Abstract
Lymphovascular invasion (LVI) is a prognostic factor in many types of human malignancies including pancreatic ductal adenocarcinoma (PDAC). However, the prognostic significance of LVI in patients with PDAC who received neoadjuvant therapy and pancreaticoduodenectomy (PD) is unclear. In this study, we analyzed LVI in 212 patients who received neoadjuvant chemoradiation and subsequent PD at our institution between January 1999 and December 2007. LVI was present in 61.8 % (131/212) of the patients. Of the 131 cases that were positive for LVI, 67 (31.6%) patients had tumor invasion into lymphovascular spaces without muscle layer (non-muscular LVS) and 64 (30.2%) had tumor invasion into muscular vessels. The presence of tumor invasion into muscular vessels correlated with higher frequencies of positive resection margin, lymph node metastasis, and locoregional/distant recurrence. Patients with tumor invasion into muscular vessels had significantly shorter disease-free survival (DFS) and overall survival (OS) than those patients who had no LVI or who had tumor invasion of non-muscular LVS (p<0.01). Tumor invasion into muscular vessels is an independent prognostic factor in patients with PDAC who received neoadjuvant therapies. Our results showed tumor invasion into muscular vessels plays an important role in the progression of PDAC and in predicting the prognosis in this group of patients.
Keywords: pancreatic cancer, muscular vessel invasion, lymphovascular invasion, survival, prognosis
Introduction
Pancreatic cancer is the 4th leading cause of cancer death in the United States and has a poor prognosis. Surgical resection remains the only hope for curative therapy for patients with pancreatic cancer. However, most patients with pancreatic ductal adenocarcinoma (PDAC) present late with locoregional advanced disease or metastatic dissemination and are not surgically resectable (17). Even in patients with PDAC who underwent surgical resection, the disease commonly recurs and long-term survival rate is only 10%-20%(16, 26). This is in part due to high frequencies of subclinical metastases at the time of diagnosis and surgery that are not detectable by radiologic imaging. Previous studies have shown that post-operative adjuvant chemoradiation improves the survival and delay tumor recurrence in patients with PDAC who underwent pancreatectomy (7, 16, 22, 24, 25). However, the overall survival for patients with PDAC has not changed significantly over the last four decades, despite significant improvements in surgical oncology and peri-operative mortality associated with pancreatectomy (12). Recently, pre-operative neoadjuvant chemoradiation therapies have been increasingly used to treat the patients with potentially resectable PDAC. In a phase II trial of neoadjuvant gemcitabine-based chemoradiation of 86 patients with PDAC, who were treated with 7 weekly intravenous (IV) infusions of gemcitabine (400 mg/m2 IV over 30 minutes) plus radiation therapy (30 Gy in 10 fractions over 2 weeks), Evans et al. reported a median survival of 34 months and 36% 5-year survival rate for the 64 patients who underwent pancreaticoduodenectomy (PD) that was better than the 22 patients who did not underwent PD (median survival of 7 months and 0% 5-year survival) (10). Similar results have been reported from another phase II trial of 79 patients who received the preoperative gemcitabine and cisplatin chemotherapy in addition to gemcitabine-based chemoradiation. In this trial, the 52 patients who completed neoadjuvant therapy and underwent PD had better survival (median survival of 31 months) than the 27 patients who did not undergo surgical resection (median survival of 10.5 months) (27). These data suggest that neoadjuvant chemoradiation is safe and may improve the survival in patients with PDAC who underwent PD (13).
Previous studies have shown that lymph node metastasis, tumor size, tumor differentiation, resection margin status, perineural and lymphovascular invasion, and the American Joint Committee on Cancer (AJCC) tumor stage correlate independently with survival in patients with PDAC who underwent PD (1, 3, 4, 6, 11, 12). However, most previous studies are based on patient populations with PDAC who did not receive pre-operative neoadjuvant therapy. Little is known about the prognostic factors in patients with PDAC who received neoadjuvant chemoradiation and PD. In our previous studies, we demonstrated that posttherapy pathologic stage, lymph node status, the number of positive regional lymph nodes, and the histologic grading of residual viable tumor are independent prognostic factors in this group of patients (5, 9). The prognostic significance of lymphovascular invasion in patients with PDAC who received neoadjuvant therapies and underwent PD is unclear. In this study, we evaluated lymphovascular invasion (LVI) by reviewing the archival hematoxylin & eosin (H&E) stained slides from 212 patients who received neoadjuvant chemoradiation therapy and underwent PD at our institution. The results of LVI were correlated with survival and other clinical and pathological parameters. Our data showed that tumor invasion into muscular vessels is an important prognostic factor and an independent predictor of overall and disease-free survival in this group of patients.
Materials and Methods
Patient population
Our study population consisted of 212 patients with PDAC who received neoadjuvant chemoradiation therapy and PD at our institution from January 1999 to December 2007. The neoadjuvant therapy regimens in this study population included five different treatment groups: group 1, fluoropyrimidine-based chemoradiation, 39 patients (18.4%); group 2, gemcitabine-based chemoradiation, 66 patients (31.1%); group 3, systemic chemotherapy followed by gemcitabine-based chemoradiation, 70 patients (33.0%); group 4, systemic chemotherapy followed by fluoropyrimidine-based chemoradiation, 32 patients (15.1%); and group 5, systemic chemotherapy alone, 5 patients (2.4%). Among these patients, 136 (64.2%, groups 2 and 3) were treated on previously published protocols (10, 27). After completion of neoadjuvant therapy, all patients underwent restaging evaluation and PD was performed only in patients with resectable disease, who did not have disease progression, metastasis or contraindications to major abdominal surgery. Patients who underwent distal pancreatectomy and those who underwent PD for other types of pancreatic tumors were excluded. There were 124 male and 88 female patients with age ranging from 39 to 85 years (median age: 63 years). The study was approved by the Institutional Review Board of the University of Texas M.D. Anderson Cancer Center.
Pathologic examination
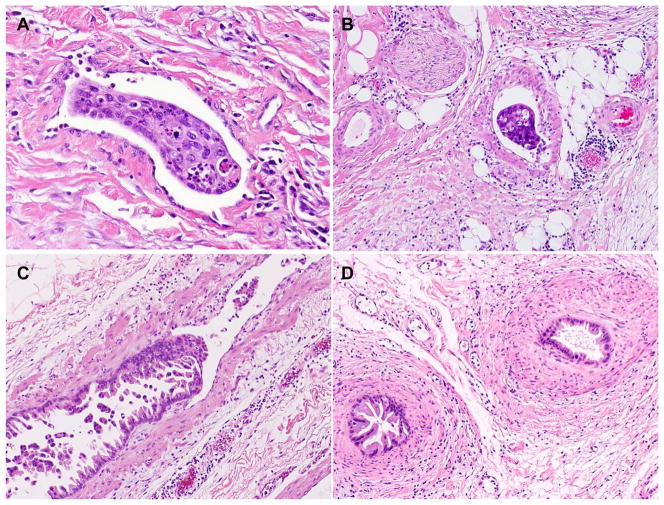
The archival H & E stained slides from all cases were uniformly reviewed by a pathologist (D.C.). In this study, we classified LVI into two types: tumor invasion into lymphovascular spaces lined by endothelium without muscle layer (non-muscular LVS) and tumor invasion into muscular vessels, which was defined by histology as the presence of tumor cells in the lumen of vascular structures containing circumferential smooth muscle layers. If both tumor invasion into muscular vessels and non-muscular LVS were present, the case was considered as positive for tumor invasion into muscular vessels. Representative micrographs of tumor invasion into non-muscular LVS and tumor invasion into muscular vessels are shown in Figure 1. In the cases with questionable LVI or to determine the type of vessels with tumor invasion, the H&E stained slides were evaluated by two additional gastrointestinal pathologists (H.W. and A.R.) and the consensus for the presence or absence of LVI and tumor invasion into non-muscular LVS or into the muscular vessels among the three pathologists was used. The presence of tumor cell invasion into the vascular wall, but no tumor cells in the lumen, was also considered as negative for LVI or tumor invasion into muscular vessels. In addition, other histologic parameters, including tumor size, differentiation, lymph node status, margin status by histology were also reviewed and recorded. If any of the resection margins of a PD specimen was involved by PDAC, the case was classified as margin positive. The total number of slides reviewed from the pancreas and tumor ranged from 3 to 45 (the mean number of slides: 14). The posttherapy pathologic staging was grouped according to the AJCC Staging Manual, 7th edition (8). The extent of residual viable tumor cells in post-therapy PD specimen was graded as previously: response group 1 (no viable tumor or less then 5% of viable tumor cells) and response group 2 (5% or more viable tumor cells) (5).
Figure 1.
Representative micrographs of tumor invasion into the lymphovascular space without muscle layer (non-muscular LVS, A) and tumor invasion into muscular vessels (B). Pancreatic ductal adenocarcinoma invades into and grows along the endothelial surface in a muscular vessel (C). D, Cross-sections of intravascular tumor growth shown in Figure C with smooth muscle layers wrapping around the tumor cells mimicking pancreatic intraepithelial neoplasia (PanIN). Hematoxylin & eosin stain, original magnifications: 200x
Clinical follow-up and Statistical analysis
Patient clinical and follow-up information through December of 2009 was extracted from a prospectively maintained database. The clinical and follow-up data from all patients has been verified by independent review of patient medical records and the U.S. Social Security Index. Local/regional or distant recurrence at first site or sites were classified based on the computer tomography (CT) scan as previously defined (5). Chi-square analysis or Fisher’s exact tests was used to compare categorical data and analysis of variance (ANOVA) was used to compare continuous variables. The Kaplan-Meier method was used to construct the survival curves and the log-rank test was used to evaluate the statistical significance of differences in survival. Disease-free survival (DFS)was calculated as the time from the date of surgery to the date of first recurrence after surgery (in patients with recurrence)or to the date of last follow-up (in patients without recurrence). Overall survival (OS) was calculated as the time from the date of diagnosis to the date of death or the date of last follow-up (if death did not occur). Univariate Cox regression analysis was used to examine the prognostic significance of LVI and other clinicopathologic characteristics. After interactions between the variables were examined, a backward stepwise procedure was used to derive the best-fitting model for multivariate analysis using Cox proportional hazards models. All statistical analyses were performed using Statistical Package for Social Sciences software (for Windows 12.0, SPSS Inc., Chicago, IL). A two-sided significance level of 0.05 was used for all statistical analyses.
Results
Correlation of LVI with clinicopathologic parameters
LVI was identified in 131 (61.8%) of 212 patients. Among the patients who had LVI, 67 (31.6%) patients had tumor invasion into non-muscular LVS and 64 (30.2%) had tumor invasion into muscular vessels. The correlations of tumor invasion into non-muscular LVS and muscular vessels in PD with other clinicopathologic features are summarized in Table 1. Compared to the patients who had no LVI, patients who had tumor invasion into non-muscular LVS showed higher frequency of lymph node metastasis and higher AJCC stage (p<0.01). However, the presence of tumor invasion into non-muscular LVS in PD specimens had no significant correlations with other clinicopathologic parameters (p>0.05). The presence of tumor invasion into muscular vessels correlated significantly with higher frequencies of positive resection margin (p=0.03), lymph node metastasis (p<0.001), posttherapy pathologic tumor stage (ypT, p=0.007), posttherapy AJCC stage (p<0.001) and worse tumor response to therapy (p=0.01) compared to those patients who were negative for LVI (Table 1). During follow-up, 58/64 (90.6%) patients who had tumor invasion into muscular vessels developed local recurrence or distant metastasis, which was significantly higher than 58.0% (47/81) for the patients who had no LVI or 67.2% (45/67) in those who had tumor invasion into non-muscular LVS (P=0.002). Patients with tumor invasion into muscular vessels also had higher frequency of positive resection margin than those patients who had tumor invasion into non-muscular LVS (p=0.04). There were no significant differences in other clinicopathologic parameters between the group with tumor invasion into muscular vessels and those with tumor invasion into non-muscular LVS (p>0.05). These data suggest that presence of tumor invasion into muscular vessels in patients with PDAC is associated with more aggressive behavior of the tumor.
Table 1.
Clinicopathologic correlation of lymphovascular invasion in patients with pancreatic ductal adenocarcinoma who received neoadjuvant therapy and pancreaticoduodenectomy
| Characteristics | Negative (%) (n=81) | Non-muscular LVS invasion (%) (n=67) | Muscular vessel invasion (%) (n=64) | P value |
|---|---|---|---|---|
| Age (yrs) | 0.27 | |||
| <60 | 28 (34.6) | 29 (43.3) | 31 (48.4) | |
| 60–70 | 29 (35.8) | 26 (38.8) | 22 (34.4) | |
| >70 | 24 (29.6) | 12 (17.9) | 11 (17.2) | |
| Gender | 0.59 | |||
| Female | 37 (45.7) | 27 (40.3) | 24 (37.5) | |
| Male | 44 (54.3) | 40 (59.7) | 40 (62.5) | |
| Neoadjuvant therapy | 0.16 | |||
| Group 1 | 15 (18.5) | 11 (16.4) | 13 (20.3) | |
| Group 2 | 30 (37.0) | 18 (26.9) | 18 (28.1) | |
| Group 3 | 23 (28.4) | 22 (32.8) | 25 (39.1) | |
| Group 4 | 11 (13.6) | 16 (23.9) | 5 (7.8) | |
| Group 5 | 2 (2.5) | 0 (0) | 3 (4.7) | |
| Tumor differentiation | 0.54 | |||
| Well-Moderate | 49 (60.5) | 41 (61.2) | 44 (68.8) | |
| Poor | 32 (39.5) | 26 (38.8) | 20 (31.2) | |
| Tumor size | 0.30 | |||
| ≤2cm | 34 (42.0) | 26 (38.8) | 19 (29.7) | |
| >2cm | 47 (58.0) | 41 (61.2) | 45 (70.3) | |
| Resection margin | 0.03 | |||
| Negative | 75 (92.6) | 62 (92.5) | 51 (79.7) | |
| Positive | 6 (7.4) | 5 (7.5) | 13 (20.3) | |
| Pathologic tumor stage | 0.007 | |||
| ypT1-2 | 10 (12.3) | 3 (4.5) | 0 (0) | |
| ypT3 | 71 (87.7) | 64 (95.5) | 64 (100) | |
| Lymph node | <0.001 | |||
| Negative | 49 (60.5) | 24 (35.8) | 14 (21.9) | |
| Positive | 32 (39.5) | 43 (64.2) | 50 (78.1) | |
| AJCC stage | <0.001 | |||
| IA and IB | 8 (9.9) | 2 (3.0) | 0 (0) | |
| IIA | 41 (50.6) | 22 (32.8) | 14 (21.9) | |
| IIB | 32 (39.5) | 43 (64.2) | 50 (78.1) | |
| Tumor response | 0.03 | |||
| Response group 1 | 18 (22.2) | 10 (14.9) | 4 (6.3) | |
| Response group 2 | 63 (77.8) | 57 (85.1) | 60 (93.7) | |
| Recurrence | 0.002 | |||
| No | 34 (42.0) | 22 (32.8) | 6 (9.4) | |
| Local | 10 (12.3) | 14 (20.9) | 16 (25.0) | |
| Distant | 37 (45.7) | 31 (46.3) | 42 (65.6) |
Correlation of LVI with survival
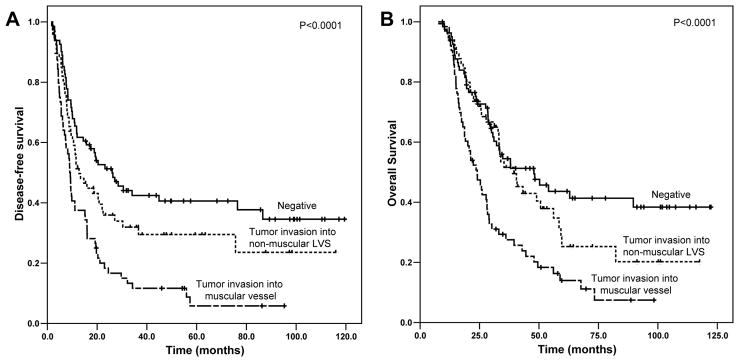
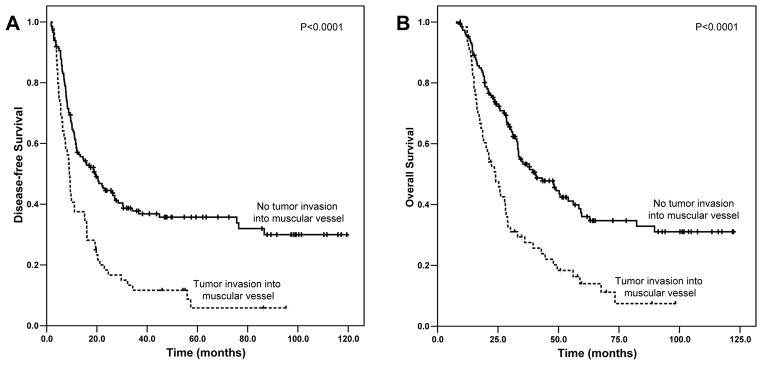
The median follow-up of all patients was 33.2 months ranging from 7.6 months to 122.3 months. Patients with tumor invasion into muscular vessels had a median DFS of 9.0 months [95% confidence interval (CI): 7.8–10.2 months], which was significantly shorter than the median DFS of 26.0 months (95% CI: 15.0–37.1 months) in patients who had no LVI (p<0.001) and the median DFS of 12.8 months (95% CI: 7.7–17.8 months) in patients who had tumor invasion into non-muscular LVS (p=0.006) (Figure 2A). Patients with tumor invasion into muscular vessels also had significantly shorter OS than patients who were negative for LVI or who had tumor invasion into non-muscular LVS (Figure 2B). The median OS for patients who had tumor invasion into muscular vessels was 24.0 months (95% CI: 19.0–29.0 months) compared to median OS of 47.9 months (95% CI: 28.6–67.2 months) in patients who had no LVI (p<0.001) and median OS of 39.5 months [95% CI: 29.2–49.7 months] for patients with tumor invasion into non-muscular LVS (p= 0.002, Figure 2B). Although patients with tumor invasion into non-muscular LVS had shorter DFS and OS compared to those patients who had no LVI, the differences in DFS and OS between these two groups were not statistically significant (p>0.05, Figure 2). Patients with tumor invasion into muscular vessels had shorter DFS and OS than those patients who had no tumor invasion into muscular vessels (Figure 3A and 3B). The median DFS and OS for patients who had tumor invasion into muscular vessels were 9.0 months (95% CI: 7.8–10.2 months) and 24.0 months (95% CI: 19.0–29.0 months) respectively compared to the median DFS of 19.3 months (95% CI: 12.1–26.5 months, P<0.0001) and median OS of 40.5 months (95% CI: 28.6–52.4 months, P<0.0001) in patients who had no tumor invasion into muscular vessels.
Figure 2.
Kaplan-Meier survival curves of disease-free survival (A) and overall survival (B) stratified by the absence of lymphovascular invasion and presence of tumor invasion into non-muscular lymphovascular spaces (LVS) or tumor invasion into muscular vessels in patients with PDAC who received neoadjuvant therapy and pancreaticoduodenectomy. The patients with tumor invasion into muscular vessels had shorter DFS and OS than those patients who were negative for lymphovascular invasion or those who had tumor invasion into non-muscular LVS.
Figure 3.
Kaplan-Meier survival curves of disease-free survival (A) and overall survival (B) stratified by the absence and presence of tumor invasion into muscular vessels in patients with PDAC who received neoadjuvant therapy and pancreaticoduodenectomy. Patients with tumor invasion into muscular vessels had shorter DFS and OS than those patients who had no tumor invasion into muscular vessels.
The results from univariate Cox’s regression analysis for DFS and OS are shown in Table 2 Both DFS and OS were significantly associated with lymph node metastasis, posttherapy AJCC tumor stage, tumor invasion into muscular vessels (p<0.05). In addition, OS was also associated with resection margin status (p=0.008) and ypT (p=0.02). There was no significant association of either DFS or OS with gender, intra-operative blood loss, neoadjuvant therapy regimens, tumor differentiation or tumor size (p>0.05, Table 2). In multivariate analysis, the presence of tumor invasion into muscular vessels was an independent prognostic factor for both OS and DFS (p<0.001, Table 3).
Table 2.
Univariate Cox Regression Analysis of Disease-free and Overall Survival in Relation to Clinicopathologic Features
| Characteristics | Number of patients | Disease -free survival
|
Overall survival
|
||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Gender | |||||
| Females (ref) | 88 | 1.00 | 1.00 | ||
| Males | 124 | 0.97 (0.70–1.34) | 0.84 | 1.04 (0.74–1.46) | 0.82 |
| Age (yrs) | 0.04 | 0.60 | |||
| <60 (ref) | 88 | 1.00 | 1.00 | ||
| 60–70 | 77 | 0.81 (0.57–1.16) | 0.25 | 0.94 (0.65–1.36) | 0.74 |
| >70 | 47 | 0.56 (0.35–0.87) | 0.01 | 0.78 (0.49– 1.26) | 0.31 |
| Blood loss | 212 | 1.00 (1.00–1.00) | 0.22 | 1.00 (1.00–1.00) | 0.10 |
| Neoadjuvant therapy | 0.98 | 0.59 | |||
| Group 1 (ref) | 39 | 1.00 | 1.00 | ||
| Group 2 | 66 | 0.80 (0.49–1.30) | 0.37 | 0.73 (0.44–1.21) | 0.23 |
| Group 3 | 70 | 1.27 (0.80–2.01) | 0.32 | 0.95 (0.59–1.55) | 0.84 |
| Group 4 | 32 | 1.50 (0.86–2.60) | 0.15 | 0.99 (0.55–1.78) | 0.97 |
| Group 5 | 5 | 1.50 (0.53–4.30) | 0.45 | 1.34 (0.47–3.85) | 0.59 |
| Tumor size | |||||
| ≤2cm (ref) | 79 | 1.00 | 1.00 | ||
| >2cm | 133 | 1.29 (0.92–1.81) | 0.14 | 1.24 (0.87– 1.76) | 0.24 |
| Margin | |||||
| Negative (ref) | 188 | 1.00 | 1.00 | ||
| Positive | 24 | 1.52 (0.95– 2.41) | 0.08 | 1.88 (1.18–3.00) | 0.008 |
| Tumor differentiation | |||||
| Well-moderate (ref) | 134 | 1.00 | 1.00 | ||
| Poor | 78 | 1.23 (0.89–1.72) | 0.21 | 1.29 (0.92–1.81) | 0.15 |
| Pathologic tumor stage | |||||
| ypT1-ypT2 (ref) | 13 | 1.00 | 1.00 | ||
| ypT3 | 199 | 1.98 (0.93–4.23) | 0.08 | 3.38 (1.25–9.15) | 0.02 |
| Lymph nodes | |||||
| Negative (ref) | 87 | 1.00 | 1.00 | ||
| Positive | 125 | 1.59 (1.14–2.22) | 0.007 | 1.77 (1.24–2.53) | 0.002 |
| AJCC Stage | 0.01 | 0.003 | |||
| Stage IA and IB (ref) | 10 | 1.00 | 1.00 | ||
| Stage IIA | 77 | 1.94 (0.77–4.86) | 0.16 | 4.57 (1.11–18.88) | 0.04 |
| Stage IIB | 125 | 2.84 (1.15–6.99) | 0.02 | 6.99 (1.72–28.45) | 0.007 |
| Tumor invasion of muscular vessels | |||||
| Absent (ref) | 148 | 1.00 | 1.00 | ||
| Present | 64 | 2.09 (1.50–2.91) | <0.001 | 2.15 (1.52– 3.04) | <0.001 |
Abbreviations: HR, hazard ratio; CI, confidence interval; ref, reference; SMV/PV, superior mesenteric vein/portal vein
Table 3.
Multivariate Cox Regression Analysis of Disease-free and Overall Survival in Relation to Clinicopathologic Features
| Characteristics | Number of patients | Disease -free survival
|
Overall survival
|
||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Age (yrs) | 0.05 | NA | |||
| <60 (ref) | 88 | 1.00 | |||
| 60–70 | 77 | 0.91 (0.64–1.30) | 0.61 | ||
| >70 | 47 | 0.57 (0.37–0.90) | 0.02 | ||
| Margin | |||||
| Negative (ref) | 188 | 1.00 | 1.00 | ||
| Positive | 24 | 1.30 (0.80–2.09) | 0.29 | 1.56 (0.97–2.51) | 0.07 |
| Pathologic stage | 0.17 | 0.03 | |||
| IA and IB (ref) | 10 | 1.00 | 1.00 | ||
| IIA | 77 | 2.04 (0.80–5.18) | 0.13 | 3.97 (0.96–16.47) | 0.06 |
| IIB | 125 | 2.35 (0.94–5.89) | 0.07 | 5.31 (1.29–21.83) | 0.02 |
| Invasion of muscular vessels | |||||
| Absent (ref) | 148 | 1.00 | 1.00 | ||
| Present | 64 | 2.08 (1.49–2.92) | <0.001 | 1.80 (1.26–2.57) | 0.001 |
Abbreviations: HR, hazard ratio; CI, confidence interval; ref, reference; SMV/PV, superior mesenteric vein/portal vein
Discussion
In this study, we examined LVI in the PD specimens from 212 patients with PDAC who received neoadjuvant therapy. We found that the presence of tumor invasion into non-muscular LVS or tumor invasion into muscular vessels correlated with lymph node metastasis and posttherapy AJCC stage. In addition, we found that tumor invasion into muscular vessels was also correlated with higher frequencies of positive resection margin, locoregional and distant recurrence and higher ypT stage. Tumor invasion into muscular vessels correlated significantly with both DFS and OS and was an independent prognostic factor for both DFS and OS in multivariate analysis. Therefore, tumor invasion into muscular vessels plays an important role in tumor progression and in predicting the prognosis in patients with PDAC who received neoadjuvant therapy and PD.
Tumor invasion into the lymphatic channels and blood vessels are an important pathway by which tumors progress and spread to the adjacent and distant tissues or organs and has been shown to be an important prognostic factor in many types of human malignancies. In patients with PDAC who underwent PD first and did not receive neoadjuvant therapy, LVI has been reported in 48% to 92% of the PD specimens (2, 15). The presence of LVI in PD specimen has been shown to be associated with peritoneal dissemination and worse survival in patients with PDAC who received surgery first but no neoadjuvant therapy (1, 6, 15). However, the frequency of LVI and its prognostic value in patients with PDAC who received neoadjuvant therapy is largely unknown. In this study, we found that LVI was present in 61.8% of the cases in a cohort of 212 patients with PDAC who received neoadjuvant therapy and PD. We further subclassified LVI into tumor invasion into non-muscular LVS and tumor invasion into muscular vessels. Our data showed that tumor invasion into muscular vessels correlated with higher frequency of positive resection margin, posttherapy pathologic tumor stage, lymph node status, posttherapy AJCC stage, worse tumor response to therapy, and higher risk of locoregional/distant recurrence compared to those who had no tumor invasion into muscular vessels in patients with PDAC who received neoadjuvant therapy. In addition, presence of tumor invasion into muscular vessels correlated with shorter DFS and OS compared to those patients who had no tumor invasion into muscular vessels. However, we did not observe significant differences in either DFS or OS between the patients who had tumor invasion into non-muscular LVS and those who had no LVI. Using univariate and multivariate analysis, we demonstrated that the presence of tumor invasion into muscular vessels was an independent prognostic factor for both DFS and OS in our patient population. Our findings are consistent with previous reports that the presence of LVI are associated with posttherapy pathologic stage and worse five-year survival rate in patients with gastric and rectal adenocarcinoma who received neoadjuvant therapy (18, 21, 23). In a cohort of 297 patients with locally advanced rectal adenocarcinoma who received neoadjuvant therapy, Guillem et al showed that the presence of LVI, along with perineural invasion, tumor response >95% and lymph node metastasis were independent prognostic factors for both DFS and OS in their patient population (14). Our results suggest that tumor invasion into muscular vessels plays an important role in tumor progression and in predicting prognosis in patients with PDAC who received neoadjuvant therapy. The shorter survival in our patients with tumor invasion into muscular vessels may be due to the higher incidence of local recurrence and distant metastasis observed in this group of patients compared to those with no LVI or those with tumor invasion into non-muscular LVS alone.
To evaluate the role of LVI in predicting the prognosis in patients with PDAC who did not receive neoadjuvant therapy, we examined lymphovascular involvement and its correlation with survival and clinicopathologic features in 60 consecutive patients who did not receive any form of neoadjuvant therapy prior to PD (untreated group) during the same time period at our institution. We found that LVI was present in 47 (78.3%) untreated cases, which is higher than those who received neoadjuvant therapy (61.8%, p=0.02). The difference in the frequency of tumor invasion into muscular vessels in the untreated group (24/60, 40.0%) was not statistically significant (p=0.16) compared to those who received neoadjuvant therapy (64/212, 30.2%). Similar to our findings in patients who received neoadjuvant therapy, the presence of LVI correlated with lymph node metastasis in untreated group (p<0.05, data not shown). In contrast to previous reports that the presence of LVI is associated with peritoneal dissemination and worse survival in patients with PDAC who received surgery first but no neoadjuvant therapy (1, 6, 15), we did not observe significant correlations of LVI or tumor invasion into muscular vessels with other clinicopathologic parameters, DFS, or OS in the untreated group (p>0.05, data not shown). This may be due in part to the highly selective patient population with PDAC who received neoadjuvant therapy in our treated group to exclude those patients whose tumor had more aggressive clinical behavior during the neoadjuvant chemoradiation treatment.
One weakness of this study is that we evaluated LVI on H&E stained sections, while other studies have used immunohistochemical markers for the lymphatic or blood vessels involvement by tumor cells, such as CD34 and CD31 for blood vessels and podoplanin/D2-40 for lymphatics (19, 20). However, immunohistochemical stains for either lymphatics or blood vessels are rarely performed for lymphovascular involvement in PD specimens in patients with PDAC during routine histopathology evaluation. In addition, the cases with questionable LVI or tumor invasion into muscular vessels in this study had been reviewed by two additional gastrointestinal pathologists and the consensus for presence or absence of lymphovascular involvement was used for statistical analysis. We felt that our approach is more practical and easily applicable by other pathologists.
In summary, the data present here show that tumor invasion into muscular vessels correlates with higher frequencies of positive resection margin, lymph node metastasis and locoregional/distant recurrence in patients with PDAC who received neoadjuvant therapy and PD. Tumor invasion into muscular vessels is associated with shorter DFS and OS and is an independent prognostic factor for both DFS and OS in our treated patient population. Therefore, tumor invasion into muscular vessels plays an important role in tumor progression and the prognosis in patients with PDAC who received neoadjuvant therapy and pancreaticoduodenectomy.
Acknowledgments
Supported by the National Institutes of Health grant (1R21CA149544-01A1) and G. S. Hogan Gastrointestinal Cancer Research Fund at The University of Texas M.D. Anderson Cancer Center
Footnotes
The author(s) have no conflicts of interest or funding to disclose
References
- 1.Badger SA, Brant JL, Jones C, et al. The role of surgery for pancreatic cancer: a 12-year review of patient outcome. Ulster Med J. 2010;79:70–75. [PMC free article] [PubMed] [Google Scholar]
- 2.Barbier L, Turrini O, Gregoire E, et al. Pancreatic head resectable adenocarcinoma: preoperative chemoradiation improves local control but does not affect survival. HPB (Oxford) 2011;13:64–69. doi: 10.1111/j.1477-2574.2010.00245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benassai G, Mastrorilli M, Quarto G, et al. Factors influencing survival after resection for ductal adenocarcinoma of the head of the pancreas. J Surg Oncol. 2000;73:212–218. doi: 10.1002/(sici)1096-9098(200004)73:4<212::aid-jso5>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 4.Cameron JL, Crist DW, Sitzmann JV, et al. Factors influencing survival after pancreaticoduodenectomy for pancreatic cancer. Am J Surg. 1991;161:120–124. doi: 10.1016/0002-9610(91)90371-j. discussion 124–125. [DOI] [PubMed] [Google Scholar]
- 5.Chatterjee D, Katz MH, Rashid A, et al. Histologic Grading the Extent of Residual Carcinoma Following Neoadjuvant Chemoradiation in Pancreatic Ductal Adenocarcinoma: A Predictor for Patient Outcome. Cancer. 2011 doi: 10.1002/cncr.26651. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen JW, Bhandari M, Astill DS, et al. Predicting patient survival after pancreaticoduodenectomy for malignancy: histopathological criteria based on perineural infiltration and lymphovascular invasion. HPB (Oxford) 2010;12:101–108. doi: 10.1111/j.1477-2574.2009.00140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Conlon KC, Klimstra DS, Brennan MF. Long-term survival after curative resection for pancreatic ductal adenocarcinoma. Clinicopathologic analysis of 5-year survivors. Ann Surg. 1996;223:273–279. doi: 10.1097/00000658-199603000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edge S, Byrd D, Compton C, et al., editors. AJCC Cancer Staging Manual. New York: Springer; 2010. [Google Scholar]
- 9.Estrella JS, Rashid A, Fleming JB, et al. Post-therapy pathologic stage and survival in patients with pancreatic ductal adenocarcinoma treated with neoadjuvant chemoradiation. Cancer. doi: 10.1002/cncr.26243. [DOI] [PubMed] [Google Scholar]
- 10.Evans DB, Varadhachary GR, Crane CH, et al. Preoperative gemcitabine-based chemoradiation for patients with resectable adenocarcinoma of the pancreatic head. J Clin Oncol. 2008;26:3496–3502. doi: 10.1200/JCO.2007.15.8634. [DOI] [PubMed] [Google Scholar]
- 11.Gao CT, Li HK, Li Q. Factors influencing survival of patients with cancer of the pancreatic head after resection. Zhonghua Zhong Liu Za Zhi. 2009;31:554–557. [PubMed] [Google Scholar]
- 12.Garcea G, Dennison AR, Pattenden CJ, et al. Survival following curative resection for pancreatic ductal adenocarcinoma. A systematic review of the literature. JOP. 2008;9:99–132. [PubMed] [Google Scholar]
- 13.Gillen S, Schuster T, Meyer Zum Buschenfelde C, et al. Preoperative/neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of response and resection percentages. PLoS Med. 2010;7:e1000267. doi: 10.1371/journal.pmed.1000267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guillem JG, Chessin DB, Cohen AM, et al. Long-term oncologic outcome following preoperative combined modality therapy and total mesorectal excision of locally advanced rectal cancer. Ann Surg. 2005;241:829–836. doi: 10.1097/01.sla.0000161980.46459.96. discussion 836–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hatzaras I, George N, Muscarella P, et al. Predictors of survival in periampullary cancers following pancreaticoduodenectomy. Annals of surgical oncology. 17:991–997. doi: 10.1245/s10434-009-0883-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hernandez JM, Morton CA, Al-Saadi S, et al. The natural history of resected pancreatic cancer without adjuvant chemotherapy. Am Surg. 2010;76:480–485. [PubMed] [Google Scholar]
- 17.Huguet F, Orthuon A, Touboul E, et al. Pancreatic cancer. Cancer Radiother. 2010;14 (Suppl 1):S94–102. doi: 10.1016/S1278-3218(10)70012-3. [DOI] [PubMed] [Google Scholar]
- 18.Mansour JC, Tang L, Shah M, et al. Does graded histologic response after neoadjuvant chemotherapy predict survival for completely resected gastric cancer? Annals of surgical oncology. 2007;14:3412–3418. doi: 10.1245/s10434-007-9574-6. [DOI] [PubMed] [Google Scholar]
- 19.Mohammed RA, Martin SG, Gill MS, et al. Improved methods of detection of lymphovascular invasion demonstrate that it is the predominant method of vascular invasion in breast cancer and has important clinical consequences. Am J Surg Pathol. 2007;31:1825–1833. doi: 10.1097/PAS.0b013e31806841f6. [DOI] [PubMed] [Google Scholar]
- 20.Mohammed RA, Martin SG, Mahmmod AM, et al. Objective assessment of lymphatic and blood vascular invasion in lymph node-negative breast carcinoma: findings from a large case series with long-term follow-up. J Pathol. 2011;223:358–365. doi: 10.1002/path.2810. [DOI] [PubMed] [Google Scholar]
- 21.Moral M, Fdez-Acenero MJ, Cuberes R, et al. Factors influencing prognosis after neo-adjuvant chemoradiation therapy for rectal carcinoma. Acta Chir Belg. 2009;109:345–351. doi: 10.1080/00015458.2009.11680437. [DOI] [PubMed] [Google Scholar]
- 22.Regine WF, Abrams RA. Adjuvant therapy for pancreatic cancer: current status, future directions. Semin Oncol. 2006;33:S10–13. doi: 10.1053/j.seminoncol.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 23.Rullier A, Laurent C, Vendrely V, et al. Impact of colloid response on survival after preoperative radiotherapy in locally advanced rectal carcinoma. Am J Surg Pathol. 2005;29:602–606. doi: 10.1097/01.pas.0000153120.80385.29. [DOI] [PubMed] [Google Scholar]
- 24.Squadroni M, Fazio N. Chemotherapy in pancreatic adenocarcinoma. Eur Rev Med Pharmacol Sci. 2010;14:386–394. [PubMed] [Google Scholar]
- 25.Stocken DD, Buchler MW, Dervenis C, et al. Meta-analysis of randomised adjuvant therapy trials for pancreatic cancer. Br J Cancer. 2005;92:1372–1381. doi: 10.1038/sj.bjc.6602513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tepper J, Nardi G, Sutt H. Carcinoma of the pancreas: review of MGH experience from 1963 to 1973. Analysis of surgical failure and implications for radiation therapy. Cancer. 1976;37:1519–1524. doi: 10.1002/1097-0142(197603)37:3<1519::aid-cncr2820370340>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 27.Varadhachary GR, Wolff RA, Crane CH, et al. Preoperative gemcitabine and cisplatin followed by gemcitabine-based chemoradiation for resectable adenocarcinoma of the pancreatic head. J Clin Oncol. 2008;26:3487–3495. doi: 10.1200/JCO.2007.15.8642. [DOI] [PubMed] [Google Scholar]