Abstract
Red meat intake is associated with increased risk of colorectal cancer. We have previously shown that haemin, haemoglobin and red meat promote carcinogen-induced preneoplastic lesions, aberrant crypt foci, in the colon of rats. We have also shown that dietary calcium phosphate inhibits haemin-induced promotion, and normalizes faecal lipoperoxides and cytotoxicity. Unexpectedly, high-calcium phosphate control diet-fed rats had more preneoplastic lesions in the colon than low-calcium control diet-fed rats. The present study was designed to find a calcium supplementation with no adverse effect, by testing several doses and types of calcium salts. One in vitro study and two short-term studies in rats identified calcium carbonate as the most effective calcium salt to bind haem in vitro and to decrease faecal biomarkers previously associated with increased carcinogenesis: faecal water cytotoxicity, thiobarbituric acid reactive substances. A long term carcinogenesis study in dimethylhydrazine-injected rats demonstrated that a diet containing 100 μmol/g calcium carbonate did not promote aberrant crypt foci, in contrast with previously tested calcium phosphate diet. The results suggest that calcium carbonate, and not calcium phosphate, should be used to reduce haem-associated colorectal cancer risk in meat-eaters. They support the concept that the nature of the associated anion to a protective metal ion is important for chemoprevention.
Keywords: Animals; Biological Markers; Calcium Carbonate; administration & dosage; pharmacology; Calcium Phosphates; administration & dosage; adverse effects; Colon; drug effects; Colonic Neoplasms; chemically induced; prevention & control; Diet; adverse effects; veterinary; Dietary Supplements; Feces; chemistry; Female; Heme; toxicity; Meat; adverse effects; Rats; Rats, Inbred F344
Keywords: Red meat, Haem, prevention, calcium, colon cancer, Calcium, Colorectal cancer, Lipid peroxidation
Introduction
Red and processed meat consumption increases the risk of colorectal cancer, according to meta-analyses of epidemiological studies [1,2]. In its 2007 report, the World Cancer Research Fund panel judges that “the evidence that red meat and processed meat are a cause of colorectal cancer is convincing” [3]. The panel thus recommends: “Limit intake of red meat and avoid processed meat”. Several meat components have been speculated to explain that meat-eaters are at a higher risk for colorectal cancer. Meat cooked at a high temperature contains mutagenic heterocyclic amines that induce colon, mammary and prostate tumours in rodents and monkeys [4]. However, heterocyclic amines might not play an important role in colorectal cancer incidence, since (i) chicken intake is a major contributor of heterocyclic amines intake, but it is not associated with the risk [5], and (ii) doses of heterocyclic amines that induce cancer in animals are 1000 to 100,000 times higher than doses ingested by humans [6]. Red meat enhances the formation of putative carcinogenic N-nitroso compounds in human and rodent faeces [7–9]. N-nitroso compounds level is associated with the promotion of colon carcinogenesis by cured meat [10]. But N-nitroso compounds brought into rat faeces by a bacon-based diet do not initiate or promote preneoplastic lesions in rat colon [11]. Red meat also contains haem, the iron-bearing prosthetic group of myoglobin. Intake of haem iron is associated with an increased risk of colon cancer among women of the Iowa Women’s Health Study [12]. Intake of black pudding, a blood sausage over-loaded with high haem iron, is associated with increased risk of colorectal cancer among women of the Swedish Mammography Cohort [13].
Experimental animal studies support the hypothesis that haem iron promotes colorectal carcinogenesis [14]. We have shown that dietary haem, in the form of haemin (haem stabilized by a chlorine atom, also called Ferriprotoporphyrin IX chloride), haemoglobin or red meat, promotes putative precancerous lesions, aberrant crypt foci (ACF) and mucin-depleted foci (MDF), in the colon of rats given a low-calcium diet [15–17]. This haem-induced promotion is associated with increased lipoperoxidation and cytotoxicity of faecal water, and linked to urinary 1,4-dihydroxynonane mercapturic acid (DHN-MA) excretion, a fat peroxidation biomarker [18]. The addition of calcium phosphate to the diet inhibits haemin-induced lipoperoxidation, cytotoxicity and promotion of carcinogenesis in rats [17], and we can explain the lack of effect of meat diets on colon cancer carcinogenesis in previous study [19] by a high concentration of calcium in the diet. The addition of calcium phosphate to beef meat diet fully prevents red meat promotion of colon carcinogenesis, but, unexpectedly, the rats given the no-meat high-calcium phosphate high fat diet had more ACF and more MDF than the rats given the no-meat low-calcium phosphate diet [16]. In the absence of meat, dietary calcium phosphate appears to promote carcinogenesis in this rodent model, which precludes its use in human volunteers.
The present study was designed to determine a calcium supplementation with no adverse effect by testing different types of calcium salts, and finding the minimum effective calcium dose.
Materials and Methods
Animals and Diets
Eighty five Fischer 344 female rats were purchased at four weeks of age from Iffa Credo (St.Germain l’Arbresle, France). Animal care was in accordance with the guidelines of the European Council on animals used in experimental studies. The animal colony and staff got official agreement #31-121 for animal studies by French government.
Short-term studies
Two short-term studies were designed to find the most effective calcium salt and the minimum effective calcium dose to suppress early biomarkers of haem toxicity. Rats were housed individually in metabolic cages. The room was kept at a temperature of 22°C on a 12-h light-dark cycle. After 5 days of acclimatization to the AIN76 powdered diet, rats were given the experimental diets. Body weights and food intake were monitored at the beginning, and at the end of each study. In the first study, forty rats were randomly allocated to 8 dietary groups (5 rats per group) for one week. Faeces were collected at day 7 and were frozen at −20°C. Animals were killed 7 d after the start of the experimental diets. In the second study, twenty five rats were randomly allocated to 5 dietary groups (5 rats per group) for two weeks. Faeces were collected at day 14 and were frozen at −20°C. Animals were killed 14 d after the start of the experimental diets.
Long-term study
A three-month study was designed to test the hypothesis that the minimum effective dose of the most effective calcium salt, selected from short-term studies results, does not promote carcinogenesis in rats. Twenty rats were housed by pairs in stainless steel wire bottomed cages. The room was kept at a temperature of 22°C on a 12-h light-dark cycle. Rats were allowed 7 d of acclimatization to the room and to the control diet, before being injected i.p. with the carcinogen 1,2 dimethylhydrazine (Sigma chemical, St.Quentin, France; 190 mg/kg body wt) in NaCl (9 g/L). In most published studies, several carcinogen injections are given to rats. We reasoned here that the first shot initiates carcinogenesis, but that the following shots would promote carcinogenesis, blurring diet-induced promotion. We thus chose a single-shot protocol, following Glauert [20]. Seven days later, rats were randomly allocated to two groups of ten, and allowed free access to calcium carbonate-based diets for 94 d. We chose to initiate all rats with the carcinogen, since the study was designed to show dietary promotion of carcinogenesis.
Diets
Experimental diets shown in Table 1 were based on a modified standard AIN-76 diet [21], prepared and formulated in a powdered form by UPAE (INRA, Jouy-en-Josas, France). For the first short-term study, high-beef meat diets were formulated to contain 60% meat (w/w, freeze-dried, 0.6 mmol haem/g of meat), and dibasic calcium phosphate was included in all diets at a low concentration of 20 μmol/g. To determine, the minimum effective dose of calcium phosphate, the effect of seven concentrations of calcium was tested in seven groups of rats given this low-calcium beef meat diet supplemented with calcium phosphate (doses range: 33 to 250 μmol/g, diet names: Phos33 … Phos250). To compare the efficacy of calcium phosphate with other salts, the effect of two calcium salts was tested in two groups of rats given calcium carbonate (Carb250) or calcium gluconate (Gluc250) at the highest tested dose (250 μmol/g). For the second short-term study, the effect of calcium carbonate was tested in five groups of rats given a haemoglobin diet, supplying 0.6 mmol/g haem to mimic the 60% beef meat diet, supplemented with calcium carbonate (doses range: 33 to 155 μmol/g, diet names: Carb33 … Carb155). For the long-term carcinogenesis study, two experimental diets were formulated to contain two levels of calcium carbonate (33 and 100 μmol/g, diet names: Control Diet and Carb100) added into the control low calcium phosphate diet.
Table 1.
Formulation of diets (g/100g)c
| First short-term study: Beef meat-based diets
|
Second short-term study: Casein-based diets
|
Long-term study
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Phos20 | Phos33 | Phos55 | Phos90 | Phos150 | Phos250 | Carb250 | Gluc250 | Carb33 | Carb55 | Carb80 | Carb110 | Carb 155 | Control Diet (Carb33) | Carb100 | |
| B Beef | 60.0 | 60.0 | 60.0 | 60.0 | 60.0 | 60.0 | 60.0 | 60.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Haemoglobin | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.63 | 0.63 | 0.63 | 0.63 | 0.63 | 0.0 | 0.0 |
| Safflower Oil | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 |
| Lard | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 |
| Caseina | 5.9 | 5.9 | 5.9 | 5.9 | 5.9 | 5.9 | 5.9 | 5.9 | 59.0 | 59.0 | 59.0 | 59.0 | 59.0 | 59.0 | 59.0 |
| Corn Starch | 6.0 | 6.0 | 6.0 | 6.0 | 6.0 | 6.0 | 6.0 | 6.0 | 7.0 | 7.0 | 7.0 | 7.0 | 7.0 | 7.0 | 7.0 |
| Sucrose | 12.8 | 12.6 | 12.3 | 11.9 | 11.1 | 9.7 | 10.6 | 2.5 | 16.0 | 15.8 | 15.5 | 15.2 | 14.8 | 16 | 15 |
| Cellulose | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5 | 5 |
| Methionine | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 |
| Mineral mixb | 3.5 | 3.5 | 3.5 | 3.5 | 3.5 | 3.5 | 3.5 | 3.5 | 3.5 | 3.5 | 3.5 | 3.5 | 3.5 | 3.5 | 3.5 |
| Vitamin mixb | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Choline bitartrate | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 |
| Ca. phosphate | 0.2 | 0.4 | 0.7 | 1.1 | 1.9 | 3.3 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 |
| Ca. carbonate | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 2.5 | 0.0 | 0.3 | 0.5 | 0.8 | 1.1 | 1.5 | 0.3 | 1.0 |
| Ca. gluconate | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 10.6 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
Low-calcium casein
AIN76 mix, but 500 g/kg of dibasic calcium phosphate replaced by sucrose in mineral mix
Phos20 to Phos250 are low-calcium beef diet supplemented with calcium phosphate from 20 μmol/g to 250 μmol/g. Carb250 and Gluc 250 are low-calcium beef diet supplemented respectively with calcium carbonate and calcium gluconate at 250 μmol/g. Carb33 to Carb155 are low-calcium haemoglobin diet supplemented with calcium carbonate from 33 μmol/g to 155 μmol/g. Control diet and Carb100 low-calcium haemoglobin diet supplemented with calcium phosphate from 33 μmol/g to 100 μmo
Preparation of Faecal Water
Faecal pellets were collected for 24 h under each cage (one rat/cage, short-term studies ; two rats/cage, long-term study), thus leading to five samples per diet. Freeze-dried faeces were used to calculate dry faecal mass and to prepare faecal water by adding 1 ml of sterilized water to 0.3 g of dry faeces. Samples were then incubated at 37°C for one hour, stirring thoroughly every 20 min, followed by centrifugation at 20 000 g for 10 min. The aqueous phase was re-centrifuged at the same speed and duration and the subsequent supernatant (faecal water) collected and stored at −20°C until use.
TBARS and Haem Assay
Thiobarbituric acid reactive substances (TBARS) were measured in faecal water according to Ohkawa et al. [22], exactly as previously described, and results are given as malondialdehyde (MDA) equivalent. [15]. Haem contents of freeze-dried faeces and of faecal water were measured by fluorescence according to Van den Berg et al. [23] and Sesink et al. [14], respectively, as already described [15]. Both TBARS and heme in faeces were measured on faecal samples from both short-term studies.
In vitro experiment
Ability of five different calcium salts (1- calcium phosphate, 2-calcium chloride, 3-calcium gluconate, 4-calcium carbonate, 5-calcium lactate) to bind and to precipitate haem from bovine haemoglobin solution was studied in vitro. Haemoglobin (Sigma chemical, St.Quentin, France) 0.36 mmol was acidified at pH 1.4 with HCl 2 mol and then heated one hour at 105°C to dissociate haem from haemoglobin. This solution was centrifuged at 20 000 g for 10 min. Supernatant with free haem was collected.
Six concentrations of each of five calcium salts were prepared (25, 50, 75, 100, 125, 150 mmol). According to Sesink [24], one ml haemoglobin added to one ml calcium salt was incubated for 90 min at 37.5°C in the dark and mixed every 10 min. The mixture was centrifuged for 2 min at 10 000 g. Supernatant (100 μl) was assayed for remaining haem after 20-folds dilution in distilled water, by absorbance measure at 400 nm with a Lambda 2 spectrophotometer (PYE Unicam-PU8600 UV/VIS-spectrophotometer Philips). Different haemoglobin concentrations were prepared as standard solutions: 1/1 (100%), 1/5, 1/10, 1/20, and 1/50 in water. The assay was repeated three times and mean values recorded.
ACF Assay
Rats were killed by CO2 asphyxiation in a random order at day 94 of the long-term study. Colons were excised from rats immediately post mortem, flushed with cold Krebs solution (Sigma chemical, St.Quentin, France), opened longitudinally and fixed flat between two sheets of filter paper in 10% formalin (Sigma chemical, St.Quentin, France), marked with a two-digit blinding code. Colons picked up in random order were stained for 6 min in a 0.05% filtered solution of methylene blue [25]. Numbers of ACF per colon, and number of crypts in each ACF, were counted under light microscope at x40 magnification in duplicate by two readers, blinded for the origin of the colon.
Statistical Analysis
Results were analyzed using Systat 10 software for Windows, and reported as mean ± SD. Long-term study data were analyzed by Student’s t test, except ACF data that were analyzed by two-way analysis of variance (ANOVA: dietary group and reader). Short-term study data were considered firstly using one-way ANOVA. If a significant difference was found between groups (P<0.05) then pairwise comparisons were made with fisher LSD test multiple comparison test.
The meta-analysis of the relation between calcium carbonate and colon cancer in carcinogen-injected rats was done as a sub-analyses of a study that we published in 2005 [26]. From this precedent meta-analysis, we selected studies where calcium carbonate had been fed to experimental groups of rats. The studies were homogeneous (Q Cochran P>0.05), we thus used the “Fixed Effects” model, using EasyMA DOS software (2001 version) to compute data.
Results
Weight and food intake during in vivo studies
Final body weight of rats was 107 ± 2 g after 7 d on experimental diets in the first short-term study, without significant differences between groups. Food intake was not significantly different (data not shown), but Gluc250-fed rats tended to eat less than the other groups (Gluc250: 7.2 ± 2.0 g/d, all other groups: 9.1 ± 1.0 g/d, P=0.09). No difference between groups body weight and diet intake was observed in the second short-term study or in the long-term study. Mean body weights were respectively 166 ± 2 g after 14 d, and 201 ± 8 g after 94 d on the experimental diets.
First short-term study: TBARS of Faecal Water
Haem can induce the formation of peroxyl radicals in fats, which may be cytotoxic and cleave DNA in vivo. This might explain the promoting effect of haem on colon carcinogenesis. Lipid peroxidation was thus measured in faecal water by TBARS assay. Calcium can prevent haem-induced promotion of colon carcinogenesis by precipitating haem in the colonic content, thus haem concentration in faecal water was measured.
Haem concentration in faecal water from beef meat fed rats was significantly reduced in rats given 150 and 250 μmol/g calcium phosphate (P<0.01; Figure 1.A), but not in rats given lower levels of calcium phosphate. Dietary calcium carbonate and gluconate 250 μmol/g produced the same effect on haem than calcium phosphate (Figure 1.A). Surprisingly, haem-induced lipid peroxidation measured by TBARS level was not significantly reduced by the calcium phosphate diets, but only the high-calcium carbonate diet decrease TBARS level in faecal water (P<0.01; Figure 1.B).
Figure 1.
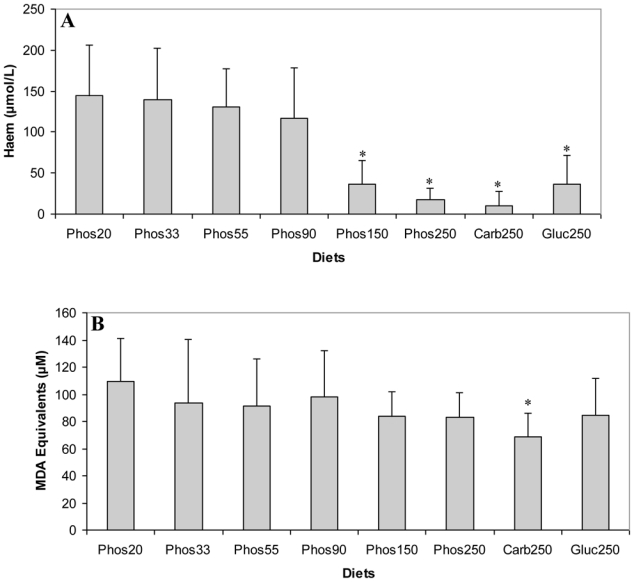
Effect of diets on haem and TBARS in faecal water after the first short term study (A) Haem in faecal water. (B) TBARS (MDA equivalents) in faecal waters as marker for lipid luminal peroxidation. * Significantly different from Phos20 (P<0.01, by fisher LSD test). Data are means, bars are standard deviations (n = 5 in each group).
Note: Phos20 to Phos250 are low-calcium beef diet supplemented with calcium phosphate from 20 μmol/g to 250 μmol/g. Carb250 and Gluc 250 are low-calcium beef diet supplemented respectively with calcium carbonate and calcium gluconate at 250 μmol/g.
In vitro study
Any tested concentration of calcium carbonate higher than 25 mMol was much more effective to precipitate haem than any other tested calcium salt. Proportion of trapped haem was more than three times higher with calcium carbonate than with calcium phosphate (100 mMol) (P<0.001; Figure 2).
Figure 2.
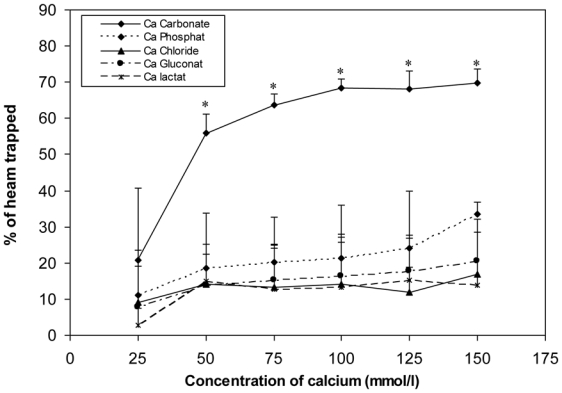
Effect of calcium salts on haem solubility in vitro. Haem (0.36 mM) was incubated with six different concentrations of calcium salts (150, 125, 100, 75, 50, 25 mMol). Data are means, bars are standard deviations (n = 3). * Significantly different from other salts.
Second short-term study: TBARS of Faecal Water
Beef meat was replaced by haemoglobin in the second study diets, since we have demonstrated previously that they produce similar effects on colon carcinogenesis and on faecal water peroxidation [15]. The high-calcium phosphate diet (250μmol/g) did not prevent haem toxicity in the 7-d study, in contrast with our previous 50-d study [17]. We suggest this duration was too short to reduce TBARs level, thus we chose the second short-term study to last for 14-d. As calcium carbonate was the only effective salt against haem-induced lipid peroxidation in vivo and the only effective salt to precipitate haem in vitro, this salt was used to determine the smallest effective calcium dose.
Haem concentration was lower in faecal water from rats given a diet containing at least 80 μmol/g of calcium carbonate (P<0.001; Figure 3.A). Accordingly, diets containing at least 80 μmol/g of calcium carbonate reduced haem-induced lipid peroxidation of faecal water (P<0.001; Figure 3.B).
Figure 3.
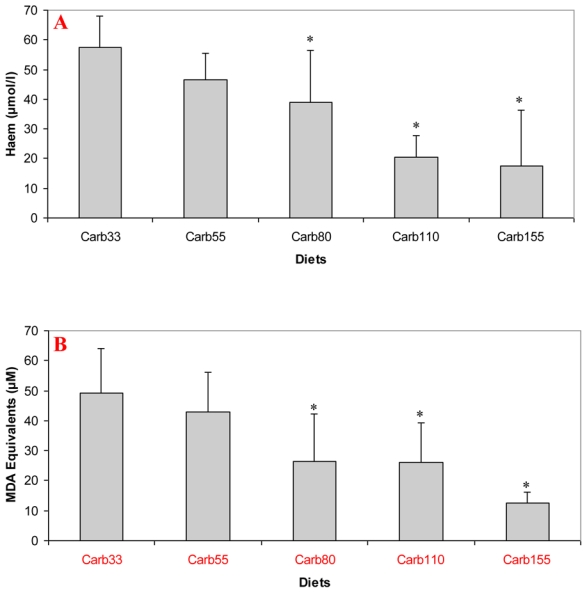
Effect of calcium carbonate-based diets (30 to 155 μmol/g) on faecal water values after the second short term study A. Haem concentration in faecal water (μmol/L) B. TBARS concentration in faecal water, expressed as μmol/L MDA equivalents. * Significantly different from Carb30 by fisher LSD test (P< 0.05). Data are mean, bars are standard deviation (n=5 in each group).
Note: Carb33 to Carb155 are low-calcium haemoglobin diet supplemented with calcium carbonate from 33 μmol/g to 155 μmol/g.
ACF Assay
We chose to test if a diet containing 100 μmol/g calcium carbonate would promote colon carcinogenesis, because the second short-term study showed that a 80 μmol/g calcium carbonate diet reduces faecal TBARS concentration, and that 110 μmol/g seemed twice more potent than 80 μmol/g to bind faecal haem. In contrast with a calcium phosphate diet [16], the consumption of a diet containing 100 μmol/g calcium carbonate for 94 days did not increase significantly the number of ACF per colon, 101 days after a dimethylhydrazine injection (Figure 4).
Figure 4.
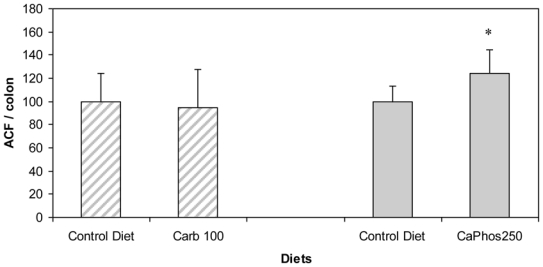
Effect of calcium salts in diet on putative precancerous lesions (ACF) per rat colon 101 d after the injection of dimethylhydrazine. Hatched bars are results of this study with 100 μmol/g of calcium carbonate. Solid bars are previous results with 250 μmol/g of calcium phosphate (Pierre et al 2008 [16]). Results are expressed after normalization of the Control Diet groups to 100. * Significantly different control group (CD).
Note: The Control Diet was a low-calcium haemoglobin diet supplemented with calcium carbonate at 33 μmol/g. Carb100 and CaPhos250 are low-calcium haemoglobin diet supplemented respectively with calcium carbonate (100μmol/g) and calcium gluconate ( 230 μmol/g).
Discussion
These data confirm that dietary calcium reduces faecal water lipoperoxides level, a biomarker linked with haem-induced carcinogenesis promotion. They also establish that the type of calcium salt is paramount in this protection, and that calcium carbonate is effective without the promoting side effect of calcium phosphate.
Red meat promotes ACF and MDF in the colon of carcinogen-induced rats, and promotion by meat is associated with faecal water haem, cytotoxicity and TBARS [15,16]. The mechanism of promotion by haem is not known, and may be linked to lipid peroxidation. We have explored the effect of faecal water from beef-fed rats on normal (Apc +/+) and premalignant colonic cells (Apc Min/+). Results show that Apc mutated cells survive haem-induced faecal lipoperoxides, notably 4-hydroxynonenal (HNE) that is toxic to normal cells. Selection of mutated cells by cytotoxic lipoperoxides may thus explain haem-promotion of colon carcinogenesis [27]. Calcium added on top of beef-meat or haemin diets normalizes the number of ACF and MDF at the same low level as in control group [17]. Calcium phosphate precipitates haem in the gut, and little haem remains available to induce lipid peroxidation [17,24]. The trapping of haem by calcium thus abolishes carcinogenesis promotion. In this study, we established that different calcium salts are able to precipitate haem in vitro and in faecal water of haem-fed rats and by consequence are able to reduce faecal water peroxidation (Figures 1 and 2). We observed that only high concentrations of calcium phosphate were able to precipitate haem (Figure 1A. Furthermore the ability of the three tested salts (phosphate, carbonate and gluconate) to trap haem was very similar in high-calcium diets (Figure 1A). However, only calcium carbonate decreased significantly the level of lipid peroxidation in faecal water (Figure 1B). So, to screen more precisely the efficiency of different calcium salts, we measured the in vitro trapping of haem: Only calcium carbonate precipitated haem significantly. In conclusion, this study shows for the first time a difference of efficiency between calcium salts in vitro (figure 2) and in vivo, in the trapping of haem and the limitation of peroxidation (figure 1B). We have no explanation for the relatively high TBARs level in controls and in rats given high doses of calcium phosphate or gluconate: we speculate the beef meat in first study may have contained already oxidized fat or oxidizing agents different from haem iron (Figure 1B).
Our previous study showed that rats eating a high-calcium control diet had more ACF and more MDF than rats eating a low-calcium control diet [16]. This promotion was observed with calcium phosphate in a high-fat diet context, but was not seen in a low-fat diet context without a clear explanation for the high-fat/low-fat discrepancy [17]. Bull et al. also showed that the promotion of tumours by calcium phosphate was more important in 30%-fat diet-fed rats than in 3%-fat diet-fed rats [28]. Phosphate, not calcium, might be the promoting nutrient, an issue already discussed by Bruce [29]. Since our in vitro study indicated that calcium carbonate was more effective than calcium phosphate to trap haem (Figure 2) and since the first in vivo study indicated that a high concentration of calcium carbonate was able to precipitate haem and to limit peroxidation in vivo, we determined the lower effective dose of calcium carbonate in a second short-term study (Figure 3). We showed that 80 μmol of calcium carbonate/g of diet is enough to trap haem and to reduce peroxidation. The first aim of this study was to identify the lower dose and type of calcium sufficient to limit the effect of haem in the colon without side effects. We thus tested the effect of an addition of 100 μmol of calcium carbonate/g of diet on the promotion of colon carcinogenesis at the level of ACF (Figure 4). In contrast with the promoting effect of calcium phosphate at 250 μmol/g [16], we did not observe a promoting effect of calcium carbonate on ACF. This study shows for the first time that a calcium salt can inhibit the toxic effect of haem in the colon without side effect on colon carcinogenesis promotion.
To confirm that calcium carbonate does not promote carcinogenesis in rats, we made a meta-analysis of published data on calcium carbonate and colon carcinogenesis (Figure 5). Our previous meta-analysis study shows that calcium (all salts) modestly decreases tumour incidence in rats and that some calcium salts are more protective than others [26]: calcium lactate, but not calcium phosphate, reduces chemically-induced carcinogenesis. Figure 5 shows data from a meta-analysis of 11 studies of high calcium carbonate diets on colon carcinogenesis in chemically-initiated rats; the combined relative risk from all of these studies was 0.983 (95% confidence interval: 0.902; 1.071, p = 0.69), compared with low calcium controls. This meta-analysis confirms the lack of promoting side-effect by calcium carbonate on colon carcinogenesis. These results on rodents are coherent with two randomized, double-blinded, placebo-controlled trials. Calcium carbonate supplementation was associated with a significant - though moderate - reduction in the risk of recurrent colorectal adenomas in the Calcium Polyp Prevention Study [30]. In contrast, daily supplementation of calcium carbonate (with vitamin D) for seven years to 36,282 postmenopausal women from 40 Women’s Health Initiative centres had no effect on the incidence of colorectal cancer among postmenopausal women [31]. Altogether, these results suggest that calcium carbonate supplementation to neutralize haem does not bear any risk. However, chelation of dietary heme iron bears the theoretical risk of iron deficiency leading to anaemia: recommendations should take this in account.
Figure 5.
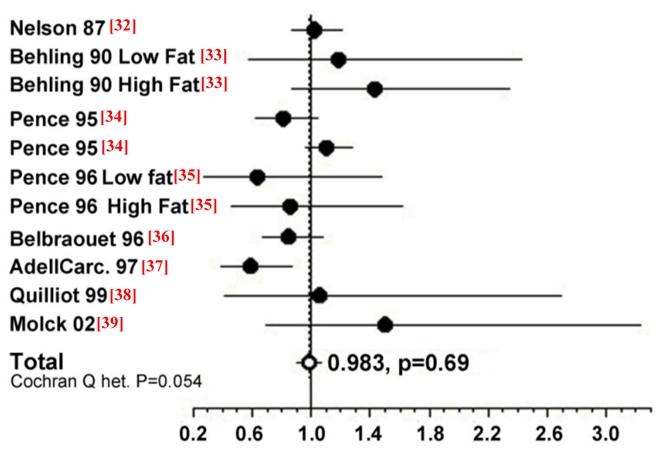
Meta-analysis of the relation between calcium carbonate and colon cancer in carcinogen-injected rats. The common relative risk with 95% confidence was calculated from 11 studies.*
* Solid black circles correspond to the study specific relative risk. The open white circle represents the common relative risk. Horizontal lines are 95% confidence intervals. References are indicated between brackets
The study also supports the concept that nutrients like calcium may inhibit the toxicity associated with the excess of another nutrient like haem. This concept differs from the classical chemoprevention concept, since calcium carbonate does not reduce carcinogenesis in rats that are not given dietary haem. This specific calcium preventive effect, by interaction, should be looked for in a cohort study by crossing haem and calcium intake with adenoma or cancer risk.
Acknowledgments
This study was supported in part by the INRA, the DGER and the French region Midi-Pyrénées. We thank Xavier Blanc (UPAE) for the preparation of experimental diets, Raymond Gazel and Florence Blas Y Estrada for the care of the animals.
Abbreviations
- ACF
aberrant crypt foci
- MDF
mucin-depleted foci
- TBARS
thiobarbituric acid reactive substances
References
- 1.Larsson SC, Wolk A. Meat consumption and risk of colorectal cancer: a meta-analysis of prospective studies. Int J Cancer. 2006;119:2657–2664. doi: 10.1002/ijc.22170. [DOI] [PubMed] [Google Scholar]
- 2.Norat T, Lukanova A, Ferrari P, Riboli E. Meat consumption and colorectal cancer risk: dose-response meta-analysis of epidemiological studies. International Journal of Cancer. 2002;98:241–256. doi: 10.1002/ijc.10126. [DOI] [PubMed] [Google Scholar]
- 3.WCRF, WCRF. Food, nutrition, physical activity, and the prevention of cancer: a global perspective. WCRF and American Institute for Cancer Research; Washington DC: 2007. pp. 1–537. [Google Scholar]
- 4.Sugimura T, Wakabayashi K, Nakagama H, Nagao M. Heterocyclic amines: Mutagens/carcinogens produced during cooking of meat and fish. Cancer Sci. 2004;95:290–9. doi: 10.1111/j.1349-7006.2004.tb03205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sinha R, Rothman N, Brown ED, Mark SD, Hoover RN, Caporaso NE, Levander OA, Knize MG, Lang NP, Kadlubar FF. Pan-fried meat containing high levels of heterocyclic aromatic amines but low levels of polycyclic aromatic hydrocarbons induces cytochrome p4501a2 activity in humans. Cancer Research. 1994;54:6154–6159. [PubMed] [Google Scholar]
- 6.Stavric B. Biological significance of trace levels of mutagenic heterocyclic aromatic amines in human diet: a critical review. Food and Chemical Toxicology. 1994;32:977–994. doi: 10.1016/0278-6915(94)90093-0. [DOI] [PubMed] [Google Scholar]
- 7.Bingham SA, Pignatelli B, Pollock JRA, Ellul A, Malaveille C, et al. Does increased endogenous formation of N-nitroso compounds in the human colon explain the association between red meat and colon cancer? Carcinogenesis. 1996;17:515–523. doi: 10.1093/carcin/17.3.515. [DOI] [PubMed] [Google Scholar]
- 8.Cross AJ, Pollock JRA, et al. Haem, not protein or inorganic iron, is responsible for endogenous intestinal n-nitrosation arising from red meat. Cancer Research. 2003;63:2358–2360. [PubMed] [Google Scholar]
- 9.Lunn JC, Kuhnle G, Mai V, Frankenfeld C, Shuker DE, Glen RC, Goodman JM, Pollock JR, Bingham SA. The effect of haem in red and processed meat on the endogenous formation of N-nitroso compounds in the upper gastrointestinal tract. Carcinogenesis. 2007;28:685–690. doi: 10.1093/carcin/bgl192. [DOI] [PubMed] [Google Scholar]
- 10.Santarelli RL, Vendeuvre JL, Naud N, Taché S, Guéraud F, Viau M, Genot C, Corpet DE, Pierre FHF. Meat processing and colon carcinogenesis: Cooked, nitrite-treated and oxidized high-heme cured meat promotes mucin depleted foci in rats. Cancer Prevention research. 2009;3:852–64. doi: 10.1158/1940-6207.CAPR-09-0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parnaud G, Pignatelli B, Peiffer G, Tache S, Corpet DE. Endogenous N-nitroso compounds, and their precursors, present in bacon, do not initiate or promote aberrant crypt foci in the colon of rats. Nutrition and Cancer. 2000;38:74–80. doi: 10.1207/S15327914NC381_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee DH, Anderson KE, Harnack LJ, Folsom AR, Jacobs DR., Jr Heme iron, zinc, alcohol consumption, and colon cancer: Iowa Women’s Health Study. J Natl Cancer Inst. 2004;96:403–7. doi: 10.1093/jnci/djh047. [DOI] [PubMed] [Google Scholar]
- 13.Larsson SC, Rafter J, Holmberg L, Bergkvist L, Wolk A. Red meat consumption and risk of cancers of the proximal colon, distal colon and rectum: the Swedish Mammography Cohort. Int J Cancer. 2005;113:829–34. doi: 10.1002/ijc.20658. [DOI] [PubMed] [Google Scholar]
- 14.Sesink ALA, Termont DSML, Kleibeuker JH, Vandermeer R. Red meat and colon cancer: the cytotoxic and hyperproliferative effects of dietary heme. Cancer Research. 1999;59:5704–5709. [PubMed] [Google Scholar]
- 15.Pierre F, Freeman A, Tache S, Van der Meer R, Corpet DE. Beef meat and blood sausage promote the formation of azoxymethane-induced mucin-depleted foci and aberrant crypt foci in rat colons. Journal of Nutrition. 2004;134:2711–2716. doi: 10.1093/jn/134.10.2711. [DOI] [PubMed] [Google Scholar]
- 16.Pierre F, Santarelli R, Tache S, Gueraud F, Corpet DE. Beef meat promotion of dimethylhydrazine-induced colorectal carcinogenesis biomarkers is suppressed by dietary calcium. Br J Nutr. 2008;99:1000–6. doi: 10.1017/S0007114507843558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pierre F, Tache S, Petit CR, Van der Meer R, Corpet DE. Meat and cancer: haemoglobin and haemin in a low-calcium diet promote colorectal carcinogenesis at the aberrant crypt stage in rats. Carcinogenesis. 2003;24:1683–90. doi: 10.1093/carcin/bgg130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pierre F, Peiro G, Tache S, Cross AJ, Bingham SA, Gasc N, Gottardi G, Corpet DE, Gueraud F. New marker of colon cancer risk associated with heme intake: 1,4-dihydroxynonane mercapturic Acid. Cancer Epidemiol Biomarkers Prev. 2006;15:2274–9. doi: 10.1158/1055-9965.EPI-06-0085. [DOI] [PubMed] [Google Scholar]
- 19.Parnaud G, Peiffer G, Tache S, Corpet DE. Effect of meat (beef, chicken, and bacon) on rat colon carcinogenesis. Nutrition and Cancer. 1998;32:165–173. doi: 10.1080/01635589809514736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karkare MR, Clark TD, Glauert HP. Effect of Dietary Calcium on Colon Carcinogenesis Induced by a Single Injection of 1,2-Dimethylhydrazine in Rats. Journal of Nutrition. 1991;121:568–577. doi: 10.1093/jn/121.4.568. [DOI] [PubMed] [Google Scholar]
- 21.American Institute of Nutrition. Report of the American Institute of Nutrition Ad Hoc Committee on standards for nutritional studies. J Nutr. 1977;107:1340–1348. doi: 10.1093/jn/107.7.1340. [DOI] [PubMed] [Google Scholar]
- 22.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Analytical Biochemistry. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 23.Van den Berg JW, Koole-Lesuis R, Edixhoven-Bosdijk A, Brouwers N. Automating the quantification of heme in feces. Clinical Chemistry. 1988;34:2125–2126. [PubMed] [Google Scholar]
- 24.Sesink ALA, Termont DSML, Kleibeuker JH, VanDerMeer R. Red meat and colon cancer: dietary haem-induced colonic cytotoxicity and epithelial hyperproliferation are inhibited by calcium. Carcinogenesis. 2001;22:1653–1659. doi: 10.1093/carcin/22.10.1653. [DOI] [PubMed] [Google Scholar]
- 25.Bird RP. Observation and quantification of aberrant crypts in murine colon treated with a colon carcinogen: preliminary findings. Cancer Lett. 1987;37:147–151. doi: 10.1016/0304-3835(87)90157-1. [DOI] [PubMed] [Google Scholar]
- 26.Corpet DE, Pierre F. How good are rodent models of carcinogenesis in predicting efficacy in humans? A systematic review and meta-analysis of colon chemoprevention in rats, mice and men. Eur J Cancer. 2005;41:1911–22. doi: 10.1016/j.ejca.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 27.Pierre F, Tache S, Gueraud F, Rerole AL, Jourdan ML, Petit C. Apc mutation induces resistance of colonic cells to lipoperoxide-triggered apoptosis induced by faecal water from haem-fed rats. Carcinogenesis. 2007;28:321–7. doi: 10.1093/carcin/bgl127. [DOI] [PubMed] [Google Scholar]
- 28.Bull A, Bird RP, Bruce WR, Nigro N, Medline A. Effect of calcium on azoxymethane induced intestinal tumors in rats. Gastroenterology. 1987;92:1332. [Google Scholar]
- 29.Bruce WR, Giacca A, Medline A. Possible mechanisms relating diet to colorectal cancer risk. Europ. Conf. Nutr. Cancer. IARC; June 21–24, 2001; Lyon France. 2001. pp. 1–7. [Google Scholar]
- 30.Baron JA, Beach M, Mandel JS, Vanstolk RU, Haile RW, et al. Calcium supplements for the prevention of colorectal adenomas. New England Journal of Medicine. 1999;340:101–107. doi: 10.1056/NEJM199901143400204. [DOI] [PubMed] [Google Scholar]
- 31.Wactawski-Wende J, Kotchen JM, Anderson GL, Assaf, et al. Calcium plus vitamin D supplementation and the risk of colorectal cancer. N Engl J Med. 2006;354:684–96. doi: 10.1056/NEJMoa055222. [DOI] [PubMed] [Google Scholar]
- 32.Nelson RL, Tanure JC, Andrianopoulos G. The effect of dietary milk and calcium on experimental colorectal carcinogenesis. Dis Colon Rect. 1987;30:947–949. doi: 10.1007/BF02554282. [DOI] [PubMed] [Google Scholar]
- 33.Behling AR, Kaup SM, Choquette LL, et al. Lipid absorption and intestinal tumour incidence in rats fed on varying levels of calcium and butterfat. Br J Nutr. 1990;64:505–513. doi: 10.1079/bjn19900050. [DOI] [PubMed] [Google Scholar]
- 34.Pence BC, Dunn DM, Zhao C, et al. Chemopreventive effects of calcium but not aspirin supplementation in cholic acid-promoted colon carcinogenesis: correlation with intermediate endpoints. Carcinogenesis. 1995;16:757–765. doi: 10.1093/carcin/16.4.757. [DOI] [PubMed] [Google Scholar]
- 35.Pence BC, Dunn DM, Zhao C, et al. Protective effects of calcium from non fat dried milk against colon carcinogenesis in rats. Nutr Cancer. 1996;25:35–45. doi: 10.1080/01635589609514426. [DOI] [PubMed] [Google Scholar]
- 36.Belbraouet S, et al. Dietary calcium salts as protective agents and laminin P1 as a biochemical marker in chemically induced colon carcinogenesis in rats. Cancer Detect Prev. 1996;20:294–299. [PubMed] [Google Scholar]
- 37.Adell-Carceller R, Segarra-Soria M, et al. Inhibitory effect of calcium on carcinogenesis at the site of colonic anastomosis: an experimental study. Dis Colon Rect. 1997;40:1376–1381. doi: 10.1007/BF02050826. [DOI] [PubMed] [Google Scholar]
- 38.Quilliot D, Belbraouet S, Pelletier X, et al. Influence of a high-calcium carbonate diet on the incidence of experimental colon cancer in rats. Nutr Cancer. 1999;34:213–219. doi: 10.1207/S15327914NC3402_13. [DOI] [PubMed] [Google Scholar]
- 39.Molck AM, Poulsen M, Meyer O. The combination of 1alpha,25(OH2)-vitamin D3, calcium and acetylsalicylic acid affects azoxymethane-induced aberrant crypt foci and colorectal tumours in rats. Cancer Lett. 2002;186:19–28. doi: 10.1016/s0304-3835(02)00285-9. [DOI] [PubMed] [Google Scholar]


