Abstract
This study compared social looking and response to novelty in preschool-aged children (47–68 mo) with or without iron deficiency anemia (IDA). Iron status of the participants from a low-income community in New Delhi, India, was based on venous hemoglobin, mean corpuscular volume, and red cell distribution width. Children’s social looking toward adults, affect, and wary or hesitant behavior in response to novelty were assessed in a semistructured paradigm during an in-home play observation. Affect and behavior were compared as a function of iron status: IDA (n = 74) vs. nonanemic (n = 164). Compared with nonanemic preschoolers, preschoolers with IDA displayed less social looking toward their mothers, moved close to their mothers more quickly, and were slower to display positive affect and touch novel toys for the first time. These results indicate that IDA in the preschool period has affective and behavioral effects similar to those reported for IDA in infancy.
Introduction
Iron deficiency is considered to be the world’s most common single-nutrient disorder. Infants in the 6 to 24-mo age range are particularly at high risk, but the entire preschool-age period may be vulnerable, especially in developing countries. According to a recent region and country summary (1,2), iron deficiency anemia (IDA)11 is widespread among children <5 y of age. Children in southern Asia and Africa are particularly affected, with over half of preschool-aged children having IDA in most countries. IDA is also a problem in Latin America, the Caribbean, the Middle East, East Asia, and the Pacific where, with the exception of a lower incidence in China, the prevalence of IDA ranges from 22 to 66%.
In contrast to a body of research showing poorer motor, cognitive, and social/emotional functioning in infants with IDA (3–5), research on the developmental and behavioral effects of IDA among preschoolers is limited. The few available studies generally show impaired motor, cognitive, and language development as well as poorer learning performance among 3 to 5-y–olds with IDA (6–9). However, there is little or no information on social or emotional alterations in the preschool-age group. The paucity of research on social and emotional development is striking, insofar as this domain has been affected in virtually every study of IDA in infancy, and changes in behavior and affective states might contribute to poorer cognitive and motor test performance (10).
Findings in social and emotional studies of iron-deficient infants provide the background for this study of preschool-aged children. Infants with chronic, severe iron deficiency have been observed to display increased fearfulness, unhappiness, fatigue, low activity, wariness, solemnity, and proximity to the mother during free play, developmental testing and at home (11–17). In a recent preventive trial in Chile (18), ratings after 30–45 min of developmental testing showed that, compared with infants who received iron supplementation, a greater percentage of unsupplemented infants never smiled, never interacted socially, and never showed social referencing. The lack of social referencing was a novel observation, as this aspect of social/emotional development had, to our knowledge, not previously been examined in iron deficiency studies. We pursued this finding, as well as other affective and behavioral alterations, in preschoolers with or without IDA.
Social referencing is an important process of emotional and instrumental communication by which infants and young children actively seek cues from an adult figure in novel and uncertain situations and use that information to regulate their emotions and behavior (19–21). For instance, infants typically look at their caregivers’ emotional expression in the presence of a new toy to decide whether to approach or avoid it. Social referencing is considered to be critical for infants’ early learning from the physical and social environment, providing important scaffolding (19,20,22,23). Individual differences in toddlers’ and preschoolers’ affect and behavior in reaction to novel situations have also been examined in previous developmental research, using behavioral inhibition paradigms (24–26). Wary or behaviorally inhibited children typically display a low approach behavior and stay in close proximity to their mothers when confronted with a range of novel stimuli, including people, objects, and situations (27). There is increasing evidence that this wary/inhibited behavioral pattern is a risk factor for future problems, such as peer problems, anxiety, depression, and negative self-perceptions of competence (28–30).
Lack of social looking toward adults and low levels of social engagement with others in novel situations might well contribute to “functional isolation,” a concept originally derived from animal models (31). According to the functional isolation hypothesis, nutritional deficiencies contribute to changes in infants’ affect and activity, which in turn means that they are less likely to seek and/or receive developmentally facilitating caregiving from their parents, such as responsiveness and verbal stimulation (14). In addition to direct effects of early IDA on brain and behavior, developmentally unsupportive caregiving has been postulated to contribute to long-lasting effects of early IDA. Altered transactional patterns between iron-deficient children and their mothers have been detected in infancy during the period of iron deficiency (14,15). Preschool follow-up studies to date have found that children with chronic, severe iron deficiency in infancy had poorer cognitive and motor development (32–35), as well as lower levels of alertness, physical activity, positive affect, and verbalization at age 5 than children with good iron status in infancy (36,37). Altered transactional patterns between former iron-deficient children and their mothers have been observed again at age 5 (37).
Integrating the functional isolation hypothesis with the reports of reduced social looking toward mothers and increased wary or hesitant behavior in infancy, we predicted that affective and behavioral differences would also be observed among preschool-aged children with IDA. Specifically, we expected that IDA preschoolers would be less likely to look at adults to elicit emotional or informational cues and would more likely show hesitant or wary behavior in a novel situation than non-IDA preschoolers.
Subjects and Methods
Design
The design of the present study was cross-sectional, although the sample was drawn from a longitudinal cohort. All methods were reviewed and approved by the Institutional Review Boards (IRB) of the University of Maryland School of Medicine, Johns Hopkins University Bloomberg School of Public Health, and Annamalai University. The follow-up protocol was also approved by the IRB of the University of Michigan.
Subjects
This study was conducted in conjunction with a follow-up of a project on the effects of micronutrient supplementation among full-term, small-for-gestational age (SGA) infants (Maureen Black, principal investigator) (38,39). The original study used a census and baseline household survey to identify pregnant women in a low-income community in New Delhi, India. Pregnant women were followed until giving birth and were advised to inform the field clinic of a birth, at which time a physician, nutritionist, and field assistant visited the home to record the neonate’s birth weight, length, and head circumference. Infants were eligible for enrollment in the micronutrient study if their gestational age was >36 wk, their birth weight was below the 10th percentile for gestational age, and they had no congenital problems, disabilities, or chronic illnesses. As infants, they were randomly assigned to 1 of 4 treatment groups: riboflavin, riboflavin + zinc, multiple micronutrients, multiple micronutrients + zinc. No blood sampling occurred in the infant study. Details of the infant study have been previously published (38). For an assessment at 15 mo, a group of children born the appropriate weight for gestational age (AGA) was added using the same census and baseline survey.
The present study was part of the preschool-age follow-up of the SGA infants in the original study and the AGA infants who joined the study at 15 mo. To relate behaviors to concurrent IDA, only those children with a blood sample taken around the time of an in-home play observation were included in this analysis: 218 SGA children and 120 AGA children. Among the SGA children in this sample, 106 had been in the riboflavin or riboflavin + zinc groups (i.e., no iron) in the original study and 112 had been in the micronutrient or micronutrient + zinc groups (i.e., iron-supplemented). Children’s mean age at the present follow-up was 4.8 ± 0.5 y, with both genders approximately equally represented. Parental mean age was 28.3 ± 4.4 y for mothers and 32.1± 4.7 y for fathers. Seventy-six percent of the participating families owned their house, and about one-third of these homeowners lived in a 1-room house. Twenty-eight percent of the mothers and 73% of the fathers had attended school. Because mothers were generally illiterate, a full explanation of the project was given verbally and verbal consent was obtained, witnessed by a third party.
Iron status
Iron status was based on a complete blood count from a venous sample obtained within a 3-mo window around the behavioral assessment. The measures of iron status included hemoglobin (Hb), mean corpuscular volume (MCV), and red cell distribution width (RDW). Seventy-four children had IDA, defined as an Hb value <110 g/L, and 2 other abnormal iron status measures: MCV <79.0 fL per CDC cut-off (40) and RDW >15.0% per laboratory cut-off (40). Children in the nonanemic group were those with Hb levels ≥ 110 g/L and no more than one abnormal iron status measure (n = 164). One hundred children did not meet criteria for either of these dichotomous groupings. The variety of combinations of abnormal hematology measures in this group precluded the possibility of viewing them as a valid “middle” group. Therefore, they were considered “indeterminate” and excluded from this analysis. Recent surveys showed that folic acid and vitamin B-12 deficiencies were uncommon in the community. Other causes of anemia, such as malaria and hemoglobinopathies, were not a problem (41,42). Descriptive statistics for the included children (n = 238) and their caregivers were presented according to iron status group (Table 1).
TABLE 1.
Child and family characteristics by iron status group1
| IDA, n = 74 | Nonanemic, n = 164 | P-value2 | |
|---|---|---|---|
| Child characteristics | |||
| Sex, % male | 54.1 | 56.1 | NS3 |
| Age, mo | 57.6 ± 0.63 | 58.0 ± 0.43 | NS |
| Hemoglobin, g/L | 94.0 ± 1.4 | 119.0 ± 0.6 | <0.001 |
| Mean corpuscular volume | 71.5 ± 0.62 | 87.0 ± 0.40 | <0.001 |
| Red cell distribution width | 18.8 ± 0.26 | 15.8 ± 0.11 | <0.001 |
| Weight-for-age, Z-score | −2.4 ± 0.12 | −2.0 ± 0.10 | <0.01 |
| Birth weight, % SGA | 66 | 63 | NS |
| Micronutrient group, % iron-supplemented | 53 | 52 | NS |
| Family characteristics | |||
| Parity of mother4 | 3.8 ± 0.18 | 3.5 ± 0.11 | NS |
| Mother's age, y | 27.8 ± 0.50 | 27.8 ± 0.31 | NS |
| Mother education, % attended | 26 | 27 | NS |
| Father education, % attended | 69 | 74 | NS |
| Home ownership, % | 78 | 74 | NS |
| House >1 room, % | 57 | 49 | NS |
Values are means ± SE or %.
Refers to overall F-test or χ2 test.
NS, not significant, P > 0.05.
Defined as the number of previous births, including still births.
Materials and data gathering
A semistructured paradigm was conducted in the child’s home to assess social looking toward adults and wariness or inhibition in reaction to novelty (24,26). Four familiarization toys were used for a free-play warm-up period, and 3 stimulus toys were used to elicit a response or requests for help from the caregiver without eliciting fear or excessive delight such as immediate laughter. All stimulus toys were battery-operated toys that moved, made sounds, or played music. Pilot testing of these toys showed that they evoked looking to mother without a strong avoidance or approach behavior.
Play observations were conducted in the home. To focus on the child’s social looking and initial reaction to the unfamiliar, the experimenter informed the mother, “We want to watch your child play with several toys. Please do not talk to your child unless he or she asks you questions or requests your help.” The assessment took place on a floor mat (2 m × 2 m) comprised of 16 squares (0.5 m × 0.5 m each). At the start, the child was 2 squares away from the mother. The familiarization toys were placed on the mat, and the child was allowed to explore these toys during the 5-min free-play warm-up period. Next, the experimenter presented the 3 stimulus toys, 1 at a time, to elicit social referencing. Each stimulus toy, presented under a box lid big enough to cover the toy, was placed 2 squares away from the child. The experimenter removed the lid and wound up the toy or turned on the sound and then stepped back. The child was then free to play with the toy. Children’s behavior toward that toy and his or her affect were observed for 2 min after a given toy was wound up or stopped making a sound. Stimulus toys were presented in the same order for all children. The maximum duration coded for any given observation was set at 750 s (12.5 min). Trained research staff conducted affect and behavior coding live using a laptop computer with The Observer software (43). A pilot phase was carried out by a medical student and an undergraduate student from the University of Michigan. Prior to the collection of data, they were trained by Angulo-Barroso and Lozoff to interobserver reliability (intraclass correlation coefficient) of ≥0.75. The students, in turn, trained research staff in India who collected data for the study. All observers were blind to children’s iron status.
Outcome variables
Social looking toward adults in this social referencing situation was recorded during the play observation. Based on previous social referencing studies (19,23), social looking was defined as the frequency of a behavioral sequence of a look first to a toy and then to an adult (i.e., mother, tester, or research assistants).
Wary or hesitant behavior was assessed following previous research on behavioral inhibition (24,25). We coded 1) the child’s latency to approach the mother, 2) the amount of time the child spent in close contact with the mother, 3) the child’s latency to first touch of a familiarization toy and a stimulus toy, and 4) the frequency of the child’s touching the familiarization and stimulus toys.
The affect outcome variables included the latency and frequency of positive affect (i.e., smiles, laughter, or positive vocalization) and negative affect (i.e., cry-face, fuss, cry, or other negative behavior), and the duration of unengaged affect (i.e., quiet and uninvolved during play session).
Maternal behavior was also assessed. The duration of the mother’s talk to the child and the frequency of maternal response with positive or negative comments were coded.
Statistical analysis
Affective and behavioral outcomes were compared in the IDA and nonanemic groups. The distribution of one of the major outcomes, frequency of social looking to mother, was highly skewed. It was therefore dichotomized into lower and upper halves, and logistic regression was used to estimate odds ratios (OR) and corresponding 95% CI for this outcome. The significance of differences in proportions was tested by a χ2 test. For all frequency and duration measures pertaining to child wariness or hesitancy and affect, we conducted the Student’s t test to test the differences between the IDA and nonanemic groups. Analysis of covariance was used when CV adjustment was indicated (see below). For all latency measures, we conducted Kaplan-Meier survival rate analyses, which measures the time until an event occurs. The events in this case were the child’s approach to mother, first touch of a familiarization and a novel toy, as well as first smile. For such latencies, there was generally a time window that characterized ~75% of the children who showed a given behavior, regardless of iron status. Thus, we did not expect group differences in median times and instead focused group comparisons on the remaining 25% (i.e., the tail of the distribution).
A preliminary step in data analysis was to consider background differences related to group iron status and outcome measures. Child’s prenatal growth status (SGA or AGA) and micronutrient supplementation did not relate to iron status (P = 0.61 and P = 0.94, respectively) or to outcome measures (P > 0.05). Therefore, these factors were not considered in further analyses. Weight-for-age Z-scores (WAZ) were computed using EpiInfo (CDC) to determine children’s current nutritional status. WAZ were significantly lower among children in the IDA group (t test, P < 0.01). However, WAZ did not correlate with the outcome variables and therefore was not included as a covariate in the analyses. There were no differences between the IDA and nonanemic groups in terms of age (t test, P = 0.57) and gender (χ2, P = 0.77). Demographic and/or family background factors (i.e., child’s and mother’s age, child’s gender, maternal literacy, and parity) even weakly correlated (P < 0.10) with a given outcome were statistically controlled in those comparisons between the IDA and nonanemic groups (41). Statistical significance was set at P < 0.05.Values in text and tables are means ± SE, unless otherwise noted. All data were analyzed with SPSS (version 13.0) software.
Results
Social looking toward adults
The iron status groups differed in the proportion of children displaying less frequent social looks to the mother (Table 2, P < 0.05). Logistic regression analysis indicated that children in the IDA group were 98% more likely than those in the nonanemic group to fall within the half that looked relatively less to their mothers (OR 1.98, CI 1.21–3.49, P < 0.05). Social looking to anyone other than the mother was so rare that it was not amenable to analysis.
TABLE 2.
Child social looking and affect or behavior by iron status group1
| Outcome variables | IDA, n = 74 | Nonanemic, n = 164 | P-value2 | Covariate(s)3 |
|---|---|---|---|---|
| Social looking toward adults | ||||
| Frequent social looks to mother, % | 42 | 57 | 0.02 | Mother's parity |
| Wary/hesitant behavior | ||||
| Time to approach mother4, s | 35.7 ± 15.4 | 270 ± 61.7 | <0.001 | |
| Time to touch a familiar toy4, s | 15.1 ± 2.1 | 16.3 ± 1.9 | 0.11 | |
| Time to touch a stimulus toy4, s | 17.1 ± 4.6 | 7.4 ± 0.7 | <0.001 | |
| Close contact with mother, s | 334.2 ± 35.6 | 300.1 ± 23.8 | 0.43 | Mother's parity and age, child age |
| Touches of familiar toys, n | 10.7 ± 1.0 | 11.9 ± 0.7 | 0.31 | Child age |
| Touches of stimulus toys, n | 6.8 ± 0.5 | 6.4 ± 0.4 | 0.52 | Child age, gender |
| Affect | ||||
| Time to first smile4, s | 267.4 ± 115.8 | 102.0 ± 27.5 | <0.001 | |
| Laughs and smiles, n | 4.7 ± 0.7 | 4.3 ± 0.5 | 0.51 | |
| Unengaged affect, s | 87.4 ± 21.1 | 55.6 ± 11.5 | 0.15 | |
| Maternal behavior | ||||
| Proportion of time spent talking, % | 6.6 ± 1.4 | 7.4 ± 1.2 | 0.70 |
Values are means ± SE or %.
P-value refers to the overall F-test/log-likelihood ratio.
Covariates were continuous variables except for gender.
Time at which 75% of each group showed a given behavior.
Wary or hesitant behavior
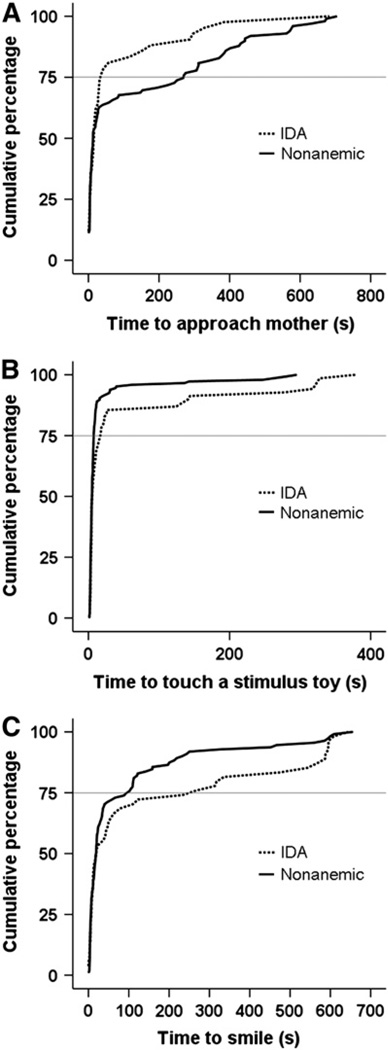
The proportion of children who approached their mother did not differ between iron status groups; overall, 60% did so. Among these children, the median time (or latency) to approach the mother was similar (15.3 ± 2.7 s for the IDA group and 12.9 ± 4.4 s for the nonanemic group). The total amount of time a child spent close to the mother also did not differ between iron status groups before or after covariate control (Table 2). However, among children who approached their mothers, but not immediately (~25%), the IDA group did so more quickly (Fig. 1A, P < 0.001). The time by which 75% of IDA children moved close to their mothers was 7.6 times faster than those of the nonanemic group (Table 2).
Figure 1.
Cumulative percentage of children to approach the mother (A), touch a stimulus toy (B), and smile (C) over time among preschoolers with and without IDA, based on Kaplan-Meier survival rate analyses.
There was no difference between the IDA and nonanemic groups with respect to latency of first touch of a familiarization toy (Table 2). The groups did not differ in the proportion of children who touched at least 1 of the stimulus toys (91% did so), in the frequency of touching familiarization or stimulus toys (Table 2), or in the median time to touch a stimulus toy (5.1 ± 0.9 s in the IDA group and 4.9 ± 0.2 s in the nonanemic group). However, among the ~25% of the sample that was slowest to touch a stimulus toy, children in the IDA group delayed longer than those in the nonanemic group (Fig. 1B, P < 0.001). The time by which 75% of IDA children had touched a stimulus toy was 2.3 times longer than for the nonanemic group (Table 2).
Affect
The proportion of children who smiled during the play observation (70% overall) did not differ between iron status groups. The median latency to smile among those who smiled was similar for both groups (19.6 ± 15.0 s for the IDA group and 19.1 ± 2.2 s for the nonanemic group). However, group differences were again observed in the tail of the distribution, i.e., among the 25% who were slowest to smile (Fig. 1C, P < 0.001). The time by which 75% of the IDA children who ever smiled had done so was 2.6 times longer than for the nonanemic group (Table 2). Negative affect (e.g., cry, fuss) was excluded from analyses, because only 2% of children in the sample showed distress during the observation. Time spent in unengaged affect did not differ between the IDA group and the nonanemic group (ANOVA, P = 0.15).
Maternal behavior
Mothers of children in the IDA and nonanemic groups did not differ significantly in the mean amount of time spent talking (Table 2). Among the mothers who talked (24% of the sample), only 2% initiated negative comments and 3% responded with negative comments.
Discussion
This study examined social looking toward adults, affect, and wary or hesitant behavior among preschool-aged Indian children with and without IDA when they were confronted with a range of novel stimuli. To our knowledge, to date, iron deficiency studies have examined these behavioral and affective aspects of social/emotional development only during infancy.
Consistent with our prediction, preschoolers with IDA showed less social looking toward their mother compared with children in the nonanemic group during a play observation that involved familiar and unfamiliar toys. Our results indicated other affective and behavioral differences in the subset of children at the tail end of the distributions. Specifically, among children who showed a given behavior but without the typical latency, those in the IDA group approached their mothers more quickly, took longer to touch a stimulus toy, and were slower to smile than those in the nonanemic group. This affective and behavioral pattern suggests more wariness, inhibition, and hesitancy in the IDA group. SGA vs. AGA, micronutrient supplementation in infancy, and current WAZ were tested as covariates and did not affect the results. Statistical control for background variables did not eliminate the significant effect of IDA on these outcomes.
Our results pertaining to the affective and behavioral alterations associated with IDA are consistent with past research on iron-deficient infants (11,12,14–18). In the present study, differences in affect and behavior occurred primarily in response to the different aspects of the observation. For instance, the only difference between the familiarization and stimulus toys was that the experimenter, an unfamiliar person, approached the mat for a few seconds to present a novel toy and then withdrew. Otherwise, the child could play with both familiarization and stimulus toys in the presence of the mother. Thus, it appears that even the slight increase in novelty when the experimenter approached with the box was sufficient to elicit a different response in children who did not touch the toy within a few seconds. Among this subset, IDA children took even longer than nonanemic children to touch the stimulus toys.
In developmental research, children with such a behavioral profile have been characterized as “wary, slow to warm up” when they are confronted with a range of novel stimuli including people and objects (44). Individual differences in wary/inhibited-child behavioral patterns can potentiate the pathway to internalizing problems (e.g., anxiety, depression), especially in the context of developmentally less-facilitating parenting such as overprotectiveness, lack of maternal warmth, responsivity, and affective reciprocity (29). In fact, other studies detected less mother-child reciprocity and maternal responsivity between iron-deficient children and their mothers in infancy during the period of iron deficiency (14,15) and again at age 5 (37). Taken together, this wary/inhibited behavior pattern in IDA infants and preschoolers may be clinically significant, especially in light of increased anxiety or depression and social problems in the only infant iron deficiency follow-up into adolescence (45).
The observed changes in response to novelty may make sense in terms of iron’s role in dopamine system function (46). Dopaminergic neurotransmission is known to play a major role in reward-seeking behaviors and in systems of behavioral activation and inhibition, especially in response to novelty (47,48). Rats with neonatal dopamine depletion are hesitant to enter a novel environment or to engage in new tasks outside the familiar environment. Neonatal dopamine terminal damage leads to a life-long hyper-sensitivity to novel objects and experiences in an unfamiliar environment (49,50). Thus, early alterations in dopamine and associated pathways seem to have long-term effects on context-dependent attentional and affective responses. This pattern of behavior is consistently observed in rat models of developmental iron deficiency (51–53). Although the neuroscience basis for behavioral alterations associated with IDA is increasingly compelling, the explanation for the behaviors observed in preschoolers in this study is still speculation.
The functional isolation framework, derived from animal models (31), postulates that several characteristics associated with IDA, such as low activity, wariness, and low positive affect, operate in a transactional fashion with less stimulating parenting, thereby leading to a child’s functional isolation from the environment and contributing to poorer behavioral and developmental outcomes over time (14). However, this study was not designed to assess mother-child interaction, and mothers were instructed to remain uninvolved unless the child initiated contact. Given that parental reactions can maintain or help overcome this behavioral pattern (29,54,55), further research is needed on the quality of the mother-child interaction in IDA preschool-aged children.
The observation that preschoolers with IDA were quicker to seek proximity to their mothers may also be interpreted within the framework of attachment theory. Proximity-seeking behavior is viewed as an adaptive pattern, especially when children are tired, sick, or frightened (56). The shorter latency of proximity-seeking behavior observed in IDA children may reflect the activation of attachment behaviors due to initial discomfort with the play observation in the presence of an unfamiliar adult experimenter. Ainsworth (57) has argued that young children may be sufficiently reassured by the mere presence of the mother, especially if they are secure and confident of her availability. Thus, the initial uncertainty of the children in the IDA group might have been resolved by the availability of their mother in the room. This could explain why IDA children did not differ from the children in the nonanemic group in overall duration of time spent close to the mother.
The results of this study must be considered within the context of its limitations. The 1st issue relates to earlier iron status. Participants in this study came from a micronutrient supplementation study that was intended to impact iron status. However, no hematologic data were obtained in infancy. Iron deficiency seems likely in this sample because a vulnerable sample of infants was recruited (WAZ was <10th percentile based on National Center for Health Statistics norms in India, a setting where infant iron deficiency is common (1,2). IDA in these preschoolers might thus have been ongoing for quite some time. As a result, we cannot attribute the differences in behavior and affect solely to children’s iron status at preschool age. Although iron supplementation was shown to enhance social interaction and affective responsivity among infants (18), there were no effects of micronutrient supplementation for 8 mo in infancy on behavior and affect in the preschool period in this sample. It is possible that the dose did not sufficiently alter the infants’ iron status in this sample, with high rates of stunting at 9 mo, indicating chronic malnutrition early on (39). Alternatively, other nutritional deficiencies might have interfered with iron absorption, thus compromising the long-term effects of iron supplementation. Furthermore, in settings like the present one, where preschool iron deficiency is widespread, it might be difficult to detect effects of iron status and iron supplementation in infancy.
The 2nd issue relates to the assessment of social referencing. Social referencing is generally considered to consist of 2 distinct components, namely, information-seeking and the subsequent use of that information to regulate emotions and guide behavior (19,20). Because the lack of social referencing during developmental testing among infants who did not receive iron supplementation was a novel finding in a recent preventive trial (18), our study specifically examined the IDA child’s social looking toward adults to seek information as they encountered an unfamiliar situation. To obtain a more complete understanding of social referencing among children with IDA, future studies should include further detailed observations of maternal affective messages (e.g., how to feel) and instrumental messages (e.g., what to do, how to act) in response to the child’s social referencing looks and the child’s subsequent responses in relation to maternal messages.
Third, developmental researchers have assessed individual differences in behavioral inhibition across different contexts that include novel nonsocial (i.e., unfamiliar toys only), adult-social (i.e., unfamiliar adults offering new toys), and peer-social (i.e., unfamiliar playmates) contexts (25). In the present study an adapted version of a classic behavioral inhibition paradigm was used to assess children’s affective and behavioral reactions in response to an unfamiliar adult experimenter in a play session involving novel toys (26). Our results for this assessment procedure indicated that the IDA children show behaviors that suggest inhibition. However, recent research indicates a lack of consistency in child wariness across novel nonsocial, adult-social, and peer-social contexts (25). A more thorough investigation of the role of IDA in relation to child wariness or inhibition in different contexts is an important avenue of research for future studies.
Future studies should also examine the domain of social and emotional development in more depth and consider biological and psychosocial processes underlying such influences, as well as the continuity and change in affective and behavioral patterns over time. Such research may point to specific psychosocial interventions in addition to iron therapy. Increasing caregivers’ sensitivity to differences in the IDA child’s emotionality and behavior might be one useful target for intervention efforts. Integrating basic research on the effects of IDA with interventions that emerge from this research has the potential to foster IDA children’s development.
In summary, preschool-aged Indian children who differed in iron status displayed differences in social looking, affect and behavior in a semistructured paradigm designed to expose children to a range of novel toys during an in-home play observation. Compared with the nonanemic group, the IDA group showed less social looking to mother. Among the ~25% of the sample in the tails of the latency distributions, IDA children displayed greater wariness and hesitancy, as evidenced by a delay in touching a novel toy, slower smiling, and quicker seeking the proximity of their mothers. Given the long-term effects of wariness, inhibition, and infant IDA, the observed behavior pattern may be clinically important, especially in developing countries where the high prevalence of IDA warrants urgent attention from researchers, clinicians, and policy makers.
Acknowledgments
We thank Ayo Badejo and Andrew Emerson for their assistance in training observers and for pilot testing the play observations. They were supported by a Minority International Research Training program grant (NIH Fogarty International Center, B. Lozoff, principal investigator).
Footnotes
Funded by the National Institute of Child Health and Human Development (R01 HD374430, Maureen Black, principal investigator).
F. Corapci and M. J. Burden performed their research as postdoctoral fellows at the Center for Human Growth and Development, University of Michigan, Ann Arbor, MI 48109-0406.
Abbreviations used: AGA, appropriate weight for gestational age; IDA, iron deficiency anemia, iron-deficient anemic; SGA, small-for-gestational age.
Literature Cited
- 1.Mason J, Bailes A, Beda-Andourou M, Copeland N, Curtis T, Deitchler M, Foster L, Hensely M, Horjus P. Recent trends in malnutrition in developing regions: vitamin A deficiency, anemia, iodine deficiency, and child underweight. Food Nutr Bull. 2005;26:59–108. doi: 10.1177/156482650502600107. [DOI] [PubMed] [Google Scholar]
- 2.UNICEF. Micronutrient initiative. A Global Progress Report. 2004 [Google Scholar]
- 3.Grantham-McGregor S, Ani C. A review of studies on the effect of iron deficiency on cognitive development in children. J Nutr. 2001;131:649S–668S. doi: 10.1093/jn/131.2.649S. [DOI] [PubMed] [Google Scholar]
- 4.Lozoff B, Georgieff MK. Iron deficiency and brain development. Semin Pediatr Neurol. 2006 doi: 10.1016/j.spen.2006.08.004. in press. [DOI] [PubMed] [Google Scholar]
- 5.Lozoff B, Beard J, Connor J, Felt B, Georgieff M, Schallert T. Long-lasting neural and behavioral effects of iron deficiency in infancy. Nutr Rev. 2006;64:S34–S43. doi: 10.1301/nr.2006.may.S34-S43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pollitt E, Leibel RL, Greenfield DB. Iron deficiency and cognitive test performance in preschool children. Nutr Behav. 1983;1:137–146. [Google Scholar]
- 7.Pollitt E, Saco-Pollitt C, Leibel RL, Viteri FE. Iron deficiency and behavioral development in infants and preschool children. Am J Clin Nutr. 1986;43:555–565. doi: 10.1093/ajcn/43.4.555. [DOI] [PubMed] [Google Scholar]
- 8.Seshadri S, Gopaldas T. Impact of iron supplementation on cognitive functions in preschool and school-aged children: the Indian experience. Am J Clin Nutr. 1989;50:675–686. doi: 10.1093/ajcn/50.3.675. [DOI] [PubMed] [Google Scholar]
- 9.Metallinos-Katsaras E, Valassi-Adam E, Dewey KG, Lonnerdal B, Stamoulakatou A, Pollitt E. Effect of iron supplementation on cognition in Greek preschoolers. Eur J Clin Nutr. 2004;58:1532–1542. doi: 10.1038/sj.ejcn.1602005. [DOI] [PubMed] [Google Scholar]
- 10.Lozoff B, Black M. Impact of micronutrient deficiencies on behavior and development. In: Pettifor J, Zlotkin SH, editors. Nutrition-micronutrient deficiencies during the weaning period and the first years of life. Basel: Karger; 2003. pp. 119–135. [Google Scholar]
- 11.Williams J, Wolff A, Daly A, MacDonald A, Aukett A, Booth IW. Iron supplemented formula milk related to reduction in psychomotor decline in infants for inner city areas: randomised study. BMJ. 1999;318:693–698. doi: 10.1136/bmj.318.7185.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Honig AS, Oski FA. A clinical risk index for iron deficient infants. Early Child Dev Care. 1984;16:69–84. [Google Scholar]
- 13.Walter T, De Andraca I, Chadud P, Perales CG. Iron deficiency anemia: adverse effects on infant psychomotor development. Pediatrics. 1989;84:7–17. [PubMed] [Google Scholar]
- 14.Lozoff B, Klein NK, Nelson EC, McClish DK, Manuel M, Chacon ME. Behavior of infants with iron deficiency anemia. Child Dev. 1998;69:24–36. [PubMed] [Google Scholar]
- 15.Lozoff B, Klein NK, Prabucki KM. Iron-deficient anemic infants at play. J Dev Behav Pediatr. 1986;7:152–158. [PubMed] [Google Scholar]
- 16.Lozoff B, Wolf AW, Urrutia JJ, Viteri FE. Abnormal behavior and low developmental test scores in iron-deficient anemic infants. J Dev Behav Pediatr. 1985;6:69–75. [PubMed] [Google Scholar]
- 17.Lozoff B, Wolf AW, Jimenez E. Iron deficiency anemia and infant development: effects of extended oral iron therapy. J Pediatr. 1996;129:382–389. doi: 10.1016/s0022-3476(96)70070-7. [DOI] [PubMed] [Google Scholar]
- 18.Lozoff B, De Andraca I, Castillo M, Smith J, Walter T, Pino P. Behavioral and developmental effects of preventing iron-deficiency anemia in healthy full-term infants. Pediatrics. 2003;112:846–854. [PubMed] [Google Scholar]
- 19.Feinman S. Social referencing and the social construction of reality in infancy. New York: Plenum Press; 1992. [Google Scholar]
- 20.Hornik R, Gunnar MR. A descriptive analysis of infant social referencing. Child Dev. 1988;59:626–634. [PubMed] [Google Scholar]
- 21.Klinnert MD. The regulation of infant behavior by maternal facial expression. Infant Behav Dev. 1984;7:447–465. [Google Scholar]
- 22.Stenberg G. Effects of maternal inattentiveness on infant social referencing. Infant Child Dev. 2003;12:399–419. [Google Scholar]
- 23.Walden TA, Kim G. Infants’ social looking toward mothers and strangers. Int J Behav Dev. 2005;29:356–360. [Google Scholar]
- 24.Garcia Coll C, Kagan J, Reznick JS. Behavioral inhibition in young children. Child Dev. 1984;55:1005–1019. [Google Scholar]
- 25.Rubin KH, Hastings PD, Stewart S, Henderson HA, Chen X. The consistency and concomitants of inhibition: some of the children, all of the time. Child Dev. 1997;68:467–483. [PubMed] [Google Scholar]
- 26.Kochanska G. Patterns of inhibition to the unfamiliar in children of normal and affectively ill mothers. Child Dev. 1991;62:250–263. doi: 10.1111/j.1467-8624.1991.tb01529.x. [DOI] [PubMed] [Google Scholar]
- 27.Kagan J. Biology and the child. In: Damon W, Eisenberg N, editors. Handbook of child psychology, vol. 3: social, emotional, and personality development. New York: Wiley; 1998. pp. 177–236. [Google Scholar]
- 28.Caspi A, Henry B, McGee RO, Moffitt TE, Silva PA. Temperamental origins of child and adolescent behavior problems: from age three to age fifteen. Child Dev. 1995;66:55–68. doi: 10.1111/j.1467-8624.1995.tb00855.x. [DOI] [PubMed] [Google Scholar]
- 29.Rubin KH, Burgess KB, Kennedy AE, Stewart SL. Social withdrawal in childhood. In: Mash EJ, Barkely RA, editors. Child psychopathology. New York: Guilford Press; 2003. pp. 372–408. [Google Scholar]
- 30.Schmitz S, Fulker DW, Plomin R, Zahn-Waxler C, Emde R, DeFries JC. Temperament and problem behaviour during early childhood. Int J Behav Dev. 1999;23:333–355. [Google Scholar]
- 31.Levitsky DA, Barnes RH. Nutritional and environmental interactions in the behavioral development of the rat: long-term effects. Science. 1972;176:68–71. doi: 10.1126/science.176.4030.68. [DOI] [PubMed] [Google Scholar]
- 32.De Andraca I, Walter T, Castillo M, Pino P, Rivera P, Cobo C. Nestle Foundation Nutrition Annual Report (1990) Vevey, Switzerland: Nestec Ltd.; 1991. Iron deficiency anemia and its effects upon psychological development at preschool age: a longitudinal study; pp. 53–62. [Google Scholar]
- 33.Palti H, Pevsner B, Adler B. Does anemia in infancy affect achievement on developmental and intelligence tests? Hum Biol. 1983;55:183–194. [PubMed] [Google Scholar]
- 34.Dommergues JP, Archambeaud B, Ducot Y, Gerval Y, Hiard C, Rossignol C, Tchernia G. Iron deficiency and psychomotor development scores: A longitudinal study between ages 10 months and 4 years. Arch Fr Pediatr. 1989;46:487–490. [PubMed] [Google Scholar]
- 35.Lozoff B, Jimenez E, Wolf AW. Long-term developmental outcome of infants with iron deficiency. N Engl J Med. 1991;325:687–694. doi: 10.1056/NEJM199109053251004. [DOI] [PubMed] [Google Scholar]
- 36.Tamura T, Goldenberg RL, Hou J, Johnston KE, Cliver SP, Ramey SL, Nelson KG. Cord serum ferritin concentrations and mental and psychomotor development of children at five years of age. J Pediatr. 2002;140:165–170. doi: 10.1067/mpd.2002.120688. [DOI] [PubMed] [Google Scholar]
- 37.Corapci F, Radan AE, Lozoff B. Iron deficiency in infancy and mother-child interaction at 5 years. J Dev Behav Pediatr. 2006;27:371–378. doi: 10.1097/00004703-200610000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sazawal S, Black RE, Menon VP, Dinghra P, Caulfield LE, Dhingra U, Bagati A. Zinc supplementation in infants born small for gestational age reduces mortality: a prospective, randomized, controlled trial. Pediatrics. 2001;108:1280–1286. doi: 10.1542/peds.108.6.1280. [DOI] [PubMed] [Google Scholar]
- 39.Black MM, Sazawal S, Black RE, Khosla S, Kumar J, Menon V. Cognitive and motor development among small for gestational age infants: Impact of zinc supplementation, birth weight, and caregiving practices. Pediatrics. 2004;113:1297–1305. doi: 10.1542/peds.113.5.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Centers for Disease Control and Prevention. Recommendations to prevent and control iron deficiency in the United States. Morb Mortal Wkly Rep. 1998;47:1–29. [PubMed] [Google Scholar]
- 41.Gomber S, Bhawna, Madan N, Lal A, Kela K. Prevalence & etiology of nutritional anaemia among school children of urban slums. Indian J Med Res. 2003;118:167–171. [PubMed] [Google Scholar]
- 42.Sarkar A, Marwah D, Dhingra U, Dhingra P, Verma P, Black RE. Prevalence of hemoglobinopathies in North Indian children and its association with anemia. Proceedings of 42nd national conference of Indian academy of pediatrics; Kolkata. 2005. p. 56. [Google Scholar]
- 43.Noldus LPJJ. The observer: a software system for collection and analysis of observational data. Behav Res Methods Instrum Comput. 1991;23:415–429. [Google Scholar]
- 44.Thomas A, Chess S. The New York longitudinal study: from infancy to early adult life. In: Plomin R, Dunn J, editors. The study of temperament. Hillsdale, NJ: Erlbaum; 1986. pp. 39–52. [Google Scholar]
- 45.Lozoff B, Jimenez E, Hagen J, Mollen E, Wolf AW. Poorer behavioral and developmental outcome more than 10 years after treatment for iron deficiency in infancy. Pediatrics. 2000;105:E51. doi: 10.1542/peds.105.4.e51. [DOI] [PubMed] [Google Scholar]
- 46.Beard JL, Connor JR. Iron status and neural functioning. Annu Rev Nutr. 2003;23:41–58. doi: 10.1146/annurev.nutr.23.020102.075739. [DOI] [PubMed] [Google Scholar]
- 47.Noble E. D2 dopamine receptor gene in psychiatric and neurologic disorders and its phenotypes. Am J Med Genet. 2003;116B:103–125. doi: 10.1002/ajmg.b.10005. [DOI] [PubMed] [Google Scholar]
- 48.Wachs TD. Linking nutrition and temperament. In: Molfese D, Molfese V, editors. Temperament and personality across the life-span. Mahwah, NJ: Lawrence Erlbaum; 2000. pp. 57–84. [Google Scholar]
- 49.Schallert T, Petrie BF, Whishaw IQ. Neonatal dopamine depletion: Spared and unspared sensorimotor and attentional disorders and effects of further depletion in adulthood. Psychobiology. 1989;17:386–396. [Google Scholar]
- 50.Schallert T, Whishaw IQ. Bilateral cutaneous stimulation of the somatosensory system in hemidecorticate rats. Behav Neurosci. 1984;98:518–540. doi: 10.1037//0735-7044.98.3.518. [DOI] [PubMed] [Google Scholar]
- 51.Beard JL, Felt B, Schallert T, Burhans M, Connor JR, Georgieff MK. Moderate iron deficiency in infancy: biology and behavior in young rats. Behav Brain Res. 2006;170:224–232. doi: 10.1016/j.bbr.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 52.Felt BT, Beard JL, Schallert T, Shao J, Aldridge JW, Connor JR, Georgieff MK, Lozoff B. Persistent neurochemical and behavioral abnormalities in adulthood despite early iron supplementation for perinatal iron deficiency anemia in rats. Behav Brain Res. 2006;171:261–270. doi: 10.1016/j.bbr.2006.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pinero D, Jones B, Beard JL. Variations in dietary iron alter behavior in developing rats. J Nutr. 2001;131:311–318. doi: 10.1093/jn/131.2.311. [DOI] [PubMed] [Google Scholar]
- 54.Wood JJ, McLeod BD, Sigman M, Hwang W, Chu BC. Parenting and childhood anxiety: theory, empirical findings, and future directions. J Child Psychol Psychiatry. 2003;44:134–151. doi: 10.1111/1469-7610.00106. [DOI] [PubMed] [Google Scholar]
- 55.Rapee RM. Potential role of childrearing practices in the development of anxiety and depression. Clin Psychol Rev. 1997;17:47–67. doi: 10.1016/s0272-7358(96)00040-2. [DOI] [PubMed] [Google Scholar]
- 56.Bowlby J. Attachment and loss, vol. 1. attachment. London: Hogarth; 1982. [Google Scholar]
- 57.Ainsworth MD. A consideration of social referencing in the context of attachment theory and research. In: Feinman S, editor. Social referencing and the social construction of reality in infancy. New York: Plenum Press; 1992. pp. 349–370. [Google Scholar]