Abstract
Angiogenesis is an indispensable mechanism in development and in many pathologies, including cancer, synovitis and aberrant wound healing. Many angiogenic stimulators and inhibitors have been investigated, and some have progressed to the clinic. A disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS) is a group of multifunctional proteinases. ADAMTS-1 and ADAMTS-8 have been reported to be anti-angiogenic. Here, we provide evidence that ADAMTS-4, like ADAMTS-1, is expressed by endothelial cells and binds to vascular endothelial groth factor (VEGF). Moreover, ADAMTS-4 inhibited human dermal microvascular endothelial cells (HuDMEC) VEGF-stimulated VEGF receptor (R) R2 phosphorylation, differentiation and migration, suggesting that ADAMTS-4 may be a novel anti-angiogenic molecule.
Keywords: ADAMTS-1, ADAMTS-4, angiogenesis, human dermal microvescular endothelial cells, VEGF
Angiogenesis, the process of forming new blood vessels from the pre-existing vasculature, is very important in development, tissue remodelling in the menstrual cycle and wound healing (Folkman 2006). The formation of new blood vessels also contributes to a number of pathologies including tumour growth and metastasis, hypertrophic scarring, keloid formation, psoriasis, choroidal neovascularization and synovitis. In these cases, angiogenesis contributes to disease progression, and inhibition of angiogenesis may be a therapeutic approach to these pathologies. Although much is known about the initiation of an angiogenic response, the process of termination of angiogenesis is less well understood, although many anti-angiogenic factors have been identified (Folkman 2006).
Vascular endothelial growth factor (VEGF)-A is known to be the most potent angiogenic stimulator identified to date, and there are eight splice variants, of which VEGF165 is the most important in angiogenesis (Stalmans et al. 2002; Kowanetz & Ferrara 2006; Ferrara 2009). VEGF165 binds to its main receptor VEGFR2 on endothelial cells, which triggers the signalling cascade leading to changes in gene expression associated with angiogenesis (Shalaby et al. 1995; Olsson et al. 2006; Staton et al. 2007).
A disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS) are secreted metalloproteinases related to the matrix metalloproteinases in family M12 of the degradome (Rawlings et al. 2010). In addition to the catalytic domain and various ancillary domains, they contain a variable number of thrombospondin-1-like motifs (Jones & Riley 2005). ADAMTS-1, -4, -5 and -8 have the capacity to cleave large aggregating chondroitin sulphate proteoglycans (Sandy et al. 1991; Nakamura et al. 2000; Jones & Riley 2005) and are sequestered in the matrix via binding to heparan sulphate (Kuno & Matsushima 1998; Vazquez et al. 1999; Longpre et al. 2009; Rodriguez-Manzaneque et al. 2009). ADAMTS-1 and ADAMTS-8 are known to have anti-angiogenic activity (Vazquez et al. 1999). ADAMTS-1 inhibits angiogenesis by two distinct mechanisms. It binds to VEGF165 and thereby prevents the growth factor from binding to its receptor (Luque et al. 2003), and it can also release anti-angiogenic peptides from thrombospondins-1 and -2 (Lee et al. 2006). ADAMTS-4 is closely related to ADAMTS-1 in the ADAMTS phylogenetic tree, and we hypothesized that it may also possess anti-angiogenic properties.
In this study, we use real-time RT-PCR to demonstrate that ADAMTS-4 and ADAMTS-1 were produced by both human dermal microvascular endothelial cells (HuDMECs) and human umbilical vein endothelial cells (HUVECs). We then assessed human recombinant ADAMTS-4 for its ability to inhibit the response of HuDMECs to VEGF stimulation and compared this directly with ADAMTS-1.
Materials and methods
Materials
Recombinant proteins: ADAMTS-1 (from Phe236 to Ala717 with a C-terminal 10-His tag), ADAMTS-4 (from Phe213 to Cys685 with a C-terminal 10-His tag) and VEGF165 (from Ala27 to Arg191) were from R&D systems (Abingdon, Oxon, UK). Growth factor-reduced Matrigel was from Scientific Laboratory Supplies Ltd., Wilford, Nottingham, UK Ultralink® Immobilized protein A/G beads were from PIERCE. I-block was from Applied Biosystems (Bedford, MA). A rabbit polyclonal anti-human VEGF antibody was from Santa Cruz (sc-152), and a monoclonal anti-His tag antibody was from QIAGEN (34660). A polyclonal normal rabbit IgG from R&D Systems (AB-105-C) was used as a negative control. Adult HuDMECs were purchased from PromoCell. EGM® endothelial cell growth medium [including endothelial basal medium (EBM-2) and all the growth factors and supplements] was obtained from Clonetics®. Starving medium was EBM-2 containing 1% (v/v) Foetal bovine serum (FBS).
HuDMEC cultures
During the course of these studies, HuDMEC from different donors was used resulting in some natural biological variability of response to pro- and anti-angiogenic factors. HuDMECs were grown in endothelial cell growth medium. The medium was changed every 3 days and subcultured when cells reached 80–90% confluence using 0.04% trypsin in PBS. They were frozen down at 5 × 105 cells/ml in freezing solution containing 50% growth medium, 45% FBS and 5% dimethyl sulphoxide (DMSO) (all v/v) and stored in liquid N2 for long-term storage. HuDMECs were used at passage 3–6 in this study.
Real-time RT-PCR
A disintegrin and metalloproteinase with thrombospondin motifs mRNA expression by cultured endothelial cells was determined by real-time RT-PCR. Total RNA was extracted using Tri-Reagent (Sigma, Poole, UK), and cDNA synthesis was performed using Superscript II (Invitrogen, Paisley, UK) primed with random hexamers. Each real-time RT-PCR amplification contained 5 μl 2× Universal PCR mastermix (Applied Biosystems, Warrington, UK), 3.5 μl water, 0.5 μl of Assay-On-Demand primer-probe mix (Applied Biosystems) and 1 μl of cDNA, and each amplification was performed in duplicate wells. The Assays-On-Demand used was as follows: Hs99999905_m1 (GAPDH), Hs00199608_m1 (ADAMTS-1), Hs00192708_m1 (ADAMTS-4), Hs00199841_m1 (ADAMTS-5), Hs00199836_m1 (ADAMTS-8) and Hs00172025_m1 (ADAMTS-9). Real-time RT-PCR was performed on an ABI_7900 sequence detection system (Applied Biosystems), and cycling parameters consisted of 40 cycles of 95 °C for 15 s, followed by 60 °C for 1 min. Relative expression was calculated using the comparative CT method (Applied Biosystems recommendations), where CT = cycle threshold, or cycle at which PCR amplification was detectable, and ΔCT = CT (housekeeping gene (GAPDH)) – CT (test gene (ADAMTS)). Data are expressed as relative expression (per cent compared with GAPDH) using the formula 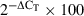 .
.
Co-Immunoprecipitation
Recombinant human (rh) ADAMTS-4 and VEGF165 (40 ng of each) were mixed together in PBS at 4 °C for 10 min followed by the addition of 40 ng of antibody against VEGF, or a normal rabbit IgG control, which were incubated with the protein mixture at 4 °C for 90 min with gentle rotation. A ‘no antibody’ control was also included; 10 μl of protein A/G beads was incubated with the protein mixture at 4 °C for 1 h. The protein complexes were washed at 4 °C with PBS or 1 M NaCl and then collected by centrifugation at 3000 g for 2 min at 4 °C thrice, followed by Western blotting analysis. In these experiments, recombinant proteins were fractioned using 10% acrylamide with 150V, for 1 h in running buffer (25 mM Tris–HCl, 192 mM glycine, 0.1% (w/v) SDS, pH 8.3), and then, proteins were transferred to a PVDF membrane with 400 mA for 30 min in transfer buffer (60 mM Tris–HCl, 40 mM CAPS (3-[cyclohexylamino]-1 propane sulfonic acid), 15% (v/v) methanol, pH 9.6). Bands were analysed by densitometry.
VEGFR2 phosphorylation
Human dermal microvescular endothelial cells were plated into six-well plates and then grown to approximately 90% confluence. For the dose–response experiment, following 6-h serum deprivation, HuDMECs were treated for 3 min with 20 ng/ml VEGF with or without ADAMTS-4 (0, 1, 3, 10, 30, 100 and 300 nM), or starving medium as control. Treatment solutions were then removed, the cells were placed on ice, and mammalian cell lysis buffer (Sigma) was immediately added to the cells. The cell lysate was sonicated for 15 s to fragment DNA, centrifuged at 12,000 g to pellet cellular debris and then stored at −40 °C awaiting Western blotting. In a second series of single-dose experiments, after serum depletion for 6 h, the cells were treated for 3 min with 30 nM ADAMTS-1, 30 nM ADAMTS-4, 20 ng/ml VEGF, 30 nM ADAMTS-1 + 20 ng/ml VEGF, 30 nM ADAMTS-4 + 20 ng/ml VEGF or starving medium. Then, the cells were lysed and stored as described earlier. Protein concentrations of the HuDMEC lysates were determined using the micro BCA protein assay (Pierce Protein Research Products) (Smith et al. 1985; Walker 1994). Equal amounts of extracted protein (60 μg) were fractionated by SDS-PAGE, electrotransferred to PVDF membranes as described earlier and exposed to anti-VEGFR2 rabbit mAb (Cell Signaling Technology, Hitchin, Herfordshire, UK diluted at 1:1000), anti-phospho-VEGFR2 rabbit mAb (Cell Signaling Technology, diluted at 1:1000) or GAPDH rabbit polyclonal antibody (Abcam, Cambridge, UK diluted at 1:2000). All blots were then incubated with secondary antibody (polyclonal horse-radish peroxidase-conjugated swine anti-rabbit immunoglobulins) (Dako Cytomation at 1:3000), and immunoreactive bands were detected by chemiluminescent exposure of X-ray films (Amersham International Little Chalfont, Buckinghamshire, UK). The intensity of detectable protein bands was semi-quantified using a GS-710 Calibrated Imaging Densitometer (Bio-Rad, Hemel Hempstead, Hertfordshire, UK) and the Quantity One software package.
HuDMEC Differentiation (Matrigel assay)
Ninety-six-well plates were coated with 35 μl growth factor-reduced Matrigel (Kleinman et al. 1986). HuDMECs were incubated in starving medium for 6 h, then were seeded at 15,000 cells per well on to Matrigel and incubated for 18 h with the following treatments: 30 nM ADAMTS-1, 30 nM ADAMTS-4, 1.0 nM (20 ng/ml) VEGF, 30 nM ADAMTS-1 + 20 ng/ml VEGF, 30 nM ADAMTS-4 + 20 ng/ml VEGF or starving medium (control). Differentiation of the cells was assessed at ×40 magnification using a phase contrast microscope and measured as the number of cord-like structures within an entire well. Cord-like structures, also referred to as tubules, were defined as elongated structures, less than the width of two cells, and ≥10 μm in length (Donovan et al., 2001). Each experiment was repeated three times and contained three replicate wells for each treatment.
HuDMEC migration (scratch assay)
This method has been described previously (Yarrow et al. 2004). Briefly, HuDMECs were seeded into 96-well plates (15,000 cells per well) to yield a confluent monolayer. The following day, unattached cells were washed off, and the remaining cells were starved for 6 h, and then, straight, even scratches were made in the HuDMEC monolayer using a 10-μl pipette tip. Cells were then washed with PBS twice before being treated with 30 nM ADAMTS-1, 30 nM ADAMTS-4, 20 ng/ml VEGF, 30 nM ADAMTS-1 + 20 ng/ml VEGF, 30 nM ADAMTS-4 + 20 ng/ml VEGF or starving medium (as control). Digital images were taken immediately after treatment as the first time point (0 h) and 18 h postscratch. The distance between the boundaries of the migrating cells was measured using ImageJ software (http://www.nih.gov) (30 measurements of each scratch were measured). Each experiment was repeated three times and contained three replicate wells per treatment.
Cell viability (flow cytometric analysis)
HuDMEC were seeded into 12-well plates at a density of 40,000/ml and allowed to grow to 90% confluence then serum-starved for 6 h. The cells were then incubated for 18 h with 30 nM ADAMTS-1, 30 nM ADAMTS-4, 20 ng/ml VEGF, 30 nM ADAMTS-1 + 20 ng/ml VEGF, 30 nM ADAMTS-4 + 20 ng/ml VEGF or starving medium alone. All cells (floating plus adherent) were then trypsinized, collected into a centrifuge tube, centrifuged at 300 g for 5 min and resuspended in 10 mM Hepes, 0.14 M NaCl, 2.5 mM CaCl2, pH 7.4. To each tube, 5 μl of propidium iodide (PI) was added before analysis on a FACSCalibur machine (Becton Dickinson, Oxford, UK) (Ludovico et al. 2001).
HuDMEC proliferation (BrdU assay)
To assess proliferation of HuDMECs, we used the bromodeoxyuridine (BrdU) assay whereby BrdU is incorporated with newly synthesized DNA of actively proliferating cells (Kee et al. 2002). HuDMECs were seeded into 96-well plates at 5000 cells/well in complete medium and incubated overnight. On the following day, cells were starved for 6 h and then treated for 24 h with 30 nM ADAMTS-1, 30 nM ADAMTS-4, 20 ng/ml VEGF, 30 nM ADAMTS-1 + 20 ng/ml VEGF, 30 nM ADAMTS-4 + 20 ng/ml VEGF or starving medium. HuDMECs were fixed and stained using the BrdU kit from Calbiochem® before spectrophotometric analysis at 450 nm.
Statistical analysis
Means of each experiment were calculated, and the unpaired t-test was used to test for statistical significance (P ≤ 0.05).
Results
HUVECs and HuDMECs express ADAMTSs
Real-time RT-PCR was used to measure the mRNA expression levels of ADAMTS-1, -4, -5, -8 and -9 in two endothelial cell types: HUVECs and HuDMECs. ADAMTS-8 mRNA was not detected in either HUVECs or HuDMECs (Figure 1). ADAMTS-1, -4 and -9 were produced by HUVECs and HuDMECs at similar levels. Although ADAMTS-5 was detected in both HUVECs and HuDMECs, the expression level was much higher in HuDMECs than in HUVECs, but still below the levels of mRNA expression of ADAMTS-1, -4 and -9.
Figure 1.
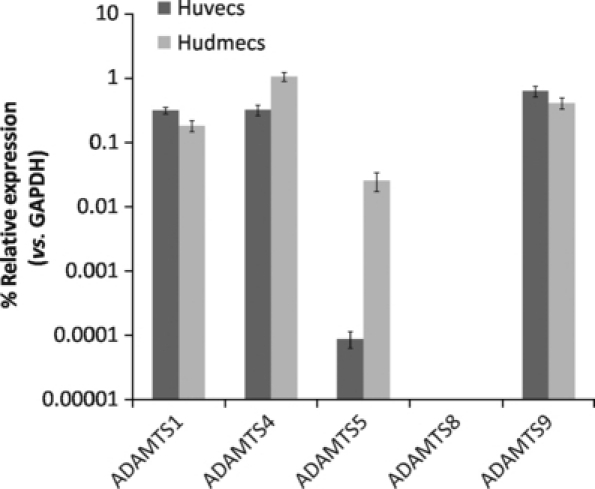
Relative mRNA expression of a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS)-1, -4, -5, -8 and -9 in human umbilical vein endothelial cells (HUVECs) and human dermal microvescular endothelial cells (HuDMECs) as determined by real-time RT-PCR. ADAMTS expression is shown compared with GAPDH expression (normalized to 1) using the formula 2−ΔCT, and data are displayed as median and range of repeat analyses.
ADAMTS-4 binds to VEGF
A disintegrin and metalloproteinase with thrombospondin motifs-1 was reported to interact with VEGF to inhibit the growth factor from binding to its receptor, VEGFR2 (Vazquez et al. 1999). Co-immunoprecipitation was used to detect the interaction between ADAMTS-4 and VEGF. Recombinant ADAMTS-4 and VEGF were mixed to form a potential complex followed by adding antibody against VEGF and protein A/G beads to pull down any protein complex. PBS and 1 M NaCl were used to wash the protein A/G beads to remove non-specific binding. ADAMTS-4 demonstrated increased binding to VEGF specifically compared with a normal rabbit IgG control and a control where no antibody was added (Figure 2). 1 M NaCl eliminated the binding (not shown), suggesting that the binding between ADAMTS-4 and VEGF may rely primarily on ionic interactions.
Figure 2.
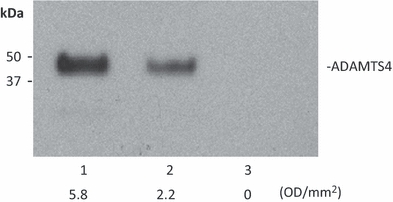
Binding of a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS)-4 and Vascular endothelial growth factor (VEGF) as detected by co-IP. Lane 1: Recombinant ADAMTS-4 and VEGF were mixed, and antibody against VEGF and protein A/G beads were added to the protein mixture to precipitate VEGF. Lane 2: As in lane 1, except an isotype control antibody was used. Lane 3: As in lane 1, but antibody was omitted. The densitometric result (OD/mm2) is shown under each band. Detection in all lanes was with an anti-His tag to identify the His-tagged ADAMTS-4.
ADAMTS-4 decreases VEGF-stimulated VEGFR2 phosphorylation
In view of the evidence for a direct interaction between ADAMTS-4 and VEGF (shown above), we investigated the impact of ADAMTS-4 and -1 on VEGF-mediated VEGFR2 phosphorylation. ADAMTS-4 inhibited VEGFR2 phosphorylation in HuDMEC cells in a dose-related manner to give a maximum of about 65% inhibition at and above 30 nM ADAMTS-4 (Figure 3A). In the absence of VEGF, no VEGFR2 phosphorylation could be detected (Figure 3B). Upon addition of VEGF, VEGFR2 phosphorylation was seen, which was inhibited by the presence of either 30 nM ADAMTS-1 or ADAMTS-4 (Figure 3B), in this series of experiments by 15% and 35%, respectively (Figure 3C). The differences between these two experiments are because of donor variations in responsiveness of the HuDMECs used.
Figure 3.
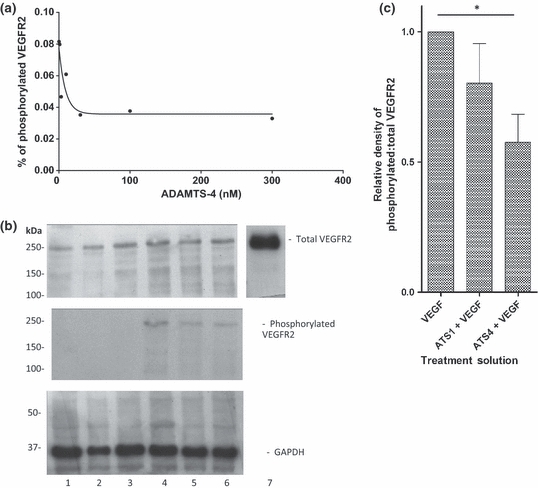
Effects of a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS)-1 and ADAMTS-4 on VEGFR2 phosphorylation. (A) VEGFR2 phosphorylation was reduced by ADAMTS-4 in a dose-dependent manner. The amount of phosphorylated VEGFR2 compared with total VEGFR2 was calculated and plotted against the concentration of ADAMTS-4. (B) A representative Western blot of VEGFR2 phosphorylation in the presence or absence of Vascular endothelial growth factor (VEGF) with or without ADAMTS-4 and -1. Lane 1: control cells (starving medium only); lane 2: 30 nM ADAMTS-1; lane 3: 30 nM ADAMTS-4; lane 4: 20 ng/ml VEGF; lane 5: ADAMTS-1 + VEGF; lane 6: ADAMTS-4 + VEGF; and lane 7: recombinant VEGFR2 control. (C) Densitometric analysis normalized to VEGF-treated cells. Data are mean ± SEM of five repeats. *P = 0.004.
ADAMTS-4 and ADAMTS-1 reduce HuDMEC differentiation
In view of the inhibition of VEGF-stimulated VEGFR2 phosphorylation by ADAMTS-4, we examined the effects of ADAMTS-4 and -1 on downstream angiogenic effects resulting from the VEGFR2 signalling pathway. In the presence of VEGF, endothelial cells on Matrigel differentiate and aggregate to form cord-like structures, which represent the first stages of tubulogenesis. ADAMTS-1 has previously been shown to inhibit VEGF-induced angiogenesis in an in vivo model (Vazquez et al., 1999), and ADAMTS-4 had a similar effect on HuDMECs (Figure 4A). In the absence of added VEGF, neither ADAMTS-1 nor ADAMTS-4 had a significant effect on HuDMEC differentiation (Figure 4B). VEGF-stimulated HuDMEC differentiation by about 1.3-fold compared with control, and both ADAMTS-1 and ADAMTS-4 significantly inhibited VEGF-stimulated HuDMEC differentiation by about 40% (Figure 4B).
Figure 4.
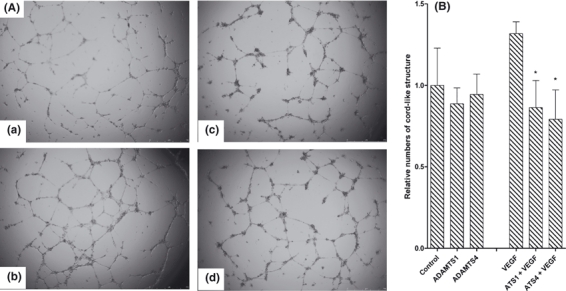
Effects of a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS)-1 and ADAMTS-4 on human dermal microvescular endothelial cells (HuDMEC) differentiation on Matrigel in vitro. (A) Representative images of Matrigel assay; (a) control cells; (b) 20 ng/ml Vascular endothelial growth factor (VEGF); (c) ADAMTS-1 + VEGF; (d) ADAMTS-4 + VEGF. (B) The relative numbers of cord-like structures per well are presented as mean ± SEM of the data normalized to the relevant control. *P < 0.05 with respect to the relevant control. N = 3.
ADAMTS-4 and ADAMTS-1 inhibit HuDMEC migration
When a confluent monolayer of HuDMECs is ‘wounded’, the cells migrate to fill the gap (Figure 5). Addition of VEGF enhanced cell migration by 1.4-fold (Figure 5B). When ADAMTS-4 combined with VEGF was added to HuDMECs, the gap filling was slower compared with the VEGF-treated HuDMECs (Figure 5A). Figure 5(B) shows the normalized scratch closure compared with the relevant control (starving medium only or starving medium with added VEGF). In the absence of added VEGF, ADAMTS-1 and ADAMTS-4 decreased HuDMEC migration by 25% and 50%, respectively, although in neither case was the difference statistically significant. In the presence of added VEGF, ADAMTS-1 and ADAMTS-4 inhibited wound closure by approximately 25% and 35%, respectively, which was a statistically significant difference in the case of ADAMTS-4.
Figure 5.
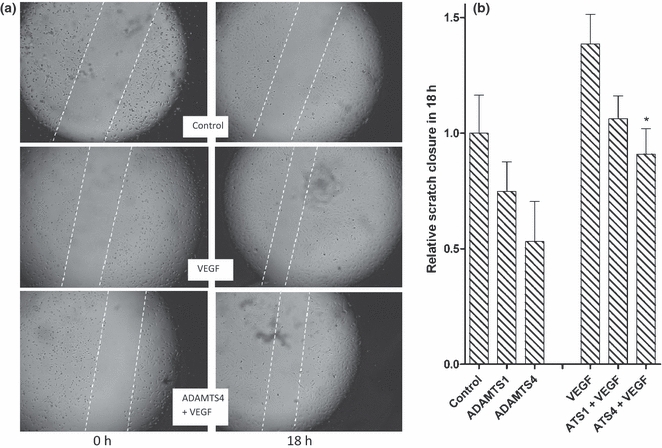
Effects of a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS)-1 and ADAMTS-4 on human dermal microvescular endothelial cells (HuDMEC) migration. (A) A set of representative images of scratch closure at time 0 (left) and 18 h later (right) in the absence of added Vascular endothelial growth factor (VEGF) and ADAMTS-4 (control, top), in the presence of added VEGF (middle) and in the presence of both added VEGF and ADAMTS-4 (bottom). The two migrating fronts of cells are highlighted with dotted lines. (B) The degree of scratch closure normalized to control or VEGF treatment, respectively. N = 4. *P < 0.05 with respect to relevant control.
HuDMEC proliferation and viability
As ADAMTS-1 and ADAMTS-4 inhibited both differentiation and migration of HuDMECs, we wished to ascertain whether these inhibitory effects were because of proliferative or cytotoxic effects. HuDMEC proliferation was assessed using the BrdU incorporation assay, and cell viability was measured using propidium iodide staining and flow cytometry. No significant effects of ADAMTS-4 or -1 on either proliferation or viability were detected, in the presence or absence of added VEGF (results not shown).
Discussion
We have demonstrated that HuDMECs express several ADAMTS enzymes including the known anti-angiogenic ADAMTS-1, as well as ADAMTS-5, ADAMTS-9 and ADAMTS-4, the latter of which we have shown to have properties consistent with an anti-angiogenic function. Kahn et al. (2000) demonstrated increased ADAMTS-4 mRNA expression in HUVECs differentiating in response to growth factors including VEGF, and detected intense ADAMTS-4 mRNA expression in vascular endothelium in areas of inflammation. However, Wimsey et al. (2006) failed to detect immunohistochemical staining of ADAMTS-4 in osteoarthritic synovium. Our data clearly demonstrate the presence of ADAMTS-4 mRNA in isolated HUVECs and HuDMECs, but the quantitative relationship between ADAMTS-4 mRNA and protein is not known. ADAMTS-4 bound to VEGF, inhibited VEGF-mediated VEGFR2 phosphorylation and VEGF-mediated HuDMEC differentiation and migration and appeared to be as potent as the previously described anti-angiogenic ADAMTS-1. Neither protein had a discernible effect on cell proliferation or viability.
Our data open up the intriguing possibility that anti-angiogenesis may be a common property of ADAMTS enzymes, all of which contain thrombospondin (TSR)-1-like motifs. Sharghi-Namini et al. (2008) showed that the first TSR-1 repeat of ADAMTS-5, but not the second, demonstrated anti-angiogenic activity that was described as being VEGF-independent. In addition to ADAMTS-1, -4 and -5, ADAMTS-8 (Vazquez et al. 1999), ADAMTS-2 (Dubail et al. 2010), ADAMTS-12 (Llamazares et al. 2007) and ADAMTS-9 (Koo et al. 2010) have now all been found to possess anti-angiogenic activity.
Thrombospondin (TSP)-1 is a known anti-angiogenic molecule whose expression has been described as being the inverse of VEGF expression in tumours (Iruela-Arispe et al. 2004). There are three different types of thrombospondin repeats in TSP-1, including three type-1 repeats. The second and third of these were demonstrated to have the ability to inhibit endothelial cell proliferation and migration in vitro and also to block blood vessel formation in vivo (Tolsma et al. 1993; Iruela-Arispe et al. 1999; Lawler 2002). All ADAMTS members contain similar sequences to the second and third repeats of type-1 thrombospondin repeats as shown by protein sequence alignment. It is possible that one or more of the three TSRs of ADAMTS-1 and the two TSRs in ADAMTS-8 contribute to their reported anti-angiogenic activity (Vazquez et al. 1999). ADAMTS-4, although phylogenetically closely related to ADAMTS-1, contains only one TSR, whereas other members of this group of metalloproteinases, such as ADAMTS-9, contain up to 15 TSRs (Jones & Riley 2005).
The recombinant ADAMTS-1 and ADAMTS-4 constructs used in this study were not the full-length proteins. Recombinant ADAMTS-1 lacked the two C-terminal TSRs and part of the spacer region, and the recombinant ADAMTS-4 lacked the spacer region at the C-terminal end (Figure 6). We can safely conclude that these regions were therefore not contributing to the anti-angiogenic effects in our experiments. The central TSR was present in both recombinant proteins and may therefore contribute to the anti-angiogenic activity of these molecules. If so, we hypothesize that the anti-angiogenic activity is at least partly mediated by the binding of this region, possibly including the cysteine-rich domain, to VEGF, an interaction that was previously reported for ADAMTS-1 (Luque et al. 2003). Karagiannis and Popel (2007) described that short peptides similar to the type I thrombospondin repeat of ADAMTS-4, ADAMTS-16 and ADAMTS-18 have anti-angiogenic activity.
Figure 6.
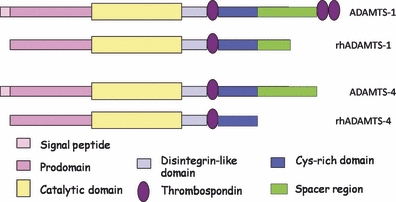
The structure of a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS)-1 and ADAMTS-4. The structures of full-length ADAMTS-1 and -4 and recombinant (rh)ADAMTS-1 and -4 are shown. Names of each domain are also identified.
Other TSR-independent anti-angiogenic mechanisms may also exist for the ADAMTSs, as ADAMTS-1 has been shown to release anti-angiogenic peptides from TSP-1 and -2, an action that requires a catalytic function and thus is not entirely dependent on the TSRs. However, ADAMTS-4 was reported not to possess this catalytic anti-angiogenic property (Lee et al. 2006), suggesting this may not be the mechanism of action for ADAMTS-4.
Our data demonstrate the inhibition of VEGFR2 phosphorylation by ADAMTS-4. However, the enzyme may also possess VEGFR2-independent anti-angiogenic mechanisms. Future work is required to determine the precise anti-angiogenic region(s) of ADAMTSs including ADAMTS-4. Such studies may reveal a common sequence or fold that could provide the lead for the development of novel anti-angiogenic strategies.
References
- Donovan D, Brown NJ, Bishop ET, Lewis CE. Comparison of three in vitro angiogenesis assays with capillaries formed in vivo. Angiogenesis. 2001;4:113–121. doi: 10.1023/a:1012218401036. [DOI] [PubMed] [Google Scholar]
- Dubail J, Kesteloot F, Deroanne C, et al. ADAMTS-2 functions as anti-angiogenic and anti-tumoral molecule independently of its catalytic activity. Cell. Mol. Life Sci. 2010;67:4213–4232. doi: 10.1007/s00018-010-0431-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrara N. VEGF-A: a critical regulator of blood vessel growth. Eur. Cytokine Netw. 2009;20:158–163. doi: 10.1684/ecn.2009.0170. [DOI] [PubMed] [Google Scholar]
- Folkman J. Angiogenesis. Annu. Rev. Med. 2006;57:1–18. doi: 10.1146/annurev.med.57.121304.131306. [DOI] [PubMed] [Google Scholar]
- Iruela-Arispe ML, Lombardo M, Krutzsch HC, Lawler J, Roberts DD. Inhibition of angiogenesis by thrombospondin-1 is mediated by 2 independent regions within the type 1 repeats. Circulation. 1999;100:1423–1431. doi: 10.1161/01.cir.100.13.1423. [DOI] [PubMed] [Google Scholar]
- Iruela-Arispe ML, Luque A, Lee N. Thrombospondin modules and angiogenesis. Int. J. Biochem. Cell Biol. 2004;36:1070–1078. doi: 10.1016/j.biocel.2004.01.025. [DOI] [PubMed] [Google Scholar]
- Jones GC, Riley GP. ADAMTS proteinases: a multi-domain, multi-functional family with roles in extracellular matrix turnover and arthritis. Arthritis Res. Ther. 2005;7:160–169. doi: 10.1186/ar1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn J, Mehraban F, Ingle G, et al. Gene expression profiling in an in vitro model of angiogenesis. Am. J. Pathol. 2000;156:1887–1900. doi: 10.1016/S0002-9440(10)65062-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karagiannis ED, Popel AS. Anti-angiogenic peptides identified in thrombospondin type I domains. Biochem. Biophys. Res. Commun. 2007;359:63–69. doi: 10.1016/j.bbrc.2007.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kee N, Sivalingam S, Boonstra R, Wojtowicz JM. The utility of Ki-67 and BrdU as proliferative markers of adult neurogenesis. J. Neurosci. Methods. 2002;115:97–105. doi: 10.1016/s0165-0270(02)00007-9. [DOI] [PubMed] [Google Scholar]
- Kleinman HK, McGarvey ML, Hassell JR, et al. Basement membrane complexes with biological activity. Biochemistry. 1986;25:312–318. doi: 10.1021/bi00350a005. [DOI] [PubMed] [Google Scholar]
- Koo B-H, Coe DM, Dixon LJ, et al. ADAMTS9 is a cell-autonomously acting, anti-angiogenic metalloprotease expressed by microvascular endothelial cells. Am. J. Pathol. 2010;176:1494–1504. doi: 10.2353/ajpath.2010.090655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowanetz M, Ferrara N. Vascular endothelial growth factor signaling pathways: therapeutic perspective. Clin. Cancer Res. 2006;12:5018–5022. doi: 10.1158/1078-0432.CCR-06-1520. [DOI] [PubMed] [Google Scholar]
- Kuno K, Matsushima K. ADAMTS-1 protein anchors at the extracellular matrix through the thrombospondin type I motifs and its spacing region. J. Biol. Chem. 1998;273:13912–13917. doi: 10.1074/jbc.273.22.13912. [DOI] [PubMed] [Google Scholar]
- Lawler J. Thrombospondin-1 as an endogenous inhibitor of angiogenesis and tumor growth. J. Cell Mol. Med. 2002;6:1–12. doi: 10.1111/j.1582-4934.2002.tb00307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee NV, Sato M, Annis DS, et al. ADAMTS1 mediates the release of antiangiogenic polypeptides from TSP1 and 2. EMBO J. 2006;25:5270–5283. doi: 10.1038/sj.emboj.7601400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llamazares M, Obaya AJ, Moncada-Pazos A, et al. The ADAMTS12 metalloproteinase exhibits anti-tumorigenic properties through modulation of the Ras-dependent ERK signalling pathway. J. Cell Sci. 2007;120:3544–3552. doi: 10.1242/jcs.005751. [DOI] [PubMed] [Google Scholar]
- Longpre JM, McCulloch DR, Koo BH, Alexander JP, Apte SS, Leduc R. Characterization of proADAMTS5 processing by proprotein convertases. Int. J. Biochem. Cell Biol. 2009;41:1116–1126. doi: 10.1016/j.biocel.2008.10.008. [DOI] [PubMed] [Google Scholar]
- Ludovico P, Sousa MJ, Silva MT, Leao C, Corte-Real M. Saccharomyces cerevisiae commits to a programmed cell death process in response to acetic acid. Microbiology. 2001;147:2409–2415. doi: 10.1099/00221287-147-9-2409. [DOI] [PubMed] [Google Scholar]
- Luque A, Carpizo DR, Iruela-Arispe ML. ADAMTS1/METH1 inhibits endothelial cell proliferation by direct binding and sequestration of VEGF165. J. Biol. Chem. 2003;278:23656–23665. doi: 10.1074/jbc.M212964200. [DOI] [PubMed] [Google Scholar]
- Nakamura H, Fujii Y, Inoki I, et al. Brevican is degraded by matrix metalloproteinases and aggrecanase-1 (ADAMTS4) at different sites. J. Biol. Chem. 2000;275:38885–38890. doi: 10.1074/jbc.M003875200. [DOI] [PubMed] [Google Scholar]
- Olsson AK, Dimberg A, Kreuger J, Claesson-Welsh L. VEGF receptor signalling – in control of vascular function. Nat. Rev. Mol. Cell Biol. 2006;7:359–371. doi: 10.1038/nrm1911. [DOI] [PubMed] [Google Scholar]
- Rawlings ND, Barrett AJ, Bateman A. MEROPS: the peptidase database. Nucleic Acids Res. 2010;38:D227–D233. doi: 10.1093/nar/gkp971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Manzaneque JC, Carpizo D, Plaza-Calonge Mdel C, et al. Cleavage of syndecan-4 by ADAMTS1 provokes defects in adhesion. Int. J. Biochem. Cell Biol. 2009;41:800–810. doi: 10.1016/j.biocel.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandy JD, Neame PJ, Boynton RE, Flannery CR. Catabolism of aggrecan in cartilage explants. Identification of a major cleavage site within the interglobular domain. J. Biol. Chem. 1991;266:8683–8685. [PubMed] [Google Scholar]
- Shalaby F, Rossant J, Yamaguchi TP, et al. Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature. 1995;376:62–66. doi: 10.1038/376062a0. [DOI] [PubMed] [Google Scholar]
- Sharghi-Namini S, Fan H, Sulochana KN, et al. The first but not the second thrombospondin type 1 repeat of ADAMTS5 functions as an angiogenesis inhibitor. Biochem. Biophys. Res. Commun. 2008;371:215–219. doi: 10.1016/j.bbrc.2008.04.047. [DOI] [PubMed] [Google Scholar]
- Smith PK, Krohn RI, Hermanson GT, et al. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Stalmans I, Ng YS, Rohan R, et al. Arteriolar and venular patterning in retinas of mice selectively expressing VEGF isoforms. J. Clin. Invest. 2002;109:327–336. doi: 10.1172/JCI14362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staton CA, Kumar I, Reed MW, Brown NJ. Neuropilins in physiological and pathological angiogenesis. J. Pathol. 2007;212:237–248. doi: 10.1002/path.2182. [DOI] [PubMed] [Google Scholar]
- Tolsma SS, Volpert OV, Good DJ, Frazier WA, Polverini PJ, Bouck N. Peptides derived from two separate domains of the matrix protein thrombospondin-1 have anti-angiogenic activity. J. Cell Biol. 1993;122:497–511. doi: 10.1083/jcb.122.2.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez F, Hastings G, Ortega MA, et al. METH-1, a human ortholog of ADAMTS-1, and METH-2 are members of a new family of proteins with angio-inhibitory activity. J. Biol. Chem. 1999;274:23349–23357. doi: 10.1074/jbc.274.33.23349. [DOI] [PubMed] [Google Scholar]
- Walker JM. The bicinchoninic acid (BCA) assay for protein quantitation. Methods Mol. Biol. 1994;32:5–8. doi: 10.1385/0-89603-268-X:5. [DOI] [PubMed] [Google Scholar]
- Wimsey S, Lien CF, Sharma S, et al. Changes in immunolocalisation of beta-dystroglycan and specific degradative enzymes in the osteoarthritic synovium. Osteoarthritis Cartilage. 2006;14:1181–1188. doi: 10.1016/j.joca.2006.04.012. [DOI] [PubMed] [Google Scholar]
- Yarrow JC, Perlman ZE, Westwood NJ, Mitchison TJ. A high-throughput cell migration assay using scratch wound healing, a comparison of image-based readout methods. BMC Biotechnol. 2004;4:21. doi: 10.1186/1472-6750-4-21. [DOI] [PMC free article] [PubMed] [Google Scholar]


