Abstract
This review emphasizes the importance of glycobiology in nature and aims to highlight, simplify and summarize the multiple functions and structural complexities of the different oligosaccharide combinatorial domains that are found in chondroitin sulphate/dermatan sulphate (CS/DS) glycosaminoglycan (GAG) chains. For example, there are 1008 different pentasaccharide sequences possible within CS, DS or CS/DS hybrid GAG chains. These combinatorial possibilities provide numerous potential ligand-binding domains that are important for cell and extracellular matrix interactions as well as specific associations with cytokines, chemokines, morphogens and growth factors that regulate cellular differentiation and proliferation during tissue development, for example, morphogen gradient establishment. The review provides some details of the large and diverse number of different enzymes that are involved in CS/DS biosynthesis and attempts to explain how differences in their expression patterns in different cell types can lead to subtle but important differences in the GAG metabolism that influence cellular proliferation and differentiation in development as well as regeneration and repair in disease. Our laboratory was the first to generate and characterize monoclonal antibodies (mAb) that very specifically recognize different ‘native’ sulphation motif/epitopes in CS/DS GAG chains. These monoclonal antibodies have been used to identify very specific spatio-temporal expression patterns of CS/DS sulphation motifs that occur during tissue and organ development (in particular their association with stem/progenitor cell niches) and also their recapitulated expression in adult tissues with the onset of degenerative joint diseases. In summary, diversity in CS/DS sulphation motif expression is a very important necessity for animal life as we know it.
Keywords: chondroitin/dermatan sulphate motifs, development, differential expression
The importance of glycobiology in nature
Glycobiology (defined here as specific sugar modifications and additions to biological molecules) is a fundamental and an essential component of all forms of life from viruses and single-cell organisms through to mammals and man. In higher order mammals, >50% of all proteins contain some ‘glyco-motif’ (Cummings 2009), and the addition of these sugar modifications to proteins and lipids and modifications thereafter (e.g. sulphation and phosphorylation) require the production and use of considerable amounts of metabolic energy (e.g the. use of ATP, GTP, UTP and PAPS) that further emphasizes the importance of ‘glyco’-modifications in normal cellular metabolism. There are nine different monosaccharides synthesized in mammals (Cummings 2009), and these can potentially produce 1012 different hexasaccharide combinations (i.e. six sugar units) through variations in their monosaccharide composition (e.g. galactose vs. N-acetylgalactosamine content), anomeric state (e.g. αvs.β linkages between sugars), glycosidic linkages (e.g. 1-3 or 1-4 hexose carbon linkages), branching (e.g. N- & O-linked oligosaccharides on glycoproteins), substituted components (e.g. sulphation and phosphorylation) and their ‘aglycone linkages’ to other proteins/peptides, lipids or nucleic acids (see Cummings 2009 for review). Thus, glycobiology is essential for life as we know it.
Glycosaminoglycans
Glycosaminoglycans (GAGs) are long-chain polysaccharides consisting of repeating disaccharide units that contain a hexosamine (i.e. glucosamine or galactosamine) and either a uronic acid (glucuronic acid or iduronic acid) or galactose (Figure 1 and Table 1). The hexosamine component of the repeating disaccharide is most often N-acetylated and both sugar components of the disaccharide can be sulphated. There are four different subgroups of GAG in mammals; they are (i) hyaluronic acid or hyaluronan (HA – the only non-sulphated GAG), (ii) keratan sulphate (KS), (iii) chondroitin/dermatan sulphate (CS/DS) and (iv) heparin/heparan sulphate (Hep/HS). The different monosaccharide components, their different linkages between each other and the different stereo-chemical positioning of sulphate and carboxyl groups on oligosaccharides from these different GAG subgroups (i.e. HA, KS, CS, DS and Hep/HS) are illustrated in the rather simplistic 2D diagrams shown in Figure 1. These GAG structural diagrams illustrate some of the potential combinatorial differences in the glycan oligosaccharides that are possible within each of these GAG subgroups (Table 1). The carboxyl groups of the two hexuronic acid isomers (i.e. glucuronic and iduronic acid) and the presence of sulphation on either or both of the disaccharide sugars provide a very strong negative charge to these GAG chains that have a high affinity for water and also bind to many cationic molecules found in cells and tissue matrices. The stereo-chemical/spacial orientation of these negatively charged groups (i.e. sulphate and carboxyl groups) within small-sized oligosaccharide components of these different GAG chains (usually 5–10 monosaccharide units) provides very specific and different stereo-chemical binding sites for numerous other cellular and matrix macromolecules including chemokines, growth factors and morphogens (Benjamin & Rapraeger 2003; Cortes et al. 2009; Heinegard 2009; Yan & Lin 2009; Kramer 2010; Rozario & DeSimone 2010; Purushothaman et al. 2011) that are very important for the regulation of tissue development and metabolism in health and disease. It is thought that these different combinations of GAG sulphation motifs can overlap with one another, termed ‘Wobble Motifs’ (Purushothaman et al. 2011), as well as having different mimetic/mimetope electron cloud presentations (Pothacharoen et al. 2007), when interacting with different morphogens, growth factors and chemokines during tissue and organ development. Consequently, there are a variable but in some cases a very large number of GAG combinatorial variants possible. For example (Cummings 2009), in just a small pentasaccharide unit for each of these four different GAG subgroups, there are only two pentasaccharide variants for HA, the only non-sulphated GAG, whereas the sulphated CS/DS GAGs provide 1008 variants and the most complex GAG subgroup Hep/HS providing 2916 different possible combinations (see Table 1). Thus, in total, there are almost 4000 different pentasaccharide combinations in these 4 GAG subgroups that can provide different overlapping Wobble and mimetope motifs to bind a wide array of different cellular and matrix regulatory molecules. This review will focus on studies illustrating the role(s) that chondroitin sulphate (CS) and dermatan sulphate (DS) GAG sulphation motifs play in tissue and organ development and also their recapitulated expression in the attempted repair and regeneration of tissues with the onset of musculoskeletal diseases. Related biological functions of Hep/HS GAGs that can act as potential mimetics of CS/DS structures are described in recent review articles (Merry & Astrautsora 2008; Turnbull 2010).
Figure 1.
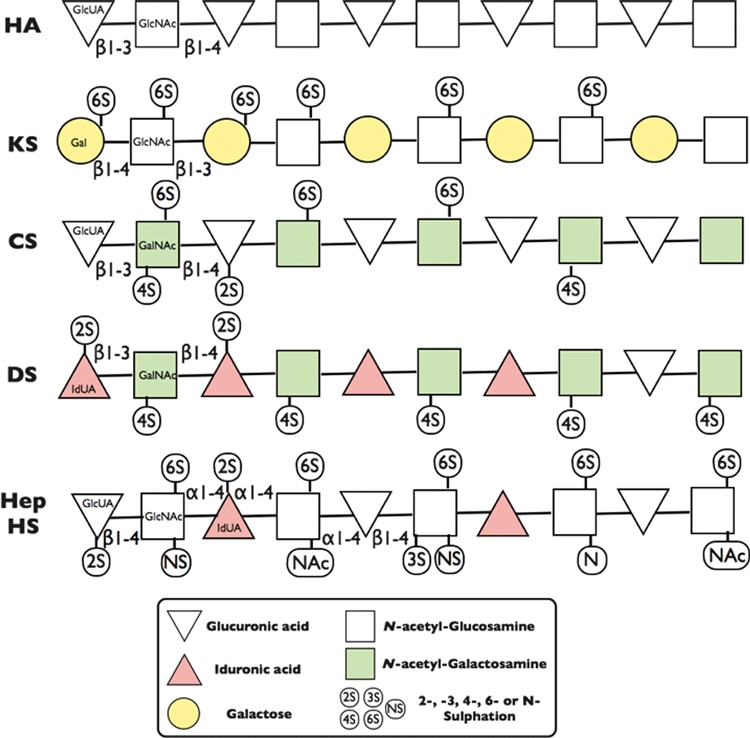
Structural Diversity of Glycosaminoglycan repeating disaccharide units. The repeating disaccharide units of Hyaluronan (HA), Keratan Sulphate (KS), Chondroitin Sulphate (CS), Dermatan Sulphate (DS), Heparin (Hep) and Heparan Sulphate (HS) consist of a hexosamine [either Glucosamine (GlcN) or Galactosamine (GalN) - Squares] and a uronic acid [either Glucuronic (GlcUA) or Iduronic acid (IdUA) - Triangles] or Galactose (Gal) –Circles. These sugar and disaccharide units are joined to one another by different anomeric glycosidic linkages (α1-4, β1-3 or β1-4) between the sugar hydroxyl groups. The hexosamine, uronic acid and galactose sugars in each disaccharide unit can also be substituted with sulphate groups on the sugar hydroxyls in the 2- (2S), 3- (3S), 4- (4S) and 6- (6S) positions. The hexosamine is usually N-acetlyated (NAc), but in Hep/HS, it can also be N-sulphated (NS) or occur as the free amine (N). HA is the only non-sulphated GAG and it is also not found covalently attached to a proteoglycan core protein.
Table 1.
Disaccharide repeat composition and potential pentasaccharide glycan combinations for Hyaluronan (HA), Keratan Sulphate (KS), Chondroitin/Dermatan Sulphate (CS/DS) and Heparin/Heparan Sulphate (Hep/HS) glycosaminoglycans (GAG)
| GAG | Disaccharide repeats | Pentasaccharide glycan combinations |
|---|---|---|
| HA | Glucuronic acid and N-acetyl-glucosamine | 2 |
| KS | Galactose and N-acetyl-glucosamine | 64 |
| CS/DS | Glucuronic or iduronic acid and N-acetylgalactosamine | 1008* |
| Hep/HS | Glucuronic and/or Iduronic acid and N-acetyl-glucosamine | 2916 |
| Total | 3990 |
Data obtained from Cummings (2009).
Errata from the reference above where the CS/DS combinations were reported as 3 × 4 × 3 × 4 × 3 = 423 instead of ‘=432’; this error adding nine more to their total of 999.
CS/DS GAG biosynthesis and their related Genes
CS/DS and Hep/HS biosynthesis is initiated by the transfer of xylose (Xyl) residues, using one of the two different xylosyl-transferases, to the hydroxyl groups of specific serine residues on the N-terminal side of a glycine residue in the core proteins of matrix, cell surface and intracellular proteoglycans (Sugahara & Mikami 2007; Watanabe & Kimata 2008; Heinegard 2009; Yan & Lin 2009). After the addition of xylose to the serine residue in the core protein, three additional sugar residues [i.e. galactose (Gal), Gal and glucuronic acid (GlcA), respectively] are attached to the xylose to form the ‘linkage region’ that is the initiation template for both CS/DS and Hep/HS GAG biosynthesis. The transfer of each of these sugar residues to the next sugar residue to form the tetrasaccharide linkage region for either CS/DS and Hep/HS GAG chains is in each case performed by separate glycosyl-transferases to produce GlcA-β1,3-Galβ-1,3-Galβ1,4-Xyl-(Serine) as the acceptor for further GAG chain elongation. This linkage region can be further modified by phosphorylation of the xylose residue on the 2-hydroxyl and sulphation of the galactose on the 4-hydroxyl positions of these additions modifying the specificity of later glucuronosyl-transferase-1 activity (Tone et al. 2008). After the linkage region synthesis has been completed, specific CS and DS GAG chain elongation occurs by the alternate addition of N-acetylgalactosamine (GalNAc) and GlcA residues by several different glycosyl-transferases to form the characteristic disaccharide repeat units of CS and DS GAG chains (Sugahara & Mikami 2007; Watanabe & Kimata 2008). The CS disaccharides are then further modified through the addition of sulphate residues to the 2-hydroxyl group of the GlcA residue and the 4- and/or 6-hydroxyl groups on the GalNAc residues using a variety of different sulphotransferase (see Kluppel 2010 for Review). These CS sulphotransferases have two forms of nomenclature in the current literature; for example, one of the enzymes that specifically transfer a sulphate residue to the 4-hydroxl group of N-acetylgalactosamine in CS GAG chains is either called Chondroitin-4-sulphotransferase-1 that is denoted with the acronym as ‘C4ST1’ or Carbohydrate sulphotransferase 11 that is denoted with the acronym ‘CHST11’. Accordingly, in animals, there are four sulphotransferases that can transfer a sulphate group to the 4-hydroxyl of N-acetylgalactosamine in CS/DS GAG biosynthesis; they are C4ST1 (CHST11), C4ST2 (CHST12), C4ST3 (CHST13) and D4ST1 (CHST14). Both C4ST1 and C4ST3 prefer chondroitin (glucuronic acid containing) disaccharides as their substrate to produce chondroitin-4-sulphate (also called CS-A) with C4ST2 and D4ST1 preferring dermatan (iduronic acid containing) disaccharides as their substrates to form dermatan sulphate (also called CS-B). There are also two other sulphotransferases that add sulphate groups to the 6-hydroxyl group of the N-acetylgalactosamine group in CS disaccharides that are denoted by C6ST1(CHST3) and C6ST2(CHST7) Furthermore, there are two additional sulphotransferases involved in CS/DS biosynthesis; one of these being GalNAc4-6ST(CHST15) that specifically catalyses 6-O-sulphation of the already 4-sulphated GalNAc(4-SO4) sugars to produce disulphated disaccharides containing GalNAc(4,6-SO4) that is also called CS-E. The other sulphotransferase is Uronyl-2-O-sulphotransferase (UST) that specifically transfers a sulphate group to the 2-hydroxyl of either glucuronic acid or iduronic acid residues in CS/DS GAG chains (Ishii & Maeda 2008). In CS biosynthesis, this 2-O-sulphation of glucuronic acid residues by CS2ST in disaccharides containing GalNAc(6-SO4) thus produces a disaccharide glucuronosyl-2-sulphate/N-acetylgalactosamine-6-sulphate (GlcA2S-GalNAc6S) that is also called CS-D.
The specific and more complex formation of DS disaccharides with large DS/iduronic acid (IdUA) containing segments (termed ‘IdUA blocks’), or CS/DS mixed GAG hybrid segments, occurs through the action of very specific DS epimerases (i.e. DSepi-1 & DSepi-2) that convert the GlcA to an IdUA residue before specific DS 4-O-sulphotransferases add a sulphate group to the hydroxyl group in the 4-position of the GalNAc residue (Maccarana et al. 2006; Pacheco et al. 2009a). Dermatan 4-O-sulfotransferase-1 (D4ST1) is also a pivotal enzyme in the formation of these ‘IdUA blocks’ in dermatan sulphate (Pacheco et al. 2009b). Several recent studies (Maccarana et al. 2009; Pacheco et al. 2009a,b,c) have shown evidence for differences in the specificity and activity of these DS epimerases and sulpho-transferases, in that, the down-regulation of one of the two epimerases (i.e. DSepi1) can cause significant reduction in the synthesis of the iduronic acid blocks in the DS GAG chains with an additional depletion of 2-sulphation in the hexuronic acid components of the CS/DS disaccharides. Interestingly, DSepi1 also requires N-glycosylation to activate its function (Pacheco et al. 2009c). Thus, differential regulation of several different epimerases and sulphotransferases markedly affects the disaccharide structure of CS, DS and CS/DS hybrid GAG chains.
In summary, in animal species, several duplications of potential genes occur that code for different glycosyl-transferases, epimerases and sulphotransferases that are used to synthesize different sulphation motif oligosaccharide domains found in CS, DS and CS/DS GAGs in cellular metabolism; that is, there are two xylosyl-transferases (XYLT1 and XYLT2), two galactose transferases (GALT1 and GALT2), two glucuronic acid transferase (GLCAT1 and GLCAT2), two GalNAc transferases (CS-GalNAcT1 and CSGalNAcT2), two epimerases (DSepi12 and DSepi2) and numerous different sulphotransferases that put sulphate residues on the 2-hydroxyl groups of the glucuronic acid and iduronic acid moiteties as well as the 4- and 6-hydroxyl groups of the N-acetly-galactosamine moieties of the CS or DS repeating disaccharide units (Sugahara & Mikami 2007; Watanabe & Kimata 2008; Kluppel 2010). Collectively, combinations of these enzymes are involved in the biosynthesis of the different subtypes of CS/DS GAG chains that are covalently attached to different proteoglycan core proteins which, yet again, are made by different cells and tissues in the body. The inherent diversity coming from the presence of these multiple genes and enzymes involved in CS/DS GAG biosynthesis that apparently produces similar but subtly different catalytic reaction products must have provided biological advantages for their selection and preservation during the evolution of our cellular metabolism in animals (Yamada et al. 2011). However, the reasons for these apparent redundancies and duplications are yet to be ascertained. Some examples of their differential expression patterns in CS/DS GAGs in tissue and organ development are provided later in this review.
Monoclonal antibodies recognizing native sulphation motif epitopes and glycosidase-generated neoepitopes in CS/DS GAGs
Our laboratory was the first to produce and characterize monoclonal antibodies (mAbs) that recognize different oligosaccharide sulphation motifs present in GAGs found on a variety of different cell and matrix proteoglycans (Caterson et al. 1983; Couchman et al. 1984). For the past 30+ years, these mAbs have underpinned research that has helped to establish the biological importance of the subtleties in the structure, function and metabolism of these GAGs during tissue development, maturation, ageing and with the onset of disease. In the late 1980s, we produced, developed and partially characterized five different monoclonal antibodies [3B3(−), 3C5, 4C3, 6C3 and 7D4] that recognized native sulphation motifs in CS/DS GAG chains. The localization and biochemical structure (Caterson et al. 1990; Sorrell et al. 1996) of some of these native CS/DS sulphation motif epitopes within oligosaccharide segments of CS/DS GAG chains has been performed by ours and other laboratories (Figure 2). Several other antibodies (i.e. CS56, MO-225, 373HD, 2H6, 846 and WF6) that recognize native CS/DS sulphation motifs have been produced by other laboratories and, with some of these, the detailed biochemical structure of the different oligosaccharides comprising their epitopes determined (Rizkalla et al. 1992; Sorrell et al. 1996; Pothacharoen et al. 2007; Purushothaman et al. 2011). Interestingly, one of these studies (Pothacharoen et al. 2007) identified mimetic/mimetope oligosaccharide alternatives for the monoclonal antibody recognition sites on CS/DS GAGs. This finding providing evidence that these CS/DS sulphation motifs can have multiple potential binding partners similar to the suggested concept of overlapping ‘Wobble Motifs’ in CS/DS GAGs that was recently reported (Purushothaman et al. 2011).
Figure 2.
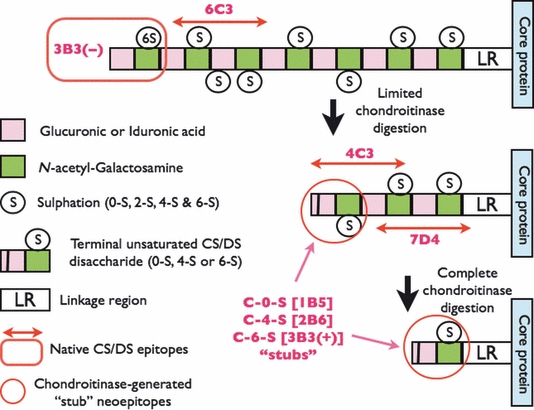
Structure and location of native epitopes [for mAbs 3-B-3(−), 4-C-3, 6-C-3 and 7-D-4] and chondroitinase-generated ‘stub’ neoepitopes [for mAbs 1-B-5, 2-B-6 and 3-B-3(+)] on chondroitin sulphate (CS) and dermatan sulphate (DS) glycosaminoglycan chains. Boxed in Red is the location of mAb 3-B-3(−) native epitope that recognizes a non-reducing terminal end saturated CS disaccharide consisting of glucuronic acid that is adjacent to N-acetylgalactosamine-6-sulphate. The native epitope for mAb 6-C-3 (Red linear arrows) is located in CS/DS oligosaccharide sequences that are located on the outer peripheral regions of the CS/DS GAG chains whilst mAbs 4-C-3 and 7-D-4 are located in oligosaccharide sequences located in the inner regions of the CS/DS GAG chains nearer to the linkage region (LR) that is the covalent attachment site that links CS/DS GAGs to the proteoglycan core protein. The chondroitinase-generated neoepitope ‘CS stubs’ are circled in Red and occur as an unsaturated uronic acid residue that is adjacent to N-acetylgalactosamine that is either non-sulphated (C-O-S; recognized by mAb 1-B-5), 4-sulphated (C-4-S; recognized by mAb 2-B-6) or 6-sulphated [C-6-S; recognized by mAb 3-B-3(+)]. CS/DS GAG chains are depicted as a sequence of disaccharides with two different coloured boxes that have either been partially (limited) or completely digested (deglycosylated) with chondroitinase. Chodroitinases are enzymes that remove CS/DS disaccharides with non-reducing unsaturated uronic acid residues from the CS/DS GAG chains eventually leaving a characteristic terminal disaccharide ‘stub’, that is, either unsulphated, 4- or 6-sulphated, and covalently attached to the proteoglycan core protein.
In studies performed in our laboratory over the past several years, we have used mAbs 3B3(−), 3C5, 4C3, 6C3 and 7D4 (Figure 2) to identify very specific patterns of CS/DS GAG sulphation motif expression in bone marrow, lymphopoietic and musculoskeletal tissues (Oguri et al. 1987a,b; Sorrell et al. 1988a,b,c; Sorrell & Caterson 1989). Furthermore, in the 1990s, when interests in stem cell-related therapies were emerging, we published several papers describing the very specific spatio-temporal expression patterns of CS/DS GAG sulphation motifs in developing skin (Caterson et al. 1990; Sorrell & Caterson 1990; Sorrell et al. 1990a,b), during normal skeletal tissue development and homoeostasis (Caterson et al. 1990) and with the onset of disease in musculoskeletal tissues in animal models and human patients (Visco et al. 1993; Roberts et al. 1994, 2001; Carlson et al. 1995; Slater et al. 1995; Lin et al. 1998; Richardson et al. 1999; Johnson et al. 2002, 2004). More recently, we have demonstrated that our native CS/DS sulphation motif mAbs can identify the location of stem/progenitor cell niches in a variety of tissues from many animal species (chickens-humans) and more specifically in the superficial zone of articular cartilage where chondrogenic progenitor cells reside (Hayes et al. 2007, 2008; Davies et al. 2008a,b). This review presents some of our findings illustrating the very specific spatio-temporal expression of CS/DS sulphation motif epitopes during tissue development and with attempted repair and regeneration of diseased tissue.
Spatio-temporal expression of CS/DS sulphation motifs during tissue development
In several studies (Sorrell et al. 1988a,b,c, 1990a; Sorrell & Caterson 1989; Caterson et al. 1990; Johnson et al. 2004), we have now shown that native CS/DS sulphation motif epitopes are relatively sparsely expressed (compared to the more generic CS and DS isomer sulphation motifs) in a variety of musculoskeletal tissues. These native CS/DS sulphation epitopes appear to be highly conserved throughout a wide variety of animal species (shark-human). In a very recent publication (Hayes et al. 2011), we also found that several of these CS/DS motifs recognized by mAbs 3B3(−), 4C3, 6C3 and 7D4 displayed very specific and dynamic spatio-temporal expression patterns during embryonic rat intervertebral disc tissue development. Similar examples of the specific expression of these CS/DS sulphation motifs in embryonic chick tissues and foetal human knee joints are shown in Figures 3 and 4, respectively. The expression of these native CS/DS sulphation motif epitopes subtlety differs from one mAb to another and also very specifically identifies and defines the potential presence of newly developing tissues prior to these tissues reaching their fully developed functional phenotype [e.g. Note: in Figures 3 and 4, the staining pattern differences between the ‘generic’ Alcian Blue (Figure 3) or Toluidine Blue (Figure 4) staining for GAGs (mainly CS) compared to the very specific tissue staining and location of the native CS/DS epitopes recognized by mAbs 3B3(−), 4C3, 6C3 and 7D4]. It is of interest to note that these very specific immunohistochemical staining patterns for CS/DS sulphation motifs that are seen in the developing rat intervertebral disc tissues (Hayes et al. 2011) very often show a similar immunostaining patterns to that of growth factor and morphogen gradient patterns that are also observed during rat intervertebral disc tissue development (Hayes & Ralphs 2011). In our ongoing research, we are performing immunohistochemical co-localization and binding-affinity studies to test the hypothesis that the specific expression of these CS/DS sulphation motifs on GAGs in cell surface and matrix proteoglycans are involved in the binding of different growth factors and morphogens during the formation of their matrix-associated gradients in tissue and organ development.
Figure 3.
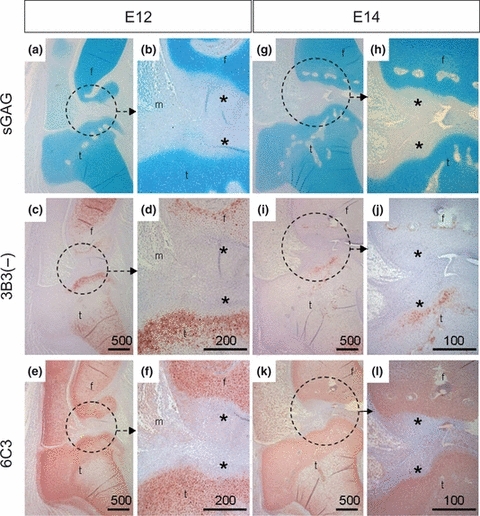
Specific spatial and temporal expression of native CS/DS sulphation motif epitopes recognized by mAbs 3B3(−) and 6C3 in developing embryonic chick tissues at stages E12 and E14. Joint tissues from stage E12 [A–F] and E14 [G–L] embryonic chicks stained with Alcian Blue [A,B,G,H] and immunostaining with mAbs 3B3(−) [C,D,I,J] and 6C3 [E,F,K,L]. Circles indicate regions shown at higher magnifications in adjacent frames. The asterisks (*) highlight unstained fibrocartilagenous articular cartilage; that is, at E12 mAb 6C3 is broadly labelling the epiphyses and not the overlaying fibrous articular cartilage, whereas 3B3(−) immunostaining is different and identifies a specific subset of cells and matrix in the developing articular cartilage.
Figure 4.
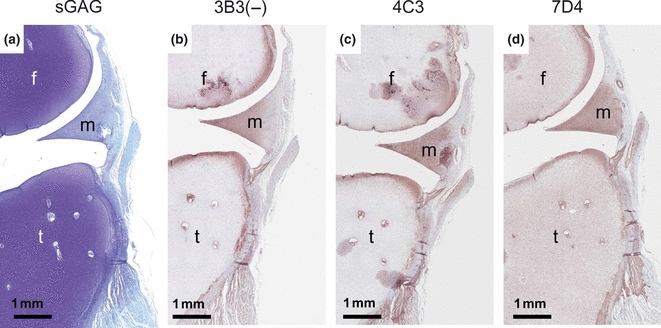
Specific spatial expression of native CS/DS sulphation motif epitopes recognized by mAbs 3B3(−), 4C3 and 7D4 in a 12-week-old human foetal knee joint. Sections have been stained with Toluidine Blue [A] and immunostained with 3B3(−) [B], 4C3 [C] and 7D4 [D]. Note that Toluidine Blue staining identifies the presence of the major populations of CS GAG chains in the developing knee joint tissues. This Toluidine Blue staining is widespread and identifies the large number of GAGs present (mainly CS) throughout the early cartilage elements of the femur (f) and tibia (t) with weaker staining of the fibrocartilagenous meniscus (m) and other fibrous connective tissues of the joint. In contrast, immunostaining with the mAbs 3B3(−), 4C3 and 7D4 shows positive staining in very specific zones of the developing joint cartilage and meniscus that clearly delineate where the future articular cartilage and inner portions of the meniscus will eventually be. Close inspection of the three different mAb staining patterns shows that there are subtle differences in the mAb staining in the matrix or on the cells themselves indicating the specific spatial expression of these CS/DS sulphation epitopes.
Expression of native CS/DS sulphation motif epitopes in osteoarthritic cartilage
In the mid-1980s and early 1990s, in collaborative studies with Helen Muir and other researchers at the Kennedy Institute of Rheumatology in London (Caterson et al. 1990; Carney et al. 1992; Ratcliffe et al. 1993; Slater et al. 1995), we noted that two of our monoclonal antibodies [i.e. 3B3(−) and 7D4] that recognized native CS/DS sulphation motif epitopes were specifically expressed on newly synthesized aggrecan extracted from human osteoarthritic (OA) cartilage. However, these native CS/DS epitopes were not expressed on similar aggrecan subpopulations isolated from normal canine or adult human cartilage (Couchman et al. 1984; Carney et al. 1992; Ratcliffe et al. 1993; Slater et al. 1995). Similar findings, showing elevated levels of mAbs 3B3(−) and 7D4 native CS epitopes, have been reported by other laboratories performing analyses of synovial fluids from human patients after exercise (Bautch et al. 2000) or in canine models of degenerative joint disease (Chu et al. 2002). In the mid-1980s, researchers from another laboratory produced a monoclonal antibody [i.e. antibody 846] that was originally reported as recognizing a protein epitope on aggrecan (Glant et al. 1986) that was extracted from foetal and young but not adult articular cartilage. This 846 mAb was subsequently found to recognize native CS sulphation motifs in the newly synthesized aggrecan from human OA cartilage (Rizkalla et al. 1992) with these findings being very similar to our earlier reported studies (Caterson et al. 1990) showing elevated 3B3(−) and 7D4 CS sulphation motif expression levels on CS GAGs in newly synthesized aggrecan extracted from OA cartilage in the Pond-Nuki dog model of osteoarthritis.
In the past, our laboratory has also performed some unpublished research using the Hartley strain Guinea Pig animal model of OA; that is, it is a genetically induced ‘natural’ animal model of OA where there is an inherent knee joint/ligament laxity that leads to biomechanical abnormalities and instability in the knee joint tissues causing the onset of cartilage degeneration. In this natural model of OA, the knee joint cartilages rapidly develop osteoarthritis in older animals aged 6–12 months, but this degeneration does not occur in the hip joint cartilage that receives similar load distributions (Kraus et al. 2010). In studies from our laboratory, using this Hartley strain Guinea Pig model, we have extracted, separated and analysed different aggrecan subpopulations [using agarose/acrylamide composite gel electrophoresis (Caterson et al. 1990; Carney et al. 1992) and Western blot analyses using mAb 3B3 – see Figure 5) on cartilage tissue extracts obtained from the hip joint (pathology absent) and the various knee cartilages (i.e. medial meniscus, medial knee and lateral knee cartilage) from a 3-month-old animal (where no overt OA is evident in knee tissues) and a 9-month-old ‘retired breeder’ (where significant surface fibrillation and loss of cartilage is evident in knee cartilages –Kraus et al. 2010; Huebner et al. 1998). Here, we found that positive immunostaining for the 3B3(−) CS sulphation motif was present on the newly synthesized aggrecan subpopulation in the extracts from the medial knee cartilage from a 3-month-old animal that showed no overt morphological or histological signs of OA (Figure 5); that is, potential OA in the knee joint cartilage could be detected very early before any overt signs of OA were evident. However, by 9 months of age, in the retired breeder animals, there was extensive expression of the 3B3(−) native epitope on newly synthesized aggrecan CS GAG chains in extracts of all the knee cartilages (i.e. the medial and lateral knee and meniscal cartilages) but not in the ‘normal’ hip joint cartilage. Interestingly, this positive immunostaining for 3B3(−) was only present in the slowest migrating aggrecan band that represents the newly synthesized aggrecan subpopulation. Western blots of nitrocellulose transfers with mAb 3B3 immunostaining after chondroitinase ABC digestion before immunoblotting showed positive staining for multiple aggrecan subpopulation containing the 3B3(+) ‘CS stub’ neoepitope (Figure 2). The 3B3(+) immunostaining also serves to show that equal amounts of sample were applied to each electrophoresis lane.
Figure 5.
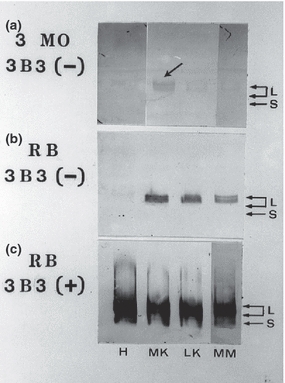
Agarose-acrylamide gel separation and Western blot analyses using mAb 3B3(−) and (+) of proteoglycan subpopulations extracted from the Hip (H) and Knee Joint cartilages (i.e. MK – medial; LK – lateral and MM – medial meniscus cartilages) of 3 month old (A) and 9 month old (B & C) retired breeder (RB) Hartley strain Guinea Pigs. Large aggregating proteoglycans (L – i.e. aggrecan subpopulations) and small leucine-rich proteoglycans (S) are identified by mAb 3B3 immunostaining either on the untreated nitrocellulose membranes [A & B where native 3B3(−) CS GAG chain epitopes are present – see Figure 2] or after the nitrocellulose membranes have been subjected to a chondroitinase ABC digestion [C] prior to their immunostaining with 3B3(+) where ‘CS-stubs’ containing a terminal unsaturated uronic acid adjacent to galactosamine-6-sulphate are present on both L and S proteoglycan subpopulations [C]. Note: only the slowest migrating (newly synthesized) aggrecan subpopulation is identified by immunostaining with 3B3(−) in [A] and [B]. The arrow points to the 3B3(−) positive staining that can only be found in the medial knee cartilage of 3-month-old animals.
Collectively, these several studies from ours and other laboratories have now established that native CS sulphation motif epitopes are increased in their expression with the early onset of OA in hyaline articular cartilage. We believe that this renewed expression of these native CS GAG motif epitopes is an attempt at cartilage repair using cellular mechanisms that have been recapitulated from those used in embryonic and adolescent tissue development. Thus, monitoring the differential expression of these CS/DS GAG sulphation motifs may provide useful biomarkers for studying the early onset and progression of OA in humans.
Conclusions
Over the past 40 years, I have had the honour and pleasure of performing basic biomedical research on the structure, function and metabolism of proteoglycans and their component GAGs in health and disease. Most of my research has focussed on cartilage metabolism, and therefore, I have been involved in more detailed studies on HA, KS, CS and DS GAG metabolism. Furthermore, my introduction to monoclonal antibody technologies in the late 1970s has facilitated much of these past and ongoing research endeavours. In this review, I have tried to provide some references and examples that emphasize the importance of glycobiology and, in particular, subtleties that occur in GAG structure, metabolism and expression in normal tissue development and with the onset of musculoskeletal tissue disease. Clearly, the number of different disaccharide combinations that are present in the four different GAG subpopulations (i.e. HA, KS, CS/DS and Hep/HS –Figure 1 and Table 1) that are found in higher order animals (e.g. chicken to man) is not just randomly synthesized and assembled in cells during tissue and organ development. These post-translational modifications in GAG biosynthesis and substitution on proteins cost the cell very significant amounts of energy through utilization of ATP, GTP, UDP and PAPS and thus their presence would not have been supported in evolutionary selection processes if they did not provide a biological advantage. The conservation of these CS/DS sulphation motifs within GAG chains on cell surface and matrix macromolecules in animals (not plants) has been in evolutionary existence over a very long and broad range of species (Yamada et al. 2011), that is, from Platyhelminthes (Planarian), Coelenterata (Hydra) through to Shellfish and Insects as well as Vertebrates (i.e. sharks, zebrafish, chickens, rodents through to humans). This early evolutionary presence and conservation of GAG structures clearly emphasizes their long-standing importance in basic biology. In conclusion, the poem presented in Figure 6 best describes my passion and conclusions regarding the diversity and importance of GAG structure, function and metabolism in living animals as we know and understand them today.
Figure 6.

The Glycosaminoglycan Poem. Inspired from the poem ‘My Country’ (2nd verse) composed by Dorothea MacKeller OBE (1885–1968).
Acknowledgments
I would very much like to acknowledge the research and scientific input of my colleagues here in Cardiff Drs Clare E. Hughes, James R. Ralphs, Anthony J. Hayes and Siyuan Li as well as collaborators Drs Chris B. Little and James Melrose in Australia (Sydney University). I would also like to acknowledge Professors Tim Hardingham (Manchester, UK), Dick Heinegard and Anders Malmstom (Lund, Sweden) for their helpful discussions with several aspects of this review. This research was supported by funding from Arthritis Research UK and the Medical Research Council UK.
Disclosures
Cardiff University has issued non-exclusive licences for the commercial sale of some of the monoclonal antibodies described in this study [i.e. 1B5, 2B6, 3B3, 3C5, 4C3, 6C3 and 7D4]. The University, School of Biosciences, Author & some of his colleagues receive royalties from these commercial sales to researchers worldwide.
References
- Bautch J, Clayton M, Chu Q, Johnson K. Synovial fluid chondroitin sulphate epitopes 3B3 and 7D4, and glycosaminoglycan in human knee osteoarthritis after exercise. Ann. Rheum. Dis. 2000;59:887–891. doi: 10.1136/ard.59.11.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin AL, Rapraeger AC. Spacial and temporal expression of heparan sulphate in mouse development regulates FGF and FGF receptor assembly. J. Cell Biol. 2003;163:637–648. doi: 10.1083/jcb.200307053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson CS, Loeser RF, Johnstone B, Tulli HM, Dobson DB, Caterson B. Osteoarthritis om cynomolgus,acaques. II. Detection of modulated proteoglycan epitopes in cartilage and synovial fluid. J. Orthop. Res. 1995;13:399–409. doi: 10.1002/jor.1100130314. [DOI] [PubMed] [Google Scholar]
- Carney SL, Billingham MEJ, Caterson B, et al. Changes in proteoglycan turnover in experimental canine osteoarthritic cartilage. Matrix. 1992;12:137–147. doi: 10.1016/s0934-8832(11)80055-7. [DOI] [PubMed] [Google Scholar]
- Caterson B, Christner JE, Baker JR. Characterization of a monoclonal antibody that specifically recognizes Corneal and Skeletal Keratan Sulfate. J. Biol. Chem. 1983;258:8848–8854. [PubMed] [Google Scholar]
- Caterson B, Mahmoodian F, Sorrell JM, et al. Modulation of native chondroitin sulfate structure in tissue development and in disease. J. Cell Sci. 1990a;97:411–417. doi: 10.1242/jcs.97.3.411. [DOI] [PubMed] [Google Scholar]
- Caterson B, Griffin J, Mahmoodian F, Sorrell JM. Monoclonal antibodies against chondroitin sulfate isomers: their use as probes for investigating proteoglycan metabolism. Biochem. Soc. Trans. 1990b;18:820–823. doi: 10.1042/bst0180820. [DOI] [PubMed] [Google Scholar]
- Chu Q, Lopez M, Hayashi K, et al. Elevation of collagenase generated type II collagen neoepitope and proteoglycan epitopes in synovial fluid following induction of joint instability in the dog. Osteoarthritis Cartilage. 2002;10:662–669. doi: 10.1053/joca.2002.0812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortes M, Baria AT, Schwartz NB. Sulfation of chondroitin sulfate proteoglycans is necessary for proper Indian hedgehog signalling in the developing growth plate. Development. 2009;136:1697–1706. doi: 10.1242/dev.030742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couchman JR, Caterson B, Christner JE, Baker JR. Mapping by monoclonal antibody detection of glycosaminoglycans in connective tissues. Nature. 1984;307:650–652. doi: 10.1038/307650a0. [DOI] [PubMed] [Google Scholar]
- Cummings RD. The repertoire of glycan determinants in the human glycome. Mol. BioSyst. 2009;5:1087–1104. doi: 10.1039/b907931a. http://www.molecularbiosystems.org. [DOI] [PubMed] [Google Scholar]
- Davies L, Blain E, Caterson B, Duance VC. Chondroitin sulphate impedes the migration of a sub-population of articular cartilage chondrocytes. Osteoarthritis Cartilage. 2008a;16:855–864. doi: 10.1016/j.joca.2007.12.005. [DOI] [PubMed] [Google Scholar]
- Davies LC, Blain EJ, Gilbert SJ, Caterson B, Duance VC. The potential of IGF-1 and TGF®1 for promoting “adult” articular cartilage repair: an in vitro study. Tissue Eng. 2008b;14:1251–1261. doi: 10.1089/ten.tea.2007.0211. [DOI] [PubMed] [Google Scholar]
- Glant TT, Mikecz K, Roughley PJ, Buzas E, Poole AR. Age-related changes in protein-related epitopes of human articular-cartilage proteoglycans. Biochem. J. 1986;236:71–75. doi: 10.1042/bj2360071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes AJ, Ralphs JR. The response of foetal annulus fibrosus cells to growth factors: modulation of matrix synthesis by TGF-β1 and IGF-1. Histochem. Cell Biol. 2011;136:163–175. doi: 10.1007/s00418-011-0835-x. [DOI] [PubMed] [Google Scholar]
- Hayes AJ, Hall A, Brown L, Tubo R, Caterson B. Macromolecular organization and in vitro growth characteristics of scaffold-free neocartilage grafts. J. Histochem. Cytochem. 2007;55:853–866. doi: 10.1369/jhc.7A7210.2007. [DOI] [PubMed] [Google Scholar]
- Hayes AJ, Tudor D, Nowell M, Caterson B, Hughes CE. Chondroitin sulfate sulfation motifs as putative biomarkers for isolation of articular cartilage progenitor cells. J. Histochem. Cytochem. 2008;56:125–128. doi: 10.1369/jhc.7A7320.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes AJ, Hughes CE, Ralphs JR, Caterson B. Chondroitin sulphate sulphation motif expression in the ontogeny of the intervertebral disc. Eur. Cell. Mater. 2011;21:1–14. http://www.ecmjournal.org/journal/papers/vol021/vol021a01.php. [PubMed] [Google Scholar]
- Heinegard D. Proteoglycans and more – from molecules to biology. Int. J. Exp. Pathol. 2009;90:575–586. doi: 10.1111/j.1365-2613.2009.00695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huebner JL, Otterness I, Freund E, Caterson B, Kraus VB. Collagenase-1 and collagenase-3 expression in a guinea pig model of OA. Arthritis Rheum. 1998;41:877–890. doi: 10.1002/1529-0131(199805)41:5<877::AID-ART16>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Ishii M, Maeda N. Oversulfated chondroitin sulfate plays critical roles in the neuronal migration in the cerebral cortex. J. Biol. Chem. 2008;283:32610–32620. doi: 10.1074/jbc.M806331200. [DOI] [PubMed] [Google Scholar]
- Johnson KA, Hay CW, Roe SC, Caterson B. Cartilage-derived biomarkers of osteoarthritis in synovial fluid of dogs with naturally acquired rupture of the cranual cruciate ligament. Am. J. Vet. Res. 2002;63:775–781. doi: 10.2460/ajvr.2002.63.775. [DOI] [PubMed] [Google Scholar]
- Johnson KA, Francis DJ, Manley PA, Chu Q, Caterson B. Comparison of the effects of caudal pole hemi-meniscectomy and complete medial meniscectomy in the canine stifle joint. Am. J. Vet. Res. 2004;65:1053–1060. doi: 10.2460/ajvr.2004.65.1053. [DOI] [PubMed] [Google Scholar]
- Kluppel M. The roles of chondroitin-4-sulfertransferase-1 in development and disease. Prog. Mol. Biol. Transl. Sci. 2010;93:113–132. doi: 10.1016/S1877-1173(10)93006-8. [DOI] [PubMed] [Google Scholar]
- Kramer KL. Specific sides to multifaceted glycosaminoglycans are observed in embryonic development. Semin. Cell Dev. Biol. 2010;21:631–637. doi: 10.1016/j.semcdb.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus VB, Huebner JL, DeGroot J, Bendele A. The OARSI histopathology initiative – recommendations for histological assessment of osteoarthritis in the guinea pig. Osteoarthritis Cartilage. 2010;18(Suppl 3):S35–S52. doi: 10.1016/j.joca.2010.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin PP, Buckwalter JA, Olmstead M, Caterson B. Expression of proteoglycan epitopes in articular cartilage repair tissue. Iowa Orthop. J. 1998;18:12–18. [PMC free article] [PubMed] [Google Scholar]
- Maccarana M, Olander B, Malmstrom J, et al. Biosynthesis of dermatan sulfate: chondroitin-glucuronate C5-epimerase is identical to SART2. J. Biol. Chem. 2006;281:11560–11568. doi: 10.1074/jbc.M513373200. [DOI] [PubMed] [Google Scholar]
- Maccarana M, Kalamajski S, Kongsgaard M, Magnusson SP, Oldberg O, Malmstrom A. Dermatan Sulfate Epimerase1-deficient mice have reduced content and changed distribution of iduronic acid in Dermatan Sulfate and an altered collagen structure in skin. Mol. Cell. Biol. 2009;29:5517–5528. doi: 10.1128/MCB.00430-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merry CL, Astrautsora SA. Glycans in evolution and development: workshop on glycoscience and development. EMBO Rep. 2008;9:617–622. doi: 10.1038/embor.2008.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oguri K, Okayama E, Caterson B, Okayama M. Isolation, characterization and localization of glycosaminoglycans in rabbit bone marrow. Blood. 1987a;70:501–510. [PubMed] [Google Scholar]
- Oguri K, Okayama E, Caterson B, Okayama M. Chondroitin-6-Sulfate proteoglycans constructing Hemopoietic microenvironment in bone marrow. Keio J. Med. 1987b;36:67–70. doi: 10.2302/kjm.36.67. [DOI] [PubMed] [Google Scholar]
- Pacheco B, Malmstrom A, Maccarana M. Two dermatansulphate epimerases from iduronic acid domains in dermatan sulfate. J. Biol. Chem. 2009a;284:9788–9795. doi: 10.1074/jbc.M809339200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacheco B, Maccarana M, Malmstrom A. Deermatan 4-O-sulfotransferase 1 is pivotal in the formation of iduronic acid blocks in dermatan sulfate. Glycobiology. 2009b;14:1–7. doi: 10.1093/glycob/cwp110. [DOI] [PubMed] [Google Scholar]
- Pacheco B, Maccarana M, Goodlett DR, Malmstrom A, Malmstrom L. Identification of the active site of DS-epimerase-1 and requirement of N-glycosylation for enzyme function. J. Biol. Chem. 2009c;284:1741–1747. doi: 10.1074/jbc.M805479200. [DOI] [PubMed] [Google Scholar]
- Pothacharoen P, Kalayanamiltra K, Deepa SS, et al. Two related but distinct chondroitin sulfate mimetope octasaccharide sequences recognised by monoclonal antibody WF6. J. Biol. Chem. 2007;282:35232–35246. doi: 10.1074/jbc.M702255200. [DOI] [PubMed] [Google Scholar]
- Purushothaman A, Sugahara K, Faissner A. Chondroitin sulfate “Wobble Motifs” modulate the maintenance and differentiation of neural stem cells and their progeny. J. Biol. Chem. 2011 doi: 10.1074/jbc.R111.298430. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratcliffe A, Shurety W, Caterson B. The quantitation of a native chondroitin sulfate epitope in synovial fluid lavages and articular cartilage from canine experimental osteoarthritis and disuse atrophy. Arthritis Rheum. 1993;36:543–551. doi: 10.1002/art.1780360416. [DOI] [PubMed] [Google Scholar]
- Richardson JB, Caterson B, Evans EH, Ashton BA, Roberts S. Human articular cartilage repair: twelve month post-operative biopsies demonstrate heterogeneous cartilage matrix morphology. J. Bone Joint Surg. 1999;81–B:1064–1068. doi: 10.1302/0301-620x.81b6.9343. [DOI] [PubMed] [Google Scholar]
- Rizkalla G, Reiner A, Bogoch E, Poole AR. Studies of the articular cartilage proteoglycan aggrecan in health and osteoarthritis. Evidence for molecular heterogeneity and extensive molecular changes in disease. J. Clin. Invest. 1992;90:2268–2277. doi: 10.1172/JCI116113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts S, Evans H, Caterson B, Elsenstein SM. Proteoglycan components of the intervertebral disc and cartilage endplate: an immunolocalization study of animal and human tissues. Histochem. J. 1994;26:402–411. doi: 10.1007/BF00160052. [DOI] [PubMed] [Google Scholar]
- Roberts S, Hollander AP, Caterson B, Menage J, Richardson JB. Matrix turnover in human cartilage repair tissue in autologous chondrocyte implantation. Arthritis Rheum. 2001;44:2586–2598. doi: 10.1002/1529-0131(200111)44:11<2586::aid-art439>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Rozario T, DeSimone DW. The extracellular matrix in development and morphogenesis: a dynamic view. Dev. Biol. 2010;341:126–140. doi: 10.1016/j.ydbio.2009.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater RR, Bayliss MT, Lachiewicz PF, Visco DM, Caterson B. Monoclonal antibodies that detect biochemical markers of arthritis in humans. Arthritis Rheum. 1995;38:655–659. doi: 10.1002/art.1780380513. [DOI] [PubMed] [Google Scholar]
- Sorrell JM, Caterson B. Detection of age-related changes in the distribution of keratan sulfates and chondroitin sulfates in developing chick limbs: an immunocytochemical study. Development. 1989;106:657–663. doi: 10.1242/dev.106.4.657. [DOI] [PubMed] [Google Scholar]
- Sorrell JM, Caterson B. Monoclonal antibodies specific for keratan sulfate detect epithelial-associated carbohydrates. Histochemistry. 1990;94:269–275. doi: 10.1007/BF00266627. [DOI] [PubMed] [Google Scholar]
- Sorrell JM, Mahmoodian F, Caterson B. Immunochemical characterization and ultrastructural localization of chondroitin sulfates and keratan sulfate in embryonic chick bone marrow. Cell Tissue Res. 1988a;252:523–531. doi: 10.1007/BF00216639. [DOI] [PubMed] [Google Scholar]
- Sorrell JM, Lintala AM, Mahmoodian F, Caterson B. Indirect immunocytochemical localization of chondroitin sulfate proteoglycans in lymphopoietic and granulopoietic compartments of developing Bursae of Fabricus. J. Immunol. 1988b;140:4263–4270. [PubMed] [Google Scholar]
- Sorrell JM, Mahmoodian F, Caterson B. Immunochemical and biochemical comparisons between embryonic chick bone marrow and epiphyseal cartilage chondroitin/dermatan sulfate proteoglycans. J. Cell Sci. 1988c;91:81–90. doi: 10.1242/jcs.91.1.81. [DOI] [PubMed] [Google Scholar]
- Sorrell JM, Mahmoodian F, Schafer IA, Davis B, Caterson B. Identification of monoclonal antibodies that recognize novel epitopes in native chondroitin-dermatan sulfate glycosaminoglycan chains: their use in mapping functionally distinct domains of human skin. J. Histochem. Cytochem. 1990a;38:393–402. doi: 10.1177/38.3.1689338. [DOI] [PubMed] [Google Scholar]
- Sorrell JM, Caterson B, Caplan AI, Davis B, Schafer IA. Human keratinocytes contain carbohydrates that are recognized by keratan sulfate specific monoclonal antibodies. J. Invest. Dermatol. 1990b;95:347–352. doi: 10.1111/1523-1747.ep12485110. [DOI] [PubMed] [Google Scholar]
- Sorrell JM, Carrino DA, Caplan AI. Regulated expression of chondroitin sulfates at sites of epithelial-mesenchymal interactions: spacio-temporal patterning identified with anti-chondroitin sulfate monoclonal antibodies. Int. J. Dev. Neurosci. 1996;14:233–238. doi: 10.1016/0736-5748(96)00010-x. [DOI] [PubMed] [Google Scholar]
- Sugahara K, Mikami T. Chondroitin/Dermatan sulfates in the central nervous system. Curr. Opin. Struct. Biol. 2007;17:536–545. doi: 10.1016/j.sbi.2007.08.015. [DOI] [PubMed] [Google Scholar]
- Tone Y, Pedersen LC, Yamamoto T, et al. 2-O-phophorylation of xylose and 6-O-sulfation of galactose in the protein linkage region of glycosaminoglycans influence the Glucuronyltransferase-1 activity involved in the linkage region synthesis. J. Biol. Chem. 2008;283:16801–16807. doi: 10.1074/jbc.M709556200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbull JE. Heparan sulfate glycomics: towards systems biology strategies. Biochem. Soc. Trans. 2010;38:1356–1360. doi: 10.1042/BST0381356. [DOI] [PubMed] [Google Scholar]
- Visco DM, Johnstone B, Hill MA, Jolly GA, Caterson B. Immunohistochemical analysis of 3-B-3(-) and 7-D-4 epitope expression in canine and human osteoarthritis. Arthritis Rheum. 1993;36:1718–1725. doi: 10.1002/art.1780361211. [DOI] [PubMed] [Google Scholar]
- Watanabe H, Kimata K. Experimental Glycosciences In. In: Taniguchi N, Suzuki A, Ito Y, Narimatsu H, Kawasaki T, Hass S, editors. Chondroitin sulphate biosynthesis and related genes. New York: Springer; 2008. Part 1, Section 1, pp 64–66. [Google Scholar]
- Yamada S, Sugahara K, Ozbek S. Evolution of glycosaminoglycans: comparative biochemical study: a mini-review. Commun. Integr. Biol. 2011;4:150–158. doi: 10.4161/cib.4.2.14547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan D, Lin X. Shaping morphogen gradients by proteoglycans. Cold Spring Harb. Perspect. Biol. 2009;1:a002493. doi: 10.1101/cshperspect.a002493. [DOI] [PMC free article] [PubMed] [Google Scholar]


