Summary
The dimeric transcription factor nuclear factor κB (NF-κB) functions broadly in coordinating cellular responses during inflammation and immune reactions, and its importance in the pathogenesis of cancer is increasingly recognized. Many of the signal transduction pathways that trigger activation of cytoplasmic NF-κB in response to a broad array of immune and inflammatory stimuli have been elaborated in great detail. NF-κB can also be activated by DNA damage, though relatively less is known about the signal transduction mechanisms that link DNA damage in the nucleus with activation of NF-κB in the cytoplasm. Here, we focus on the conserved signaling pathway that has emerged that promotes NF-κB activation following DNA damage. Post-translational modification of NF-κB essential modulator (NEMO) plays a central role in linking the cellular DNA damage response to NF-κB via the ataxia telangiectasia mutated (ATM) kinase. Accumulating evidence suggests that DNA damage-dependent NF-κB activation may play significant biological roles, particularly during lymphocyte differentiation and progression of human malignancies.
Keywords: NF-κB, NEMO, ATM, SUMO, DNA damage, Genotoxic stress
Personal and historical narrative commemorating 25 years of NF-κB
As part of this special issue commemorating the 25th anniversary of the discovery of nuclear factor κB (NF-κB), I (S. Miyamoto) was asked to include a brief account of my scientific interests relating to activation of NF-κB by DNA-damaging agents. After obtaining an independent faculty position, I wished to start a new line of research. However, as with any other scientific quests, identifying and deciding what specific puzzle to tackle was challenging. Obviously, the task needed to be carefully weighed against limited amounts of time (ticking tenure clock) and financial and personnel resources. The answer came in the form of a side project that then led to a much bigger puzzle. One of my colleagues in the department at that time, David Boothman, suggested that we run a pilot experiment to test whether NF-κB could be activated by β-lapachone, a chemical that was thought to inhibit topoisomerase I (Top I) activity. We used a pre-B-cell line and gel shift assays (EMSA) to test this. β-lapachone did activate NF-κB but did so very poorly in comparison to our positive control lipopolysaccharide (LPS) stimulation. However, another inhibitor of Top I, Camptothecin (CPT), induced a robust activation of NF-κB in this cell system.
At this point, a light went on with a basic question: how could nuclear DNA damage induce activation of NF-κB that was known at the time to be sequestered and activated in the cytoplasm? This is backward of a typical signaling pathway that begins at a cell surface receptor. Had this been described by others? We quickly collected and eagerly read all the papers that we could identify in the literature describing NF-κB activation by different DNA-damaging agents. At that time (early 1996), the concept of ‘nuclear-to-cytoplasmic’ signaling to activate NF-κB by ultraviolet-C (UV-C) radiation exposure had been presented in 1989 by Peter Herrlich’s group (1). However, we could not find any mechanistic solution to this interesting question. Moreover, although the functional significance of NF-κB activation by various DNA-damaging agents had been continually investigated, it appeared that the mechanism regarding nuclear-to-cytoplasmic signaling was not being tackled in the literature. It also appeared that ‘DNA damage’ was vaguely used to describe the signaling event. However, it was totally unclear which types of DNA damage were actually involved, what exactly happened after DNA damage, and whether DNA damage was sufficient to induce such a signaling pathway. Furthermore, mitochondria harbor DNA outside the nucleus, so it was not even clear whether the nucleus was involved or not. It was possible that mitochondrial DNA damage could be a cytoplasmic signal-initiation event. In the meantime, there was already accumulated evidence that nuclear DNA damage was probably not even involved in activation of NF-κB by UV-C (2-4). Thus, even if we hypothesized nuclear DNA damage as being the cause of such an activation pathway, it was unclear what exactly the ‘nuclear signal’ was that left the nucleus to communicate with latent cytoplasmic NF-κB. Was it a protein, nucleic acid, or other macromolecules? Could it be a second messenger like calcium? Existing literature did not reveal known signaling pathways that involved a nuclear-initiated signal that communicated with cytoplasmic targets. The only thing that could remotely fit this idea was mRNAs that are generated in the nucleus and exported to the cytoplasm to produce encoded protein products. However, it did not fit the notion of ‘signal transduction’, like those involved in growth factor signaling or cytokine signaling—classical models of intracellular cell signaling.
The next several years were spent analyzing different cell lines and cell systems (including almost all available normal human cell types that could be purchased from Clonetics) with different classes of DNA-damaging agents using gel shift assays to first understand what efficiently activated NF-κB and in which cell systems. Based on these initial analyses, we selected model cell systems to tackle the mechanism(s) involved. We experimentally evaluated the requirement of nuclear DNA damage by assessing activation in cytoplasts that contained mitochondria but were devoid of intact nuclei (5). Given that our initial observations utilized the Top I inhibitor CPT, we used a cell system developed by Yves Pommier that harbored mutated nuclear Top I that was defective for CPT interaction but with intact mitochondrial Top I that maintained normal CPT interaction to test the requirement of nuclear versus mitochondrial TopI. We assessed NF-κB activation in different cell cycle phases using different DNA-damaging agents to ask whether this activation was coupled to specific cell cycle phases or not. We employed restriction enzymes capable of producing different DNA end structures and electroporated different forms of DNA (naked double-stranded-DNA, mono-nucleosomes, and poly-nucleosomes) (6). These early studies led to the general conclusion that nuclear DNA double strand breaks (DSBs) are among the most potent DNA damage signals to activate NF-κB and revealed no requirement for cell cycle progression. Moreover, this activation did not require new protein synthesis and occurred within 1-2 h after break induction through the activation of the canonical inhibitor of κB (IκB) kinase (IKK) complex and IκBα degradation. However, we still did not have any idea how nuclear DSBs affected these cytoplasmic events to activate NF-κB. Although other DNA-damaging agents, like aphidicolin or hydroxyurea, could induce NF-κB very weakly after prolonged exposures, we focused our attention on dissecting early activation mechanisms that did not require prolonged chemical exposure to avoid complications of potential secondary events.
Very early on we learned that the DSB-responsive nuclear kinase, ataxia telangiectasia mutated (ATM), was required for this signaling process, but we could not obtain any evidence for the involvement of any known downstream effectors of ATM, including the canonical downstream checkpoint kinase Chk2, suggesting the possibility of a specific ATM substrate designated for NF-κB signaling. Critical insight came from reconstitution experiments with 1.3E2 NEMO-deficient mouse pre-B cell system that we obtained from Alan Israel’s group. In particular, we obtained a robust phenotype with a C-terminal zinc finger (ZF) deletion mutant of NEMO, which gave completely defective NF-κB signaling with all DNA-damaging agents we tested while LPS-induced activation was relatively normal (7). Additional investigation led to a breakthrough—small ubiquitin like modifier-1 (SUMO-1) modification of NEMO that was selectively induced in response to DNA-damaging agents in a manner dependent on the ZF but induced not by lipopolysaccharide (LPS) or tumor necrosis factor (TNF) stimulation (8). Characterization of the events associated with NEMO SUMOylation began to unravel how this DSB-dependent NF-κB signaling pathway operates.
The following sections elaborate on key aspects of this NF-κB signaling pathway induced by nuclear DSBs and provide an update on additional mechanisms and insights that have emerged from the efforts of multiple laboratories. Elsewhere in this special issue of Immunological Reviews (Volume 246), the reader may find a more general introduction to NF-κB signaling. We also touch upon newly emerging biological and pathological roles for this pathway and finally address some perspectives focusing on outstanding questions.
Brief overview of DNA damage response
Cells are continuously faced with threats of damage to their genomes through endogenous sources as well as exposures to exogenous agents. In order to maintain the integrity of their genetic material, an elaborate signal transduction network, collectively termed the DNA damage response (DDR) has evolved in eukaryotes. DDR components collectively sense damage, amplify the signal, and activate downstream effectors through coordinated cascades of post-translational modifications that serve specific functions to repair damage and modulate appropriate cellular responses (9,10). The DDR is choreographed by a family of phosphatidylinositol 3-kinase-like proteins—ATM, ATR and DNA-PK—whose actions coordinate downstream effectors in part through phosphorylation. Recruitment to break sites is mediated by specific phosphopeptide binding motifs (e.g. FHA) (11) present in many DDR components that themselves contain additional enzymatic functions. These components include enzymes that catalyze a host of post-translational modifications, including ubiqutination, SUMOylation and poly(ADP-ribosylation) that are all important for efficient DDR signaling (12,13). Double strand breaks are among the most toxic forms of DNA damage and are principally sensed and responded to by the kinase ATM. ATM has a major functional role in promoting transcriptional programs to DSBs through direct phosphorylation of transcription factors such as the tumor suppressor p53 (14,15). It has been known for some time that DNA damage was capable of activating the transcription factor NF-κB, and multiple early reports clearly implicated ATM as a requirement for its activation (8,16-18), though the mechanism remained obscure. In the years since, it has emerged that signaling pathways involving ATM and NEMO cooperate to directly link DNA damage events in the nucleus with the activation of NF-κB in the cytoplasm.
Mechanism of DNA damage-dependent NF-κB activation
Nuclear events: post-translational modifications of NEMO
Many known stimuli that activate NF-κB require signaling through the conserved IKK complex consisting of at least two catalytic subunits, IKKα and IKKβ, and a regulatory subunit NEMO (aka IKKγ) (Reviewed in Liu et al., this volume). Studies in cell lines deficient in IKK components demonstrated that IKK is also required for NF-κB activation following DNA damage. Significant progress regarding the role of IKK has been made utilizing the NEMO-deficient 1.3E2 pre-B-cell line, which is unresponsive to many known NF-κB activators (19-21). When stably reconstituted with wildtype NEMO, NF-κB activation in this cell line is restored (7,21), enabling their use as a robust somatic genetic model system to address the functional requirements of NEMO in vivo. NEMO consists of two coiled coil domains (designated CC1 and CC2), a leucine zipper, and a C-terminal zinc finger (ZF) domain (22). Efforts to determine the domain(s) required for NEMO function revealed that deletion or mutation of the C-terminal ZF domain completely abrogated NF-κB activation following treatment with DNA-damaging agents. Surprisingly, the ZF domain was not required for NF-κB activation following treatment with canonical stimuli (e.g. LPS). This led to the hypothesis that NEMO has a unique IKK-independent role mediated by the ZF domain that is required for DNA damage-dependent NF-κB activation.
Consistently, the ZF domain is required for an essential cascade of post-translational modifications of NEMO that occur in the nucleus following exposure to DNA damage that include SUMOylation, phosphorylation, and ubiquitination.Upon genotoxic stress, NEMO is modified by SUMO-1 at lysine residues 277 and 309 by the SUMO E3 ligase protein inhibitor of activated STATy (PIASy aka PIAS4) (8, 23, 24). Mutation of the SUMO-1 acceptor lysines has minimal impact on canonical NF-κB signaling but completely abrogates DNA damage-induced NF-κB activation. Genetic analysis suggests that the NEMO ZF domain is necessary to promote NEMO SUMOylation. Surprisingly, in-frame fusion of SUMO-1 to the N-terminus of NEMO ZF mutants is sufficient to complement the DNA damage-induced NF-κB activation defect observed in ZF mutant alleles. Together, these data suggest the necessary and sufficient function of the NEMO ZF domain is to promote NEMO SUMOylation in response to DNA damage. It is currently unclear how the ZF domain controls SUMOylation. One attractive hypothesis was that the ZF domain is required for mediating the interaction between NEMO and the SUMO E3 ligase. However the interaction of NEMO with PIASy does not require the ZF but rather is mediated by the CC1 region of NEMO located at the N-terminus. Interestingly, the location of NEMO:PIASy interaction overlaps the IKK kinase-binding domain of NEMO, and interaction between NEMO, PIASy and IKKβ are mutually exclusive (24). This underscores the unique role that NEMO plays in mediating nuclear signal transduction events that are independent of IKK binding and of its role as an IKK subunit.
Consistent with other reported SUMO-1 substrates (25-27), SUMOylation appears to alter the intracellular distribution of NEMO to promote its nuclear localization. Whereas wildtype NEMO can localize to the nucleus upon stimulation, non-SUMOylatable NEMO is observed to be almost exclusively cytoplasmic following DNA damage and is thus unable to promote NF-κB activation. Further, in-frame SUMO-1 fusion leads to constitutive nuclear localization of NEMO, even in the absence of DNA damage. The mechanism by which SUMOylated NEMO is localized to the nucleus is still obscure.
Building on these initial works, new molecular players have been introduced that in some cellular contexts can modulate NEMO SUMOylation. P53 inducible death domain-containing protein (PIDD), initially shown to activate caspase-2 following DNA damage, was reported to nucleate a nuclear signaling complex containing receptor-interacting protein 1 (RIP1) that promotes SUMOylation of NEMO (28). The physiologic relevance of this so-called PIDDosome complex was bolstered by the observation that RIP1 was required for NF-κB activation by genotoxic agents (29). However, a recent report has revealed an essential cytoplasmic function for RIP1 (discussed below) (30). Further, apoptotic responses to DNA-damaging agents in cells derived from Pidd–/– mice are largely unperturbed, suggesting Pidd is not essential (31).
Poly(ADP-Ribose) polymerase-1 (PARP-1) was recently shown to be necessary for NEMO SUMOylation following DNA damage (23). PARP-1 is capable of directly sensing DNA damage and catalyzes the formation of polyADP-ribose (PAR) on target substrates—including on PARP-1 itself—that can serve as scaffolds for recruitment of additional signaling and repair proteins. Automodified PARP-1 was shown to rapidly assemble a signaling complex containing NEMO, PIASy ,and ATM following DNA damage that promotes NEMO SUMOylation. Complex formation is dependent on both the catalytic activity and DNA binding domains of PARP-1, as well as a PAR binding domain present in the C-terminus of PIASy. The interaction of NEMO with PARP-1 and PIASy maps to similar regions in the NEMO N-terminus that are exclusive of IKK binding (23, 24). Consistent with the formation of a PAR-mediated signaling complex, the interactions of NEMO with both PARP-1 and PIASy appear to be co-dependent and require PAR synthesis following DNA damage in cells. However, binary interactions of NEMO:PIASy and NEMO:PARP-1 can be observed with recombinant components in vitro, even in the absence of PAR chains, suggesting PAR scaffolds may be required to enhance binding among these proteins in vivo. The work on PARP-1-mediated NEMO SUMOylation is discordant with several previous reports highlighting the role of PARP-1 in DNA damage induced NF-κB activation as an essential transcriptional co-factor of the p65 subunit of previously activated NF-κB (32-35). In these studies, PARP-1 was shown to be essential for DNA-damage induced NF-κB transcriptional activity (32), but its function in this capacity is independent of both PARP-1
DNA binding and catalytic activity (34). Furthermore, both IκBα degradation and p65 nuclear translocation occurred normally in Parp1–/– MEFs following DNA damage (32,36). PARP-1 binding to p65 overlaps the Rel homology domain (RHD), which might be expected to directly inhibit p65 DNA binding function. Moreover, PARP-1 may function in a cell-type or stimulus-specific manner, as clinically relevant PARP-1 inhibitors fail to inhibit NF-κB activation in leukemic T cells following DNA damage (37). Future experiments are required to reconcile these apparent incompatibilities and delineate the precise functional role of PARP-1 in DNA damage induced NF-κB activation.
Multiple lines of evidence demonstrate that NEMO is also phosphorylated on serine 85 (S85) by the DSB-responsive kinase ATM following DNA damage (8, 38). This observation corroborated multiple early reports demonstrating an essential function for ATM in mediating NF-κB activation following ionizing radiation or treatment with chemical DNA-damaging agents (8,16-18). Indeed, intact NEMO S85 is required for DNA damage-induced NF-κB activation and cell survival, providing a mechanistic link between the cellular DNA damage response and the stress-responsive transcription factor NF-κB. The precise function of NEMO phosphorylation is currently unclear, though ATM activity and intact NEMO S85 are prerequisites for subsequent NEMO monoubiquitination (discussed below). It is unclear if NEMO phosphorylation and SUMOylation are directly coupled, as some cellular stresses that activate ATM kinase activity do not result in NEMO SUMOylation or NF-κB activation (6). Additionally, NEMO SUMOylation is not sensitive to ATM kinase inhibitors, and NEMO alleles that cannot be phosphorylated (e.g. S85A) are still SUMOylated following DNA damage (8, 38). Nevertheless, NEMO SUMOylation and phosphorylation are both required for NF-κB activation following DNA damage. Collectively, these data suggest a model where coincident SUMOylation and phosphorylation of NEMO represents a necessary point of convergence for independent nuclear signal transduction pathways required for subsequent NF-κB activation in the cytoplasm.
The only other currently known function of NEMO phosphorylation is as a prerequisite for monoubiquitination of NEMO on lysine residues 277 and 309 (8,38). It has been suggested that the E3 ubiquitin ligase required for NEMO monoubiquitination is cellular inhibitor of apoptosis-1 (cIAP1) (39). NEMO monoubiquitination also requires intact NEMO S85 and ATM kinase activity, suggesting prior phosphorylation of NEMO is essential. However, in vitro ubiquitination assay using recombinant cIAP1 clearly showed specificity to lysine 277and 309 but did not appear to show this phosphorylation requirement. In-frame fusion of ubiquitin to the N-terminus of NEMO-S85A is able to fully complement the NF-κB activation defect observed in NEMO S85A cells, consistent with the idea that phosphorylation is upstream of monoubiquitination in vivo (38). Given that NEMO monoubiquitination is dependent on the same acceptor lysines as SUMOylation, it has been difficult to clearly establish if NEMO deSUMOylation is required prior to ubiquitination or if both modifications can coexist. However, kinetic analysis of NEMO modifications indicates that NEMO proceeds through transient SUMOylation prior to phosphorylation and consequent ubiquitination (8, 38). Though the function of NEMO monoubiquitination is not fully elucidated, available data suggest that ubiquitination, like SUMOylation, regulates the subcellular localization of NEMO. However in contrast to SUMOylation, ubiquitination appears to promote nuclear export of NEMO to promote downstream signal transduction events. Whereas the non-ubiquitinated NEMO S85A remains nuclear following DNA damage, in-frame fusion of ubiquitin to the NEMO N-terminus distributes NEMO S85A preferentially to the cytoplasm. NEMO monoubiquitination correlates with the emergence of a cytoplasmic NEMO:ATM:IKK complex that promotes subsequent NF-κB activation (38). Whether NEMO monoubiquitination is directly involved in NEMO nuclear export to promote downstream signal transduction remains a significant unanswered question.
Cytoplasmic events: activation of TAK1 and IKK
Although ubiquitin fusion rescues the function of NEMO-S85A, NF-κB activation following DNA damage remains dependent on ATM kinase activity in cells expressing Ub-NEMO-S85A. Moreover, ubiquitinated NEMO can partition a small fraction of ATM to the cytoplasm. These observations suggest that additional signaling event(s) can be coordinated by ATM in the cytoplasm (38). Several recent reports are beginning to shed light on ATM’s putative cytoplasmic roles in mediating NF-κB activation following DNA damage through an additional kinase transforming growth factor β-activated kinase-1 (TAK1). TAK1 was previously known to be required for NF-κB activation in response to multiple stimuli (40). TAK1 exists in a multisubunit kinase complex with its binding proteins TAB1 and TAB2, or the TAB2-related TAB3 (41). The TAK1 complex is activated through TAB2/3-mediated binding to K63-linked polyubiquitin chains following activation of multiple cell surface receptors (42). In the canonical NF-κB activation pathway, TAK1 mediates IKK activation through phosphorylation of T-loop serines 177 and 181 of IKKβ. Indeed, TAK1 appears also to be required for activation of IKK and NF-κB following DNA damage stimuli. Jin et al. (39) first suggested that TAK1 is involved in DNA damage-induced NF-κB activation. Three groups (30, 43, 44) have independently demonstrated in ATM- and TAK1-deficient cells that ATM-dependent TAK1 activation is required for NF-κB activation following DNA damage. However, several alternative models have been proposed that each address the mechanism of DNA damage induced TAK1 activation.
Jin et al. (39) demonstrated that TAK1 activation requires the function of the ubiquitin E3 ligase X-linked inhibitor of apoptosis (XIAP). XIAP has been previously implicated in TAK1 activation, as the BIR domain of XIAP can directly interact with the TAB1 subunit of the TAK1 complex (45, 46). However, while expression of full length XIAP in XIAP-deficient cells was able to restore TAK1 activation following DNA damage, deletion of either the BIR domain or the catalytic RING domain led to defective TAK1 activation (39). This finding suggests that both direct XIAP:TAB1 interaction as well as additional signaling events mediated by the ubiquitin ligase function of XIAP are required.
TAK1 activation also requires polyubiquitination of ELKS [a protein rich in glutamate, leucine, lysine (K), and serine]. ELKS had been previously demonstrated to be required for NF-κB activation following DNA damage and TNF stimulation though the mechanism was initially unclear (38, 47). Utilizing cells derived from ELKS–/– mice, Wu et al. (43) demonstrated that TAK1 activation is defective in the absence of ELKS. The interaction between ELKS and TAK1 is dependent on NEMO and ATM. Consistent with XIAP’s function as a ubiquitin ligase, polyubiquitination of ELKS is dependent on both XIAP and the K63-specific E2 enzyme Ubc13. Polyubiquitinated ELKS nucleates a complex containing NEMO, ATM, ELKS, and TAK1 in the cytoplasm. Furthermore, binding of TAK1 to ELKS is dependent on the polyubiquitin binding function of the TAB2 subunit of TAK1. Though TAK1 activation ultimately appears dependent upon NEMO, the ubiquitin binding function mediated by the UBAN (aka NOA or NUB) domain of NEMO is dispensable for TAK1 activation. This favors the interpretation that direct interaction with NEMO and ELKS mediates an essential but insufficient step in TAK1 activation that further relies on XIAP-dependent ubiquitination of ELKS to recruit and assemble TAK1.
An alternative model of ATM-mediated TAK1 activation relies on the DNA damage-dependent automodification of the ubiquitin E3 ligase TNF receptor (TNFR)-associated factor 6 (TRAF6) (44). In this case, ATM is exported to the cytoplasm in a calcium-dependent manner, which can then activate TRAF6 autoubiquitination. This is presumed to occur through direct binding mediated by a putative consensus TRAF6 binding motif present in ATM. The mechanism by which ATM binding stimulates TRAF6 catalytic activity is unclear; TRAF6 dimerization has been shown to be crucial for TRAF6 activation and autoubiquitination (48).
Another mechanism of TAK1 activation has been proposed recently implicating a cytoplasmic role for RIP1 (30), a protein previously demonstrated to be essential for DNA damage induced NF-κB activation (29). In this case, RIP1 is polyubiquitinated by K63-linked chains. RIP1 ubiquitination requires prior DNA damage-dependent SUMOyation of RIP1 by the SUMO ligase PIASy. Modification of RIP1 correlates with the assembly of a cytoplasmic complex containing at least NEMO, ATM, RIP1, and TAK1 and IKKβ. A RIP1 mutant (RIP1-4KR) that cannot be modified by SUMOylation fails to interact with TAK1, whereas in-frame fusion of SUMO1 to RIP1-4KR restores polyubiquitination of RIP1 and its interaction with TAK1(30).
It is currently difficult to reconcile these models of TAK1 activation into a coherent unified picture of TAK1 activation following DNA damage. Nevertheless, highlighting several points of overlap in the above studies may promote future experiments to clarify the mechanism(s) by which TAK1 is activated. First, TAK1 is required for DNA damage induced NF-κB activation based on multiple studies utilizing TAK1 knockdown or TAK1-deficient cells (30, 39, 43, 44). Second, TAK1 activation requires ATM activity (30, 43, 44). There is currently no evidence that ATM directly phosphorylates TAK1; rather, available data suggest it is required for mediating signal transduction events that trigger TAK1 activation. Third, the shared observations that TAK1 activation is dependent on Ubc13 and the K63-specific ubiquitin binding subunit TAB2 collectively argue for a general role of K63 polyubiquitination in mediating TAK1 activation (43, 44). This mirrors the canonical pathway of NF-κB activation, where TAK1 is activated by TAB2-mediated recruitment to K63-linked polyubiquitin chains attached to target substrates following cell surface receptor stimulation (42). Different E3 ligases and substrates of ubiquitination in the DNA damage pathway have been implicated in different studies. Importantly, where differences do exist in the above studies, it is worth noting that all groups have relied on different cell types and varying doses of genotoxic agents. Thus, there may exist cell type-specific pathways of TAK1 activation following DNA damage, where different cell types could employ a unique cohort of signaling proteins to promote TAK1 activation. This may reflect an important point of cell type specification in the mechanism of TAK1 activation.
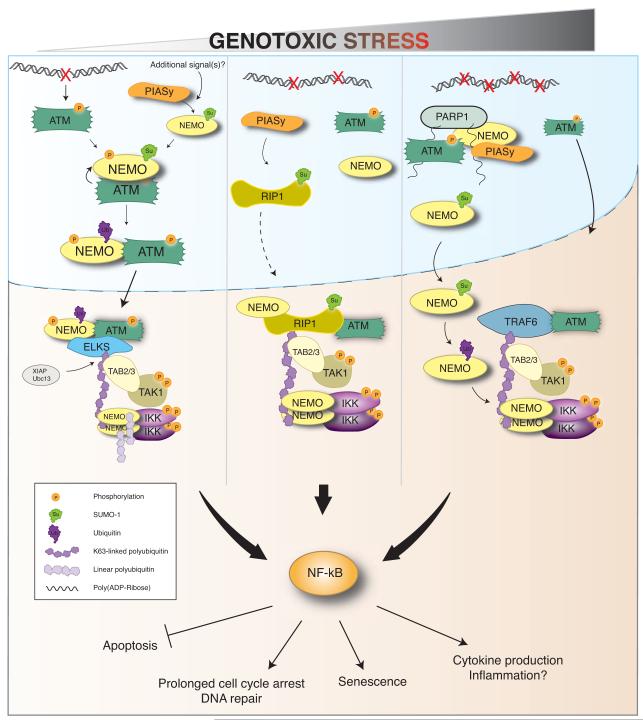
NF-κB activation additionally requires the function of the conserved cytoplasmic IKK complex. IKK is essential for NF-κB activation following DNA damage and is required for viability of mice following total body irradiation (49). The precise mechanism by which IKK activation occurs in the cytoplasm—even in the relatively more studied canonical NF-κB pathway—is still not clear (22). There is similar lack of clarity regarding the mechanism of IKK activation downstream of DNA damage. Here again, several groups have postulated alternative mechanistic explanations of IKK activation. Based on their observation that XIAP was required for both TAK1 and IKK activation, Jin et al. (39) postulated that XIAP is required for recruitment of activated TAK1 to the IKK complex. Consistent with this, ELKS polyubiquitination mediated by XIAP appears to facilitate recruitment of the IKK complex to activated TAK1 (43). Hinz et al. (44) observed cytoplasmic monoubiquitination of NEMO K285, dependent on TRAF6 and cIAP1 that is required for subsequent IKK activation, though the mechanism remained obscure. More recently, Niu et al. (50) have uncovered a requirement for the LUBAC linear ubiquitination complex in DNA damage induced NF-κB activation that expands on several reports that have suggested LUBAC-mediated linear ubiquitination of NEMO is important for canonical IKK activation (51-55). Following DNA damage, NEMO can be linear ubiquitinated on K285/309, in a manner dependent on previous ELKS K63 polyubiquitination. LUBAC also appears to be required for full TAK1 activation following DNA damage, presumably through recruitment via the ubiquitin-binding TAB2 subunit (50). It has been hypothesized that NEMO linear ubiquitination triggers a conformational change that is transmitted to the NEMO kinase binding domain to directly activate IKK (56). Alternatively, NEMO can also associate with linear ubiquitin chains through its UBAN domain which could cluster IKK in close proximity with active TAK1 to promote phosphorylation of serines in the activation loop of IKK. Additional experiments will likely shed light on the mechanistic function of these complex patterns of ubiquitination that can be observed following DNA damage. Clearly, post-translational modifications play a pervasive role in multiple steps leading to NF-κB activation following DNA damage. Fig. 1 outlines a proposed schematic model to incorporate the observations from multiple groups where different doses of genotoxic agents are employed to observe the different mechanisms.Table 1 summarizes the available information regarding substrates, sites of modification, and responsible enzymes for various components in this pathway.
Fig. 1. Proposed schematic model for DNA damage dependent NF-κB activation.
Following DNA damage, nuclear events trigger activation of ATM and post-translational modification of NEMO and RIP1. These nuclear events are transduced to cytoplasmic signaling complexes that mediate the activation of TAK1 and ultimately IKK. Depending on the severity of genotoxic stress (and cell type), multiple signal transduction mechanisms may be engaged to promote NF-κB activation. Several cellular fates can result to encourage cell survival. In the event of overwhelming damage, additional mechanisms— both dependent and independent of NF-κB—may be employed to promote programmed cell death (e.g. Ripoptosome assembly).
Table 1.
Summary of post-translational modifications implicated in DNA damage-dependent NF-κB activation.
| Substrate | Modification | Site | Modifying Enzyme(s) | Function | References |
|---|---|---|---|---|---|
| NEMO | SUMO-1 | Lysines 277/309 | PIASy | Altered subcellular localization | (8, 23, 24) |
| Phosphorylation | Serine 85 | ATM | Unknown; Permits ubiquitination | (8, 38) | |
| Monoubiquitination | Lysines 277/309 | cIAP1 | Altered subcellular localization | (8, 38, 39) | |
| Monoubiquitination | Lysine 285 | TRAF6 and/or cIAP1 | Unknown | (44) | |
|
| |||||
| ATM | Phosphorylation | Serine 1981 | ATM | ATM activation | (6, 38) |
|
| |||||
| RIP1 | SUMOylation | Lysines 105/140/305/565 |
PIASy | TAK1 interaction; Permits ubiquitination |
(30) |
| K63-linked polyubiquitination |
Unknown | E2: Ubc13 E3: Unknown |
Unknown | (30) | |
|
| |||||
| ELKS | K63-linked polyubiquitination |
Unknown | E2: Ubc13; E3: XIAP |
TAK1 interaction; IKK interaction | (43) |
|
| |||||
| TRAF6 | K63-linked polyubiquitination |
Unknown | E2: Ubc13; E3: TRAF6 (automodification) |
TAK1 activation; Permits NEMO K285 monoubiquitination |
(44) |
|
| |||||
| IKKε | SUMOylation | Lysine 231 | TOPORS | Localization to PML nuclear bodies | (26) |
| p65 | Phosphorylation | Serine 468 | IKKε | Transcriptional Activation | (26) |
Activation of noncanonical NF-κB pathways by DNA damage
While much previous work has focused on delineating the mechanisms that link DNA damage to the canonical IKK-NF-κB pathway, DNA damage can also lead to activation of noncanonical NF-κB pathways. Nuclear localization of RelB is observed in prostate cancer cells following ionizing radiation and correlates with poor prognosis and protection from cell death (57-59). Though the mechanism of p100 processing and consequent RelB nuclear localization following DNA damage is unclear, available data suggest multiple upstream kinases may be involved (60). In osteosarcoma cell lines, p100 phosphorylation and subsequent processing to p52 can be observed following multiple forms of DNA damage. Based on short interfering RNA knockdown data, p100 phosphorylation is dependent on ATM, IKKα, and NEMO. Nuclear targets of IKKα have been observed in other contexts (61, 62); however, it is unknown if ATM and/or NEMO are involved in transducing DNA damage signals directly to IKKα to mediate p100 processing. Renner et al. (26) observe that the IKK-related kinase IKKε is inducibly SUMOylated and translocated to the nucleus in a manner dependent on IKKε kinase activity and lysine 231 as the SUMO acceptor residue. Moreover, SUMOylation is mediated by the SUMO E3 ligase TOPORS and targets IKKε to PML nuclear bodies. IKKε retained in PML nuclear bodies promotes phosphorylation of serine 468 in the transactivation domain of the p65 NF-κB subunit to promote transcription of target genes.
Negative regulation of DNA damage-dependent NF-κB activation
Downregulation of NF-κB activation is critical for limiting constitutive NF-κB activity. Several modes of negative regulation are intrinsic to the NF-κB pathway, and more continue to emerge (63). A well-established mechanism of negative regulation includes the direct NF-κB-dependent induction of IκBα that mediates post-activation repression of NF-κB following stimulation with multiple stimuli, including following DNA damage (64, 65). This strong negative feedback suppresses NF-κB activation globally, without altering upstream signaling events such as IKK activation. Additional levels of negative regulation that attenuate signal transduction pathways that lead to IKK activation after DNA damage are beginning to emerge. SUMOylation is an important post-translational modification in multiple signal transduction pathways, including as an essential event in DNA damage-dependent NF-κB activation. Cells have evolved mechanisms to counteract SUMOylation through the action of SUMO proteases—SENPs—that can hydrolyze the isopeptide linkage between the SUMO C-terminus and modified lysines in SUMOylated substrates. SENP2 has been identified as the primary SUMO protease that deSUMOylates NEMO in vivo (66). Overexpression of SENP2 leads to NEMO deSUMOylation and decreased NF-κB activation following DNA damage. Importantly, MEFs from SENP2–/– mice show more rapid activation kinetics, heightened maximal NF-κB activation, and a more prominent second wave of NF-κB activation following DNA damage as compared to control cells. Moreover, SENP2–/– MEFs are protected from apoptotic cell death following DNA damage. More surprisingly, SENP2 and the related SENP1 were revealed as direct transcriptional targets of NF-κB following DNA damage but not TNF stimulation. How DNA damage specifically promotes SENP2 induction is explained in part by ATM-dependent changes in histone modifications overlapping the NF-κB binding sites in the regulatory region of this locus. In addition to NEMO and RIP1, SUMOylation of proteins involved in the DDR has been shown to be required for their accumulation at sites of DNA damage (12,67). Thus, NF-κB-dependent SENP induction may be a generalized self-limiting response to DNA damage.
The pervasive role of ubiquitination in multiple NF-κB activation pathways has led to the discovery of multiple deubiquitinases (DUBs) that can negatively regulate NF-κB activation (68-71). The CYLD tumor suppressor gene encodes a DUB that has been shown to antagonize ubiquitination of ELKS and NEMO and limits NF-κB activation following DNA damage. Whether CYLD expression can be induced by NF-κB specifically following DNA damage as a means of feedback inhibition remains to be determined.
Multiple additional mechanisms of negative regulation of NF-κB activation following DNA damage may exist, but they remain uncharacterized. For example, the action of PARP-1 can be antagonized by Poly(ADP-ribose) glycohydrolases (PARGs) that disassemble PAR chains synthesized by PARP-1. A final step in apoptotic cell death includes the caspase-mediated cleavage of PARP-1. In cells fated to undergo cell death following DNA damage, PARP-1 cleavage may limit any further activation of NF-κB. Undoubtedly, future investigation will uncover additional modes of negative regulation that limit chronic NF-κB activation following DNA damage.
Functional significance of DNA damage induced NEMO/ATM signaling and NF-κB activation
Given that NF-κB has well described roles in coordinating innate and adaptive immunity, a question arises: what is the functional significance of DNA damage dependent activation of NF-κB? As outlined above, much has been learned regarding the molecular players mediating DNA damage-induced NF-κB activation; however, our understanding of the in vivo functions of this pathway remains in its infancy. Many recent studies are continuing to advance our understanding of the role and importance of this pathway for normal homeostasis in response to a variety of cellular stresses including, but not merely limited to, DNA damage.
Cell fate decisions following DNA damage
A major function of NF-κB activation following DNA damage is protecting damaged cells from apoptotic cell death. Baldwin and colleagues (72) were the first to directly demonstrate NF-κB activation by DNA damage served to protect cells from apoptosis in vivo. Similarly, expression of the non-phosphorylatable IκBα-SS32/36AA ‘super repressor’ (IκBα-SR) in a mouse fibrosarcoma xenograft model showed absent NF-κB activation, increased apoptosis, and reduced tumor growth following treatment with the clinical topoisomerase I poison CPT-11 (73). In LoVo colonic adenocarcinoma xenograft models, treatment with either CPT-11 or ionizing radiation robustly activated NF-κB. Inhibition of NF-κB via treatment with the proteasome inhibitor bortezomib resulted in increased cellular apoptosis and reduced tumor growth in vivo (74, 75). Additional genetic evidence for the requirement of NF-κB in mediating protection from apoptosis in vivo was obtained from mice with intestinal epithelial cell-specific knockout of IKKβ (49). Following total body irradiation, activation of NF-κB requires the presence of IKKβ for protection of intestinal epithelial cells from radiation-induced death. Additionally, prior activation of NF-κB through treatment with LPS before irradiation leads to protection of intestinal epithelium from radiation-induced apoptotic death in control but not IKKβ-deleted animals. An additional study utilizing genome-wide analysis of ATM-dependent transcriptional responses to irradiation in lymphoid tissues in mice suggests that the primary anti-apoptotic transcription factor activated by DNA damage is NF-κB (64). Collectively, these studies argue for a general role of DNA damage dependent NF-κB activation in mediating protection of normal and malignant cells from DNA damage-induced apoptotic death.
Cell culture studies have revealed that the specific NEMO/ATM signaling pathway outlined above is a primary mediator of NF-κB-mediated protection from apoptosis. Reconstitution with wildtype NEMO promotes clonogenic survival of NEMO-deficient murine B cells following ionizing radiation, while mutation of the ATM phosphorylation site in NEMO abrogates this observation (38). This effect is dependent in part on NF-κB-dependent induction of anti-apototic genes such as Bcl-XL and Bcl-2 family members (76). Similarly, PIDD and PARP1 have been demonstrated to be required for expression of the anti-apoptotic NF-κB target genes and protection of cells from apoptotic cell death following DNA damage (23, 28). Additionally, a recent RNA interference screen revealed that silencing of TAK1 can significantly enhance apoptosis in some breast and colon cancer cell lines treated with CPT (77).
Several recent reports have revealed that DNA damage-dependent signaling through ATM/NEMO/RIP1 can serve as a critical point of regulation for modulating cell fate decisions in the face of overwhelming DNA damage following unusually high doses or prolonged exposure to genotoxic chemotherapeutics. Biton and Ashkenazi uncovered a unique role for RIP1 in mediating two waves of ATM-dependent NF-κB activation following prolonged or high-dose exposure to the topoisomerase poison etoposide. Consistent with other reports, the first wave of NF-κB activation is independent of RIP1 kinase activity as treatment with a RIP1 kinase inhibitor had no impact on early NF-κB activation. In contrast, the secondary activation can be blunted in response to RIP1 inhibition. The second wave of NF-κB activation following prolonged etoposide treatment correlates with autophosphorylation and ubiquitination of RIP1. RIP1 further mediates the assembly of a signaling complex containing NEMO, RIP1, and the pro-apoptotic factors FADD and caspase-8 that ultimately leads to activation of caspase-8 and apoptosis. Moreover, this secondary wave of NF-κB activation correlates with the induction and secretion of the cytokines TNF and IL-8. Given that TNF can itself both activate NF-κB and trigger apoptosis through the TNFR, the authors suggested a model whereby autocrine TNF secretion following prolonged etoposide exposure creates a feed-forward loop that triggers apoptosis in response to insurmountable DNA damage. This occurs by engagement of TNFRs following extensive DNA damage and assembly of a RIP1/NEMO complex that recruits FADD and caspase-8, ultimately activating caspase-8 and triggering apoptosis through the extrinsic pathway. As this mechanism is contingent on the NF-κB-dependent synthesis and secretion of cytokines such as IL-8 and TNF, the authors suggest the exciting possibility that cells destined for death as a consequence of DNA damage are able to signal surrounding cells of their impending fate.
Two groups (78, 79) also independently reveal the presence of a TNFR-independent RIP1 cell-death inducing platform termed the ‘Ripoptosome’ to differentiate it from the previously characterized TNFR-associated ‘Complex-II’. In part confirming the previous work on RIP1-mediated recruitment of FADD and caspase-8, etoposide treatment induces formation of the ~2 MDa Ripoptosome complex. The presence of NEMO was not specifically addressed, thus whether NEMO is incorporated into the Ripoptosome is unknown. In contrast to Biton and Ashkenazi, multiple modes of inhibiting TNFR signaling had no impact on Ripoptosome assembly or cell death, and no increase in TNF synthesis was observed following high dose etoposide exposure. Instead, the authors implicated the ubiquitin ligases cIAP1, cIAP2, and XIAP as critical suppressors of Ripoptosome assembly; intriguingly, intracellular levels of IAPs can be depleted by etoposide treatment (80). Under conditions where cIAP1, cIAP2, and XIAP are limiting, Ripoptosome formation is enhanced, and caspase-8-mediated apoptosis is increased. Given that cIAP1, cIAP2 and XIAP have been implicated as essential in DNA damage-induced NF-κB activation, it is tempting to speculate that following DNA damage, the intracellular levels of these signaling components may dictate whether cells can survive through NF-κB-dependent anti-apoptotic gene induction or die through RIP1-mediated caspase-8 activation. DNA damage can clearly activate additional signaling pathways (e.g. p38MAPK, JNK, ERK) in a NEMO- and RIP1-dependent manner (30), and the integration of these complex signaling networks is likely to be a critical mode of regulation for determining cell fate decisions following DNA damage. Future experiments will be required to determine the generalizability of these observations, as they are likely to have important ramifications for clinical responses to anti-cancer therapies.
DNA damage-dependent NF-κB activation may also play additional roles in promoting cellular functions and cell fate specification following DNA damage, including DNA repair, cell cycle arrest to promote recovery from damage, and induction of senescence. In breast cancer cells, pre-treatment with IKK inhibitors prior to irradiation leads to a dose-dependent reduction in the resolution of DNA repair foci, suggesting that IKK function is required for efficient repair of DNA damage (81). Similar defects can be observed in MEFs deficient for specific IKK subunits. Surprisingly, this effect appears to be predominantly dependent on IKKβ and not IKKα; the mechanism(s) of IKKβ-facilitated DNA repair remain to be investigated. Defective DNA repair following irradiation can also be observed in tissues and MEFs derived from mice deficient for the p65 subunit of NF-κB, suggesting a general role for IKK/NF-κB signaling in DNA repair (82). Concomitant with DNA repair, elaborate mechanisms—initiated and coordinated by ATM— trigger cell cycle arrest to allow cells ample opportunity for genome repair prior to replication. Cell cycle arrest is clearly dependent on the function of p53, Chk1, Chk2, and many additional members of the DNA damage response (83). However, in leukemic T cells, induction of prolonged cell cycle arrest at the G2/M border following DNA damage can be blocked by expression of dominant negative IκBα-SR (84). Moreover, individual cells that fail to activate NF-κB following CPT treatment fail to undergo sustained cell cycle arrest and subsequently undergo cell death. This correlates with the induction of the cyclin dependent kinase inhibitor p21waf1/cip1. Expression of p21waf1/cip1 in this context specifically requires DNA damage-dependent NF-κB activation as activation of the canonical NF-κB pathway through TNF does not result in p21waf1/cip1 induction. This further hints at the complexity of transcriptional programs capable of being initiated by NF-κB following different stimuli (85, 86).
Senescence is an important cell fate that serves to limit the transmission of mutated DNA in response to cellular aging, oncogenic stress, or DNA damage (87). Though NF-κB has not been widely linked to this process, DNA damage-dependent NF-κB activation may play a previously underappreciated role in induction of cellular senescence, specifically following DNA damage. Following irradiation, human cells undergo senescence and develop an autonomous senescence-associated secretory phenotype (SASP) that triggers release of inflammatory cytokines. Intriguingly, SASP is dependent on ATM and is associated with numerous NF-κB target genes including IL-6 (88). This immediately suggests the hypothesis that ATM/NEMO-dependent NF-κB activation following DNA damage could participate in SASP induction following irradiation. Consistent with this idea, NF-κB appears necessary for maintaining cellular senescence in some contexts (82). Moreover, senescent melanoma cells harbor a pro-invasive secretome that is dependent on PARP-1 and NF-κB (89). Recently, p38MAPK has been implicated in SASP induction in response to genotoxic stress (90). Here, the authors specifically interrogated the potential role of NF-κB and conclude that NF-κB is directly implicated in SASP induction. Chromatin immunoprecipitation revealed that p65 is bound to the promoter region of at least 3 SASP genes and that p65 occupancy was dependent on p38MAPK and ATM activity. Whether p65 recruitment and SASP induction is specifically dependent on NEMO/ATM-mediated NF-κB activation following DNA damage awaits further investigation.
DNA damage-dependent NF-κB activation and B-cell differentiation
Endogenous DSBs are an obligate cosequence of B-lymphocyte development due to somatic rearrangement of immunoglobulin loci that is necessary for generation of antibody diversity and isotype class switching. These DSBs engage the DDR machinery in a manner reminiscent of the cellular response to exogenous DNA damage (91). As a consequence, mice defective for DDR components, specifically in genes involved in sensing and repair of DSBs (e.g. ATM) show defects in normal B-lymphocyte function and responses to pathogens (92). Similarly in humans, many primary immunodeficiencies are attributable to monogenic defects in DDR signaling (93), and the study of rare diseases like ataxia telangectasia have provided insight into the mammalian DDR (94). Interestingly, NF-κB has been shown to be activated by endogenous DSBs that are formed during lymphocyte differentiation in mice (95). As is the case for exogenous DSBs, NF-κB activation by endogenous DSBs requires the function of NEMO and ATM signaling. In this case, NF-κB activation correlates with upregulation of a cohort of genes whose expression is involved in protection from apoptosis and B-cell development, including Pim2 and CD40, respectively.
Human patients with mutations in the gene encoding NEMO also display defects in B-lymphocyte differentiation. The X-linked NEMO locus (OMIM ID:300248) displays considerable allelic heterogeneity with at least 23 allelic variants. Depending on the specific allele, affected patients display disorders ranging from severe incontinentia pigmenti in females and embryonic lethality in hemizygous males, to the relatively milder hypohydrotic ectodermal dysplasia with immunodeficiency. Many mutations in NEMO map to the C-terminal ZF domain that is essential for DNA damage induced NF-κB activation. All tested NEMO ZF alleles are specifically defective for the DNA damage NF-κB pathway, whereas canonical NF-κB activation by LPS stimulation remains largely intact. A prominent feature of B lymphocytes from patients with NEMO ZF mutations is failure to undergo class switch recombination and an almost complete absence of memory B cells (96, 97). Notably, microarray analysis suggests a general failure to upregulate expression of genes involved in class switch recombination and proliferation in patient B cells stimulated to undergo class switching in vitro (97). This defect may be due, in part, to the modest defects in CD40 signaling that are also observed in these samples. However, corroborating the observations of a DNA damage-specific role for the NEMO ZF, coupled with the observation of a NEMO/ATM dependent NF-κB transcriptional program induced during murine B-cell differentiation, we speculate that the defect in B-cell functions observed in patients with NEMO ZF mutations also specifically requires DNA damage dependent NF-κB activation. Additional analyses will be required to provide further support for this hypothesis.
Additional roles for NEMO/ATM-mediated NF-κB activation
NEMO/ATM signaling has been demonstrated to play additional roles in some cellular contexts that heighten the potential clinical importance of this signaling pathway. Myelodysplastic syndrome (MDS) is a disease of hematopoietic stem cells that results in bone marrow failure due to ineffective hematopoiesis. MDS cases are grouped into prognostic groups that predict the likelihood of progression to acute myeloid leukemia (AML). A larger proportion of myeloblasts from high-risk MDS as well as AML patients harbor constitutive NF-κB activity that serves as a mitogen and protects cells from apoptosis. Moreover, inhibition of NF-κB is sufficient to promote apoptosis in these cells (98, 99). Constitutive NF-κB in these cells appears to be dependent on the NEMO/ATM pathway of NF-κB activation (100). Additionally, AML cells display constitutive nuclear NEMO and PIDD localization, with NEMO exhibiting constitutive association with ATM. ATM kinase inhibition leads to loss of NF-κB activation, re-localization of NEMO and PIDD out of the nucleus, and ultimately induces apoptosis. Treatment of high-risk MDS and AML cells with peptide inhibitors of NEMO function has also shown some promise as a therapeutic modality for treating this disease (101).
Histone deacetylase inhibitors (HDACi) such as panobinostat are being evaluated in multiple phase III clinical trials for efficacy against both hematologic and epithelial cancer types. Recently, panobinostat was shown to activate NF-κB as a consequence of HDACi-mediated reactive oxygen species and DNA damage (102). Panobinostat induces ATM phosphorylation and NEMO nuclear localization, leading to NEMO:ATM association in the nucleus. Importantly, NF-κB activation also appears to depend on NEMO SUMOylation, as cells expressing NEMO double lysine 277 and 309 mutant display reduced NF-κB activation and undergo cell death more readily than cells expressing wildtype NEMO. The anti-apoptotic function of NF-κB activity appears to be a major culprit in treatment failures with HDACi (103,104). These results highlight an additional clinically relevant context for NEMO/ATM-dependent NF-κB activation and suggest a new target that could be exploited for therapeutic synergy.
Perspectives: conclusions and outstanding questions
Reflecting on 20 years following the initial observation that NF-κB activation can be induced by ionizing radiation (105), much has been learned and yet more remains to be discovered. Key biochemical questions linger, whose answers will clarify mechanistic details; experiments utilizing mouse genetics will expand the functional significance in vivo of this still-emerging pathway.
A central—and not fully resolved—question regarding the activation of NF-κB by DNA-damaging agents is the molecular nature of the signal initiation event. As early as 1989, it was postulated that nuclear DNA damage could directly result in activation of the cytoplasmic NF-κB system following UV irradiation (1). Subsequent reports that ionizing radiation or treatment with topoisomerase inhibitors were also capable of activating NF-κB suggested that specific signal transduction events were required between DNA damage occurring in the nucleus and the NF-κB components present in the cytoplasm (37, 105). A robust body of evidence collectively suggests that DNA DSBs, not other forms of DNA damage, represent a necessary molecular event leading to NF-κB activation through the conserved signal transduction pathway outlined in the preceding sections: (i) activation by Top I poison CPT requires nuclear Top I and correlates with S phase-dependent DSB formation caused by Top I inhibition (5, 106); (ii) enucleated cytoplasts fail to activate NF-κB following treatment with CPT (5); (iii) the DSB-responsive ATM kinase is essential for NF-κB activation following DNA damage (8,16-18); (iv) DNA breaks generated by electroporation of restriction enzymes robustly activate IKK kinase activity and NF-κB, whereas electroporation of heat-inactivated enzymes does not (6).
However, our combined observations have led us to a postulate a ‘two signal’ model that requires coincident NEMO SUMOylation (that is independent of DNA breaks per se), and ATM activation by DSBs to permit robust NF-κB activation following DNA damage. First, SUMOylation of NEMO can be observed in the absence of DSBs but does not lead to NF-κB activation. Second, neither DSBs nor activated ATM alone are sufficient for NF-κB activation (6). This model draws conceptual parallel from two signal models in immunology as in the case of lymphocyte activation during the adaptive immune response. In this context, lymphocyte activation is restricted to its proper cellular context only in the presence of specific antigen and costimulatory signals. NF-κB signaling following DNA damage may similarly respond to the combined presence of DNA DSBs and additional ‘stress’ or ‘danger signals’ to ensure that activation is limited only to a specific cellular context. It has been hypothesized that PARP-1 activation serves as the second signal, though these authors invoke DNA damage-induced PARP-1 activation as the necessary initiating event (23). This is inconsistent with the observation that NEMO SUMOylation can occur in the absence of DNA damage (6). Thus, further investigation into the necessary and sufficient signaling events that can trigger DNA damage dependent NF-κB may uncover additional molecular players that coordinate PIASy-mediated SUMOylation and ATM-mediated phosphorylation of NEMO.
The concept of IKK-free NEMO was initially met with some derision, though NEMO present in the nucleus has materialized as a central regulator of this pathway. Nevertheless, little is understood about the mechanism(s) governing the emergence of ‘free’ NEMO. At least two additional independent signaling pathways have emerged that require NEMO without notable involvement of IKKα or IKKβ, including MAPK activation downstream of the CD40 receptor (107) and activation of the transcription factor IRF-3 during viral infection (108). Can NEMO be actively dissociated in a signal-inducible manner from the IKK complex following stimulation, or do latent pools of free NEMO exist that can serve these functions? Though SUMOylation of NEMO is essential for competent signaling, still unclear is the precise molecular consequence or function of this post-translational modification. One possibility is that SUMOylation of NEMO promotes interaction with additional signaling component(s) through so-called SUMO interacting motifs (SIMs) that may serve some essential function. One of the SUMO acceptor residues in NEMO overlaps the UBAN domain that is involved in linking IKK to polyubiquitin chains during canonical IKK activation. One possible function of SUMOylation is to disrupt NEMO’s ability to bind polyubiquitin as it participates in nuclear signal transduction events. NEMO has been shown to interact with multiple proteins through an overlapping region in the NEMO N-terminus, including PIASy, PARP-1, SENP2, ATM, cIAP1, and TRAF6; many of these interactions have been shown to be mutually exclusive with IKKβ. How NEMO can integrate association with all these partner proteins to promote additional signaling events remains to be determined.
Available data are consistent with a model whereby nuclear NEMO is engaged in proximal events (e.g. SUMOylation, phosphorylation) that occur in the nucleus and then exits the nucleus to participate in additional signal transduction events in the cytoplasm. Some hints into the mechanism underlying nuclear export of NEMO are available (109), but additional work is required to identify nuclear export receptors responsible for mediating this intermediate step. Clearly NEMO is required for additional functions once present in the cytoplasm, including in promoting IKK activation. Does the once-nuclear NEMO re-assemble into the IKK complex in the cytoplasm, or does NEMO transduce the nuclear signal via intermediates (e.g. ELKS, TAK1) to an independent pool of IKK-bound NEMO?
One important consideration for establishing the functional significance of this pathway lies in the varying doses of agents that have been utilized to induce DNA damage. For example, many studies rely on doses of etoposide as high as 100 micromolar or ionizing radiation up to 80 Gray (Gy). We estimate from available pharmacokinetic data that the peak concentration of etoposide in the blood of patients exposed to routinely administered doses of etoposide to be in the range of 10 to 20 micromolar (110). Moreover, exposure of cells to radiation as high as 80 Gy likely triggers additional biochemically and biologically relevant events, such as excessive heat, oxidative damage to all biological macromolecules, and perturbations to mitochondrial function, among others. How these other events impact the DSB-initiated NF-κB signaling is unclear at present. Thus, the universality of specific signal transduction events established through studies utilizing such supraphysiological doses of DNA-damaging agents should be considered with some caution. Moreover, little is currently known about the consequences of prolonged exposure to DNA-damaging agents and their effects on NF-κB activation. Given the multitude of cell fates that can result from DNA damage—both NF-κB-dependent and NF-κB-independent damage—cells may employ many distinct mechanisms that together modulate overall cell behaviors. We hypothesize that many of the functions for NF-κB may be contextual based on the degree of DNA damage and cellular injury (Fig. 1). Moreover, there may be differences among cell and tissue types in their mechanisms employed to respond to these varying degrees of DNA damage. Undoubtedly, additional signal transduction pathways can be activated following DNA damage, and it is likely that significant cross-talk among these many different pathways is key to establish the contextual importance of NF-κB activation following DNA damage. A potentially exciting future area of investigation may be in understanding the mechanisms of integration among these pathways.
Furnished with the identities of many of the key molecular players involved in DNA damage-dependent NF-κB activation, future work to establish the in vivo regulation and significance of this pathway will require manipulating genetic models in the mouse. Given the clear and growing evidence of the links between cancer and NF-κB (Reviewed in Didonato et al. and Lim et al., this volume), it seems plausible that a more thorough understanding of the consequences of NF-κB activation, specifically mediated by endogenous and exogenous DNA damage, will provide new insight and expand our appreciation for the clinical importance of NF-κB.
Acknowledgements
The authors thank members of the Miyamoto laboratory for critical review and suggestions. The authors’ work was funded by NIH R01 CA77474 and GM083681 to S.M.
Footnotes
The authors declare no conflicts of interest.
References
- 1.Stein B, Rahmsdorf HJ, Steffen A, Litfin M, Herrlich P. UV-induced DNA damage is an intermediate step in UV-induced expression of human immunodeficiency virus type 1, collagenase, c-fos, and metallothionein. Mol Cell Biol. 1989 Nov.9(11):5169–5181. doi: 10.1128/mcb.9.11.5169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tsuchiya Y, Asano T, Nakayama K, Kato T, Karin M, Kamata H. Nuclear IKKbeta is an adaptor protein for IkappaBalpha ubiquitination and degradation in UV-induced NF-kappaB activation. Mol Cell. 2010 Aug. 2739(4):570–582. doi: 10.1016/j.molcel.2010.07.030. [DOI] [PubMed] [Google Scholar]
- 3.Devary Y, Rosette C, DiDonato JA, Karin M. NF-kappa B activation by ultraviolet light not dependent on a nuclear signal. Science. 1993 Sep. 10261(5127):1442–1445. doi: 10.1126/science.8367725. [DOI] [PubMed] [Google Scholar]
- 4.Bender K, Göttlicher M, Whiteside S, Rahmsdorf HJ, Herrlich P. Sequential DNA damage-independent and -dependent activation of NF-kappaB by UV. EMBO J. 1998 Aug. 3117(17):5170–5181. doi: 10.1093/emboj/17.17.5170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang TT, Wuerzberger-Davis SM, Seufzer BJ, Shumway SD, Kurama T, Boothman DA, et al. NF-kappaB activation by camptothecin. A linkage between nuclear DNA damage and cytoplasmic signaling events. J Biol Chem. 2000 Mar. 31275(13):9501–9509. doi: 10.1074/jbc.275.13.9501. [DOI] [PubMed] [Google Scholar]
- 6.Wuerzberger-Davis SM, Nakamura Y, Seufzer BJ, Miyamoto S. NF-kappaB activation by combinations of NEMO SUMOylation and ATM activation stresses in the absence of DNA damage. Oncogene. 2007 Feb. 126(5):641–651. doi: 10.1038/sj.onc.1209815. [DOI] [PubMed] [Google Scholar]
- 7.Huang TT, Feinberg SL, Suryanarayanan S, Miyamoto S. The zinc finger domain of NEMO is selectively required for NF-kappa B activation by UV radiation and topoisomerase inhibitors. Mol Cell Biol. 2002 Aug.22(16):5813–5825. doi: 10.1128/MCB.22.16.5813-5825.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang TT, Wuerzberger-Davis SM, Wu Z-H, Miyamoto S. Sequential modification of NEMO/IKKgamma by SUMO-1 and ubiquitin mediates NF-kappaB activation by genotoxic stress. Cell. 2003 Nov. 26115(5):565–576. doi: 10.1016/s0092-8674(03)00895-x. [DOI] [PubMed] [Google Scholar]
- 9.Ciccia A, Elledge SJ. The DNA damage response: making it safe to play with knives. Mol Cell. 2010 Oct. 2240(2):179–204. doi: 10.1016/j.molcel.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jackson SP, Bartek J. The DNA-damage response in human biology and disease. Nature. 2009 Oct. 22461(7267):1071–1078. doi: 10.1038/nature08467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Durocher D, Henckel J, Fersht AR, Jackson SP. The FHA domain is a modular phosphopeptide recognition motif. Mol. Cell. 1999 Sep.4(3):387–394. doi: 10.1016/s1097-2765(00)80340-8. [DOI] [PubMed] [Google Scholar]
- 12.Galanty Y, Belotserkovskaya R, Coates J, Polo S, Miller KM, Jackson SP. Mammalian SUMO E3-ligases PIAS1 and PIAS4 promote responses to DNA double-strand breaks. Nature. 2009 Dec. 17462(7275):935–939. doi: 10.1038/nature08657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bergink S, Jentsch S. Principles of ubiquitin and SUMO modifications in DNA repair. Nature. 2009 Mar. 26458(7237):461–467. doi: 10.1038/nature07963. [DOI] [PubMed] [Google Scholar]
- 14.Banin S. Enhanced Phosphorylation of p53 by ATM in Response to DNA Damage. Science. 1998 Sep. 11281(5383):1674–1677. doi: 10.1126/science.281.5383.1674. [DOI] [PubMed] [Google Scholar]
- 15.Canman CE. Activation of the ATM Kinase by Ionizing Radiation and Phosphorylation of p53. Science. 1998 Sep. 11281(5383):1677–1679. doi: 10.1126/science.281.5383.1677. [DOI] [PubMed] [Google Scholar]
- 16.Li N, Karin M. Ionizing radiation and short wavelength UV activate NF-kappaB through two distinct mechanisms. Proc Natl Acad Sci USA. 1998 Oct. 2795(22):13012–13017. doi: 10.1073/pnas.95.22.13012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Piret B, Schoonbroodt S, Piette J. The ATM protein is required for sustained activation of NF-kappaB following DNA damage. Oncogene. 1999 Mar. 3118(13):2261–2271. doi: 10.1038/sj.onc.1202541. [DOI] [PubMed] [Google Scholar]
- 18.Lee SJ, Dimtchev A, Lavin MF, Dritschilo A, Jung M. A novel ionizing radiation-induced signaling pathway that activates the transcription factor NF-kappaB. Oncogene. 1998 Oct. 817(14):1821–1826. doi: 10.1038/sj.onc.1202088. [DOI] [PubMed] [Google Scholar]
- 19.Rooney JW, Emery DW, Sibley CH. 1.3E2, a variant of the B lymphoma 70Z/3, defective in activation of NF-kappa B and OTF-2. Immunogenetics. 1990;31(2):73–78. doi: 10.1007/BF00661216. [DOI] [PubMed] [Google Scholar]
- 20.Courtois G, Whiteside ST, Sibley CH, Israel A. Characterization of a mutant cell line that does not activate NF-kappaB in response to multiple stimuli. Mol Cell Biol. 1997 Feb. 2817(3):1441–1449. doi: 10.1128/mcb.17.3.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamaoka S, Courtois G, Bessia C, Whiteside ST, Weil R, Agou F, et al. Complementation cloning of NEMO, a component of the IkappaB kinase complex essential for NF-kappaB activation. Cell. 1998 Jun. 2693(7):1231–1240. doi: 10.1016/s0092-8674(00)81466-x. [DOI] [PubMed] [Google Scholar]
- 22.Israël A. The IKK Complex, a Central Regulator of NF-B Activation. Cold Spring Harbor Perspectives in Biology. 2010 Mar. 12(3):a000158–a000158. doi: 10.1101/cshperspect.a000158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stilmann M, Hinz M, Arslan SC, Zimmer A, Schreiber V, Scheidereit C. A nuclear poly(ADP-ribose)-dependent signalosome confers DNA damage-induced IkappaB kinase activation. Mol. Cell. 2009 Nov. 1336(3):365–378. doi: 10.1016/j.molcel.2009.09.032. [DOI] [PubMed] [Google Scholar]
- 24.Mabb AM, Wuerzberger-Davis SM, Miyamoto S. PIASy mediates NEMO sumoylation and NF-kappaB activation in response to genotoxic stress. Nat Cell Biol. 2006 Sep.8(9):986–993. doi: 10.1038/ncb1458. [DOI] [PubMed] [Google Scholar]
- 25.Cai Q, Robertson ES. Ubiquitin/SUMO modification regulates VHL protein stability and nucleocytoplasmic localization. PLoS ONE. 2010;5(9) doi: 10.1371/journal.pone.0012636. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 26.Renner F, Moreno R, Schmitz ML. SUMOylation-dependent localization of IKKepsilon in PML nuclear bodies is essential for protection against DNA-damage-triggered cell death. Mol Cell. 2010 Feb. 2637(4):503–515. doi: 10.1016/j.molcel.2010.01.018. [DOI] [PubMed] [Google Scholar]
- 27.Du JX, Bialkowska AB, McConnell BB, Yang VW. SUMOylation regulates nuclear localization of Krüppel-like factor 5. J Biol Chem. 2008 Nov. 14283(46):31991–32002. doi: 10.1074/jbc.M803612200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Janssens S, Tinel A, Lippens S, Tschopp J. PIDD mediates NF-kappaB activation in response to DNA damage. Cell. 2005 Dec. 16123(6):1079–1092. doi: 10.1016/j.cell.2005.09.036. [DOI] [PubMed] [Google Scholar]
- 29.Hur GM. The death domain kinase RIP has an essential role in DNA damage-induced NF-kappa B activation. Genes & Development. 2003 Mar. 3117(7):873–882. doi: 10.1101/gad.1062403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang Y, Xia F, Hermance N, Mabb A, Simonson S, Morrissey S, et al. A Cytosolic ATM/NEMO/RIP1 Complex Recruits TAK1 To Mediate the NF-{kappa}B and p38 Mitogen-Activated Protein Kinase (MAPK)/MAPK-Activated Protein 2 Responses to DNA Damage. Mol Cell Biol. 2011 Jul.31(14):2774–2786. doi: 10.1128/MCB.01139-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Manzl C, Krumschnabel G, Bock F, Sohm B, Labi V, Baumgartner F, et al. Caspase-2 activation in the absence of PIDDosome formation. The Journal of Cell Biology. 2009 Apr. 20185(2):291–303. doi: 10.1083/jcb.200811105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hassa PO, Hottiger MO. A role of poly (ADP-ribose) polymerase in NF-kappaB transcriptional activation. Biol. Chem. 1999 Jun.380(7-8):953–959. doi: 10.1515/BC.1999.118. [DOI] [PubMed] [Google Scholar]
- 33.Hassa PO, Haenni SS, Buerki C, Meier NI, Lane WS, Owen H, et al. Acetylation of poly(ADP-ribose) polymerase-1 by p300/CREB-binding protein regulates coactivation of NF-kappaB-dependent transcription. J Biol Chem. 2005 Dec. 9280(49):40450–40464. doi: 10.1074/jbc.M507553200. [DOI] [PubMed] [Google Scholar]
- 34.Hassa PO, Covic M, Hasan S, Imhof R, Hottiger MO. The enzymatic and DNA binding activity of PARP-1 are not required for NF-kappa B coactivator function. J Biol Chem. 2001 Dec. 7276(49):45588–45597. doi: 10.1074/jbc.M106528200. [DOI] [PubMed] [Google Scholar]
- 35.Hassa PO, Hottiger MO. The functional role of poly(ADP-ribose)polymerase 1 as novel coactivator of NF-kappaB in inflammatory disorders. Cell Mol Life Sci. 2002 Sep.59(9):1534–1553. doi: 10.1007/s00018-002-8527-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Veuger SJ, Hunter JE, Durkacz BW. Ionizing radiation-induced NF-kappaB activation requires PARP-1 function to confer radioresistance. Oncogene. 2009 Feb. 1128(6):832–842. doi: 10.1038/onc.2008.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Piret B, Piette J. Topoisomerase poisons activate the transcription factor NF-kappaB in ACH-2 and CEM cells. Nucleic Acids Research. 1996 Nov. 124(21):4242–4248. doi: 10.1093/nar/24.21.4242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu Z-H, Shi Y, Tibbetts RS, Miyamoto S. Molecular linkage between the kinase ATM and NF-kappaB signaling in response to genotoxic stimuli. Science. 2006 Feb. 24311(5764):1141–1146. doi: 10.1126/science.1121513. [DOI] [PubMed] [Google Scholar]
- 39.Jin H-S, Lee D-H, Kim D-H, Chung J-H, Lee S-J, Lee TH. cIAP1, cIAP2, and XIAP act cooperatively via nonredundant pathways to regulate genotoxic stress-induced nuclear factor-kappaB activation. Cancer Res. 2009 Mar. 169(5):1782–1791. doi: 10.1158/0008-5472.CAN-08-2256. [DOI] [PubMed] [Google Scholar]
- 40.Sato S, Sanjo H, Takeda K, Ninomiya-Tsuji J, Yamamoto M, Kawai T, et al. Essential function for the kinase TAK1 in innate and adaptive immune responses. Nat Immunol. 2005 Nov.6(11):1087–1095. doi: 10.1038/ni1255. [DOI] [PubMed] [Google Scholar]
- 41.Wang C, Deng L, Hong M, Akkaraju GR, Inoue J, Chen ZJ. TAK1 is a ubiquitin-dependent kinase of MKK and IKK. Nature. 2001 Jul. 19412(6844):346–351. doi: 10.1038/35085597. [DOI] [PubMed] [Google Scholar]
- 42.Kanayama A, Seth RB, Sun L, Ea C-K, Hong M, Shaito A, et al. TAB2 and TAB3 activate the NF-kappaB pathway through binding to polyubiquitin chains. Mol Cell. 2004 Aug. 2715(4):535–548. doi: 10.1016/j.molcel.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 43.Wu Z-H, Wong ET, Shi Y, Niu J, Chen Z, Miyamoto S, et al. ATM- and NEMO-dependent ELKS ubiquitination coordinates TAK1-mediated IKK activation in response to genotoxic stress. Mol Cell. 2010 Oct. 840(1):75–86. doi: 10.1016/j.molcel.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hinz M, Stilmann M, Arslan SC, Khanna KK, Dittmar G, Scheidereit C. A cytoplasmic ATM-TRAF6-cIAP1 module links nuclear DNA damage signaling to ubiquitin-mediated NF-κB activation. Mol Cell. 2010 Oct. 840(1):63–74. doi: 10.1016/j.molcel.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 45.Lu M, Lin S-C, Huang Y, Kang YJ, Rich R, Lo Y-C, et al. XIAP induces NF-kappaB activation via the BIR1/TAB1 interaction and BIR1 dimerization. Mol Cell. 2007 Jun. 826(5):689–702. doi: 10.1016/j.molcel.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yamaguchi K, Nagai S, Ninomiya-Tsuji J, Nishita M, Tamai K, Irie K, et al. XIAP, a cellular member of the inhibitor of apoptosis protein family, links the receptors to TAB1-TAK1 in the BMP signaling pathway. EMBO J. 1999 Jan. 418(1):179–187. doi: 10.1093/emboj/18.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sigala JL Ducut, Bottero V, Young DB, Shevchenko A, Mercurio F, Verma IM. Activation of transcription factor NF-kappaB requires ELKS, an IkappaB kinase regulatory subunit. Science. 2004 Jun. 25304(5679):1963–1967. doi: 10.1126/science.1098387. [DOI] [PubMed] [Google Scholar]
- 48.Yin Q, Lin S-C, Lamothe B, Lu M, Lo Y-C, Hura G, et al. E2 interaction and dimerization in the crystal structure of TRAF6. Nat Struct Mol Biol. 2009 Jun.16(6):658–666. doi: 10.1038/nsmb.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Egan LJ, Eckmann L, Greten FR, Chae S, Li Z-W, Myhre GM, et al. IkappaB-kinasebeta-dependent NF-kappaB activation provides radioprotection to the intestinal epithelium. Proc Natl Acad Sci USA. 2004 Feb. 24101(8):2452–2457. doi: 10.1073/pnas.0306734101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Niu J, Shi Y, Iwai K, Wu Z-H. LUBAC regulates NF-κB activation upon genotoxic stress by promoting linear ubiquitination of NEMO. EMBO J. 2011 Aug. 2 doi: 10.1038/emboj.2011.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tokunaga F, Sakata S-I, Saeki Y, Satomi Y, Kirisako T, Kamei K, et al. Involvement of linear polyubiquitylation of NEMO in NF-kappaB activation. Nat Cell Biol. 2009 Feb.11(2):123–132. doi: 10.1038/ncb1821. [DOI] [PubMed] [Google Scholar]
- 52.Haas TL, Emmerich CH, Gerlach B, Schmukle AC, Cordier SM, Rieser E, et al. Recruitment of the linear ubiquitin chain assembly complex stabilizes the TNF-R1 signaling complex and is required for TNF-mediated gene induction. Mol Cell. 2009 Dec. 1136(5):831–844. doi: 10.1016/j.molcel.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 53.Ikeda F, Deribe YL, Skånland SS, Stieglitz B, Grabbe C, Franz-Wachtel M, et al. SHARPIN forms a linear ubiquitin ligase complex regulating NF-κB activity and apoptosis. Nature. 2011 Mar. 31471(7340):637–641. doi: 10.1038/nature09814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tokunaga F, Nakagawa T, Nakahara M, Saeki Y, Taniguchi M, Sakata S-I, et al. SHARPIN is a component of the NF-κB-activating linear ubiquitin chain assembly complex. Nature. 2011 Mar. 31471(7340):633–636. doi: 10.1038/nature09815. [DOI] [PubMed] [Google Scholar]
- 55.Kirisako T, Kamei K, Murata S, Kato M, Fukumoto H, Kanie M, et al. A ubiquitin ligase complex assembles linear polyubiquitin chains. EMBO J. 2006 Oct. 1725(20):4877–4887. doi: 10.1038/sj.emboj.7601360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rahighi S, Ikeda F, Kawasaki M, Akutsu M, Suzuki N, Kato R, et al. Specific recognition of linear ubiquitin chains by NEMO is important for NF-kappaB activation. Cell. 2009 Mar. 20136(6):1098–1109. doi: 10.1016/j.cell.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 57.Josson S, Xu Y, Fang F, Dhar SK, St Clair DK, St Clair WH. RelB regulates manganese superoxide dismutase gene and resistance to ionizing radiation of prostate cancer cells. Oncogene. 2006 Mar. 925(10):1554–1559. doi: 10.1038/sj.onc.1209186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xu Y, Fang F, St Clair DK, Sompol P, Josson S, St Clair WH. SN52, a novel nuclear factor-kappaB inhibitor, blocks nuclear import of RelB:p52 dimer and sensitizes prostate cancer cells to ionizing radiation. Mol. Cancer Ther. 2008 Aug.7(8):2367–2376. doi: 10.1158/1535-7163.MCT-08-0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lessard L, Bégin LR, Gleave ME, Mes-Masson A-M, Saad F. Nuclear localisation of nuclear factor-kappaB transcription factors in prostate cancer: an immunohistochemical study. Br. J. Cancer. 2005 Oct. 3193(9):1019–1023. doi: 10.1038/sj.bjc.6602796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xu Y, Fang F, Sun Y, St Clair DK, St Clair WH. RelB-dependent differential radiosensitization effect of STI571 on prostate cancer cells. Mol. Cancer Ther. 2010 Apr.9(4):803–812. doi: 10.1158/1535-7163.MCT-09-1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Anest V, Hanson JL, Cogswell PC, Steinbrecher KA, Strahl BD, Baldwin AS. A nucleosomal function for IkappaB kinase-alpha in NF-kappaB-dependent gene expression. Nature. 2003 Jun. 5423(6940):659–663. doi: 10.1038/nature01648. [DOI] [PubMed] [Google Scholar]
- 62.Yamamoto Y, Verma UN, Prajapati S, Kwak Y-T, Gaynor RB. Histone H3 phosphorylation by IKK-alpha is critical for cytokine-induced gene expression. Nature. 2003 Jun. 4423(6940):655–659. doi: 10.1038/nature01576. [DOI] [PubMed] [Google Scholar]
- 63.Renner F, Schmitz ML. Autoregulatory feedback loops terminating the NF-kappaB response. Trends Biochem Sci. 2009 Mar.34(3):128–135. doi: 10.1016/j.tibs.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 64.Rashi-Elkeles S, Elkon R, Weizman N, Linhart C, Amariglio N, Sternberg G, et al. Parallel induction of ATM-dependent pro- and antiapoptotic signals in response to ionizing radiation in murine lymphoid tissue. Oncogene. 2006 Mar. 925(10):1584–1592. doi: 10.1038/sj.onc.1209189. [DOI] [PubMed] [Google Scholar]
- 65.Chiao PJ, Miyamoto S, Verma IM. Autoregulation of I kappa B alpha activity. Proc Natl Acad Sci USA. 1994 Jan. 391(1):28–32. doi: 10.1073/pnas.91.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lee MH, Mabb AM, Gill GB, Yeh ETH, Miyamoto S. NF-kB Induction of the SUMO Protease SENP2: A Negative Feedback Loop to Attenuate Cell Survival Response to Genotoxic Stress. Mol Cell. 2011 Jul. 2243(2):180–191. doi: 10.1016/j.molcel.2011.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Morris JR, Boutell C, Keppler M, Densham R, Weekes D, Alamshah A, et al. The SUMO modification pathway is involved in the BRCA1 response to genotoxic stress. Nature. 2009 Dec. 17462(7275):886–890. doi: 10.1038/nature08593. [DOI] [PubMed] [Google Scholar]
- 68.Kovalenko A, Chable-Bessia C, Cantarella G, Israël A, Wallach D, Courtois G. The tumour suppressor CYLD negatively regulates NF-kappaB signalling by deubiquitination. Nature. 2003 Aug. 14424(6950):801–805. doi: 10.1038/nature01802. [DOI] [PubMed] [Google Scholar]
- 69.Trompouki E, Hatzivassiliou E, Tsichritzis T, Farmer H, Ashworth A, Mosialos G. CYLD is a deubiquitinating enzyme that negatively regulates NF-kappaB activation by TNFR family members. Nature. 2003 Aug. 14424(6950):793–796. doi: 10.1038/nature01803. [DOI] [PubMed] [Google Scholar]
- 70.Brummelkamp TR, Nijman SMB, Dirac AMG, Bernards R. Loss of the cylindromatosis tumour suppressor inhibits apoptosis by activating NF-kappaB. Nature. 2003 Aug. 14424(6950):797–801. doi: 10.1038/nature01811. [DOI] [PubMed] [Google Scholar]
- 71.Wertz IE, O’Rourke KM, Zhou H, Eby M, Aravind L, Seshagiri S, et al. De-ubiquitination and ubiquitin ligase domains of A20 downregulate NF-kappaB signalling. Nature. 2004 Aug. 4430(7000):694–699. doi: 10.1038/nature02794. [DOI] [PubMed] [Google Scholar]
- 72.Wang CY, Mayo MW, Baldwin AS. TNF- and cancer therapy-induced apoptosis: potentiation by inhibition of NF-kappaB. Science. 1996 Nov. 1274(5288):784–787. doi: 10.1126/science.274.5288.784. [DOI] [PubMed] [Google Scholar]
- 73.Wang CY, Cusack JC, Liu R, Baldwin AS. Control of inducible chemoresistance: enhanced anti-tumor therapy through increased apoptosis by inhibition of NF-kappaB. Nat Med. 1999 Apr.5(4):412–417. doi: 10.1038/7410. [DOI] [PubMed] [Google Scholar]
- 74.Russo SM, Tepper JE, Baldwin AS, Liu R, Adams J, Elliott P, et al. Enhancement of radiosensitivity by proteasome inhibition: implications for a role of NF-kappaB. Int. J. Radiat. Oncol. Biol. Phys. 2001 May 1;50(1):183–193. doi: 10.1016/s0360-3016(01)01446-8. [DOI] [PubMed] [Google Scholar]
- 75.Cusack JC, Liu R, Houston M, Abendroth K, Elliott PJ, Adams J, et al. Enhanced chemosensitivity to CPT-11 with proteasome inhibitor PS-341: implications for systemic nuclear factor-kappaB inhibition. Cancer Res. 2001 May 1;61(9):3535–3540. [PubMed] [Google Scholar]
- 76.Wu Z-H, Miyamoto S. Induction of a pro-apoptotic ATM-NF-kappaB pathway and its repression by ATR in response to replication stress. EMBO J. 2008 Jul. 2327(14):1963–1973. doi: 10.1038/emboj.2008.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Martin SE, Wu Z-H, Gehlhaus K, Jones TL, Zhang Y-W, Guha R, et al. RNAi Screening Identifies TAK1 as a Potential Target for the Enhanced Efficacy of Topoisomerase Inhibitors. Curr Cancer Drug Targets. 2011 Aug. 12 doi: 10.2174/156800911797264734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Feoktistova M, Geserick P, Kellert B, Dimitrova DP, Langlais C, Hupe M, et al. cIAPs Block Ripoptosome Formation, a RIP1/Caspase-8 Containing Intracellular Cell Death Complex Differentially Regulated by cFLIP Isoforms. Mol Cell. 2011 Aug. 543(3):449–463. doi: 10.1016/j.molcel.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tenev T, Tenev T, Bianchi K, Bianchi K, Darding M, Darding M, et al. The Ripoptosome, a Signaling Platform that Assembles in Response to Genotoxic Stress and Loss of IAPs. Mol Cell. 2011 Jul. doi: 10.1016/j.molcel.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 80.Yang Y, Fang S, Jensen JP, Weissman AM, Ashwell JD. Ubiquitin protein ligase activity of IAPs and their degradation in proteasomes in response to apoptotic stimuli. Science. 2000 May 5;288(5467):874–877. doi: 10.1126/science.288.5467.874. [DOI] [PubMed] [Google Scholar]
- 81.Wu L, Shao L, An N, Wang J, Pazhanisamy S, Feng W, et al. IKKβ Regulates the Repair of DNA Double-Strand Breaks Induced by Ionizing Radiation in MCF-7 Breast Cancer Cells. PLoS ONE. 2011 Apr. 76(4):e18447. doi: 10.1371/journal.pone.0018447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang J, Jacob NK, Ladner KJ, Beg A, Perko JD, Tanner SM, et al. RelA/p65 functions to maintain cellular senescence by regulating genomic stability and DNA repair. EMBO Rep. 2009 Sep. 2510(11):1272–1278. doi: 10.1038/embor.2009.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Stracker TH, Usui T, Petrini JHJ. Taking the time to make important decisions: The checkpoint effector kinases Chk1 and Chk2 and the DNA damage response. DNA Repair (Amst) 2009 Sep. 28(9):1047–1054. doi: 10.1016/j.dnarep.2009.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wuerzberger-Davis SM, Chang P-Y, Berchtold C, Miyamoto S. Enhanced G2-M arrest by nuclear factor-{kappa}B-dependent p21waf1/cip1 induction. Mol. Cancer Res. 2005 Jun.3(6):345–353. doi: 10.1158/1541-7786.MCR-05-0028. [DOI] [PubMed] [Google Scholar]
- 85.Hoffmann A, Natoli G, Ghosh G. Transcriptional regulation via the NF-kappaB signaling module. Oncogene. 2006 Oct. 3025(51):6706–6716. doi: 10.1038/sj.onc.1209933. [DOI] [PubMed] [Google Scholar]
- 86.Perkins ND. Integrating cell-signalling pathways with NF-kappaB and IKK function. Nat Rev Mol Cell Biol. 2007 Jan.8(1):49–62. doi: 10.1038/nrm2083. [DOI] [PubMed] [Google Scholar]
- 87.Campisi J, d’Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol. 2007 Sep.8(9):729–740. doi: 10.1038/nrm2233. [DOI] [PubMed] [Google Scholar]
- 88.Rodier F, Coppé J-P, Patil CK, Hoeijmakers WAM, Muñoz DP, Raza SR, et al. Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion. Nat Cell Biol. 2009 Jul. 1311(8):973–979. doi: 10.1038/ncb1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ohanna M, Giuliano S, Bonet C, Imbert V, Hofman V, Zangari J, et al. Senescent cells develop a PARP-1 and nuclear factor-B-associated secretome (PNAS) Genes & Development. 2011 Jun. 1725(12):1245–1261. doi: 10.1101/gad.625811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Freund A, Patil CK, Campisi J. p38MAPK is a novel DNA damage response-independent regulator of the senescence-associated secretory phenotype. EMBO J. 2011 Mar. 1130(8):1536–1548. doi: 10.1038/emboj.2011.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bassing CH, Alt FW. The cellular response to general and programmed DNA double strand breaks. DNA Repair (Amst) 2004 Jul.3(8-9):781–796. doi: 10.1016/j.dnarep.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 92.Lumsden JM. Immunoglobulin Class Switch Recombination Is Impaired in Atm-deficient Mice. Journal of Experimental Medicine. 2004 Nov. 1200(9):1111–1121. doi: 10.1084/jem.20041074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Slatter MA, Gennery AR. Primary immunodeficiencies associated with DNA-repair disorders. Expert Rev. Mol. Med. 2010 Mar. 12 doi: 10.1017/S1462399410001419. [DOI] [PubMed] [Google Scholar]
- 94.Lavin MF. Ataxia-telangiectasia: from a rare disorder to a paradigm for cell signalling and cancer. Nat Rev Mol Cell Biol. 2008 Oct.9(10):759–769. doi: 10.1038/nrm2514. [DOI] [PubMed] [Google Scholar]
- 95.Bredemeyer AL, Helmink BA, Innes CL, Calderon B, Mcginnis LM, Mahowald GK, et al. DNA double-strand breaks activate a multi-functional genetic program in developing lymphocytes. Nature. 2008 Dec. 11456(7223):819–823. doi: 10.1038/nature07392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jain A, Ma CA, Liu S, Brown M, Cohen J, Strober W. Specific missense mutations in NEMO result in hyper-IgM syndrome with hypohydrotic ectodermal dysplasia. NatImmunol. 2001 Mar.2(3):223–228. doi: 10.1038/85277. [DOI] [PubMed] [Google Scholar]
- 97.Jain A, Ma CA, Lopez-Granados E, Means G, Brady W, Orange JS, et al. Specific NEMO mutations impair CD40-mediated c-Rel activation and B cell terminal differentiation. J Clin Invest. 2004 Dec.114(11):1593–1602. doi: 10.1172/JCI21345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Birkenkamp KU, Geugien M, Schepers H, Westra J, Lemmink HH, Vellenga E. Constitutive NF-kappaB DNA-binding activity in AML is frequently mediated by a Ras/PI3-K/PKB-dependent pathway. Leukemia. 2004 Jan.18(1):103–112. doi: 10.1038/sj.leu.2403145. [DOI] [PubMed] [Google Scholar]
- 99.Guzman ML, Neering SJ, Upchurch D, Grimes B, Howard DS, Rizzieri DA, et al. Nuclear factor-kappaB is constitutively activated in primitive human acute myelogenous leukemia cells. Blood. 2001 Oct. 1598(8):2301–2307. doi: 10.1182/blood.v98.8.2301. [DOI] [PubMed] [Google Scholar]
- 100.Grosjean-Raillard J, Tailler M, Adès L, Perfettini J-L, Fabre C, Braun T, et al. ATM mediates constitutive NF-kappaB activation in high-risk myelodysplastic syndrome and acute myeloid leukemia. Oncogene. 2009 Feb. 2628(8):1099–1109. doi: 10.1038/onc.2008.457. [DOI] [PubMed] [Google Scholar]
- 101.Carvalho G, Fabre C, Braun T, Grosjean J, Adès L, Agou F, et al. Inhibition of NEMO, the regulatory subunit of the IKK complex, induces apoptosis in high-risk myelodysplastic syndrome and acute myeloid leukemia. Oncogene. 2007 Apr. 526(16):2299–2307. doi: 10.1038/sj.onc.1210043. [DOI] [PubMed] [Google Scholar]
- 102.Rosato RR, Kolla SS, Hock SK, Almenara JA, Patel A, Amin S, et al. Histone deacetylase inhibitors activate NF-kappaB in human leukemia cells through an ATM/NEMO-related pathway. Journal of Biological Chemistry. 2010 Mar. 26285(13):10064–10077. doi: 10.1074/jbc.M109.095208. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 103.Dai Y, Rahmani M, Dent P, Grant S. Blockade of Histone Deacetylase Inhibitor-Induced RelA/p65 Acetylation and NF-B Activation Potentiates Apoptosis in Leukemia Cells through a Process Mediated by Oxidative Damage, XIAP Downregulation, and c-Jun N-Terminal Kinase 1 Activation. Mol Cell Biol. 2005 Jun. 1625(13):5429–5444. doi: 10.1128/MCB.25.13.5429-5444.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kim YK, Lee EK, Kang JK, Kim JA, You J-S, Park JH, et al. Activation of NF-κB by HDAC inhibitor apicidin through Sp1-dependent de novo protein synthesis: its implication for resistance to apoptosis. Cell Death Differ. 2006 Apr. 2113(12):2033–2041. doi: 10.1038/sj.cdd.4401915. [DOI] [PubMed] [Google Scholar]
- 105.Brach MA, Hass R, Sherman ML, Gunji H, Weichselbaum R, Kufe D. Ionizing radiation induces expression and binding activity of the nuclear factor kappa B. J Clin Invest. 1991 Aug.88(2):691–695. doi: 10.1172/JCI115354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Habraken Y, Piret B, Piette J. S phase dependence and involvement of NF-kappaB activating kinase to NF-kappaB activation by camptothecin. Biochem Pharmacol. 2001 Sep. 162(5):603–616. doi: 10.1016/s0006-2952(01)00709-2. [DOI] [PubMed] [Google Scholar]
- 107.Matsuzawa A, Tseng P-H, Vallabhapurapu S, Luo J-L, Zhang W, Wang H, et al. Essential cytoplasmic translocation of a cytokine receptor-assembled signaling complex. Science. 2008 Aug. 1321(5889):663–668. doi: 10.1126/science.1157340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zeng W, Xu M, Liu S, Sun L, Chen ZJ. Key role of Ubc5 and lysine-63 polyubiquitination in viral activation of IRF3. Mol Cell. 2009 Oct. 2336(2):315–325. doi: 10.1016/j.molcel.2009.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Berchtold CM, Wu Z-H, Huang TT, Miyamoto S. Calcium-dependent regulation of NEMO nuclear export in response to genotoxic stimuli. Mol Cell Biol. 2007 Jan.27(2):497–509. doi: 10.1128/MCB.01772-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hande KR, Wedlund PJ, Noone RM, Wilkinson GR, Greco FA, Wolff SN. Pharmacokinetics of high-dose etoposide (VP-16-213) administered to cancer patients. Cancer Res. 1984 Jan.44(1):379–382. [PubMed] [Google Scholar]