellisville, MO) or an equal volume of water (basal activity) or 10 μM unlabelled GTPγS (non-specific binding) in each well of the 96-well filter plate. 10 μg of membrane was added per well and equilibrated for 20 minutes. GTP-binding was initiated by the addition of 10 nM GTP-Eu, a hydrolysis-resistant, fluorescent GTP analogue (PerkinElmer, Waltham, MA) to a final total volume of 100 μl per reaction. After 40 minutes, the reaction was terminated by filtration on a vacuum manifold and washed four times with 200 μl of ice-cold 1× GTP wash buffer and read on a Victor3 fluorimeter (PerkinElmer Life, Shelton, CT).
2.6. Western blotting
Unless otherwise noted, all reagents used for gel electrophoresis were purchased from Bio-Rad (Hercules, CA) and all steps were performed at room temperature. Membrane proteins were denatured and reduced in Laemmli sample buffer with 5% (v/v) β-mercaptoethanol (Fisher, Pittsburgh, PA) and heated at 95°C for 5 minutes. 10 μg of protein per lane were electrophoretically separated on a 4–15% Tris-HCl gel at 200 V for 55 minutes then transferred to nitrocellulose membranes using a semi-dry transfer apparatus (iBlot; Invitrogen, Carlsbad, CA) for 7 minutes at 20 V. Blots were washed 3 times with tris-buffered saline (TBS; pH 7.4) then blocked for 1 hour in blocking buffer (Rockland, Gilbertsville, PA). Blots were then incubated overnight at 4°C with anti-GABABR1 or anti-GABABR2 diluted 1:1000 (Cell Signaling Technology, Beverly, MA) diluted in blocking buffer with 0.1% Tween-20. Blots were then washed three times with TBS and incubated with the appropriate AlexaFluor 680-conjugated anti-IgG (Invitrogen) diluted 1:20,000 in TBS with 0.1% Tween-20 (Bio-Rad) for 1 hour. Following three additional TBS washes, blots were scanned on an Odyssey imaging system (LI-COR Biosciences, Lincoln, NE). A total of four experiments were conducted for each subunit.
2.7. Data analysis
All data are presented as the mean ± standard error of the mean. The student’s t-test, one-way or repeated measures ANOVAs were performed where appropriate. When necessary, Fisher’s least-significant difference post-hoc tests were conducted to determine significant differences between groups. In all statistical comparisons, values of p<0.05 were considered significant.
Behavioral data was analyzed using SPSS (Cary, NC). The Spatial Learning Index (SLI) was calculated by weighting and summing mean search error from the interpolated probe trials to provide an overall measure of spatial learning performance for each rat (Gallagher et al., 1993; Bizon et al., 2009). The SLI was used to perform correlational analyses with neurobiological parameters (i.e. GTP-binding or protein levels); partial correlations correcting for the effects of age were used to test significant relationships between SLI and each neurobiological measure across all ages, while bivariate correlations were performed to test relationships separately for each age group.
GTP-binding parameters were analyzed using GraphPad Prism 5 (La Jolla, CA). Non-specific binding values were subtracted from each reaction and the specific stimulation by baclofen was normalized to young (e.g. basal=0% and EMAX of young=100%). These data were log-transformed and curves were fitted using a sigmoidal, non-linear regression with variable slope.
Digitized images of immunoblots were converted to gray scale and analyzed using ImageJ software. Specific bands were identified by creating a threshold mask and excluding pixels that fell below threshold values. Integrated density, the product of mean optical density and area, was measured for each band and these values were normalized to young controls. To analyze R1 isoform protein, expression was normalized to the R1b isoform, as this is the most abundant isoform in the adult brain (Fritschy et al., 1999).
3. Results
3.1. Spatial learning performance
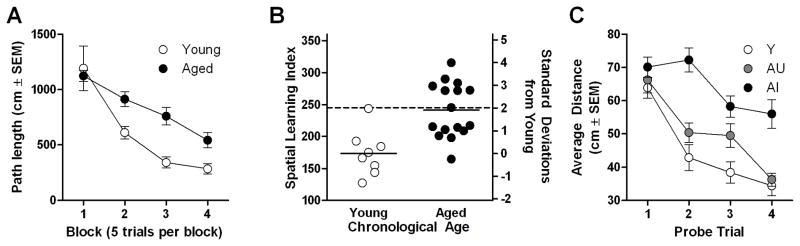
Young rats and aged rats perfomed similarly on the first training trial (F(1,22)=2.66, ns) and while both groups improved over the course of training (F(3,66)=23.64, p<0.05), a two-way repeated measures ANOVA (age × training trial block) revealed a significant main effect of age (F(1,22)=8.96, p<0.05; Fig. 1A) indicating that aged rats were not as proficient in locating the hidden platform compare to young rats. In agreement with training trial performance, a two-way repeated measures ANOVA (age × probe trial) showed that search accuracy for the platform improved over the course of training (F(6,63)=30.93, p<0.0001) and that there was a main effect of age on probe trial performance (F(1,22)=29.91, p<0.0001). In contrast to the effects of age on spatial reference learning abilities, there was no impairment in the ability of aged rats to locate a visible escape platform during cue (visible platform) training. A one-way ANOVA revealed no significant difference between pathlength of young or aged rats (F(1,22)=1.95, ns) on the visible cued task.
Figure 1. Spatial learning in young and aged rats.
While young and aged rats improved as a function of training, aged rats were impaired relative to young at learning to swim to a hidden, submerged platform within the water maze [A]. Spatial learning index (SLI) scores (left y-axis) were greater, on average (noted by horizontal line), for aged rats than young indicating that aged rats were less proficient in searching for the platform location, however, the distribution of individual index scores demonstrates that aged rat performance spans a range which includes aged rats that are unimpaired relative to young (AU) and those exhibiting impaired performance (AI; greater than 2 standard deviations from young on right y-axis) [B]. When subgrouped according to SLI score, AI rats were significantly impaired on probe trial performance compared to young (Y) and age-matched controls without spatial learning impairment (AU) [C].
In order to relate biochemical measures to cognitive abilities, probe trial data were used to calculate an overall “index” of spatial learning for each individual subject (Fig. 1B; described in Gallagher et al., 1993 and Bizon et al., 2009). Learning index scores have been shown to correlate with a number of age-related changes in neurobiological substrates of spatial memory (Bizon et al., 2001; Nicolle et al., 1999; Smith et al., 2000). For some analyses, aged rats were subgrouped based on their spatial learning indices (SLI). The top 50% of aged rats (SLI<245) were classified “aged cognitively-unimpaired” (AU) and the bottom 50% (SLI>245) were classified as “aged cognitively-impaired” (AI; see Bizon et al., 2009; Baxter and Gallagher, 1996). Using this subgroup designation, all AI rats were at least 2 standard deviations outside the mean SLI of young rats (Fig 1B) and all performed outside the upper limit of individual young performance. A two-way repeated measures ANOVA (cognitive group × probe trial) performed on sub-grouped data revealed that all cognitive groups improved over the course of training (F(3,63)=30.93, p<0.0001), and that there was a main effect of cognitive group (F(2,21)=12.26, p<0.005; Fig. 1C). Post hoc analyses revealed that both young and AU groups were significantly different from the AI group (young vs AI: F(1,14)=44.36, p<0.0001; AU vs. AI: F(1,14)=64.33, p<0.0001) but not from each other (young vs. AU: F(1,14)=3.6, ns; Fig. 1C). A planned comparison performed on the final block of training trial data confirmed that, even at the conclusion of training, AI rats were less proficient than AU rats at finding the platform (F(1,14)= 5.48, p<0.05).
3.2. Baclofen-stimulated GTP-binding in hippocampus
To assess functionality of GABABRs in the hippocampus, GTP-binding was measured following stimulation with baclofen, a selective GABABR agonist. Non-specific and basal GTP-binding values were comparable between young and aged rats. Agonist stimulated parameters, maximal GTP-binding (EMAX), agonist affinity (EC50) and Hill slope coefficients (NH), did not differ between young and aged rats or between cognitive groups (Fs<0.75, p>0.05, Table 1 and Fig. 2). Correlation analyses between maximal GTP-binding (determined by curve-fit) and SLI confirmed that binding was stable across a range of cognitive abilities in young (r=−0.08, ns) and aged rats (r=0.04, ns). Furthermore, there were no differences between ages or cognitive groups at any dose of baclofen (Fs<1.0, p>0.05).
Table 1.
Parameters of baclofen-stimulated GTP-binding in the hippocampus of young and aged F344 rats
| Parameter | Young | Aged-Total | Aged-Unimpaired | Aged-Impaired |
|---|---|---|---|---|
| EMAX (% of young) | 100.00 ± 10.33 | 99.30 ± 11.35 | 89.48 ± 12.88 | 107.50 ± 16.42 |
| Log EC50 (M) | −4.78 ± 0.18 | −5.01 ± 0.24 | −4.89 ± 0.26 | −5.14 ± 0.36 |
| Hill Slope (NH) | 1.00 ± 0.34 | 0.69 ± 0.21 | 0.96 ± 0.47 | 0.59 ± 0.23 |
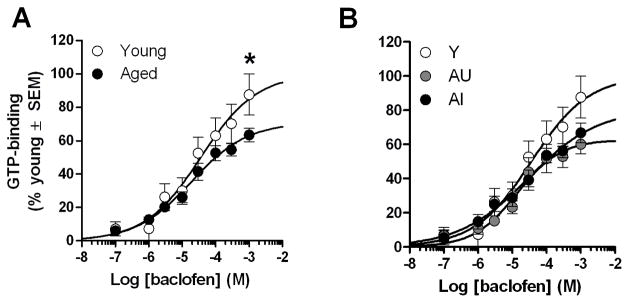
Figure 2. Baclofen-stimulated GTP-binding in the hippocampus of young and aged rats.
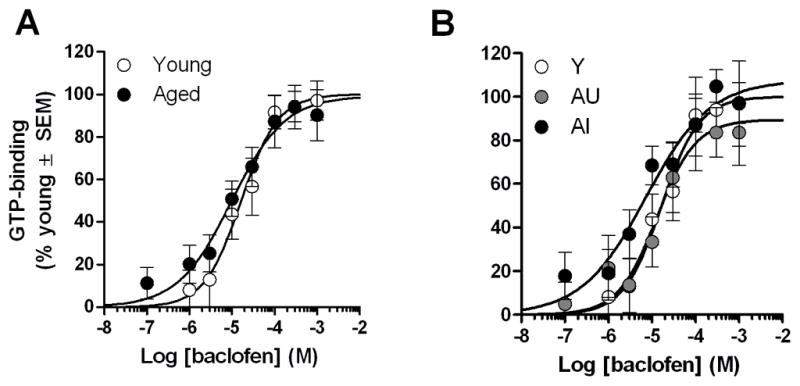
Dose-response curves were not different between age groups [A] or cognitive groups [B]. The line of best-fit determined by non-linear, four-parameter fit is illustrated for young and aged rats.
3.3. Baclofen-stimulated GTP-binding in prefrontal cortex
As in the hippocampus, non-specific and basal values obtained from PFC were not significantly different between young and aged rats. In contrast, while the fitted parameters did not differ (Table 2), two-way ANOVAs (age or cognitive group × concentration) comparing the mean GTP-binding values (i.e. not fitted data) obtained for each concentration of baclofen revealed a significant interaction between age and baclofen concentration (F(7,154)=3.04, p<0.05) and between cognitive group and baclofen concentration (F(14,147)= 1.76, p<0.05). Post hoc analyses revealed that the interaction between age and concentration was attributable to a marked 28% decrease in baclofen-stimulated GTP binding in aged relative to young rats (p<0.05) at the highest concentration of baclofen tested (1 mM; Fig. 3A). A post hoc one-way ANOVA (cognitive group) demonstrated a similar trend towards decreased GTP binding in AU and AI relative to young at the 1 mM baclofen dose but this change did not quite reach statistical significance (F(2,21)= 2.89, p=0.08; Figure 3B). In agreement with the observation that binding was reduced by a similar magnitude in both AU and AI rats (31% and 24%, respectively; Fig. 3B), no significant correlations were observed between GTP-binding stimulated by 1 mM baclofen in PFC and SLI in young (r=−0.18, ns) or aged rats (r=0.20, ns).
Table 2.
Parameters of baclofen-stimulated GTP-binding in the PFC of young and aged F344 rats
| Parameter | Young | Aged-Total | Aged-Unimpaired | Aged-Impaired |
|---|---|---|---|---|
| EMAX (% of young) | 100.00 ± 26.08 | 70.90 ± 8.13 | 62.81 ± 7.66 | 80.62 ± 16.58 |
| Log EC50 (M) | −4.44 ± 0.57 | −4.73 ± 0.26 | −4.86 ± 0.25 | −4.53 ± 0.52 |
| Hill Slope (NH) | 0.52 ± 0.20* | 0.52 ± 0.10*** | 0.68 ± 0.20a | 0.43 ± 0.12** |
p <0.05,
p <0.01,
p <0.001 versus NH=1.
p =0.15 versus NH=1.
Figure 3. Baclofen-stimulated GTP-binding in the prefrontal cortex of young and aged rats.
Aged rats exhibited a 28% decrease in GTP-binding elicited by 1 mM baclofen in the prefrontal cortex [A] and this decrease was similar in aged-unimpaired (AU) and aged-impaired (AI) rats [B]. The line of best-fit determined by non-linear, four-parameter fit is illustrated for young and aged rats. * p<0.05 young versus aged.
3.4. Regional differences in cooperativity of binding
The discrepancy in the magnitude of the fitted and observed maximal values in the PFC may be a consequence of low slope factors (Hill coefficient; NH) apparent in this region. As lower slope factors (i.e. NH <1) indicate decreased cooperativity or efficiency of receptor:G-protein coupling, we compared the Hill slope coefficients in both the PFC and hippocampus. Indeed, while the Hill slope coefficients (NH) did not differ with age in PFC, the slope coefficients for both young and aged rats were significantly less than 1.0 (young: t(7)=2.45, p<0.05; aged: t(15)=4.63, p<0.001; Table 2). This is in contrast to hippocampus in which the slope factors did not deviate from unity (NH=1.0) in either age group (young: t(7)=0.0003, ns; aged: t(13)= 1.46, ns).
3.5. GABABR subunit expression in hippocampus
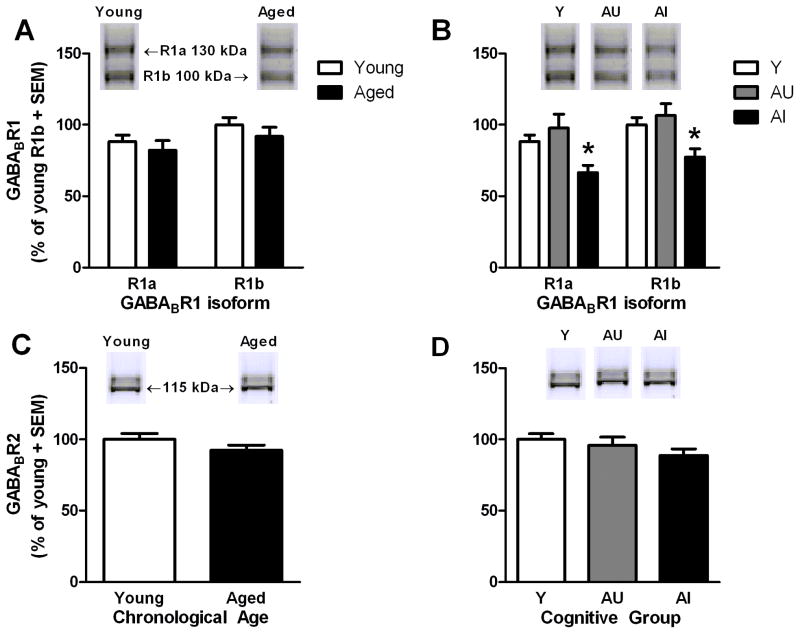
A two-way ANOVA (isoform × age) revealed no main effect of age (F(1,22)=0.54, ns; Fig. 4A) but a significant effect of isoform (F(1,22)=29.75, p<0.001) such that GABABR1b was modestly but significantly more abundant in hippocampus than was GABABR1a (~1.14-fold greater; p<0.001). These differences in isoform expression did not differentially interact with age (F(1,22)=0.29, ns); however, a two-way isoform × cognitive group ANOVA revealed a main effect of cognitive group (F(2,21)=5.72, p<0.05; Fig. 4B). Post-hoc analyses demonstrated significantly lower protein levels in AI rats relative to young (p<0.05) and AU rats (p<0.01). One-way ANOVAs comparing effects of cognitive group separately for each isoform demonstrated that both GABABR1a (F(2,21)=5.31, p<0.05) and GABABR1b (F(2,21)=5.48, p<0.05) were significantly reduced in AI rats relative to young (−25% GABABR1a and −23% GABABR1b; p<0.05 for both) while levels of both isoforms were comparable between young and AU rats. Among aged rats, there was a trend towards lower GABABR1a protein levels being associated with greater SLI scores (GABABR1a: r=−0.46, p=0.07; GABABR1b: r=−0.41, p>0.1).
Figure 4. GABABR1 and GABABR2 protein levels in hippocampus of young and aged rats.
Levels of hippocampal GABABR1 isoforms were similar between age groups [A] but aged-impaired rats expressed less of both isoforms than young [B]. Levels of GABABR2 were not changed by age [C] or cognitive group [D]. Insets of A–D demonstrate representative immunoreactive bands observed for young and aged PFC samples when incubated with the indicated antibody. *p<0.05 versus young.
In contrast to the GABABR1 subunits, levels of hippocampal GABABR2 did not reliably differ as a function of age (t(22)=−1.31, ns; Fig. 4C) or cognitive status (F(2,21)=1.42, ns; Fig. 4D) and no significant relationship between levels of this protein and SLI were observed (aged: r=−0.20, ns; young: r=−0.10, ns).
3.6 GABABR subunit expression in prefrontal cortex
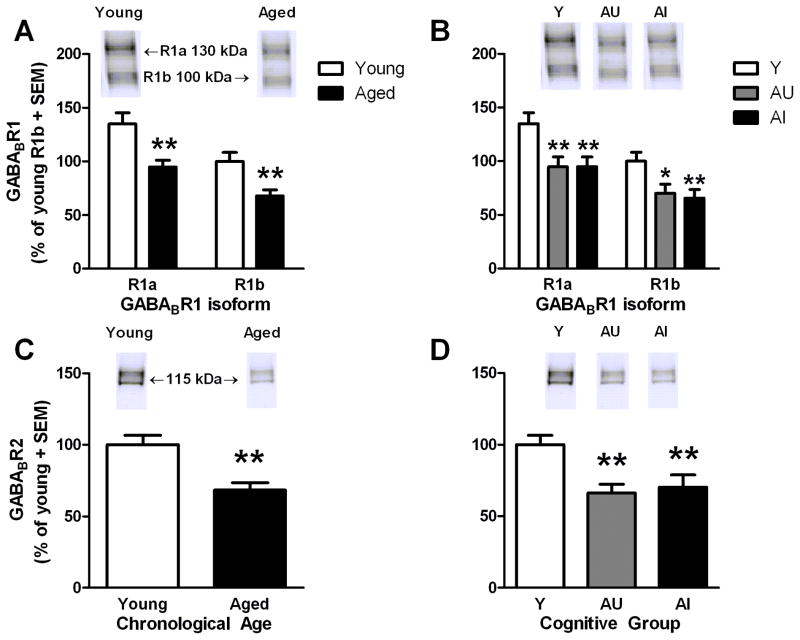
Comparisons between ages revealed main effects of GABABR1 isoform (F(1,22)=819.64, p<0.001) and age (F(1,22)=11.44, p<0.01; Fig. 5A). First, in contrast to hippocampus, GABABR1a was present in greater levels than GABABR1b (1.38-fold; p<0.001) in the PFC. In relation to age, both GABABR1a and GABABR1b protein levels were significantly reduced by a similar magnitude in aged rats relative to young (−30% and −32%, respectively; p<0.01 for both). A two-way isoform × cognitive group ANOVA also revealed main effects of isoform (F(2,21)=956.93, p<0.001) and cognitive group (F(2,21)=5.48, p<0.05). Protein levels were significantly decreased in both AU (p<0.05) and AI rats (p<0.01) relative to young, but were not significantly different between these two aged groups. When isoforms were analyzed separately, each isoform was significantly reduced in both AU (p<0.01 for alpha and p<0.05 for beta; Fig. 5B) and AI rats (p<0.01 for both) relative to young, but neither isoform was significantly different when comparing between the two aged groups. Futhermore, proteins levels of either isoform were not associated with SLI (aged: r≤0.14, ns).
Figure 5. GABABR1 and GABABR2 protein levels in the prefrontal cortex of young and aged rats.
Specific GABABR1 isoforms were similarly reduced by aging [A], but not differentially changed among cognitive groups [B]. Levels of GABABR2 are decreased by 32% in the aged PFC [C] irrespective of cognitive group [D]. Insets of A–D demonstrate representative immunoreactive bands observed for young and aged hippocampal samples when incubated with the indicated antibody. ** p<0.01 versus young.* p<0.05, ** p<0.01 versus young.
In PFC, GABABR2 protein levels were reduced in aged relative to young rats (- 32%); t(22)= 3.65, p<0.01; Fig. 5C). There was also a main effect of cognitive group (F(2,21)=6.49, p<0.01; Fig. 5D) but as with GABABR1, post-hoc comparisons revealed a similar magnitude reduction of GABABR2 levels in AU and AI rats relative to young (−34% and −30%, respectively, p<0.01 for both). GABABR2 protein levels did not differ between AU and AI rats (p>0.05) nor was there a significant relationship between reduced GABABR2 protein levels and SLI scores (aged: r=0.15, ns).
3.7. Regional differences in GABABR1 isoform expression
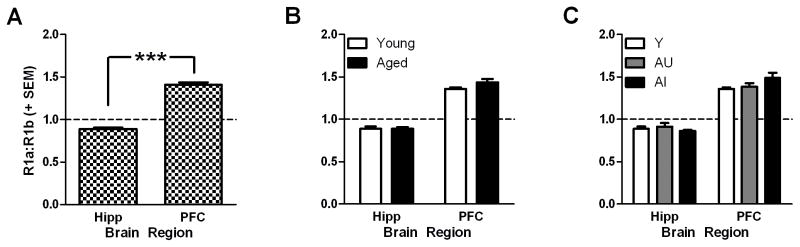
Main effects of isoform were observed, independent of age or cognitive status, demonstrating that the expression of each isoform was not equivalent within either region (Figs 4 and 5). The antibody used to detect GABABR1 was raised against an antigenic epitope common to both GABABR1a and GABABR1b isoforms thus enabling quantification of both isoforms within a single immunoblot due to their differing molecular weights. While direct comparisons between isoform levels between brain regions are not possible in our current study, we computed the ratios of alpha-to-beta for each animal for both regions to formally compare the relative abundance of each isoform between the hippocampus and PFC. The ratio of R1a:R1b was significantly different between the PFC and hippocampus (t(46)=16.60, p<0.001; Fig. 6A), and these ratios were not significantly changed when dividing the experimental cohort between young and aged rats (F(1,22)=1.187, ns; Fig. 6B) or among cognitive groups (F(2,21)=0.797, ns; Fig. 6C). Futhermore, the PFC ratio was significantly greater than 1 (t(23)=15.547, p<0.001; Fig. 6A) whereas the hippocampus ratio was significantly less than 1 (t(23)=−6.638, p<0.001; Fig. 6A). The results of these comparisons formally support the within-region conclusions: R1a is the dominant GABABR1 isoform in PFC while R1b is dominant in hippocampus.
Figure 6. Ratios of GABABR1a:GABABR1b across PFC and hippocampus of young and aged rats.
Relative levels of GABABR1a and GABABR1b were significantly different between the PFC and hippocampus (Hipp) when results from young and aged rats were pooled into a single analysis [A]. Relative expression ratios did not change as a function of age [B] or cognitive group [C]. Dashed line indicates a hypothetical value of 1 where the relative levels of expression of both isoforms are equivalent. *** p<0.001 PFC versus hippocampus.
4. Discussion
An emerging concept in both human and rodent cognitive aging is that age-related decline in hippocampal and frontal cortical systems occur somewhat independently and that consideration of both systems is essential for a thorough understanding of cognitive dysfunction and the development of effective interventions to promote successful cognitive aging. Findings from the current study in which we report differential effects of age on GABABR expression and signaling in hippocampus and PFC supports this concept as signaling via this receptor is markedly attenuated in PFC but largely unaltered in hippocampus.
4.1. Age-associated changes to GABAB receptors in the prefrontal cortex
Baclofen-stimulated GTP-binding as well as both GABABR subunits were significantly decreased in the aged PFC. While decreased GABABR function and protein expression in the aged PFC was not specifically associated with the severity of cognitive impairment, this result is not entirely unexpected as the watermaze task used for behavioral characterization is hippocampal-dependent and may not have been sufficiently sensitive to age-related alterations in PFC function. While some investigations have revealed significant alterations to PFC GPCRs in spatial learning-impaired aged rats (Parent et al., 1995; Zhang et al., 2007), impaired performance on frontal cortical dependent tasks (e.g. working memory, attentional set-shifting) is largely dissociable from hippocampal-dependent spatial reference memory deficits in aged rats and mice (Schoenbaum et al., 2002; Barense et al., 2002; Lambert et al., 2005; Bizon et al., 2009). Within this context, it is interesting to consider that the same signaling cascades downstream of receptor:G-protein complexes can exert unique (and opposing) actions on cognition supported by PFC and hippocampus. Protein kinase A (PKA) is a downstream effector of the GABABR and reduced inhibitory drive by these receptors would be expected to result in a failure to inhibit PKA activity. In agreement with this prediction, there is evidence that the cAMP-PKA pathway becomes disinhibited in PFC at advanced ages, and moreover, that such disinhibition contributes to loss of PFC-dependent cognition (Ramos et al., 2003). While drugs directed at increasing activation of the cAMP-PKA pathway generally enhance hippocampal-dependent plasticity, disinhibiting this signaling pathway and increasing PKA activity in PFC can instead impair working memory in young adult rats (Taylor et al., 1999). These data, together with the current findings, suggest that decreased modulation of this signaling pathway within PFC in aging, but not hippocampus, could contribute to specific cognitive deficits. Consequently, it will be important in the future to further explore the relationship between behaviors that are mediated by frontal cortical nuclei and the status of this receptor system and its effectors in frontal regions in young and aged rats, and specifically to determine the extent to which alterations to the GABABR system described here contribute to age-related changes in PFC-dependent cognition (Schoenbaum et al., 2002; Barense et al., 2002; Rodefer and Nguyen, 2008; Simon et al., 2010).
Although baclofen-stimulated efficacy was decreased in the aged PFC, affinity for the receptor (EC50 values) did not change with age. In contrast to the binding of antagonists, agonists preferentially bind to receptors in their G-protein coupled, or high affinity, state. Levels of Gαi or Gαo, the G-proteins that couple to GABABRs, were not directly measured in the current study, however, the lack of a change in EC50 suggests that sufficient quantities of Gαi and Gαo are present in aged PFC to facilitate normal agonist binding. In agreement, an autoradiographic study demonstrated decreased affinity of GABA for GABABRs in between 2 and 3 months of age in F344 rat cortex, but no change between 3 and 23 months of age suggesting that early development, but not advancing age, modulates GABABR:G-protein coupling (Turgeon and Albin, 1994). Similarly, levels of Gαq/11, another G-protein subtype, are not changed by aging in the PFC (Zhang et al., 2007) suggesting that G-protein expression is largely resistant to changes by the normal aging process. Instead, a marked reduction in receptor proteins (−30%) was associated with the attenuation in maximal receptor response, providing evidence that blunted GTP-binding in aged PFC is largely mediated by loss of functional GABABR receptor proteins.
While the changes in GABABR signaling in PFC reported here adds to a growing body of work indicating that GPCRs are altered in the normal aging process, unique mechanisms appear to underlie changes in G-protein signaling across neurotransmitter systems. While mAChR GTP-binding is also attenuated in the PFC of aged Long-Evans rats (Zhang et al., 2007), in contrast to the current findings, mAChRs in the aged PFC become functionally decoupled from their cognate G-proteins without outright loss of receptors. Other evidence indicates that mAChR- and mGluR-mediated production of inositol phosphates is actually elevated in spatially-impaired aged F344 rats relative to aged-matched spatially-unimpaired cohorts (Parent et al., 1995). While the reasons for discrepancies in mAChR status across studies are not entirely clear, the data to date do support varied effects of age on GPCRs. Such differences can be likely attributed in part to distinct G-proteins and downstream effectors that are activated by individual receptors. For example, whereas a subset of mAChRs and mGluRs signal via activation of the effector phospholipase C, GABABRs inhibit adenylyl cyclase. A better understanding of the specific mechanisms that mediate age-related alterations in GPCR signaling in PFC across receptor/effector systems is an important topic for future study, particularly with respect to the development of pharmacotherapies targeting GPCRs.
4.2. GABABR1 subunit is selectively lost in the hippocampus of rats with cognitive impairment
In contrast to the PFC, no difference in baclofen-stimulated GTP-binding was observed between the young and aged hippocampus. However, in this same tissue, expression of the GABABR1 protein was selectively reduced in spatially-impaired aged rats while spatially-unimpaired aged-matched controls expressed this protein at levels that were indistinguishable from young. The basis for the loss of this receptor protein may be attributable, in part, to the well-described phenotypic changes that are associated with GABAergic interneurons within the aged hippocampus. While the number of GAD-67-expressing cells declines with age, this change appears to be largely mediated by specific subclasses of interneurons such as somatostatin- and calbindin-immunopositive cells whereas those interneurons that express other molecular markers like calretinin and parvalbumin are relatively unaffected (Shetty and Turner, 1998; Stanley and Shetty, 2004; Stanley et al., 2011). Notably, cytosolic expression of GABABR1 is observed within these same susceptible somatostatin- and calbindin-immunopositive cells but generally is not associated with resistant parvalbumin- or calretinin-expressing interneurons (Sloviter et al., 1999). Thus, reduced GABABR1 protein may be secondary to functional loss of interneurons or, alternatively, decreased GABABR1 expression may increase the vulnerability of these neurons to the effects of aging.
Despite the significant, yet selective changes to GABABR1 in the aged hippocampus, preserved receptor activity in this region may be of consequence to hippocampal-dependent behaviors. Post-synaptic excitatory receptors or associated functions are depressed in the aged hippocampus, including NMDA receptor expression (Wenk and Barnes, 2000; Clayton and Browning, 2001; Adams et al., 2001), NMDAR associated plasticity (Lee et al., 2005; Boric et al., 2008), and GPCR mediated activity dependent on M1 muscarinic receptors (Chouinard et al., 1995; Nicolle et al., 2001; Zhang et al., 2007) and Group I metabotropic glutamate receptors (Nicolle et al., 1999). Thus, the relative sparing of inhibitory GABABR signaling in hippocampus suggests an imbalance between excitation and inhibition of hippocampal synaptic activity that might in turn contribute to cognitive deficits. In fact, apart from the ability of GABABRs to regulate post-synaptic excitability directly or indirectly via inhibition of glutamate release, GABABRs suppress NMDA receptor calcium signals in a G-protein/PKA dependent manner (Chalifoux and Carter, 2010) and produce heterosynaptic depression at mossy fiber-CA3 synapses (Guetg et al., 2009) which are spared relative to perforant path inputs in the aged hippocampus (Smith et al., 2000). If the contributions of the GABABR become more pronounced with age in the hippocampus, moderate antagonism of the GABABR might be expected to restore a more favorable ratio of excitatory-to-inhibitory neurotransmission and to facilitate normal learning and memory. Indeed, GABAB antagonists have shown potential to enhance working and reference memory in animal models and preliminary clinical studies (Froestl et al., 2004; Lasarge et al., 2009), but no candidates have approved for use in patients (Sabbagh, 2009), underscoring the need for additional basic science work to more clearly elucidate the role for this system in the context of the aged brain and postulate a mechanism of action for GABAB-based pharmacotherapies.
4.3. Pharmacological characteristics and relative composition of GABABRs are region-specific
While the primary aim of our study was to assess age-related changes in the hippocampus and PFC, there are also notable differences in the pharmacology and relative abundance of GABABR1 isoforms between the PFC and hippocampus. While binding parameters did not deviate from unity in the hippocampus, slope factors in the PFC were significantly lower. Negative cooperativity observed with baclofen-stimulated GTP-binding in PFC suggests these GABABRs, but not those in hippocampus, form unique complexes possibly with other GPCRs, effectors such as K+ channels, or allosteric modulators of G-protein activity (i.e. RGS4; David et al., 2006; Fowler et al., 2007; Ciruela et al., 2010). The negative modulation of binding observed in the PFC is consistent with emerging evidence that suggests that GABABR heterodimers can form complexes with other GABAB heterodimers to negatively modulate ligand-binding:G-protein coupling (Maurel et al., 2008).
Additionally, we compared the relative levels of GABABR1 isoforms and found that GABABR1a is the dominant isoform in the PFC, while GABABR1b is the dominant isoform in the hippocampus. Our findings in the hippocampus closely match the observed ratios reported in a previous experiment using a similar approach to quantify relative levels of each isoform (Fritschy et al., 1999). However, ours is the first study to specifically quantify relative levels of each isoform in the PFC, rather than the cerebral cortex as a whole. While homogenates prepared from whole brain or whole cortex demonstrate greater levels of GABABR1b relative to GABABR1a, the PFC, a heterogeneous grouping of brain nuclei including the infralimbic/prelimbic, orbitofrontal, and frontal association areas, shows the reverse trend.
Collectively, these region-specific observations suggest that systemically administered compounds may activate GABABRs less efficiently in the PFC than the hippocampus and preferentially modulate presynaptic release in the PFC and post-synaptic excitation in the hippocampus. While it is not possible to infer how interactions between brain regions will ultimately influence complex behaviors in intact animals based upon our current data, these region-specific characteristics of GABABRs will help to better inform lead optimization when screening GABAB compounds and interpretation of behavioral endpoints in preclinical studies.
5. Conclusion
The present experiments investigated functions and protein levels of PFC and hippocampal GABABRs and revealed significant regionally-specific differences in expression of GABABR complexes in adult and aged brains. Specifically, significant age-related reductions in GABABR mediated GTP-binding were accompanied by decreased levels of GABABR proteins in the prefrontal cortex. Conversely, there was no change in GABABR GTP-binding in the hippocampus, but there was a selective loss of the GABABR1 subunit in aged rats with spatial learning impairment. Overall, these results indicate that normal aging differentially modulates the expression of GABABRs between the PFC and hippocampus and these changes have significant effects on signaling efficacy within the PFC.
Acknowledgments
This work was supported by the National Institute on Aging grants R01-AG020572 to MMN and R01-AG029421 to JLB and the McKnight Brain Research Foundation. The authors also wish to thank Allyn C. Howlett for expert advice offered in the interpretation of the GTP-binding results.
Footnotes
Disclosure/Conflict of Interest
The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams MM, Smith TD, Moga D, Gallagher M, Wang Y, Wolfe BB, Rapp PR, Morrison JH. Hippocampal dependent learning ability correlates with N-methyl-D-aspartate (NMDA) receptor levels in CA3 neurons of young and aged rats. J Comp Neurol. 2001;432:230–243. doi: 10.1002/cne.1099. [DOI] [PubMed] [Google Scholar]
- Barense MD, Fox MT, Baxter MG. Aged rats are impaired on an attentional set-shifting task sensitive to medial frontal cortex damage in young rats. Learn Mem. 2002;9:191–201. doi: 10.1101/lm.48602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter MG, Gallagher M. Neurobiological substrates of behavioral decline: Models and data analytic strategies for individual differences in aging. Neurobiol Aging. 1996;17:491–495. doi: 10.1016/0197-4580(96)00011-5. [DOI] [PubMed] [Google Scholar]
- Biermann B, Ivankova-Susankova K, Bradaia A, Abdel AS, Besseyrias V, Kapfhammer JP, Missler M, Gassmann M, Bettler B. The Sushi domains of GABAB receptors function as axonal targeting signals. J Neurosci. 2010;30:1385–1394. doi: 10.1523/JNEUROSCI.3172-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bizon JL, Helm KA, Han J, Chun H, Pucilowska J, Lund PK, Gallagher M. Hypothalamic–pituitary–adrenal axis function and corticosterone receptor expression in behaviourally characterized young and aged Long–Evans rats. European Journal of Neuroscience. 2001;14:1739–1751. doi: 10.1046/j.0953-816x.2001.01781.x. [DOI] [PubMed] [Google Scholar]
- Bizon JL, Lasarge CL, Montgomery KS, McDermott AN, Setlow B, Griffith WH. Spatial reference and working memory across the lifespan of male Fischer 344 rats. Neurobiol Aging. 2009;30:646–655. doi: 10.1016/j.neurobiolaging.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boric K, Muñoz P, Gallagher M, Kirkwood A. Potential Adaptive Function for Altered Long-Term Potentiation Mechanisms in Aging Hippocampus. J Neurosci. 2008;28:8034–8039. doi: 10.1523/JNEUROSCI.2036-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalifoux JR, Carter AG. GABAB Receptors Modulate NMDA Receptor Calcium Signals in Dendritic Spines. Neuron. 2010;66:101–113. doi: 10.1016/j.neuron.2010.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chouinard ML, Gallagher M, Yasuda RP, Wolfe BB, McKinney M. Hippocampal muscarinic receptor function in spatial learning-impaired aged rats. Neurobiol Aging. 1995;16:955–963. doi: 10.1016/0197-4580(95)02015-2. [DOI] [PubMed] [Google Scholar]
- Ciruela F, Fernandez-Duenas V, Sahlholm K, Fernandez-Alacid L, Nicolau JC, Watanabe M, Lujan R. Evidence for oligomerization between GABAB receptors and GIRK channels containing the GIRK1 and GIRK3 subunits. Eur J Neurosci. 2010;32:1265–1277. doi: 10.1111/j.1460-9568.2010.07356.x. [DOI] [PubMed] [Google Scholar]
- Clayton DA, Browning MD. Deficits in the expression of the NR2B subunit in the hippocampus of aged Fisher 344 rats. Neurobiol Aging. 2001;22:165–168. doi: 10.1016/s0197-4580(00)00196-2. [DOI] [PubMed] [Google Scholar]
- David M, Richer M, Mamarbachi AM, Villeneuve LR, Dupre DJ, Hebert TE. Interactions between GABA-B1 receptors and Kir 3 inwardly rectifying potassium channels. Cell Signal. 2006;18:2172–2181. doi: 10.1016/j.cellsig.2006.05.014. [DOI] [PubMed] [Google Scholar]
- Fowler CE, Aryal P, Suen KF, Slesinger PA. Evidence for association of GABA(B) receptors with Kir3 channels and regulators of G protein signalling (RGS4) proteins. J Physiol. 2007;580:51–65. doi: 10.1113/jphysiol.2006.123216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritschy J, Meskenaite V, Weinmann O, Honer M, Benke D, Mohler H. GABAB-receptor splice variants GB1a and GB1b in rat brain: developmental regulation, cellular distribution and extrasynaptic localization. Eur J Neurosci. 1999;11:761–768. doi: 10.1046/j.1460-9568.1999.00481.x. [DOI] [PubMed] [Google Scholar]
- Froestl W, Gallagher M, Jenkins H, Madrid A, Melcher T, Teichman S, Mondadori CG, Pearlman R. SGS742: the first GABAB receptor antagonist in clinical trials. Biochem Pharmacol. 2004;68:1479–1487. doi: 10.1016/j.bcp.2004.07.030. [DOI] [PubMed] [Google Scholar]
- Gallagher M, Burwell R, Burchinal M. Severity of spatial learning impairment in aging: development of a learning index for performance in the Morris water maze. Behav Neurosci. 1993;107:618–626. doi: 10.1037//0735-7044.107.4.618. [DOI] [PubMed] [Google Scholar]
- Guetg N, Seddik R, Vigot R, Turecek R, Gassmann M, Vogt KE, Bräuner-Osborne H, Shigemoto R, Kretz O, Frotscher M, et al. The GABAB1a Isoform Mediates Heterosynaptic Depression at Hippocampal Mossy Fiber Synapses. J Neurosci. 2009;29:1414–1423. doi: 10.1523/JNEUROSCI.3697-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KA, Borowsky B, Tamm JA, Craig DA, Durkin MM, Dai M, Yao WJ, Johnson M, Gunwaldsen C, Huang LY, et al. GABA(B) receptors function as a heteromeric assembly of the subunits GABA(B)R1 and GABA(B)R2. Nature. 1998;396:674–679. doi: 10.1038/25348. [DOI] [PubMed] [Google Scholar]
- Kaupmann K, Huggel K, Heid J, Flor PJ, Bischoff S, Mickel SJ, McMaster G, Angst C, Bittiger H, Froestl W, et al. Expression cloning of GABA(B) receptors uncovers similarity to metabotropic glutamate receptors. Nature. 1997;386:239–246. doi: 10.1038/386239a0. [DOI] [PubMed] [Google Scholar]
- Kaupmann K, Malitschek B, Schuler V, Heid J, Froestl W, Beck P, Mosbacher J, Bischoff S, Kulik A, Shigemoto R, et al. GABA(B)-receptor subtypes assemble into functional heteromeric complexes. Nature. 1998;396:683–687. doi: 10.1038/25360. [DOI] [PubMed] [Google Scholar]
- Lambert TJ, Fernandez SM, Frick KM. Different types of environmental enrichment have discrepant effects on spatial memory and synaptophysin levels in female mice. Neurobiol Learn Mem. 2005;83:206–216. doi: 10.1016/j.nlm.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Lasarge CL, Banuelos C, Mayse JD, Bizon JL. Blockade of GABA(B) receptors completely reverses age-related learning impairment. Neuroscience. 2009;164:941–947. doi: 10.1016/j.neuroscience.2009.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H-K, Min SS, Gallagher M, Kirkwood A. NMDA receptor-independent long-term depression correlates with successful aging in rats. Nat Neurosci. 2005;8:1657–1659. doi: 10.1038/nn1586. [DOI] [PubMed] [Google Scholar]
- Luscher C, Jan LY, Stoffel M, Malenka RC, Nicoll RA. G protein-coupled inwardly rectifying K+ channels (GIRKs) mediate postsynaptic but not presynaptic transmitter actions in hippocampal neurons. Neuron. 1997;19:687–695. doi: 10.1016/s0896-6273(00)80381-5. [DOI] [PubMed] [Google Scholar]
- Margeta-Mitrovic M, Jan YN, Jan LY. A trafficking checkpoint controls GABA(B) receptor heterodimerization. Neuron. 2000;27:97–106. doi: 10.1016/s0896-6273(00)00012-x. [DOI] [PubMed] [Google Scholar]
- Maurel D, Comps-Agrar L, Brock C, Rives ML, Bourrier E, Ayoub MA, Bazin H, Tinel N, Durroux T, Prezeau L, et al. Cell-surface protein-protein interaction analysis with time-resolved FRET and snap-tag technologies: application to GPCR oligomerization. Nat Methods. 2008;5:561–567. doi: 10.1038/nmeth.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara RK, Skelton RW. Baclofen, a selective GABAB receptor agonist, dose-dependently impairs spatial learning in rats. Pharmacol Biochem Behav. 1996;53:303–308. doi: 10.1016/0091-3057(95)02025-x. [DOI] [PubMed] [Google Scholar]
- Nicolle MM, Colombo PJ, Gallagher M, McKinney M. Metabotropic Glutamate Receptor-Mediated Hippocampal Phosphoinositide Turnover Is Blunted in Spatial Learning-Impaired Aged Rats. J Neurosci. 1999;19:9604–9610. doi: 10.1523/JNEUROSCI.19-21-09604.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolle MM, Gallagher M, McKinney M. Visualization of muscarinic receptor-mediated phosphoinositide turnover in the hippocampus of young and aged, learning-impaired Long Evans rats. Hippocampus. 2001;11:741–746. doi: 10.1002/hipo.1089. [DOI] [PubMed] [Google Scholar]
- Odagaki Y, Koyama T. Identification of galpha subtype(s) involved in gamma-aminobutyric acid(B) receptor-mediated high-affinity guanosine triphosphatase activity in rat cerebral cortical membranes. Neurosci Lett. 2001;297:137–141. doi: 10.1016/s0304-3940(00)01692-x. [DOI] [PubMed] [Google Scholar]
- Odagaki Y, Nishi N, Koyama T. Functional coupling of GABA(B) receptors with G proteins that are sensitive to N-ethylmaleimide treatment, suramin, and benzalkonium chloride in rat cerebral cortical membranes. J Neural Transm. 2000;107:1101–1116. doi: 10.1007/s007020070024. [DOI] [PubMed] [Google Scholar]
- Parent A, Rowe W, Meaney MJ, Quirion R. Increased production of inositol phosphates and diacylglycerol in aged cognitively impaired rats after stimulation of muscarinic, metabotropic-glutamate and endothelin receptors. J Pharmacol Exp Ther. 1995;272:1110–1116. [PubMed] [Google Scholar]
- Pin JP, Comps-Agrar L, Maurel D, Monnier C, Rives ML, Trinquet E, Kniazeff J, Rondard P, Prezeau L. G-protein-coupled receptor oligomers: two or more for what? Lessons from mGlu and GABAB receptors. J Physiol. 2009;587:5337–5344. doi: 10.1113/jphysiol.2009.179978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos BP, Birnbaum SG, Lindenmayer I, Newton SS, Duman RS, Arnsten AFT. Dysregulation of Protein Kinase A Signaling in the Aged Prefrontal Cortex: New Strategy for Treating Age-Related Cognitive Decline. Neuron. 2003;40:835–845. doi: 10.1016/s0896-6273(03)00694-9. [DOI] [PubMed] [Google Scholar]
- Robbins MJ, Calver AR, Filippov AK, Hirst WD, Russell RB, Wood MD, Nasir S, Couve A, Brown DA, Moss SJ, et al. GABA(B2) is essential for g-protein coupling of the GABA(B) receptor heterodimer. J Neurosci. 2001;21:8043–8052. doi: 10.1523/JNEUROSCI.21-20-08043.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodefer JS, Nguyen TN. Naltrexone reverses age-induced cognitive deficits in rats. Neurobiol Aging. 2008;29:309–313. doi: 10.1016/j.neurobiolaging.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Sabbagh MN. Drug development for Alzheimer’s disease: where are we now and where are we headed? Am J Geriatr Pharmacother. 2009;7:167–185. doi: 10.1016/j.amjopharm.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G, Nugent S, Saddoris MP, Gallagher M. Teaching old rats new tricks: age-related impairments in olfactory reversal learning. Neurobiol Aging. 2002;23:555–564. doi: 10.1016/s0197-4580(01)00343-8. [DOI] [PubMed] [Google Scholar]
- Shetty AK, Turner DA. Hippocampal interneurons expressing glutamic acid decarboxylase and calcium-binding proteins decrease with aging in Fischer 344 rats. J Comp Neurol. 1998;394:252–269. [PubMed] [Google Scholar]
- Simon NW, LaSarge CL, Montgomery KS, Williams MT, Mendez IA, Setlow B, Bizon JL. Good things come to those who wait: Attenuated discounting of delayed rewards in aged Fischer 344 rats. Neurobiol Aging. 2010;31:853–862. doi: 10.1016/j.neurobiolaging.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloviter RS, Ali-Akbarian L, Elliott RC, Bowery BJ, Bowery NG. Localization of GABAB (R1) receptors in the rat hippocampus by immunocytochemistry and high resolution autoradiography, with specific reference to its localization in identified hippocampal interneuron subpopulations. Neuropharmacology. 1999;38:1707–1721. doi: 10.1016/s0028-3908(99)00132-x. [DOI] [PubMed] [Google Scholar]
- Smith TD, Adams MM, Gallagher M, Morrison JH, Rapp PR. Circuit-specific alterations in hippocampal synaptophysin immunoreactivity predict spatial learning impairment in aged rats. J Neurosci. 2000;20:6587–6593. doi: 10.1523/JNEUROSCI.20-17-06587.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley DP, Shetty AK. Aging in the rat hippocampus is associated with widespread reductions in the number of glutamate decarboxylase-67 positive interneurons but not interneuron degeneration. J Neurochem. 2004;89:204–216. doi: 10.1111/j.1471-4159.2004.02318.x. [DOI] [PubMed] [Google Scholar]
- Stanley EM, Fadel JR, Mott DD. Interneuron loss reduces dendritic inhibition and GABA release in hippocampus of aged rats. Neurobiol Aging [Internet] 2011 doi: 10.1016/j.neurobiolaging.2010.12.014. In Press, Corrected Proof. Available from: http://www.sciencedirect.com/science/article/B6T09-522YC1J-1/2/0d0119ed33d9307305d6294c76f17a43. [DOI] [PMC free article] [PubMed]
- Stranahan AM, Jiam NT, Stocker AM, Gallagher M. Aging reduces total neuron number in the dorsal component of the rodent prefrontal cortex. The Journal of Comparative Neurology. 2011 doi: 10.1002/cne.22790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Kajikawa Y, Tsujimoto T. G-Protein-coupled modulation of presynaptic calcium currents and transmitter release by a GABAB receptor. J Neurosci. 1998;18:3138–3146. doi: 10.1523/JNEUROSCI.18-09-03138.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JR, Birnbaum S, Ubriani R, Arnsten AFT. Activation of cAMP-Dependent Protein Kinase A in Prefrontal Cortex Impairs Working Memory Performance. J Neurosci. 1999;19:RC23. doi: 10.1523/JNEUROSCI.19-18-j0001.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turgeon SM, Albin RL. GABAB binding sites in early adult and aging rat brain. Neurobiol Aging. 1994;15:705–711. doi: 10.1016/0197-4580(94)90052-3. [DOI] [PubMed] [Google Scholar]
- Vigot R, Barbieri S, Brauner-Osborne H, Turecek R, Shigemoto R, Zhang YP, Lujan R, Jacobson LH, Biermann B, Fritschy JM, et al. Differential compartmentalization and distinct functions of GABAB receptor variants. Neuron. 2006;50:589–601. doi: 10.1016/j.neuron.2006.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldmeier PC, Kaupmann K, Urwyler S. Roles of GABAB receptor subtypes in presynaptic auto- and heteroreceptor function regulating GABA and glutamate release. J Neural Transm. 2008;115:1401–1411. doi: 10.1007/s00702-008-0095-7. [DOI] [PubMed] [Google Scholar]
- Wenk GL, Barnes CA. Regional changes in the hippocampal density of AMPA and NMDA receptors across the lifespan of the rat. Brain Research. 2000;885:1–5. doi: 10.1016/s0006-8993(00)02792-x. [DOI] [PubMed] [Google Scholar]
- White JH, Wise A, Main MJ, Green A, Fraser NJ, Disney GH, Barnes AA, Emson P, Foord SM, Marshall FH. Heterodimerization is required for the formation of a functional GABA(B) receptor. Nature. 1998;396:679–682. doi: 10.1038/25354. [DOI] [PubMed] [Google Scholar]
- Zhang H-Y, Watson ML, Gallagher M, Nicolle MM. Muscarinic receptor-mediated GTP-Eu binding in the hippocampus and prefrontal cortex is correlated with spatial memory impairment in aged rats. Neurobiol Aging. 2007;28:619–626. doi: 10.1016/j.neurobiolaging.2006.02.016. [DOI] [PubMed] [Google Scholar]