Abstract
Background and aims
Knowledge on the etiology of exocrine pancreatic cancer (EPC) is scant. The best established risk factor for EPC is tobacco smoking. Among other carcinogens, tobacco contains cadmium, a metal previously associated with an increased risk of EPC. We evaluated the association between concentrations of trace elements in toenails and EPC risk.
Methods
The study included 118 EPC cases and 399 hospital controls from Eastern Spain. Levels of twelve trace elements were determined in toenail samples by inductively coupled plasma - mass spectrometry. Odds ratios (ORs) and 95% confidence intervals (CIs), adjusted for potential confounders, were calculated using logistic regression.
Results
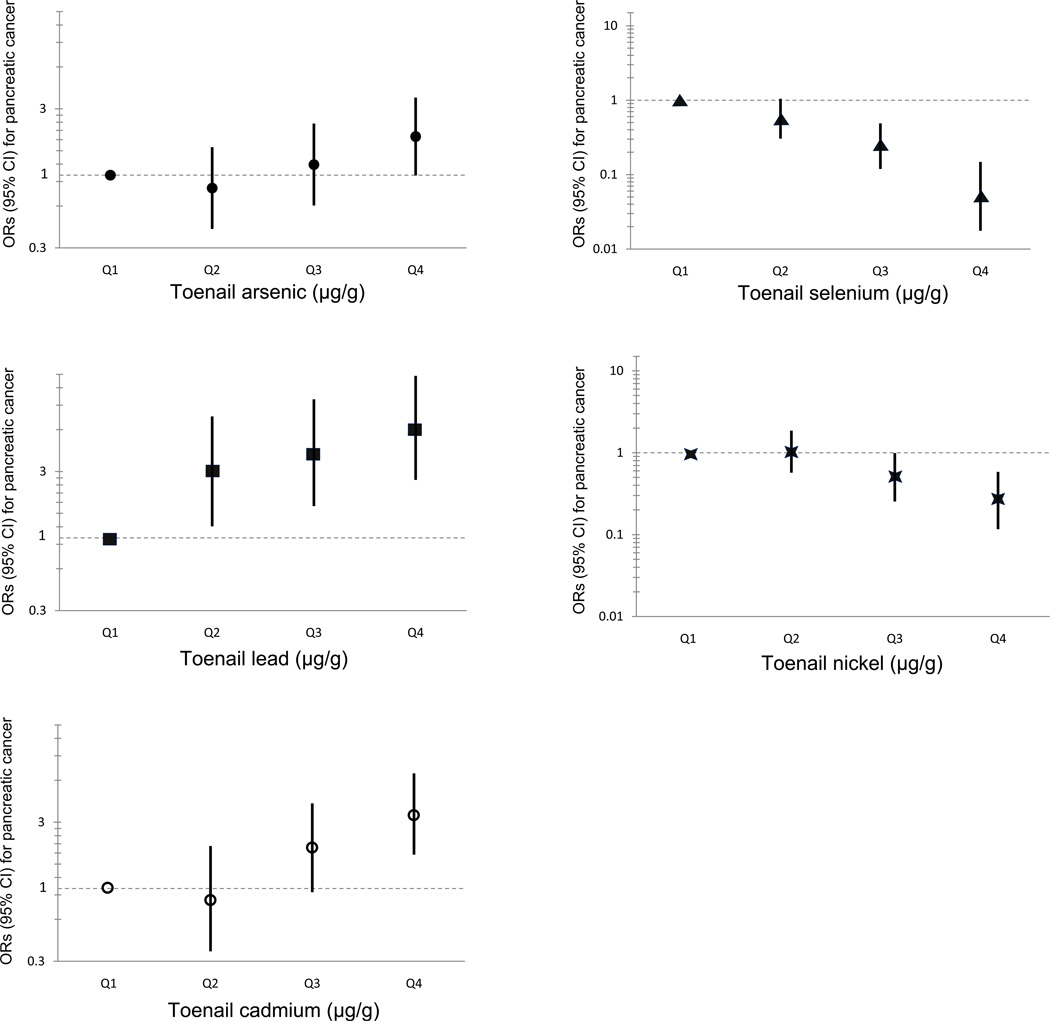
Significantly increased risks of EPC were observed among subjects whose concentrations of cadmium (OR=3.58, 95%CI 1.86–6·88; Ptrend=5×10−6), arsenic (OR=2.02, 95%CI 1.08–3.78; Ptrend=0.009), and lead (OR=6.26, 95%CI 2.71–14.47; Ptrend=3×10−5) were in the highest quartile. High concentrations of selenium (OR=0.05, 95%CI 0.02–0.15; Ptrend=8×10−11) and nickel (OR=0.27, 95%CI 0.12–0.59; Ptrend=2×10−4) were inversely associated with risk of EPC.
Conclusion
We report novel associations of lead, nickel, and selenium toenail concentrations with pancreas cancer risk. Furthermore, results confirm previous associations with cadmium and arsenic. These novel findings, if replicated in independent studies, would point to an important role of trace elements in pancreatic carcinogenesis.
Keywords: Pancreas, Arsenic, Cadmium, Lead, Selenium
INTRODUCTION
In spite of decades of research, the etiology of exocrine pancreatic cancer (EPC) remains largely unknown.[1] The best established risk factor for EPC is tobacco smoking, which may account for up to one third of cases.[2] A personal history of diabetes, chronic pancreatitis, and high body-mass index, as well as family history of cancer have also been consistently associated with an increased risk of EPC.[1] In addition to aromatic amines, one of the main carcinogens involved in pancreatic carcinogenesis,[3] tobacco contains other carcinogens, including trace metals, such as cadmium.[4] High levels of cadmium have been associated with an increased risk of EPC.[5, 6] Recently, a potential link between pancreatic cancer mortality and childhood exposure to milk powder contaminated with arsenic has also been suggested.[7, 8] The objective of the present study was to assess the risk of EPC associated with concentrations of selected trace elements measured in toenails.
METHODS
Study populations
We conducted a case-control study with incident cases of EPC included in the PANKRAS II Study,[9] and hospital-based controls from the Spanish Bladder Cancer/EPICURO Study.[10] The two studies had overlapping geographic recruitment areas and were performed close together in time. Case enrollment occurred during 1992–1995 at five general hospitals from the Mediterranean coast in Spain. An epidemiologic questionnaire was administered by trained monitors through a face-to-face interview during the first hospital admission. Sociodemographic information as well as data on tobacco smoking and past history of diabetes was considered in this analysis. Out of 185 patients with EPC included in the study, 118 (63.8%) provided pre-treatment toenail samples. Controls, recruited during 1998–2001 in 18 Spanish hospitals, were patients with diagnoses unrelated to the exposures of interest.[10] For the present analysis, only controls (n=441) admitted to hospitals from the same regions as those participating in the PANKRAS II Study were considered. Information on known or potential cancer risk factors, as well as toenail samples, were obtained from 399 (90.5%) controls during their inpatient hospital stay, as was done with cases. The final study sample was mainly composed of men, with a high prevalence of tobacco smokers. Cases were slightly older than controls (Suppl. Table 1). Informed consent from all subjects and ethical approval from local Institutional Review Boards were obtained.
Trace element assessment
Toenails were stored at room temperature until the time of the analysis. After careful cleaning and washing to remove external contaminants, trace elements were quantified at the Trace Element Analysis Core (Dartmouth College, NH, USA), using inductively coupled plasma-mass spectrometry.[11] Toenails were acid digested with Optima HNO3 (Fisher Scientific, St. Louis, MO) at 105 °C followed by addition of H2O2 and further heating the dilution with deionized water. All sample preparation steps were recorded gravimetrically. As a quality control, each batch of analyses included six standard reference material samples with known trace element content (SRM; GBW 07601, powdered human hair) and six analytic blanks, along with the study samples. The within- and the between-assay coefficients of variation for SRM replicates were <15% for arsenic, manganese, lead, selenium, and zinc; 15–25% for aluminum, cadmium, copper, and nickel; 25–40% for iron, vanadium; and >40% for chromium. The amount of SRM used ranged from < 10 mg – 50 mg to mimic the mass of toenails. This small SRM sample mass may be the cause of some of the variability seen in the within- and between-batch SRM results. The case-control status of study participants was not disclosed to the testing laboratory.
Statistical analysis
Mann-Whitney’s U test was used to assess differences in median toenail concentrations of the trace elements between cases and controls. For association analyses, participants were divided into quartiles based on the distribution of trace element concentrations among controls and logistic regression was applied to estimate adjusted odds ratios (ORa) and their 95% confidence intervals (CIs), with the lowest quartile as the reference category. Basic models for each trace element included age at interview (continuous), gender (dichotomous), region (three categories), and smoking status (ever/never) as covariates. Controls were classified as ever or never smokers according to the definition of smoking status of cases.[12] Further adjustment was made for potential confounders such as educational level (high vs. low, [13]), pack-years and total duration of cigarette smoking (continuous), past medical history of diabetes status (dichotomous), and for trace elements (categorical) for which an association was observed in the basic model. Tests for linear trend were computed by including the median of each quartile of the trace element concentration as a continuous variable. Since controls from Balearic Islands were not available, in a first round of analyses, cases from these islands were grouped with those of Barcelona due to their similarities and proximity. In a second round, those cases were excluded. Results were considered statistically significant with a two-sided P < 0.05. Statistical analyses were performed using STATA/SE v·10·1.
RESULTS
The toenail concentrations of cadmium and lead were significantly higher in cases than in controls (P<0.001). In contrast, the concentrations of nickel and selenium were lower in cases than in controls (Suppl. Table 2). Comparing the highest quartile versus the lowest quartile of concentrations, an increased risk of EPC was associated with lead (ORa = 6.26, 95%CI 2.71–14.47; Ptrend= 3×10−5), cadmium (ORa = 3.58, 95%CI 1.86–6.88; Ptrend = 5×10−6), and arsenic (ORa = 2.02, 95%CI 1.08–3.78; Ptrend= 0.009). By contrast, levels of nickel (ORa = 0.27, 95%CI 0.12–0.59; Ptrend= 2×10−4) and selenium (ORa = 0.05, 95%CI 0.02–0.15 Ptrend= 8×10−11) were inversely associated with EPC risk (Table 1, Figure 1). No statistically significant associations were observed for the other trace elements (Suppl. Table 3).
Table 1.
Odds ratios (OR), 95% confidence intervals (CI), and P for the associations between pancreatic cancer risk and concentrations in toenails of selected trace elements (arsenic, cadmium, lead, nickel, and selenium). Estimates are adjusted for age (continuous), gender, region, and smoking status (ever/never).
| Controls | % | Cases | % | ORa (95% CI) | P | |
|---|---|---|---|---|---|---|
| Arsenic, µg.g−1 | ||||||
| ≤ 0.0518 | 100 | 25 | 34 | 30 | 1.00 | |
| 0.0519–0.0709 | 100 | 25 | 21 | 19 | 0.81 (0.42–1.56) | 0.526 |
| 0.0710–0.1061 | 100 | 25 | 23 | 20 | 1.22 (0.63–2.36) | 0.551 |
| > 0.1061 | 98 | 25 | 35 | 31 | 2.02 (1.08–3.78) | 0.029 |
| Trend* | 0.009 | |||||
| Cadmium, µg.g−1 | ||||||
| ≤ 0.0080 | 100 | 25 | 17 | 15 | 1.00 | |
| 0.0081–0.0134 | 99 | 25 | 11 | 9 | 0.87 (0.37–2.03) | 0.745 |
| 0.0135–0.0291 | 100 | 25 | 27 | 24 | 2.04 (1.00–4.17) | 0.051 |
| > 0.0291 | 99 | 25 | 59 | 52 | 3.58 (1.86–6.88) | 1×10−4 |
| Trend* | 5×10−6 | |||||
| Lead, µg.g−1 | ||||||
| ≤ 0.2534 | 99 | 25 | 8 | 7 | 1.00 | |
| 0.2535–0.4692 | 100 | 25 | 23 | 20 2.88 | (1.19–6.99) | 0.019 |
| 0.4693–0.9750 | 100 | 25 | 33 | 29 4.07 | (1.72–9.61) | 0.001 |
| > 0.9750 | 99 | 25 | 49 | 44 6.26 | (2.71–14.47) | 2×10−5 |
| Trend* | 3×10−5 | |||||
| Nickel, µg.g−1 | ||||||
| ≤ 0.1856 | 100 | 25 | 40 | 35 | 1.00 | |
| 0.1857–0.3855 | 99 | 25 | 42 | 37 | 1.10 (0.63–1.92) | 0.738 |
| 0.3856–0.8852 | 100 | 25 | 21 | 18 | 0.51 (0.27–0.98) | 0.042 |
| > 0.8852 | 99 | 25 | 11 | 10 | 0.27 (0.12–0.59) | 9×10−4 |
| Trend* | 2×10−4 | |||||
| Selenium, µg.g−1 | ||||||
| ≤ 0.5198 | 100 | 25 | 59 | 52 | 1.00 | |
| 0.5199–0.5965 | 100 | 25 | 30 | 27 | 0.57 (0.32–1.01) | 0.054 |
| 0.5966–0.6829 | 99 | 25 | 19 | 17 | 0.25 (0.13–0.50) | 6×10−5 |
| > 0.6829 | 99 | 25 | 5 | 4 | 0.05 (0.02–0.15) | 2×10−8 |
| Trend* | 8×10−11 | |||||
Tests for linear trend were carried out by including the median of each quartile of the toenail trace element concentration as a continuous variable in the logistic regression model.
Figure 1.
Odds ratios (OR), 95% confidence intervals (CI) for pancreatic cancer risk according to the concentrations in toenails of arsenic, cadmium, lead, nickel, and selenium.
Further adjustment for diabetes (Suppl. Table 3) and other potential confounders did not substantially change results (Suppl. Tables 4–5). Additional adjustment for the elements found significant in the basic model (arsenic, cadmium, lead, nickel, and selenium) did not markedly change the OR estimates, although the association with arsenic was no longer statistically significant (highest vs. lowest quartile: ORa = 1.72, 95%CI 0.77–3.86; Ptrend= 0.201) when lead concentration was included in the model (Suppl. Table 6). Results were similar after excluding the Balearic cases from the analysis (data not shown).
DISCUSSION
The study shows, for the first time, highly significant associations between EPC risk and toenail concentrations of lead, selenium, and nickel, and confirms associations with cadmium and arsenic exposure, previously reported by a few studies.[5, 6, 7, 8] Importantly, a dose-response effect was observed for each of these associations. Cadmium has previously been associated with increased risk of lung, prostate, and kidney cancers,[14, 15] in addition to pancreatic cancer risk and mortality.[5, 6] Cadmium is a well established carcinogen that acts on different steps of carcinogenesis, inhibiting DNA repair, and causing genomic instability.[16, 17, 18] Furthermore, it causes transdifferentiation of pancreatic cells, inhibits DNA repair, and induces or regulates the activity of several oncogenes or tumor-suppressor proteins that are expressed in human pancreatic cancer.[5, 19, 20]
Arsenic exposure has been associated with increased risk of cancer.[21] Regarding its association with pancreatic cancer risk, little has been published. However, a potential relation between childhood exposure to milk powder contaminated with arsenic and an almost twofold excess mortality due to pancreatic cancer was recently reported.[7, 8] Some studies on the relationship between arsenic exposure and type 2 diabetes, which is a potential risk factor for pancreatic cancer, obtained conflicting results.[22, 23] In the present study, diabetes did not confound the association between arsenic and EPC. Inorganic arsenic is a highly toxic and carcinogenic metalloid, which can induce oxidative stress leading to inhibition of DNA repair.[15, 21, 24] Arsenic-induced oxidative stress also causes DNA strand breaks, alkali-labile sites, and eventually DNA adducts.[25] Moreover, alterations in the methylation status of oncogenes and tumor-suppressor genes, mediated by arsenic, may also play a role in carcinogenesis.[26] To our knowledge, this is the first epidemiologic study showing an association between lead and EPC risk. The International Agency for Research on Cancer classifies inorganic lead as “probably carcinogenic to humans”, and several studies found it linked to cancer.[27, 28, 29] The possible involvement of lead in pancreatic cancer development cannot be ruled out, given the long residence time of this metal in the bone and the regular exchange between this matrix and blood and soft tissues.[30, 31] Lead induces chromosome aberrations, micronuclei and sister chromatid exchanges.[32, 33] It can activate EGFR and SFK tyrosine kinases and increase Ras-GTP levels, leading to cell proliferation and differentiation.[34] Furthermore, a direct association has recently been observed between bone lead levels and LINE-1 DNA hypomethylation,[35] which in turn has been linked to cancer risk.[36, 37]
Epidemiologic evidence on nickel carcinogenicity to humans is limited and controversial. High occupational exposure to nickel has been associated with an increased risk of lung and prostate cancers,[21, 38, 39, 40, 41] but several studies found no association with bladder, colorectal, gastric or lung cancers.[42, 43, 44, 45] A meta-analysis of occupational exposures and pancreatic cancer reported an increased risk with nickel exposure.[46] However, in occupational settings nickel may be associated with high concentrations of polychlorinated biphenyls, and the latter compounds could account for the observed increased risk.[9, 47] Furthermore, no measurement of nickel concentrations in biological samples was performed in previous studies.[47] Nickel may increase DNA methylation, inhibit DNA repair, and induce apoptosis through the generation of reactive oxygen species.[48, 49, 50, 51]
Selenium is an essential micronutrient,[52, 53] and high levels of this trace element have been inversely associated with several cancers.[54, 55, 56, 57, 58] While a small study published in 1989 showed a strong inverse association between serum selenium levels and pancreatic cancer risk,[59] no replication studies have been published. Aberrant expression patterns of some selenoproteins show that they are relevant in scavenging reactive oxygen species and diminishing oxidative damage.[60] The protection against cancer given by selenium has also been linked to the activities of hydrogen selenide and selenomethionine present in cells leading to higher methylating efficiency of RNA and thiols.[61] In addition, selenium may boost p53 activity, leading to either DNA repair or apoptosis.[62] Selenium seems also to play a role as antagonist of arsenic, cadmium and lead, decreasing the oxidative stress caused by exposure to these elements.[63, 64] In the present study, the association between selenium and pancreatic cancer risk was not affected by the concentrations of those three elements. Limitations of our study include its retrospective design, relatively small sample size, and slight difference in the recruitment period of cases and controls. Nevertheless, the appropriateness of this control group is supported by the replication of the association between smoking and EPC risk (OR = 2.08, 95%CI 1.09–3.99; P = 0.027). Regarding lead, the banning of leaded gasoline took place in Spain in January 2002, after samples from controls had been collected. Also, we did not observe differences in toenail concentration of lead by year of recruitment among controls. Furthermore, diet is the main source of lead exposure in adults, with the exception of individuals occupationally exposed.[29, 31] Therefore a potential decrease of concentrations of lead in the air would not necessarily imply a direct decrease in toenail lead levels in the participants of the present study. Also, lead has a long half-life in bone, from where it goes back to the blood stream and to soft tissues,[30, 31] which means that levels of lead in the organism and those measured in toenails do not reflect recent environmental changes; rather, they mirror past and chronic exposures. The study was not designed to identify the environmental sources for the trace elements found. Among the potential sources of these elements are air and water pollution, though it should be taken into account that both cases and controls share the area of residence and drinking water is publicly supplied. We adjusted for smoking, which is one source for some metals. Adjusting for diet or occupation was not possible, and these factors cannot be ruled out as sources for some of the trace elements relevant in this study. Finally, the possibility that even under similar environmental exposures, different genetic profiles between cases and controls might account for pancreatic cancer risk cannot be excluded.
The study also has important strengths, including the matching on area of residence, the similar age distributions of cases and controls, and the simultaneous quantification of the trace elements in the same laboratory and under the same quality control procedures. Furthermore, toenails are not altered with long-term storage and they are reliable matrices to assess past exposures.[65, 66, 67, 68, 69, 70]
In conclusion, our results support an increased risk of pancreatic cancer associated with higher levels of cadmium, arsenic, and lead, as well as an inverse association with higher levels of selenium and nickel. While our findings need to be replicated in independent studies, they suggest a role of trace elements in pancreatic cancer pathogenesis, and justify further research.
Significance of the study.
What is already known about this subject?
-
>
Little is known about the etiology of pancreatic cancer.
-
>
Some trace elements, such as arsenic and cadmium, are carcinogenic for humans and may enter the organism trough different routes.
-
>
A few studies have found a link between exposure to arsenic and cadmium and pancreatic cancer risk.
What are the new findings?
-
>
Individuals with the highest levels of selenium or nickel in toenails present a lower risk of pancreatic cancer.
-
>
The study confirms the increased risk of pancreas cancer among subjects with the highest levels of arsenic or cadmium in toenails.
-
>
Besides arsenic and cadmium, high levels of lead may also be a risk factor for pancreatic cancer.
How might it impact on clinical practice in the foreseeable future?
-
>
Selenium intake could be administered as a chemopreventive intervention to reduce the risk of pancreatic cancer. Understanding the role of trace elements in pancreatic cancer pathogenesis could lead to preventive measures or treatments.
Supplementary Material
Acknowledgements
We acknowledge the coordinators, field and administrative workers, technicians and patients of both PANKRAS II and Spanish Bladder Cancer/EPICURO studies.
Funding
This work was partially supported by the Association for International Cancer Research (AICR09-0780); Fondo de Investigación Sanitaria, Spain (#PI09-02102); Red Temática de Investigación Cooperativa en Cáncer (RTICC) and CIBER de Epidemiología y Salud Pública (CIBERESP), Instituto de Salud Carlos III, Ministry of Health, Spain; Fundación Científica de la Asociación Española Contra el Cáncer (AECC); and the Intramural Research Program of the Division of Cancer Epidemiology and Genetics, National Cancer Institute, USA. The Dartmouth Trace Element Core is partially supported by NIH Grant Number P42 ES007373 from the National Institute of Environmental Health Sciences.
Footnotes
The Corresponding Author has the right to grant on behalf of all authors and does grant on behalf of all authors, an exclusive licence (or non exclusive for government employees) on a worldwide basis to the BMJ Publishing Group Ltd and its Licensees to permit this article (if accepted) to be published in Gut editions and any other BMJPGL products to exploit all subsidiary rights, as set out in our licence.
Competing interests
None
Contributors
AFSA participated in statistical analysis, writing and editing of the manuscript, and preparation of artwork and tables. MP participated in study design, interpretation of results, and writing. DTS participated in study design. RLM participated in statistical analysis, and writing. MK participated in study design. NR participated in study design. KPC participated in writing. BPJ supervised the quantification of trace elements, and participated in writing. JAP, and TL participated in statistical analysis, and interpretation of results. AC, and LG provided samples and support in the enrollment of study participants. FXR participated in study design and writing. NM participated in study design, statistical analysis, writing and editing of the manuscript, and oversight of the study.
REFERENCES
- 1.Li D, Xie K, Wolff R, et al. Pancreatic cancer. Lancet. 2004;363:1049–1057. doi: 10.1016/S0140-6736(04)15841-8. [DOI] [PubMed] [Google Scholar]
- 2.Silverman DT, Dunn JA, Hoover RN, et al. Cigarette smoking and pancreas cancer: a case-control study based on direct interviews. J Natl Cancer Inst. 1994;86:1510–1516. doi: 10.1093/jnci/86.20.1510. [DOI] [PubMed] [Google Scholar]
- 3.Weisburger JH. Comments on the history and importance of aromatic and heterocyclic amines in public health. Mutat Res. 2002;506–507:9–20. doi: 10.1016/s0027-5107(02)00147-1. [DOI] [PubMed] [Google Scholar]
- 4.Pappas RS, Polzin GM, Zhang L, et al. Cadmium, lead, and thallium in mainstream tobacco smoke particulate. Food Chem Toxicol. 2006;44:714–723. doi: 10.1016/j.fct.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 5.Schwartz GG, Reis IM. Is cadmium a cause of human pancreatic cancer? Cancer Epidemiol Biomarkers Prev. 2000;9:139–145. [PubMed] [Google Scholar]
- 6.Kriegel AM, Soliman AS, Zhang Q, et al. Serum cadmium levels in pancreatic cancer patients from the East Nile Delta region of Egypt. Environ Health Perspect. 2006;114:113–119. doi: 10.1289/ehp.8035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yorifuji T, Tsuda T, Doi H, et al. Cancer excess after arsenic exposure from contaminated milk powder. Environ Health Prev Med. 2011;16:164–170. doi: 10.1007/s12199-010-0182-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yorifuji T, Tsuda T, Grandjean P. Unusual cancer excess after neonatal arsenic exposure from contaminated milk powder. J Natl Cancer Inst. 2010;102:360–361. doi: 10.1093/jnci/djp536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Porta M, Malats N, Jariod M, et al. Serum concentrations of organochlorine compounds and K-ras mutations in exocrine pancreatic cancer. Lancet. 1999;354:2125–2129. doi: 10.1016/s0140-6736(99)04232-4. [DOI] [PubMed] [Google Scholar]
- 10.Garcia-Closas M, Malats N, Silverman D, et al. NAT2 slow acetylation, GSTM1 null genotype, and risk of bladder cancer: results from the Spanish Bladder Cancer Study and meta-analyses. Lancet. 2005;366:649–659. doi: 10.1016/S0140-6736(05)67137-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hopkins WA, Staub BP, Baionno JA, et al. Trophic and maternal transfer of selenium in brown house snakes (Lamprophis fuliginosus) Ecotoxicol Environ Saf. 2004;58:285–293. doi: 10.1016/S0147-6513(03)00076-9. [DOI] [PubMed] [Google Scholar]
- 12.Crous-Bou M, Porta M, Lopez T, et al. Lifetime history of tobacco consumption and K-ras mutations in exocrine pancreatic cancer. Pancreas. 2007;35:135–141. doi: 10.1097/mpa.0b013e31805d8fa4. [DOI] [PubMed] [Google Scholar]
- 13.Castano-Vinyals G, Cantor KP, Villanueva CM, et al. Socioeconomic status and exposure to disinfection by-products in drinking water in Spain. Environ Health. 2011;10:18. doi: 10.1186/1476-069X-10-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.IARC. Beryllium, cadmium, mercury, and exposures in the glass manufacturing industry. Lyon: International Agency for Research on Cancer; 1994. [Google Scholar]
- 15.Straif K, Benbrahim-Tallaa L, Baan R, et al. A review of human carcinogens--part C: metals, arsenic, dusts, and fibres. Lancet Oncol. 2009;10:453–454. doi: 10.1016/s1470-2045(09)70134-2. [DOI] [PubMed] [Google Scholar]
- 16.Hartwig A. Mechanisms in cadmium-induced carcinogenicity: recent insights. Biometals. 2010;23:951–960. doi: 10.1007/s10534-010-9330-4. [DOI] [PubMed] [Google Scholar]
- 17.Schwerdtle T, Ebert F, Thuy C, et al. Genotoxicity of soluble and particulate cadmium compounds: impact on oxidative DNA damage and nucleotide excision repair. Chem Res Toxicol. 2010;23:432–442. doi: 10.1021/tx900444w. [DOI] [PubMed] [Google Scholar]
- 18.Bertin G, Averbeck D. Cadmium: cellular effects, modifications of biomolecules, modulation of DNA repair and genotoxic consequences (a review) Biochimie. 2006;88:1549–1559. doi: 10.1016/j.biochi.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 19.Candeias S, Pons B, Viau M, et al. Direct inhibition of excision/synthesis DNA repair activities by cadmium: analysis on dedicated biochips. Mutat Res. 2010;694:53–59. doi: 10.1016/j.mrfmmm.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 20.Waalkes MP, Cherian MG, Ward JM, et al. Immunohistochemical evidence of high concentrations of metallothionein in pancreatic hepatocytes induced by cadmium in rats. Toxicol Pathol. 1992;20:323–326. doi: 10.1177/019262339202000302. [DOI] [PubMed] [Google Scholar]
- 21.IARC. Overall evaluations of carcinogenicity: an updating of IARC Monographs volumes 1 to 42. Lyon: International Agency for Research on Cancer; 1987. [PubMed] [Google Scholar]
- 22.Navas-Acien A, Silbergeld EK, Pastor-Barriuso R, et al. Arsenic exposure and prevalence of type 2 diabetes in US adults. JAMA. 2008;300:814–822. doi: 10.1001/jama.300.7.814. [DOI] [PubMed] [Google Scholar]
- 23.Chen Y, Ahsan H, Slavkovich V, et al. No association between arsenic exposure from drinking water and diabetes mellitus: a cross-sectional study in Bangladesh. Environ Health Perspect. 2010;118:1299–1305. doi: 10.1289/ehp.0901559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qin XJ, Hudson LG, Liu W, et al. Dual actions involved in arsenite-induced oxidative DNA damage. Chem Res Toxicol. 2008;21:1806–1813. doi: 10.1021/tx8001548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pu YS, Jan KY, Wang TC, et al. 8-Oxoguanine DNA glycosylase and MutY homolog are involved in the incision of arsenite-induced DNA adducts. Toxicol Sci. 2007;95:376–382. doi: 10.1093/toxsci/kfl166. [DOI] [PubMed] [Google Scholar]
- 26.Reichard JF, Puga A. Effects of arsenic exposure on DNA methylation and epigenetic gene regulation. Epigenomics. 2010;2:87–104. doi: 10.2217/epi.09.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boffetta P, Fontana L, Stewart P, et al. Occupational exposure to arsenic, cadmium, chromium, lead and nickel, and renal cell carcinoma: a case-control study from Central and Eastern Europe. Occup Environ Med. 2011 doi: 10.1136/oem.2010.056341. [DOI] [PubMed] [Google Scholar]
- 28.Steenland K, Boffetta P. Lead and cancer in humans: where are we now? Am J Ind Med. 2000;38:295–299. doi: 10.1002/1097-0274(200009)38:3<295::aid-ajim8>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 29.IARC. Inorganic and organic lead compounds. Lyon: International Agency for Research on Cancer; 2006. [Google Scholar]
- 30.Rabinowitz MB, Wetherill GW, Kopple JD. Kinetic analysis of lead metabolism in healthy humans. J Clin Invest. 1976;58:260–270. doi: 10.1172/JCI108467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Skerfving S, Bergdahl IA, Gunnar FN, et al. Handbook on the Toxicology of Metals. Third Edition. Burlington: Academic Press; 2007. Lead; pp. 599–643. [Google Scholar]
- 32.Wu FY, Chang PW, Wu CC, et al. Correlations of blood lead with DNA-protein cross-links and sister chromatid exchanges in lead workers. Cancer Epidemiol Biomarkers Prev. 2002;11:287–290. [PubMed] [Google Scholar]
- 33.Silbergeld EK, Waalkes M, Rice JM. Lead as a carcinogen: experimental evidence and mechanisms of action. Am J Ind Med. 2000;38:316–323. doi: 10.1002/1097-0274(200009)38:3<316::aid-ajim11>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 34.Wang CY, Wang YT, Tzeng DW, et al. Lead acetate induces EGFR activation upstream of SFK and PKCalpha linkage to the Ras/Raf-1/ERK signaling. Toxicol Appl Pharmacol. 2009;235:244–252. doi: 10.1016/j.taap.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 35.Wright RO, Schwartz J, Wright RJ, et al. Biomarkers of lead exposure and DNA methylation within retrotransposons. Environ Health Perspect. 2010;118:790–795. doi: 10.1289/ehp.0901429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takai D, Yagi Y, Habib N, et al. Hypomethylation of LINE1 retrotransposon in human hepatocellular carcinomas, but not in surrounding liver cirrhosis. Jpn J Clin Oncol. 2000;30:306–309. doi: 10.1093/jjco/hyd079. [DOI] [PubMed] [Google Scholar]
- 37.Wilhelm CS, Kelsey KT, Butler R, et al. Implications of LINE1 methylation for bladder cancer risk in women. Clin Cancer Res. 2010;16:1682–1689. doi: 10.1158/1078-0432.CCR-09-2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jarup L, Bellander T, Hogstedt C, et al. Mortality and cancer incidence in Swedish battery workers exposed to cadmium and nickel. Occup Environ Med. 1998;55:755–759. doi: 10.1136/oem.55.11.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grimsrud TK, Berge SR, Martinsen JI, et al. Lung cancer incidence among Norwegian nickel-refinery workers 1953–2000. J Environ Monit. 2003;5:190–197. doi: 10.1039/b211722n. [DOI] [PubMed] [Google Scholar]
- 40.Sorahan T, Waterhouse JA. Mortality study of nickel-cadmium battery workers by the method of regression models in life tables. Br J Ind Med. 1983;40:293–300. doi: 10.1136/oem.40.3.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Andersen A, Berge SR, Engeland A, et al. Exposure to nickel compounds and smoking in relation to incidence of lung and nasal cancer among nickel refinery workers. Occup Environ Med. 1996;53:708–713. doi: 10.1136/oem.53.10.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Elinder CG, Kjellstrom T, Hogstedt C, et al. Cancer mortality of cadmium workers. Br J Ind Med. 1985;42:651–655. doi: 10.1136/oem.42.10.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sorahan T. Mortality from lung cancer among a cohort of nickel cadmium battery workers:1946–84. Br J Ind Med. 1987;44:803–809. doi: 10.1136/oem.44.12.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Karjalainen S, Kerttula R, Pukkala E. Cancer risk among workers at a copper/nickel smelter and nickel refinery in Finland. Int Arch Occup Environ Health. 1992;63:547–551. doi: 10.1007/BF00386344. [DOI] [PubMed] [Google Scholar]
- 45.Pang D, Burges DC, Sorahan T. Mortality study of nickel platers with special reference to cancers of the stomach and lung, 1945–93. Occup Environ Med. 1996;53:714–717. doi: 10.1136/oem.53.10.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ojajarvi IA, Partanen TJ, Ahlbom A, et al. Occupational exposures and pancreatic cancer: a meta-analysis. Occup Environ Med. 2000;57:316–324. doi: 10.1136/oem.57.5.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bosch de Basea M, Porta M, Alguacil J, et al. Relationships between occupational history and serum concentrations of organochlorine compounds in exocrine pancreatic cancer. Occup Environ Med. 2011;68:332–338. doi: 10.1136/oem.2009.054197. [DOI] [PubMed] [Google Scholar]
- 48.Hartwig A, Mullenders LH, Schlepegrell R, et al. Nickel(II) interferes with the incision step in nucleotide excision repair in mammalian cells. Cancer Res. 1994;54:4045–4051. [PubMed] [Google Scholar]
- 49.Kasprzak KS. The role of oxidative damage in metal carcinogenicity. Chem Res Toxicol. 1991;4:604–615. doi: 10.1021/tx00024a002. [DOI] [PubMed] [Google Scholar]
- 50.Lee YW, Klein CB, Kargacin B, et al. Carcinogenic nickel silences gene expression by chromatin condensation and DNA methylation: a new model for epigenetic carcinogens. Mol Cell Biol. 1995;15:2547–2557. doi: 10.1128/mcb.15.5.2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ahamed M, Akhtar MJ, Siddiqui MA, et al. Oxidative stress mediated apoptosis induced by nickel ferrite nanoparticles in cultured A549 cells. Toxicology. 2011;283:101–108. doi: 10.1016/j.tox.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 52.Brown KM, Arthur JR. Selenium, selenoproteins and human health: a review. Public Health Nutr. 2001;4:593–599. doi: 10.1079/phn2001143. [DOI] [PubMed] [Google Scholar]
- 53.Papp LV, Lu J, Holmgren A, et al. From selenium to selenoproteins: synthesis, identity, and their role in human health. Antioxid Redox Signal. 2007;9:775–806. doi: 10.1089/ars.2007.1528. [DOI] [PubMed] [Google Scholar]
- 54.Amaral AF, Cantor KP, Silverman DT, et al. Selenium and bladder cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2010;19:2407–2415. doi: 10.1158/1055-9965.EPI-10-0544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bardia A, Tleyjeh IM, Cerhan JR, et al. Efficacy of antioxidant supplementation in reducing primary cancer incidence and mortality: systematic review and meta-analysis. Mayo Clin Proc. 2008;83:23–34. doi: 10.4065/83.1.23. [DOI] [PubMed] [Google Scholar]
- 56.Bjelakovic G, Nikolova D, Simonetti RG, et al. Antioxidant supplements for preventing gastrointestinal cancers. Cochrane Database Syst Rev. 2008:CD004183. doi: 10.1002/14651858.CD004183.pub3. [DOI] [PubMed] [Google Scholar]
- 57.Zhuo H, Smith AH, Steinmaus C. Selenium and lung cancer: a quantitative analysis of heterogeneity in the current epidemiological literature. Cancer Epidemiol Biomarkers Prev. 2004;13:771–778. [PubMed] [Google Scholar]
- 58.Etminan M, FitzGerald JM, Gleave M, et al. Intake of selenium in the prevention of prostate cancer: a systematic review and meta-analysis. Cancer Causes Control. 2005;16:1125–1131. doi: 10.1007/s10552-005-0334-2. [DOI] [PubMed] [Google Scholar]
- 59.Burney PG, Comstock GW, Morris JS. Serologic precursors of cancer: serum micronutrients and the subsequent risk of pancreatic cancer. Am J Clin Nutr. 1989;49:895–900. doi: 10.1093/ajcn/49.5.895. [DOI] [PubMed] [Google Scholar]
- 60.Murawaki Y, Tsuchiya H, Kanbe T, et al. Aberrant expression of selenoproteins in the progression of colorectal cancer. Cancer Lett. 2008;259:218–230. doi: 10.1016/j.canlet.2007.10.019. [DOI] [PubMed] [Google Scholar]
- 61.Jackson MI, Combs GF., Jr Selenium and anticarcinogenesis: underlying mechanisms. Curr Opin Clin Nutr Metab Care. 2008;11:718–726. doi: 10.1097/MCO.0b013e3283139674. [DOI] [PubMed] [Google Scholar]
- 62.Smith ML, Lancia JK, Mercer TI, et al. Selenium compounds regulate p53 by common and distinctive mechanisms. Anticancer Res. 2004;24:1401–1408. [PubMed] [Google Scholar]
- 63.Fowler BA, Whittaker MH, Lipsky M, et al. Oxidative stress induced by lead, cadmium and arsenic mixtures: 30-day, 90-day, and 180-day drinking water studies in rats: an overview. Biometals. 2004;17:567–568. doi: 10.1023/b:biom.0000045740.52182.9d. [DOI] [PubMed] [Google Scholar]
- 64.Schrauzer GN. Anticarcinogenic effects of selenium. Cell Mol Life Sci. 2000;57:1864–1873. doi: 10.1007/PL00000668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wickre JB, Folt CL, Sturup S, et al. Environmental exposure and fingernail analysis of arsenic and mercury in children and adults in a Nicaraguan gold mining community. Arch Environ Health. 2004;59:400–409. doi: 10.3200/AEOH.59.8.400-409. [DOI] [PubMed] [Google Scholar]
- 66.Slotnick MJ, Nriagu JO. Validity of human nails as a biomarker of arsenic and selenium exposure: A review. Environ Res. 2006;102:125–139. doi: 10.1016/j.envres.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 67.Slotnick MJ, Meliker JR, AvRuskin GA, et al. Toenails as a biomarker of inorganic arsenic intake from drinking water and foods. J Toxicol Environ Health A. 2007;70:148–158. doi: 10.1080/15287390600755232. [DOI] [PubMed] [Google Scholar]
- 68.Longnecker MP, Stampfer MJ, Morris JS, et al. A 1-y trial of the effect of high-selenium bread on selenium concentrations in blood and toenails. Am J Clin Nutr. 1993;57:408–413. doi: 10.1093/ajcn/57.3.408. [DOI] [PubMed] [Google Scholar]
- 69.Garland M, Morris JS, Rosner BA, et al. Toenail trace element levels as biomarkers: reproducibility over a 6-year period. Cancer Epidemiol Biomarkers Prev. 1993;2:493–497. [PubMed] [Google Scholar]
- 70.Hunter DJ, Morris JS, Chute CG, et al. Predictors of selenium concentration in human toenails. Am J Epidemiol. 1990;132:114–122. doi: 10.1093/oxfordjournals.aje.a115623. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.