Abstract
Context
Mindfulness Based Cognitive Therapy (MBCT) is a group-based psychosocial intervention designed to enhance self-management of prodromal symptoms associated with depressive relapse.
Objective
To compare rates of relapse in remitted depressed patients receiving MBCT against maintenance antidepressant pharmacotherapy, the current standard of care
Design
Patients who met remission criteria following 8 months of algorithm informed antidepressant treatment were randomized to either: Maintenance Antidepressant Medication (M-ADM), MBCT or placebo (PLA) and were followed for 18 months.
Setting
Outpatient clinics at the Centre for Addiction and Mental Health, Toronto and St. Joseph’s Healthcare, Hamilton.
Participants
One hundred sixty patients aged 18 to 65 meeting DSM-IV for major depressive disorder with a minimum of 2 past episodes. Of these, 84 achieved remission (52.5%) and were assigned to one of the 3 study conditions.
Interventions
Remitted patients either discontinued their antidepressants and attended eight weekly group sessions of MBCT, continued on their therapeutic dose of antidepressant medication or discontinued active medication onto placebo.
Main Outcome Measure
Relapse was defined as a return, for at least 2 weeks, of symptoms sufficient to meet the criteria for major depression on Module A of the SCID.
Results
Intention to treat analyses revealed a significant interaction between the quality of acute phase remission and subsequent prevention of relapse in randomized patients (p = .03). Among unstable remitters (defined as 1 or more HRSD >7 during remission) patients in both MBCT and M-ADM showed a 73% decrease in hazard compared to PLA (p = .03), whereas for stable remitters (all HRSD ≤ 7 during remission) there were no group differences in survival. Findings remained significant after accounting for the effects of past depressive episodes on relapse.
Conclusion
For depressed patients who are unwilling or unable to tolerate long term maintenance antidepressant treatment, MBCT offers equivalent protection from relapse.
Relapse and recurrence following recovery from Major Depressive Disorder (MDD) are common and debilitating outcomes that carry enormous person, familial and societal costs1. Maintenance antidepressant monotherapy (M-ADM), the current standard for depressive relapse prophylaxis2 is effective as long as it is continued, yet in practice, this plan is compromised by rates of patient noncompliance that can reach 40%3,4. Alternatives to long term antidepressant monotherapy, especially those that address mood outcomes in a broader context of well-being, may appeal to patients wary of continued intervention.
One such approach involves the use of sequenced, phase-specific depression treatments within an envelope spanning both acute phase and post-remission care5. Such models involve treating patients to remission pharmacologically and then providing psychotherapy aimed at preventing relapse by teaching affect regulation and self-management skills to be used during recovery. Implicit here is the view that the mechanisms underlying the onset of a depressive episode differ from those responsible for its return6 and that unique interventions are required to address each. Prevention outcomes from the sequential treatment of mood disorders are largely supportive of the approach. Fava7,8 reported lower relapse rates at 4 year follow up and fewer multiple relapses for remitted patients who discontinued medication and received CBT, compared to clinical management. Frank et al.,9 found that time to recurrence was greater for patients who discontinued antidepressants at remission and received Interpersonal Therapy (IPT) or IPT and pill placebo (PLA) versus PLA alone.
Similar findings have been obtained with Mindfulness-Based Cognitive Therapy (MBCT), a group intervention designed to train recovered, recurrently depressed patients to disengage from dysphoria-activated depressogenic thinking that increases risk for relapse/recurrence. In addition, MBCT’s emphasis on the daily practice of health enhancing behaviours such as meditation or yoga is a positive incentive for the type of long term engagement required by any maintenance therapy. To date, this intervention designed to be suitable for patients achieving remission via antidepressant treatment, has been evaluated in 3 RCTs with outcomes suggesting a 50% reduction in relapse for patients receiving MBCT compared to treatment as usual10,11 or no difference in survival compared to maintenance pharmacotherapy12.
These data, while encouraging, do not address the frequently encountered clinical scenario where a remitted patient wishes, whether for reasons of preference13, side effect burden14 or suitability15, (e.g. pregnancy) to discontinue antidepressant treatment but requires additional prophylactic care. Although previous studies have enrolled patients who were already in remission, no study has explicitly treated patients to remission pharmacologically with the aim of testing MBCT’s prevention effects directly following discontinuation, against active treatment or a placebo (PLA) control. Addressing this question would help determine MBCT’s generalizability to real world clinical settings and evaluate, more broadly, the sequential staging through which both treatments are delivered.
The present study was designed to test the relative efficacy of MBCT and M-ADM (versus PLA and clinical management) for prevention of relapse in patients with recurrent depression who have achieved remission through antidepressant pharmacotherapy. We predicted that both MBCT and M-ADM would offer effective protection when compared against PLA and that the level of protection achieved by MBCT would not differ from that provided by M-ADM.
METHODS
The study protocol was approved by respective institutional review boards at the Centre for Addiction and Mental Health (CAMH), Toronto and St. Joseph’s Healthcare, Hamilton. All participants provided written consent prior to any research activity. Subjects were recruited through clinical referrals, physician outreach and from media announcements that described the Mood Disorders Clinics at CAMH and St. Josesph’s, There were two study phases. During the acute phase, all patients received open label, 2 step antidepressant pharmacotherapy in accord with the Texas Medication Algorithm Project guidelines16. Patients who met the criteria for remission were treated for five additional months and then randomly assigned to one of the three study arms.
Diagnostic eligibility for the study was determined using the Structured Clinical Interview for DSM- IV diagnosis (Axis I and II)17,18. In addition, the first 17 items of the Hamilton Depression Rating Scale (HDRS)19 were used to determine whether the severity of depressive symptoms warranted inclusion in the trial.
Inclusion criteria were: (1) diagnosis of Major Depressive Disorder (MDD) according to DSM-IV criteria, (2) a score of ≥ 16 on the Hamilton Depression Rating Scale (HRSD-17), (3) ≥ 2 previous episodes of MDD [to ensure that those randomized would have a minimum of 3 past episodes], (4) between 18 and 65 years of age and (5) English speaking and the ability to provide informed consent. Exclusion criteria were: (1) a current diagnosis of Bipolar Disorder, Substance Abuse Disorder, Schizophrenia or Borderline Personality Disorder, (2) a trial of ECT within the past six months (3) depression secondary to a concurrent medical disorder, (4) current or planned pregnancy within the 6 months of acute phase treatment, (5) current practice of meditation more than once per week or yoga more than twice per week.
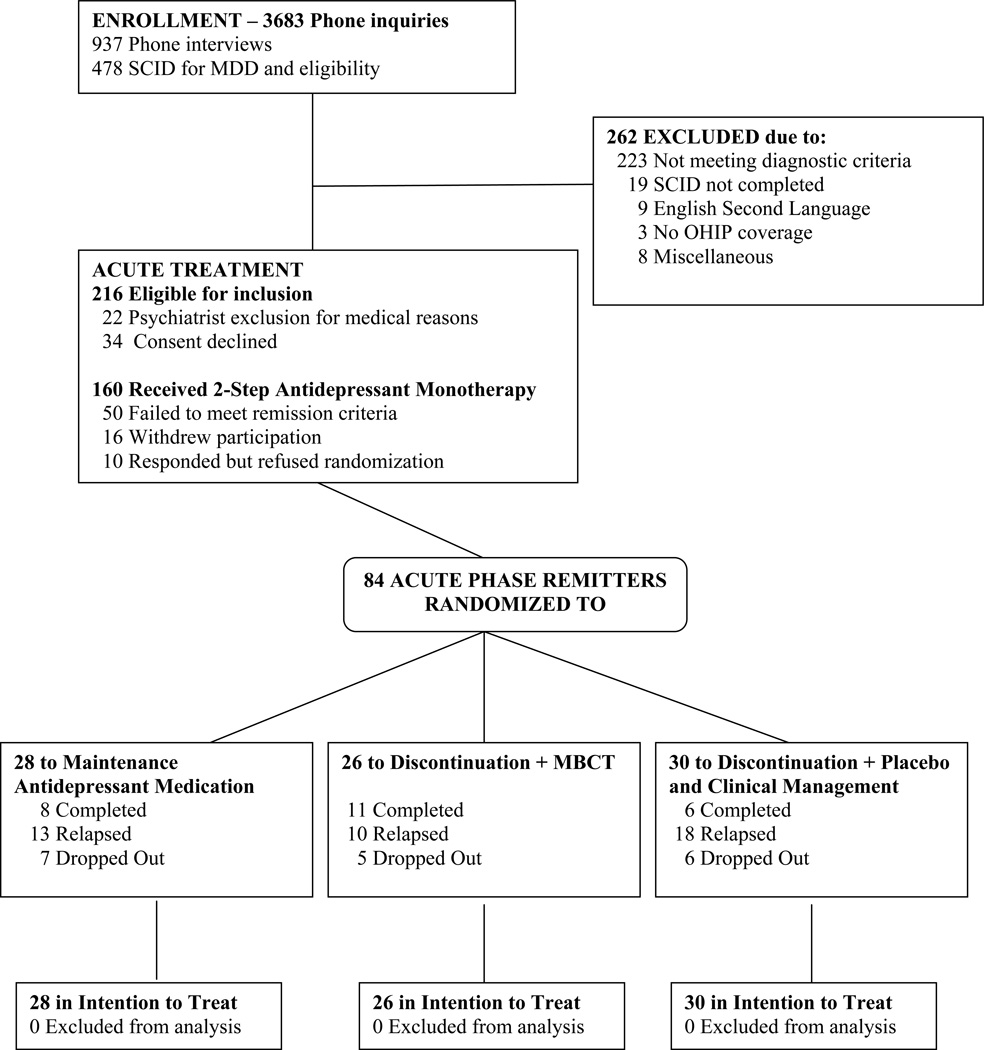
A total of 478 patients were evaluated for the study, 112 of whom either did not meet diagnostic criteria for MDD or did not achieve HDRS scores of 16 or higher at both the screen and baseline study visits. Another 150 patients met the following exclusion criteria: (1) history of bipolar disorder (n=14); (2) substance abuse or dependence judged to require treatment (n=33); (3) current or past psychosis (n=3); (4) another DSM-IV Axis I disorder judged to require treatment in preference to the depression (n=36); (5) DSM-IV Axis II disorders deemed to be poorly suited to the treatments under investigation (n=23); (6) suicide risk requiring immediate hospitalization (n=1); or (7) other exclusion criteria (n=40). This left a final sample of 216 patients eligible for acute treatment and of these 22 were ruled out for medical reasons and 34 declined consent, leaving a final sample of 160 patients that entered the open label study (see Figure 1).
Figure 1.
Study Flow of Patients from Screening to Analysis
Study Phases
Open Label, Acute Phase
All patients were treated with a 2 step, standardized monotherapy algorithm informed by the Texas Medication Algorithm Project16 designed to maximize the likelihood of treatment response. Patients in Step 1 started treatment with citalopram at a target dose of 20mg that was increased in 10mg steps if needed to a maximum of 60 mg, until either response was achieved or dose-limiting side effects emerged. In patients who could not tolerate citalopram, a trial of sertraline starting at 50mg/day with 50mg increments per week was initiated with a target dose of at least 100 mg and a maximum of 200mg/day. Patients with documented SSRI failure in this episode over at least an eight week trial were switched to a novel antidepressant, either venlafaxine or mirtazapine based on symptom profile and patient preference. Patients in Step 2 were started after no more than 24 hours washout following the taper of Step 1 medication. Venlafaxine was started at 37.5mg per day for one week, increased to 75mg the next week, 150mg (the minimum target dose) the following week and then in 75mg increments until the patient showed a full response (HRSD-17 < 8) or was unable to tolerate side effects (to a maximum of 375mg). For patients who could not tolerate venlafaxine, mirtazapine was started at 15mg per day for one week and increased in 15mg increments per week to a minimum target dose of 30mg and a maximum of 45mg based on response and tolerability. Patients meeting criteria20 for treatment response (50% reduction in HRSD) and clinical remission (HRSD ≤ 7 for 8 weeks) were treated for five additional months to ensure full remission. Patients who did not respond to or tolerate the treatment options allowed in the protocol were withdrawn from the study and offered treatment, based on clinical profile and preference in the respective Mood Disorders Clinic.
Medication was prepared by the pharmacy at CAMH according to CAMH formulary standards and dispensed in blister packs containing patients’ daily dosage for the time between visits. Patients met with their study psychiatrist biweekly for the first 8 weeks and monthly thereafter. Study psychiatrists inquired about compliance during the interval between visits and patients were asked to return unused pills. Raters noted the number of unused pills on a Medication Dosage Record Form. Patients who had not taken at least 75% of the prescribed dose in any two week period were considered to be non-compliant.
Clinical Remission during the Acute Phase
Prior work has demonstrated that the quality of acute phase remission strongly influences the risk of subsequent relapse20,21. To examine this relationship across our two study phases, we classified all remitters as having had either an unstable or stable remission, based on the presence or absence of ‘symptom flurries’20 during the approximately 5 months between initial remission and randomization. Patients who had a stable remission were those who maintained an HRSD score of ≤ 7 across this interval, while unstable remitters achieved the same HRSD threshold but had occasional elevated scores across this interval. These patients were considered in remission if 1) their score subsequent to an elevation was ≤ 7 and 2) the range of elevated scores fell between 8 and 14. This classification divided the entire sample in half (49% stable remitters and 51% unstable remitters; see Table 2).
Table 2.
Baseline Characteristics of Clinical Remitters
| Variable | Stable Remission (n=41) |
Unstable Remission (n=43) |
|---|---|---|
| HRSD score at entry, mean ± SD | 18.7 ± 3.2 | 19.5 ± 2.9 |
| HRSD score at randomization | 2.12 ± 2.3 | 3.42 ± 3.0* |
| QIDS at entry | 13.6 ± 4.4 | 14.4 ± 3.7 |
| QIDS at randomization | 2.8 ± 2.2 | 3.4 ± 2.0 |
| Female, % | 59 | 67 |
| White, % | 84 | 73 |
| Age, yrs | 44 ± 11.6 | 44 ± 10.4 |
| Married/cohabitating, % | 33 | 44 |
| Employed, % | 65 | 79 |
| Age of first onset | 33 ± 11.4 | 29 ± 11.6 |
| No. of prior episodes | 4.9 ± 2.6 | 4.6 ± 2 |
| Duration current episode in weeks | 63.3 ± 84.2 | 100.3 ± 113.3 |
| Days in acute phase | 217.1 ± 56.9 | 248.8 ± 66.9* |
| Days to reach remission | 72.7 ± 51.76 | 86.8 ± 61.1 |
| Days in remission | 144.4 ± 32.7 | 162 ± 39.7* |
| Hx of prior antidepressant % | 45 | 65 |
| Hx of psychiatric hospitalization, % | 10 | 5 |
| Any Axis I comorbidity, % | 39 | 28 |
| Hx substance abuse/dependence, % | 10 | 2 |
| Any Axis II comorbidity, % | 37 | 37 |
p < .05
Double/Single Blind Maintenance Phase
Following a minimum of seven months clinical remission (8 weeks to meet criteria and 5 months of additional treatment) patients entered the maintenance phase, where they were randomly assigned to one of the three study arms: maintenance antidepressant medication, medication taper plus MBCT, medication taper plus PLA. Block randomization, utilizing a block size of 4 was performed at CAMH by an independent statistician (TB) using computer generated quasi-random numbers. Details of group assignment were contained in sealed envelopes which were opened by the statistician and communicated to the coordinator once a patient was deemed suitable for study entry. Patients in the M-ADM condition remained on the same drug at the maximum tolerated and effective dose as outlined above. With respect to M-ADM and PLA+CLIN, study psychiatrists were blind to treatment assignment, whereas once patients in MBCT completed their taper they no longer took any pills. Patients in both the PLA+CLIN and MBCT conditions were tapered gradually, over a 4 week period, via placebo substitution and reduced pill count, respectively, at the recommended rate for their specific medication to minimize the risk of discontinuation syndrome22,23. Prescription of additional medication for sleep complaints or anxiety symptoms was also permitted during this period (e.g. zopiclone and benzodiazepines). Study psychiatrists met with patients biweekly for the first four weeks of both acute and maintenance treatment phases, then monthly for the next three months and bimonthly thereafter. Meeting frequency with study psychiatrists was identical in all three conditions.
MBCT was delivered according to the protocol described in Segal et al.,24. Patients attended 8 weekly group meetings of 2 hours duration and a retreat day held between sessions 6 and 7. In addition, patients had the option of attending a monthly one hour mindfulness mediation class that was offered throughout the maintenance phase. MBCT is based on empirical work showing that relapse is associated with the reinstatement of automatic modes of thinking and feeling that are characteristic of the depressed state25 (e.g. rumination and avoidance). By deliberately monitoring and observing their thinking patterns when they feel sad, patients develop skills in metacognition or decentering that serve to render this type of automatic processing more accessible to effortful reflection26,27. This is accomplished through daily homework exercises featuring 1) guided (taped) awareness exercises directed at increasing moment-by-moment non-judgmental awareness of bodily sensations, thoughts and feelings, 2) accepting difficulties with a stance of self-compassion and 3) in later sessions, developing an ‘action plan’ comprised of strategies for responding to early warning signs of relapse/recurrence. A key theme stressed throughout the program is the transfer of these awareness skills into patients’ everyday lives.
Outcome Measures
Patients were assessed by clinical evaluators blind to treatment allocation at randomization, biweekly for the first eight weeks, monthly for the next three months and bimonthly for the remainder of the 18 month maintenance phase.
The primary outcome measure was time to relapse/recurrence of DSM-IV major depressive episode, using the depression module of the SCID. Patients who scored 16 or greater on the HRSD-17 at a scheduled physician visit were re-interviewed in a week's time and if their scores were in the same range, they were then assessed with the SCID to determine whether their level of symptomatology met criteria for MDD. In addition, patients were encouraged to call the clinic if they were concerned that depressive symptoms were reemerging, in which case, an ad hoc assessment was scheduled as soon as possible. In those cases when a patient did not attend a scheduled visit or failed to notify study staff when they began to experience new symptoms, they could be judged to have relapsed based on the Longitudinal Interval Follow Up Evaluation28. A patient was judged to have an episode of major depression if they had a score of ≥ 5 for 2 consecutive weeks at any time during the maintenance phase. All interviews were autdiotaped. Interviewers’ ratings of a subset of taped assessments using the 17 item HRSD yielded an intraclass correlation coefficient of 0.94 (n=18) and the reliability of the major depressive episode diagnosis based on the SCID, in a subset of taped interviews, yielded a K29 coefficient of 0.82 (n=22). Diagnoses were also confirmed by an experienced research psychiatrist.
Data Analysis
Tests of potential differences across study groups on demographic and clinical history variables were performed using ANOVA for continuous measures and Pearson Chi Square for categorical variables. Where applicable, post-hoc testing for continuous variables was performed with Tukey’s HSD. To examine whether receiving preferred maintenance treatment was associated with relapse, we assessed treatment preference via the Treatment Preference Index Form (TXPR)10. Survival curves and relapse rates testing the main effect of intervention and potential effects of quality of acute phase remission and number of past episodes were estimated using the Cox proportional hazards regression model30. Patients unavailable for follow-up and those who accessed non-study depression treatment without a documented relapse were treated as censored observations. Survival rates for the three conditions were compared with the log-rank test.
RESULTS
Patient Flow and Dropout
One hundred and sixty patients enrolled in the open label, acute treatment phase. Of these, 50 failed to reach remission, 16 withdrew participation and 10 responded but declined consent for moving to the next study phase. During the maintenance phase, 18 patients dropped out of the protocol, 7 from M-ADM, 5 from MBCT and 6 from PLA. Attrition was evenly distributed across the 18 month follow up interval, with 50% of drop outs occurring by the 9th month. Some patients missed 1 or more physician visits but did complete the LIFE interviews at subsequent meetings. We, therefore, have complete information on 66 (75%) of the 84 remitted patients.
Demographic and Clinical Characteristics
Patients in the overall sample had a mean age at study entry of 44 years (11.49) and 58% of the sample was female, with 20% self-identified as a member of an ethnic/racial minority group. Differences on baseline demographic and clinical history variables between clinical remitters and patients who were not randomized are shown in (Table 1). As expected, randomized patients showed significant decreases in pre to post treatment scores on the HRSD (P < .001) and the Quick Inventory of Depressive Symptoms31 (QIDS) (P < .001). Non-randomized patients had higher QIDS scores (P<.05) and spent fewer days in the acute phase (P<.001) than remitters. During open label, acute phase treatment, 43 patients (51%) were classified as unstable remitters while 41 (49%) met criteria for stable remission, essentially dividing the remitter sample in half. As shown in Table 2, unstable remitters had higher HRSD scores (P<.05), took longer to reach remission (P<.05) and spent more days in remission than stable remitters (P<.05), but interestingly, there was no difference in the time taken by each group to reach remission. Table 3 shows that there were no differences in baseline characteristics between the three prevention arms, with the only exception being a greater percentage of Axis II comorbidity in MBCT (P<.05).
Table 1.
Baseline Characteristics
| Variable | Whole Sample (n=160) |
Randomized (n=84) |
Non- Randomized (n=76) |
|---|---|---|---|
| HRSD score at entry, mean ± SD | 19.4 ± 3.5 | 19.1 ± 3.1 | 19.7 ± 3.9 |
| HRSD score at randomization | 2.8 ± 2.8 | ||
| QIDS at entry | 14.5 ± 3.9 | 14 ± 4.0 | 15.3 ± 3.8* |
| QIDS at randomization | 3.11 ± 2.13 | ||
| Female, % | 58 | 63 | 53 |
| White, % | 80 | 79 | 82 |
| Age, yrs | 44 ± 11 | 44 ± 11 | 45 ± 12 |
| Married/cohabitating, % | 40 | 38 | 42 |
| Employed, % | 67 | 72 | 61 |
| Age of onset first | 31 ± 12.3 | 31 ± 11.6 | 31 ± 13.3 |
| No. of prior episodes | 4.3 ± 3.5 | 4.7 ± 2.3 | 3.9 ± 4.6 |
| Duration current episode in weeks | 100 ± 128.8 | 83 ± 101.6 | 119 ± 151 |
| Days in acute phase | 188.0 ± 85.9 | 233.3 ± 63.8 | 138 ± 79.2** |
| Days to reach remission | N/A | 79.9 ± 56.8 | |
| Days in remission | N/A | 153.4 ±37.3 | N/A |
| Hx prior Antidepressant, % | 54 | 55 | 52 |
| Hx psychiatric hospitalization, % | 8 | 7 | 9 |
| Any Axis I comorbidity, % | 38 | 33 | 42 |
| Hx substance abuse/depend. % | 9 | 6 | 12 |
| Any Axis II comorbidity, % | 39 | 37 | 41 |
Comparisons between randomized and non randomized patients:
p < .05,
p <.001
Table 3.
Baseline Characteristics of Treatment Groups
| Variable | M-ADM (n=28) |
MBCT (n=26) |
PLA+Clin (n=30) |
|---|---|---|---|
| HRSD score at entry, mean ± SD | 19.2 ± 3 | 18.9 ± 3.5 | 19.2 ± 2.8 |
| HRSD score at randomization | 2 ± 2.3 | 3 ± 2.8 | 3.3 ± 3 |
| QIDS at entry | 14.3 ± 4.6 | 13.6 ± 3.7 | 14.1 ± 3.9 |
| QIDS at randomization | 3 ± 1.7 | 3.4 ± 2.4 | 2.9 ± 2.3 |
| Unstable remission in acute phase | N = 11 | N = 18 | N = 14 |
| Stable remission in acute phase | N = 17 | N = 8 | N = 16 |
| Female, % | 71.4 | 50.0 | 66.7 |
| White, % | 85.7 | 73.1 | 76.7 |
| Age, yrs | 45.8 ± 11.4 | 44.8 ± 9.4 | 41.9 ± 11.6 |
| Married/cohabitating, % | 36 | 39 | 40 |
| Employed, % | 79 | 77 | 62 |
| Age of first onset | 34.6 ± 12.7 | 28.78 ± 10 | 29.9 ± 11.3 |
| No. of prior episodes | 4.9 ± 2.6 | 4.5 ± 2.2 | 4.8 ± 2.1 |
| Duration of current episode in weeks | 80.7 ± 111.6 | 102.6 ± 92.2 | 67.8 ± 101.1 |
| Days in acute phase | 231.4 ± 59.7 | 228 ± 52.6 | 239.7 ± 34.2 |
| Days to reach remission | 80.1 ± 60 | 68.1 ± 51.9 | 90 ± 57.8 |
| Days in remission | 151.3 ± 31.7 | 160 ± 34.2 | 149.7 ± 44.5 |
| Hx of prior antidepressant, % | 61 | 54 | 52 |
| Hx psychiatric hospitalization, % | 7 | 4 | 10 |
| Any Axis I comorbidity, % | 39 | 35 | 27 |
| Hx substance abuse/dependence, % | 4 | 4 | 10 |
| Any Axis II comorbidity, % | 18 | 58 | 37* |
p < .05
Preliminary Analyses
Patients were asked at study entry to indicate which condition they would prefer being assigned to in the maintenance phase. An analysis was performed using Chi Square, on 70 of the 84 randomized patients who completed the TXPR. Of these, 23 (32.9%) stated a preference for medication during the maintenance phase, 35 (50%) stated a preference to receive MBCT, 1 (1.4%) stated a preference for PLA and 11 (15.7%) stated no preference. Chi square analyses revealed no significant difference in relapse rate between matched (12/19 or 63% relapse) and mismatched patients (20/40 or 50% relapse) suggesting no effect of preference matching on the key outcome measure.
The three MBCT therapists were two PhD level psychologists and one Master’s level social worker, each of whom had attended a 7 day residential training workshop with Dr. Segal and taught the MBCT program in their respective clinical workplaces. All MBCT group sessions were videotaped and therapist performance was monitored using the Mindfulness-Based Cognitive Therapy Adherence Scale32 (MBCT-AS) a 17 item scale describing specific mindfulness exercises and cognitive therapy content. Scores range from 0 to 2 for each item describing one of the therapeutic tasks included in the protocol (0 = no evidence for item, 1 = slight evidence, 2 = definite evidence). A rating of 0 on any item indicates unsatisfactory performance and calls for specific supervisory intervention. Across all groups, study patients attended an average of 6 out of the 8 weekly MBCT sessions. An independent rater viewed all MBCT sessions and rated them for treatment adherence. His score of 1.8 indicated that adherence was very good across all groups.
Study psychiatrists were trained by Dr. Young in accordance with the manual used in the Treatment of Depression Collaborative Research Project33. Pharmacotherapy sessions were 20 minutes in duration and emphasized both medication management (education, dosage adjustment, dosage scheduling and side effects) and clinical management (discussion of functionality, support and limited advice). Psychotherapeutic strategies, especially CBT techniques were prohibited. Monthly and informal consultation continued throughout the study to address any issues that arose as a result of pharmacological treatment.
Relapse
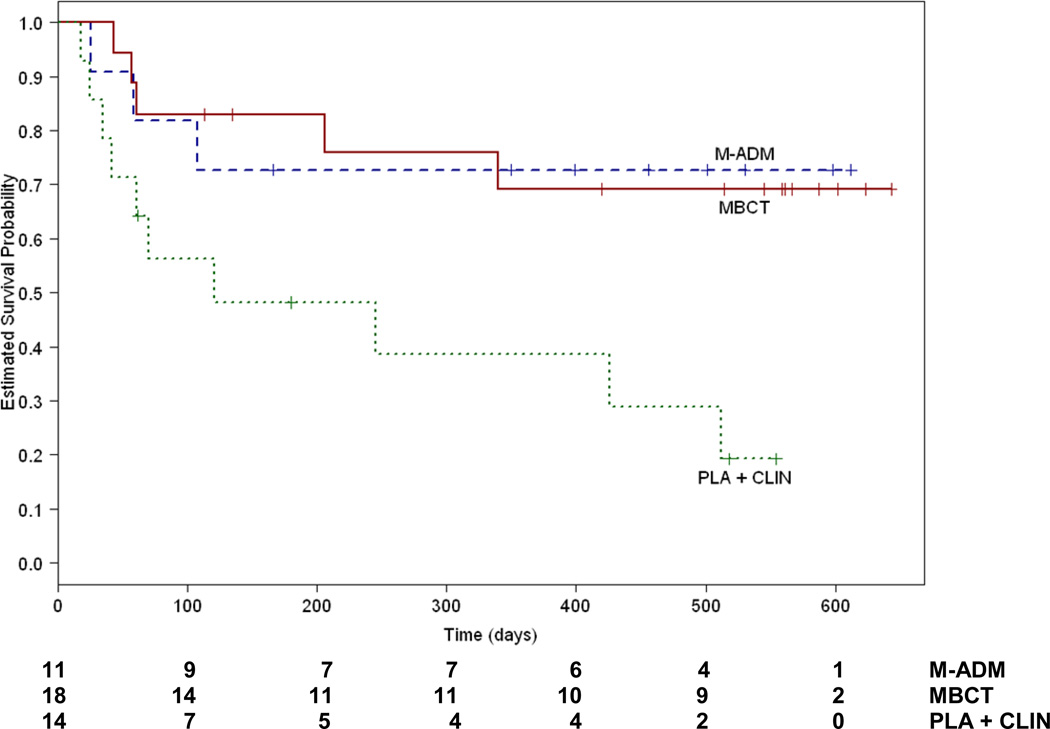
In the Intention to Treat sample, the model we used to test the association between predictors of interest and the hazard of relapse included separate terms for the number of past depressive episodes, treatment group, quality of remission and the interaction of treatment group by quality of remission. The overall model was significant (X2 = 13.70, df=6, P=.03) and there was a significant interaction between the quality of acute phase remission (stable or unstable) and treatment group (X2 = 7.27, df=2, P=.03) but no main effects for treatment group (X2 = 0.84, df=2, P=.66 - relapse rates: MBCT 38%, M-ADM 46%, PLA 60%) or quality of acute phase remission (X2 = 2.40, df=1, P=0.12 - relapse rates: unstable remitters 42% and stable remitters 56%). As shown in Figure 2, for unstable remitters, MBCT reduced the risk for subsequent relapse relative to PLA (X2 = 6.01, df=1, P=.01) as did M-ADM (X2 = 4.55, df=1, P=.03). MBCT and M-ADM did not differ from each other in their protective effects (X2 = 1.07, df=1, P=.93). Adjusted relapse rates for each condition were 27% for M-ADM, 28% for MBCT and 71% for PLA. Hazard ratios were calculated between PLA and each of the active treatments. Exposure to MBCT was associated with a hazard ratio for subsequent relapse of .26 (95% CI 0.09–0.79) relative to PLA which means that MBCT reduced risk by 74%. Maintenance antidepressant pharmacotherapy was associated with a hazard ratio of .24 (95% CI 0.07–0.89) indicating a 76% reduction in risk relative to PLA. The hazard associated with the comparison of MBCT to M-ADM was 1.07 (95% CI 0.25–4.49) indicating no change in risk status.
Figure 2.
Cumulative proportion of unstable remitters who survived without relapse during maintenance/follow up. M-ADM indicates maintenance antidepressant pharamacotherapy, MBCT indicates taper + Mindfulness Based Cognitive Therapy and PLA+CLIN indicates taper + pill placebo and clinical management.
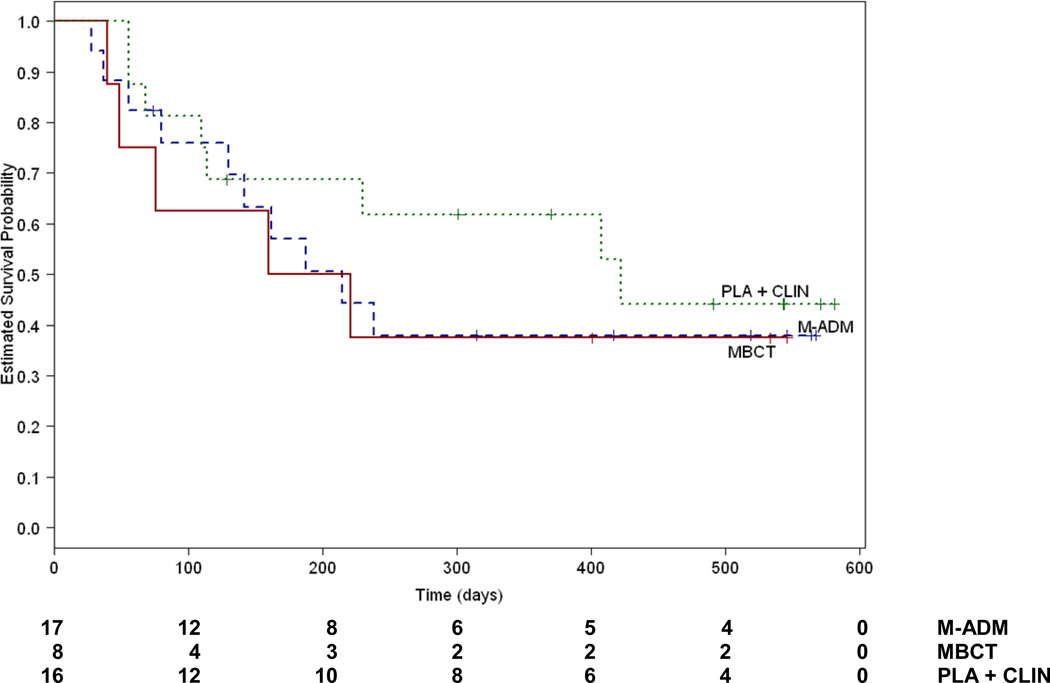
For stable remitters, there was no difference between the treatments in relapse rates (Figure 3). MBCT did not differ from PLA (X2 = .73, df=1, P=.39) and neither did M-ADM (X2 = .47, df=1, P=.49) in reducing risk for subsequent relapse. In patients showing a stable remission, MBCT and M-ADM did not differ in their effects (X2 = .08, df=1, P=.77). Adjusted relapse rates for each condition were 59% for M-ADM, 62% for MBCT and 50% for PLA.
Figure 3.
Cumulative proportion of stable remitters who survived without relapse during maintenance/follow up. M-ADM indicates maintenance antidepressant pharamacotherapy, MBCT indicates taper + Mindfulness Based Cognitive Therapy and PLA+CLIN indicates taper + pill placebo and clinical management.
Examining the combined outcomes of patients receiving any active treatment compared to PLA, we found that the overall model was significant (X2 = 13.70, df=4, P=.009) and that the interaction between the quality of acute phase remission and the type of prevention treatment patients received was also significant (X2 = 7.23, df=1, P=.007). For patients with an unstable remission during the acute phase, active treatment during the maintenance phase reduced the risk for subsequent relapse relative to PLA (X2 = 8.26, df=1, P=.004). Adjusted relapse rates for each condition were 28% for active treatment and 71% for PLA. Prior exposure to active treatment was associated with a hazard ratio of .25 (95% CI 0.10–0.65). In sum, these data suggest that providing a long-term active treatment to unstable remitters allowed them to maintain their treatment gains over time, a finding previously reported in studies of patients with residual symptoms8. Surprisingly, for stable remitters, there was no difference in relapse rates between active treatment or PLA (X2 = .74, df=1, P=.39). Adjusted relapse rates for each condition were 60.5% for active treatment and 50% for PLA.
As has been reported previously34, the number of past depressive episodes at study entry was a significant predictor of relapse during the maintenance phase in analyses with either MBCT and M-ADM examined singly (X2 = 5.55, df=1 P=.02) or combined (X2 = 5.51, df=1 P=.02). Each additional episode of depression was associated with a 16% increase in hazard (HR = 1.15, 95% CI 1.02–1.30). Inclusion of past depressive episodes in the larger statistical model did not alter the pattern of results reported above.
COMMENT
Naturalistic studies of depressed outpatients suggest that many will stop medication prematurely on their own despite recommendations for continuation4. Another group of patients may be unsuitable for long term antidepressant treatment because of emergent clinical issues such as pregnancy or drug interactions5,15,35. We studied a preventive MBCT intervention in recurrently depressed patients who were discontinued from antidepressant medication after achieving full remission and compared their long term outcomes to those who stayed on medication or received placebo. Our findings indicated that the quality of remission achieved during the acute phase interacted with the type of prevention treatment patients received to determine relapse outcomes during the subsequent maintenance phase. For patients whose acute phase remission was marked by periodic symptoms flurries20,21, discontinuing ADM and receiving MBCT or continuing with M-ADM significantly lowered relapse risk compared to discontinuation to PLA. These results are in accord with previous reports that the temporal features of remission or the presence of residual symptoms are correlated with poorer acute and maintenance phase outcomes36,37 and that reduction of this risk with targeted treatment is beneficial34,38,39. Of note, in this group of patients in need of continued intervention, MBCT and M-ADM were equally effective.
Surprisingly, for patients whose acute phase remission was stable, there was no differential impact on survival between the treatments we studied. While the 50% relapse rate in PLA is in line with other studies in which antidepressants were discontinued following continuation treatment (e.g. Keller et al,40: 47.3%, Montgomery et al.,41: 55%), the protective effects of active treatment were smaller. What explains this discrepancy? First, since sample characteristics are known to vary across studies it makes it difficult to compare absolute relapse rates from other trials. Second, stable and unstable remitters did not differ on a number of demographic or clinical history variables known to impact relapse risk. One possibility is that stable remitters may have had lower motivation to comply with treatment in the maintenance phase where, having shown a robust clinical response, they faced a two out of three chance of discontinuation. Secondary analyses of treatment mediators may help to clarify these group differences.
In spite of its growing evidence base, exactly how MBCT exerts its preventive effect is not fully understood. Because the daily practice of mindfulness invariably cues exposure to negative emotions, patients learn how to uncouple their habitual responses to dysphoria-triggering cues26,42 in favor of responses informed by a metacognitive relationship to the very same mental contents. Data on the neural changes associated with mindfulness training support this view. Mindfulness practitioners demonstrated less neural reactivity to sadness provocation relative to a group of novices, as seen via both reduced activation of posterior cortical midline structures and in reduced suppression of right viscerosomatic networks such as the insula and right lateral prefrontal cortex27. Reduced suppression in the insula and subgenual ACC have also been observed in depressed patients treated with cognitive therapy43 and may point to a common locus of effect. The affect regulation afforded by these growing capacities, may make it easier for patients to adopt lifestyle and behavioural strategies that support recovery, a sine qua non of any effective maintenance treatment.
This study had a number of limitations. Reporting a lack of difference between M-ADM and MBCT in both stable and unstable remitters raises the risk of Type II error, in which, due to low power, an important effect may be missed. One way to address this involves calculating E44, the expected number of relapse events required to replicate the reported hazard ratios for the comparisons between M-ADM and MBCT. The analysis revealed that in both stable and unstable groups, detecting hazard ratios different from one would require extremely large samples (N > 1000). This suggests that the lack of significance between M-ADM and MBCTs is less likely attributable to under sampling, and more likely due to a very small measured effect.
As with any long term treatment study, there is a possibility for bias through differential retention of patients. Only slightly more than half of the patients initially enrolled were eligible for randomization into the maintenance phase, most due to nonresponse but also because once having achieved remission they declined to move into the maintenance phase. This raises the question of how closely the randomized sample represents the initial group. Although we relied on randomization to equate the treatment groups on baseline characteristics, differential retention cannot be fully ruled out for clinical features we did not consider.
It is well established that patients with recurrent depression require care past the point of episode remission. For those, unwilling or unable to tolerate maintenance antidepressant treatment our data suggest that MBCT offers equal protection from relapse over 18 months and highlights the importance of maintaining at least one active long term treatment in unstable remitters.
Acknowldegements
We thank the following colleagues for contributing to this research. Richard Bloch MA, Shelly Ferris, MA, Kate Szacun-Shimizu, MA and Karyn Hood, MA served as study coordinators. Susan Woods, MA and Theresa Casteels, PhD and Peter Bieling, PhD served as MBCT study therapists. Robert Levitan MD, Trevor Young MD, Glenda MacQueen MD, Lawrence Martin MD, Robert Cooke MD and Jennifer Brasch MD served as study psychiatrists. Lori Hoar, MA, Joanne Nault, MA, Rebecca Pedersen MA and Zoe Laksman, MA served as project interviewers, Bao Chau Du and Heidy Morales provided research support. Tom Buis, MA, and Mr Andrew Pedersen provided programming and data analytic support. David Streiner PhD provided statistical and study design consultation.
This study was funded by Grant #066992 (R01: Dr. Segal) from the National Institute of Mental Health, Bethesda, MD and was presented at the Association for Behavioural and Cognitive Therapies, New York, November 2009.
Contributor Information
Zindel V. Segal, Centre for Addiction and Mental Health, Toronto, Ontario.
Peter Bieling, St. Joseph’s Healthcare, Hamilton, Ontario.
Trevor Young, University of British Columbia.
Glenda MacQueen, University of Calgary.
Robert Cooke, Centre for Addiction and Mental Health, Toronto, Ontario.
Lawrence Martin, St. Joseph’s Healthcare, Hamilton, Ontario.
Richard Bloch, Centre for Addiction and Mental Health, Toronto, Ontario.
Robert Levitan, Centre for Addiction and Mental Health, Toronto, Ontario.
REFERENCES
- 1.Judd LL, Akiskal HS, Zeller PJ, Paulus M, Leon AC, Maser JD, Endicott J, Coryell W, Kunovac JL, Mueller TI, Rice JP, Keller MB. Psychosocial disability during the long-term course of unipolar major depressive disorder. Arch Gen Psychiatry. 2000;57:375–380. doi: 10.1001/archpsyc.57.4.375. [DOI] [PubMed] [Google Scholar]
- 2.Practice guideline for the treatment of patients with major depressive disorder (revision) Am J Psychiatry. 2000;157(4 suppl):1–45. [PubMed] [Google Scholar]
- 3.ten Doesschate MC, Bockting CL, Schene AH. Adherence to continuation and maintenance antidepressant use in recurrent depression. J Affect Disord. 2009;115:167–170. doi: 10.1016/j.jad.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 4.Simon GE, Von Korff M, Rutter CM, Peterson DA. Treatment process and outcomes for managed care patients receiving new antidepressant prescriptions from psychiatrists and primary care physicians. Arch Gen Psychiatry. 2001;58:395–401. doi: 10.1001/archpsyc.58.4.395. [DOI] [PubMed] [Google Scholar]
- 5.Fava GA, Ruini C, Rafanelli C. Sequential treatment of mood and anxiety disorders. J Clin Psychiatry. 2005;66:1392–1400. doi: 10.4088/jcp.v66n1108. [DOI] [PubMed] [Google Scholar]
- 6.Post RM. Transduction of psychosocial stress into the neurobiology of recurrent affective disorder. Am J Psychiatry. 1992;149:999–1010. doi: 10.1176/ajp.149.8.999. [DOI] [PubMed] [Google Scholar]
- 7.Fava GA, Rafanelli C, Grandi S, Conti S, Belluardo P. Prevention of recurrent depression with cognitive behavioral therapy: preliminary findings. Arch Gen Psychiatry. 1998;55:816–820. doi: 10.1001/archpsyc.55.9.816. [DOI] [PubMed] [Google Scholar]
- 8.Fava GA, Ruini C, Rafanelli C, Finos L, Conti S, Grandi S. Six-year outcome of cognitive behavior therapy for prevention of recurrent depression. Am J Psychiatry. 2004;161:1872–1876. doi: 10.1176/ajp.161.10.1872. [DOI] [PubMed] [Google Scholar]
- 9.Frank E, Kupfer DJ, Perel JM, Cornes C, Jarrett DB, Mallinger AG, Thase ME, McEachran AB, Grochocinski VJ. Three-year outcomes for maintenance therapies in recurrent depression. Arch Gen Psychiatry. 1990;47:1093–1099. doi: 10.1001/archpsyc.1990.01810240013002. [DOI] [PubMed] [Google Scholar]
- 10.Teasdale JD, Segal ZV, Williams JM, Ridgeway VA, Soulsby JM, Lau MA. Prevention of relapse/recurrence in major depression by mindfulness-based cognitive therapy. J Consult Clin Psychol. 2000;68:615–623. doi: 10.1037//0022-006x.68.4.615. [DOI] [PubMed] [Google Scholar]
- 11.Ma SH, Teasdale JD. Mindfulness-based cognitive therapy for depression: replication and exploration of differential relapse prevention effects. J Consult Clin Psychol. 2004;72:31–40. doi: 10.1037/0022-006X.72.1.31. [DOI] [PubMed] [Google Scholar]
- 12.Kuyken W, Byford S, Taylor RS, Watkins E, Holden E, White K, Barrett B, Byng R, Evans A, Mullan E, Teasdale JD. Mindfulness-based cognitive therapy to prevent relapse in recurrent depression. J Consult Clin Psychol. 2008;76:966–978. doi: 10.1037/a0013786. [DOI] [PubMed] [Google Scholar]
- 13.Raue PJ, Schulberg HC, Heo M, Klimstra S, Bruce ML. Patients' depression treatment preferences and initiation, adherence, and outcome: a randomized primary care study. Psychiatr Serv. 2009;60:337–343. doi: 10.1176/appi.ps.60.3.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clayton A, Keller A, McGarvey EL. Burden of phase-specific sexual dysfunction with SSRIs. J Affect Disord. 2006;91:27–32. doi: 10.1016/j.jad.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 15.Pedersen LH, Henriksen TB, Vestergaard M, Olsen J, Bech BH. Selective serotonin reuptake inhibitors in pregnancy and congenital malformations: population based cohort study. BMJ. 2009;339:b3569–b3569. doi: 10.1136/bmj.b3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trivedi MH, Rush AJ, Crimson M, Kashner T, Toprac M, et al. Clinical results for patients with major depressive disorder in the Texas Medication Project. Arch Gen Psychiatry. 2004;61:669–680. doi: 10.1001/archpsyc.61.7.669. [DOI] [PubMed] [Google Scholar]
- 17.First M, Spitzer R, Gibbon M, Williams J. Structured clinical interview for Axis I DSM IV disorders- -Patient edition. Washington, DC: American Psychiatric Press Inc; 1994. [Google Scholar]
- 18.First MB, Gibbon M, Spitzer RL, Williams JB, Benjamin LS. Structured Clinical Interview for DSM-IV Axis II Personality Disorders, (SCID-II) Washington, DC: American Psychiatric Press, Inc; 1997. [Google Scholar]
- 19.Hamilton M. Rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frank E, Prien RF, Jarrett RB, Keller MB, Kupfer DJ, Lavori PW, Rush AJ, Weissman MM. Conceptualization and rationale for consensus definitions of terms in major depressive disorder: remission, recovery, relapse, and recurrence. Arch Gen Psychiatry. 1991;48:851–855. doi: 10.1001/archpsyc.1991.01810330075011. [DOI] [PubMed] [Google Scholar]
- 21.Jarrett RB, Kraft D, Doyle J, Foster BM, Eaves GG, Silver PC. Preventing recurrent depression using cognitive therapy with and without a continuation phase: a randomized clinical trial. Arch Gen Psychiatry. 2001;58:381–388. doi: 10.1001/archpsyc.58.4.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haddad P. The SSRI discontinuation syndrome. J Psychopharmacol. 1998;12:305–313. doi: 10.1177/026988119801200311. [DOI] [PubMed] [Google Scholar]
- 23.Rosenbaum JF, Zajecka J. Clinical management of antidepressant discontinuation. J Clin Psychiatry. 1997;58 Suppl 7:37–40. [PubMed] [Google Scholar]
- 24.Segal ZV, Williams JM, Teasdale JD. Mindfulness-based cognitive therapy for depression: A new approach to preventing relapse. New York: Guilford Press; 2002. [Google Scholar]
- 25.Segal ZV, Kennedy S, Gemar M, Hood K, Pedersen R, Buis T. Cognitive reactivity to sad mood provocation and the prediction of depressive relapse. Arch Gen Psychiatry. 2006;63:749–755. doi: 10.1001/archpsyc.63.7.749. [DOI] [PubMed] [Google Scholar]
- 26.Michalak J, Heidenreich T, Meibert P, Schulte D. Mindfulness predicts relapse/recurrence in major depressive disorder after mindfulness-based cognitive therapy. J Nerv Ment Dis. 2008;196:630–633. doi: 10.1097/NMD.0b013e31817d0546. [DOI] [PubMed] [Google Scholar]
- 27.Farb N, Anderson A, Mayberg H, Bean J, McKeon D, Segal Z. Mindfulness training is associated with altered neural response to sad mood provocation. Emotion. In press. [Google Scholar]
- 28.Keller MB, Lavori PW, Friedman B, Nielsen E, Endicott J, McDonald-Scott P, Andreasen NC. The Longitudinal Interval Follow-up Evaluation. A comprehensive method for assessing outcome in prospective longitudinal studies. Arch Gen Psychiatry. 1987;44:540–548. doi: 10.1001/archpsyc.1987.01800180050009. [DOI] [PubMed] [Google Scholar]
- 29.Fleiss JL, Cohen J. Statistical Methods for Rates and Proportions. New York, NY: John Wiley & Sons Inc; 1973. [Google Scholar]
- 30.Cox DR, Oakes D. Analysis of Survival Data. London, England: Chapman & Hal; 1984. [Google Scholar]
- 31.Rush AJ, Trivedi MH, Ibrahim HM, Carmody TJ, Arnow B, Klein DN, Markowitz JC, Ninan PT, Kornstein S, Manber R, Thase ME, Kocsis JH, Keller MB. The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry. 2003;54:573–583. doi: 10.1016/s0006-3223(02)01866-8. [DOI] [PubMed] [Google Scholar]
- 32.Segal ZV, Teasdale JD, Williams JM, Gemar MC. The mindfulness-based cognitive therapy adherence scale: Inter-rater reliability, adherence to protocol and treatment distinctiveness. Clin Psychol Psychother, 2002;9:131–138. [Google Scholar]
- 33.Fawcett J, Epstein P, Fiester SJ, Elkin I, Autry JH. Clinical management: imipramine/placebo administration manual: NIMH Treatment of Depression Collaborative Research Program. Psychopharmacol Bull. 1987;23:309–324. [PubMed] [Google Scholar]
- 34.Paykel ES, Scott J, Teasdale JD, Johnson AL, Garland A, Moore R, Jenaway A, Cornwall PL, Hayhurst H, Abbott R, Pope M. Prevention of relapse in residual depression by cognitive therapy: a controlled trial. Arch Gen Psychiatry. 1999;56:829–835. doi: 10.1001/archpsyc.56.9.829. [DOI] [PubMed] [Google Scholar]
- 35.Solomon DA, Leon AC, Mueller TI, Coryell W, Teres JJ, Posternak MA, Judd LL, Endicott J, Keller MB. Tachyphylaxis in unipolar major depressive disorder. J Clin Psychiatry. 2005;66:283–290. doi: 10.4088/jcp.v66n0302. [DOI] [PubMed] [Google Scholar]
- 36.Bech P, Lönn SL, Overø KF. Relapse prevention and residual symptoms: A closer analysis of placebo-controlled continuation studies with escitalopram in major depressive disorder, generalized anxiety disorder, social anxiety disorder, and obsessive-compulsive disorder. J Clin Psychiatry. doi: 10.4088/JCP.08m04749blu. in press. [DOI] [PubMed] [Google Scholar]
- 37.Nierenberg AA, Husain MM, Trivedi MH, Fava M, Warden D, Wisniewski SR, Miyahara S, Rush AJ. Residual symptoms after remission of major depressive disorder with citalopram and risk of relapse: a STAR*D report. Psychol Med. 2010;40:41–50. doi: 10.1017/S0033291709006011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Judd LL, Paulus MJ, Schettler PJ, Akiskal HS, Endicott J, et al. Does incomplete recovery from first lifetime major depressive episode herald a chronic course of illness? Am J Psychiatry. 2000;157:1501–1504. doi: 10.1176/appi.ajp.157.9.1501. [DOI] [PubMed] [Google Scholar]
- 39.Dew MA, Reynolds CF, 3rd, Mulsant B, Frank E, Houck PR, et al. Initial recovery patterns may predict which maintenance therapies for depression will keep older adults well. J Affect Disord. 2001;65:155–166. doi: 10.1016/s0165-0327(00)00280-9. [DOI] [PubMed] [Google Scholar]
- 40.Keller MB, Trivedi MH, Thase ME, Shelton RC, Kornstein SG, et al. The prevention of recurrent episodes of depression with venlafaxine for two years (PREVENT) Study: Outcomes from the 2-year and combined maintenance phases. J Clin Psychiatry. 2007;68:1246–1256. doi: 10.4088/jcp.v68n0812. [DOI] [PubMed] [Google Scholar]
- 41.Montgomery SA, Entsuah R, Hackett D, Kunz NR, Rudolph RL Venlafaxine 335 Study Group. Venlafaxine versus placebo in the preventive treatment of recurrent major depression. J Clin Psychiatry. 2004;65:328–336. doi: 10.4088/jcp.v65n0307. [DOI] [PubMed] [Google Scholar]
- 42.Kuyken W, Dalgleish T, Watkins E, Holden E, White K, et al. How does mindfulness based cognitive therapy work? Manuscript under review. [DOI] [PubMed] [Google Scholar]
- 43.Goldapple K, Segal Z, Garson C, Lau M, Bieling P, Kennedy S, et al. Modulation of cortical-limbic pathways in major depression: Treatment-specific effects of cognitive behavior therapy. Archives of General Psychiatry. 2004;61:34–41. doi: 10.1001/archpsyc.61.1.34. [DOI] [PubMed] [Google Scholar]
- 44.Schoenfeld DA Sample-size formula for the proportional-hazards regression model. Biometrics. 1983;39:499–453. [PubMed] [Google Scholar]