Abstract
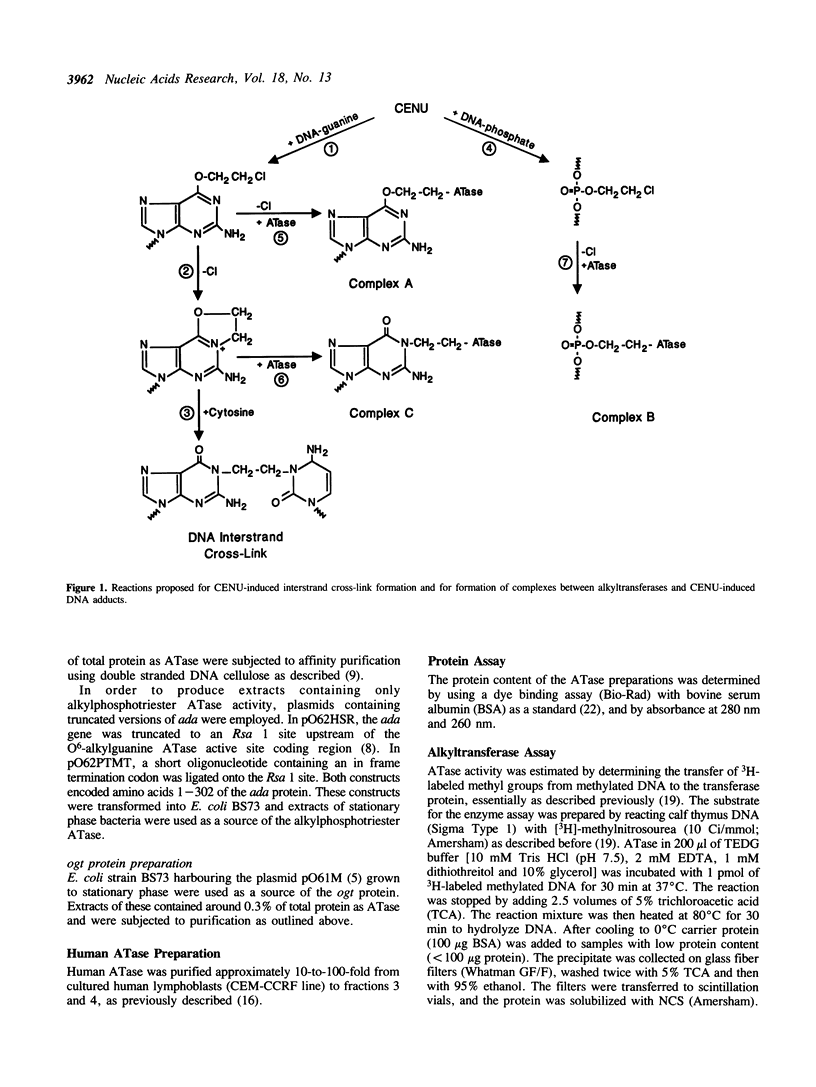
Chloroethylnitrosoureas (CENUs) are thought to induce cytotoxic DNA interstrand cross-links via an initial reaction at O6-position of guanine, yielding a rearranged intermediate, O6,N1-ethanoguanine. Repair of these adducts by mammalian and bacterial DNA alkyltransferases blocks the formation of cross-links. Human alkyltransferase can form a covalent complex with DNA containing BCNU-induced cross-link precursors, but the nature of the DNA-protein linkage remains unknown. Using E. coli alkyltransferases expressed by the ada and ogt genes, we now demonstrate that both enzymes can form such complexes with CENU-treated DNA. We attribute this reaction to the O6-alkylguanine repair function, because an N-terminal fragment of the ada protein, which has only alkylphosphotriester repair activity, failed to form a similar complex. This result is consistent with the idea that complex formation requires an alkyltransferase reaction with a guanine adduct, such as O6,N1-ethanoguanine. It tends to exclude the possibility that such reactions simply involve alkylation of the enzyme by reactive DNA adducts such as chloroethylphosphate or chloroethylguanine.
Full text
PDF
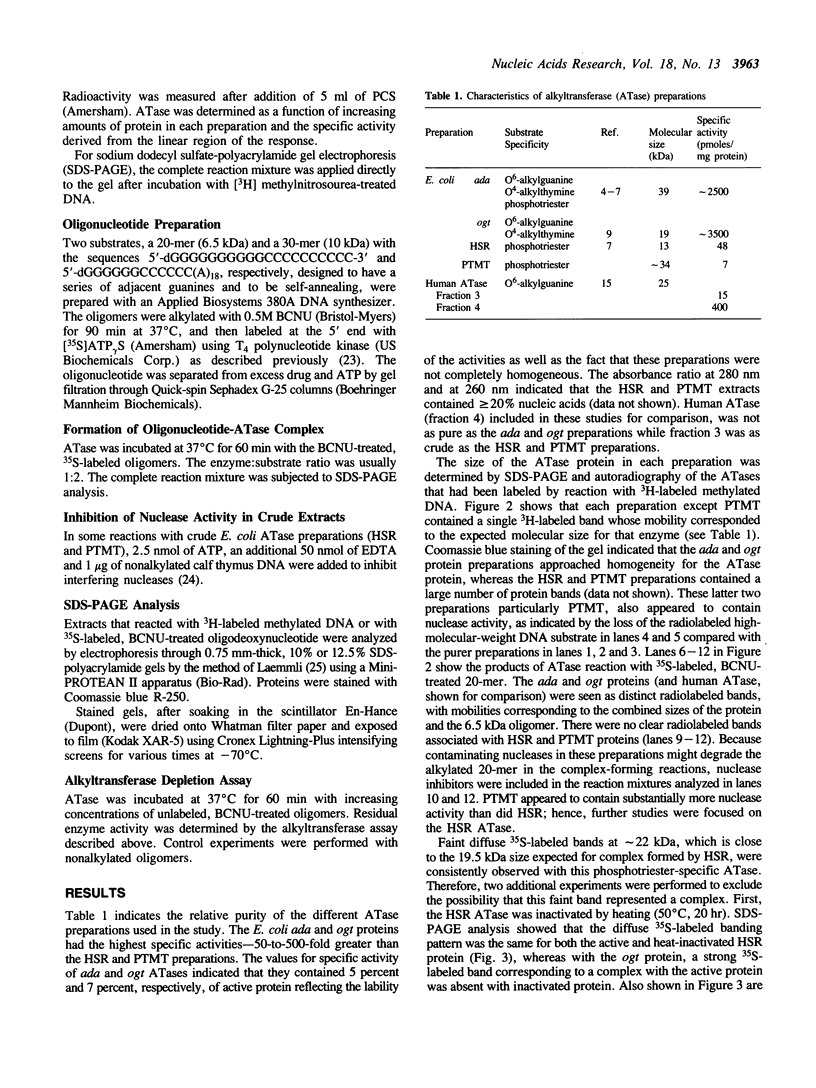
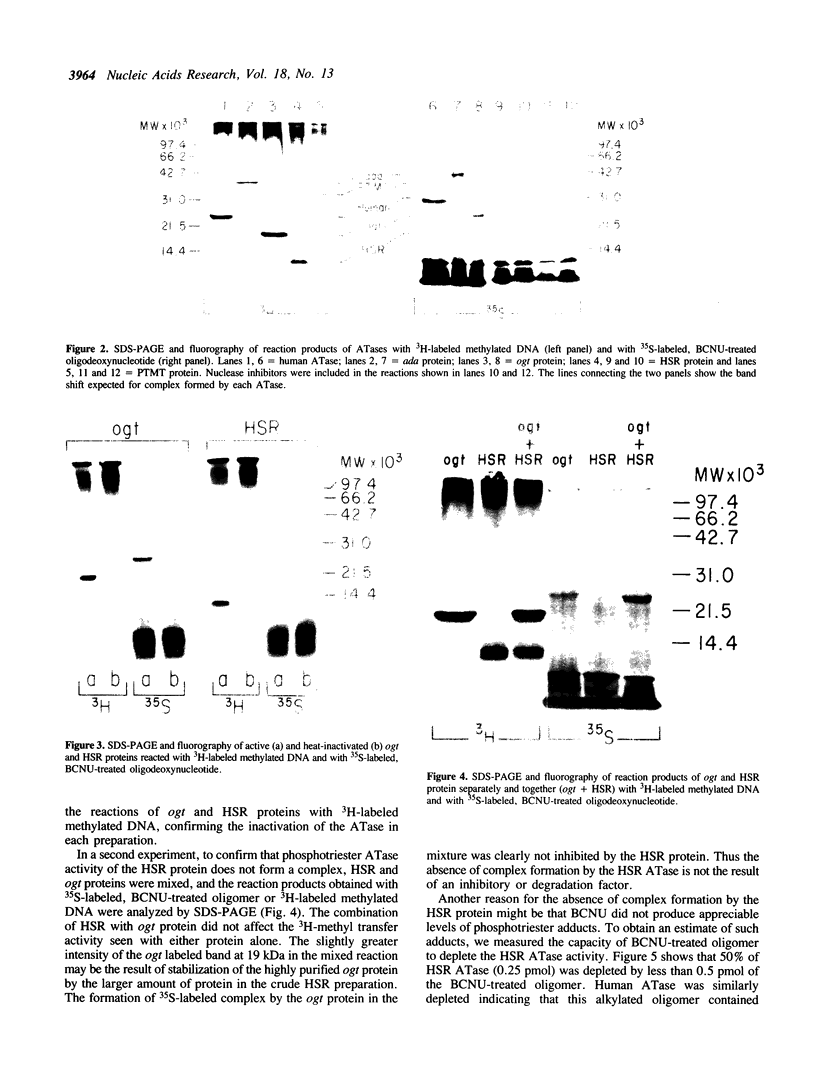
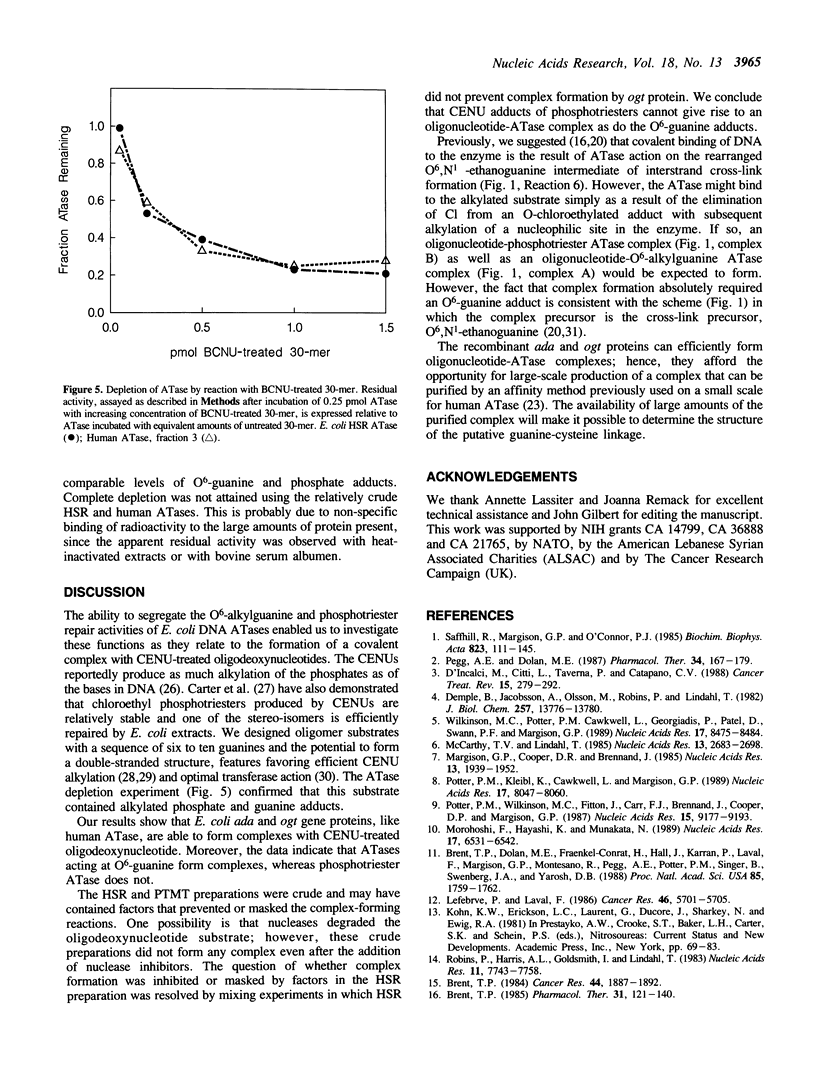

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bodell W. J., Tokuda K., Ludlum D. B. Differences in DNA alkylation products formed in sensitive and resistant human glioma cells treated with N-(2-chloroethyl)-N-nitrosourea. Cancer Res. 1988 Aug 15;48(16):4489–4492. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brent T. P., Dolan M. E., Fraenkel-Conrat H., Hall J., Karran P., Laval L., Margison G. P., Montesano R., Pegg A. E., Potter P. M. Repair of O-alkylpyrimidines in mammalian cells: a present consensus. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1759–1762. doi: 10.1073/pnas.85.6.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brent T. P. Isolation and purification of O6-alkylguanine-DNA alkyltransferase from human leukemic cells. Prevention of chloroethylnitrosourea-induced cross-links by purified enzyme. Pharmacol Ther. 1985;31(1-2):121–140. doi: 10.1016/0163-7258(85)90040-3. [DOI] [PubMed] [Google Scholar]
- Brent T. P., Remack J. S. Formation of covalent complexes between human O6-alkylguanine-DNA alkyltransferase and BCNU-treated defined length synthetic oligodeoxynucleotides. Nucleic Acids Res. 1988 Jul 25;16(14B):6779–6788. doi: 10.1093/nar/16.14.6779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brent T. P., Remack J. S., Smith D. G. Characterization of a novel reaction by human O6-alkylguanine-DNA alkyltransferase with 1,3-bis(2-chloroethyl)-1-nitrosourea-treated DNA. Cancer Res. 1987 Dec 1;47(23):6185–6188. [PubMed] [Google Scholar]
- Brent T. P., Smith D. G., Remack J. S. Evidence that O6-alkylguanine-DNA alkyltransferase becomes covalently bound to DNA containing 1,3-bis(2-chloroethyl)-1-nitrosourea-induced precursors of interstrand cross-links. Biochem Biophys Res Commun. 1987 Jan 30;142(2):341–347. doi: 10.1016/0006-291x(87)90279-8. [DOI] [PubMed] [Google Scholar]
- Brent T. P. Suppression of cross-link formation in chloroethylnitrosourea-treated DNA by an activity in extracts of human leukemic lymphoblasts. Cancer Res. 1984 May;44(5):1887–1892. [PubMed] [Google Scholar]
- Briscoe W. T., Duarte S. P. Preferential alkylation by 1,3-bis(2-chloroethyl)-1-nitrosourea (BCNU) of guanines with guanines as neighboring bases in DNA. Biochem Pharmacol. 1988 Mar 15;37(6):1061–1066. doi: 10.1016/0006-2952(88)90511-4. [DOI] [PubMed] [Google Scholar]
- Carter C. A., Kirk M. C., Ludlum D. B. Phosphotriester formation by the haloethylnitrosoureas and repair of these lesions by E. coli BS21 extracts. Nucleic Acids Res. 1988 Jun 24;16(12):5661–5672. doi: 10.1093/nar/16.12.5661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Incalci M., Citti L., Taverna P., Catapano C. V. Importance of the DNA repair enzyme O6-alkyl guanine alkyltransferase (AT) in cancer chemotherapy. Cancer Treat Rev. 1988 Dec;15(4):279–292. doi: 10.1016/0305-7372(88)90026-6. [DOI] [PubMed] [Google Scholar]
- Demple B., Jacobsson A., Olsson M., Robins P., Lindahl T. Repair of alkylated DNA in Escherichia coli. Physical properties of O6-methylguanine-DNA methyltransferase. J Biol Chem. 1982 Nov 25;257(22):13776–13780. [PubMed] [Google Scholar]
- Gonzaga P. E., Brent T. P. Affinity purification and characterization of human O6-alkylguanine-DNA alkyltransferase complexed with BCNU-treated, synthetic oligonucleotide. Nucleic Acids Res. 1989 Aug 25;17(16):6581–6590. doi: 10.1093/nar/17.16.6581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley J. A., Gibson N. W., Kohn K. W., Mattes W. B. DNA sequence selectivity of guanine-N7 alkylation by three antitumor chloroethylating agents. Cancer Res. 1986 Apr;46(4 Pt 2):1943–1947. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lefebvre P., Laval F. Enhancement of O6-methylguanine-DNA-methyltransferase activity induced by various treatments in mammalian cells. Cancer Res. 1986 Nov;46(11):5701–5705. [PubMed] [Google Scholar]
- Ludlum D. B., Mehta J. R., Tong W. P. Prevention of 1-(3-deoxycytidyl),2-(1-deoxyguanosinyl)ethane cross-link formation in DNA by rat liver O6-alkylguanine-DNA alkyltransferase. Cancer Res. 1986 Jul;46(7):3353–3357. [PubMed] [Google Scholar]
- Margison G. P., Cooper D. P., Brennand J. Cloning of the E. coli O6-methylguanine and methylphosphotriester methyltransferase gene using a functional DNA repair assay. Nucleic Acids Res. 1985 Mar 25;13(6):1939–1952. doi: 10.1093/nar/13.6.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy T. V., Lindahl T. Methyl phosphotriesters in alkylated DNA are repaired by the Ada regulatory protein of E. coli. Nucleic Acids Res. 1985 Apr 25;13(8):2683–2698. doi: 10.1093/nar/13.8.2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morohoshi F., Hayashi K., Munakata N. Bacillus subtilis gene coding for constitutive O6-methylguanine-DNA alkyltransferase. Nucleic Acids Res. 1989 Aug 25;17(16):6531–6543. doi: 10.1093/nar/17.16.6531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegg A. E., Dolan M. E. Properties and assay of mammalian O6-alkylguanine-DNA alkyltransferase. Pharmacol Ther. 1987;34(2):167–179. doi: 10.1016/0163-7258(87)90010-6. [DOI] [PubMed] [Google Scholar]
- Pegg A. E., Scicchitano D., Morimoto K., Dolan M. E. Specificity of O6-alkylguanine-DNA alkyltransferase. IARC Sci Publ. 1987;(84):30–34. [PubMed] [Google Scholar]
- Potter P. M., Kleibl K., Cawkwell L., Margison G. P. Expression of the ogt gene in wild-type and ada mutants of E. coli. Nucleic Acids Res. 1989 Oct 25;17(20):8047–8060. doi: 10.1093/nar/17.20.8047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter P. M., Wilkinson M. C., Fitton J., Carr F. J., Brennand J., Cooper D. P., Margison G. P. Characterisation and nucleotide sequence of ogt, the O6-alkylguanine-DNA-alkyltransferase gene of E. coli. Nucleic Acids Res. 1987 Nov 25;15(22):9177–9193. doi: 10.1093/nar/15.22.9177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins P., Harris A. L., Goldsmith I., Lindahl T. Cross-linking of DNA induced by chloroethylnitrosourea is presented by O6-methylguanine-DNA methyltransferase. Nucleic Acids Res. 1983 Nov 25;11(22):7743–7758. doi: 10.1093/nar/11.22.7743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg N. L. ATP as an alternative inhibitor of bacterial and endogenous nucleases and its effect on native chromatin compaction. Mol Cell Biochem. 1987 Aug;76(2):113–121. doi: 10.1007/BF00223476. [DOI] [PubMed] [Google Scholar]
- Saffhill R., Margison G. P., O'Connor P. J. Mechanisms of carcinogenesis induced by alkylating agents. Biochim Biophys Acta. 1985 Dec 17;823(2):111–145. doi: 10.1016/0304-419x(85)90009-5. [DOI] [PubMed] [Google Scholar]
- Tong W. P., Kirk M. C., Ludlum D. B. Mechanism of action of the nitrosoureas--V. Formation of O6-(2-fluoroethyl)guanine and its probable role in the crosslinking of deoxyribonucleic acid. Biochem Pharmacol. 1983 Jul 1;32(13):2011–2015. doi: 10.1016/0006-2952(83)90420-3. [DOI] [PubMed] [Google Scholar]
- Wilkinson M. C., Potter P. M., Cawkwell L., Georgiadis P., Patel D., Swann P. F., Margison G. P. Purification of the E. coli ogt gene product to homogeneity and its rate of action on O6-methylguanine, O6-ethylguanine and O4-methylthymine in dodecadeoxyribonucleotides. Nucleic Acids Res. 1989 Nov 11;17(21):8475–8484. doi: 10.1093/nar/17.21.8475. [DOI] [PMC free article] [PubMed] [Google Scholar]