Abstract
Objective
The adventitia is increasingly recognized as an important player during the development of intimal hyperplasia. However, the mechanism of adventitial cell recruitment to the subintimal space remains largely undefined. We have shown previously that gene transfer of protein kinase C-delta (PKCδ) increases apoptosis of smooth muscle cells (SMCs) following balloon injury. In the current study, we investigated a potential role of PKCδ in regulating the recruitment of adventitial cells.
Methods and results
Conditioned media from PKCδ-overexpressing SMCs stimulated migration and CCR2 expression of adventitial fibroblasts through a MCP-1 dependent mechanism. Following balloon injury of rat carotid arteries, overexpression of PKCδ in SMCs significantly increased MCP-1 and CCR2 expression and the number of adventitia-originated cells detected in the neointima. Administration of an anti-MCP-1 antibody markedly diminished the recruitment of adventitial cells. Combined PKCδ overexpression and anti-MCP-1 inhibited initmal hyperplasia more effectively than either approach alone.
Conclusions
Our data suggest that PKCδ regulates recruitment of adventitial cells to the neointima via a mechanism involving upregulation of the MCP-1/CCR2 signaling axis in injured arteries. Blockage of MCP-1 while enhancing apoptosis may serve as a potential therapeutic strategy to attenuate intimal hyperplasia.
Keywords: PKCδ, MCP-1, CCR2, intimal hyperplasia, myofibroblasts
INTRODUCTION
Intimal hyperplasia is a primary cause of restenosis after vascular interventions, including balloon angioplasty, stenting, endarterectomy, and bypass.1 Neointimal formation is a complex process, triggered by injury to endothelial cells as well as vascular smooth muscle cells (SMCs). Sensing the intimal and medial injury, the tunica adventitia responds with robust cell proliferation, matrix remodeling and elaboration of inflammatory cells and cytokines.2, 3 In addition to fibroblasts/myofibroblasts and residential immune cells, stem cells or progenitor cells are now believed to reside within the adventitia.4–6 Evidence supporting the potential migration of adventitial cells through the media and into the neointima is derived mostly from animal studies involving selective labeling of adventitial cells or transplanting cultured adventitial cells onto the adventitial side and monitoring movement of these cells following arterial injury. Although several cell types residing within the adventitia have the potential to directly contribute to the intimal lesion through migration, adventitial fibroblasts have been investigated most extensively in this content. Substantial experimental data suggest that adventitial fibroblasts may respond to arterial injury by transforming to a migratory SMC-like myofibroblast phenotype.7–9 However, the mechanism of adventitial fibroblasts sensing endothelial injury and subsequently initiating migration is poorly understood. Since adventitial fibroblasts do not have direct cell-cell contact with endothelial or smooth muscle cells, it is plausible to propose that fibroblasts are recruited into the subintimal space by soluble factors released by injured endothelial or/and smooth muscle cells.
We have shown previously that PKCδ, a member of the protein kinase C (PKC) family of serine-threonine kinases, plays a critical role in mediating vascular injury response. PKCδ expression is upregulated in human restenotic lesions where it colocalizes with apoptotic SMCs. Mice that lack PKCδ develop exacerbated intimal hyperplasia associated with diminished medial SMC apoptosis when subjected to vein grafting or carotid ligation.10, 11 Furthermore, gene transfer of PKCδ in the rat carotid angioplasty model inhibits intimal hyperplasia which is associated with a profound upregulation of apoptotic activity within medial SMCs.10 Recent data from our laboratory suggest that PKCδ also regulates the production of monocyte chemoattractant protein-1(MCP-1) in vitro.12 Being able to mediate both apoptosis and chemokine expression, PKCδ is poised as a potential mechanism underlying the recruitment of adventitial cells by injured medial SMCs.
MCP-1 is a member of the small inducible gene (SIG) family. Increased MCP-1 expression has been reported in injured arteries.13 Blocking MCP-1 signaling through the use of neutralizing antibodies or gene deletion of the MCP-1 receptor CCR2 attenuates intimal hyperplasia in experimental artery injury models.13, 14 Interestingly, inhibition of the MCP-1 signaling pathway was found to reduce intimal hyperplasia without altering macrophage infiltration,13, 15, 16 although the MCP-1/CCR2 axis is mostly known for its role in the recruitment of monocytes and other types of inflammatory cells. Similarly, we did not detect any significant alterations in inflammatory infiltration following PKCδ gene transfer and arterial injury. This observation further motivated the present study in which we examined whether PKCδ through MCP-1 regulates the cell-cell communication between SMCs and fibroblasts in vitro and in vivo. In addition, we investigated whether inhibition of the MCP-1 signaling attenuates migration of the adventitial cells through the media and into the subintimal space.
Materials and Methods
Animal model
After induction of anesthesia with 2.5% isoflurane, arterial injury was induced in male Sprague-Dawley rats (2~3 months, ~350g) by means of carotid balloon angioplasty as described before.10, 17 Gene transfer to medial SMCs was achieved by intraluminal perfusion with adenoviruses that expresses PKCδ (AdPKCδ) or empty vector (AdNull) as described previously.10 Cells in the perivascular zone or adventitia were labeled with an adenovirus expressing LacZ (AdLacZ) administered via pluronic gel (2.5×109 pfu/ml AdLacZ in 200μl 25% w/v gel) on the adventitial surface prior to wound closure as described elsewhere.18 A sham group underwent surgery without balloon angioplasty/intraluminal viral infection. In a subset of studies, two sequential survival surgery procedures were performed. During the first procedure, AdLacZ was administered to the adventitial surface of left common carotid arteries. Three days later, the second procedure was performed to administer angioplasty balloon injury to the AdLacZ treated arterial segment. In the antibody administration study, mice receiving either AdNull or AdPKCδ perfusion were assigned randomly to administration of either an anti-MCP-1 antibody or an IgG control group. Anti-MCP-1 (Biolegend, CA, 1mg/kg) or equivalent amounts of nonimmunized hamster IgG (Biolegend, CA, 1mg/kg) were injected via tail vein 30 minutes before arterial injury, followed by two additional injections 12 and 24 hours, as described by Furukawa et al.14 Arteries were harvested on days 3, 7, and 14 by perfusion fixation with 4% paraformaldehyde at physiologic pressure (90 mmHg). X-gal staining was performed by using X-gal staining assay kit (Genlantis, CA). Unless it stated otherwise, 3 animals were used for each group. All experimental protocols were approved by the Institute Animal Care and Use Committee at University of Wisconsin Madison (#M002285) and conformed to the Guide for the Care and Use of Laboratory Animals published by the NIH Publication No. 85-23, 1996 revision.
Statistical analyses
Values were expressed as mean±SEM derived from at least 3 independent experiments unless stated otherwise. Differences between two groups were analyzed by 2-tailed Student’s t test since all data conformed to the constraints of parametric analyses. For comparisons between more than two sets of experimental conditions, one-way analysis of variance (ANOVA) was performed with post hoc testing using a Fisher least significant difference test. p values less than 0.05 were considered as statistically significant. Other methods are detailed in the Supplemental Materials and Methods.
RESULTS
PKCδ-expressing SMCs attracted adventitial fibroblast cells through MCP-1
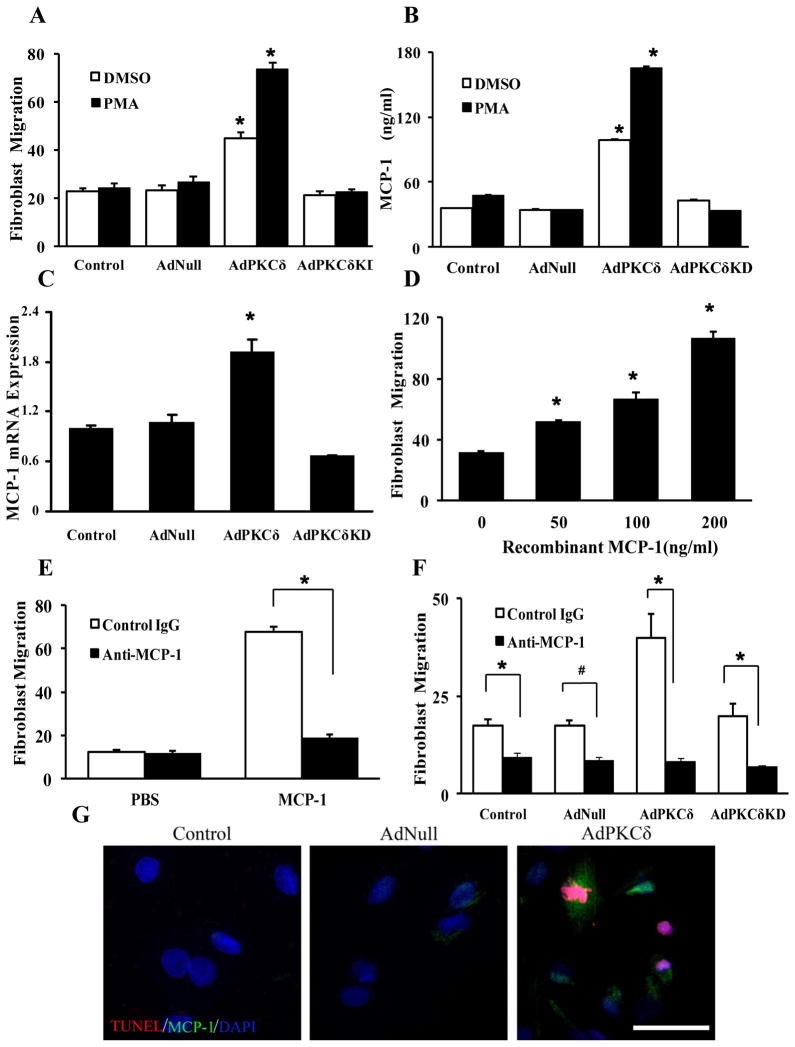
To determine the molecular mechanism underlying cell-cell communication between medial SMCs and adventitial cells, we isolated SMCs and fibroblasts from the media and adventitia of rat carotid arteries. Isolated arterial SMCs were characterized by immunostaining of smooth muscle-specific α-actin (SMA) and calponin (Supplemental Figure 1). Adventitial fibroblasts were positive for the fibroblast marker ER-TR7 and mesenchymal marker thy1.1, but negative for calponin (Supplemental Figure 1). A large portion of cultured fibroblasts expressed SMA, albeit to a lesser degree compared to SMCs (Supplemental Figure 1), indicating spontaneous transformation to myofibroblasts during in vitro manipulations. However, expression of CD68, a macrophage marker, was undetectable in either SMC or fibroblast cultures (Supplemental Figure 1).
A transwell chemotaxis assay was used to study fibroblast migration in vitro. Compared to control media (DMEM supplemented with 0.5% FBS), media conditioned by SMCs significantly increased the number of adventitial fibroblasts migrated through a porous membrane (7.4±3.1 versus 22.3±6.9). To mimic high PKCδ expression in SMCs of injured arteries, we infected SMCs with AdNull (empty viral vector), AdPKCδ, or a kinase dead mutant of PKCδ (AdPKCδKD). Compared to AdNull-infected SMCs, SMCs infected with AdPKCδ but not its kinase dead form stimulated migration of fibroblasts by 80% (Figure 1A). We further activated exogenous PKCδ with 1nM PMA. At this concentration, PMA, by itself, had minimum effects on SMCs but further increased PKC-associated chemoattractant properties (Figure 1A). In parallel, AdPKCδ-infected SMCs had substantially increased abundance of MCP-1 protein and mRNA compared to the AdNull controls (Figure 1B&C). We also analyzed mRNA expression of several other chemokines and cytokines including SDF-1 (CXCL12), RANTES (CCL5), TNF-α, PDGF-BB, IFN-γ, IL-6, and IL-1β that are known to be associated with vascular injury and remodeling. In this small panel of factors, only IL-6 mRNA was upregulated significantly by PKCδ (Supplemental Figure 2). However, the magnitude of IL-6 induction was much less than that of MCP-1.
Figure 1.
Next, we determined the role of MCP-1 in communication between SMCs and fibroblast by determining whether purified MCP-1 is capable of attracting fibroblasts. As shown in Figure 1D, recombinant MCP-1 concentrations-dependently stimulated migration of fibroblasts. To further validate that migration of fibroblasts is indeed caused by MCP-1, we repeated the migration assay in the presence of a neutralizing antibody specific to MCP-1. Neutralizing MCP-1 greatly diminished the ability of recombinant MCP-1 or SMCs to attract fibroblasts (Figure 1E&F).
We have shown previously that overexpression of PKCδ causes SMC apoptosis.19 To determine whether MCP-1 is produced by apoptotic SMCs or their surviving neighbors, we co-immunostained cultured SMCs for MCP-1 and TUNEL. Confocal analysis showed that overexpression of PKCδ increased MCP-1 expression in both TUNEL positive- and negative-cells (Figure 1G).
To exclude the possibility that conditioned media may contain significant amount of adenovirus lingered from infection of SMCs, we evaluated the expression of exogenous PKCδ in fibroblasts using an antibody against the FLAG tag that is engineered in AdPKCδ. No FLAG-tagged PKCδ band was detected in fibroblasts while it was abundantly present in AdPKCδ-infected SMCs (Supplemental Figure 3). These results indicated that the effects of PKCδ on fibroblasts migration, at least in our transwell assay, is indirect and is achieved through secretion of chemoattractant molecules by SMCs.
MCP-1 attracted fibroblasts through induction of CCR2
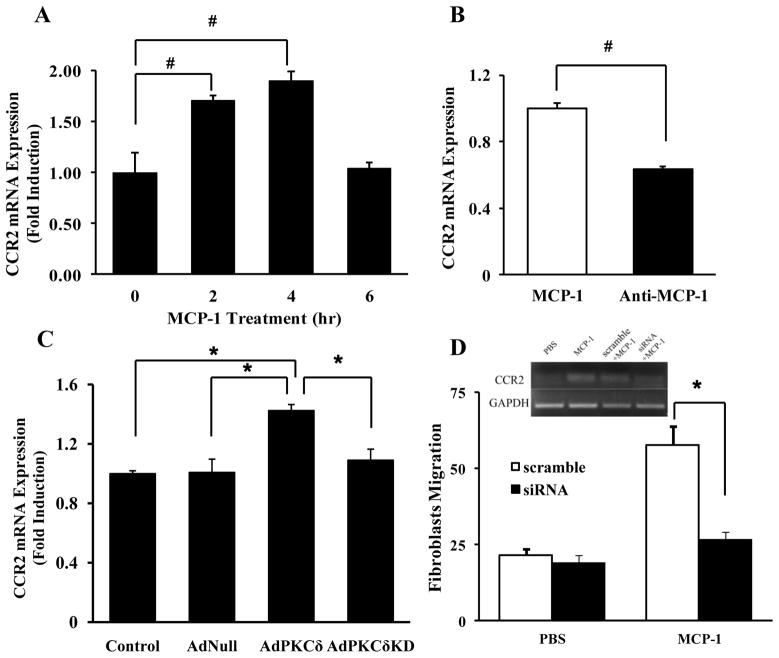
The chemoattractant effect of MCP-1 has been extensively studied in monocytes and macrophages and is believed to be mediated predominantly by its receptor CCR2.20, 21 We therefore examined CCR2 expression in migrated fibroblasts collected from the bottom wells at the end of the trasnswell assay. As shown in Supplemental Figure 4, migrated fibroblasts were positive for CCR2, SMA, and thy1.1 but negative for calponin and CD68. However, CCR2 protein was undetectable in the naïve or general population of adventitial fibroblasts (Supplemental Figure 4). We then determined whether exposure to MCP-1 during the chemotaxis assay induces CCR2 expression in adventitial fibroblasts. As shown in Figure 2A, incubating with recombinant MCP-1 triggered a rapid and transient increase in CCR2 mRNA abundance. This induction was blocked by incubation with an anti-MCP-1 neutralizing antibody (Figure 2B). Conditioned media derived from AdPKCδ-infected SMCs produced a similar effect on CCR2 mRNA abundance in fibroblasts (Figure 2C). Finally, siRNA knocking down of CCR2 mRNA abundance in fibroblasts markedly reduced MCP-1 induced migration (Figure 2D).
Figure 2.
In contrast, similar amounts of SMA were detected in fibroblasts incubated with conditioned media made by SMCs infected with AdNull, AdPKCδ or AdPKCδKD (Supplemental Figure 5), suggesting the stimulatory effect of PKCδ-expressing SMCs on CCR2 mRNA abundance was likely to be specific, and not secondary to phenotypic transformation.
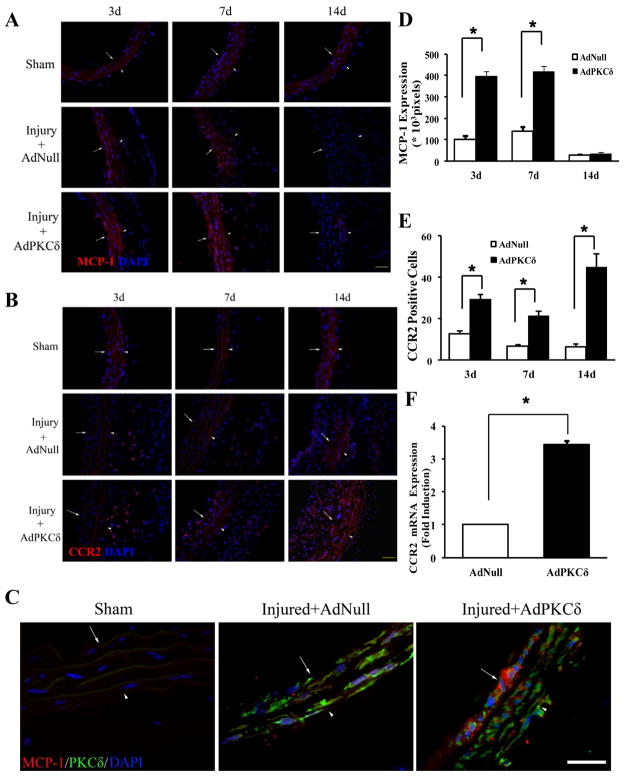
The MCP-1/CCR2 signaling axis was upregulated by PKCδ in injured arteries
After established a role of PKCδ-mediated MCP-1 expression in cell-cell communication between medial SMCs and adventitial fibroblasts, we determined whether the MCP-1/CCR2 signaling axis was upregulated by PKCδ in injured arteries. To this end, we administered AdPKCδ or AdNull through luminal infusion following carotid balloon injury, a method that has been demonstrated previously to transfer genes predominantly in the medial layer of the carotid artery wall.17 Indeed, elevated PKCδ expression produced by AdPKCδ luminal infusion was predominantly in the tunica media (Supplemental Figure 6A&B). Consistent with our previous report, AdPKCδ reduced the I/M ratio by 33.8% and caused extensive SMC apoptosis (Supplemental Figure 6C&D). After validating the effectiveness of PKCδ gene transfer, we evaluated whether PKCδ overexpression upregulated the MCP-1/CCR2 axis following arterial injury. Non-injured arteries (Sham) contained undetectable MCP-1 or CCR2 protein (Figure 3A&B), which was confirmed by qPCR (data not shown). Both MCP-1 and CCR2 protein became clearly noticeable after injury (Figure 3A&B). Additionally, PKCδ gene transfer further increased abundance of MCP-1 and CCR2 protein by 4 and 2.4 fold, respectively. The MCP-1 upregulation peaked around 3 days and subsided by 14 days post injury (Figure 3A&D). The presence of MCP-1 was detected predominantly in the media, although a few MCP-1 positive cells were detectable in adventitia. Confocal imaging confirmed the spatial association between MCP-1 and PKCδ-overexpressing cells (Figure 3C). Interestingly, CCR2 induction showed different kinetics. Temporally, CCR2 induction persisted to at least 14 days after injury (Figure 3B&E). Spatially, the CCR2-positive cells first accumulated in the adventitia (Figure 3B). As the arterial injury response proceeded, significant number of CCR2-positive cells became detectable in the media-intima region (Figure 3B), presumably due to migration of adventitial cells toward the luminal side. To quantify the CCR2 induction, we harvested the injured carotid arteries and analyzed CCR2 mRNA abundance by quantitative RT-PCR. As shown in Figure 3F, AdPKCδ increased the CCR2 mRNA abundance by 3.5 fold.
Figure 3.
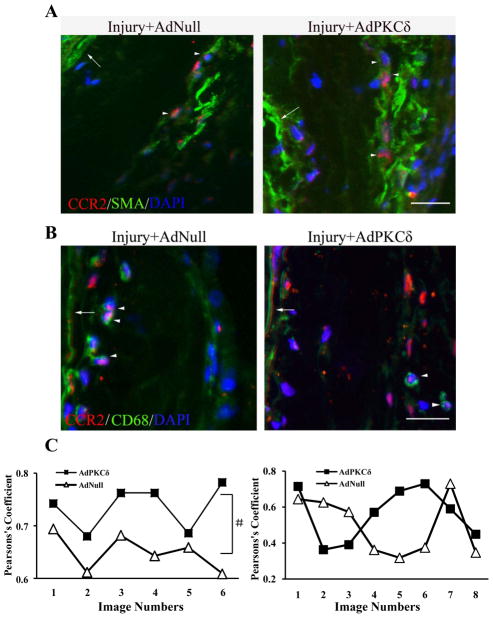
We next determined whether adventitial myofibroblasts of injured arteries were a source of CCR2 stimulated by injury and PKCδ gene transfer. Arterial sections harvested 7 days after the carotid injury were co-stained with antibodies specific to CCR2 and SMA. Although abundant in vascular SMCs, SMA is also expressed in myofibroblasts in the adventitia and has been widely used as a marker for this population of fibroblasts.22 Confocal microscopic analyses showed that significant SMA/CCR2 double positive cells were present in the adventitial of arteries (Figure 4A). As expected, infiltrated CD68+ macrophages expressed CCR2 as well (Figure 4B). Pearson’s coefficiency analyses indicated that colocalization between CCR2 and SMA was significantly higher in AdPKCδ-arteries compared to AdNull group (Figure 4C). However, PKCδ gene transfer had no effect on macrophage infiltration or CCR2/CD68 colocalization (Figure 4D and Supplemental Figure 7).
Figure 4.
The MCP-1/CCR2 axis mediated adventitial cell migration and contributed to intimal hyperplasia
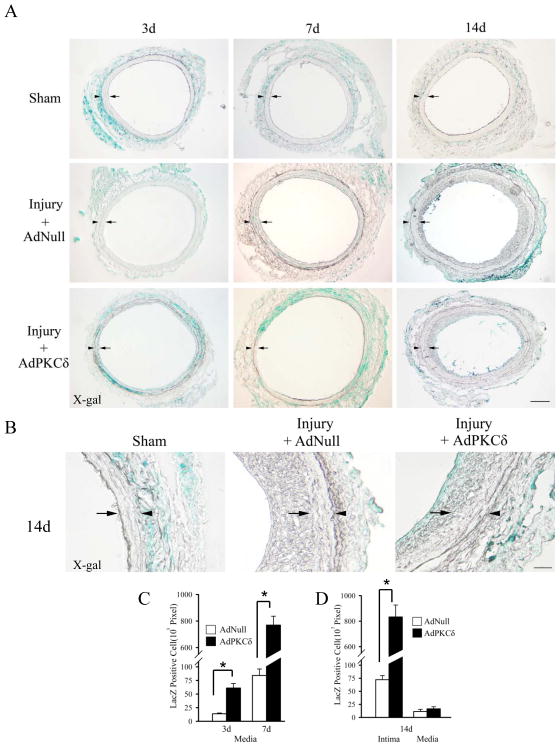
To study the migration of adventitial cells in injured arteries, we labeled adventitial cells by applying AdLacZ to the adventitial surface of balloon injured arteries as described previously by Siow and colleagues.23 In agreement with this report, we detected LacZ positive cells only in the adventitia of non-injured (sham) arteries (Figure 5A&B), suggesting that perivascular administration of AdLacZ did not lead to significant gene transfer in medial SMCs or endothelial cells. In contrast, LacZ positive cells were readily recognized throughout the arterial wall of injured arteries presumably due to active migration of adventitial cells such as myofibroblasts (Figure 5A&B). To rule out the possibility that AdLacZ may also migrate from the adventitia to inner side through increased vasa vasorum of injured arteries and directly infect medial SMCs, we performed balloon injury 3 days after viral infection when excessive viral particles have been cleared or become inactivated. A similar intimal/medial distribution of LacZ+ cells was observed in injured, but not in sham arteries (Supplemental Figure 8), confirming that the intimal/medial LacZ expression was primarily resulted from migration of adventitial cells rather than adenoviruses.
Figure 5.
Using LacZ as a marker for adventitial originated cells, we determined the effects of PKCδ gene transfer on adventitial cell migration 3, 7, and 14 days following balloon angioplasty. PKCδ gene transfer to medial SMCs markedly increased the number of adventitia-originated cells in the medial-intimal region (Figure 5A–D). At day 7, the number of LacZ+ cells detected in the medial-intimal area of AdPKCδ-infected arteries was over 7-fold higher than that of the control virus (AdNull)-infected arteries (X-gal positive areas in pixels: 763±178 AdPKCδ versus 90±31AdLacZ, p<0.01, n=3) (Figure 5A&C). By day 14, the majority of LacZ+ or adventitia-originated cells were found to accumulate in the neointima (Figure 5A, B&D).
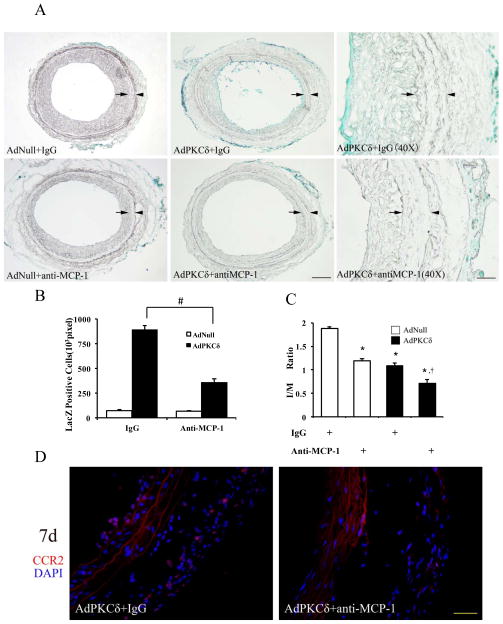
To test whether PKCδ-expressing SMCs recruited adventitial myofibroblasts through the MCP-1/CCR2 axis, we administrated a MCP-1 neutralizing antibody before and after carotid injury and adenovirus infection. Compared to the group administered control IgG, the anti-MCP-1 antibody profoundly reduced the number of adventitia-originated cells detected in the medial/intimal region of injured arteries (Figure 6A&B), indicating a role of MCP-1 in regulation of adventitial cell migration. Additionally, neutralizing MCP-1 caused further reduction of intimal hyperplasia. The combination of PKCδ gene transfer and anti-MCP-1 inhibited the I/M ratio by 62% as compared to a 36.3% reduction produced by AdPKCδ alone. (p<0.01, n=3) (Figure 6C). Of note, inhibition of MCP-1, without PKCδ overexpression, reduced I/M ratio by 30.8%. Finally, CCR2 expression was also diminished by the anti-MCP-1 antibody (Figure 6D), further supporting the notion that arterial expression of CCR-2 was MCP-1 dependent.
Figure 6.
DISCUSSION
The importance of the adventitia in intimal hyperplasia and vascular remodeling has been illustrated by experimental data accumulated through the past 10 years.4, 5 Adventitial fibroblasts, as well as residential progenitor cells, have been shown to migrate through the media to neointima in experimental models of intimal hyperplasia.7, 24 However, other than the involvement of MMPs,25, 26 molecular mechanisms underlying adventitial cell activation and migration in response to injuries of endothelium and SMCs remain to be delineated. To this end, we examined the effect of overexpressing the pro-apoptotic factor, PKCδ, on the migration of adventitial cells using a rat carotid balloon injury model. Our results provide evidence that adventitial cells are recruited to the neointima, at least in part, through a PKCδ-MCP-1-CCR2 pathway. As a key mediator of apoptosis, PKCδ has been shown to be upregulated in apoptotic arterial SMCs following balloon injury as well as human restenotic lesion.10 Manipulation of PKCδ, either through gene deletion or adenovirus-mediated overexpression, altered the prevalence of apoptosis in the injury vessel wall thus the size of neointimal lesion. The current study demonstrated a new function of PKCδ, that is to mediate the recruitment of adventitial cells through MCP-1. As a result of this dual function, gene transfer of PKCδ to medial SMCs following balloon injury increased not only SMC apoptosis but also the migration of adventitial cells. With an anti-MCP-1 antibody, we separated PKCδ’s pro-apoptotic function from its role in the recruitment of adventitial cells. In the presence of a MCP-1 neutralizing antibody, gene transfer of PKCδ promoted SMC apoptosis without trigging migration of adventitial cells and therefore produced a significantly greater inhibitory effect on neointimal development as compared to PKCδ gene therapy alone.
We were surprised by the lack of alteration in CD68+ macrophage infiltration in PKCδ-gene transferred arteries since the upregulation of MCP-1 in these arteries was profound. MCP-1 is a well-established inflammatory chemokine that is expressed by SMCs, macrophages, endothelial cells, and fibroblasts. The primary receptor of MCP-1 is CCR2, a member of the family of G-protein coupled chemokine receptors.27 The MCP-1/CCR2 axis plays a critical role in chemotaxis of monocytes and other inflammatory cells and has been implicated in a variety of inflammatory disease processes including rheumatoid arthritis.28 MCP-1 is induced in medial SMCs during early atherosclerotic lesion development27 and in animal models of arterial injury.14 Mice deficient of MCP-1 or CCR2 developed smaller intimal lesions,13, 15 albeit the effect of CCR2 deficiency appears to be more dramatic. Additionally, blocking the MCP-1 signaling with a neutralizing antibody or a dominant negative mutant inhibits intimal hyperplasia in various models of arterial injuries, including balloon or wire injury in rodent arteries,13, 14 vein graft,16 cardiac allografts29 and in-stent stenosis.30 However, whether blocking the MCP-1 signaling affects vascular inflammation appears to differ amongst these early studies. Inhibition of the MCP-1/CCR2 axis generally does not alter the inflammatory response in vascular injury associated balloon angioplasty of rat carotid and wire injury of C57B6 mice.13, 14 It is possible that the MCP-1/CCR2 axis contributes to intimal hyperplasia by stimulating SMC proliferation as suggested by several in vitro studies.31, 32 However, the mitogenic effect of MCP-1 has not been demonstrated in vivo. Using a rat carotid balloon injury model, Furukawa showed that an anti-MCP-1 antibody did not alter intimal medial proliferation although the number of neointimal cells was reduced.14 These authors also reported that recombinant rat MCP-1 failed to stimulate chemotactic activities of culture SMCs. Using a mouse wire injury model, Kim and colleagues found that MCP-1 deficiency inhibited intimal hyperplasia without affecting medial DNA synthesis.15 Here, we provide a novel mechanism in which MCP-1, secreted by high PKCδ-expressing SMCs, prompts adventitial cells migration to the neointima. This theory was based on the following observations: 1) recombinant MCP-1 initiated migration of adventitial fibroblasts in vitro; 2) SMCs, through a PKCδ- and MCP1/CCR2-dependent mechanism, caused migration of adventitial fibroblasts in vitro; 3) both MCP-1 and CCR2 were upregulated in PKCδ-overexpressing arteries following carotid balloon injury; and 4) neutralization of MCP-1 with an antibody attenuated the trans-media migration of adventitial cells in injured carotid arteries.
Our immunostaining and qPCR analyses revealed no detectable levels of CCR2 protein and mRNA in adventitial fibroblasts isolated from the carotid arteries of healthy adult rats, which is in agreement with previously published data on adventitia of young normal blood vessels.26, 33, 34 However, when adventitial fibroblasts were incubated with either recombinant MCP-1 or MCP-1-rich media conditioned by PKCδ overexpressing SMCs, CCR2 mRNA expression was induced rapidly. In support of this in vitro finding, blocking MCP-1 in vivo with a neutralizing antibody diminished CCR2 protein abundance in PKCδ-overexpressing arteries. The migration of fibroblasts toward MCP-1 or SMC-conditioned media was sensitive to siRNA knockdown of CCR2, suggesting that CCR2 is the primary receptor that transmits the extracellular chemotactic signal into adventitial fibroblasts. The critical role of adventitial CCR2 in intimal hyperplasia has been previously demonstrated by Eefting and colleagues who showed that perivascular overexpression of short hairpin RNA against CCR2 inhibits vein graft thickening in hypercholesterolemic apolipoprotein E3-Leiden mice.33
In addition to MCP-1, several other chemokines and their receptors are implicated in the recruitment of progenitor cells during arterial remodeling.27 Most noticeably, CXC-chemokine stromal cell-derived factor (SDF)-1α is upregulated in injured arteries and contribute to intimal hyperplasia through a CXCR4-dependent recruitment of smooth muscle progenitor cells.35 However, our qPCR analysis suggests that PKCδ did not alter SDF-1α mRNA expression in SMCs, at least in vitro.
One limitation of our study is that perivascular application of AdLacZ non-distinguishably labels cells residing in the adventitia. Additionally, we cannot rule out the possibility that bone marrow derived progenitor cells become infected by AdLacZ upon entering the adventitia. Of note, we observed a subpopulation of CCR2+ cells in the adventitia that are negative for SMA or CD68. The MCP-1/CCR2 axis has been implicated in the adhesion of bone marrow-derived endothelial progenitor cells (EPCs) during re-endothelialization following carotid angioplasty.36 Moors et al also reported that the MCP-1/CCR2 axis is important for recruitment of bone marrow derived fibrocytes to the alveolar space after pulmonary fibrotic injury.37 Furthermore, we have shown in vitro that MCP-1 stimulates migration of mesenchymal stem cells (MSC).38 Although we observed some adventitia-derived cells in the intima that were also positive for CD31 (data not shown), future studies employing cell lineage-specific labeling and tracing methods are necessary to prove the role of adventitial cells in endothelium regeneration.
In conclusion, we have demonstrated that PKCδ plays a dual function in arterial injury response. Upregulated in medial SMCs following injury, PKCδ stimulates apoptosis of SMCs and increases MCP-1 expression. While the PKCδ-mediated SMC apoptosis results in diminished intimal hyperplasia, PKCδ-induction of MCP-1 promotes the repair mechanism by activating the CCR2-mediated migration of myofibroblasts and possibly progenitors from the adventitia to the neointima. These findings reiterate the complexity of arterial injury response. Stimulating apoptosis of SMCs may be a logical approach to reduce intimal hyperplasia, however, considerations should be given to potential repair mechanisms including the recruitment of progenitor cells or/and myofibroblasts evoked by pro-apoptotic genes/factors. Future studies aimed to delineate the molecular link between cell injury and repair are necessary for designing effective therapeutic strategies to treat intimal hyperplasia.
Supplementary Material
Acknowledgments
The authors like to thank Stephanie Morgan, Stephen Seedial, Justin Lengfeld and Dai Yamanouchi for scientific discussions and Drew Allen Roenneburg and Glen Leverson for technical assistance.
Source of Funding
This work was supported in part by a Public Health Service Grant R01 HL-81424 (B.Liu and KC Kent) from the National Heart Lung, Blood Institute and an American Heart Association grant-in-aid 10GRANT3020052 (B.Liu) and Scholarship from China Scholarship Council (J Ren).
Footnotes
Disclosures:
None.
References
- 1.Kent KC, Liu B. Intimal hyperplasia--still here after all these years! Ann Vasc Surg. 2004;18:135–137. doi: 10.1007/s10016-004-0019-4. [DOI] [PubMed] [Google Scholar]
- 2.Maeng M, Olesen PG, Emmertsen NC, Thorwest M, Nielsen TT, Kristensen BÃ, Falk E, Andersen HR. Time course of vascular remodeling, formation of neointima and formation of neoadventitia after angioplasty in a porcine model. Coronary Artery Disease. 2001;12:285–293. doi: 10.1097/00019501-200106000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Wilcox JN, Okamoto E-I, Nakahara K-I, Vinten-Johansen J. Perivascular responses after angioplasty which may contribute to postangioplasty restenosis. Annals of the New York Academy of Sciences. 2001;947:68–92. doi: 10.1111/j.1749-6632.2001.tb03931.x. [DOI] [PubMed] [Google Scholar]
- 4.Majesky MW, Dong XR, Hoglund V, Mahoney WM, Jr, Daum G. The adventitia: A dynamic interface containing resident progenitor cells. Arterioscler Thromb Vasc Biol. 2011;31:1530–1539. doi: 10.1161/ATVBAHA.110.221549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hu Y, Xu Q. Adventitial biology: Differentiation and function. Arterioscler Thromb Vasc Biol. 2011;31:1523–1529. doi: 10.1161/ATVBAHA.110.221176. [DOI] [PubMed] [Google Scholar]
- 6.Tsai S, Butler J, Rafii S, Liu B, Kent KC. The role of progenitor cells in the development of intimal hyperplasia. J Vasc Surg. 2009;49:502–510. doi: 10.1016/j.jvs.2008.07.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sartore S, Chiavegato A, Faggin E, Franch R, Puato M, Ausoni S, Pauletto P. Contribution of adventitial fibroblasts to neointima formation and vascular remodeling: From innocent bystander to active participant. Circ Res. 2001;89:1111–1121. doi: 10.1161/hh2401.100844. [DOI] [PubMed] [Google Scholar]
- 8.Smith JD, Bryant SR, Couper LL, Vary CP, Gotwals PJ, Koteliansky VE, Lindner V. Soluble transforming growth factor-beta type ii receptor inhibits negative remodeling, fibroblast transdifferentiation, and intimal lesion formation but not endothelial growth. Circ Res. 1999;84:1212–1222. doi: 10.1161/01.res.84.10.1212. [DOI] [PubMed] [Google Scholar]
- 9.Poole JC, Cromwell SB, Benditt EP. Behavior of smooth muscle cells and formation of extracellular structures in the reaction of arterial walls to injury. Am J Pathol. 1971;62:391–414. [PMC free article] [PubMed] [Google Scholar]
- 10.Yamanouchi D, Kato K, Ryer EJ, Zhang F, Liu B. Protein kinase c delta mediates arterial injury responses through regulation of vascular smooth muscle cell apoptosis. Cardiovasc Res. 2010;85:434–443. doi: 10.1093/cvr/cvp328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leitges M, Mayr M, Braun U, Mayr U, Li C, Pfister G, Ghaffari-Tabrizi N, Baier G, Hu Y, Xu Q. Exacerbated vein graft arteriosclerosis in protein kinase cdelta-null mice. J Clin Invest. 2001;108:1505–1512. doi: 10.1172/JCI12902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schubl S, Tsai S, Ryer EJ, Wang C, Hu J, Kent KC, Liu B. Upregulation of protein kinase cdelta in vascular smooth muscle cells promotes inflammation in abdominal aortic aneurysm. J Surg Res. 2009;153:181–187. doi: 10.1016/j.jss.2008.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roque M, Kim WJ, Gazdoin M, Malik A, Reis ED, Fallon JT, Badimon JJ, Charo IF, Taubman MB. Ccr2 deficiency decreases intimal hyperplasia after arterial injury. Arterioscler Thromb Vasc Biol. 2002;22:554–559. doi: 10.1161/hq0402.105720. [DOI] [PubMed] [Google Scholar]
- 14.Furukawa Y, Matsumori A, Ohashi N, Shioi T, Ono K, Harada A, Matsushima K, Sasayama S. Anti-monocyte chemoattractant protein-1/monocyte chemotactic and activating factor antibody inhibits neointimal hyperplasia in injured rat carotid arteries. Circ Res. 1999;84:306–314. doi: 10.1161/01.res.84.3.306. [DOI] [PubMed] [Google Scholar]
- 15.Kim WJ, Chereshnev I, Gazdoiu M, Fallon JT, Rollins BJ, Taubman MB. Mcp-1 deficiency is associated with reduced intimal hyperplasia after arterial injury. Biochem Biophys Res Commun. 2003;310:936–942. doi: 10.1016/j.bbrc.2003.09.088. [DOI] [PubMed] [Google Scholar]
- 16.Schepers A, Eefting D, Bonta PI, Grimbergen JM, de Vries MR, van Weel V, de Vries CJ, Egashira K, van Bockel JH, Quax PH. Anti-mcp-1 gene therapy inhibits vascular smooth muscle cells proliferation and attenuates vein graft thickening both in vitro and in vivo. Arterioscler Thromb Vasc Biol. 2006;26:2063–2069. doi: 10.1161/01.ATV.0000235694.69719.e2. [DOI] [PubMed] [Google Scholar]
- 17.Kundi R, Hollenbeck ST, Yamanouchi D, Herman BC, Edlin R, Ryer EJ, Wang C, Tsai S, Liu B, Kent KC. Arterial gene transfer of the tgf-beta signalling protein smad3 induces adaptive remodelling following angioplasty: A role for ctgf. Cardiovasc Res. 2009;84:326–335. doi: 10.1093/cvr/cvp220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mallawaarachchi CM, Weissberg PL, Siow RC. Smad7 gene transfer attenuates adventitial cell migration and vascular remodeling after balloon injury. Arterioscler Thromb Vasc Biol. 2005;25:1383–1387. doi: 10.1161/01.ATV.0000168415.33812.51. [DOI] [PubMed] [Google Scholar]
- 19.Ryer EJ, Sakakibara K, Wang C, Sarkar D, Fisher PB, Faries PL, Kent KC, Liu B. Protein kinase c delta induces apoptosis of vascular smooth muscle cells through induction of the tumor suppressor p53 by both p38-dependent and p38-independent mechanisms. J Biol Chem. 2005;280:35310–35317. doi: 10.1074/jbc.M507187200. [DOI] [PubMed] [Google Scholar]
- 20.Leonard EJ, Skeel A, Yoshimura T. Biological aspects of monocyte chemoattractant protein-1 (mcp-1) Adv Exp Med Biol. 1991;305:57–64. doi: 10.1007/978-1-4684-6009-4_7. [DOI] [PubMed] [Google Scholar]
- 21.Yoshimura T, Leonard EJ. Human monocyte chemoattractant protein-1: Structure and function. Cytokines. 1992;4:131–152. [PubMed] [Google Scholar]
- 22.Desmouliere A, Geinoz A, Gabbiani F, Gabbiani G. Transforming growth factor-beta 1 induces alpha-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J Cell Biol. 1993;122:103–111. doi: 10.1083/jcb.122.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Siow RCM, Mallawaarachchi CM, Weissberg PL. Migration of adventitial myofibroblasts following vascular balloon injury: Insights from in vivo gene transfer to rat carotid arteries. Cardiovascular Research. 2003;59:212–221. doi: 10.1016/s0008-6363(03)00292-x. [DOI] [PubMed] [Google Scholar]
- 24.Hu Y, Zhang Z, Torsney E, Afzal AR, Davison F, Metzler B, Xu Q. Abundant progenitor cells in the adventitia contribute to atherosclerosis of vein grafts in apoe-deficient mice. J Clin Invest. 2004;113:1258–1265. doi: 10.1172/JCI19628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gautier EL, Jakubzick C, Randolph GJ. Regulation of the migration and survival of monocyte subsets by chemokine receptors and its relevance to atherosclerosis. Arterioscler Thromb Vasc Biol. 2009;29:1412–1418. doi: 10.1161/ATVBAHA.108.180505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weber KS, Nelson PJ, Grone HJ, Weber C. Expression of ccr2 by endothelial cells : Implications for mcp-1 mediated wound injury repair and in vivo inflammatory activation of endothelium. Arterioscler Thromb Vasc Biol. 1999;19:2085–2093. doi: 10.1161/01.atv.19.9.2085. [DOI] [PubMed] [Google Scholar]
- 27.Schober A. Chemokines in vascular dysfunction and remodeling. Arterioscler Thromb Vasc Biol. 2008;28:1950–1959. doi: 10.1161/ATVBAHA.107.161224. [DOI] [PubMed] [Google Scholar]
- 28.Iwamoto T, Okamoto H, Toyama Y, Momohara S. Molecular aspects of rheumatoid arthritis: Chemokines in the joints of patients. FEBS J. 2008;275:4448–4455. doi: 10.1111/j.1742-4658.2008.06580.x. [DOI] [PubMed] [Google Scholar]
- 29.Saiura A, Sata M, Hiasa K, Kitamoto S, Washida M, Egashira K, Nagai R, Makuuchi M. Antimonocyte chemoattractant protein-1 gene therapy attenuates graft vasculopathy. Arterioscler Thromb Vasc Biol. 2004;24:1886–1890. doi: 10.1161/01.ATV.0000141045.49616.6f. [DOI] [PubMed] [Google Scholar]
- 30.Egashira K, Nakano K, Ohtani K, Funakoshi K, Zhao G, Ihara Y, Koga J, Kimura S, Tominaga R, Sunagawa K. Local delivery of anti-monocyte chemoattractant protein-1 by gene-eluting stents attenuates in-stent stenosis in rabbits and monkeys. Arterioscler Thromb Vasc Biol. 2007;27:2563–2568. doi: 10.1161/ATVBAHA.107.154609. [DOI] [PubMed] [Google Scholar]
- 31.Porreca E, Di Febbo C, Reale M, Castellani ML, Baccante G, Barbacane R, Conti P, Cuccurullo F, Poggi A. Monocyte chemotactic protein 1 (mcp-1) is a mitogen for cultured rat vascular smooth muscle cells. J Vasc Res. 1997;34:58–65. doi: 10.1159/000159202. [DOI] [PubMed] [Google Scholar]
- 32.Viedt C, Vogel J, Athanasiou T, Shen W, Orth SR, Kubler W, Kreuzer J. Monocyte chemoattractant protein-1 induces proliferation and interleukin-6 production in human smooth muscle cells by differential activation of nuclear factor-kappab and activator protein-1. Arterioscler Thromb Vasc Biol. 2002;22:914–920. doi: 10.1161/01.atv.0000019009.73586.7f. [DOI] [PubMed] [Google Scholar]
- 33.Eefting D, Bot I, de Vries MR, Schepers A, van Bockel JH, Van Berkel TJ, Biessen EA, Quax PH. Local lentiviral short hairpin rna silencing of ccr2 inhibits vein graft thickening in hypercholesterolemic apolipoprotein e3-leiden mice. J Vasc Surg. 2009;50:152–160. doi: 10.1016/j.jvs.2009.03.027. [DOI] [PubMed] [Google Scholar]
- 34.Spinetti G, Wang M, Monticone R, Zhang J, Zhao D, Lakatta EG. Rat aortic mcp-1 and its receptor ccr2 increase with age and alter vascular smooth muscle cell function. Arterioscler Thromb Vasc Biol. 2004;24:1397–1402. doi: 10.1161/01.ATV.0000134529.65173.08. [DOI] [PubMed] [Google Scholar]
- 35.Schober A, Knarren S, Lietz M, Lin EA, Weber C. Crucial role of stromal cell-derived factor-1alpha in neointima formation after vascular injury in apolipoprotein e-deficient mice. Circulation. 2003;108:2491–2497. doi: 10.1161/01.CIR.0000099508.76665.9A. [DOI] [PubMed] [Google Scholar]
- 36.Fujiyama S, Amano K, Uehira K, Yoshida M, Nishiwaki Y, Nozawa Y, Jin D, Takai S, Miyazaki M, Egashira K, Imada T, Iwasaka T, Matsubara H. Bone marrow monocyte lineage cells adhere on injured endothelium in a monocyte chemoattractant protein-1-dependent manner and accelerate reendothelialization as endothelial progenitor cells. Circ Res. 2003;93:980–989. doi: 10.1161/01.RES.0000099245.08637.CE. [DOI] [PubMed] [Google Scholar]
- 37.Moore BB, Kolodsick JE, Thannickal VJ, Cooke K, Moore TA, Hogaboam C, Wilke CA, Toews GB. Ccr2-mediated recruitment of fibrocytes to the alveolar space after fibrotic injury. Am J Pathol. 2005;166:675–684. doi: 10.1016/S0002-9440(10)62289-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang F, Tsai S, Kato K, Yamanouchi D, Wang C, Rafii S, Liu B, Kent KC. Transforming growth factor-beta promotes recruitment of bone marrow cells and bone marrow-derived mesenchymal stem cells through stimulation of mcp-1 production in vascular smooth muscle cells. J Biol Chem. 2009;284:17564–17574. doi: 10.1074/jbc.M109.013987. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.