Abstract
Numerous studies have identified age differences in brain structure and function that correlate with declines in motor performance. While these investigations have typically focused on activity in isolated regions of the brain, resting state functional connectivity MRI and diffusion tensor imaging allow for more integrative assessments of spatially disparate neural networks. The novel contribution of the current study is to combine both resting state functional connectivity and diffusion tensor imaging to examine motor cortico-cortical circuits in young and older adults. We find that relatively greater functional connectivity between the primary motor cortices was strongly associated with decreased structural connectivity and poorer motor performance solely in older adults. We suggest that greater functional connectivity in older adults may be reflective of a release from the normally predominantly inhibitory interhemispheric communication associated with the primary motor cortices.
1. Introduction
Numerous studies have identified age differences in brain structure, function, and biochemistry that correlate with declines in motor (cf. Seidler et al., 2010) and cognitive (cf. Reuter-Lorenz and Lustig, 2005) performance. However, these investigations have typically focused on isolated regions of interest. In contrast, recent approaches in resting state functional connectivity MRI (fcMRI) and diffusion tensor imaging (DTI) have been used to assess brain functional (fcMRI) and structural (DTI) network connectivity, allowing for more integrative assessments of distributed neural systems. There is currently scant information about whether structural and functional connectivity are affected by age in a parallel fashion and whether the two are related to motor behavior in unique or overlapping ways.
FcMRI studies have shown that brain regions with similar functions and known anatomical connections exhibit strong correlations in the low-frequency blood oxygen level dependent (BOLD) signal when individuals are at rest (cf. Biswal et al., 1995; Fox, 2007, Vincent et al., 2007). Intriguingly, while most patient groups exhibit reduced functional network connectivity (e.g. Jelsone-Swain et al., 2010), recent work provides compelling evidence that older adults demonstrate increased connectivity of interhemispheric motor cortical networks compared to young adults (Langan et al., 2010; Zuo et al., 2010). Furthermore, older adults with greater functional connectivity between the motor cortices exhibit less ipsilateral sensorimotor cortex activation during a unimanual motor task (Langan et al., 2010). One notable limitation of fcMRI is that correlated activity between two structures can reflect both direct and indirect connections (Fox, 2007). Moreover, a change in coupling as measured with fcMRI may also reflect changes in signal-to-noise ratio within a given structure (Donahue et al., 2011). Thus integrating fcMRI with measures of structural connectivity can provide additional insight.
In organized tissue such as white matter in the brain and muscle, diffusion of water is anisotropic, i.e. direction dependent (cf. Chanraud et al., 2010). DTI has been used to map and assess structural integrity of motor cortical network tracts (Fling et al., in press; Hofer and Frahm, 2006). A growing body of literature indicates that not only is the quantity of white matter reduced in older adults, but the quality of remaining white matter is compromised as well (reviewed in Seidler et al., 2010). Specifically, older adults demonstrate a marked loss of myelinated fibers (Marner et al., 2003), which can be assessed with radial diffusivity (measured with DTI; Gulani et al., 2001) a sensitive measure of white matter microstructural decline with age. Recent work from our lab has demonstrated that the microstructural integrity of callosal regions connecting sensorimotor cortical targets is related to motor performance in a differential fashion for young and older adults. Specifically, on a unimanual tapping task greater callosal microstructure was related to better performance solely in older adults (Fling et al., 2011).
There is a gap in understanding how brain structural and functional network connectivity are interrelated, how they change with age, and how such changes contribute to older adults’ sensorimotor deficits. The goal of the current study was to integrate across measures of functional and structural connectivity to determine how they relate to motor function in young and older adults. We hypothesized that advanced age would be associated with decreased integrity of interhemispheric structural connectivity (DTI) and a concomitant increase in the strength of resting state functional connectivity (fcMRI) between primary motor cortices. Further, we predicted that relatively poorer structural cortico-cortical fiber tract integrity would be associated with greater functional connectivity solely in older adults, reflective of an age-related shift towards greater interhemispheric facilitatory communication between motor cortices. Finally, based upon previous work (Fling et al., 2011; Langan et al., 2010) we predicted that both poorer structural and greater functional connectivity would be negatively related to motor performance in older but not young adults.
2. Methods
2.1 Participants
Twelve young adults (7 males; mean age 22.1 ± 2.8 years; range 18–28 years) were recruited from the student population at the University of Michigan (average education: 14.9 years). Fifteen community-dwelling older adults (8 males; 67.2 ± 5.2 years; range 65–76 years) also participated in this study (average education: 15.3 years). Participants were strongly right-handed as determined by the Edinburgh Handedness Inventory (YA: 0.87; OA:0.9 Oldfield, 1971).
2.2 Motor Paradigm (Unimanual tapping)
Thirty minutes prior to their MRI scan, participants performed a finger tapping task consisting of four 30-second tapping trials, with 20 seconds of visual fixation at the start and end of each of these trials (Fling et al., 2011). Participants were asked to focus on a fixation crosshair in the center of a computer monitor (LabView 6.1 National Instruments). Red circles (13 mm diameter) flashed 2cm from the right side of fixation at 1Hz to pace participants. Participants tapped with their right (dominant) index finger at 1 Hz and were instructed not to use the circle appearance as a cue to tap; instead they were to tap in synchrony with its onset.
2.3 Image acquisition
We collected whole brain high-resolution structural MR images on a 3T MRI scanner (General Electric, Waukesha, WI, USA) using a spoiled gradient echo sequence (124 slices, field-of-view: 24 cm, voxel size: 0.94 × 0.94 × 1.4 mm, TR: 10.2 ms and TE: 3.4 ms).
Functional Connectivity (fcMRI)
240 T2* - weighted BOLD images (TR = 2 s, TE = 30 ms, flip angle = 90°, FOV = 220 × 220 mm, voxel size = 3.4 × 3.4 × 3.2 mm, 40 axial slices) were collected using a single-shot gradient-echo reverse spiral pulse sequence. A visual fixation cross was presented using a rear projection visual display. Participants were instructed to keep their eyes centered on the cross and to not think about anything in particular. A pressure belt was placed around the abdomen of each participant to monitor the respiratory signal. A pulse oximeter was placed on the participant’s finger to monitor the cardiac signal. The respiratory, cardiac and fMRI data collection were synchronized.
Structural Connectivity (DTI)
Diffusion weighted images were collected using a single shot echo-planar sequence in the axial plane (39 slices; TE/TR: 82.8 ms/9000 ms; image field of view: 220 mm × 220 mm; voxel size 3 × 3 × 3 mm; b-value = 800 s/mm2; 15 diffusion-sensitizing directions). Images were motion and eddy-current corrected. Using the averaged images with b = 0 and b = 800 s/mm2, the diffusion tensor was calculated and fractional anisotropy (FA) images were constructed off-line using ExploreDTI (Tournier et al., 2011). Diffusion tensors were calculated from the 15 DW images based upon a simple least squares fit of the tensor model to the diffusion data (Basser, et al. 2000). Diagonalization of the tensor yields three voxel-specific eigenvalues (λ1 > λ2 > λ3) representing diffusivities along the three principle directions of the tensor. The three eigenvectors were then used to construct fiber tracts and the resultant diffusion properties as described below.
Fiber Tractography
Interhemispheric fiber tractography between bilateral primary motor cortices (M1s) was performed using a previously described technique (Fling et al., in press). Briefly, each participant’s FA map was normalized into MNI space, aligned along the anterior/posterior commissure line with the coordinate 0, 0, 0 placed at the brain’s center of mass, and voxel size was re-sampled to 2 × 2 × 2 mm through the use of ExploreDTI (Tournier et al., 2011). The Human Motor Area Template (HMAT; Mayka et al., 2006) was co-registered to each individual’s MNI-normalized FA image and subsequently used as a mask of the M1. Interhemispheric fiber tracts were identified by placing seed and target ROIs in homologous M1 regions (e.g. right and left) as identified by the HMAT. Fiber tracts were constructed based upon deterministic streamline tractography (cf. Mori and van Zijl, 2002).
2.4 Data Analysis
Motor Performance
Motor performance was defined as the standard deviation of the intertap interval. This is a typical measure used to describe tapping consistency for visually-paced unimanual tapping (Bangert et al., 2010; Fling et al., 2011; Helmuth & Ivry, 1996) where a lower value is indicative of better performance.
Functional Connectivity (fcMRI)
The preprocessed data were normalized to MNI space using SPM5 (Wellcome Department of Cognitive Neurology, London, UK; http://www.fil.ion.ucl.ac.uk) and low-pass filtered with a 0.08 Hz cutoff frequency in order to examine the frequency band of interest, and to exclude higher frequency sources of noise such as heart rate and respiration (Biswal et al., 1995). The HMAT (Mayka et al., 2006) was co-registered to each individual’s MNI-normalized fMR image and subsequently used as a mask of the M1. To create the individual interhemispheric M1 fcMRI map, the following procedures were taken: for each voxel within the left (or right) M1, correlation coefficients with the time course from all the other voxels in the contra-lateral M1 were averaged. The average correlation value was Z transformed and assigned to the left (or right) M1 voxel. Thus each voxel of the interhemispheric fcMRI map indicates how strongly a particular voxel is connected to the contralateral M1. The left and right interhemispheric fcMRI maps (restricted to the HMAT-defined M1s) were entered into the group-level random effects analyses, which were carried out in SPM5 using a threshold of p < 0.05 family-wise error (FWE) correction and an extent voxel threshold of 10. Interhemispheric M1 connectivity was determined by placing a spherical ROI with a radius of 5 mm, centered on the peak voxel identified in the group-level random effects analyses (Nichols and Hayasaka, 2003). Z scores averaged across all voxels within the ROI were used as an index of interhemispheric M1 connectivity for the left and the right M1.
Structural Connectivity (DTI)
Descriptive metrics were calculated for the microstructure of fiber tracts connecting homologous M1s, specifically fractional anisotropy, mean diffusivity, longitudinal diffusivity (λ1), and radial diffusivity ((λ2 + λ3)/2). Radial diffusivity describes water diffusion along the off-principle eigenvalues; therefore lower radial diffusivity is interpreted as being indicative of better tract microstructural integrity (Basser et al., 2000).
2.5 Statistical Analysis
Independent t-tests were performed to compare between-group measures of tapping variability, functional connectivity strength and fiber tract microstructure. Additionally within each group we performed linear regression to investigate the relationship between motor performance (assessed by tapping variability), fiber tract microstructure, and functional connectivity strength while correcting for multiple comparisons. For all significant relationships we used a Fisher r-to-Z transformation to identify differences in the strength of correlations between age groups. All data are presented as mean ± standard deviation unless otherwise noted.
3. Results
3.1 Motor Performance
Young adults had significantly less (P < 0.05) unimanual tapping variability (79.0 ± 12.1 ms) than older adults (89.6 ± 19.8 ms).
3.2 Functional Connectivity (fcMRI)
Comparable peak coordinates of M1 functional connectivity were found within the left M1 for the young (-28, -28, 46) and older adults (-26, -28, 48), whereas in the right M1 the young adult hotspot was more lateral (44, -20, 60) than that of the older participants (24, -32, 72). In both right and left M1, the young adults’ hotspot was located more inferiorly. No differences in functional connectivity strength were observed when performing a between group contrast of either YA > OA or the converse, OA > YA, using a corrected FWE 0.05 threshold. When performing exploratory analysis with the threshold relaxed to an uncorrected 0.001, older adults display significantly stronger functional connectivity than young adults in both the right and left M1. With this less stringent threshold, young adults still show no areas of greater functional connectivity than their older counterparts in either M1.
3.3 Structural Connectivity (DTI)
A significant age group difference was noted for radial diffusivity (P < 0.001) with lower radial diffusivity of fiber tracts connecting bilateral M1s in the young (0.54 × 10−3 mm2/s) versus older adults (0.58 × 10−3 mm2/s), indicative of better fiber tract microstructure for the young adults. Significantly greater fractional anisotropy (P < 0.001) was observed in young adults as well. Finally, no group differences were found for mean or longitudinal diffusivity (P > 0.10 for both).
3.4 Relationships between behavior and metrics of connectivity
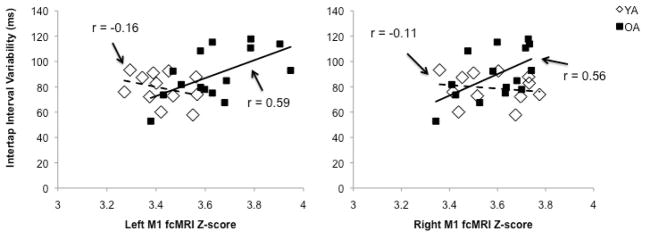
No relationship (P > 0.1) was observed between fiber tract microstructure and motor performance in either young or older adults. Nor was any association found between functional connectivity and performance in young adults (P > 0.2). Conversely, greater right (r = 0.56; P < 0.02) and left M1 (r = 0.59; P< 0.01) functional connectivity was significantly correlated with tapping variability in older adults. The strength of these correlations was significantly different between age groups for both the right (Z = 2.4; P < 0.01) and left M1 (Z = 1.9; P < 0.04). Thus, more functional connectivity between primary motor cortices was predictive of poorer performance in older, but not young, adults (Figure 1).
Figure 1.
Stronger functional connectivity of both the right and left M1 was significantly associated with poorer unimanual motor performance in older (solid lines), but not young adults (dashed lines).
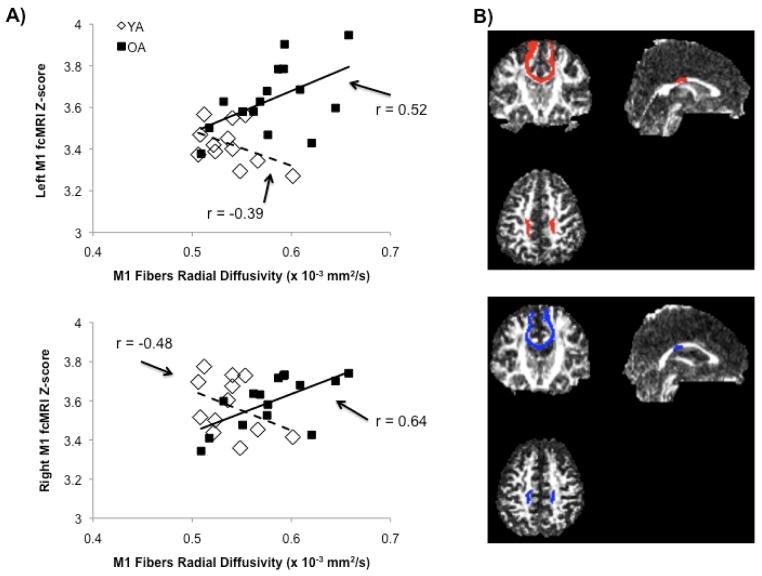
Radial diffusivity of fiber tracts connecting the primary motor cortices was significantly and positively correlated with functional connectivity of the right (r = 0.64; P < 0.01) and left M1 (r = 0.52; P < 0.03) in older but not young (P > 0.1) adults. The strength of these correlations was significantly different between age groups for both the right (Z = 3.07; P < 0.001) and left M1 (Z = 2.3; P < 0.02). That is to say, poorer structural connectivity was related to greater functional connectivity between motor cortices solely in older adults (Figure 2). Finally, we report no relationships between metrics of structural connectivity (FA, mean, radial or longitudinal diffusivity) and motor behavior.
Figure 2.
Poorer fiber tract microstructure (i.e. higher radial diffusivity) was positively correlated with the strength of functional connectivity for both the right and left M1 in older (solid lines), but not young (dashed lines) adults (A). Interhemispheric fiber tracts connecting bilateral primary motor cortices in a representative young (red) and older (blue) adult. Coordinates for both images are (x = 0, y = -2, z = 30) in normalized MNI space (B).
4. Discussion
This is the first study to combine structural and functional connectivity to provide a comprehensive description of the effects of age on the neuromotor system. We report that stronger functional connectivity between the primary motor cortices is related to poorer structural connectivity and poorer motor performance in older adults. None of these relationships were observed in young adults.
The goals of the current study were to integrate across neuroimaging techniques to begin to elucidate the complex relationships between patterns of functional and structural connectivity. Poorer structural connectivity was related to stronger functional connectivity between motor cortices in older adults. While it is not possible to disambiguate the BOLD signal to identify inhibitory or excitatory processes, recent studies indicate that both inter- and intra-hemispheric inhibition are decreased in the aging cortex (Peinemann et al., 2001; Talelli et al., 2008). There has yet to be definitive evidence indicating how the aging process affects interhemispheric facilitation; however, multiple studies have documented age differences in brain recruitment patterns during motor task performance.
Older adults exhibit over-recruitment of bilateral motor cortices relative to young adults, even when performing unimanual movements (Riecker et al., 2006; Ward and Frackowiak, 2003). This has oft been posited to be the result of non-selective recruitment, suggesting that brain structure–function relationships become less precise with age, resulting in older adults inefficiently recruiting additional regions of the brain compared to young adults (Logan et al., 2002; Riecker et al., 2006). Further, the extent of brain activation in the ipsilateral sensorimotor cortex during unimanual actions (“motor overflow”) is associated with the degree of change in interhemispheric inhibition from rest to muscle contraction in older adults (Talelli et al., 2008). Taken together with the current results, these findings suggest that increased connectivity in older adults may be reflective of a release from the normally predominantly inhibitory interhemispheric communication associated with the primary motor cortices (Lenzi et al., 2007; Netz et al., 1999), and signify a shift towards more facilitatory interhemispheric communication. Future work would benefit from investigating these relationships during the performance of bimanual tasks where interhemispheric communication demands are increased.
Finally, our previous work has shown that unimanual motor performance is positively associated with callosal microstructural integrity in older adults (Fling et al., 2011). It is worth noting the lack of such a relationship in either young or older adults in the current manuscript. While our previous work focused solely on microstructural integrity of the corpus callosum from a single mid-sagittal slice, the current work uses a more comprehensive fiber tractography approach. The methodological differences may explain the lack of structure-behavior correlations observed here, but this remains unclear.
Acknowledgments
This work was supported by National Institutes of Health [T32-AG00114-21] and the UM National Institutes of Health Claude D. Pepper Older Americans Independence Center [AG08808 pilot grant and human subjects cores].
Footnotes
Disclosure Statement
There is no conflict of interest including any financial, personal or other relationships with other people or organizations. The Medical Institutional Review Board (IRBMED) of the University of Michigan approved all procedures and human participant involvement in this study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bangert AS, Reuter-Lorenz PA, Walsh CM, Schachter AB, Seidler RD. Bimanual coordination and aging: neurobehavioral implications. Neuropsychologia. 2010;48:1165–1170. doi: 10.1016/j.neuropsychologia.2009.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basser PJ, Pajevic S, Pierpaoli C, Duda J, Aldroubi A. In vivo fiber tractography using DT-MRI data. Magn Reson Med. 2000;44:625–632. doi: 10.1002/1522-2594(200010)44:4<625::aid-mrm17>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34(4):537–41. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Bonzano L, Tacchino A, Roccatagliata L, Abbruzzese G, Mancardi GL, Bove M. Callosal contributions to simultaneous bimanual finger movements. J Neurosci. 2008;28(12):3227–3233. doi: 10.1523/JNEUROSCI.4076-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanraud S, Zahr N, Sullivan EV, Pfefferbaum A. MR diffusion tensor imaging: a window into white matter integrity of the working brain. Neuropsychol Rev. 2010;20:209–225. doi: 10.1007/s11065-010-9129-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahue MJ, Hoogduin H, Smith SM, Siero JC, Jezzard P, Luljten PR, Hendrikse J. Spontaneous BOLD fMRI signal is modulate by behavioral state and correlates with evoked response in sensorimotor cortex. Hum Brain Map. doi: 10.1002/hbm.21228. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fling BW, Benson BL, Seidler RD. Transcallosal sensorimotor fiber tract structure-function relationships. Hum Br Map. doi: 10.1002/hbm.21437. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fling BW, Walsh CM, Bangert A, Reuter-Lorenz PA, Welsh RC, Seidler RD. Differential Callosal Contributions To Bimanual Control in Young and Older Adults. J of Cog Neurosci. 2011;23(9):2171–85. doi: 10.1162/jocn.2010.21600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Gulani V, Webb AG, Duncan ID, Lauterbuy PC. Apparent diffusion tensor measurements in myelin-deficient rat spinal cords. Magn Reson Med. 2001;45(2):191–5. doi: 10.1002/1522-2594(200102)45:2<191::aid-mrm1025>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Helmuth LL, Ivry RB. When two hands are better than one: reduced timing variability during bimanual movements. J Exp Psychol Hum Percept Perform. 1996;22(2):278–93. doi: 10.1037//0096-1523.22.2.278. [DOI] [PubMed] [Google Scholar]
- Hofer S, Frahm J. Topography of the human corpus callosum revisited--comprehensive fiber tractography using diffusion tensor magnetic resonance imaging. Neuroimage. 2006;32(3):989–994. doi: 10.1016/j.neuroimage.2006.05.044. [DOI] [PubMed] [Google Scholar]
- Jelsone-Swain L, Fling BW, Seidler RD, Hovatter R, Gruis K, Welsh RC. Reduced interhemispheric functional connectivity in the motor cortex during rest in limb-onset amyotrophic lateral sclerosis. Front Syst Neurosci. 2010;4(158) doi: 10.3389/fnsys.2010.00158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langan J, Peltier S, Bo J, Fling BW, Welsh RC, Seidler RD. Functional implications of age differences in motor system connectivity. Front Syst Neurosci. 2010;4(17) doi: 10.3389/fnsys.2010.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenzi D, Conte A, Mainero C, Frasca V, Fubelli F, Totaro P, Caramia F, Inghilleri M, Pozzilli C, Pantano P. Effect of corpus callosum damage on ipsilateral motor activation in patients with multiple sclerosis: a functional and anatomical study. Hum Brain Mapp. 2007;28(7):636–644. doi: 10.1002/hbm.20305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan JM, Sanders AL, Snyder AZ, Morris JC, Buckner RL. Under-recruitment and nonselective recruitment: dissociable neural mechanisms associated with aging. Neuron. 2002;33(5):827–40. doi: 10.1016/s0896-6273(02)00612-8. [DOI] [PubMed] [Google Scholar]
- Marner L, Nyengaard JR, Tang Y, Pakkenberg B. Marked loss of myelinated nerve fibers in the human brain with age. J Comp Neurol. 2003;462:144–152. doi: 10.1002/cne.10714. [DOI] [PubMed] [Google Scholar]
- Mayka MA, Corcos DM, Leurgans SE, Vaillancourt DE. Three-dimensional locations and boundaries of motor and premotor cortices as defined by functional brain imaging: a meta-analysis. Neuroimage. 2006;31(4):1453–74. doi: 10.1016/j.neuroimage.2006.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S, van Zijl PC. Fiber tracking: principles and strategies - a technical review. NMR Biomed. 2002;15(7–8):468–80. doi: 10.1002/nbm.781. [DOI] [PubMed] [Google Scholar]
- Netz J. Asymmetry in transcallosal inhibition. Electroencephalogr Clin Neurophysiol, Suppl. 1999;51:137–44. [PubMed] [Google Scholar]
- Nichols T, Hayasaka S. Controlling the familywise error rate in functional neuroimaging: a comparative review. Stat Methods Med Res. 2003;12(5):419–46. doi: 10.1191/0962280203sm341ra. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Peinemann A, Lehner C, Conrad B, Siebner HR. Age-related decrease in paired-pulse intracortical inhibition in the human primary motor cortex. Neurosci Lett. 2001;313(1–2):33–36. doi: 10.1016/s0304-3940(01)02239-x. [DOI] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, Lustig C. Brain aging: reorganizing discoveries about the aging mind. Curr Opin Neurobiol. 2005;15(2):245–51. doi: 10.1016/j.conb.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Riecker A, Groschel K, Ackermann H, Steinbrink C, Witte O, Kastrup A. Functional significance of age- related differences in motor activation patterns. Neuroimage. 2006;32(3):1345–1354. doi: 10.1016/j.neuroimage.2006.05.021. [DOI] [PubMed] [Google Scholar]
- Seidler RD, Bernard JA, Burutolu TB, Fling BW, Gwin JT, Kwak Y, Lipps DB. Motor control and aging: links to age-related brain structural, functional, and biochemical effects. Neurosci Biobehav Rev. 2010;34:721–733. doi: 10.1016/j.neubiorev.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talelli P, Ewas A, Waddingham W, Rothwell JC, Ward NS. Neural correlates of age-related changes in cortical neurophysiology. Neuroimage. 2008;40:1772–81. doi: 10.1016/j.neuroimage.2008.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tournier JD, Mori S, Leemans A. Diffusion tensor imaging and beyond. Magn Reson Med. 2011;65(6):1532–56. doi: 10.1002/mrm.22924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JL, Patel GH, Fox MD, Snyder AZ, Baker JT, Van Essen DC, Zempel JM, Snyder LH, Corbetta M, Raichle ME. Intrinsic functional architecture in the anaesthetized monkey brain. Nature. 2007;447:83–86. doi: 10.1038/nature05758. [DOI] [PubMed] [Google Scholar]
- Ward NS, Frackowiak RS. Age-related changes in the neural correlates of motor performance. Brain. 2003;126:873–88. doi: 10.1093/brain/awg071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo X, Kelly C, DiMartino A, Mennes M, Margulies DS, Bangaru S, Grzadzinski R, Evans AC, Zang Y, Castellanos FX, Milham MP. Growing together and growing apart: regional and sex differences in the lifespan developmental trajectories of functional homotopy. J Neurosci. 2010;30(45):15034–43. doi: 10.1523/JNEUROSCI.2612-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]