Abstract
An efficacious tuberculosis (TB) vaccine will probably need to induce both CD4 and CD8 T-cell responses specific to a protective Mycobacterium tuberculosis antigen(s). To achieve this broad cellular immune response we tested a heterologous DNA/protein combination vaccine strategy. We used a purified recombinant protein preparation of a unique M. tuberculosis antigen (rMT1721) found in the urine of TB patients, an optimized plasmid DNA expressing this protein (DNA-MT1721), and a Toll-like receptor 4 agonist adjuvant. We found that priming mice with DNA-MT1721 and subsequently boosting with rMT1721 elicited high titres of specific IgG1 and IgG2a antibodies as well as high magnitude and polyfunctional CD4+ T-cell responses. However, no detectable CD8+ T-cell response was observed using this regimen of immunization. In contrast, both CD4+ and CD8+ T-cell responses were detected after a prime/boost vaccination regimen using rMT1721 as the priming antigen and DNA-MT1721 as the boosting immunogen. These findings support the exploration of heterologous DNA/protein immunization strategies in vaccine development against TB and possibly other infectious diseases.
Keywords: Mycobacterium tuberculosis, prime/boost, vaccine
Introduction
Tuberculosis (TB) remains a major infectious cause of morbidity and mortality worldwide.1 Incidence of the disease remains high and is increasing in many parts of the world partly because of its association with HIV infection.2 Despite the availability of specific anti-TB drugs and the worldwide administration of the bacille Calmette–Guérin (BCG) vaccine, it is estimated that one-third of the world’s population is infected with Mycobacterium tuberculosis,3 with 8 million newly diagnosed cases of TB and up to 2·5 million deaths occurring each year. BCG, the only commercially available vaccine, only protects children from disseminated TB and unfortunately does not prevent pulmonary disease,4 the most common and contagious form of TB. Moreover, the efficacy of BCG is limited and highly variable in geographically distinct populations.4 Unequivocally, a better vaccination strategy is needed to control TB infections worldwide.
The correlates of protective immunity against TB are not entirely clear. Based on human studies and those in animal model systems, both CD4 and CD8 T cells and certain effector cytokines will most likely be required to contain the M. tuberculosis pathogen. Production of interferon-γ (IFN-γ), primarily by T helper type 1 (Th1) CD4+ T cells, appears to be essential for M. tuberculosis infection and disease prevention,5–7 and expression of tumour necrosis factor-α (TNF-α) and its corresponding p55 receptor is associated with TB resistance.8
The MHC class I restricted T cells apparently also participate in resistance to M. tuberculosis.9,10 However, mice lacking perforin and Fas/CD95 receptor, two important mediators of CD8+ T-cell cytotoxicity, are able to control the infection with either Mycobacterium bovis BCG or M. tuberculosis,11 suggesting that cytolysis of infected target cells may not be involved in the resistance conferred by these cells. Alternatively, it has been proposed that CD8+ cells augment resistance to TB by providing an additional source of IFN-γ.
The identification of relevant vaccine antigens is key in TB vaccine development. A number of vaccine candidate antigens are being pursued and the primary approaches to their discoveries have used the immune response of patients or of resistant hosts (e.g. healthy purified protein derivatives of tuberculin-positive individuals) as the readout of the antigen discovery to select the candidate molecules. Our novel approach is to identify M. tuberculosis antigens in the urine of patients with active pulmonary TB. We have now identified several antigens in the urine of humans with TB using reversed-phase HPLC and mass spectrometry.12,13 One such antigen, MT1721, is a highly interesting vaccine candidate molecule in that its gene and coded protein are unique to the M. tuberculosis complex organisms and is apparently absent in other representative members of the Mycobacterium genus.12 In addition, the rMT1721 is strongly recognized by peripheral blood mononuclear cells from healthy purified protein derivative of tuberculin-positive individuals and to a lesser extent from patients with TB.
In this study, we evaluated a heterologous DNA/protein prime/boost immunization protocol as a potential vaccination strategy against TB. We employed a novel combination using an optimized DNA vaccine vector14 and recombinant protein adjuvanted with a synthetic Toll-like receptor 4 agonist.15 The DNA vaccine vector was used to express a novel and unique TB antigen, MT1721. The results show that the heterologous prime/boost immunization protocol elicited robust CD4+ and CD8+ T-cell responses to rMT1721 antigen, and therefore warrant further assessment of this immunization regimen in protection experiments against TB.
Materials and methods
Generation of recombinant DNA and protein
The VRC8400 expression plasmid (kindly donated by Dr Gary Nabel, National Institutes of Health) was constructed as follows.14 The parental 1012 DNA vaccine plasmid contains the human cytomegalovirus (CMV) immediate early (IE) enhancer, promoter, and intron. To construct the CMV/R regulatory element, a SacII/HpaI fragment of the 1012 plasmid containing the majority of the CMV IE intron was replaced with a 227-bp EcoRV/HpaI fragment of the HTLV-1 R region.16 The resulting VRC8400 plasmid therefore contains the human CMV IE enhancer/promoter, followed by the HTLV-1 R region and a 123-bp fragment of CMV IE 3 intron. The splice donor in the R region and the splice acceptor in the CMV IE 3 intron serve as the pair of splicing signals. The MT1721 gene was cloned from M. tuberculosis genomic DNA by PCR and subsequently inserted into the VRC8400 plasmid.
Mice
Female C57BL/6 mice were purchased from Charles River Laboratories (Wilmington, MA) and kept under specific pathogen-free conditions. At the time of experiments mice were 8–12 weeks of age. All experiments were carried out under the guidelines of the Institutional Animal Care and Use Committee at the Forsyth Institute.
M. tuberculosis antigens and adjuvant
The native M. tuberculosis protein used here, named MT1721, was first identified in the urine of patients with TB by mass spectrometry as described previously in detail.12 Characteristically, the MoA-related protein coded for by the MT_1721 gene is present only in the members of the M. tuberculosis complex. The gene was initially subcloned into the pET-14b expression vector (Novagen-EMD Chemicals, Gibbstown, NJ); it is now subcloned in the pET-29b plasmid vector (Qiagen, Valencia, CA), which showed an improved expression rate. Recombinant protein MT1721 (rMT1721) was expressed in BL21(DE3)pLysS Escherichia coli host (Invitrogen, Carlsbad, CA) and purified by ion-exchange chromatography. The adjuvant glucopyranosyl lipid A (GLA) purchased from the Infectious Disease Research Institute (Seattle, WA), contains a synthetic version of the monophosphoryl lipid A derivative of lipopolysaccharide obtained from Salmonella minnesota, and it is formulated as a stable oil-in-water emulsion. Culture filtrate antigen of M. tuberculosis H37Rv was prepared at and obtained from Colorado State University through the National Institutes of Health/National Institute of Allergy and Infectious Diseases Tuberculosis Vaccine Testing and Research Materials contract HHSN266200400091c.
Western blot
Culture filtrate of M. tuberculosis H37Rv strain and purified rMT1721 were fractionated in 4–20% SDS–PAGE and transferred to PVDF membrane (Millipore, Medford, MA). Blotting was blocked with 1% BSA in Tris-buffered saline with 0·1% Tween-20 (TBS-T) and subsequently incubated with rabbit anti-rMT1721 overnight at 4°C. After several rinses with TBS-T, goat anti-rabbit IgG labelled with horseradish peroxidase (ThermoScientific Pierce, Rockford, IL) was added. After additional washings, bound conjugates were detected using an ECL enhanced chemiluminescence system (Amersham/GE Healthcare, Piscataway, NJ) and proteins were visualized by autoradiography (Kodak BioMax, Rochester, NY).
Experimental groups
Priming–boosting combinations using recombinant plasmid DNA carrying the gene encoding MT_1721 (henceforth known as DNA-MT1721) or rMT1721 protein in the context of GLA adjuvant were studied. Homologous or heterologous priming–boosting vaccinations consisted of a priming injection followed by two boosts, 4 weeks apart. Mice were inoculated intramuscularly with 50 μg DNA-MT1721 in each quadriceps muscle or subcutaneously with a solution containing 5 μg rMT1721 and 20 μg GLA adjuvant. Control mice were administered saline.
Antibody ELISA
IgG1 and IgG2a antibodies raised against rMT1721 were titrated by standard ELISA. High-binding 96-well microplates (Costar, Lowell, MA) were coated with purified rMT1721 (2 μg/ml) prepared in 0·2 m sodium carbonate/bicarbonate buffer (pH 9·6) and incubated overnight at 4°C. Wells were washed with PBS containing 0·05% Tween-20 (PBS-T) and blocked with 1% BSA in PBS-T for 2 hr. Serum samples were added at twofold serial dilutions in PBS-T containing 0·1% BSA and plates were incubated for 1 hr at room temperature. After another washing step, biotinylated rat anti-mouse IgG1 or IgG2a (2 μg/ml, BD Biosciences, San Diego, CA) was added and incubated for 1 hr. Streptavidin-horseradish peroxidase conjugate (BD Biosciences) was used at 1/2000 followed by addition of ready-to-use substrate solution containing tetramethylbenzidine (KPL, Gaithersburg, MD). Colour development was stopped using 1 m HCl. Optical density data were recorded as absorbance at 450 nm.
Interferon-γ detection
Twelve days after the last boost, spleens were excised and homogenized, and the cells were harvested using 70-μm cell strainers. Splenocyte suspensions were centrifuged over Histopaque (Sigma, St Louis, MO) and washed. Mononuclear cell suspensions were prepared in RPMI-1640 supplemented with 10% fetal bovine serum (Hyclone; Thermo Scientific, Rockford, IL), 100 μg/ml streptomycin, 100 U/ml penicillin, 25 mm HEPES, 2 mm l-glutamine, 0·05 mm 2-mercaptoethanol (all Sigma). Cell viability was estimated with Trypan blue 0·4% (Sigma); 2 × 105 cells were added to the wells of a 96-well flat-bottomed culture microplate (Costar). Cells were stimulated with 10 μg/ml rMT1721 for 72 hr, then supernatants were collected for IFN-γ assessment. Cells cultured in the presence of concanavalin A (5 μg/ml) or complete medium alone were included as controls. Cytokine concentration in the supernatants was measured using specific sandwich ELISA kit (R&D Systems, Minneapolis, MN).
Intracellular cytokine staining analysis
Splenocytes were cultured at 37°C in a 5% CO2 environment for 6 hr in the presence of RPMI-1640/10% fetal calf serum alone (unstimulated), or with 10 μg/ml rMT1721. All cultures contained Monensin (GolgiStop; BD Biosciences). The cultured cells were cell-surface stained with the following monoclonal antibodies purchased from BD Biosciences: anti-CD3-FITC (145-2C11), anti-CD4-allophycocyanin-Cychrome7 (GK1.5), anti-CD8α-perdinin chlorophyll protein-Cychrome 5·5 (53–6.7). After fixing with Cytofix/Cytoperm solution (BD Biosciences), cells were permeabilized and stained with anti-IFN-γ/allophycocyanin (XMG1.2), anti-TNF-α/phycoerythrin-Cychrome 7 (MP6-XT22), and anti-interleukin-2 (IL-2) -phycoerythrin (JES6-5H4). Labelled cells were fixed in 1% formaldehyde-PBS. Samples were collected on a BD LSRII flow cytometer (BD Biosciences) and analysed using flowjo software (Tree Star, Ashland, OR). Approximately 500 000–1 000 000 events were collected per sample. Doublets were excluded by forward scatter-area versus forward scatter-height. The CD4+ and CD8+ T cells were determined by their expression of CD3, CD4 or CD8. Functional analysis was performed by plotting the expression of each cytokine molecule against another, and a Boolean combination of single functional gates was generated using flowjo software (version 7·6·3; Tree Star). The frequency of cells producing IFN-γ, TNF-α and IL-2, either individually or in any combination, was determined using flowjo (Tree Star). All values used for analysis are background subtracted. Responses were considered positive when the percentage of total cytokine-producing cells was at least twice that of the background.
Statistical analysis
Student’s t-test was used to compare the cellular immune responses between groups of experimental animals. A P-value of < 0·05 was considered significant.
Results
Recognition of MT1721 as a native protein in M. tuberculosis
To assess the E. coli-expressed and purified rMT1721 (Fig. 1a) as a true M. tuberculosis protein, culture filtrate of M. tuberculosis with the recombinant protein were immunoblotted and then probed with polyclonal rabbit anti-rMT1721 antiserum (Fig. 1b). The results indicate that the polyclonal antiserum was able to recognize a protein in the culture filtrate of bacteria with a molecular weight similar to the recombinant protein, strongly suggesting the presence of the native MT1721 in the secreted antigen fraction of M. tuberculosis. Two extra bands of higher molecular weight (∼ 50 000 and 90 000) also reacted with the rabbit antiserum, which may represent polymerized/aggregate/conjugated forms of the native MT1721 protein. Alternatively, these bands were detected by non-specific interaction of the rabbit antiserum with other M. tuberculosis antigens.
Figure 1.
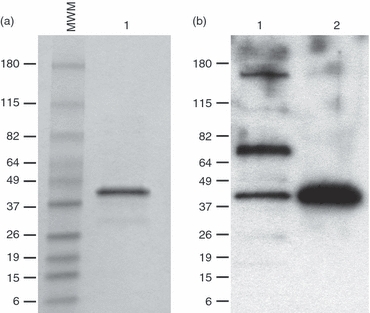
Identification of native MT1721 antigen in the culture filtrate of Mycobacterium tuberculosis. Purified rMT1721 was electrophoresed under reducing conditions in 4–20% gradient SDS–PAGE and stained with Coomassie Blue (a). In parallel, rMT1721 and culture filtrate samples were run in SDS–PAGE under similar conditions and transferred to a PVDF membrane followed by probing with rabbit anti-rMT1721 antiserum. Reactivity was detected with horseradish peroxidase-labelled goat anti-rabbit IgG and developed using the enhanced chemiluminescent reagent (b). Lane 1, culture filtrate; lane 2, purified rMT1721.
Heterologous prime/boost immunization induces a strong IgG2a antibody response to rMT721
To begin the characterization of the immune responses induced by heterologous prime/boost immunizations, mice were primed with either DNA-MT1721 and boosted with the recombinant protein, or vice versa. Both IgG1 and IgG2a antibody responses were evaluated by ELISA using specific anti-mouse isotype antibodies (Fig. 2). Immunization with only plasmid DNA containing the MT_1721 gene resulted in little or no detectable antibody response of either isotype. In contrast, a single immunization with rMT1721 mixed with the adjuvant GLA induced both IgG1 and IgG2a antibody responses. In contrast, heterologous prime/boost vaccination protocols clearly favoured an IgG2a response against rMT1721. Interestingly, priming the mice with rMT1721 followed by boosting with DNA-MT1721 resulted in a clearly more robust IgG2a response than that observed in mice primed with DNA-MT1721 followed by boosting with rMT1721. Because class switch to the IgG2a isotype is dependent on IFN-γ production these results suggest that a DNA prime/protein boost regimen of immunization is a potentially useful protocol for the generation of Th1-biased immune responses.
Figure 2.
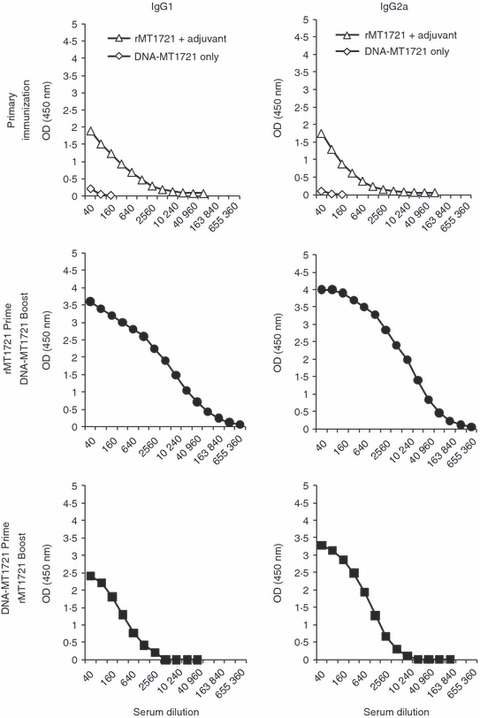
rMT1721-specific antibody response. At 12 days after the last boosting dose, each mouse was anaesthetized for eye bleeding. Serum was obtained and screened for IgG1 and IgG2a by ELISA. The data point at each dilution represents the average absorbance in sera obtained from 10 mice in each group.
Specific antigen stimulation of IFN-γ production following heterologous prime/boost immunization
The acquired cell-mediated immune response is considered the main arm of immunity in TB and IFN-γ is an essential mediator of resistance.5–7,17 Therefore, we next tested for antigen-stimulated IFN-γ production in mice following heterologous prime/boost immunization regimens. The results showed that priming mice with DNA-MT1721 and boosting with the rMT1721/adjuvant was highly effective in generating IFN-γ responses upon in vitro stimulation of spleen cells with rMT1721 (Fig. 3). Although to a lesser extent, a similar response was observed in mice that were primed with rMT1721/adjuvant followed by boost with DNA-MT1721. Single immunization with DNA-MT1721 or rMT1721/adjuvant elicited little or no detectable IFN-γ (not shown). These results support the former observation (Fig. 2), which indicated that both heterologous prime/boost immunization protocols used in these studies stimulated high titres of IgG2a.
Figure 3.
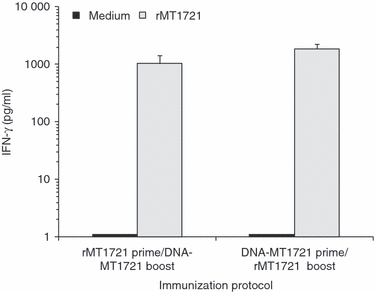
Interferon-γ (IFN-γ) production by mouse splenocytes. Groups of three mice were immunized either subcutaneously or intramuscularly according to the vaccination strategy described in the Materials and Methods. Twelve days after the last boost, spleens were excised and processed for cell cultures. Supernatants from cultures of cells stimulated with 10 μg/ml rMT1721 were collected after 72 hr of incubation. Data are shown as the average of triplicates ± SEM. No statistical differences were found between the two heterologous immunization groups. Open bars: medium control; filled bars, rMT1721 (10 μg/ml).
Functional profiles of M. tuberculosis antigen-specific T-cell responses after heterologous prime/boost immunization in mice
The magnitude and quality of the cellular immune response are suggested to contribute to the control of infections caused by infectious disease pathogens.18,19 We therefore assessed the functional profile of the cellular immune responses elicited by the DNA-MT1721 prime/rMT1721 boost and vice versa. Splenocytes were isolated from mice followed by stimulation in vitro with the rMT1721, and production of IFN-γ, TNF-α and IL-2 cytokines by T cells was measured by intracellular cytokine staining. The production of each of these three cytokines by either CD4+ or CD8+ T cells is shown in Figs 4 and 5. Mice receiving the DNA-MT1721 prime/rMT1721 boost generated markedly higher CD4 T-cell responses for all three cytokines (IFN-γ, P < 0.021; TNF-α, P < 0.015; and IL-2, P < 0.026) compared with rMT1721 prime/DNA-MT1721 boost (Fig. 4).
Figure 4.
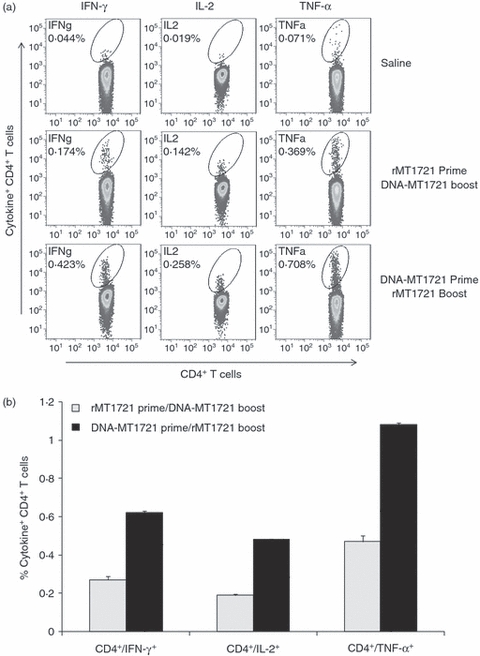
Heterologous DNA and recombinant protein prime/boost immunization induces robust Mycobacterium tuberculosis rMT1721-specific CD4+ cells producing interferon-γ (IFN-γ), tumour necrosis factor-α (TNF-α) and interleukin-2 (IL-2). Splenocytes from three mice per group were isolated 12 days after immunization. The splenocytes were exposed to rMT1721 and cytokine production was measured by monoclonal antibody staining and flow cytometric analysis. (a) Flow cytometer plot of results obtained from one representative individual mouse from each group. (b), Average (± SEM) of the percentage of IFN-γ-producing, TNF-α-producing and IL-2-producing CD4+ T cells obtained from three mice per group following stimulation with rMT1721. Mice receiving the DNA-MT1721 prime/rMT1721 boost generated markedly higher CD4 T-cell responses for all three cytokines (IFN-γ, P < 0.021; TNF-α, P < 0.015; and IL-2, P < 0.026) compared with rMT1721 prime/DNA-MT1721 boost.
Figure 5.
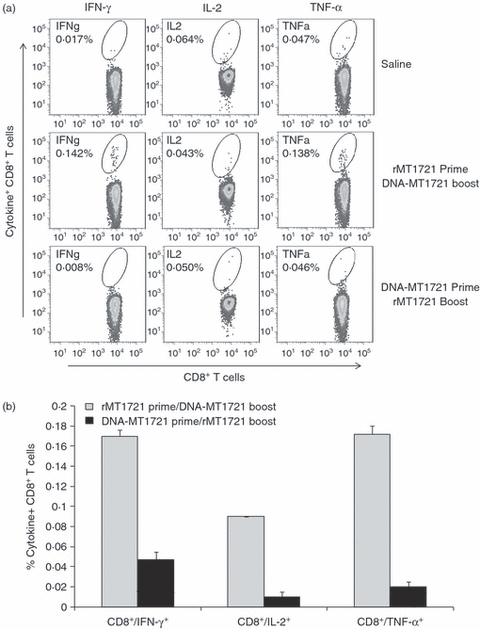
Heterologous recombinant protein and DNA prime/boost immunization induces Mycobacterium tuberculosis rMT1721-specific CD8+ cells producing interferon-γ (IFN-γ), tumour necrosis factor-α (TNF-α) and interleukin-2 (IL-2). Splenocytes from three mice per group were isolated 12 days after immunization. The splenocytes were exposed to rMT1721 and cytokine production was measured by monoclonal antibody staining and flow cytometric analysis. (a) Flow cytometer plot of results obtained from one representative individual mouse from each group. (b), Average (± SEM) of the percentage of IFN-γ-producing, TNF-α-producing and IL-2-producing CD8+ T cells obtained from three mice per group following stimulation with rMT1721. Mice that received rMT1721 prime/DNA-MT1721 boost produced significantly higher IFN-γ (P < 0.05) and TNF-α (P < 0.03), but not IL-2 (P < 0.15) compared with mice receiving DNA-MT1721 prime/rMT1721 boost immunization.
Regarding the CD8+ responses, mice receiving rMT1721 prime/DNA-MT1721 boost produced, although to a lesser extent than CD4+ T cells, after in vitro stimulation with rMT1721, significantly higher IFN-γ (P < 0·05) and TNF-α (P < 0·03) but not IL-2 (P < 0·15) compared with mice receiving DNA-MT1721 prime/rMT1721 boost immunization (Fig. 5).
Polyfunctional T cells, those capable of producing two or more cytokines, have been associated with effective control of infections caused by infectious disease pathogens.18,20,21 Therefore, we next assessed the rMT1721-stimulated production of these three cytokines by individual cells to characterize the functional profiles of the elicited T-cell responses. Total M. tuberculosis-specific multi-cytokine-producing T cells were divided into three distinct populations based on their production of IFN-γ, TNF-α and IL-2, those that could produce only one cytokine, two cytokines or all three cytokines. The profiles of the functional capacities of the cells are shown by expressing each type of cytokine response as a proportion of the total response. The mean values for the animals in each group are shown in a series of pie charts (Fig. 6). The M. tuberculosis-specific CD4+ T-cell responses induced by DNA-MT1721 prime/rMT1721 boost as well as by rMT1721 prime/DNA-MT1721 boost were highly polyfunctional, with roughly 65% of the CD4 T cells producing two or three cytokines and 8% of the cells producing all three cytokines. A similar profile of polyfunctional cell response was observed for M. tuberculosis-specific CD8+ T cells elicited by the rMT1721 prime/DNA-MT1721 boost immunization regimen. No such observation could be performed with cells obtained from mice immunized with DNA-MT1721 prime/rMT1721 because this regimen did not induce detectable M. tuberculosis-specific CD8+ T cells.
Figure 6.
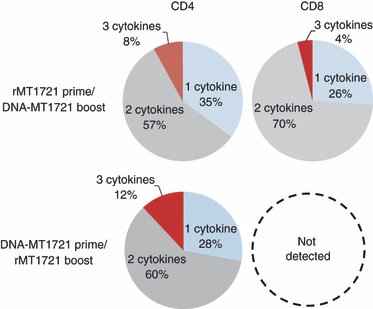
Heterologous recombinant DNA and protein prime/boost immunization elicits polyfunctional Mycobacterium tuberculosis rMT1721-specific CD4+ and CD8+ T cells. Splenocytes were isolated 12 days after immunization. The splenocytes were exposed to the rMT1721 and cytokine production was measured by monoclonal antibody staining and flow cytometric analysis. The antigen-specific T cells were divided into three distinct populations based on their ability to produce interferon-γ (IFN-γ), tumour necrosis factor-α (TNF-α) and interleukin-2 (IL-2) individually (one cytokine), or any combination of two cytokines (two cytokines) or all three cytokines (three cytokines). The cytokine profiles were determined by expressing each cytokine response as a proportion of the total antigen-specific cytokine-producing T-cell response. Data were analysed using the flowjo 7.6 software (Tree Star) and are presented as the mean values from the groups rMT1721 prime/DNA-MT1721 boost and DNA-MT1721 prime/rMT1721 in a pie chart.
Taken together, these findings show that the optimized VRC8400 DNA vector and subunit protein with the potent TLR4 agonist GLA as adjuvant in a combination prime/boost strategy are highly immunogenic and can generate both CD4 and CD8 T-cell responses, particularly strong CD4 T-cell responses, with a polyfunctional phenotype.
Discussion
Heterologous prime/boost vaccination regimens employing recombinant bacteria, viruses and proteins have been shown to generate more robust and more diverse cellular immune responses than vaccine strategies employing a simple (homologous) vaccine prime/boost modality.20,22–27 The rationale for the present work was built from these former observations. Our goal was to evaluate the immunogenicity of a heterologous prime/boost strategy targeting one novel M. tuberculosis antigen that is abundantly produced in vivo during active pulmonary TB and eliminated in the patient’s urine. This antigen is encoded by the gene MT_1721 and is unique to the M. tuberculosis complex, so it is an interesting vaccine candidate. The two components of the heterologous prime/boost strategy of the present studies were an optimized DNA vaccine vector, VRC8400, into which the gene MT_1721 was sub-cloned, and a highly purified rMT1721 protein produced and expressed by E. coli BL21(DE3)pLysS transformed with pET-29b. The VRC8400-based DNA vaccines were previously shown to induced markedly higher cellular immune responses in mice and monkeys than conventional DNA vaccine vectors containing the CMV promoter. This enhanced immunogenicity is thought to be the result of fivefold to 10-fold greater expression of vaccine antigens as a result of the introduction of a regulatory sequence from the R region of the long terminal repeat from HTLV-1 to the CMV enhancer/promoter in the expression plasmid.14
To enhance the immunogenicity of the rMT1721 we opted to use the adjuvant GLA, a synthetic version of the Toll-like receptor 4 agonist monophosphoryl lipid A. This adjuvant has been previously used as potent adjuvant for subunit protein vaccine candidates and was found to effectively improve the vaccine-elicited immune responses and protection against experimental murine leishmaniasis.15
The results clearly demonstrated that heterologous immunization using these two formats of antigen delivery stimulates a robust IgG2a response specific to rMT1721. The preferential stimulation of this immunoglobulin isotype was not strictly dependent on the order in which the two formulations were delivered to the animals. However, protein priming and DNA boost clearly caused a substantial increase in the IgG1 and IgG2a antibody titres to rMT1721. In contrast, when mice were primed with DNA-MT1721 and boosted with rMT1721 there was only a small change in IgG2a antibody response and no boosting effect was seen on the IgG1 antibody response. In fact, these results are consistent and supported by the findings that, regardless of the order of the prime/boost immunizations, spleen cells obtained from the immunized mice produced high levels of IFN-γin vitro after stimulation with rMT1721. It is well-known that induction of isotype switch to IgG2a is strictly dependent on IFN-γ production.28–30
However, the order of immunizations had a clear influence on the T-cell response to rMT1721. We found that priming with recombinant DNA and subsequently boosting with rMT1721 generated M. tuberculosis-specific CD4+ and little or undetectable CD8+ T-cell responses in immunized mice. In contrast, priming with rMT1721 and boosting with the recombinant DNA, elicited both CD4+ and CD8+ T-cell responses. At this point, we do not have a clear explanation for this distinct pattern of immune responses. However, these results are in consonance with the general patterns of immune responses induced by immunization with soluble protein antigens (primarily CD4+ T-cell response) and bacterial plasmid DNA (both CD4+ and CD8+ T-cell responses). Hence, initial immunization with DNA-MT1721 primes both CD4+ and CD8+ T cells. The heterologous boost with rMT1721 will then boost primarily CD4+ T cells, resulting in robust cytokine production by these cells upon stimulation in vitro with the antigen. Because single DNA priming is not sufficient to induce strong responses, few or no CD8+ T cells are detected by this regimen of antigen delivery. In contrast, initial immunization with rMT1721 primes strong CD4+ T cells and subsequent heterologous boost with DNA-MT1721 will boost these CD4+ T cells and will concomitantly prime CD8+ T cells. The boost of CD4+ T cells will provide a strong help to the CD8+ T cells, resulting in highly functional CD8+ T-cell responses specific to the immunizing antigen. This suggestion is supported by well-known observations showing that CD8+ cell responses are strongly amplified by CD4+ helper T cells.31–35 Indeed, this possibility is supported by the observation that indicated that primary immunization with the protein resulted in clear priming of the immune system to produce both IgG1 and IgG2a antibodies. In contrast, primary immunization with the DNA format of the antigen barely primed the immune system to produce antibodies. As production of IgG antibodies for most protein antigens is strictly dependent on the CD4+ T-cell response, these results help to explain the observed more robust immune responses induced by protein priming followed by DNA boost as opposed to DNA priming and protein boosting.
It is important to mention that we used whole soluble rMT1721 antigen for in vitro stimulation of splenocytes to detect CD8 T-cell responses. It is known that extracellular soluble protein antigens in general do not induce a CD8+ T-cell response in vivo. Usually these molecules do not have access to the endosomic compartment of the antigen-presenting cells and are not processed within the MHC class I compartment of the antigen-processing machinery, which is an essential pathway to prime CD8+ T cells. However, it is possible that soluble MHC class I binding peptides derived from degradation of the rMT1721 could be present in the protein solution used to stimulate the mouse spleen cells in vitro. These exogenous peptides could bind directly to empty MHC class I molecules and therefore be presented to specific CD8+ T cells, as has been described for other M. tuberculosis antigens.36 Alternatively, several studies have shown that CD8+ T cells can indeed be stimulated in vitro by particulate or soluble exogenous antigens internalized by dendritic cells using a process known as cross-priming.37–42 Although dendritic cells can efficiently cross-present to CD8+ T cells peptides derived from internalized particulate substrates, cross-presentation of exogenous soluble proteins occurs with substantially lower efficiency.40,41 Therefore, the somewhat low levels of cytokines detected in CD8+ T cells stimulated with soluble rMT1721 are indeed not surprising.
In summary, the heterologous prime/boost strategies used in these studies, regardless of the order in which the two different formulation of the antigen are delivered, are excellent at stimulating preferentially IgG2a antibody response (Th1 response) and at inducing CD4+ T-cell responses, of which approximately 90% are polyfunctional. In contrast, generation of CD8+ T-cell responses was highly dependent on the sequential order of immunization with the different formats of antigen delivery, i.e. CD8+ T-cell response only occurred when animals were first primed with the recombinant protein and boosted with DNA.
Finally, these immunization protocols establish a rational foundation for protection experiments not only against TB but for other diseases like leishmaniasis, for which immunity is mediated by Th1 CD4+ T cells as well as by CD8+ T cells.
Acknowledgments
We thank Dr John Beslile, Dr Angelo Izzo and Dr Karen Dobos, Colorado State University (NIAID/NIH Tuberculosis Research Materials contract no. 1-A125174), for kindly supplying M. tuberculosis culture filtrate proteins and Dr Lizeng Qin for his expert help with the intracellular cytokine staining assay and analysis.
Disclosures
None of the authors has any financial conflict of interest.
Financial support
This work was supported by the following grant from the National Institutes of Health: R01 AI076425 to A. Campos-Neto.
References
- 1.Dye C, Scheele S, Dolin P, Pathania V, Raviglione MC. Consensus statement. Global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. WHO Global Surveillance and Monitoring Project. JAMA. 1999;282:677–86. doi: 10.1001/jama.282.7.677. [DOI] [PubMed] [Google Scholar]
- 2.The current global situation of the HIV/AIDS pandemic. Wkly Epidemiol Rev. 1994;69:191–2. [PubMed] [Google Scholar]
- 3.Raviglione MC, Snider DE, Jr, Kochi A. Global epidemiology of tuberculosis. Morbidity and mortality of a worldwide epidemic. JAMA. 1995;273:220–6. [PubMed] [Google Scholar]
- 4.Colditz GA, Brewer TF, Berkey CS, et al. Efficacy of BCG vaccine in the prevention of tuberculosis. Meta-analysis of the published literature. JAMA. 1994;271:698–702. [PubMed] [Google Scholar]
- 5.Cooper AM, Dalton DK, Stewart TA, Griffen JP, Russel DG, Orme IM. Disseminated tuberculosis in interferon-g gene-disrupted mice. J Exp Med. 1993;178:2243–7. doi: 10.1084/jem.178.6.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flynn JL, Chan J, Triebold KJ, Dalton DK, Stewart TA, Bloom BR. An essential role for IFN-γ in resistance to Mycobacterium tuberculosis infection. J Exp Med. 1993;178:2248–53. doi: 10.1084/jem.178.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Newport MJ, Huxley CM, Huston S, et al. A mutation in the interferon-γ-receptor gene and susceptibility to mycobacterial infection. N Engl J Med. 1996;335:1941–9. doi: 10.1056/NEJM199612263352602. [DOI] [PubMed] [Google Scholar]
- 8.Flynn JL, Goldstein MM, Chan J, et al. Tumor necrosis factor-α is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity. 1995;2:561–72. doi: 10.1016/1074-7613(95)90001-2. [DOI] [PubMed] [Google Scholar]
- 9.Flynn JL, Goldstein MM, Triebold KJ, Koller B, Bloom BR. Major histocompatibility complex I-restricted T cells are required for resistance to Mycobacterium tuberculosis infection. Proc Natl Acad Sci USA. 1992;89:12013–7. doi: 10.1073/pnas.89.24.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muller I, Cobbold SP, Waldmann H, Kaufmann SH. Impaired resistance to Mycobacterium tuberculosis infection after selective in vivo depletion of L3T4a and Lyt-2+ T cells. Infect Immun. 1987;55:2037–41. doi: 10.1128/iai.55.9.2037-2041.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laochumroonvorapong P, Wang J, Liu CC, et al. Perforin, a cytotoxic molecule which mediates cell necrosis, is not required for the early control of mycobacterial infection in mice. Infect Immun. 1997;65:127–32. doi: 10.1128/iai.65.1.127-132.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kashino SS, Pollock N, Napolitano DR, Rodrigues V, Jr, Campos-Neto A. Identification and characterization of Mycobacterium tuberculosis antigens in urine of patients with active pulmonary tuberculosis: an innovative and alternative approach of antigen discovery of useful microbial molecules. Clin Exp Immunol. 2008;153:56–62. doi: 10.1111/j.1365-2249.2008.03672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Napolitano DR, Pollock N, Kashino SS, Rodrigues V, Jr, Campos-Neto A. Identification of Mycobacterium tuberculosis ornithine carboamyltransferase in urine as a possible molecular marker of active pulmonary tuberculosis. Clin Vaccine Immunol. 2008;15:638–43. doi: 10.1128/CVI.00010-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barouch DH, Yang ZY, Kong WP, et al. A human T-cell leukemia virus type 1 regulatory element enhances the immunogenicity of human immunodeficiency virus type 1 DNA vaccines in mice and nonhuman primates. J Virol. 2005;79:8828–34. doi: 10.1128/JVI.79.14.8828-8834.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bertholet S, Goto Y, Carter L, et al. Optimized subunit vaccine protects against experimental leishmaniasis. Vaccine. 2009;27:7036–45. doi: 10.1016/j.vaccine.2009.09.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seiki M, Hattori S, Hirayama Y, Yoshida M. Human adult T-cell leukemia virus: complete nucleotide sequence of the provirus genome integrated in leukemia cell DNA. Proc Natl Acad Sci U S A. 1983;80:3618–22. doi: 10.1073/pnas.80.12.3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jouanguy E, Altare F, Lamhamedi S, et al. Interferon-γ-receptor deficiency in an infant with fatal bacille Calmette–Guérin infection. N Engl J Med. 1996;335:1956–61. doi: 10.1056/NEJM199612263352604. [DOI] [PubMed] [Google Scholar]
- 18.Betts MR, Nason MC, West SM, et al. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood. 2006;107:4781–9. doi: 10.1182/blood-2005-12-4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun Y, Santra S, Schmitz JE, Roederer M, Letvin NL. Magnitude and quality of vaccine-elicited T-cell responses in the control of immunodeficiency virus replication in rhesus monkeys. J Virol. 2008;82:8812–9. doi: 10.1128/JVI.00204-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goonetilleke NP, McShane H, Hannan CM, Anderson RJ, Brookes RH, Hill AV. Enhanced immunogenicity and protective efficacy against Mycobacterium tuberculosis of bacille Calmette–Guérin vaccine using mucosal administration and boosting with a recombinant modified vaccinia virus Ankara. J Immunol. 2003;171:1602–9. doi: 10.4049/jimmunol.171.3.1602. [DOI] [PubMed] [Google Scholar]
- 21.Darrah PA, Patel DT, De Luca PM, et al. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat Med. 2007;13:843–50. doi: 10.1038/nm1592. [DOI] [PubMed] [Google Scholar]
- 22.McShane H, Brookes R, Gilbert SC, Hill AV. Enhanced immunogenicity of CD4+ T-cell responses and protective efficacy of a DNA-modified vaccinia virus Ankara prime-boost vaccination regimen for murine tuberculosis. Infect Immun. 2001;69:681–6. doi: 10.1128/IAI.69.2.681-686.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McShane H, Pathan AA, Sander CR, et al. Recombinant modified vaccinia virus Ankara expressing antigen 85A boosts BCG-primed and naturally acquired antimycobacterial immunity in humans. Nat Med. 2004;10:1240–4. doi: 10.1038/nm1128. [DOI] [PubMed] [Google Scholar]
- 24.Romano M, D’Souza S, Adnet PY, et al. Priming but not boosting with plasmid DNA encoding mycolyl-transferase Ag85A from Mycobacterium tuberculosis increases the survival time of Mycobacterium bovis BCG vaccinated mice against low dose intravenous challenge with M. tuberculosis H37Rv. Vaccine. 2006;24:3353–64. doi: 10.1016/j.vaccine.2005.12.066. [DOI] [PubMed] [Google Scholar]
- 25.Santosuosso M, McCormick S, Zhang X, Zganiacz A, Xing Z. Intranasal boosting with an adenovirus-vectored vaccine markedly enhances protection by parenteral Mycobacterium bovis BCG immunization against pulmonary tuberculosis. Infect Immun. 2006;74:4634–43. doi: 10.1128/IAI.00517-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vordermeier HM, Rhodes SG, Dean G, et al. Cellular immune responses induced in cattle by heterologous prime-boost vaccination using recombinant viruses and bacille Calmette–Guérin. Immunology. 2004;112:461–70. doi: 10.1111/j.1365-2567.2004.01903.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Williams A, Goonetilleke NP, McShane H, et al. Boosting with poxviruses enhances Mycobacterium bovis BCG efficacy against tuberculosis in guinea pigs. Infect Immun. 2005;73:3814–6. doi: 10.1128/IAI.73.6.3814-3816.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bossie A, Vitetta ES. IFN-γ enhances secretion of IgG2a from IgG2a-committed LPS-stimulated murine B cells: implications for the role of IFN-γ in class switching. Cell Immunol. 1991;135:95–104. doi: 10.1016/0008-8749(91)90257-c. [DOI] [PubMed] [Google Scholar]
- 29.Collins JT, Dunnick WA. Germline transcripts of the murine immunoglobulin gamma 2a gene: structure and induction by IFN-γ. Int Immunol. 1993;5:885–91. doi: 10.1093/intimm/5.8.885. [DOI] [PubMed] [Google Scholar]
- 30.Chu RS, Targoni OS, Krieg AM, Lehmann PV, Harding CV. CpG oligodeoxynucleotides act as adjuvants that switch on T helper 1 (Th1) immunity. J Exp Med. 1997;186:1623–31. doi: 10.1084/jem.186.10.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kumamoto Y, Mattei LM, Sellers S, Payne GW, Iwasaki A. CD4+ cells support cytotoxic T lymphocyte priming by controlling lymph node input. Proc Natl Acad Sci U S A. 2011;108:8749–54. doi: 10.1073/pnas.1100567108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khanolkar A, Badovinac VP, Harty JT. CD8 T cell memory development: CD4 T cell help is appreciated. Immunol Res. 2007;39:94–104. doi: 10.1007/s12026-007-0081-4. [DOI] [PubMed] [Google Scholar]
- 33.Rocha B, Tanchot C. Towards a cellular definition of CD8+ T-cell memory: the role of CD4+ T-cell help in CD8+ T-cell responses. Curr Opin Immunol. 2004;16:259–63. doi: 10.1016/j.coi.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 34.Bourgeois C, Tanchot C. Mini-review CD4 T cells are required for CD8 T cell memory generation. Eur J Immunol. 2003;33:3225–31. doi: 10.1002/eji.200324576. [DOI] [PubMed] [Google Scholar]
- 35.Huber B, Cantor H, Shen FW, Boyse EA. Independent differentiative pathways of Ly1 and Ly23 subclasses of T cells. Experimental production of mice deprived of selected T-cell subclasses. J Exp Med. 1976;144:1128–33. doi: 10.1084/jem.144.4.1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zugel U, Schoel B, Kaufmann SH. Beta 2-microglobulin independent presentation of exogenously added foreign peptide and endogenous self-epitope by MHC class I α-chain to a cross-reactive CD8+ CTL clone. J Immunol. 1994;153:4070–80. [PubMed] [Google Scholar]
- 37.Maecker HT, Ghanekar SA, Suni MA, He XS, Picker LJ, Maino VC. Factors affecting the efficiency of CD8+ T cell cross-priming with exogenous antigens. J Immunol. 2001;166:7268–75. doi: 10.4049/jimmunol.166.12.7268. [DOI] [PubMed] [Google Scholar]
- 38.Brossart P, Bevan MJ. Presentation of exogenous protein antigens on major histocompatibility complex class I molecules by dendritic cells: pathway of presentation and regulation by cytokines. Blood. 1997;90:1594–9. [PMC free article] [PubMed] [Google Scholar]
- 39.Carbone FR, Bevan MJ. Class I-restricted processing and presentation of exogenous cell-associated antigen in vivo. J Exp Med. 1990;171:377–87. doi: 10.1084/jem.171.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ackerman AL, Cresswell P. Cellular mechanisms governing cross-presentation of exogenous antigens. Nat Immunol. 2004;5:678–84. doi: 10.1038/ni1082. [DOI] [PubMed] [Google Scholar]
- 41.Ackerman AL, Kyritsis C, Tampe R, Cresswell P. Access of soluble antigens to the endoplasmic reticulum can explain cross-presentation by dendritic cells. Nat Immunol. 2005;6:107–13. doi: 10.1038/ni1147. [DOI] [PubMed] [Google Scholar]
- 42.Cresswell P, Ackerman AL, Giodini A, Peaper DR, Wearsch PA. Mechanisms of MHC class I-restricted antigen processing and cross-presentation. Immunol Rev. 2005;207:145–57. doi: 10.1111/j.0105-2896.2005.00316.x. [DOI] [PubMed] [Google Scholar]


