Abstract
Many severely hypoxic cells fail to initiate DNA replication, but the mechanism underlying this observation is unknown. Specifically, while the ataxia-telangiectasia-rad3 related (ATR) kinase has been shown to be activated in hypoxic cells, several studies have not been able to document down-stream consequences of ATR activation in these cells. By clearly defining the DNA replication initiation checkpoint in hypoxic cells, we now demonstrate that ATR is responsible for activating this checkpoint. We show that the hypoxic activation of ATR leads to the phosphorylation dependent degradation of the cdc25a phosphatase. Down regulation of cdc25a protein by ATR in hypoxic cells decreases CDK2 phosphorylation and activity, which results in the degradation of cdc6 by APC/CCdh1. These events do not occur in hypoxic cells when ATR is depleted, and the initiation of DNA replication is maintained. We therefore present a novel mechanism of cdc6 regulation in which ATR can play a central role in inhibiting the initiation of DNA replication via the regulation of cdc6 by APC/CCdh1. This model provides insight into the biology and therapy of hypoxic tumors.
Keywords: Hypoxia, ATR, cdc6, DNA replication, APC/CCdh1
Introduction
A wide variety of solid and hematological malignancies contain severely hypoxic cells, with both animal and human studies documenting oxygen levels below 0.1% in up to 35% of tumors (Olive et al 2002, Vaupel et al 1991). Hypoxic cells respond to this stress by a variety of transcriptional and post-transcriptional mechanisms which result in an increase in glycolytic flux, a suppression of oxidative metabolism and protein translation, and an increase in angiogenesis and autophagy (reviewed in (Gardner and Corn 2008)). Hypoxic cells also adapt to their microenvironment by suppressing proliferation, a general phenomenon in non-transformed cells that is absent in several transformed cell lines (Gardner et al 2001, Gardner et al 2003, Schmaltz et al 1998). Because the hypoxic-activation of cell cycle checkpoints may help to explain phenomena such as tumor dormancy, and the absence of these checkpoints in tumors may help explain the poor prognosis of those with hypoxic tumors, the characteristics and mechanisms of the hypoxia-induced growth arrest have been intensively studied (reviewed in (Green and Giaccia 1998)).
The cell cycle checkpoints activated in hypoxic cells vary depending on the extent of cellular hypoxia (which is both a function of the duration of hypoxia and the degree of hypoxia). In addition to a G1 cell cycle arrest that occurs with moderate (~1% oxygen) hypoxia (Gardner et al 2001, Graeber et al 1994, Koshiji et al 2004), we and others have described a distinct S phase arrest that occurs in severely hypoxic cells (Gardner et al 2003, Green et al 2001, Hammond et al 2003b, Olcina et al 2010). Specifically, we noted that cell progression through S phase did not occur in hypoxic cells, but that the elongation component of DNA replication was not altered, suggesting that the initiation of DNA replication is defective in severely hypoxic cells. However the mechanism underlying this S phase checkpoint is unknown.
The normal initiation of DNA replication is a highly orchestrated event. Prior to DNA replication, pre-replicative complexes are formed by the recruitment of cdc6 to origins of replication, followed by the loading and phosphorylation of the minichromosome maintenance proteins (MCMs). cdc6 plays a critical role in regulating DNA replication, as it is both limiting and essential for S phase entry, and depletion of cdc6 inhibits new origin firing (Hateboer et al 1998, Lau et al 2006, Stoeber et al 1998). The CDK2 kinase phosphorylates cdc6 and prevents its degradation, therefore promoting pre-replicative complex assembly (Cook et al 2002, Mailand and Diffley 2005). Cdk2 kinase activity, in turn, is regulated by cyclin dependent kinase inhibitors and the cdc25A phosphatase which activates CDK2 (Mailand et al 2000). A variety of kinases, including CDK2 and Cdc7-Dbf4, phosphorylate MCM2 and promotes MCM helicase activity, which is also necessary for the initiation of DNA replication (Jiang et al 1999, Montagnoli et al 2006).
This initiation of DNA replication can be repressed by an S phase checkpoint in response to a variety of cellular stresses. This checkpoint can be activated by either the ataxia telangectasia mutated (ATM) and/or the ATM-Rad9 related (ATR) kinases. ATM activation occurs in response to double strand DNA breaks, which occurs when hypoxic cells are reoxygenated, but is unlikely to be responsible for the S phase checkpoint observed in pure hypoxic conditions (Hammond et al 2003b). In contrast, several studies have convincingly demonstrated that ATR is activated in hypoxic cells (Hammond et al 2002, Hammond et al 2003a). The activation of ATR in hypoxic cells is presumably due to the documented accumulation of single strand DNA in hypoxic cells (Hammond and Giaccia 2004). Activation of ATR and its downstream target Chk1 have been demonstrated to play an important role in the S phase checkpoint during UV irradiation, and indeed ATR has been described to promote the degradation of the cdc25a protein (Busino et al 2003, Heffernan et al 2002, Heffernan et al 2007). However, in hypoxic cells the depletion of ATR has been reported to not affect thymidine incorporation into DNA (Hammond et al 2004). In addition, several well described consequences of ATR activation by other stresses (e.g. UV irradiation) have not been observed in hypoxic cells. For example, while cdc25a is down-regulated in hypoxic cells, this has been attributed to alterations in the transcript level, not ATR activation and cdc25a protein stability (de Oliveira et al 2009, Hammer et al 2007). Thus the biological consequences of ATR activation in hypoxic cells are unknown.
Previous studies that have assessed the role of ATR activation in hypoxic cells have utilized conditions that markedly suppress general DNA replication (Hammond et al 2004). We reasoned that any participation of ATR to the inhibition of DNA replication would be best appreciated when the initiation of DNA replication, as opposed to other hypoxia-induced checkpoints, is suppressed. Therefore we focused on those conditions which selectively inhibit the initiation of DNA replication and investigated both known and novel consequences of ATR activation which contribute to regulating DNA replication in hypoxic cells, as well as the upstream events required for ATR activation in hypoxic cells.
Results
ATR participates in the hypoxic suppression of DNA replication
To identify the conditions in which the initiation of DNA replication is most affected in hypoxic cells, we pulsed hypoxic cells with tritiated thymidine to tag the ends of replicating DNA, and then separated nascent DNA molecules according to size in alkaline sucrose gradients. Lighter, smaller molecules represent those initiated during the pulse, and larger, heavier, fragments represent elongating fragments. This technique, for example, has demonstrated that low dose UV irradiation inhibits the initiation of DNA replication, while higher doses suppress both DNA initiation and elongation (Heffernan et al 2002, Kaufmann et al 1980). We noted a similar pattern in hypoxic cells, where cells rendered severely hypoxic for short periods of time selectively inhibited DNA replication initiation, and DNA synthesis in general was inhibited after longer periods of time (Fig 1A).
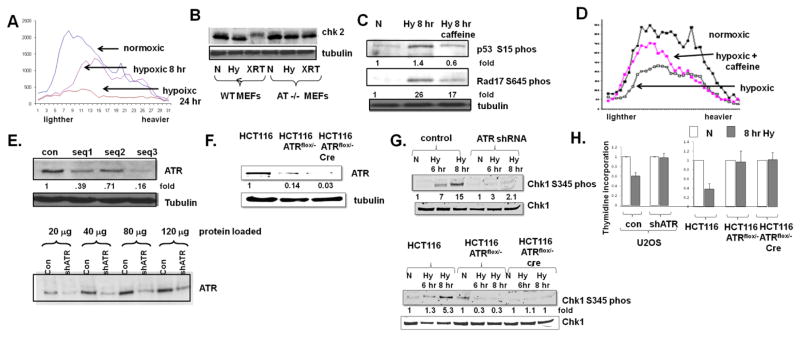
Fig 1.
ATR inhibits the initiation of DNA replication in hypoxic cells. A. U2OS cells were rendered hypoxic for 8 hrs or 24 hrs, and pulsed with thymidine prior to the collection of DNA and fractionation as described in the text. Lighter fractions represent smaller, newly synthesized DNA molecules. B. Chk2 expression in normoxic (N), hypoxic (Hy) and 10 Gy irradiated (XRT) MEFs and, as control, AT−/− MEFs. Phosphorylation of Chk2 manifests as a mobility shift. C. Expression of Chk1-serine 345 phosphorylation and rad17-serine 645 phosphorylation in cells rendered hypoxic and also rendered hypoxic and treated with 10 mM caffeine. D. Incoroporation of thymidine into DNA in cells rendered hypoxic for eight hours, with and without 10 mM caffeine. Experiments were replicated twice; fold changes are displayed. E. ATR expression in U2OS cells expressing three different shRNA sequences (top) and extent of ATR depletion as indicated by increasing loading of proteins (bottom). F. ATR expression in wild-type HCT116 cells, HCT116 ATRflox/− cells expressing a tamoxifen inducible cre recombinase, and HCT116 ATRflox/− cells treated with tamoxifen for 24 hrs. G. Chk1-serine 345 phosphorylation in cells described in (F) rendered hypoxic. H. Thymidine incorporation in U2OS and HCT116 depleted cells rendered hypoxic for eight hours. Experiments were replicated three times. Average ± standard error is displayed.
We therefore elected to focus further studies on DNA replication in cells rendered severely hypoxic for a relatively brief period, when initiation is most hampered. During this period we confirmed that a specific ATM substrate, Chk2, is not phosphorylated (Fig 1B) but that the ATM/ATR targets rad17-serine 645 and p53-serine 15 are phosphorylated (Fig 1C). Caffeine, a non-selective inhibitor of both ATM and ATR attenuated these phosphorylation events (Fig 1C). When cells were rendered hypoxic for short periods of time and then treated with caffeine, we also noted a restoration in the initiation component of DNA replication (Fig 1D).
Several studies have specifically investigated, and have not found a role for ATR activation in hypoxia-induced cell cycle arrests (Hammond et al 2004). In addition, caffeine has many effects in addition to the inhibition of ATR. We therefore used two separate strategies to specifically deplete ATR and assess the hypoxic suppression of DNA replication. While complete deletion of ATR is incompatible with long term proliferation (Brown and Baltimore 2000) we generated several shRNA sequences against ATR and identified one where we achieved ~80% depletion of ATR in the osteosarcoma cell line, U2OS, without any gross effect on proliferation (1E). We also generated a system to express an inducible cre recombinase in previously described colon cancer HCT116 ATRflox/− cells (Cortez et al 1999)); cre induction led to a dramatic decrease in ATR expression in these cells after two days (Fig 1F). Compared to control hypoxic cells, the hypoxia-induced phosphorylation of Chk1-serine 345, a specific ATR target, was blunted in both U2OS and HCT116 ell lines after ATR expression was diminished (Fig 1G). When ATR depleted cells were rendered hypoxic for durations that impair the initiation of DNA replication, there was no significant suppression of DNA replication, in contrast to their respective matched control cells (Fig 1H). Of note, under our conditions even the heterozygous HCT116 ATRflox/− cells, which express ~15% of wild-type levels of ATR (Fig. 1F) had marked deficiencies in the hypoxic phosphorylation of Chk1 compared to wild type HCT116 cells (Fig 1G). In addition, there was no attenuation of DNA replication in hypoxic HCT116 ATRflox/− flox cells with or without cre induction (Fig 1H), confirming that even moderate decreases of ATR can promote proliferation in hypoxic cells.
The hypoxic activation of ATR down-regulates cdc25a and promotes CDK2 phosphorylation
We next sought to identify the mechanisms by which the activation of ATR suppresses the initiation of DNA replication in hypoxic cells. It should be noted that the activation of ATR in hypoxic cells does not necessarily have the same consequences as the activation of ATR in, for example, UV irradiated cells. For example, p53 is induced in hypoxic cells, but in contrast to its function in irradiated cells, hypoxia-induced p53 is neither transcriptionally active nor participates in any hypoxia-induced cell cycle checkpoints (Graeber et al 1994). Similarly, although cdc25a protein has been well described to be degraded as a consequence of UV mediated ATR activation (Busino et al 2003), the decrease in cdc25a protein expression in hypoxic cells has been attributed to transcriptional repression and/or RNA destabilization by a microRNA (de Oliveira et al 2009, Hammer et al 2007).
However, when we examined hypoxic U2OS and HCT116 cells under conditions that selectively suppress the initiation of DNA replication in an ATR dependent manner, we noted a marked down-regulation of cdc25a protein expression (Fig 2A) in the absence of a significant decrease in cdc25a mRNA expression (Fig 2B). A cdc25a construct without a 3′ untranslated region expressed from an exogenous promoter was also down-regulated in hypoxic cells, confirming that cdc25a is down-regulated in a post-transcriptional mechanism (Fig 2C). In both U2OS and HCT116 cells cdc25a protein was not down-regulated in the absence of ATR (Fig 2A). Chk1 is known to phosphorylate cdc25a on serine 76, and cdc25a constructs which cannot be phosphorylated on this residue are not susceptible to ATR mediated destruction (Busino et al 2003). Accordingly, we found that, in contrast to wild-type cdc25a, cdc25a S76A is stable when expressed in hypoxic cells (Fig 2C).
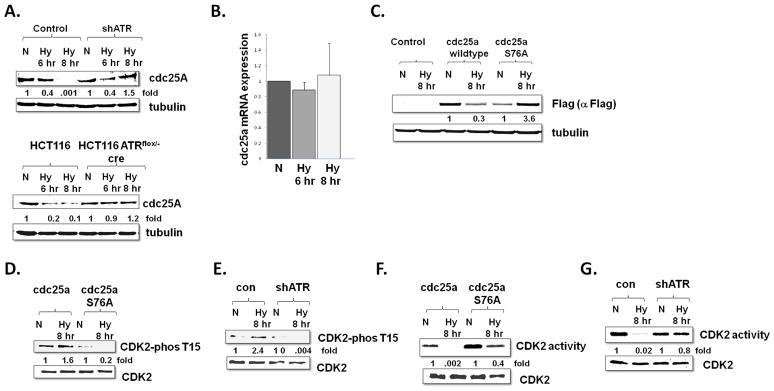
Fig 2.
cdc25a protein and CDK2 activity are regulated by ATR in hypoxic cells. A. U2OS and HCT117cells with and without ATR depletion as demonstrated in Fig 1, were rendered hypoxic (Hy) or maintained as normoxic (N) and cdc25a protein expression was assessed. Experiments were replicated and fold changes displayed. B. cdc25a mRNA expression in U2OS cells rendered hypoxic for four and eight hours. Experiments were done in triplicate and average ± standard error displayed. C. Expression of Cdc25a in normoxic and hypoxic cells stably expressing a wild-type cdc25a construct or a cdc25a S76A construct. Experiments were done in triplicate and fold changes displayed. D. CDK2 tyrosine 15 phosphorylation in normoxic and hypoxic U2OS cells expressing either wild-type cdc25a or cdc25a S76A, and in E. normoxic and hypoxic control and ATR depleted U2OS cells. Experiments were replicated and fold changes displayed F. CDK2 activity and expression in normoxic and hypoxic U2OS cells expressing wild-type cdc25a or cdc25a S76A G. CDK2 activity and expression in normoxic and hypoxic control and ATR depleted U2OS cells.
Cdc25a activates CDK2 by removing an inhibitory phosphate on CDK2 tyrosine 15 (Mailand et al 2000), so to determine the significance of cdc25a down-regulation in hypoxic cells we assessed the phosphorylation status of CDK2. As expected, the phosphorylation of tyrosine 15 on cdk2 phosphorylation correlated to the level of cdc25a expression in hypoxic and normoxic cells. Specifically CDK2 tyrosine phosphorylation increased in hypoxic cells compared to normoxic cells, and this phosphorylation was diminished in hypoxic cells that expressed a cdc25a S76A, which is not degraded in hypoxic cells (Fig 2D). Consistent with the stabilization of cdc25a in hypoxic ATR depleted cells, CDK2 phosphorylation was also diminished in hypoxic cells with ATR depletion, in contrast to control hypoxic cells (Fig 2E). Finally, CDK2 activity also correlated to cdc25a expression and CDK2 tyrosine 15 phosphorylation status; it decreased in hypoxic cells and was de-repressed in hypoxic cells expressing a stable cdc25a (Fig 2F) and in hypoxic cells with ATR depletion (Fig 2G). Together these data suggest that the hypoxic activation of ATR decreases CDK2 activity via phosphorylation and degradation of cdc25a.
The hypoxic activation of ATR leads to degradation of cdc6 via APC/CCdh1
While CDK2 has many substrates that play important roles in proliferation, we elected to focus on the hypoxic regulation of cdc6, a protein with a well described role in the initiation of DNA replication. Both total (soluble) cdc6 and chromatin bound cdc6 protein expression decreased in hypoxic cells (Fig 3A) in the absence of any decrease in cdc6 mRNA (Fig 3B). This decrease in cdc6 protein expression was attenuated in hypoxic cells when ATR was depleted (Fig 3A, 3C). CDK2 stabilizes cdc6 by phosphorylating cdc6 on several sites, including serine 106 (Mailand and Diffley 2005), and because of our data demonstrating a role for ATR in regulating CDK2 activity in hypoxic cells we examined the phosphorylation of cdc6 in hypoxic cells. Cdc6 serine 106 phosphorylation decreased in hypoxic cells, and along with a rescue of total cdc6 expression with the depletion of ATR, we noted a rescue of cdc6 serine 106 phosphorylation (Fig 3C). In addition, in contrast to a wild-type cdc6 construct, expression of a cdc6 S54D, S74D, S106D phosphomimetic construct was stable in hypoxic cells (Fig 3D).
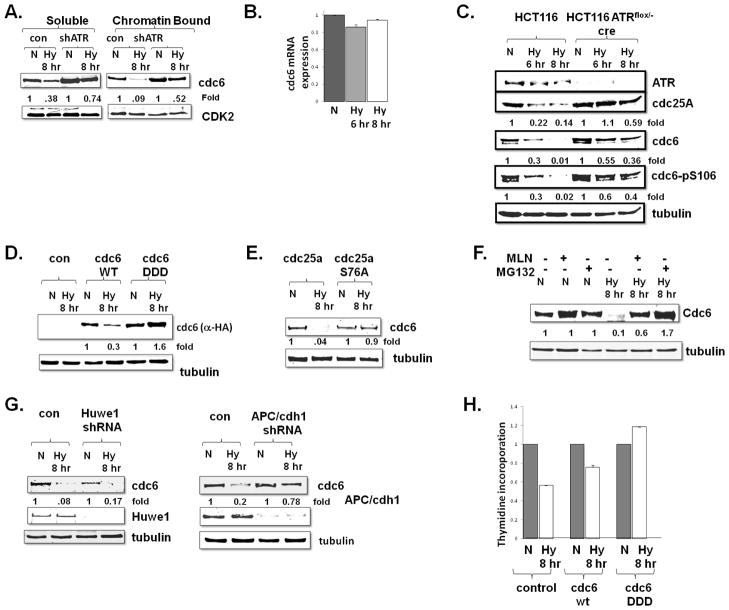
Fig 3.
ATR down-regulates Cdc6 in hypoxic cells by APC/CCdh1. A. Soluble and chromatin bound cdc6 protein expression in normoxic (N) and hypoxic (Hy) control and ATR depleted U2OS cells. Experiments were done in triplicate and fold changes displayed. B. cdc6 mRNA expression in hypoxic U2OS cells. Experiments were done in triplicate average ± standard error is displayed. C. Cdc6 expression and phosphorylation of cdc6 serine 106 in normoxic and hypoxic wild-type and ATR deleted cells. Note lanes have been removed from this figure, but exposure is consistent. D. Transient transfected 293T cells, expressing either wild-type or a phosphomimetic (DDD) form of cdc6 were rendered hypoxic and assessed for cdc6 expression Experiments were replicated and fold changes displayed E. cdc6 protein expression in normoxic and hypoxic U2OS cells expressing wild-type cdc25a or a stable cdc25a S76A construct. Experiments were done in triplicate and fold changes displayed F. U2OS cells were rendered hypoxic or normoxic for eight hours, the last three with either MG132 (10 mM) or MLN, and cdc6 expression was assessed. G. Normoxic and hypoxic U2OS cells were depleted for Huwe1 or cdh1 and cdc6 expression was assessed. Experiments were replicated and fold changes displayed H. 293T cells, expressing either control plasmid, wild-type or a phosphomimetic (DDD) form of cdc6 were rendered hypoxic and thymidine incorporation was assessed. Experiments were done in triplicate and average ± is displayed.
To assess whether this hypoxia-induced, ATR-mediated cdc6 down-regulation was mediated by a decrease in CDK2 activity, we examined cdc6 expression in hypoxic cells expressing cdc25aS76A, which is stabilized in hypoxic cells and thus rescues CDK2 activity in hypoxic cells (Fig 2). The expression of this stable cdc25a in hypoxic cells phenocopied ATR depletion by stabilizing cdc6, thus suggesting that decreased CDK2 activity is responsible for the downregulation of cdc6 in hypoxic cells (Fig 3E). These data suggest that in hypoxic cells the activation of ATR, down-regulation of cdc25a, decrease in cdk2 activity, and diminished phosphorylation of cdc6, leads to cdc6 down-regulation.
Downregulation of cdc6 was rescued by the treatment of hypoxic cells with a MG132, a proteasome inhibitor, suggesting that ATR led to the ubiqutination and degradation of cdc6 protein (Fig 3F). Two ubiquitin ligases have been implicated in the degradation of cdc6, APC/CCdh1 and Huwe1 (Hall et al 2007, Mailand and Diffley 2005). Huwe1 has specifically found to degrade cdc6 with DNA damage (Hall et al 2007) and APC/CCdh1 is thought to regulate cdc6 to prevent the re-replication of DNA (Mailand and Diffley 2005). Intiguingly, in addition to proteasome inhibition, cd6 degradation was also inhibited by MLN, an inhibitor of neddylation that affects neither Huwe1 nor APC/CCdh11 directly (Fig 3F). However, MLN does lead to an accumulation of the APC/CCdh1 inhibitor Emi1 (due to inhibition of trcp/cul1) (Benmaamar and Pagano 2005, Guardavaccaro et al 2003, Reimann et al 2001). Consistent with this possibility, specific depletion of Huwe1 had no effect on the expression of cdc6 in hypoxic cells (3G, left panel), whereas the knock-down of cdh1 blocked the down-regulation of cdc6 in hypoxic cells (Fig 3G, right panel).
Finally, we assessed the functional significance of cdc6 degradation in hypoxic cells. Expression of wild-type cdc6 partially rescued DNA replication in hypoxic cells (Fig 3H), consistent with the previous report that high expression of even wild-type cdc6 can bind to chromatin, avoid degradation, and promote proliferation (Cook et al 2002). However, expression of the phophomimentic cdc6 DDD mutant, stable in hypoxic cells, was sufficient to fully restore DNA replication in hypoxic cells (Fig 3H). Together these data suggest that degradation of cdc6 by APC1 in hypoxic cells plays a critical role in inhibiting the initiation of DNA replication.
MCM2 loading and phosphorylation is inhibited in hypoxic cells
cdc6 plays an important role on loading the MCM proteins into a replication complex, a process necessary for the initiation of DNA replication (Cook et al 2002). We therefore examined the extent of chromatin bound MCM2 in hypoxic cells, and the contribution the hypoxic activation of ATR plays in this loading. We noted that while total MCM2 was not greatly altered in hypoxic cells, the fraction of chromatin bound MCM2 was moderately decreased, and released into the nucleoplasm (Fig 4A). This hypoxic down-regulation of chromatin bound MCM2 was rescued in ATR depleted cells (Fig 4B).
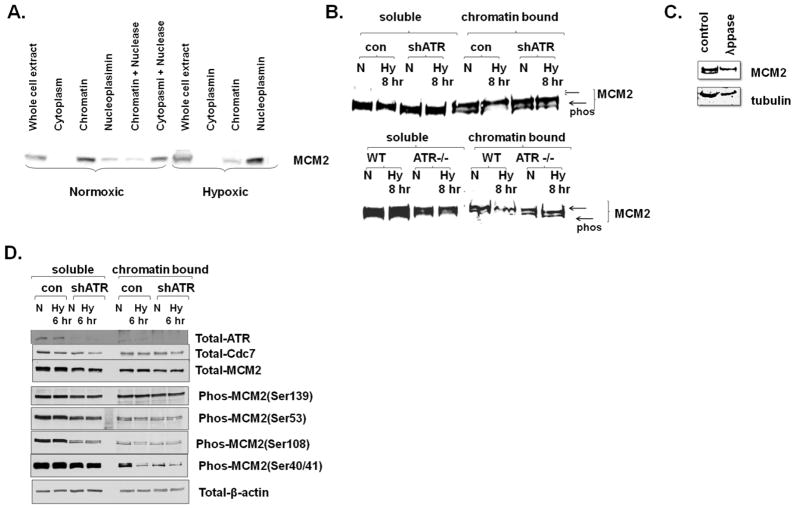
Fig 4.
Chromatin bound MCM2 expression and phosphorylation is affected by hypoxic activation of ATR. A. MCM2 expression in hypoxic (Hy) and normoxic (N) cells was assessed in cytoplasmic, chromatin bound, and nucleoplasm cell compartments. Chromatin bound MCM2 was also treated with nuclease to confirm proper fractionation. B. Soluble and chromatin bound MCM2 in normoxic and hypoxic U2OS control and ATR depleted cells (note, panel is a composite). Proteins were resolved so that two bands were apparent, the bottom one consistent with a phosphorylated form, as C. demonstrated by its disappearance with lambda phosphatase treatment. D. Soluble and Chromatin bound phosphorylated forms of MCMC2 in normoxic and six hours hypoxic U2OS control and ATR depleted cells.
After recruitment of the MCM complex by cdc6, MCMs can be activated by phosphorylation on multiple sites by cdc7, CDK2, and CDK1, which activate MCM helicase activity (Montagnoli et al 2006). In xeonpus, ATR has been reported to decrease MCM2 phosphorylation via inhibition of the cdc7 kinase (Costanzo et al 2003) while separate studies have indicated that ATR can directly phosphorylate MCM2 on serine 108 (Cortez et al 2004). When we fully resolved proteins with electrophoresis, we noted that along with the decrease in chromatin bound MCM2 in hypoxic cells, there was a shift in mobility of the chromatin bound MCM2 (Fig 4B) consistent with altered phosphorylation levels. Indeed phosphates treatment caused MCM2 to migrate as a single band (Fig 4C). This hypoxia dependent change in MCM2 mobility was rescued by depletion of ATR (Fig 4B).
In order to get a better insight into the events required for MCM2 phosphorylation in hypoxic cells, we used phosphospecific MCM2 antibodies on normoxic and hypoxic control and ATR depleted cells. To simplify the comparisons, we utilized extracts from less severely hypoxic cells, where total chromatin bound MCM2 was not greatly altered (Fig 4D). There was no dramatic difference in the phosphorylation status of serine 53 (which is cdc7 dependent) or serine 139 (which is thought to be cdc7 independent) in hypoxic cells (Fig 4D). While soluble serine 108 phosphorylated MCM2 was diminished with ATR depletion, the amount of chromatin bound serine 108 phosphorylated MCM2 was not dramatically altered in hypoxic cells (where ATR is activated). In contrast, chromatin bound MCM2 serine 40/41 phosphorylation was strongly decreased in hypoxic cells. This decrease of serine 40/41 was marginal in hypoxic ATR depleted cells, although the absence of ATR itself led to a dramatic decrease in serine 40/41 phosphorylation. The unaltered phosphorylation status of serine 53, as well as the stable expression of chromatin bound cdc7 in hypoxic cells, suggests that cdc7 kinase activity is not obviously altered in hypoxic cells, despite ATR activation. Thus the down-regulation of Serine 40/41 phosphorylation in hypoxic cells could be either due to loss of a priming kinase that has been reported to be necessary for Cdc7 dependent Serine 40 phosphorylation in vitro (Montagnoli et al 2006) or hyper activation of a phosphatase acting on MCM2 Serine 40/41.
Discussion
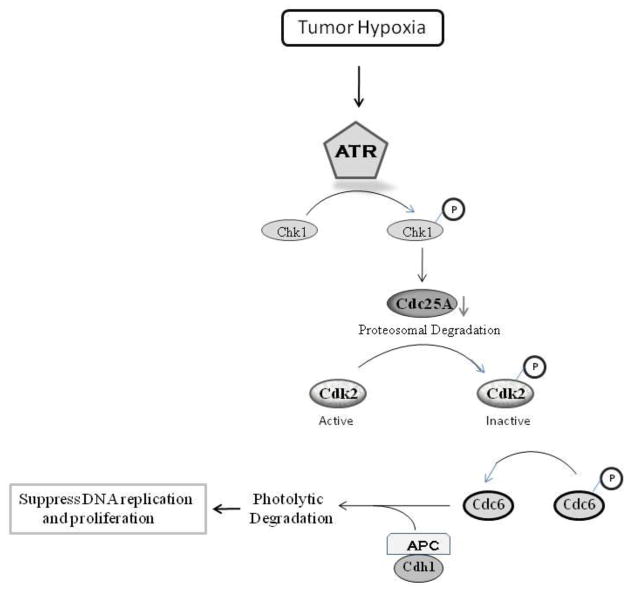
Our studies indicate that the hypoxic activation of ATR plays an important role in suppressing DNA replication, in large part through the degradation of cdc25a, resulting inactivation of CDK2 activity, and the down-regulation of cdc6 via APC/cdh1 mediated degradation (Fig 5). The central role for the hypoxic down-regulation of cdc6 in the inhibition of DNA replication is demonstrated by the restoration of DNA replication in hypoxic cells expressing a stable cdc6. Although APC/CCdh1 has been referred to as the master controller origin licensing (Sivaprasad et al 2007), the important link between ATR activation, APC/CCdh1, and cdc6 has not previously been appreciated. Our clear documentation that APC/cdh1 plays an important role in the initiation of DNA replication in hypoxic cells, emphasizes both the well known regulation of ubiqutin/proteasome system in hypoxic cells and the established of this system in controlling the cell cycle. Indeed, APC/CCdh1 regulation by Emi1 has been found to be particularly important for the physiological regulation of the initiation of DNA replication (Machida and Dutta 2007). The fact that, in contrast to the events seen in DNA damage, Huwe1 is not responsible for degrading cdc6 in hypoxic cells, highlights the important differences between the cellular response to hypoxic stress and to DNA damage.
Fig 5.
A model for the hypoxic regulation of the initiation of DNA replication.
While previous studies have demonstrated diminished CDK2 activity and decreased cdc25a expression in hypoxic cells, the mechanisms for these observations did not include a role for ATR (de Oliveira et al 2009, Gardner et al 2001, Green et al 2001, Hammer et al 2007, Koshiji et al 2004). Indeed, deletion of ATR was previously not found to play a role in the proliferation of hypoxic cells (Hammond et al 2004). These discrepancies are best resolved by noting that our studies focused on conditions in which the initiation of DNA replication is most affected. For example, during the hypoxic conditions we utilized, total thymidine incorporation decreased 40–50%, whereas previous studies used conditions which suppressed total thymidine incorporation up to 90% (Hammond et al 2004), suggesting the strategies used in those studies rendered cells more hypoxic and almost completely ceased proliferation. Accordingly, we have previously noted (Gardner et al 2003) that the duration and degree of cellular hypoxia can result in the activation of distinct checkpoints, as confirmed from our experiments here. For example, moderate hypoxia does not result in activation of ATR, and prolonged hypoxia is expected to result in decreased transcriptional activation of cdc25a due to hypophosphorylation of the Rb protein and decreased E2F expression (Gardner et al 2001, Gardner et al 2003, Vigo et al 1999).
We did not observe any tumor growth in nude mice injected subcutaneously with control U2OS cells, U2OS cells with cdc6 wild-type over-expression, U2OS cells with cdc6 DDD over-expression, U2OS cells with cdc25a over-expression or cdc25a S76A over-expression (data not shown). This observation suggests that manipulation of the cdc25a or cdc6 pathways is not sufficient to promote tumorigenesis, a finding readily explained by the metabolic, angiogenic, and many other pathways that need to be activated to support three dimensional tumor growth. In HCT116 cells manipulated to express the above constructs (Fig S1A) the manipulation of cdc25a or cdc6 did not have a significant effect on cell proliferation in tissue culture (i.e. not hypoxic) (Fig S1B), or in tumorigenesis in nude mice (Fig S1C). Thus the manipulation of the ATR downstream pathway alone cannot alter growth in hypoxic xenografts, again consistent with the complex requirements for three dimensional tumor growth (which include the bypass of several distinct cell cycle checkpoints we and others have described). Thus additional studies, including the assessment of ATR activation, cdc25a expression, and activity of the DNA replication complex in hypoxic regions of tumors, are required to fully determine the biological relevance of our findings.
However, because many tumors contain areas of severely hypoxic cells, and because ATR and downstream targets including cdc6 and cdc25a have been both noted to be dysregulated in cancer and to be targets for cancer therapy (Borlado and Mendez 2008), the hypoxic regulation of ATR is likely to have biological and therapeutic implications in cancer. The de-regulation of ATR signaling would be expected to allow at least the initiation of DNA replication to occur in hypoxic cancer cells. We and others have noted that some malignant cells have the capacity to proliferate, despite being rendered severely hypoxic (Gardner et al 2003). Oncoproteins have been reported to activate cdc6 (Sotillo et al 2009), and indeed we have determined that over-expression of the E1a oncoprotein allows hypoxic cells to proliferate (Gardner et al 2003). Finally, our current studies demonstrate that activation of ATR in hypoxic cells leads to the degradation of cdc6 by APC/CCdh1, and this is inhibited by both proteasome inhibitors, as well as inhibitors of neddylation, two classes of agents that have been proven clinically active in a variety of malignancies. While bypass of hypoxic checkpoints could permit cancers to escape a dormant state, it is possible that the therapeutic manipulation of these checkpoints could render cells more prone to death from a hostile microenvironment, or from proliferation dependent therapeutics.
Methods
Cell lines
U2OS, HCT116 and 293 cell lines were obtained from ATCC. U2OS cells were maintained in DMEM, 10% fetal calf serum (FCS), and HCT116 and HCT116 ATRflox/− (Zou and Elledge 2003) were cultured in McCoy’s 5A containing Geneticin (G418). Cells were incubated at 37°C in 5% CO2 and room air (standard tissue culture incublator) or in a Plas-Labs environmental chamber with 5% CO2 and <0.1% oxygen. HCT116 ATRflox/− cells were infected with a tamoxifen inducible pBABE-Cre recombinase retrovirus, and for cre was induced with 40 μM tamoxifen for 48 hrs prior to experimentation.
Plasmids
cdc25a wild-type and S76A mutated plasmids (Busino et al 2003) were cloned into the retroviral construct pQCXIN. Flag tagged RPA2 wild-type, A and D mutants (Vassin et al 2004) were cloned into the pLPC retrovirus. pCDNA3.1 expressing Cdc6 wild type and DDD mutants have been described (Mailand and Diffley 2005). Hairpin sequences directed against ATR were derived computationally and cloned into the pSUPER retrovirus; the sequence GAGCTTACCTTGCGTAGGGC was deemed most effective, and utilized. LKO.1 shRNA lentiviruses directed against RPA2 (TRCN5896), Huwe1 (TRCN73306) and APC/CCdh1 (TRCN231902) were obtained from Sigma.
Retroviral Generation and Transfections
Cdc6 vectors were transfected into 293T cells with calcium phosphate. Retroviruses and lentiviruses were generated as previously reported (Martin et al 2010). For APC/CCdh1 knock-down experiments, cells were infected twice with lentivirus over 12 hrs and experiments were performed 24 hrs later.
Immunoblots
Whole cell extracts were prepared as previously reported (Martin et al 2010). Complete compartmental extraction experiments were performed as reported (Mendez and Stillman 2000). For soluble and insoluble chromatin bound fractionation, cells were suspended in CSK buffer containing 0.5% Triton X-100, 10mM Pipes pH 6.8, 100mM NaCl, 1.5mM MgCl2, 300mM sucrose, 1mM of protease inhibitors, 1mM PMSF, 10mM NaF and 1mM ATP, incubated on ice for 15 mins and then centrifuged at 3200 rpm. The supernatant containing soluble protein and the pellet containing insoluble chromatin bound protein were separated and resuspended in equal volumes of 2X Laemelli buffer, Membranes were blocked and probed as described previously (Martin et al 2010)with antibodies for ATR (A300-138A, Bethyl laboratories, Inc), CHK1 (sc-7898), phosphor CHK1, Cdc25A (sc-7157), Cdk2 (sc-163), p-Cdc2 p34 (Thr14/Tyr15)(sc-12340), Cdc6 (sc-9964), Huwe1, Cdh1, Flag (F1804, Sigma), HA (H6908, Sigma), Tubulin (T9026, Sigma), Chk1-Serine 345, Rad17 ser645, p53 serine 15, H2AX serine 139 were obtained from Cell Signaling. Anti-phospho MCM2 antibodies were previously described (Montagnoli et al 2006)
Real Time PCR
RNA was isolated, cDNA prepared, and real-time PCR performed as previously described (Martin et al 2010). Cdc25a primers were F; ACCGTCACTATGGACCAGC R: TTCAGACTGGACTACATC. cdc6 primers were CTTAAGCCGGATTCTGCAAG, R: CAGTCCTCAAGGACATGCAA
Thymidine incorporation Assay
Cells (300,000/35mM) were split 24 hrs prior to a change to fresh media and either normoxic or hypoxic incubation. After cells were incubated for seven hours, they were pulse labeled with media (or deoxygenated media) containing 3H-Thymidine (30μCi/mL) for 15 minutes. Cells were then washed with ice cold PBS and lysed (while normoxic or hypoxic) with 1M NaOH. DNA was precipitated with salmon testes and 1mL 17% TCA, precipitated DNA was filtered through GF/C Whatman 24mm filters, and 3H-Thymidine was counted.
Alkaline Sucrose Gradient Analysis
Hypoxic and normoxic cells were pulsed with 3H-Thymidine and fractionated on a sucrose gradient as reported (Heffernan et al 2002).
In vitro kinase assay
CDK2 was immunoprecipitated from cells, and H1 kinase activity was assessed as previously reported (Gardner et al 2001).
Supplementary Material
Acknowledgments
We appreciate the kind gifts of reagents from John Dillfey and Stephen Elledge. Luca Busino, Michele Pagano, Vincenzo D’Angiolella provided reagents and helpful advice. This work was supported in part by DK08164 and the Feldstein Foundation. L.B.G. is the Saul J. Farber Assistant Professor of Medicine.
Footnotes
Conflicts of Interest
The authors declare no conflicts of interest
Refrences
- Benmaamar R, Pagano M. Involvement of the SCF complex in the control of Cdh1 degradation in S-phase. Cell Cycle. 2005;4:1230–1232. doi: 10.4161/cc.4.9.2048. [DOI] [PubMed] [Google Scholar]
- Borlado LR, Mendez J. CDC6: from DNA replication to cell cycle checkpoints and oncogenesis. Carcinogenesis. 2008;29:237–243. doi: 10.1093/carcin/bgm268. [DOI] [PubMed] [Google Scholar]
- Brown EJ, Baltimore D. ATR disruption leads to chromosomal fragmentation and early embryonic lethality. Genes Dev. 2000;14:397–402. [PMC free article] [PubMed] [Google Scholar]
- Busino L, Donzelli M, Chiesa M, Guardavaccaro D, Ganoth D, Dorrello NV, et al. Degradation of Cdc25A by beta-TrCP during S phase and in response to DNA damage. Nature. 2003;426:87–91. doi: 10.1038/nature02082. [DOI] [PubMed] [Google Scholar]
- Cook JG, Park CH, Burke TW, Leone G, DeGregori J, Engel A, et al. Analysis of Cdc6 function in the assembly of mammalian prereplication complexes. Proc Natl Acad Sci U S A. 2002;99:1347–1352. doi: 10.1073/pnas.032677499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortez D, Wang Y, Qin J, Elledge SJ. Requirement of ATM-dependent phosphorylation of brca1 in the DNA damage response to double-strand breaks. Science. 1999;286:1162–1166. doi: 10.1126/science.286.5442.1162. [DOI] [PubMed] [Google Scholar]
- Cortez D, Glick G, Elledge SJ. Minichromosome maintenance proteins are direct targets of the ATM and ATR checkpoint kinases. Proc Natl Acad Sci U S A. 2004;101:10078–10083. doi: 10.1073/pnas.0403410101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costanzo V, Shechter D, Lupardus PJ, Cimprich KA, Gottesman M, Gautier J. An ATR- and Cdc7-dependent DNA damage checkpoint that inhibits initiation of DNA replication. Mol Cell. 2003;11:203–213. doi: 10.1016/s1097-2765(02)00799-2. [DOI] [PubMed] [Google Scholar]
- de Oliveira PE, Zhang L, Wang Z, Lazo JS. Hypoxia-mediated regulation of Cdc25A phosphatase by p21 and miR-21. Cell Cycle. 2009;8:3157–3164. doi: 10.4161/cc.8.19.9704. [DOI] [PubMed] [Google Scholar]
- Gardner LB, Li Q, Park MS, Flanagan WM, Semenza GL, Dang CV. Hypoxia inhibits G1/S transition through regulation of p27 expression. J Biol Chem. 2001;276:7919–7926. doi: 10.1074/jbc.M010189200. [DOI] [PubMed] [Google Scholar]
- Gardner LB, Li F, Yang X, Dang CV. Anoxic fibroblasts activate a replication checkpoint that is bypassed by E1a. Mol Cell Biol. 2003;23:9032–9045. doi: 10.1128/MCB.23.24.9032-9045.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner LB, Corn PG. Hypoxic regulation of mRNA expression. Cell Cycle. 2008;7:1916–1924. doi: 10.4161/cc.7.13.6203. [DOI] [PubMed] [Google Scholar]
- Graeber TG, Peterson JF, Tsai M, Monica K, Fornace AJ, Jr, Giaccia AJ. Hypoxia induces accumulation of p53 protein, but activation of a G1-phase checkpoint by low-oxygen conditions is independent of p53 status. Mol Cell Biol. 1994;14:6264–6277. doi: 10.1128/mcb.14.9.6264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green SL, Giaccia AJ. Tumor hypoxia and the cell cycle: implications for malignant progression and response to therapy. Cancer J Sci Am. 1998;4:218–223. [PubMed] [Google Scholar]
- Green SL, Freiberg RA, Giaccia AJ. p21(Cip1) and p27(Kip1) regulate cell cycle reentry after hypoxic stress but are not necessary for hypoxia-induced arrest. Mol Cell Biol. 2001;21:1196–1206. doi: 10.1128/MCB.21.4.1196-1206.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guardavaccaro D, Kudo Y, Boulaire J, Barchi M, Busino L, Donzelli M, et al. Control of meiotic and mitotic progression by the F box protein beta-Trcp1 in vivo. Dev Cell. 2003;4:799–812. doi: 10.1016/s1534-5807(03)00154-0. [DOI] [PubMed] [Google Scholar]
- Hall JR, Kow E, Nevis KR, Lu CK, Luce KS, Zhong Q, et al. Cdc6 stability is regulated by the Huwe1 ubiquitin ligase after DNA damage. Mol Biol Cell. 2007;18:3340–3350. doi: 10.1091/mbc.E07-02-0173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer S, To KK, Yoo YG, Koshiji M, Huang LE. Hypoxic suppression of the cell cycle gene CDC25A in tumor cells. Cell Cycle. 2007;6:1919–1926. doi: 10.4161/cc.6.15.4515. [DOI] [PubMed] [Google Scholar]
- Hammond EM, Denko NC, Dorie MJ, Abraham RT, Giaccia AJ. Hypoxia links ATR and p53 through replication arrest. Mol Cell Biol. 2002;22:1834–1843. doi: 10.1128/MCB.22.6.1834-1843.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond EM, Dorie MJ, Giaccia AJ. ATR/ATM targets are phosphorylated by ATR in response to hypoxia and ATM in response to reoxygenation. J Biol Chem. 2003a;278:12207–12213. doi: 10.1074/jbc.M212360200. [DOI] [PubMed] [Google Scholar]
- Hammond EM, Green SL, Giaccia AJ. Comparison of hypoxia-induced replication arrest with hydroxyurea and aphidicolin-induced arrest. Mutat Res. 2003b;532:205–213. doi: 10.1016/j.mrfmmm.2003.08.017. [DOI] [PubMed] [Google Scholar]
- Hammond EM, Dorie MJ, Giaccia AJ. Inhibition of ATR leads to increased sensitivity to hypoxia/reoxygenation. Cancer Res. 2004;64:6556–6562. doi: 10.1158/0008-5472.CAN-04-1520. [DOI] [PubMed] [Google Scholar]
- Hammond EM, Giaccia AJ. The role of ATM and ATR in the cellular response to hypoxia and re-oxygenation. DNA Repair (Amst) 2004;3:1117–1122. doi: 10.1016/j.dnarep.2004.03.035. [DOI] [PubMed] [Google Scholar]
- Hateboer G, Wobst A, Petersen BO, Le Cam L, Vigo E, Sardet C, et al. Cell cycle-regulated expression of mammalian CDC6 is dependent on E2F. Mol Cell Biol. 1998;18:6679–6697. doi: 10.1128/mcb.18.11.6679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffernan TP, Simpson DA, Frank AR, Heinloth AN, Paules RS, Cordeiro-Stone M, et al. An ATR- and Chk1-dependent S checkpoint inhibits replicon initiation following UVC-induced DNA damage. Mol Cell Biol. 2002;22:8552–8561. doi: 10.1128/MCB.22.24.8552-8561.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffernan TP, Unsal-Kacmaz K, Heinloth AN, Simpson DA, Paules RS, Sancar A, et al. Cdc7-Dbf4 and the human S checkpoint response to UVC. J Biol Chem. 2007;282:9458–9468. doi: 10.1074/jbc.M611292200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang W, McDonald D, Hope TJ, Hunter T. Mammalian Cdc7-Dbf4 protein kinase complex is essential for initiation of DNA replication. EMBO J. 1999;18:5703–5713. doi: 10.1093/emboj/18.20.5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann WK, Cleaver JE, Painter RB. Ultraviolet radiation inhibits replicon initiation in S phase human cells. Biochim Biophys Acta. 1980;608:191–195. doi: 10.1016/0005-2787(80)90147-1. [DOI] [PubMed] [Google Scholar]
- Koshiji M, Kageyama Y, Pete EA, Horikawa I, Barrett JC, Huang LE. HIF-1alpha induces cell cycle arrest by functionally counteracting Myc. EMBO J. 2004;23:1949–1956. doi: 10.1038/sj.emboj.7600196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau E, Zhu C, Abraham RT, Jiang W. The functional role of Cdc6 in S-G2/M in mammalian cells. EMBO Rep. 2006;7:425–430. doi: 10.1038/sj.embor.7400624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machida YJ, Dutta A. The APC/C inhibitor, Emi1, is essential for prevention of rereplication. Genes Dev. 2007;21:184–194. doi: 10.1101/gad.1495007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mailand N, Falck J, Lukas C, Syljuasen RG, Welcker M, Bartek J, et al. Rapid destruction of human Cdc25A in response to DNA damage. Science. 2000;288:1425–1429. doi: 10.1126/science.288.5470.1425. [DOI] [PubMed] [Google Scholar]
- Mailand N, Diffley JF. CDKs promote DNA replication origin licensing in human cells by protecting Cdc6 from APC/C-dependent proteolysis. Cell. 2005;122:915–926. doi: 10.1016/j.cell.2005.08.013. [DOI] [PubMed] [Google Scholar]
- Martin L, Kimball SR, Gardner LB. Regulation of the unfolded protein response by eif2bdelta isoforms. J Biol Chem. 2010;285:31944–31953. doi: 10.1074/jbc.M110.153148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez J, Stillman B. Chromatin association of human origin recognition complex, cdc6, and minichromosome maintenance proteins during the cell cycle: assembly of prereplication complexes in late mitosis. Mol Cell Biol. 2000;20:8602–8612. doi: 10.1128/mcb.20.22.8602-8612.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagnoli A, Valsasina B, Brotherton D, Troiani S, Rainoldi S, Tenca P, et al. Identification of Mcm2 phosphorylation sites by S-phase-regulating kinases. J Biol Chem. 2006;281:10281–10290. doi: 10.1074/jbc.M512921200. [DOI] [PubMed] [Google Scholar]
- Olcina M, Lecane PS, Hammond EM. Targeting hypoxic cells through the DNA damage response. Clin Cancer Res. 2010 doi: 10.1158/1078-0432.CCR-10-0286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olive PL, Banath JP, Durand RE. The range of oxygenation in SiHa tumor xenografts. Radiat Res. 2002;158:159–166. doi: 10.1667/0033-7587(2002)158[0159:troois]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Reimann JD, Freed E, Hsu JY, Kramer ER, Peters JM, Jackson PK. Emi1 is a mitotic regulator that interacts with Cdc20 and inhibits the anaphase promoting complex. Cell. 2001;105:645–655. doi: 10.1016/s0092-8674(01)00361-0. [DOI] [PubMed] [Google Scholar]
- Schmaltz C, Hardenbergh PH, Wells A, Fisher DE. Regulation of proliferation-survival decisions during tumor cell hypoxia. Mol Cell Biol. 1998;18:2845–2854. doi: 10.1128/mcb.18.5.2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivaprasad U, Machida YJ, Dutta A. APC/C--the master controller of origin licensing? Cell Div. 2007;2:8. doi: 10.1186/1747-1028-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotillo E, Garriga J, Padgaonkar A, Kurimchak A, Cook JG, Grana X. Coordinated activation of the origin licensing factor CDC6 and CDK2 in resting human fibroblasts expressing SV40 small T antigen and cyclin E. J Biol Chem. 2009;284:14126–14135. doi: 10.1074/jbc.M900687200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoeber K, Mills AD, Kubota Y, Krude T, Romanowski P, Marheineke K, et al. Cdc6 protein causes premature entry into S phase in a mammalian cell-free system. EMBO J. 1998;17:7219–7229. doi: 10.1093/emboj/17.24.7219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassin VM, Wold MS, Borowiec JA. Replication protein A (RPA) phosphorylation prevents RPA association with replication centers. Mol Cell Biol. 2004;24:1930–1943. doi: 10.1128/MCB.24.5.1930-1943.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaupel P, Schlenger K, Knoop C, Hockel M. Oxygenation of human tumors: evaluation of tissue oxygen distribution in breast cancers by computerized O2 tension measurements. Cancer Res. 1991;51:3316–3322. [PubMed] [Google Scholar]
- Vigo E, Muller H, Prosperini E, Hateboer G, Cartwright P, Moroni MC, et al. CDC25A phosphatase is a target of E2F and is required for efficient E2F-induced S phase. Mol Cell Biol. 1999;19:6379–6395. doi: 10.1128/mcb.19.9.6379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou L, Elledge SJ. Sensing DNA damage through ATRIP recognition of RPA-ssDNA complexes. Science. 2003;300:1542–1548. doi: 10.1126/science.1083430. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.