Abstract
Signal regulatory protein α (SIRPα/CD172a), expressed by myeloid cells including CD11b+ dendritic cells, interacts with ubiquitously expressed CD47 to mediate cell–cell signalling and therefore, may be pivotal in the development of tolerance or immunity. We show that in mice deficient in CD47 (CD47−/−) the cellularity in gut-associated lymphoid tissues is reduced by 50%. In addition, the frequency of CD11b+ CD172a+ dendritic cells is significantly reduced in the gut and mesenteric lymph nodes, but not in Peyer’s patches. Activation of ovalbumin (OVA)-specific CD4+ T cells in the mesenteric lymph nodes after feeding OVA is reduced in CD47−/− mice compared with wild-type however, induction of oral tolerance is maintained. The addition of cholera toxin generated normal serum anti-OVA IgG and IgA titres but resulted in reduced intestinal anti-OVA IgA in CD47−/− mice. Replacing the haematopoietic compartment in CD47−/− mice with wild-type cells restored neither the cellularity in gut-associated lymphoid tissues nor the capacity to produce intestinal anti-OVA IgA following immunization. This study demonstrates that CD47 signalling is dispensable for oral tolerance induction, whereas the expression of CD47 by non-haematopoietic cells is required for intestinal IgA B-cell responses. This suggests that differential CD4 T cell functions control tolerance and enterotoxin-induced IgA immunity in the gut.
Keywords: antibody responses, dendritic cells, mucosal immunity, tolerance and IgA
Introduction
The intestinal immune system has dual and opposing roles as it must discriminate between harmful substances, to generate an effector response, and benign food antigens, to maintain tolerance. A prominent feature of the intestinal immune system is the generation of IgA-producing plasma cells. Oral immunization with the powerful adjuvant cholera toxin (CT) is dependent on CD4+ T cells to generate antigen-specific IgA.1,2 Dendritic cells (DC) strategically placed beneath intestinal epithelial cells have been shown to be important for the induction of oral tolerance.3 They are essential for immunogenic functions including CD4+ T-cell activation and subsequent generation of antigen-specific antibodies following oral immunization with adjuvants.4
CD47 is a ubiquitously expressed cell surface immunoglobulin superfamily protein that was first identified as a protein associated with αvβ3 integrins.5 It is involved in regulating a range of functions including phagocytosis, cell adhesion and migration.6–8 CD47 was also found to be a receptor for the extracellular matrix protein thrombospondin,6 and to function as the ligand for signal regulatory protein α (SIRPα/CD172a).7,9 CD172a is a cell surface immunoglobulin superfamily member expressed by most myeloid cells, but also by non-haematopoietic cells such as vascular endothelial cells and smooth muscle cells.10,11 The cytoplasmic tail of CD172a contains immunoreceptor tyrosine-based inhibitory motifs that, upon phosphorylation, are able to recruit the tyrosine phosphatases SHP-1 or SHP-2. These phosphatases in turn modulate phagocytosis, cell migration and cellular responses to growth factors and other soluble signalling molecules.12 Not only interaction between CD47 and CD172a, but also integrin-mediated cell adhesion,10,11 leads to phosphorylation of the CD172a immunoreceptor tyrosine-based inhibitory motifs and regulation of these cellular functions.
Blood monocytes, macrophages, granulocytes and CD11b+ (CD4+) DC express CD172a.13,14 The expression of both CD47 and CD172a has recently been shown to be required for the homeostasis of CD11b+ DC in lymphoid organs,15 and also to regulate migration of this DC subset from skin to the draining lymph nodes (LN).13,14,16 In intestinal tissues, CD172a–CD47 interactions are also required for the regulation of eosinophil degranulation and homeostasis.17 CD47 is crucial for cellular recruitment to sites of intestinal inflammation, as mice lacking CD47 (CD47−/−) fail to recruit CD172a+ CD11c+ cells to the gut and are therefore protected from trinitrobenzenesulphonic acid-induced colitis.18 Moreover, CD47 has been demonstrated to negatively regulate inducible Foxp3+ T regulatory cells expressing CD103, resulting in increased proliferation and accumulation of the T regulatory cells with age in CD47−/− mice.19 However, the role of CD47 in both the induction of immune responses following oral immunization with adjuvants and the maintenance of oral tolerance has not been investigated.
In this study we use CD47−/− mice to explore the role of CD47 and gut-associated lymphoid tissue (GALT) -resident CD172a+ antigen-presenting cells in the induction of oral tolerance and following immunization with the adjuvant CT. We observe that CD47−/− mice exhibit reduced total cell numbers selectively in the GALT. In addition, we show that the frequency of CD11b+ CD172a+ DC is reduced by 50% in the small intestine and draining mesenteric lymph nodes (MLN) but not in the Peyer’s patches (PP). Although MLN are required for oral tolerance induction, CD47−/− mice maintain this capacity despite their diminished cell numbers. In contrast, production of antigen-specific intestinal IgA following oral immunization is significantly reduced in CD47−/− mice, although normal antigen-specific systemic IgG and total IgA levels are maintained. Finally, we show that replacement of the haematopoietic compartment in CD47−/− mice with wild-type (WT) cells (WT → CD47−/−) restores the frequency of CD11b+ DC, but not the cellularity in GALT or the capacity to generate intestinal IgA following oral immunization. Therefore, the defect in ovalbumin (OVA) -specific IgA production is unlikely to be linked to the reduced frequency of CD11b+ DC but rather would be linked to the lack of CD47 expression by non-haematopoietic cells.
Materials and methods
Mice
CD47−/− BALB/c (back-crossed for 16 generations) and DO11.10 mice were bred in specific pathogen-free conditions at the Experimental Biomedicine Animal Facility, University of Gothenburg. BALB/c (WT) mice were purchased from Taconic, Ry, Denmark. To generate bone marrow (BM) chimeric mice, BM cells from donor WT mice were filtered, red blood cells were lysed, and the remaining cells were resuspended in PBS. Recipient WT or CD47−/− mice were irradiated (1000 rad) before 2 × 106 to 5 × 106 donor BM cells were transferred intravenously to generate WT → CD47−/− (WT/CD47) chimeras or CD47−/− → CD47−/− irradiation controls (CD47/CD47) and WT → WT (WT/WT). Irradiated mice and mice which underwent mesenteric lymphadenectomy were left to recover for 6 weeks before being included in experiments. The chimerism was confirmed by flow cytometry. All experiments performed were approved by the Swedish government’s Animal Ethics Committee and followed institutional animal use and care guidelines.
Isolation of cells
Cells were isolated from LN and spleen by mechanical disruption. For DC isolation, tissues were pre-treated with liberase (0·4 mg/ml; Roche, Indianapolis, IN) in Hank’s buffered saline solution (HBSS, GIBCO/Invitrogen, Leek, The Netherlands) supplemented with 2% fetal bovine serum (FBS) at 37° for 30 min. Small intestines were flushed with calcium-free and magnesium-free HBSS (GIBCO/Invitrogen) and cut into smaller pieces. The PP were excised from intestinal tissue and washed. For removal of epithelial cells, tissues were incubated at 37° for 15 min with HBSS containing EDTA (5 mm), FBS (2%) and antibiotics, and then shaken vigorously. The procedure was repeated twice for small intestinal lamina propria (LP) and once for PP. The LP was then digested with collagenase D (100 U/ml; Roche) in RPMI-1640 medium supplemented with FBS (10%), HEPES (15 mm) and antibiotics during two 1 hr incubations. The PP were digested with liberase (0·4 mg/ml) in HBSS containing polymycin B (10 U/ml) at 37° for 27 min. Remaining tissue was disrupted over nylon mesh and counted using a cell counter (Sysmex, Kungsbacka, Sweden) or manually using trypan blue to exclude dead cells.
Immunohistochemistry
Mesenteric lymph nodes and small intestines were frozen in OCT compound, then 8-μm cryosections were collected on gelatin-coated slides, air-dried and fixed in 1% paraformaldehyde for 5 min. Sections were washed three times in PBS, incubated with glucose oxidase (Sigma Aldrich, Stockholm, Sweden) to quench endogenous peroxidase, then endogenous biotin was blocked using an Avidin-Biotin Blocking Kit (Zymed Laboratories, Invitrogen). Non-specific binding was blocked using 10% goat serum in TBST (0·1 m Tris–HCl, pH 7·5; 0·15 m NaCl; 0·1% Tween-20) for 30 min. Sections were then incubated for 60 min with the following primary antibodies: CD3e-biotin, CD11b, CD11c-allophycocyanin (APC), CD103-phycoerythrin (PE), CD11c-biotin (BD Biosciences, Stockholm, Sweden) and with IgD (Biolegend, San Diego, CA), diluted in TBST. Unlabelled antibodies were detected using Cy5-conjugated anti-rat IgG (Jackson ImmunoResearch, West Grove, PA), and biotinylated antibodies were detected using fluorophore tyramide (PerkinElmer, Waltham, MA). Tissue sections were mounted in Vectashield with DAPI (Vector Laboratories, Burlingame, CA), and analysed using laser scanning confocal microscopy (Leica TSP-2; Leica, Heidelberg, Germany). Images were analysed using leica lcs software (Leica, San Jose, CA) and Adobe Photoshop CS3.
Flow cytometry
Intracellular staining for Foxp3 was carried out using a Mouse Regulatory T Cell Staining kit (eBioscience, San Diego, CA). 7-Amino-actinomycin D (7AAD) was used to exclude dead cells. The following conjugated antibodies were used for surface staining: CD3e-APC, CD4-Alexa-700, CD8a-PE-Cy7, CD11b-APC-Cy7, CD11c-Pacific blue, CD45R-Pacific blue, CD45R-Alexa Fluor 488, MHC-II-Alexa-700, KJ1-26-PE and Foxp3-PE (eBioscience), CD19-APC, CD25-APC-Cy7, CD62L-APC, CD103-PE (BD Bioscience), and streptavidin-Qdot 605 (Invitrogen). CD172a antibody was provided by Dr Karl Lagenaur and biotinylated in-house. Flow cytometry was performed on an LSR:II (BD Bioscience) and results were analysed using flowjo software (Tree Star, Ashland, OR).
Adoptive transfer
CD4+ T cells were enriched from spleens and LN of DO11.10 mice by positive selection magnetic separation using a MACS LS-column (Miltenyi Biotec, BergischGladbach, Germany). CD4+ cells were stained with 2·5 μm 5,6-carboxyfluorescein diacetate succinimidyl ester (CFSE; Invitrogen) and 2·5 × 106 to 5 × 106 cells were injected intravenously into recipient CD47−/− and WT mice. The following day, mice were fed 10 mg OVA (grade V; Sigma, Stockholm, Sweden) in the presence or absence of 10 μg CT (Sigma) in 3% NaHCO3, or injected with 100 μg OVA intravenously. After 3 days, organs were harvested and CD4+ T-cell proliferation was analysed by CFSE profiling.
Oral tolerance
CD47−/− and WT mice were fed PBS or OVA (5 or 50 mg). Ten days later, all mice were challenged subcutaneously with 100 μg OVA in incomplete Freund’s adjuvant (IFA). Draining LN (inguinal) were harvested 1 week later and cells were re-stimulated with low-endotoxin OVA. Three days later, [3H]thymidine was added for 6 hr, then cells were harvested, and thymidine incorporation was measured using a β-counter. The stimulation index was defined as cellular proliferation in the OVA-fed group in relation to the PBS-fed group normalized to 0%. Wild-type mice that received PBS were used as reference for OVA-fed WT mice, and PBS-fed CD47−/− mice were reference for OVA-fed CD47−/− mice.
In vivo immunization
CD47−/− and WT mice were fed PBS or 300 μg OVA + 10 μg CT on three occasions at 10 day intervals. One week after the last immunization, mice were killed, blood was taken and, following perfusion, intestinal samples were collected using the perfusion-extraction (PERFEXT) technique.20 Ovalbumin-specific IgG and IgA titres were determined by ELISA.
Measurement of antibody titres by ELISA
Ninety-six-well plates (Greiner Bioscience, Frickenhausen, Germany) were coated with OVA (20 μg/ml) and blocked with PBS/BSA. Serially diluted serum and intestinal samples were added followed by goat anti-mouse horseradish peroxidase-conjugated IgA or IgG (SouthernBiotech, Birmingham, AL). Plates were developed with o-phenylenediamine dihydrochloride, stopped with 0·1 m H2SO4 and absorbance was read at 490 nm. Titres of IgG and IgA were determined from the sample dilution giving an optical density value above 0·4.
Statistical analysis
Data were statistically analysed in Prism (graphpad software) using the Student’s t-test, in which *P < 0·05, **P < 0·01 and ***P < 0·001.
Results
Reduced number of cells in GALT of CD47−/− mice
Although systemic immune compartments and skin-draining LN of CD47−/− mice have been extensively studied, the GALT has not been carefully characterized. We therefore enumerated cells in the GALT of CD47−/− mice and revealed a 50% reduction of total cell numbers in MLN, LP and PP, compared with those in WT mice (Table 1). In contrast, the number of cells in skin-draining LN and spleen was not significantly different between WT and CD47−/− mice (Table 1). Although immunohistochemical analysis showed normal localization of T and B cells in MLN and PP of CD47−/− mice (see supplementary material, Fig. S1a), and both CD47−/− and WT CD4+ T cells in PP and MLN were found to express similar levels of CD44 and CD62L (data not shown), the frequency of CD4+ T cells in MLN and PP of CD47−/− mice was significantly reduced compared with that in WT mice (Fig. S1b). In contrast, the frequency of Foxp3+ CD4+ T cells in PP, but not in MLN, was significantly increased in CD47−/− compared with WT mice (Fig. S1c).
Table 1.
Total number of cells in different organs
| Organ | ||||||
|---|---|---|---|---|---|---|
| Strain | ALN | ILN | Spleen | MLN | LP | PP |
| Wild-type | 6·98 | 3·51 | 82·14 | 25·00 | 7·60 | 8·53 |
| ± 2·58 | ± 1·43 | ± 16·12 | ± 7·60*** | ± 3·75** | ± 3·58** | |
| Knockout | 6·85 | 3·18 | 63·23 | 14·25 | 3·25 | 5·04 |
| ± 2·49 | ± 0·92 | ± 19·54 | ± 3·77 | ± 2·54 | ± 3·01 | |
ALN, axillary lymph node; ILN, inguinal lymph node; MLN, mesenteric lymph node; LP, lamina propria; PP, Peyer’s patches.
The organs were harvested and digested in liberase or collagenase (LP) after EDTA-treatment (LP + PP) and the total number of live cells in the cell suspensions was determined as non-Trypan blue stained cells. Data shown are median × 106 ± SD, and represent data from at least three experiments with four mice in each.
P < 0.005 and
P < 0.001 using Student’s t-test.
Decreased frequency of CD11b+ DC in MLN and LP, but not in PP, of CD47−/− mice
Impaired DC migration from the skin and subset-specific alterations in splenic DC at steady state have previously been reported in CD47−/− mice13,14 therefore, we next assessed populations of antigen-presenting cells in the GALT of these mice. As the total number of cells in the GALT of CD47−/− mice was reduced by 50%, frequency rather than total number of cells within cell populations was determined. Flow cytometric analysis showed a significant reduction in the frequency of CD11c+ MHC-II+ conventional DC (cDC) in MLN, but not in LP or PP, of CD47−/− mice (Fig. 1a). In contrast, no significant change in the frequency of CD172a+ CD11clow MHC-IIlow SSClow cells was detected (Fig. 1b). Further phenotypic characterization was therefore focused on cDC and identified two populations of cDC in MLN (see supplementary material, Fig. S2a). The CD11c+ MHC-IIbright cDC mostly expressed CD103, an integrin shown to be preferentially expressed by gut-derived DC in the MLN,21–23 whereas CD11c+ MHC-II+ cDC were largely negative for CD103 (Fig. S2b). The frequency of these two subsets among cDC in MLN of CD47−/− and WT mice did not differ significantly (Fig. S2c). CD11c+ MHC-IIbright cells could be further separated into two subsets based on their co-expression of CD11b and the CD47 ligand CD172a (Fig. S2d). Expression of CD172a by CD11b+ DC was also confirmed in other tissues of GALT (for PP, Fig. S3d). Analysis of multiple mice revealed a significant reduction in the frequency of CD103+ CD11b+ CD172a+ MLN cDC in CD47−/− mice compared with WT mice (Fig. 1c). CD103− cDC were further divided based on their mutually exclusive expression of CD8 and CD11b (Fig. S2e). Comparison of these populations showed a significant reduction in the frequency of CD103− CD11b+ CD8− cDC in CD47−/− mice compared with WT mice (Fig. 1d).
Figure 1.
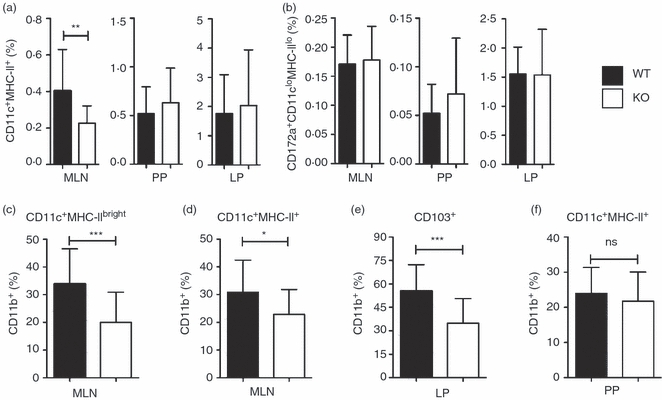
Alterations in frequencies of dendritic cell (DC) populations in the gut-associated lymphoid tissue (GALT) and intestine of CD47−/− mice. Frequency of (a) CD11c+ MHC-II+ and (b) SSClow CD172a+ CD11clow MHC-IIlow within total cells in GALT and intestine. Frequency of CD11b+ DC among (c) CD11c+ MHC-IIbright mesenteric lymph node (MLN) DC, (d) CD11c+ MHC-II+ MLN DC, (e) CD11c+ MHC-II+ CD103+ intestinal DC and (f) CD11c+ MHC-II+ Peyer’s patch (PP) DC. Results are pooled from flow cytometric analysis of at least three experiments with two to four mice/group. Error bars show SD.
Small intestinal LP CD11c+ MHC-II+ cells were next analysed for CD103 expression (see supplementary material, Fig. S3a,b). The frequency of CD103− cells, which all expressed CD11b, was significantly reduced in CD47−/− mice (42 ± 15% in CD47−/− mice versus 55 ± 11% in WT, P < 0·05). When the CD103+ population was further divided into CD8+ CD11b− and CD11b+ CD8− cells (Fig. S3a; right panels), we found that the frequency of the latter cDC population was also significantly reduced in CD47−/− mice (Fig. 1e). These differences were not the result of an increase in CD103+ or CD103+ CD8+ CD11b− cDC, because the frequency of total CD11c+ MHC-II+ cells in LP did not differ between CD47−/− and WT mice (Fig. 1a). Immunohistochemical staining showed no apparent difference in the localization of CD11c+ cells in the small intestinal LP, but suggested a decrease of CD11c+ CD103+ CD11b+ (white) cells in CD47−/− mice, compared with WT mice (Fig. S3c). In contrast to our findings in MLN and LP, CD47−/− mice had a normal frequency of CD11b+ cDC in PP (Fig. 1f and Fig. S3d), and a normal distribution of this population in the subepithelial dome region (Fig. S3e), when compared with WT mice.
These results show that CD47−/− mice have a reduced frequency of cDC in MLN, but not in LP or PP, compared with WT mice. Moreover, while DC subsets are unaltered in PP of CD47−/− mice, a specific decrease of CD11b+ cDC is apparent in LP and MLN.
Reduced proliferation of CD4+ T cells in GALT of CD47−/− mice after oral immunization
After observing GALT-specific lymphopenia and subset-specific defects in LP and MLN cDC of CD47−/− mice, we next assessed CD4+ T cell activation in the GALT of these mice after oral immunization. CFSE-labelled OVA-transgenic (DO11.10) CD4+ T cells were adoptively transferred to CD47−/− and WT mice. The use of CD47+ DO11.10 T cells eliminated possible intrinsic defects in responding T cells. After confirming that mesenteric lymphadenectomy completely abrogates oral tolerance induction in mice fed 50 mg OVA (see supplementary material, Fig. S4a), but that it does not reduce the generation of intestinal or serum anti-OVA IgA and IgG in mice fed OVA + CT (Fig. S4b),3,24 we focused on MLN T cells in mice fed OVA, and on PP T cells in mice fed OVA + CT. In control CD47−/− and WT mice fed PBS, a similar frequency of adoptively transferred cells was found in MLN (Fig. 2a). Three days after feeding OVA, the fraction of DO11.10 T cells that had entered division was reduced by 50% in the MLN of CD47−/− mice, when compared with WT mice (Fig. 2b,c). However, intravenous OVA administration did not affect proliferation of DO11.10 T cells in the spleen of CD47−/− mice (Fig. 2d). Addition of CT did not alter the reduced proliferation of DO11.10 T cells in MLN (data not shown) or PP of CD47−/− mice (Fig. 2e,f).
Figure 2.
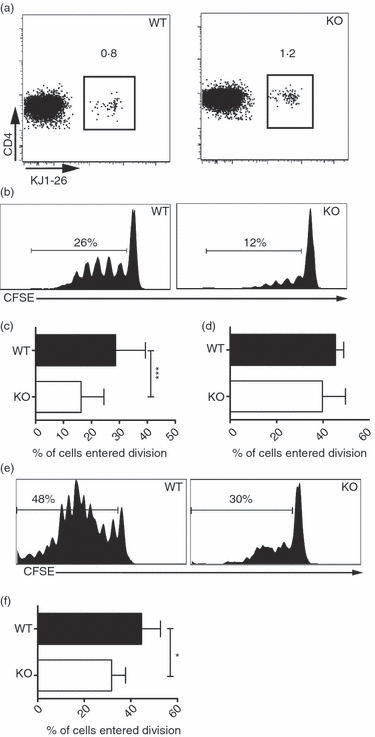
Reduced proliferation of CD4+ T cells in gut-associated lymphoid tissue (GALT) of CD47−/− mice after oral immunization. CFSE-labelled ovalbumin (OVA) -specific CD4+ T cells were transferred into CD47−/− or wild-type (WT) mice. Three days after oral immunization with (a) PBS, (b–c) OVA or (e, f) OVA + cholera toxin (CT) or (d) intravenous immunization with OVA, cells from (a–c) mesenteric lymph nodes (MLN), (d) spleen and (e, f) Peyer’s patches (PP) were analysed using flow cytometry. (b, e) Representative flow cytometric analysis of 7AAD− CD4+ KJ1-26+ cells. (c, d, f) Proliferation presented as frequency of T cells entering division from two to six pooled experiments. Each group consists of at least six individual mice. Error bars show SD.
These experiments show that CD47−/− mice have a reduced ability to induce proliferation of CD47-expressing CD4+ T cells in GALT after feeding OVA in the presence or absence of an adjuvant. However, the expansion of CD4+ T cells in CD47−/− mice is not compromised after parenteral immunization.
Maintained ability to induce oral tolerance in CD47−/− mice
We next assessed the capability of CD47−/− mice to induce oral tolerance. CD47−/− and WT mice were fed 50 mg OVA or PBS. Two weeks later, mice were challenged subcutaneously with OVA + IFA, and 1 week later draining LN were harvested. The antigen-specific proliferative response of LN cells was then determined in vitro after re-stimulation with OVA. The OVA-fed CD47−/− and WT mice exhibited a similar capacity to inhibit the OVA-specific proliferative response in vitro (approximately 75% suppression; Fig. 3a). As feeding a high dose of OVA may conceal differences in the efficacy of tolerance induction between mouse strains, the experiment was repeated using a 10-fold lower dose of OVA. This reduced antigen dose resulted in efficient tolerance induction in CD47−/− mice that was not significantly different from what was seen in WT mice (Fig. 3b). These results show that although CD47−/− mice have a reduced frequency of CD11b+ DC in LP and MLN, and a reduced capacity to induce T cell proliferation in the MLN following OVA feeding, they maintain the capacity to induce oral tolerance.
Figure 3.
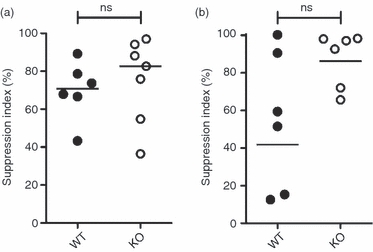
Maintained ability to induce oral tolerance in CD47−/− mice. CD47−/− and wild-type (WT) mice were fed (a) 50 mg ovalbumin (OVA), (b) 5 mg OVA or (a, b) PBS, challenged with OVA + incomplete Freund’s adjuvant (IFA) subcutaneously, and OVA-specific proliferation was measured in the draining lymph nodes after restimulation ex vivo. Suppression index is calculated as the proliferation (counts/min) of T cells from OVA-fed mice in relation to PBS-fed animals that were normalized to 0%. Results are (a) pooled from two experiments with two to four mice in each or (b) from one experiment with six mice per group. Bars show grand median.
OVA-specific intestinal IgA titres, but not serum IgG or IgA titres, are reduced in CD47−/− mice after oral immunizations
CD4+ T cell help is required for the generation of antigen-specific antibodies following oral immunization with CT.1,2 As feeding OVA + CT resulted in reduced proliferation of OVA-specific CD4+ T cells in PP of CD47−/− mice, we next assessed OVA-specific antibody titres in intestinal tissues and serum after three oral immunizations with OVA + CT. CD47−/− mice generated significantly lower intestinal anti-OVA IgA titres than WT mice (Fig. 4a), whereas total intestinal IgA and OVA-specific serum IgA and IgG titres did not differ between CD47−/− and WT mice (Fig. 4b–d). In support of this, the frequency of OVA-specific IgA-producing cells in the intestine is reduced in CD47−/− mice following immunization with OVA and CT (531 ± 102/1 × 106 cells in WT and 219 ± 49/1 × 106 cells in CD47−/− mice, n = 10 and P < 0·05). Adoptive transfer of OVA-specific (CD47+) CD4+ T cells to CD47−/− and WT mice before immunizations did not overcome the reduced capacity of CD47−/− mice to generate OVA-specific intestinal IgA (data not shown).
Figure 4.
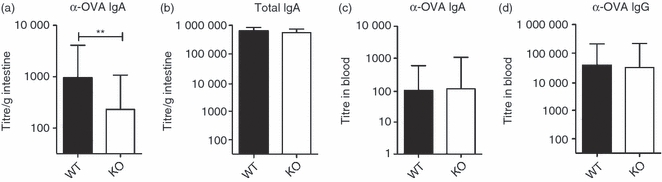
Ovalbumin (OVA) -specific intestinal IgA but not serum IgA or IgG titres are reduced in CD47−/− mice. Wild-type (WT) and CD47−/− mice were fed OVA and cholera toxin (CT) three times. One week later (a) anti-OVA-specific IgA and (b) total IgA in perfused intestines, or (c) anti-OVA-specific IgA and (d) IgG titres in serum were determined. The graphs show titres from four experiments with (a, b, d) at least three mice or (c) two or three mice in each. Error bars show SD.
These results show that CD47−/− mice have a reduced ability to generate antigen-specific intestinal IgA following oral immunization. However, this does not reflect a general defect in antibody production, as CD47−/− mice exhibit normal levels of total intestinal IgA and a maintained capacity to generate antigen-specific serum IgG and IgA following oral immunizations.
Expression of CD47 on non-haematopoietic cells is required for normal cellularity in GALT and for the generation of OVA-specific intestinal IgA after oral immunizations
To determine if expression of CD47 by haematopoietic cells was sufficient to restore cellularity in GALT, the frequency of CD11b+ DC and the capacity to generate OVA-specific intestinal IgA following immunization, we irradiated CD47−/− mice and introduced WT BM to generate WT/CD47 chimeras. Irradiation controls (CD47/CD47 and WT/WT) were also generated but not CD47/WT, as WT macrophages would phagocytose the CD47-deficient BM cells after transfer.25 Oral immunization with CT influenced neither the total number of cells in GALT nor the frequency of CD11b+ DC 2 weeks after immunization, as no significant differences in either parameter were observed when comparing unimmunized WT mice and mice fed CT three times (data not shown). The three groups of chimeric mice were immunized with OVA and CT three times then the level of OVA-specific intestinal IgA, the cellularity in GALT and the frequency of CD11b+ DC were assessed. Intestinal anti-OVA IgA titres and the total number of cells in the MLN of WT/CD47 mice were significantly lower than in WT/WT mice, but not significantly different from CD47/CD47 mice (Fig. 5a and b). In contrast, the frequency of CD11b+ DC in the spleen of WT/CD47 reached the same level as in WT/WT mice and was significantly higher than in CD47/CD47 mice (Fig. 5c). When the frequency of CD11b+ cells among MHC-IIbright DC in the MLN was determined, although the trend was the same as in the spleen, the individual variance between the mice was too large to obtain a significant difference between the groups (Fig. 5d). These results show that the expression of CD47 on non-haematopoietic cells is required for normal cellularity in GALT and for the generation of OVA-specific intestinal IgA after oral immunizations.
Figure 5.
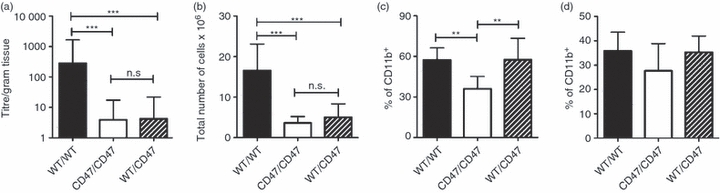
Expression of CD47 by non-haematopoietic cells is required for normal cellularity in gut-associated lymphoid tissue (GALT) and production of ovalbumin (OVA) -specific intestinal IgA following oral immunization. Wild-type (WT)/WT, CD47/CD47 and WT/CD47 mice were fed OVA and cholera toxin (CT) three times. Two weeks later (a) anti-OVA-specific IgA levels in perfused intestines, (b) total number of cells in mesenteric lymph nodes (MLN), frequency of CD11b+ among (c) splenic dendritic cells (DC) and (d) CD11c+ MHC-IIbright MLN DC were determined. The groups contain 6–10 mice. The graphs show results from one representative experiment out of three (a) or two (b, d). Error bars show SD.
Discussion
Intestinal antigen-presenting cells, in particular DC, are key cells for the induction of oral tolerance as well as for generation of protective IgA antibodies secreted into the lumen of the gut.3,4 CD4+ T cells are required in these processes, and recent results suggest that regulatory T cells also play an important role.26 Previous studies have shown that mice lacking CD47 have reduced numbers of CD11b+ DC, an accumulation of regulatory T cells with age, and reduced susceptibility to induced colitis.13,14,18,19 In this study we show that oral immunizations of CD47−/− mice with OVA and CT result in a significantly reduced intestinal anti-OVA IgA response compared with WT mice. It has been shown that PP, and not MLN or isolated lymphoid follicles, are the major site for generation of specific IgA following oral immunization with CT.27 The capacity to activate CD4+ T cells in PP following oral CT administration is reduced in CD47−/− mice, and the antibody responses to CT and co-administered proteins is completely dependent on T cell help;1,2 this could contribute to reduced levels of OVA-specific intestinal IgA in these mice. Interestingly, although loss of CD11b+ DC in the subepithelial dome of the PP has been suggested to cause an incapacity to mount antigen-specific IgA responses in CCR6−/− mice,28 PP is the only GALT in CD47−/− mice that does not have a reduced frequency of this DC subset (before and after administration of CT). In addition, in CD47−/− chimeric mice reconstituted with WT BM, the frequency of DC is restored to WT levels in the spleen with a similar trend in the MLN. Despite this the capacity to generate OVA-specific intestinal IgA following oral immunization with OVA and CT is not regained. Therefore, the defect in OVA-specific IgA production is unlikely to be linked to the reduced frequency of CD11b+ DC, but is rather the result of the lack of CD47 expression by non-haematopoietic cells.
In addition to defective activation of CD4+ T cells in CD47−/− mice, another reason for the reduced levels of OVA-specific intestinal IgA could be that IgA-secreting plasma cells generated in the PP do not properly home to the intestine in CD47−/− mice. This is consistent with the fact that the frequency of OVA-specific IgA-producing cells in the intestine is reduced in CD47−/− mice following immunization with OVA and CT. Entry of plasma cells into peripheral tissues requires extravasation across the blood endothelial wall. As endothelial cells express CD172a, it is possible that interactions between leucocyte CD47 and CD172a on vascular endothelial cells is important for leucocyte transmigration, resulting in impaired ability of plasma cells generated in GALT to leave the circulation and efficiently home to the intestinal tissue in the absence of this bi-directional interaction. In addition, it has been shown that integrin-mediated phosphorylation of CD172a in endothelial cells is greatly reduced if the cells also lack CD47, which could have an impact on endothelial permeability.12 Hence, integrin-mediated transmigration could be hampered even if the leucocyte expresses CD47 if the endothelial cell still lacks this protein. This could possibly explain why reduced levels of anti-OVA-specific IgA are still generated in CD47−/− mice whose haematopoietic compartment is replaced with CD47-sufficient cells. This is also consistent with the normal levels of OVA-specific serum IgA and IgG in CD47−/− mice, as plasma cells secreting these immunoglobulins can reside in the BM without homing to the intestine.
A third explanation for the reduced levels of intestinal anti-OVA IgA is the reduced number of cells in the intestinal tissue in CD47−/− mice. The reduction of cells in GALT was not due to one specific cell type. The only case in which a similar selective reduction or atrophy in GALT has been reported is in mice receiving total parenteral nutrition.29 In contrast to CD47−/− mice, these animals also showed a reduced level of total intestinal IgA. A defect in extravasation from blood vessels into the intestine and GALT, as suggested above for OVA-specific plasma cells, could be applied to all leucocytes and could explain the decreased number of total cells in CD47−/− mice. Maintained levels of total intestinal IgA in CD47−/− mice could be the result of a homeostatic mechanism in place to ensure normal levels of IgA, possibly through generation of IgA-producing cells directly in the intestinal LP.30
We have previously shown that DC are required for activation of CD4+ T cells after antigen feeding.4 In this study, we show a significant reduction in the frequency of CD11b+ cells among CD103+ and CD103− DC in the MLN of CD47−/− mice. We additionally confirm that removal of MLN completely abrogates the capacity to induce oral tolerance.3 It is the CD103+ MLN DC that exclusively present orally administered antigen to T cells ex vivo,21 and this subset has been shown to be gut-derived.23 Furthermore, migration of DC from the gut to the MLN is crucial for the initiation of oral tolerance, as CCR7-deficient mice fail to generate this response.3 However, although CD47−/− mice have reduced cell numbers in their GALT, reduced DC frequencies in MLN, a reduced proportion of CD103+ CD11b+ DC in the LP and MLN, and decreased activation of antigen-specific CD4+ T cells following antigen feeding, their capacity to induce oral tolerance is still maintained. Additionally, in preliminary experiments the capacity to generate OVA-specific FoxP3 regulatory T cells following feeding of OVA was not different between CD47−/− and WT mice (data not shown). These results indicate that the remaining CD11b+ and/or CD11b− DC are sufficient for the induction of oral tolerance in CD47−/− mice. Alternatively, DC are not completely necessary. We have recently shown that feeding high doses of antigen can result in efficient proliferation of CD4+ T cells in DC-depleted mice.4 However, even when a 10-fold lower antigen dose was given orally, the CD47−/− mice were efficiently tolerized.
Our study demonstrates reduced numbers of gut-derived CD11b+ CD172a+ DC and a blunted capacity to expand CD4+ T cells following oral immunization in CD47−/− mice. Importantly, these impairments do not influence the capacity to induce oral tolerance. This shows that decreased T cell proliferation does not necessarily equate to reduced T cell-mediated function. However, CD47−/− mice have a gut-specific defect in total immune cell numbers, and following oral immunization they show reduced levels of antigen-specific intestinal IgA but normal systemic IgA and IgG. Replacing the haematopoietic compartment with CD47-expressing cells does not restore cellularity or the capacity to produce intestinal IgA. This shows that CD47 expressed by non-haematopoietic cells is critical for intestinal B cell IgA responses, most likely by regulating the entry of cells into intestinal tissue, but not for oral tolerance. This suggests that dissimilar CD4 T cell functions control tolerance and enterotoxin-induced IgA immunity in the gut.
Acknowledgments
This study was supported by grants from the Swedish Foundation for Strategic Research, through its support of the Mucosal Immunobiology and Vaccine Centre, the Swedish Research Council (2006-6441, to U.Y. and 2010-4286, to P.A.O.), Jeansson Foundation, Åke Wiberg Foundation, Clas Grochinsky Foundation, Magnus Bergvall Foundation, Golje Foundation, Hierta Foundation, the Royal Arts and Society of Arts and Science in Göteborg, the Umeå University Faculty of Medicine Foundations, and a Young Researcher Award from Umeå University (to P.A.O.).
Disclosures
The authors have no conflict of interest.
Supporting information
Figure S1. Analysis of cell populations in thegut-associated lymphoid tissue of CD47−/− mice.
Figure S2. Reduced frequency ofCD11b+ dendritic cells in the mesenteric lymph nodes ofCD47−/− mice.
Figure S3. Reduced frequency ofCD11b+ conventional dendritic cells in the smallintestinal lamina propria but not Peyer’s patches ofCD47−/− mice.
Figure S4. Mesenteric lymph nodes are required for oral tolerance but not for the generation of antigen-specific IgA following oral immunization.
References
- 1.Hornquist CE, Ekman L, Grdic KD, Schon K, Lycke NY. Paradoxical IgA immunity in CD4-deficient mice. Lack of cholera toxin-specific protective immunity despite normal gut mucosal IgA differentiation. J Immunol. 1995;155:2877–87. [PubMed] [Google Scholar]
- 2.Lycke N, Eriksen L, Holmgren J. Protection against cholera toxin after oral immunization is thymus-dependent and associated with intestinal production of neutralizing IgA antitoxin. Scand J Immunol. 1987;25:413–9. doi: 10.1111/j.1365-3083.1987.tb02208.x. [DOI] [PubMed] [Google Scholar]
- 3.Worbs T, Bode U, Yan S, Hoffmann MW, Hintzen G, Bernhardt G, Forster R, Pabst O. Oral tolerance originates in the intestinal immune system and relies on antigen carriage by dendritic cells. J Exp Med. 2006;203:519–27. doi: 10.1084/jem.20052016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fahlen-Yrlid L, Gustafsson T, Westlund J, et al. CD11chigh dendritic cells are essential for activation of CD4+ T cells and generation of specific antibodies following mucosal immunization. J Immunol. 2009;183:5032–41. doi: 10.4049/jimmunol.0803992. [DOI] [PubMed] [Google Scholar]
- 5.Lindberg FP, Gresham HD, Schwarz E, Brown EJ. Molecular cloning of integrin-associated protein: an immunoglobulin family member with multiple membrane-spanning domains implicated in α v beta 3-dependent ligand binding. J Cell Biol. 1993;123:485–96. doi: 10.1083/jcb.123.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown EJ, Frazier WA. Integrin-associated protein (CD47) and its ligands. Trends Cell Biol. 2001;11:130–5. doi: 10.1016/s0962-8924(00)01906-1. [DOI] [PubMed] [Google Scholar]
- 7.Jiang P, Lagenaur CF, Narayanan V. Integrin-associated protein is a ligand for the P84 neural adhesion molecule. J Biol Chem. 1999;274:559–62. doi: 10.1074/jbc.274.2.559. [DOI] [PubMed] [Google Scholar]
- 8.Oldenborg PA, Zheleznyak A, Fang YF, Lagenaur CF, Gresham HD, Lindberg FP. Role of CD47 as a marker of self on red blood cells. Science. 2000;288:2051–4. doi: 10.1126/science.288.5473.2051. [DOI] [PubMed] [Google Scholar]
- 9.Seiffert M, Cant C, Chen Z, et al. Human signal-regulatory protein is expressed on normal, but not on subsets of leukemic myeloid cells and mediates cellular adhesion involving its counterreceptor CD47. Blood. 1999;94:3633–43. [PubMed] [Google Scholar]
- 10.Barclay AN. Signal regulatory protein α (SIRPα)/CD47 interaction and function. Curr Opin Immunol. 2009;21:47–52. doi: 10.1016/j.coi.2009.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matozaki T, Murata Y, Okazawa H, Ohnishi H. Functions and molecular mechanisms of the CD47-SIRPα signalling pathway. Trends Cell Biol. 2009;19:72–80. doi: 10.1016/j.tcb.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 12.Johansen ML, Brown EJ. Dual regulation of SIRPα phosphorylation by integrins and CD47. J Biol Chem. 2007;282:24219–30. doi: 10.1074/jbc.M701565200. [DOI] [PubMed] [Google Scholar]
- 13.Hagnerud S, Manna PP, Cella M, Stenberg A, Frazier WA, Colonna M, Oldenborg PA. Deficit of CD47 results in a defect of marginal zone dendritic cells, blunted immune response to particulate antigen and impairment of skin dendritic cell migration. J Immunol. 2006;176:5772–8. doi: 10.4049/jimmunol.176.10.5772. [DOI] [PubMed] [Google Scholar]
- 14.Van VQ, Lesage S, Bouguermouh S, et al. Expression of the self-marker CD47 on dendritic cells governs their trafficking to secondary lymphoid organs. EMBO J. 2006;25:5560–8. doi: 10.1038/sj.emboj.7601415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saito Y, Iwamura H, Kaneko T, et al. Regulation by SIRPα of dendritic cell homeostasis in lymphoid tissues. Blood. 2010;116:3517–25. doi: 10.1182/blood-2010-03-277244. [DOI] [PubMed] [Google Scholar]
- 16.Fukunaga A, Nagai H, Noguchi T, et al. Src homology 2 domain-containing protein tyrosine phosphatase substrate 1 regulates the migration of Langerhans cells from the epidermis to draining lymph nodes. J Immunol. 2004;172:4091–9. doi: 10.4049/jimmunol.172.7.4091. [DOI] [PubMed] [Google Scholar]
- 17.Verjan Garcia N, Umemoto E, Saito Y, et al. SIRPα/CD172a regulates eosinophil homeostasis. J Immunol. 2011;187:2268–77. doi: 10.4049/jimmunol.1101008. [DOI] [PubMed] [Google Scholar]
- 18.Fortin G, Raymond M, Van VQ, Rubio M, Gautier P, Sarfati M, Franchimont D. A role for CD47 in the development of experimental colitis mediated by SIRPα+ CD103– dendritic cells. J Exp Med. 2009;206:1995–2011. doi: 10.1084/jem.20082805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van VQ, Darwiche J, Raymond M, Lesage S, Bouguermouh S, Rubio M, Sarfati M. Cutting edge: CD47 controls the in vivo proliferation and homeostasis of peripheral CD4+ CD25+ Foxp3+ regulatory T cells that express CD103. J Immunol. 2008;181:5204–8. doi: 10.4049/jimmunol.181.8.5204. [DOI] [PubMed] [Google Scholar]
- 20.Villavedra M, Carol H, Hjulstrom M, Holmgren J, Czerkinsky C. “PERFEXT”: a direct method for quantitative assessment of cytokine production in vivo at the local level. Res Immunol. 1997;148:257–66. doi: 10.1016/s0923-2494(97)80867-x. [DOI] [PubMed] [Google Scholar]
- 21.Jaensson E, Uronen-Hansson H, Pabst O, et al. Small intestinal CD103+ dendritic cells display unique functional properties that are conserved between mice and humans. J Exp Med. 2008;205:2139–49. doi: 10.1084/jem.20080414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johansson-Lindbom B, Svensson M, Pabst O, Palmqvist C, Marquez G, Forster R, Agace WW. Functional specialization of gut CD103+ dendritic cells in the regulation of tissue-selective T cell homing. J Exp Med. 2005;202:1063–73. doi: 10.1084/jem.20051100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schulz O, Jaensson E, Persson EK, Liu X, Worbs T, Agace WW, Pabst O. Intestinal CD103+, but not CX3CR1+, antigen sampling cells migrate in lymph and serve classical dendritic cell functions. J Exp Med. 2009;206:3101–14. doi: 10.1084/jem.20091925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hahn A, Thiessen N, Pabst R, Buettner M, Bode U. Mesenteric lymph nodes are not required for an intestinal immunoglobulin A response to oral cholera toxin. Immunology. 2010;129:427–36. doi: 10.1111/j.1365-2567.2009.03197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blazar BR, Lindberg FP, Ingulli E, Panoskaltsis-Mortari A, Oldenborg PA, Iizuka K, Yokoyama WM, Taylor PA. CD47 (integrin-associated protein) engagement of dendritic cell and macrophage counterreceptors is required to prevent the clearance of donor lymphohematopoietic cells. J Exp Med. 2001;194:541–9. doi: 10.1084/jem.194.4.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cong Y, Feng T, Fujihashi K, Schoeb TR, Elson CO. A dominant, coordinated T regulatory cell-IgA response to the intestinal microbiota. Proc Natl Acad Sci U S A. 2009;106:19256–61. doi: 10.1073/pnas.0812681106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamamoto M, Kweon MN, Rennert PD, Hiroi T, Fujihashi K, McGhee JR, Kiyono H. Role of gut-associated lymphoreticular tissues in antigen-specific intestinal IgA immunity. J Immunol. 2004;173:762–9. doi: 10.4049/jimmunol.173.2.762. [DOI] [PubMed] [Google Scholar]
- 28.Cook DN, Prosser DM, Forster R, et al. CCR6 mediates dendritic cell localization, lymphocyte homeostasis, and immune responses in mucosal tissue. Immunity. 2000;12:495–503. doi: 10.1016/s1074-7613(00)80201-0. [DOI] [PubMed] [Google Scholar]
- 29.Keith Hanna M, Zarzaur BL, Jr, Fukatsu K, Chance DeWitt R, Renegar KB, Sherrell C, Wu Y, Kudsk KA. Individual neuropeptides regulate gut-associated lymphoid tissue integrity, intestinal immunoglobulin A levels, and respiratory antibacterial immunity. JPEN J Parenter Enteral Nutr. 2000;24:261–8. doi: 10.1177/0148607100024005261. discussion 8–9. [DOI] [PubMed] [Google Scholar]
- 30.Fagarasan S, Kinoshita K, Muramatsu M, Ikuta K, Honjo T. In situ class switching and differentiation to IgA-producing cells in the gut lamina propria. Nature. 2001;413:639–43. doi: 10.1038/35098100. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


