Abstract
Early studies that used parasite-infected interleukin-4 (IL-4) reporter animals led us to identify basophils as the primary source of IL-4 and hence propose the hypothesis that basophils trigger the development of antigen-specific T helper type 2 (Th2) immune responses in vivo. These findings appeared to resolve a long-standing puzzle underlying Th2 immunity, that is, ‘what is the source of the initial IL-4 necessary for CD4 T-cell differentiation into Th2 effector cells?’. However, results from extensive investigations of the contribution of basophils to Th2 immunity unveiled some controversial data that cast doubt on the initial hypothesis. In this review, the consensus and the controversy regarding the roles of basophils in infection and immunity, as well as outstanding questions for the future, are discussed.
Keywords: antibody responses; basophils; mast cells, CD4; helper T cells (Th cells)
Introduction
Basophils are the least abundant leucocytes primarily found in the circulation. They comprise only a small percentage (∼ 0·5%) of circulating blood cells under steady-state conditions, but rapidly expand in the bone marrow in response to inflammatory signals and are mobilized to the blood, spleen, lung and liver. They are generated from the granulocyte–monocyte progenitors in the bone marrow and populate the periphery as fully mature cells.1 The lifespan of basophils is short; recently estimated to be in the range of 1–2 days.2 For many years basophils have been a somewhat enigmatic immune cell type and questions regarding their role in protective immunity as well as the specific pathogens or insults that elicit basophil responses are not fully answered.3,4 In addition to their scarcity and short lifespan, studies of basophils have been hindered by the lack of information about how basophils can be distinguished both phenotypically and functionally from the developmentally related mast cells. Both mast cells and basophils express the high-affinity receptor for IgE and can release a similar spectrum of mediators upon IgE cross-linking. A panel of markers that define basophils (e.g. ckit−FcεRI+, CD11b+, IL-3Rhi, etc.) have now been identified that can distinguish these cell lineages and the list continues to expand.5,6 The recent development of basophil-depleting monoclonal antibodies7–9 and transgenic mouse strains that are deficient in basophils10–12 have enabled us to further explore the relationship between basophils and host immunity.
A major breakthrough in the understanding of basophil effector functions came from studies using interleukin-4 (IL-4) -reporter animals, and led to the discovery that basophils are a primary source of IL-4 in vivo.3,13 It was therefore hypothesized that basophils direct the development of robust IL-4-producing CD4 T cells. It is well accepted that upon initial antigen encounter, naive CD4 T-cell differentiation into IL-4-producing T helper type 2 (Th2) effector cells occurs with the help of IL-4 present in the microenvironment.14 The initial source of IL-4 remained elusive for many years. However, unlike CD4 T cells, which must undergo rounds of differentiation to express significant IL-4,15 basophils immediately secrete IL-4 following activation, and this is readily achieved even in immature basophil precursor cells.16
Immunomodulatory functions of basophils
Because T helper cell differentiation takes place in the lymphoid tissues, a key question was how could circulating basophils affect Th2 differentiation? Recent data showing that basophils are recruited into the immune-active draining lymph nodes and locate close to antigen-activated naive CD4 T cells offers a likely answer.9 The recruitment of basophils occurring between 3 and 4 days after immunization/infection is transient, and is observed with similar kinetics in several Th2 immunity models. These include immunization with protease antigen such as papain9 or house dust mite antigen,17 non-viable parasite injection,18,19 and infection with live parasites such as Nippostrongylus brasiliensis,2,3,19Schistosoma mansonii18,20 and Trichuris muris.20 Accumulation and activation of basophils at sites of infection by parasites such as N. brasiliensis is well documented,3,10,13 Importantly, IL-3 produced by activated parasite-specific CD4 T cells plays a key role in the timing of basophil arrival and the numbers of basophils recruited to the sites.21 A cellular mechanism underlying IL-3-mediated basophil recruitment is not well understood, although the targets of IL-3 are of bone marrow-derived cell origin.22
Subsequent reports from three independent studies that basophils can function as professional antigen-presenting cells further support the hypothesis that basophils are critical for the induction of Th2 immunity.23–26 Immunization of mice with ovalbumin protein antigen together with papain results in the induction of ovalbumin-specific IL-4-producing CD4 T cells. However, injection of the basophil-depleting anti-FcεRI monoclonal antibody, MAR-1, abolishes this response.27 Basophils can capture antigen–IgE complexes leading to the differentiation of antigen-specific naive CD4 T cells into Th2 type cells.28 In the context of helminth infection, dendritic cell depletion does not affect parasite-specific Th2 immunity; instead, MAR-1-mediated basophil depletion significantly impairs Th2 cytokine responses as well as parasite expulsion.20 Therefore, these findings strongly suggest that Th2 immunity arises with the help of specialized antigen-presenting basophils and the basophil-derived cytokine IL-4.
However, this conclusion was recently challenged by several contradictory reports demonstrating that basophils are dispensable for Th2 immunity. Using CD11c-DTR transgenic mice in which dendritic cells can be selectively ablated by injecting diphtheria toxin (DT), Phythian-Adams et al.18 show that dendritic cell depletion in mice is sufficient to disrupt Th2 immunity induced by Schistosoma egg antigen challenge or live Schistosoma infection, despite efficient recruitment of basophils to the lymph nodes. In contrast to earlier studies, they demonstrate that depleting basophils has no effect on the Th2 induction. In a separate study, Hammad et al. identified a novel ‘inflammatory’ DC subset that also expresses FcεRI.17 Upon intranasal challenge of mice with protease allergen, house dust mite antigen, TLR4/MyD88-dependent recruitment of basophils as well as FcεRI-expressing dendritic cells into the draining lymph node tissues was observed. However, it was found that basophils did not take up inhaled antigen, and most importantly did not express molecules involved in antigen presentation nor were they able to activate T cells.17 Instead, a population of FcεRI-expressing inflammatory dendritic cells are necessary and sufficient to induce Th2 immunity and all the features of asthmatic inflammation.17
Novel mouse models to examine basophil functions in vivo
Although the contradictory results may be attributed to the different animal models used, some of these studies generated opposing results using an identical experimental system (e.g. immunization with ovalbumin plus papain).17,27,29 The recent development of novel mouse models that allows selective basophil ablation has begun to resolve some of these controversies and unveil novel functions of basophils. Three independent conditional basophil-deficient mice have been established so far, and studies with these mice have yielded similar conclusions regarding the contribution of basophils to Th2-dependent immune responses. Ohnmacht et al.12 generated Mcpt8Cre mice that express the Cre recombinase under the control of the mast cell protease 8 (mcpt8) gene, which is expressed by basophil lineage cells. Unexpectedly, more than 90% of basophils, but not other cells, were spontaneously depleted in these mice. Using these Mcpt8Cre mice, it was shown that active systemic anaphylaxis, passive systemic IgE-mediated anaphylaxis, and passive IgG1-mediated anaphylaxis were normally induced in these mice, although IgE-mediated chronic allergic skin inflammation was attenuated indicating that basophils are essential in this disease model.12 Notably, papain-induced Th2 differentiation, ovalbumin/alum-induced allergic lung inflammation, and the primary immune response after N. brasiliensis infection were also normal in Mcpt8Cre mice.12 Interestingly, N. brasiliensis expulsion following secondary infection was significantly compromised in these mice, suggesting a protective role for basophils.12
Locksley and colleagues took a similar approach to generate basophil-deficient animals by replacing the mcpt8 gene with a YFP-IRES-Cre cassette (referred as basoph8 mice).10 Highly efficient depletion of basophils was achieved upon crossing the basoph8 mice with the Rosa-DTα mice. Similar to the Mcpt8Cre mice, immunization of basoph8 × Rosa-DTα mice with Schistosoma egg antigen or papain-induced wild-type level IL-4-producing CD4 T-cell responses, indicating a dispensable role of basophils during these responses.10 In contrast to earlier studies, however, intravital image analysis of YFP-positive basophils in the draining lymph node of the basoph8 mice did not find evidence of interaction between antigen-specific CD4 T cells and basophils. Instead, prolonged serial interaction between T cells and basophils was noted in the lung tissues, although the biological significance underlying this interaction remains to be elucidated. Interestingly, this study reported somewhat different results from studies using the Mcpt8Cre mice. Mice infected both primarily and secondarily with N. brasiliensis did not display any defects in clearing parasites.10 Consistent with this observation, mice deficient in IL-3 or IL-3Rβ developed normal Th2 immune responses following N. brasiliensis infection, despite the fact that basophil lymph node recruitment was completely abolished.22 Interestingly, targeted deletion of IL-4 and IL-13 in either CD4 T cells or basophils had a minimal effect on parasite clearance. However, a non-redundant role for basophils in moderating parasite burden could be discerned in situations where IL-4/IL-13 deletion occurred in both basophils and CD4 T cells, where higher N. brasiliensis intestinal worm burdens were observed.10 This would argue for a potentially supportive role for basophils in anti-parasite immune responses through their activation by immune serum and production of cytokines.10 This view is supported by a recent study demonstrating that the increased susceptibility of juvenile animals to helminth infection could be explained in part by the finding that basophils in juvenile animals are inherently less functional than basophils in adult mice.30
The results of early observational studies, based largely in guinea pigs, suggested that basophils are involved in mediating immunity against ectoparasites such as ticks.31–34 These studies were initially prompted by the finding of increased basophil numbers at the site of tick feeding and the demonstration that histamine antagonists can ablate immunity to ticks. It was subsequently shown that c-kitW/Wv mast-cell-deficient mice are able to develop protective immunity to ticks similar to wild-type mice, thereby excluding a role for mast cells and, by default, supporting a role for basophils in immunity against tick infection.31 Basophils may play a more predominant role in conferring immunity against repeated tick infestation as shown in a recent study using the basophil-deficient Mcpt8DTR mice, where DT receptor is transgenically expressed on basophils and DT injection results in a transient depletion of basophils.11 While wild-type mice are able to restrict the growth and development of ticks upon repeated tick infestation, the absence of basophils in DT-treated Mcpt8DTR mice impairs this response. Notably, the infiltration of eosinophils and neutrophils at tick feeding sites remains unaffected in these mice, arguing for a key non-redundant role for basophils for immunity against this type of ectoparasite.11 Future studies using other skin-localized pathogens should determine whether protective basophil-mediated contribution can be more generalized to skin immunity.
Pathways involved in basophil development
Although regulatory roles of basophils have been extensively studied, there is still much to be learned about the developmental programmes that give rise to basophils. Most studies have relied on the use of in vitro differentiation assays using either whole bone marrow or isolated bone marrow precursor cells cultured with IL-3, which exerts effects at several stages of stem cell and basophil development.35,36 Because of phenotypic and functional similarities between basophils and mast cells, it is not surprising that the molecular pathways regulating lineage commitment and differentiation of these cells during normal haematopoiesis are interconnected.37 Basophils and mast cells can be derived from the multipotent, lineage-restricted granulocyte–monocyte progenitor (IL-7Rα− Lin− Sca-1− c-Kit+ CD34+ FcγRII/IIIhi β7lo) in the bone marrow where they differentiate into either c-kit− FcεRI+ CD11b+ basophil precursors (BaP) or c-kithi FcεRI+ CD11b− mast cell precursors (MCp).37 A common bipotent basophil–mast cell precursor (BMCP: Lin− c-Kit+ FcεRII/IIIhiβ7hi), which is thought to arise from granulocyte–monocyte progenitors in the bone marrow, was recently identified in the spleen.38 This precursor displays high expression of the intestinal-homing integrin, α4β7, gives rise exclusively to BaP and MCp in culture and is able to reconstitute intestinal MCp when transferred to mast-cell-deficient mice. Whether these splenic BMCP are also the source of cells that are precursors to the rapidly expanded and mobilized population of basophils in infection settings remains to be determined.
Haematopoietic cell lineage decisions are facilitated by cell–cell interactions through molecules such as notch–notch ligands as well as by the growth factors that elicit a complex interplay of transcription factors in the common precursors.39,40 The precise relationship of the cell–cell interactions, growth factor signals and transcription factors is still unknown, but the relative level as well as the timing of transcription factor expression appear to be critical in this regard.40 There is substantial evidence that CCAAT-enhancer-binding protein α (C/EBPα) is a key switch factor in basophil development and that it acts in an antagonistic as well as cooperative fashion with GATA-2 to drive lineage choice.37,38 Granulocyte–monocyte progenitors constitutively express C/EBPα and basophil differentiation is accompanied by increased expression of C/EBPα coupled with a concomitant reduction in GATA-2.37,40 Co-expression of GATA-2 and C/EBPα in granulocyte–monocyte progenitors promotes eosinophil development, while GATA-2 expression in the absence of C/EBPα generates BMCP.40 It was shown that enforced expression of C/EBPα in BMCP also results in exclusive differentiation into basophils and that conditional deletion of C/EBPα supports differentiation of BMCP exclusively into mast cells. Furthermore, ectopic expression of C/EBPα in MCp reprogrammes them into basophils.38
Recent studies have implicated Ikaros in basophil–mast cell lineage decisions. Ikaros is a family of zinc finger-containing transcription factors best studied in lymphoid cell development.41 Ikaros−/− mice have significantly reduced numbers of intestinal mast cells. The in vitro differentiation of mast cells from Ikaros−/− bone marrow cells is also profoundly altered and results in skewing towards a basophil-like population. Consistent with this predominance of basophils observed in vitro, Ikaros−/− mice exhibit profound basophilia in the absence of infection. These data indicate that Ikaros may act to promote mast cell lineage choice by suppressing C/EBPα expression and its absence results in a default to a basophil differentiation-dominated pathway (K.N. Rao et al., unpublished data).
Perspectives and outstanding questions
Although IL-3 is known to promote the differentiation of BaPs (and BMCPs) into basophils both in vitro and in vivo,2,10,42 it is also involved in multiple steps of basophil expansion and function Fig. 1. Interleukin-3 primes basophils to increase IL-4 secretion following stimulation,4,16,21,43 enhances basophil generation after parasite infection,3,13 and recruits circulating basophils into the lymphoid tissues.12,17,18,22 Nonetheless, the homeostatic maintenance of basal level basophils in vivo is not altered by the lack of IL-3.21,44,45 This paradox might be explained by a recent study from Artis and colleagues who identified the thymic stromal lymphopoietin (TSLP) as a key regulator of basophil haematopoiesis that acts independently of IL-3.46 Most strikingly, they also demonstrated that basophils elicited by either IL-3 or TSLP in vivo appear to be phenotypically and functionally distinct, suggesting that functionally heterogeneous basophils may exist in vivo and play unique roles in Th2 immunity and in other basophil-mediated responses.46 There are many questions related to these observations that need to be examined. For example, what circumstances favour the generation of functionally distinct basophils? What are the target cells of these distinct basophil responses? Finally, what are the underlying mechanisms by which IL-3 and TSLP confer multiple and distinct functions to basophils?
Figure 1.
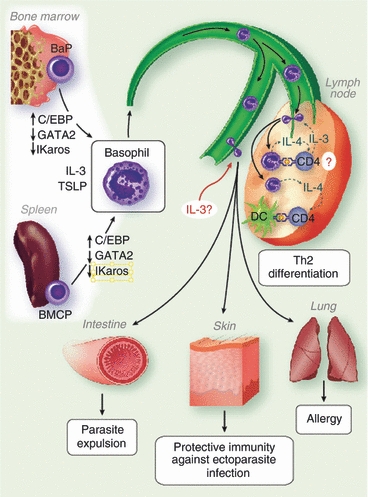
Pathways of basophil responses. Basophils are generated from the progenitors by coordinated expression of transcription factors as well as haematopoietic cytokines, interleukin-3 (IL-3) and thymic stromal lymphopoietin (TSLP). Mature basophils enter the circulation and mobilized into either lymphoid and non-lymphoid tissues. Recruited basophils are expected to modulate the induction of adaptive immunity or to carry out effector functions. BaP, basophil progenitor; BMCP, basophil–mast cell precursor; c/EBP, CCAAT-enhancer-binding protein; DC, dendritic cell; Th2, T helper type 2.
In addition to IL-3 and TSLP, there are many other factors that regulate basophil biology. These include positive regulators that enhance basophil generation and functions (immune complexes,28,47,48 IL-18,43,49 IL-25,50,51 IL-33,49,52,53 lipopolysaccharide,51,54 complement C5a,55,56 HIV gp12057) and negative regulators that inhibit basophil functions and expansion (interferon regulatory factor 2 [IRF2],58 lyn kinase,59 SH2 domain containing inositol phosphatase [SHIP]60,61). Interestingly, all positive regulators identified so far are soluble/secretory proteins, whereas all negative regulators are intracellular enzymes that influence basophil expansion and receptor-mediated activation, which result in marked basophilia. Mechanisms underlying the regulation by these factors also remain to be identified.
Perhaps the most exciting findings come from a recent study showing that adoptive transfer of basophils is sufficient to induce Th2 immunity in an environment where Th2 immunity is impaired.11,46 This suggests the possibility that basophil transfer could be used therapeutically to modulate immune responses mediated by pathological pro-inflammatory Th1/Th17 type cells. Indeed, parasite infection is currently being used to treat patients with inflammatory bowel disease.62,63 Whether basophils mediate the protection in this case is still unclear, but if they do then understanding how basophils modulate these responses will be an excellent question for future study.
Acknowledgments
Supported by National Institutes of Health grants AI080908 (B.M.) and AI088299 (M.A.B), Marjorie Barclay Trust (G.L.G.), and Health Research Council of New Zealand (G.L.G.)
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Falcone FH, Zillikens D, Gibbs BF. The 21st century renaissance of the basophil? Current insights into its role in allergic responses and innate immunity. Exp Dermatol. 2006;15:855–64. doi: 10.1111/j.1600-0625.2006.00477.x. [DOI] [PubMed] [Google Scholar]
- 2.Ohnmacht C, Voehringer D. Basophil effector function and homeostasis during helminth infection. Blood. 2009;113:2816–25. doi: 10.1182/blood-2008-05-154773. [DOI] [PubMed] [Google Scholar]
- 3.Min B, Prout M, Hu-Li J, et al. Basophils produce IL-4 and accumulate in tissues after infection with a Th2-inducing parasite. J Exp Med. 2004;200:507–17. doi: 10.1084/jem.20040590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ben-Sasson SZ, Le Gros G, Conrad DH, Finkelman FD, Paul WE. Cross-linking Fc receptors stimulate splenic non-B, non-T cells to secrete interleukin 4 and other lymphokines. Proc Natl Acad Sci U S A. 1990;87:1421–5. doi: 10.1073/pnas.87.4.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ugajin T, Kojima T, Mukai K, et al. Basophils preferentially express mouse Mast Cell Protease 11 among the mast cell tryptase family in contrast to mast cells. J Leukoc Biol. 2009;86:1417–25. doi: 10.1189/jlb.0609400. [DOI] [PubMed] [Google Scholar]
- 6.Min B. Basophils: what they ‘can do’ versus what they ‘actually do’. Nat Immunol. 2008;9:1333–9. doi: 10.1038/ni.f.217. [DOI] [PubMed] [Google Scholar]
- 7.Obata K, Mukai K, Tsujimura Y, et al. Basophils are essential initiators of a novel type of chronic allergic inflammation. Blood. 2007;110:913–20. doi: 10.1182/blood-2007-01-068718. [DOI] [PubMed] [Google Scholar]
- 8.Denzel A, Maus UA, Rodriguez Gomez M, et al. Basophils enhance immunological memory responses. Nat Immunol. 2008;9:733–42. doi: 10.1038/ni.1621. [DOI] [PubMed] [Google Scholar]
- 9.Sokol CL, Barton GM, Farr AG, Medzhitov R. A mechanism for the initiation of allergen-induced T helper type 2 responses. Nat Immunol. 2008;9:310–8. doi: 10.1038/ni1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sullivan BM, Liang HE, Bando JK, et al. Genetic analysis of basophil function in vivo. Nat Immunol. 2011;12:527–35. doi: 10.1038/ni.2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wada T, Ishiwata K, Koseki H, et al. Selective ablation of basophils in mice reveals their nonredundant role in acquired immunity against ticks. J Clin Invest. 2010;120:2867–75. doi: 10.1172/JCI42680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ohnmacht C, Schwartz C, Panzer M, Schiedewitz I, Naumann R, Voehringer D. Basophils orchestrate chronic allergic dermatitis and protective immunity against helminths. Immunity. 2010;33:364–74. doi: 10.1016/j.immuni.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 13.Voehringer D, Shinkai K, Locksley RM. Type 2 immunity reflects orchestrated recruitment of cells committed to IL-4 production. Immunity. 2004;20:267–77. doi: 10.1016/s1074-7613(04)00026-3. [DOI] [PubMed] [Google Scholar]
- 14.Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations (*) Annu Rev Immunol. 2010;28:445–89. doi: 10.1146/annurev-immunol-030409-101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bird JJ, Brown DR, Mullen AC, et al. Helper T cell differentiation is controlled by the cell cycle. Immunity. 1998;9:229–37. doi: 10.1016/s1074-7613(00)80605-6. [DOI] [PubMed] [Google Scholar]
- 16.Le Gros G, Ben-Sasson SZ, Conrad DH, et al. IL-3 promotes production of IL-4 by splenic non-B, non-T cells in response to Fc receptor cross-linkage. J Immunol. 1990;145:2500–6. [PubMed] [Google Scholar]
- 17.Hammad H, Plantinga M, Deswarte K, et al. Inflammatory dendritic cells – not basophils – are necessary and sufficient for induction of Th2 immunity to inhaled house dust mite allergen. J Exp Med. 2010;207:2097–111. doi: 10.1084/jem.20101563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Phythian-Adams AT, Cook PC, Lundie RJ, et al. CD11c depletion severely disrupts Th2 induction and development in vivo. J Exp Med. 2010;207:2089–96. doi: 10.1084/jem.20100734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Panhuys N, Prout M, Forbes E, Min B, Paul WE, Le Gros G. Basophils are the major producers of IL-4 during primary helminth infection. J Immunol. 2011;186:2719–28. doi: 10.4049/jimmunol.1000940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perrigoue JG, Saenz SA, Siracusa MC, et al. MHC class II-dependent basophil-CD4+ T cell interactions promote TH2 cytokine-dependent immunity. Nat Immunol. 2009;10:697–705. doi: 10.1038/ni.1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shen T, Kim S, Do JS, et al. T cell-derived IL-3 plays key role in parasite infection-induced basophil production but is dispensable for in vivo basophil survival. Int Immunol. 2008;20:1201–9. doi: 10.1093/intimm/dxn077. [DOI] [PubMed] [Google Scholar]
- 22.Kim S, Prout M, Ramshaw H, Lopez AF, LeGros G, Min B. Cutting edge: basophils are transiently recruited into the draining lymph nodes during helminth infection via IL-3, but infection-induced Th2 immunity can develop without basophil lymph node recruitment or IL-3. J Immunol. 2010;184:1143–7. doi: 10.4049/jimmunol.0902447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakanishi K. Basophils as APC in Th2 response in allergic inflammation and parasite infection. Curr Opin Immunol. 2010;22:814–20. doi: 10.1016/j.coi.2010.10.018. [DOI] [PubMed] [Google Scholar]
- 24.Siracusa MC, Perrigoue JG, Comeau MR, Artis D. New paradigms in basophil development, regulation and function. Immunol Cell Biol. 2010;88:275–84. doi: 10.1038/icb.2010.1. [DOI] [PubMed] [Google Scholar]
- 25.Sokol CL, Medzhitov R. Role of basophils in the initiation of Th2 responses. Curr Opin Immunol. 2010;22:73–7. doi: 10.1016/j.coi.2010.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wynn TA. Basophils trump dendritic cells as APCs for TH2 responses. Nat Immunol. 2009;10:679–81. doi: 10.1038/ni0709-679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sokol CL, Chu NQ, Yu S, Nish SA, Laufer TM, Medzhitov R. Basophils function as antigen-presenting cells for an allergen-induced T helper type 2 response. Nat Immunol. 2009;10:713–20. doi: 10.1038/ni.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yoshimoto T, Yasuda K, Tanaka H, et al. Basophils contribute to TH2-IgE responses in vivo via IL-4 production and presentation of peptide-MHC class II complexes to CD4+ T cells. Nat Immunol. 2009;10:706–12. doi: 10.1038/ni.1737. [DOI] [PubMed] [Google Scholar]
- 29.Ohnmacht C, Voehringer D. Basophils protect against reinfection with hookworms independently of mast cells and memory Th2 cells. J Immunol. 2010;184:344–50. doi: 10.4049/jimmunol.0901841. [DOI] [PubMed] [Google Scholar]
- 30.Nel HJ, Hams E, Saunders SP, et al. Impaired basophil induction leads to an age-dependent innate defect in type 2 immunity during helminth infection in mice. J Immunol. 2011;186:4631–9. doi: 10.4049/jimmunol.1002995. [DOI] [PubMed] [Google Scholar]
- 31.Steeves EB, Allen JR. Tick resistance in mast cell-deficient mice: histological studies. Int J Parasitol. 1991;21:265–8. doi: 10.1016/0020-7519(91)90020-8. [DOI] [PubMed] [Google Scholar]
- 32.Brown SJ, Barker RW, Askenase PW. Bovine resistance to Amblyomma americanum ticks: an acquired immune response characterized by cutaneous basophil infiltrates. Vet Parasitol. 1984;16:147–65. doi: 10.1016/0304-4017(84)90016-5. [DOI] [PubMed] [Google Scholar]
- 33.Brown SJ, Galli SJ, Gleich GJ, Askenase PW. Ablation of immunity to Amblyomma americanum by anti-basophil serum: cooperation between basophils and eosinophils in expression of immunity to ectoparasites (ticks) in guinea pigs. J Immunol. 1982;129:790–6. [PubMed] [Google Scholar]
- 34.Brown SJ, Graziano FM, Askenase PW. Immune serum transfer of cutaneous basophil-associated resistance to ticks: mediation by 7SIgG1 antibodies. J Immunol. 1982;129:2407–12. [PubMed] [Google Scholar]
- 35.Saito H, Hatake K, Dvorak AM, et al. Selective differentiation and proliferation of hematopoietic cells induced by recombinant human interleukins. Proc Natl Acad Sci U S A. 1988;85:2288–92. doi: 10.1073/pnas.85.7.2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Valent P, Schmidt G, Besemer J, et al. Interleukin-3 is a differentiation factor for human basophils. Blood. 1989;73:1763–9. [PubMed] [Google Scholar]
- 37.Arinobu Y, Iwasaki H, Akashi K. Origin of basophils and mast cells. Allergol Int. 2009;58:21–8. doi: 10.2332/allergolint.08-RAI-0067. [DOI] [PubMed] [Google Scholar]
- 38.Arinobu Y, Iwasaki H, Gurish MF, et al. Developmental checkpoints of the basophil/mast cell lineages in adult murine hematopoiesis. Proc Natl Acad Sci U S A. 2005;102:18105–10. doi: 10.1073/pnas.0509148102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Radtke F, Wilson A, Ernst B, MacDonald HR. The role of Notch signaling during hematopoietic lineage commitment. Immunol Rev. 2002;187:65–74. doi: 10.1034/j.1600-065x.2002.18706.x. [DOI] [PubMed] [Google Scholar]
- 40.Iwasaki H, Mizuno S, Arinobu Y, et al. The order of expression of transcription factors directs hierarchical specification of hematopoietic lineages. Genes Dev. 2006;20:3010–21. doi: 10.1101/gad.1493506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.John LB, Ward AC. The Ikaros gene family: transcriptional regulators of hematopoiesis and immunity. Mol Immunol. 2011;48:1272–8. doi: 10.1016/j.molimm.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 42.Ohmori K, Luo Y, Jia Y, et al. IL-3 induces basophil expansion in vivo by directing granulocyte-monocyte progenitors to differentiate into basophil lineage-restricted progenitors in the bone marrow and by increasing the number of basophil/mast cell progenitors in the spleen. J Immunol. 2009;182:2835–41. doi: 10.4049/jimmunol.0802870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yoshimoto T, Tsutsui H, Tominaga K, et al. IL-18, although antiallergic when administered with IL-12, stimulates IL-4 and histamine release by basophils. Proc Natl Acad Sci U S A. 1999;96:13962–6. doi: 10.1073/pnas.96.24.13962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lantz CS, Boesiger J, Song CH, et al. Role for interleukin-3 in mast-cell and basophil development and in immunity to parasites. Nature. 1998;392:90–3. doi: 10.1038/32190. [DOI] [PubMed] [Google Scholar]
- 45.Lantz CS, Min B, Tsai M, Chatterjea D, Dranoff G, Galli SJ. IL-3 is required for increases in blood basophils in nematode infection in mice and can enhance IgE-dependent IL-4 production by basophils in vitro. Lab Invest. 2008;88:1134–42. doi: 10.1038/labinvest.2008.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Siracusa MC, Saenz SA, Hill DA, et al. TSLP promotes interleukin-3-independent basophil haematopoiesis and type 2 inflammation. Nature. 2011;477:229–33. doi: 10.1038/nature10329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Charles N, Hardwick D, Daugas E, Illei GG, Rivera J. Basophils and the T helper 2 environment can promote the development of lupus nephritis. Nat Med. 2010;16:701–7. doi: 10.1038/nm.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Anthony RM, Kobayashi T, Wermeling F, Ravetch JV. Intravenous gammaglobulin suppresses inflammation through a novel TH2 pathway. Nature. 2011;475:110–3. doi: 10.1038/nature10134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kroeger KM, Sullivan BM, Locksley RM. IL-18 and IL-33 elicit Th2 cytokines from basophils via a MyD88- and p38alpha-dependent pathway. J Leukoc Biol. 2009;86:769–78. doi: 10.1189/jlb.0708452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang H, Mobini R, Fang Y, et al. Allergen challenge of peripheral blood mononuclear cells from patients with seasonal allergic rhinitis increases IL-17RB, which regulates basophil apoptosis and degranulation. Clin Exp Allergy. 2010;40:1194–202. doi: 10.1111/j.1365-2222.2010.03542.x. [DOI] [PubMed] [Google Scholar]
- 51.Yoshimoto T, Nakanishi K. Roles of IL-18 in basophils and mast cells. Allergol Int. 2006;55:105–13. doi: 10.2332/allergolint.55.105. [DOI] [PubMed] [Google Scholar]
- 52.Pecaric-Petkovic T, Didichenko SA, Kaempfer S, Spiegl N, Dahinden CA. Human basophils and eosinophils are the direct target leukocytes of the novel IL-1 family member IL-33. Blood. 2009;113:1526–34. doi: 10.1182/blood-2008-05-157818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Suzukawa M, Iikura M, Koketsu R, et al. An IL-1 cytokine member, IL-33, induces human basophil activation via its ST2 receptor. J Immunol. 2008;181:5981–9. doi: 10.4049/jimmunol.181.9.5981. [DOI] [PubMed] [Google Scholar]
- 54.Bieneman AP, Chichester KL, Chen YH, Schroeder JT. Toll-like receptor 2 ligands activate human basophils for both IgE-dependent and IgE-independent secretion. J Allergy Clin Immunol. 2005;115:295–301. doi: 10.1016/j.jaci.2004.10.018. [DOI] [PubMed] [Google Scholar]
- 55.Eglite S, Pluss K, Dahinden CA. Requirements for C5a receptor-mediated IL-4 and IL-13 production and leukotriene C4 generation in human basophils. J Immunol. 2000;165:2183–9. doi: 10.4049/jimmunol.165.4.2183. [DOI] [PubMed] [Google Scholar]
- 56.Ochensberger B, Rihs S, Brunner T, Dahinden CA. IgE-independent interleukin-4 expression and induction of a late phase of leukotriene C4 formation in human blood basophils. Blood. 1995;86:4039–49. [PubMed] [Google Scholar]
- 57.Patella V, Florio G, Petraroli A, Marone G. HIV-1 gp120 induces IL-4 and IL-13 release from human Fc epsilon RI+ cells through interaction with the VH3 region of IgE. J Immunol. 2000;164:589–95. doi: 10.4049/jimmunol.164.2.589. [DOI] [PubMed] [Google Scholar]
- 58.Hida S, Tadachi M, Saito T, Taki S. Negative control of basophil expansion by IRF-2 critical for the regulation of Th1/Th2 balance. Blood. 2005;106:2011–7. doi: 10.1182/blood-2005-04-1344. [DOI] [PubMed] [Google Scholar]
- 59.Charles N, Watford WT, Ramos HL, et al. Lyn kinase controls basophil GATA-3 transcription factor expression and induction of Th2 cell differentiation. Immunity. 2009;30:533–43. doi: 10.1016/j.immuni.2009.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kuroda E, Ho V, Ruschmann J, et al. SHIP represses the generation of IL-3-induced M2 macrophages by inhibiting IL-4 production from basophils. J Immunol. 2009;183:3652–60. doi: 10.4049/jimmunol.0900864. [DOI] [PubMed] [Google Scholar]
- 61.Kuroda E, Antignano F, Ho VW, et al. SHIP represses Th2 skewing by inhibiting IL-4 production from basophils. J Immunol. 2011;186:323–32. doi: 10.4049/jimmunol.1002778. [DOI] [PubMed] [Google Scholar]
- 62.Wang LJ, Cao Y, Shi HN. Helminth infections and intestinal inflammation. World J Gastroenterol. 2008;14:5125–32. doi: 10.3748/wjg.14.5125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Weinstock JV, Elliott DE. Helminths and the IBD hygiene hypothesis. Inflamm Bowel Dis. 2009;15:128–33. doi: 10.1002/ibd.20633. [DOI] [PubMed] [Google Scholar]


