Abstract
The vascular endothelium is the inner lining of blood vessels serving as autocrine and paracrine organ that regulates vascular wall function. Endothelial dysfunction is recognized as initial step in the atherosclerotic process and is well advanced in diabetes, even before the manifestation of end-organ damage. Strategies capable of assessing changes in vascular endothelium at the preclinical stage hold potential to refine cardiovascular risk. In vitro cell culture is useful in understanding the interaction of endothelial cells with various mediators; however, it is often criticized due to the uncertain relevance of results to humans. Although circulating endothelial cells, endothelial microparticles, and progenitor cells opened the way for ex vivo studies, a recently described method for obtaining primary endothelial cells through endovascular biopsy allows direct characterization of endothelial phenotype in humans. In this article, we appraise the use of endothelial cell-based methodologies to study vascular inflammation in diabetes and atherosclerosis.
Keywords: Inflammation, Diabetes, Atherosclerosis, Adhesion, Apoptosis, Vascular biology, Endothelium, Endothelial function, Endothelial cells, Vein, Cell culture, Stem cells
Introduction
According to the World Health Organization, there are over 220 million people with diabetes worldwide with at least 1.1 million deaths from diabetes in 2005; this number is projected to double by 2030 [1]. Diabetes has classically been associated with multiple microvascular and macrovascular complications. According to National Institute of Diabetes, Digestive and Kidney Diseases (NIDDK), heart disease accounts for 65% of diabetic deaths each year and adults with diabetes have heart disease death rates two to four times higher than adults without diabetes. Accordingly, patients with diabetes have a reduced life expectancy than subjects without diabetes [2] with a higher incidence of cardiac, cerebrovascular, and peripheral vascular diseases [3].The vascular endothelium serves as an important autocrine and paracrine organ and maintains vascular homeostasis by modulating blood vessel tone, by regulating local cellular growth and extracellular matrix deposition, and controlling homeostatic as well as inflammatory responses. The endothelial cells (ECs) produce a variety of vasculoregulatory and vasculotropic molecules that act locally or at distant sites. Alteration of the vascular endothelium is a primary event in the pathogenesis of vascular diseases (e.g., atherosclerosis) and is a critical target for preventing or slowing the progression of vascular disease. Endothelial dysfunction is recognized as the initial step in the atherosclerotic process and is well described in patients with diabetes and atherosclerosis [3–7]. For these reasons, the study of human endothelium has become central in cardiovascular research and clinical research has focused on elucidating the role of endothelial dysfunction in influencing vascular disease progression [4].
Methods capable of assessing changes in vascular endothelium and its function at a preclinical stage would hold potential to refine cardiovascular risk stratification and would serve as a guide to monitor the effects of therapeutic interventions. Hyperglycemia represents a physiologic challenge that can further alter endothelial function, gene and protein expression. However, availability of human endothelial tissue has been a major constraint when investigating the cellular mechanisms of vascular dysfunction. A key step in bridging the gap between clinical and basic science is a suitable method to sample and study human endothelial tissue. In this review, we provide an overview of the different approaches studied to date in diabetes and vascular inflammation by using human vascular ECs.
The Inflammatory State of Vascular Endothelium in Association with Diabetes and Atherosclerosis
Inflammation participates in atherosclerosis from its inception onwards and participates pivotally in all stages of atherosclerosis, from lesion initiation to progression and destabilization. In addition, inflammation regulates both the “solid-state” thrombotic potential in the plaque itself and the prothrombotic and antifibrinolytic capacity of blood in the fluid phase. Oxidatively modified low-density lipoprotein can induce a local inflammatory response [5]. Circulating leukocytes adhere poorly to the normal endothelium under normal conditions. When the endothelium becomes inflamed, it expresses adhesion molecules that bind cognate ligands on leukocytes. Selectins mediate a loose rolling interaction of leukocytes with the inflammatorily activated ECs [6]. Integrins mediate firm attachment. Chemokines expressed provide a chemotactic stimulus to the adherent leukocytes, directing their diapedesis and migration into the intima, where they take residence and divide. Progression of atherosclerosis occurs, when macrophages augment the expression of scavenger receptors in response to inflammatory mediators, transforming them into lipid-laden foam cells following the endocytosis of modified lipoprotein particles. Macrophage-derived foam cells drive lesion progression by secreting proinflammatory cytokines. Leukocytes, as well as ECs, secrete additional cytokines and growth factors that promote the migration and proliferation of vascular smooth muscle cells (SMCs). In response to inflammatory stimulation, SMCs express specialized enzymes that can degrade elastin and collagen, allowing their penetration into the expanding lesion [3, 4, 7].
Diabetes is a major accelerator of macrovascular disease and its complications and is an independent risk factor for atherosclerosis. Fundamental pathogenetic mechanisms in diabetes-associated macrovascular disease include endothelial dysfunction, accentuated vascular inflammation, and increased oxidative stress. Hyperglycemia and its immediate biochemical sequelae directly alter endothelial function or influence ECs functioning indirectly by the synthesis of growth factors, cytokines, and vasoactive agents in other cells [3, 7]. Advanced glycation end products (AGEs) accumulate in diabetic tissues and are the products of nonenzymatic glycation/oxidation of proteins and lipids. Endothelial activation, via receptor for AGEs (RAGE), has been implicated in the pathogenesis of atherosclerosis in animal and in vitro studies. Hyperglycemia promotes endothelial activation and monocyte chemoattractant protein-1 (MCP-1) expression in cultured ECs and MCP-1 has a pivotal role in the pathogenesis of diabetic vasculopathy [8]. Glucose is known to induce the expression of early growth response-1 (EGR-1), which in turn induce RAGE [9]. In type 1 diabetes mellitus (T1DM), endothelial dysfunction precedes and may cause diabetic microangiopathy, but it is not clear whether endothelial dysfunction is a feature of the diabetic state itself. In type 2 diabetes mellitus (T2DM), endothelial function is impaired from the onset of the disease and is strongly related to adverse outcomes. It is not clear whether impaired endothelial function is caused by hyperglycemia or by other factors. Impaired endothelial function is closely associated with and may contribute to insulin resistance regardless of the presence of diabetes (Fig. 1) [3]. However, limited availability of endothelial tissue is a major constraint when studying the cellular mechanisms of diabetic vasculopathy in humans.
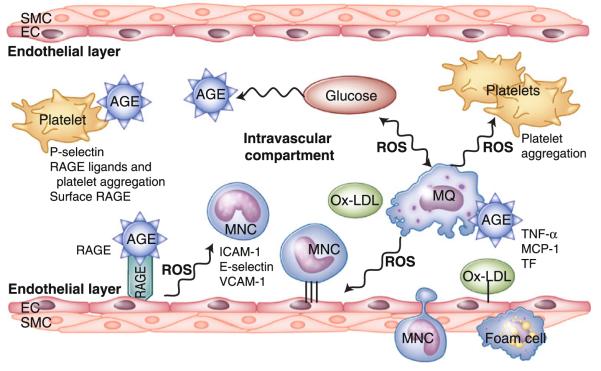
Fig. 1.
Schematic representation of the effects of diabetes on vascular inflammation and atherosclerosis. Hyperglycemia induces vascular injury through complex overlapping pathways: formation of advanced glycation end products (AGEs) and generation of reactive oxygen species (ROS), among others. Sources of ROS include the mitochondria, auto-oxidation of glucose, and enzymatic pathways. AGEs generate ROS directly or through receptors for AGE (RAGE), whereas ROS, in turn, promote formation of AGEs. ROS increase oxidized low-density lipoprotein (ox-LDL). RAGE is present on endothelial cells (ECs), fibroblasts, smooth muscle cells (SMCs), monocyte (MNC), and macrophages (MQ), which are involved in the genesis or development of vascular diseases. Tissue factor (TF), monocyte chemoattractant protein-1 (MCP-1), tumor necrosis factor-α (TNF-α), vascular cell adhesion molecule-1 (VCAM-1), and intercellular adhesion molecule-1 (ICAM-1) are all increased and further promote formation of foam cells and progression of atherosclerosis in diabetes
Methods for In Vitro Evaluation of Human Vascular Endothelial Cells for the Assessment of Inflammatory Status in Diabetes and Atherosclerosis
Human Vascular Endothelial Cells in Culture
Culture of ECs was first reported nearly four decades ago [10] and until recently, the progress in the knowledge of endothelial physiology has been mainly the consequence of investigations performed with ECs in culture. Due to the limited availability of human vascular endothelial tissue, EC functions have been modified in cell culture to mimic alterations of vascular wall properties as a relevant experimental model in understanding endothelial physiology and the study of the interaction of ECs with blood cells and various mediators.
Replication Index of Endothelial Cells
In normal conditions, ECs proliferation in vivo occurs with an extremely low turnover rate. Glucose toxicity causes delayed replication, disturbed cell cycle, and accelerated death in human ECs in culture in elevated glucose concentrations [11]. Cell culture studies showed that cytokines, such as tumor necrosis factor-α, interleukin-1β, interferon-γ, and monocyte-derived endothelial cell inhibitory factor, cause inhibition of EC replication. Thiamine, a coenzyme, decreases intracellular glycolysis metabolites and corrects delayed replication caused by high glucose concentrations in cultured human umbilical vein endothelial cells (HUVEC) [12].
Measurement of Prostacyclin in Vascular Homeostasis
Prostacyclin (PGI2) is a member of the family of lipid molecules, derived from arachidonic acid metabolism, and a major vascular mediator, which is continuously generated by endothelium. By means of its effect on cell proliferation, cell adhesion, permeability, and vascular tone, PGI2 is involved in the cardiovascular homeostasis in relation to vascular damage. Endothelial dysfunction in patients suffering from atherosclerosis or T2DM is associated with suppressed release of PGI2. It is shown that there are differences between microvascular ECs and HUVEC with respect to secretory functions and their modulation by glucose, indicating regional specificity of these functions [13]. Beraprost sodium, a prostaglandin I2 analogue, reduced mononuclear cell adhesion to HUVEC and repressed the expression of the adhesion molecule vascular cell adhesion molecule-1 (VCAM-1) in HUVEC [14].
Expression of Adhesion Molecules in Endothelial Cells
Cell adhesion molecules of the immunoglobulin superfamily, selectins, and integrins regulate endothelium-cell interactions in the intravascular compartment. Leukocyte adhesion molecules are expressed in pathologic processes and inflammatory stimuli cause ECs to facilitate adhesion and trans-endothelial migration of leukocytes [6]. Adhesion molecules constitutively present on the EC surface, such as intracellular adhesion molecule-1 (ICAM-1), increase expression after cell activation; whereas E-selectin and VCAM-1 are induced only after cell activation. E-selectin and platelet endothelial cell adhesion molecule have been shown to be expressed in atherosclerotic lesions, and might be involved in mononuclear cell adhesion to the vascular endothelium [6, 7]. It is demonstrated that hyperglycemia results in the expression of adhesion molecules: endothelial-leukocyte adhesion molecule-1, VCAM-1 and ICAM-1 in HUVEC [15]. However, inflammation determines the pro-adhesive properties of high glucose in human ECs in vitro as the elevation of extracellular D-glucose levels was not sufficient to promote vascular inflammation and to induce ICAM-1 and VCAM-1 expression, or to promote leukocyte adhesion to HUVEC [16].
AGE/RAGE Interaction in Endothelial Cells in Diabetes
AGEs result from nonenzymatic glycation of aldose sugars with a free amino group supported by macromolecules. The AGE/RAGE interaction, by binding of AGE to its receptor dramatically modifies the biological properties of ECs. It is shown that Red blood cells (RBCs) of patients with diabetes induced oxidant stress on ECs, which is prevented when ECs are preincubated with antioxidant probucol or antibody against RAGE, or when diabetic RBCs are preincubated with anti-AGE IgG [17, 18]. The AGE/RAGE interaction is shown to increase tissue factor release, endothelial interleukin-6 secretion, VCAM-1 expression, and to alter endothelial barrier permeability [17, 18]. VCAM-1 expression accompanied by an increase in monocyte adhesion and consequently could contribute to generation of atherosclerosis. A dose-dependent increase of endothelial permeability was observed when ECs were incubated with diabetic RBCs, whereas normal RBCs did not modify endothelial permeability [17]. This diabetes-induced endothelial hyperpermeability was prevented by the use of a blocking agent such as soluble RAGE and anti-RAGE IgG or antioxidants. The central role of the RAGE in the development of diabetic disease is supported by its presence on ECs, fibroblasts, SMCs, and monocyte/macrophages [5, 7]. RAGE is present on the surface of both HUVEC and human aortic ECs [15, 17].
Apoptosis
It has been previously shown that hyperglycemia enhances reactive oxygen species (ROS) production, which activates pathways implicated in apoptosis and necrosis. There is overwhelming evidence for the involvement of ROS in the pathogenesis of diabetes-associated vascular complications [5]. A study demonstrated that intermittent high glucose is more dangerous than constant high glucose medium for HUVEC in culture, because in the former condition, there was a marked increase in cellular apoptosis [19]. They further verified that hyperglycemia, both stable and oscillating, increases oxidative stress and EC apoptosis through ROS overproduction at the mitochondrial transport chain level. Another group showed that glucose upregulated cyclooxygenase-2 (COX-2) expression in HUVEC, via phosphatidylinositol 3-kinase/Akt signaling, which triggered the caspase-3 activity facilitating apoptosis [20]. This glucose-induced apoptosis was inhibited by quercetin sulfate/glucuronide [21] or by 3,4,5,6-tetrahydroxyxanthone by increasing Bcl-2 protein expression [22].
Methods for Ex Vivo Evaluation of Human Vascular Endothelial Cells for the Assessment of Inflammatory Status in Diabetes and Atherosclerosis
Circulating Endothelial Cells: Apoptosis and Vascular Injury in Diabetes
The existence of circulating endothelial cells (CECs) in the bloodstream was first described in 1978 [23], and the clinical and pathophysiologic meaning of these cells has gained renewed attention. In normal conditions, the number of CECs in the bloodstream is very low, due to the fact that endothelial turnover is a very slow process in the absence of pathologic stimuli. CECs are believed to be mature cells derived from the vessel wall, by shedding of resident ECs into the circulation, as part of their normal turnover process, or as an effect of pathological conditions, either mechanical (e.g., high blood pressure) or chemical (e.g., high glucose) [24–26]. CECs might be viable integral cells or cell fragments displaying apoptotic features [25]. As a result, CECs have been reported to be an ex vivo indicator of vascular injury to identify subjects at higher risk for cardiovascular events [26] and increased in patients with diabetes [27–29]. However, correlation of CECs with hemoglobin A1c (HbA1c) levels may differ in T1DM and T2DM. It is shown that patients with T2DM had an elevated number of CECs compared with healthy controls, which did not correlate with HbA1c levels and were elevated regardless of glucose levels, suggesting that, even with control of glucose levels, there is increased ECs sloughing [28]. Another group showed that patients with T1DM had higher number of CECs and endothelial dysfunction compared to controls with a positive correlation between CEC numbers and HbAlc levels, which was dependent on long-term glucose treatment [29]. CECs were isolated with anti-CD146–coated immunomagnetic Dynabeads [30] or selected by flow cytometry protocol stained with CD45 (to exclude hematopoietic cells), Syto16 (nuclear dye), CD31 (to exclude platelets), and with the CD146 (for the expression of the endothelial antigen). Negative staining for CD133 distinguishes between CECs and differentiating endothelial progenitor cells (EPCs) [31••]. Another group performed electron microscopy imaging on Syto16+CD45-CD31+CD146+ cells isolated by a fluorescence-activated cell sorter [32].
Endothelial Microparticles: Apoptosis and Vascular Injury in Diabetes
Endothelial microparticles (EMPs) are small vesicles ranging in size from 0.1 to 2 μm, originating from plasma membranes of disturbed ECs. EMPs are heterogeneous: those released in activation versus apoptosis are distinctive in phenotypic markers and procoagulant properties (tissue factor-dependent procoagulant assay/Annexin V staining). Some EMPs carry functional von Willebrand factor (vWF) with properties different from soluble vWF. Certain EMPs bind to and activate monocytes; EMP-monocyte conjugates were found to be a marker of inflammatory disease such as multiple sclerosis, and to enhance migration of leukocytes in vitro. Other circulating microparticles originating from plasma membranes of platelets, leukocytes, and erythrocytes also are involved in inflammation, coagulation, and cardiovascular diseases [33]. Elevated EMP reflects cellular injury as a surrogate marker of vascular dysfunction [34] in cardiovascular disease [35] and T2DM [36]. Like CECs, increased EMPs would reflect ongoing endothelial damage.
EMPs can further impact negatively on cardiovascular biology by stimulating inflammation, endothelial adhesiveness, and thrombosis. In T1DM, the procoagulant potential of EMPs was elevated and correlated with the degree of glycemic control [37]. In contrast, although total numbers of EMPs were elevated in T2DM, there was no associated increase in their procoagulant potential. Elevated EMP levels are more significant independent risk factors than length of diabetic disease, lipid levels, or presence of hypertension. Interestingly, elevated EMP levels are predictive in identifying a subpopulation of diabetic patients without typical anginal symptoms who have angiographic evidence of coronary artery disease [37]. Circulating EMP levels can be assayed by flow cytometry analysis of platelet-poor plasma based on CD31 or CD144 expression and enzyme-linked immunosorbent assay method [37].
Circulating Endothelial Progenitor Cells: Adhesion and Vascular Regeneration in Diabetes
EPCs were discovered in 1997 [38], as circulating cells originated from the bone marrow and migrating into the peripheral circulation and as progenitor cells being able to differentiate into mature ECs in vitro and in vivo. EPCs incorporate into the sites of physiologic and pathologic neovascularization and differentiate into mature vascular ECs. EPCs have two important functions in the cardiovascular system: regeneration of the endothelial layer and formation of new blood vessels. Thus, unlike CECs, EPCs are considered to be vasculoprotective, and a general inverse correlation is believed to exist between CEC levels and EPC levels. Despite profound methodologic differences among studies, EPCs appear to be consistently reduced or impaired in the setting of virtually all risk factors for cardiovascular disease [38]. It is shown that the migratory function of EPCs is impaired in patients with T2DM, and EPCs are reduced in peripheral vascular complications of T2DM [39]. These findings presented an insight into the pathogenesis of impaired neovascularization and critical limb ischemia in diabetic patients. Another group investigating the relationship between the number of circulating EPCs before and after the treatment showed that treatment of diabetes significantly increases the number of EPCs, which may be involved in angiogenesis and atherosclerosis in diabetes [40]. There are two main methods to study EPCs: quantification of EPCs by flow cytometry based on cells expressing immaturity/stem cell antigen (CD34 or CD133) plus endothelial antigen (KDR or CD31), or qualitative and functional data by prolonged EPC culture [41••]. Studies suggest that CD34+KDR+ is the best EPC phenotype for clinical analyses [41••].
Endothelial Biopsy: AGE/RAGE Interactions, Adhesion Molecules, Apoptosis, Vascular Inflammation/Dysfunction in Atherosclerosis
In the past decade, we validated an innovative and minimally invasive approach that allows collection of vascular ECs and characterization of their molecular phenotype in human subjects. In 1999, a novel method was described to sample human aortic ECs using coaxial stainless steel guide wires inserted into an iliac artery in patients undergoing routine endovascular procedures [42]. The wire sampled ECs from the intima of aorta by scraping the vessel wall. The tip of the wire was transferred to a lab tube and treated with a dissociation buffer to release the attached cells. About 50–75 viable arterial ECs were collected in each procedure. Endothelial identity is confirmed by immunoreactivity to vWF, thrombomodulin, and angiotensin-converting enzyme. Adhesion molecules, VCAM, and ICAM are detected by immunocytochemical analysis and E-selectin is measured by single-cell reverse transcriptase polymerase chain reaction (RT-PCR) method [42]. We improved this technique by immunomagnetic isolation of CD146+ ECs [43, 45] and it is shown that MCP-1 and EGR-1 are induced in the arterial endothelium of T2DM patients undergoing cardiac catheterization [43]. This study had several limitations, such as small sample size, invasive nature of sampling arterial ECs, limited selection of subjects, and semiquantitative nature of PCR approach.
To overcome these limitations, we introduced a novel venous EC harvesting approach by inserting 20-gauge angiocatheters into a forearm vein and sampled ECs by gentle scraping of the intima [44]. By quantitative immunofluorescence analysis on ECs harvested from a superficial forearm vein or the radial artery, we showed similar protein expressions of COX-2, nitrotyrosine level, and nuclear factor-κB nuclear translocation in the arterial and venous ECs of chronic heart failure (CHF) patients, suggesting that changes in venous ECs might mimic arterial EC alteration during advanced cardiovascular disease [44]. This study was limited with the small number of proteins that can be measured in each endothelial biopsy and needed further evaluation of EC phenotype by quantitative approaches. Accordingly, we have increased wires used in each endothelial biopsy (up to five wires to be sequentially inserted through 20-gauge angiocatheters), improved immunomagnetic enrichment steps by enclosed and shorter washing steps at 4°C (to increase cell viability and reduce RNA degradation), and finally amplified total RNA to couple the method with quantitative Real-Time PCR (qPCR) analysis [45]. By this method, we increased the final EC yield from 40–75 viable ECs [42–44] to 50–200 [45] viable ECs after immunomagnetic enrichment steps. As evidenced by Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA) quantitation of total RNA, and mRNA, we isolated a minimum of 200-pg total RNA/biopsy prior RNA amplification and up to 100 μg aRNA/biopsy, after RNA amplification [45–47]. We showed that venous ECs sampled from subjects with vascular disease had significantly higher expression of proatherosclerotic genes, such as EGR-1 and MCP-1, confirming venous endothelial sampling to be a reliable method in the study of cardiovascular disease. Furthermore, we showed that this method can be safely repeated in 1-week intervals, providing reproducible results [45]. Thus, we were able to demonstrate for the first time with a direct approach that endothelial dysfunction associated with cardiovascular risk is characterized by increased EC inflammation. More recently, with the advancement in microarray studies allowing smaller input aRNA (750 ng–100 μg), our group and collaborators were able to couple venous endothelial biopsy approach with microarray studies [46, 47].
Current limitations of venous endothelial biopsy are as follows: venous ECs are not subjected to the same hemodynamics of the arterial compartment, and therefore venous ECs may give important, but possibly incomplete information, and total RNA of ECs obtained from endothelial biopsy should be amplified to perform quantitative analysis of qPCR and microarray studies. However, this method provides a novel outlook on human vascular ECs as close as possible to their natural environment with quantitative analysis of protein and gene expression. Moreover, current methodologies allow standardized RNA isolation/amplification methods and quantitative analysis of RNA in as little as 50-pg input [45–47]. As it is recognized by others and demonstrated by us, this study casts the basis for a more comprehensive evaluation of the potential data that can be drawn from patient’s own ECs. With the improvement in catheterization, immunomagnetic isolation, and RNA amplification methods, we were able to standardize our model for minimally invasive and reproducible results [45-49]. Data from our laboratory and other investigators indicate that a common molecular pattern of activation of the oxidative and inflammatory programs are present in venous ECs collected from patients with chronic disease states such as but not limited to CHF, sleep apnea, obesity, lupus, and aging [44–49].
Conclusions
Despite the prevalence and debilitating nature of diabetic vascular complications, cellular/molecular/genetic mechanisms underlying vascular dysfunction remain unclear; however, the literature to date reflect that endothelial dysfunction is the key initiator preceding vascular complications in diabetes. Unfortunately, endothelium has limited ability to repair itself; thus, methods to prevent or counter endothelial dysfunction/damage are widely pursued.
The information gathered with ECs in culture shows that ECs isolated from different vascular beds can function differently and it is shown that the success of primary HUVEC culture is influenced by maternal and fetal factors [50]. Moreover, high glucose medium may not reflect the whole physiologic conditions present in vivo, as other cells secrete cytokines, increase ROS, and RAGE [3-7]. The molecular mechanisms triggered on cultured ECs by periodically changing glucose concentrations are not known and during chronic exposure to high glucose, some metabolic variations might change or feedback regulatory cell controls [13], partially counteracting the glucose toxic effect [11]. Furthermore, it is not clear that intensive blood glucose control per se favorably affects atherosclerotic vascular diseases. These limitations create uncertain relevance of culture studies to humans (Table 1). As a result, cell culture studies should be evaluated by a systemic in vivo investigation to validate the model. However, due to the limited availability of human vascular endothelial tissue until recently, the progress in the knowledge of endothelial physiology has been mainly the consequence of investigations performed with ECs in culture. Therefore, ECs in culture can be considered as a useful, convenient, and complementary technique to present ex vivo methods.
Table 1.
Studies for in vitro evaluation of diabetic vascular inflammation and atherosclerosis
| End points examined | Design | Cell type | Findings | Limitations of method | Reference |
|---|---|---|---|---|---|
| Proliferation and endothelial barrier |
-ECs culture in high glucose medium -[3H] thymidine incorporation or cell count -Inhibition of glucose toxicity by thiamine |
HUVEC/ HAEC |
-Suppressed cell number -Delayed replication and disturbed cell cycle -Compromised endothelial barrier and accelerated death |
-Uncertain relevance to humans -Physiologic situation is more complex |
[10–12] |
| Vascular homeostasis |
-ECs culture in high glucose medium -PGI2 secretion in cell proliferation, adhesion, permeability, and vascular tone -Beraprost sodium, a PGI2 analogue |
HUVEC/ HREC |
-Regional specificity of PGI2 secretory functions and modulation by glucose -PGI2 role in EC dysfunction |
-The molecular mechanisms triggered on by changing glucose concentrations are not known |
[13, 14] |
| Adhesion | -ECs cultures in high glucose medium activated by IL-1, TNF, or treated with inhibitors -Adhesion molecules measured by ELISA, flow cytometry, or by flow chamber assays -Co-culture of monocyte-endothelial cells or use of monocyte-conditioned medium |
HUVEC | -Increased adhesion, and expressions of VCAM and E-selectin from ECs -Monocytes culture increased VCAM, ICAM, E-selectin, expressions of ECs |
-Uncertain relevance to humans -Physiologic situation is more complex -No clear effect of intensive blood glucose control on atherosclerosis |
[15, 16] |
| AGE/RAGE and ROS |
-ECs cultures in high glucose medium/AGEs -Use of a blocking agent such as sRAGE and 18] anti-RAGE IgG or antioxidants -RAGE on the cell surface studied by immunohistochemistry |
HUVEC/ HAEC |
-Increased ROS, TF, eIL-6, VCAM-1 ex- pression, and monocyte adhesion -ROS prevented by anti-RAGE IgG or antioxidants |
-No clear effect of intensive blood glucose control on atherosclerosis -Physiologic situation is more complex |
[15, 17, 18] |
| Apoptosis | -Induced by stable or oscillating high glucose -Medium enriched by SOD, TTFA, MnTBAP, nitrotyrosine, 8OHdG, or quercetin metabolites -Bcl-2 and caspase-3 expression -Hoechst staining, Annexin V/propidium iodide staining, DNA ladder formation, or flow cytometry |
HUVEC | -Intermittent high glucose is more dangerous -Increase ROS and apoptosis; caspase-3 activity through PI3K -Inhibition by quercetin and 3,4,5,6- tetrahydroxyxanthone -Changes in fatty acids |
-The molecular mechanisms triggered on by changing glucose concentrations are not known -Possible counteracting of the glucose toxic effect |
[19-22] |
AGE advanced glycation end products; COX-2 cyclooxygenase-2; ECs endothelial cells; eIL-6 endothelial interleukin-6; ELISA enzyme-linked immunosorbent assay; HAEC human aortic endothelial cells; HREC human retinal microvascular endothelial cells; HUVEC human umbilical vein endothelial cells; ICAM intracellular adhesion molecule; IL-1 interleukin-1; MnTBAP Mn(III) tetrakis (4-benzoic acid) porphyrin chloride; MQ macrophages; PGI2 prostacyclin; PI3K phosphatidylinositol 3-kinase; RAGE receptor for advanced glycation end products; ROS reactive oxygen species; SOD superoxide dismutase; sRAGE soluble receptor for advanced glycation end products; TF tissue factor; TNF tumor necrosis factor; TTFA 2-thenoyltrifluoroacetone; VCAM vascular cell adhesion molecule.
Up-to-date ex vivo cell-based methodologies are based on the analysis of patient-derived ECs obtained by endothelial biopsy and the analysis of patients’ circulating CECs, EMPs, and EPCs (Table 2). However, these methods also have their limitations, such as low circulating levels of CECs, EMPs, and EPCs, lack of single marker to identify each cell type [41••] or expressing same markers [31••], which in turn cause incomplete separation of these cells making it difficult to standardize their techniques. Furthermore, circulating markers will not produce functional or biochemical studies and ex vivo culture of EPCs might not closely reflect in vivo pathophysiologic mechanism [41••]. Conversely, endothelial biopsy method will allow reproducible and quantitative molecular/biochemical analysis of ECs, but it may require a second measure of endothelial dysfunction such as flow-mediated dilation or circulating EC analysis to confirm the correlation of venous EC dysfunction to ongoing arterial EC dysfunction. Despite these limitations, circulating CECs, EMPs, and EPCs would provide novel complementary analysis of vascular injury/degeneration, whereas endothelial biopsy would allow a quantitative measurement of atherosclerosis-related readouts on patients’ own vascular ECs; thus, long sought after biomarkers of therapeutic responses can be investigated well before the frank changes in end-organ damage.
Table 2.
Studies for ex vivo evaluation of diabetic vascular inflammation and atherosclerosis
| End points examined | Design | Cell type | Findings | Limitations of method | Reference |
|---|---|---|---|---|---|
| Apoptosis and vascular injury | -Immunomagnetic isolation and staining of CD146 + vWF+, UEA-1+ CD133-cells -Syto16+CD45-CD31+CD146+ cells isolated by flow cytometry or by a fluorescence-activated cell sorter and electron microscopy imaging |
CECs | -Opposite correlation with HbA1c in T1DM and T2DM -Increased in diabetes -Have apoptotic cell features |
-Incomplete separation from EPCs -Low circulating levels -No functional or biochemical studies -No single marker to identify -Site of endothelial damage unknown -The correlation with HbA1c is unclear |
[23-30] [31••] [32] |
| Apoptosis and vascular injury | -Flow cytometry analysis of platelet-poor plasma, apoptotic microparticles by Annexin V staining -TF-dependent procoagulant assay, ELISA, or prothrombinase assay -EC identity based on CD31/CD144 expression |
EMPs | -Involved in cardiovascular diseases, inflammation, and coagulation -Cardiovascular risk marker in diabetes |
-Incomplete standardization -Low circulating levels -No functional or biochemical studies -In diabetes, released by large panel of cells |
[33-37] |
| Adhesion and vascular regeneration | -Cells with CD34+ or CD133+ and with KDR+ or CD31+ isolated by flow cytometry -EPC ex vivo cell culture protocol (≥ 14 days) in endothelial differentiation medium |
EPCs | -Reduced or impaired in cardiovascular disease and diabetes -Form colonies, adhere to a monolayer of ECs in culture -Diabetic PBMCs can be differentiated into EPCs |
-Incomplete separation from CECs/HSCs -Poor or incomplete standardization, -No functional/biochemical studies -Semiquantitative analysis -Low circulating levels -Extrapolation from in vivo environment |
[38-40] [41••] |
| Adhesion, apoptosis, AGE/RAGE and ROS, endothelial activation/dysfunction, vascular inflammation/injury |
-CD146+ ECs collected by angiocatheterization and enriched by immunomagnetic isolation -RNA amplified for quantitative PCR and microarray analysis -ECs fixed and then stained with vWF+ and desired markers for protein analysis |
human aortic ECs/ human venous ECs |
-Patients own aorta and/or venous ECs are sampled and showed ECs activation in chronic inflammation (e.g., in diabetes, cardiovascular diseases, sleep apnea, lupus and aging) |
-Sampling of aorta replaced by venous for minimally invasive and repeatable sampling -Venous ECs give important, but incomplete information -RNA amplification needed for quantitative analysis |
[42-44] [45] [46-49] |
AGE advanced glycation end products; CECs circulating endothelial cells; ECs endothelial cells; ELISA enzyme-linked immunosorbent assay; EMPs endothelial microparticles; EPCs endothelial progenitor cells; HbA1c hemoglobin A1c; HSCs hematopoietic stem cells; PBMCs peripheral blood mononuclear cells; PCR polymerase chain reaction; RAGE receptor for advanced glycation end products; ROS reactive oxygen species; T1DM type 1 diabetes; T2DM type 2 diabetes; νWF von Willebrand factor.
The recent discovery of ex vivo methodologies advanced the field of cell-based technologies for a comprehensive evaluation of EC biology in humans, which in turn can be correlated to clinical development of vascular disease in diabetes and would provide a novel perspective on the endothelium with pathophysiologic and therapeutic implications targeted to the reduction of vascular inflammation.
Footnotes
Disclosure Conflicts of interest: D. Onat: received a grant from the American Heart Association (Postdoctoral Fellowship Award #0425894T); D. Brillon: none; P.C. Colombo: received a grant (NIH-K23 Award # HL-72758); A.M. Schmidt: none.
Contributor Information
Duygu Onat, Department of Medicine, Division of Cardiology, College of Physicians and Surgeons, Columbia University Medical Center, 630 West, 168th Street, PS-17-401, New York, NY 10032, USA.
David Brillon, Department of Medicine, Division of Endocrinology, Diabetes and Metabolism, Weill Cornell Medical College of Cornell University, 525 East, 68th Street, F-2008, New York, NY 10065, USA, djbrillo@med.cornell.edu.
Paolo C. Colombo, Department of Medicine, Division of Cardiology, College of Physicians and Surgeons, Columbia University Medical Center, 622 West, 168th Street, PH12-134, New York, NY 10032, USA, pcc2001@columbia.edu
Ann Marie Schmidt, Diabetes Research Program, Department of Medicine, Division of Endocrinology, NYU Langone Medical Center, 550 First Avenue, Smilow 9, New York, NY 10016, USA, AnnMarie.Schmidt@nyumc.org.
References
Papers of particular interest, published recently, have been highlighted as:
•• Of major importance
- 1.Setacci C, de Donato G, Setacci F, et al. Diabetic patients: epidemiology and global impact. J Cardiovasc Surg. 2009;50:263–73. [PubMed] [Google Scholar]
- 2.Gu K, Cowie CC, Harris MI. Mortality in adults with and without diabetes in a national cohort of the U.S. population, 1971–1993. Diab Care. 1998;21:1138–45. doi: 10.2337/diacare.21.7.1138. [DOI] [PubMed] [Google Scholar]
- 3.Schalkwijk CG, Stehouwer CDA. Vascular complications in diabetes mellitus: the role of endothelial dysfunction. Clin Sci. 2005;109:143–59. doi: 10.1042/CS20050025. [DOI] [PubMed] [Google Scholar]
- 4.Packard RRS, Libby P. Inflammation in atherosclerosis: from vascular biology to biomarker discovery and risk prediction. Clin Chem. 2008;54:124–38. doi: 10.1373/clinchem.2007.097360. [DOI] [PubMed] [Google Scholar]
- 5.Son SM. Role of vascular reactive oxygen species in development of vascular abnormalities in diabetes. Diabetes Res Clin Pract. 2007;(Suppl 1):S65–70. doi: 10.1016/j.diabres.2007.01.036. [DOI] [PubMed] [Google Scholar]
- 6.Maugeri N, Rovere-Querini P, Baldini M, et al. Translational mini-review series on immunology of vascular disease: mechanisim of vascular inflammation and remodeling in systemic vasculitis. Clin Exp Immunol. 2009;156:395–404. doi: 10.1111/j.1365-2249.2009.03921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chavakis T, Bierhaus A, Nawroth PP. RAGE (receptor for advanced glycation end products): a central player in the inflammatory response. Microbes Infect. 2004:1219–25. doi: 10.1016/j.micinf.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 8.Takaishi H, Taniguchi T, Takahashi A, et al. High glucose accelerates MCP-1 production via p38 MAPK in vascular endothelial cells. Biochem Biophys Res Commun. 2003;305:122–30. doi: 10.1016/s0006-291x(03)00712-5. [DOI] [PubMed] [Google Scholar]
- 9.Hasan RN, Phukan S, Harada S. Differential regulation of early growth response Gene-1 expression by insulin and glucose in vascular endothelial cells. Arterioscler Thromb Vasc Biol. 2003;23:988–95. doi: 10.1161/01.ATV.0000071351.07784.19. [DOI] [PubMed] [Google Scholar]
- 10.Jaffe E, Nachman R, Becker C, et al. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest. 1973;52:2745–56. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lorenzi M, Cagliero E, Toledo S. Glucose toxicity for human endothelial cells in culture. Delayed replication, disturbed cell cycle, and accelerated death. Diabetes. 1985;34:621–7. doi: 10.2337/diab.34.7.621. [DOI] [PubMed] [Google Scholar]
- 12.La Selva M, Beltramo E, Pagnozzi F, et al. Thiamine corrects delayed replication and decreases production of lactate and advanced glycation end-products in bovine retinal and human umbilical vein endothelial cells cultured under high glucose conditions. Diabetologia. 1996;39:1263–8. doi: 10.1007/s001250050568. [DOI] [PubMed] [Google Scholar]
- 13.Rymaszewski Z, Szymanski PT, Abplanalp WA, et al. Human retinal vascular cells differ from umbilical cells in synthetic functions and their response to glucose. Proc Soc Exp Biol Med. 1992;199:183–91. doi: 10.3181/00379727-199-43345. [DOI] [PubMed] [Google Scholar]
- 14.Goya K, Otsuki M, Xu X, et al. Effects of the prostaglandin I2 analogue, beraprost sodium, on vascular cell adhesion molecule-1 expression in human vascular endothelial cells and circulating vascular cell adhesion molecule-1 level in patients with type 2. Diab Mellitus Metab. 2003;52:192–8. doi: 10.1053/meta.2003.50025. [DOI] [PubMed] [Google Scholar]
- 15.Altannavch TS, Roubalová K, Kučera P, et al. Effect of high glucose concentrations on expression of ELAM-1, VCAM-1 and ICAM-1 in HUVEC with and without cytokine activation. Physiol Res. 2004;53:77–82. [PubMed] [Google Scholar]
- 16.Azcutia V, Abu-Taha M, Romacho T, et al. Inflammation determines the pro-adhesive properties of high extracellular D-glucose in human endothelial cells in vitro and rat microvessels in vivo. PLoA One. 2010;5:e1009. doi: 10.1371/journal.pone.0010091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wautier JL, Wautier MP, Schmidt AM, et al. Advanced glycation end products (AGEs) on the surface of diabetic erythrocytes bind to the vessel wall via a specific receptor inducing oxidant stress in the vasculature: a link between surface-associated AGEs and diabetic complications. Proc Natl Acad Sci USA. 1994;91:7742–6. doi: 10.1073/pnas.91.16.7742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schmidt AM, Hori O, Chen JX, et al. Advanced glycation end products interacting with their endothelial receptor induce expression of vascular cell adhesion molecule-1 (VCAM-1) in cultured human endothelial cells and in mice. A potential mechanism for the accelerated vasculopathy of diabetes. J Clin Invest. 1995;96:1395–403. doi: 10.1172/JCI118175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Piconi L, Quagliaro L, Assaloni R, et al. Constant and intermittent high glucose enhances endothelial cell apoptosis through mitochondrial superoxide overproduction. Diab Metab Res Rev. 2006;22:198–203. doi: 10.1002/dmrr.613. [DOI] [PubMed] [Google Scholar]
- 20.Sheu ML, Ho FM, Yang RS, et al. High glucose induces human endothelial cell apoptosis through a phosphoinositide 3-kinase-regulated cyclooxygenase-2 pathway. Arterioscler Thromb Vasc Biol. 2005;25:539–45. doi: 10.1161/01.ATV.0000155462.24263.e4. [DOI] [PubMed] [Google Scholar]
- 21.Chao CL, Hou YC, Chao PD, et al. The antioxidant effects of quercetin metabolites on the prevention of high glucose-induced apoptosis of human umbilical vein endothelial cells. Br J Nutr. 2009;101:1165–70. doi: 10.1017/S0007114508073637. [DOI] [PubMed] [Google Scholar]
- 22.Dai Z, Liao DF, Jiang DJ, et al. 3, 4, 5, 6-Tetrahydroxyxanthone prevents vascular endothelial cell apoptosis induced by high glucose. Naunyn Schmiedebergs Arch Pharmacol. 2004;370:314–9. doi: 10.1007/s00210-004-0973-y. [DOI] [PubMed] [Google Scholar]
- 23.Hladovec J. Circulating endothelial cells as a sign of vessel wall lesions. Physiol Bohemoslov. 1978;27:140–4. [PubMed] [Google Scholar]
- 24.Strijbos MH, Verhoef C, Gratama JW, et al. On the origin of (CD105+) circulating endothelial cells. Thromb Haemost. 2009;102:347–51. doi: 10.1160/TH08-11-0762. [DOI] [PubMed] [Google Scholar]
- 25.Mariucci S, Rovati B, Chatzileontiadou S, et al. A six-colour flow cytometric method for simultaneous detection of cell phenotype and apoptosis of circulating endothelial cells. Scand J Clin Lab Invest. 2009;69:433–8. doi: 10.1080/00365510802673175. [DOI] [PubMed] [Google Scholar]
- 26.Boos CJ, Lip GY, Blann AD. Circulating endothelial cells in cardiovascular disease. J Am Coll Cardiol. 2006;48:1538–47. doi: 10.1016/j.jacc.2006.02.078. [DOI] [PubMed] [Google Scholar]
- 27.Egawhary DN, Swoboda BE, Chen J, et al. Damage to the vascular endothelium of diabetic patients can be assessed by analysing blood samples for the number of circulating endothelial cells with mitochondrial DNA deletions. Biochem Soc Trans. 1995;23:402S. doi: 10.1042/bst023402s. [DOI] [PubMed] [Google Scholar]
- 28.McClung JA, Naseer N, Saleem M, et al. Circulating endothelial cells are elevated in patients with type 2 diabetes mellitus independently of HbA(1)c. Diabetologia. 2005;48:345–50. doi: 10.1007/s00125-004-1647-5. [DOI] [PubMed] [Google Scholar]
- 29.Asicioglu E, Gogas Yavuz D, Koc M, et al. Circulating endothelial cells are elevated in patients with type 1 diabetes mellitus. Eur J Endocrinol. 2010;162:711–7. doi: 10.1530/EJE-09-0795. [DOI] [PubMed] [Google Scholar]
- 30.Woywodt A, Blann AD, Kirsch T, et al. Isolation and enumeration of circulating endothelial cells by immunomagnetic isolation: proposal of a definition and a consensus protocol. J Thromb Haemost. 2006;4:671–7. doi: 10.1111/j.1538-7836.2006.01794.x. [DOI] [PubMed] [Google Scholar]
- 31. ••.Mancuso P, Antoniotti P, Quarna J, et al. Validation of a standardized method for enumerating circulating endothelial cells and progenitors: flow cytometry and molecular and ultrastructural analyses. Clin Cancer Res. 2009;15:267–73. doi: 10.1158/1078-0432.CCR-08-0432. This is an excellent study about the validation of flow cytometry methods for the circulating ECs and progenitors.
- 32.Delorme B, Basire A, Gentile C, et al. Presence of endothelial progenitor cells, distinct from mature endothelial cells, within human CD146+ blood cells. Thromb Haemost. 2005;94:1270–9. doi: 10.1160/TH05-07-0499. [DOI] [PubMed] [Google Scholar]
- 33.Puddu P, Puddu GM, Cravero E, et al. The involvement of circulating microparticles in inflammation, coagulation and cardiovascular diseases. Can J Cardiol. 2010;26:140–5. doi: 10.1016/s0828-282x(10)70371-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Horstman LL, Jy W, Jimenez JJ, et al. Endothelial microparticles as markers of endothelial dysfunction. Front Biosci. 2004;9:1118–35. doi: 10.2741/1270. [DOI] [PubMed] [Google Scholar]
- 35.Jimenez JJ, Jy W, Mauro LM, et al. Endothelial microparticles (EMP) as vascular disease markers. Adv Clin Chem. 2005;39:131–57. doi: 10.1016/s0065-2423(04)39005-0. [DOI] [PubMed] [Google Scholar]
- 36.Leroyer AS, Tedgui A, Boulanger CM. Microparticles and type 2 diabetes. Diab Metab. 2008;34(Suppl 1):S27–32. doi: 10.1016/S1262-3636(08)70100-9. [DOI] [PubMed] [Google Scholar]
- 37.Nomura S, Ozaki Y, Ikeda Y. Function and role of microparticles in various clinical settings. Thromb Res. 2008;123:8–23. doi: 10.1016/j.thromres.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 38.Asahara T, Murohara T, Sullivan A, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–7. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 39.Chen MC, Sheu JJ, Wang PW, et al. HW complications impaired endothelial progenitor cell function in Type 2 diabetic patients with or without critical leg ischaemia: implication for impaired neovascularization in diabetes. Diabet Med. 2009;26:134–41. doi: 10.1111/j.1464-5491.2008.02649.x. [DOI] [PubMed] [Google Scholar]
- 40.Kusuyama T, Omura T, Nishiya D, et al. Effects of treatment for diabetes mellitus on circulating vascular progenitor cells. J Pharmacol Sci. 2006;102:96–102. doi: 10.1254/jphs.fp0060256. [DOI] [PubMed] [Google Scholar]
- 41. ••.Ruiter MS, van Golde JM, Schaper NC, et al. Diabetes impairs arteriogenesis in the peripheral circulation: review of molecular mechanisims. Clinical Sci. 2010;119:225–38. doi: 10.1042/CS20100082. This is an excellent review about the circulating ECs and progenitors in diabetes.
- 42.Feng L, Stern DM, Pile-Spellman J. Human endothelium: endovascular biopsy and molecular analysis. Radiology. 1999;212:655–64. doi: 10.1148/radiology.212.3.r99au28655. [DOI] [PubMed] [Google Scholar]
- 43.Feng L, Matsumoto C, Schwartz A, et al. Chronic vascular inflammation in patients with type 2 diabetes: endothelial biopsy and RT-PCR analysis. Diab Care. 2005;28:379–84. doi: 10.2337/diacare.28.2.379. [DOI] [PubMed] [Google Scholar]
- 44.Colombo PC, Ashton AW, Celaj S, et al. Biopsy coupled to quantitative immunofluorescence: a new method to study the human vascular endothelium. J Appl Physiol. 2002;92:1331–8. doi: 10.1152/japplphysiol.00680.2001. [DOI] [PubMed] [Google Scholar]
- 45.Onat D, Jelic S, Schmidt AM, et al. Vascular endothelial sampling and analysis of gene transcripts: a new quantitative approach to monitor vascular inflammation. J Appl Physiol. 2007;103:1873–8. doi: 10.1152/japplphysiol.00367.2007. [DOI] [PubMed] [Google Scholar]
- 46.Colombo PC, Onat D, Kebschull M, et al. Expression profiling of the vascular endothelium in patients with heart failure using a novel methodology: human endothelial sampling coupled with microarray analysis. J Am Coll Cardiol. 2009:1031–119. A439. [Google Scholar]
- 47.Goldenberg D, Olferiey M, Onat D, et al. Expression profiling of the vascular endothelium in patients with SLE using a novel methodology: human endothelial sampling coupled with micro-array analysis. Arthritis Rheum. 2009;60:117. [Google Scholar]
- 48.Jelic S, Padeletti M, Kawut SM, et al. Inflammation, oxidative stress and repair capacity of the vascular endothelium in obstructive sleep apnea. Circulation. 2008;117:2270–8. doi: 10.1161/CIRCULATIONAHA.107.741512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Donato AJ, Eskurza I, Silver AE, et al. Direct evidence of endothelial oxidative stress with aging in humans: relation to impaired endothelium-dependent dilation and upregulation of nuclear factor-kappaB. Circ Res. 2007;100:1659–66. doi: 10.1161/01.RES.0000269183.13937.e8. [DOI] [PubMed] [Google Scholar]
- 50.Balconi G, Pietra A, Busacca M, et al. The success of primary human endothelial cell culture from umbilical cords is influenced by maternal and fetal factors and interval from delivery. In Vitro. 1983;19:807–10. doi: 10.1007/BF02618159. [DOI] [PubMed] [Google Scholar]