Abstract
Here, the literature was reviewed to evaluate whether a population of mesenchymal stromal cells derived from Wharton’s jelly cells (WJCs) is a primitive stromal population. A clear case can be made for WJCs as a stromal population since they display the characteristics of MSCs as defined by the International Society for Cellular Therapy; for example, they grow as adherent cells with mesenchymal morphology, they are self-renewing, they express cell surface markers displayed by MSCs, and they may be differentiated into bone, cartilage, adipose, muscle, and neural cells. Like other stromal cells, WJCs support the expansion of other stem cells, such as hematopoietic stem cells, are well-tolerated by the immune system, and they have the ability to home to tumors. In contrast to bone marrow MSCs, WJCs have greater expansion capability, faster growth in vitro, and may synthesize different cytokines. WJCs are therapeutic in several different pre-clinical animal models of human disease such as neurodegenerative disease, cancer, heart disease, etc. The preclinical work suggests that the WJCs are therapeutic via trophic rescue and immune modulation. In summary, WJCs meet the definition of MSCs. Since WJCs expand faster and to a greater extent than adult-derived MSCs, these findings suggest that WJCs are a primitive stromal cell population with therapeutic potential. Further work is needed to determine whether WJCs engraft long-term and display self-renewal and multipotency in vivo and, as such, demonstrate whether Wharton’s jelly cells are a true stem cell population.
Keywords: Mesenchymal stromal cells, Perinatal cells, Discarded tissue, Stromal cells
Introduction
Stroma, derived from the Greek word for bed, is defined as: “The framework, usually consisting of connective tissue, of an organ, gland, or other structure, as distinguished from the parenchyma of specific substance of the part …” [1]. The stroma and stromal cells contain cues and signals driving the differentiation and maturation of hematopoietic cells and provide physical support for them [2]. To the physiologist, stromal cells function as supportive cells both physically, such as by providing a three-dimensional scaffold consisting of collagen(s), fibroblasts, vascular cells, and immune cells, and nutritively, such as by providing blood supply (endothelial cells and blood vessels), growth factors, and chemokines. To the hematologist, bone marrow is an enriched source of both hematopoietic stem cells (HSCs; or blood forming cells) and mesenchymal stromal cells (MSCs; or stroma-forming cells).
More than forty years ago, Friedenstein showed that cells isolated from the stroma of bone marrow were capable of osteogenesis [3]. Bone-marrow-derived MSCs (BMSCs) have been functionally defined as non-hematopoietic, multipotential cells that support HSC expansion in vitro and can differentiate into cells of various connective tissues. Within the scientific literature, the acronym MSC has been used to represent (bone) marrow stromal cells, mesenchymal stem cells, and multipotent mesenchymal stromal cells, and this causes confusion. The International Society for Cellular Therapy (ISCT) differentiates between MSCs and mesenchymal stem cells based upon in vivo characterization; for example, mesenchymal stem cells undergo self-renewal and multipotential differentiation following engraftment. Currently, there is not a consensus marker set that permits prospective identification of mesenchymal stem cells from the in vitro MSC population. The matter is further complicated because these stem cells have poorly characterized growth conditions, such as low glucose DMEM containing fairly high concentrations of fetal bovine serum (FBS, 10–20%) and because not all lots of FBS are equal in terms of their ability to maintain MSCs. The problems identifying mesenchymal stem cells are likely due to differences between individuals (e.g., the donor’s age), limited expansion capability of MSCs in vitro, changes in potency and phenotype related to in vitro expansion, or the inability to identify definitive surface markers that discriminates the stem cells in a bone marrow isolate.
Regarding the latter, four markers were recently suggested. First, the neural ganglioside GD2 and the key enzyme involved in its synthesis, GD2 synthase, were shown to distinguish MSCs from all other cells within marrow [4]. Second, the early embryonic antigen SSEA-4 was reported to identify an adult mesenchymal stem cell population [5]. Third, recently, several markers of mesenchymal stem cells have been suggested, and these markers are not yet widely available for evaluation [6, 7]. Finally, nucleostemin was reported to identify undifferentiated bone marrow stromal cells [8]. Further work is needed to confirm whether a single population is labeled by all these markers and to determine whether those cells are stem or stromal cells.
Immunophenotype of Mesenchymal Stromal Cells
In 2006, The ISCT convened a working group to discuss immunophenotypic analysis MSCs. This group defined bone-marrow-derived MSCs, in a clear, minimalist fashion, as a plastic-adherent cell population isolated from the bone marrow cavity with the following surface markers: CD13, CD44, CD90, CD73, CD105+, CD14, CD11b, CD79, CD 34, CD45 and HLA-DR− [9].
MSCs Are Multipotent Cells
MSCs derived from bone marrow and from fat self-renew and differentiate into specialized cells in vitro. MSCs have been shown to differentiate into bone [10–12], muscle [13], adipose tissue [14], cartilage [12] and tendon [15]. There is evidence that MSCs differentiate into neural cells, such as neurons and glial cells. However, these cells possess many, but not all, of the properties of mature neurons [16].
MSC and Stromal Support of the Stem Cell Niche
MSCs provide a supportive role and a microenvironment that enables engraftment/culturing of HSCs. For example, MSCs support HSC expansion ex vivo (most likely via release of diffusible factors) [17, 18], and MSCs support HSC engraftment in vivo when co-grafted [19, 20]. Nilsson et al. suggested that hyaluronic acid (HA) may be an important factor for the HSC niche [21].
Defining Primitive Stromal Cells: Differences Between Fetal and Adult MSCs
MSCs may be harvested from bone marrow of the human fetus or from adults. Although the literature is sparse, several differences between fetal MSCs and adult MSCs are noted. First, fetal MSCs appear to have greater expansion capacity in vitro and faster doubling time than adult MSCs, which may be due to their having longer telomeres than adult MSCs [22, 23]. Second, fetal MSCs appear to lack some of the immune suppression properties observed in adult MSCs [24]. Third, fetal MSCs appear to lack class II human leukocyte antigens (HLA), in contrast to adult MSCs [25]. Similarly, fetal MSCs appear to synthesize HLA-G, which is absent in adult MSCs [24]. Fourth, fetal MSCs express a slightly different cytokine profile than adult MSCs. In summary, primitive MSCs have a greater ability to expand in culture, perhaps due to their relative youth, and have a different physiology that is likely due to their naïve status. These differences are similar to those observed between umbilical cord blood and adult peripheral blood.
Umbilical Cord MSCs
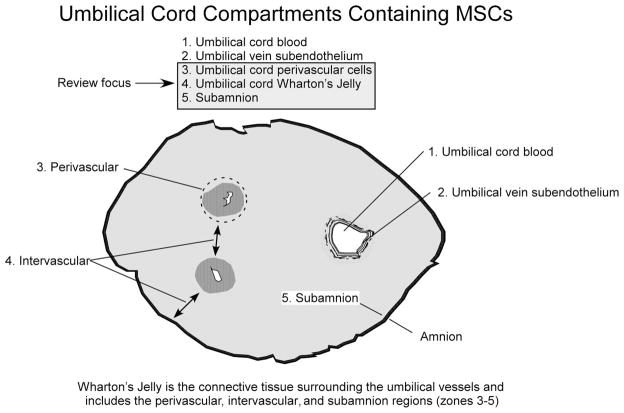
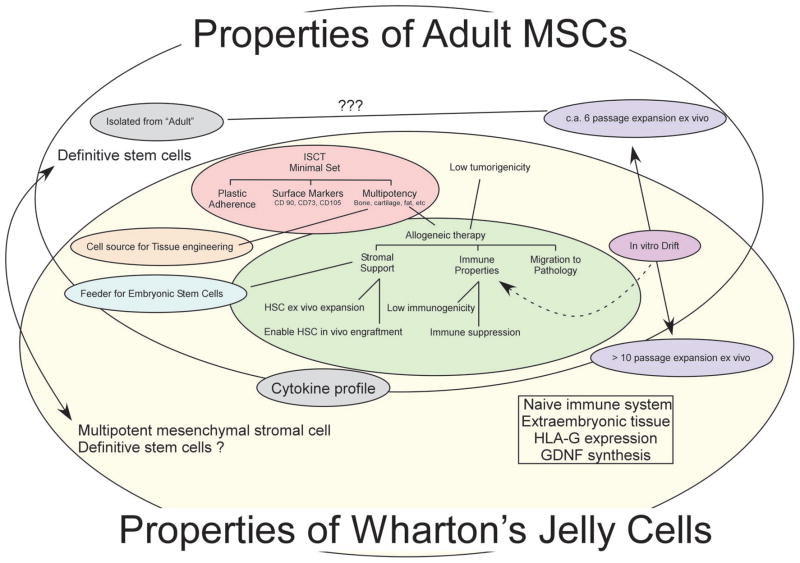
MSCs have been isolated from several compartments of the umbilical cord (Fig. 1 and Table 1). Specifically, the MSCs have been isolated from umbilical cord blood, umbilical vein subendothelium, and the Wharton’s jelly (Fig. 1). Within Wharton’s jelly, MSCs have been isolated from three relatively indistinct regions: the perivascular zone, the intervascular zone, and the subamnion. It is unknown whether MSCs isolated from the different compartments of the umbilical cord represent different populations [26]. This discussion is confined to the MSCs isolated from Wharton’s jelly cells (WJCs; zones 3–5 in Fig. 1). WJCs display MSC surface markers, suggesting that they are of the MSC family. A broad comparison of adult-derived MSCs and those from the umbilical cord is shown in Figure 2.
Figure 1.
Compartments within the umbilical cord. Five separate regions have been shown to contain mesenchymal stromal cells: (1) MSCs can be isolated from 20–50% of freshly prepared mononuclear cell fractions from umbilical cord blood; (2) MSCs have been isolated from umbilical vein subendothelial layer; (3) MSCs can be isolated following enzymatic digestion of the outer layers of umbilical vessels, for example, the perivascular region; (4) intravascular space consistently produces MSCs in healthy individuals; (5) the subamnion region. Wharton’s jelly includes zones 3 through 5. This review focuses on Wharton’s jelly-derived cells and not on MSCs derived from umbilical cord blood (zone 1) or umbilical vein subendothelium (zone 2).
Table 1.
MSCs in umbilical cord and cord blood
| Zone | Designation | Acronym | Reference | Differentiation potential in vitro | Differentiation in vivo | Cells supported by UCMS | Trophic effect in vivo |
|---|---|---|---|---|---|---|---|
| 1 | Umbilical cord blood-derived multilineage progenitor cells | MLPC | Berger MJ, et al., 2006 | Endodermal differentiation, specifically respiratory epithelium type II alveolar cells | |||
| 1 | Mesenchymal stem cell-like cells | MSC-like cells | Bieback K, et al., 2004 | A (weak), B, C | |||
| 1 | Mesenchymal-like cells | MLCs | Erices A, et al., 2000 | A, B | |||
| 1 | Umbilical cord blood-derived mesenchymal stem cells | UCB-derived MSCs | Gang EJ, et al., 2004 | SM | |||
| 1 | Umbilical cord blood-derived mesenchymal stem cells | UCB-derived MSCs | Gang EJ, et al., 2006 | Endothelial markers | Expanded CD34+ cells in vitro | ||
| 1 | Umbilical cord blood-derived stromal cell | Gao L, et al., 2006 | |||||
| 1 | Non-hematopoietic progenitors | NHPs | Goodwin HS, et al., 2001 | A (weak), B, N | |||
| 1 | Hong SH, et al., 2005 | ||||||
| 1 | MSC from umbilical cord blood | MSCs | Hou L, et al., 2003 | N | |||
| 1 | Umbilical cord blood-derived mesenchymal stem cells | UCB-derived MSCs | Jang YK, et al., 2006 | A, B, C | Expanded total and CD34+ cells in vitro | ||
| 1 | Human umbilical cord blood-derived mesenchymal stem cells | MSCs | Jeong JA, et al., 2005 | ||||
| 1 | Kang XQ, et al., 2006 | ||||||
| 1 | AC133-CD14+ umbilical cord blood-derived cells | AC133-CD14+ cells | Kim SY, et al., 2005 | Endothelial markers | |||
| 1 | Umbilical cord blood-derived mesenchymal stem cells | UCB-MSCs | Kern S, et al., 2006 | A (could not differentiate to A), B, C | |||
| 1 | Unrestricted somatic stem cells from human cord blood | USSC | Kogler G, et al., 2004 | A, B, C, N | B, C, hematopoietic, cardiac tissue, liver cells, neural cells | ||
| 1 | Multipotent mesenchymal stem cells from umbilical cord blood | Lee OK, et al., 2004 | A, B, C, N, hepatogenic differentiation | ||||
| 1 | Umbilical cord blood-derived mesenchymal stem cells | UCB-MSCs | Lu FZ, et al., 2005 | A, B | |||
| 1 | Cord blood derived-mesenchymal stem cells | CB-MSC | Wagner W, et al., 2005 | A (reduced/absent), B, C | |||
| 1 | Human mesenchymal stem/progenitor cell | Wang J-F, et al., 2004 | C | Expanded CD34+ cells in vitro | |||
| 1 | Human mesenchymal stem/progenitor cell | MSPC | Yang S-E, et al., 2004 | ||||
| 1 | Human umbilical cord blood stromal cells | HUCB-derived adherent layer cultures | Ye ZQ, et al., 1994 | Reduced or lacked adipocytes | Expanded total MNCs in vitro | ||
| 1 | Fetal blood derived mesenchymal stem cells | - | Yu M, et al., 2004 | A (reduced), B, N | |||
|
| |||||||
| 2 | Umbilical vein mesenchymal stem cells | Covas DT, et al., 2003 | A, B | ||||
| 2 | Mesenchymal progenitor or stem cells, fibroblastic and endothelial cells isolated | Kim JW, et al., 2004 | A, B | ||||
| 2 | Umbilical cord-mesenchymal stem cells | UC-MSCs | Panepucci RA, et al., 2004 | A, B, C | |||
| 2 | Mesenchymal stem cell-like cells | MSC-like cells | Romanov YA, et al., 2003 | A, B | |||
| 2 | Umbilical fibroblast-like cells | Yarygin KN, et al, 2006 | Endothelial precursors | 2 wk survival no disorders noted | Hematopoietic stem cells from cord blood | ||
|
| |||||||
| 3 | Human umbilical cord perivascular cells | HUCPVCs | Baksh K, et al., 2007 | A, B, C | |||
| 3 | Human umbilical cord perivascular cells | HUCPV cells | Sarugaser R, et al., 2005 | B | |||
|
| |||||||
| 4 | Human umbilical cord matrix cells | HUCM | Bailey MM, et al., 2007 | Cartilage | |||
| 4 | CD105+/CD31−/KDR− cells | None given | Conconi MT, et al., 2006 | A, B, SM | SM for 14 d | ||
| 4 | Umbilical cord mesenchymal cells | None given | Fu Y-S, et al., 2004 | Neuron-like and glia | |||
| 4 | Human umbilical mesenchymal stem cells | HUMSCs | Fu Y-S, et al., 2006 | N (dopaminergic, noradrenergic, GABAergic marker expression) | N (TH+, DBH+, GD+ staining 20 wks survival) | Behavioral recovery in PD model | |
| 4 | Rat umbilical cord matrix cells | RUCMs | Jomura, S, et al., 2006 | Rescue of CA1 neurons following stroke | |||
| 4 | Umbilical cord cells | UCC | Kadner A et al., 2002 | ||||
| 4 | Whole umbilical cord | WUCC | Kadner A et al., 2004 | ||||
| 4 | Human umbilical cord stroma cell, intervascular cells (IVCs) vs perivascular cells (PVCs) | HUCSCs | Karahuseyinoglu, S, et al., 2006 | A (reduced), B, C, N | |||
| 4 | Mesenchymal stem cells derived from umbilical cord | UC-MSC | Lu L-L, et al, 2006 | A, B, N | Expanded allogeneic cord blood CD34+ cells in vitro | ||
| 4 | Human umbilical cord-derived cells | hUTC | Lund RD, et al., 2007 | Rescue of retinal photoreceptor cells | |||
| 4 | Wharton’s Jelly mesenchymal stem cells | Ma L, et al., 2005 | N | ||||
| 4 | Pig umbilical cord matrix’ stem cells | UCM cells | Medicetty S, et al., 2004 | N (TH+ cells), brain engraftment 8 wk survival | |||
| 4 | Wharton’s jelly cells | Mitchell K, et al., 2003 | N (neuronal and glial markers) | ||||
| 4 | Umbilical cord matrix stem cells | UCMSCs | Rachakatla RS, et al., 2007 | Homing to lung cancer tumors | |||
| 4 | Bovine umbilical cord-derived fibroblasts | Saito S, et al., 2003 | Supported equine ES- like cells | ||||
| 4 | Wharton’s Jelly-derived myofibroblast cells | WMFs | Schmidt D, et al., 2006 | Subendothelial marker, collagen | |||
| 4 | Mesenchymal cells in Wharton’s jelly | Wang H-S, et al., 2004 | A (varying degrees), B, C, cardio (markers) | ||||
| 4 | Umbilical cord matrix cells | UCM cells | Weiss ML, et al., 2003 | N (neuronal markers) | N (neural markers), engraftment in kidney and muscle 3wk survival | ||
| 4 | Umbilical matrix stem cells | UCMS cells | Weiss ML, et al., 2006 | N (neuronal markers) | Rescue of SN neurons in PD model | ||
| 4 | Umbilical cord-derived stem cells | UCDS | Wu KH, et al., 2007 | A (variable), B, endothelial cells | Endothelial cells 4 wk survival (CD34+ cells) | Supported revascularization of ischaemic limb | |
|
| |||||||
| 5 | Coppi P, et al., 2007 | A, B, SM, N, endothelial and hepatic markers | Brain engraftment 8-week survival, hepatic engraftment with marker expression, bone engraftment with calcification | ||||
| 5 | Chiavegato A, et al., 2007 | ||||||
Abbreviations: A, adipose; B, bone; C, cartilage; Cardio, cardiac muscle; H, hematopoietic stem cells; N, neural endothelial markers; SM, skeletal muscle.
Figure 2.
Venn diagram illustrating common and differing properties between adult mesenchymal stromal cells (MSCs; gray oval) and Wharton’s jelly-derived cells (WJCs, umbilical cord matrix cells; yellow oval). Adult MSCs are defined by International Society for Cellular Therapy working group as cells that are plastic adherent, possess specific surface markers, and are capable of differentiating into multiple mesenchymal lineages, (e.g., bone, cartilage, muscle, tendon, adipose, etc.). As indicated by the overlap, the WJCs share these properties. Similarly, as discussed within, stromal support, specific immune properties of low immunogenicity and immune suppression, and the ability to migrate to pathology are taken to be properties of adult MSCs. These properties are observed in WJCs. Also indicated, adult MSCs have limited expansion capability in vitro before their multipotency is compromised. In contrast, Wharton’s jelly cells can be expanded >15 passages. WJCs, like MSCs from other sources, can serve as feeders for embryonic-like stem cells and for hematopoietic stem cells. In contrast to adult MSCs, WJCs may be derived from extra-embryonic tissue; this may explain why those cells express human leukocyte antigen-G (HLA-G) isoform and glial derived neurotrophic factor (GDNF). Abbreviation: ???, unknown.
WJCs have stromal support properties. For example, extra-embryonic mesenchyme, that is, primitive Wharton’s jelly, surrounds the migrating embryonic blood island cells during their migration to the aorta-gonad mesonephros (AGM) from the yolk sac region prior to day E10.5 [27]. WJCs retain this property as demonstrated by their role in ex vivo hematopoietic expansion [28] and in vivo engraftment of HSCs [29]. Lu and colleagues reported that WJCs produced cytokines similar to those of BMSCs and that WJCs synthesized granulocyte macrophage colony stimulating factors (GM-CSF) and granulocyte colony stimulating factors (G-CSF) that BMSCs did not [28]. WJCs differ from BMSCs because WJCs are slower to differentiate to adipocytes [26, 28]. Since this and other features (listed below) are shared with MSCs derived from umbilical cord blood (UCB), it is unclear whether the MSCs derived from UCB differ from those found in Wharton’s jelly (Table 1).
UCB-MSCs and WJCs have several common properties, such as poor ability to differentiate to adipocytes [26, 30, 31], shorter doubling times than BMSCs, and greater numbers of passages to senescence [26, 30–33]. Like WJCs, UCB-MSCs may make GM-CSF [34], although this has not been consistently found [35, 36]. It is clear that HSCs can be expanded by MSCs from both UCB and WJC [28, 34–37].
There are differences between BMSCs, UCB-MSCs and WJCs. First, the isolation frequency of colony forming units (CFU)-F from bone marrow is estimated to be in the range of 1–10 CFU-F per 106 mononuclear cells (MNCs) and in umbilical cord blood is reported to be around one CFU-F clone per 108 MNCs [30] to 1–3 CFU-F per 106 MNCs [35, 38]. [Note: The isolation frequency from first semester fetal blood-derived MSCs was 8.2 CFU-F per 106 MNCs [22]]. In contrast, cells derived from Wharton’s jelly have a higher frequency of CFU-F [26, 39]. Thus, an order of magnitude more of MSCs than of bone marrow or umbilical cord blood may be found in the initial isolation from WJ. Second, coupled with the greater CFU-F frequency, the doubling time of WJCs and UCB-MSCs is shorter than adult bone marrow-derived MSC (BMSC)s [26, 32, 33]. Faster doubling time is a common feature for MSCs derived from fetal blood [22], cord blood, and Wharton’s jelly, and this common feature is thought to reflect the relatively primitive nature of these MSCs compared to adult BMSCs. An important difference between UCB-MSCs and WJCs is that WJCs can be isolated from close to 100% of the samples, even from umbilical cords that are delayed in their processing up to 48-hour [40]. UCB-MSCs have been more difficult to isolate, and with optimized procedures, the success rate has reached 63% for optimal samples [30].
Furthermore, osteopontin has been shown to be a major component of the hematopoietic stem cell niche and a regulator of hematopoietic progenitor cells [41], and WJCs express the osteopontin gene [40]. As mentioned above, HA is also important in the HSC niche. WJCs are a likely source of HA, since Wharton’s jelly is rich in HA [42]; in fact, it is often a source of commercially available HA.
The role of embryonic fibroblasts to support embryonic stem cells is well-established. WJCs appear to support ESCs and ESC-like cells. Specifically, equine ESC-like cells may be derived and maintained using bovine fibroblasts isolated from Wharton’s jelly as a feeder layer [43]. Histologically, cells in Wharton’s jelly are fibroblastic in appearance [26]; this suggests that Saito’s lab was isolating WJCs [43]. Similarly, human ESCs may be maintained using human WJCs in co-culture [44] or by using conditioned medium from human WJCs (A. Toujmade and J. Auerbach, unpublished observations). Further evidence for the supportive function of WJCs for primitive stem cell populations is suggested by (a) their proximity during embryonic germ cell migration and (b) the extracellular release of glial-derived neurotrophic factor (GDNF), an important factor in maintaining spermatogonial stem cells in the undifferentiated state [45] by WJCs [40].
Characterization of Umbilical Cord Matrix Stem Cells
WJCs are not derived from umbilical blood but from the cushioning matrix between the umbilical blood vessels [46]. Previously, we termed these cells “umbilical cord matrix stem cells” to distinguish them from cells isolated from other umbilical vein endothelial cells or umbilical cord blood. The WJCs meet the criteria for stem cells: they self-renew and can be induced to differentiate into various cell types. There are numerous descriptions and references to these stem cells in the literature since the original report [46], and they have been given different names by different groups. Examples of these are shown in Table 1. The term Wharton’s jelly cells (WJCs) is used throughout this review and includes cells derived from the perivascular space, the intravascular space, and the subamnion (Fig. 1) [26].
This source of stem cells allows the rapid initial isolation of large numbers of cells, avoiding the necessity of extensive multiplication and potential epigenetic damage [47, 48]. This source has the advantage that cells are isolated from fetal structure in the perinatal period and, perhaps like umbilical cord blood, may be better tolerated following transplantation with less incidence of graft versus host disease.
Other MSC-Like Cells from Umbilical Cord
Four MSC populations have been identified in the umbilical cord. First, MSC cells have been identified in Wharton’s jelly. Second, MSCs cells have been identified surrounding the umbilical vessels. Third, MSCs have been isolated from umbilical cord blood. Fourth, MSCs have been isolated from the subendothelium of umbilical vein.
Some authors have focused on a cell population that is isolated from around the umbilical vessels [32, 39]. These have been termed human umbilical cord perivascular cells (HUCPV cells). Whereas the non-vascular components of the umbilical cord may harbor more than one cell population, the Wharton’s jelly is a continuum from the subamnion to the perivascular region [26]. Karahusevinoglu compared the expansion ability of Wharton’s jelly stromal cells and the HUCPV cells and reported that the Wharton’s jelly stromal cells had greater expansion capability and faster doubling times than HUCPV cells. Furthermore, the HUCPV cells stain for pancytokeratin more strongly than Wharton’s jelly stromal cells. This group suggested that HUCPV cells are more differentiated than Wharton’s jelly stromal cells and this explains why the HUCPVs may not differentiate to neuronal cells [26].
Other laboratories have isolated MSCs from umbilical cord blood (Table 1). There are difficulties in isolating MSCs from umbilical cord blood and high consistency has not yet been obtained. Recently, it was reported that MSC culture must be initiated less than five hours after cord blood collection to increase the chances of MSC isolation. The clinical application of MSCs derived from cord blood may be limited due to the inconsistency initiating cultures and the required expansion time of the relatively small numbers of cells.
Human umbilical vein subendothelial cells are another source of MSC-like cells. The isolation of MSCs from umbilical vein was first described by Romanov’s lab [49]. This source has been shown to be similar to bone-marrow-derived MSCs, has osteogenic capability, and multilineage potential [49–52].
Comparison of WJCs to Adult-Derived MSCs
WJCs are CD45, CD 34, and HLA class II negative; CD73, CD90, CD105 are HLA-class I positive; WJCs are plastic adherent, and multipotent. Similar to early passage MSCs, WJCs grow robustly, can be frozen and thawed, and can be engineered to express exogenous proteins. Thus, the similarities of WJCs and MSCs can be summarized as follows: WJCs share the basic criteria used to define adult-derived MSCs. Additionally, WJCs express GD2 synthase, a marker that has been proposed to uniquely identify MSCs in a bone marrow aspirate (D. Troyer, C. Ganta, M.L. Weiss, unpublished data).
In all reports, WJCs have faster proliferation and greater ex vivo expansion capabilities than BMSC. This may be due to the expression of telomerase by WJCs [46]. Karahuseyinoglu et al. were able to expand WJC numbers over 300-fold over seven passages [26]. Other groups have expanded WJC to more than 1015 cells [33]. MSCs are generally used within six passages. In contrast to MSCs, WJCs have a significantly higher CFU-F frequency [28]. A subset of WJCs expresses nestin, a marker for neural and other stem cells [40, 53, 54]. Others have reported that WJCs may be superior to MSCs for repair of photoreceptor damage [33] or for tissue engineering [55].
WJCs appear to be similar to bone marrow stromal and other MSCs, since in addition to the three surface markers mentioned above, they express CD10, CD13, CD29, and CD44 [26, 28, 40, 53, 56]. Like BMSCs, WJCs express the stem cell factor gene [28]. However, some of these markers appear to be downregulated as passage number increases [40] and unpublished data. WJCs are negative for CD34, CD45, CD14, CD33, CD56, CD31 and HLA-DR [40, 56]. Karahuseyinoglu et al. [26] divided WJCs into (a) Type I cells, which were more fusiform in appearance but also expressed cytokeratins and other differentiation markers, and (b) Type II cells, which were more elongated but were more efficiently induced into neural cells. These authors reported that WJCs in chondrogenic media were strongly positive for type II collagen, whereas BMSCs in the same induction media only showed weak positive type II collagen immunofluorescence.
In Vitro Differentiation of WJCs
WJCs, like BMSCs, can be induced in vitro to become cells with morphologic and biochemical characteristics of neural cells [46, 56, 57]. When they were cultured in media conditioned by primary rat brain neurons, WJCs could be invoked with glutamate to have an inward current and express neuronal proteins [53]. Exposure of WJCs to primary neuron-conditioned medium upregulated the astrocyte protein GFAP [53]. Interestingly, this group also showed they could increase CD11b (microglial) cell numbers from 1.7% to 3.0% by subjecting WJCs to neuronal-conditioned media. Ma and colleagues induced WJCs to express neuronal markers β-tubulin III, neurofilament, and GFAP by treatment with Salvia miltorrhiza [57].
Mesenchymal stromal cells isolated from Wharton’s jelly have been induced to form bone, cartilage and adipose cells [26, 39, 53, 56, 58]. WJCs were spun into poly(lactic-co-glycolic acid) scaffolds and cultured in media containing chondrogenic growth factors and were compared to similarly treated cells isolated from condylar cartilage from the temporomandibular joint [55]. Interestingly, these authors found that the WJCs outperformed the cartilage cells with regard to collagens I and II, glycosaminoglycans and cellularity of the constructs after 4 weeks. WJCs can also be induced toward heart cells. After 5-azacytidine treatment for 3 weeks, WJCs expressed the cardiomyocyte markers cardiac troponin I, connexin 43, and desmin, and exhibited cardiac myocyte morphology [56]. They have been used along with tissue engineering to generate artificial blood vessels and heart valves [59–61]. WJCs can be directed toward skeletal muscle cells; when they were placed in myogenic media, they expressed Myf5 from day 7, Myo-D from day 11, and formed elongated, multinucleated cells [58]. After undifferentiated WJCs were injected into rat muscle damaged by bupivacaine chloridrate, elongated cells expressing sarcomeric tropomyosin that were HLA immunopositive were identified within the muscle [58]. Finally, it has been shown that human WJCs can be differentiated successfully into endothelial cells [62] after the addition of vascular endothelial growth factor (VEGF) and basic fibroblast growth factor to cultures. Moreover, the authors reported that undifferentiated human WJCs injected into a murine ischemic model differentiated into endothelial cells.
Comparison of WJCs to Fetal MSCs
In contrast to what has been observed for adult MSCs, WJCs share several of the properties unique to fetal-derived MSCs. First, they have greater expansion potential in vitro than adult MSCs (reviewed above). Second, WJCs express HLA-class I and do not express HLA class-II surface markers [28, 39, 40]. In contrast to what has been published for fetal MSCs, WJCs are immune suppressive in mixed lymphocyte assays and inhibit T-cell proliferation ([63]; Ennis et al., abstract from ISSCR 2007). WJCs are tolerated following allogenic transplant and stimulate an immune response following multiple injections or injection of WJCs exposed to interferon [63]. Human WJCs express markers of primitive stem cells, such as leukemia inhibitory factor receptor pathway, embryonic stem cell specific gene 1, and telomerase reverse transcriptase [40]. Porcine WJCs express the ESC markers Oct4, Sox-2, and Nanog at a low level relative to embryonic stem cells and are alkaline phosphatase positive [64]. Flow cytometry indicates that a subpopulation of WJCs express the primitive stem cell markers SSEA4 and TRA-1–60 [65]. When the Hoechst dye exclusion test is used to identify dye-excluding, side-population cells, about 20% of human WJCs exclude dye. Flow-sorting to enrich the Hoechstdim population resulted in cells that appeared morphologically smaller than the parent population, and many of the selected cells expressed CD44, the HA receptor [40].
Transplantation of WJCs
Human WJCs ameliorate apomorphine-induced behavioral deficits in a hemiparkinsonian rat model [40]. There was a significant decrease in apomorphine-induced rotations at 4 weeks continuing up to 12 weeks post-transplantation in Parkinson’s disease (PD) rats that received human WJC transplants compared to the PD rats that received a sham transplant. The behavioral findings correlated with the numbers of tyrosine hydroxylase-positive cell bodies observed in the midbrain following sacrifice at 12 weeks, indicating a “rescue from a distance” phenomenon. One explanation for this effect may be that WJCs synthesize GDNF, a potent survival factor for dopaminergic neurons, as well as other trophic factors such as VEGF and ciliary neurotrophic factor. In another report, WJCs were first induced toward dopaminergic neurons using neuron-conditioned media, sonic hedgehog, and fibroblast growth factor 8, and then transplanted into hemiparkinsonian rats [66]. Despite the fact that the rats were not immune suppressed, WJCs were identified 5 months later and prevented the progressive degeneration/behavioral deterioration seen in control rats with unilateral lesions. Similarly, rat Wharton’s jelly cells transplanted into the brains of rats with global cerebral ischemia caused by cardiac arrest and resuscitation significantly reduced neuronal loss, apparently due to a rescue phenomenon [67].
Lund et al. administered WJCs into the eyes of a rodent model of retinal disease. Here, WJCs were compared to BMSCs and placental stem cells. They reported that the WJCs exhibited the best histological evidence of photoreceptor rescue [33]. In addition, they reported that WJCs significantly increased production of trophic factors such as brain-derived neurotrophic factor and fibroblast growth factor 2 compared to the other two cell types. Interestingly, WJCs transplanted into the vitreous demonstrated a rescue effect, indicating that they could enhance survival of photoreceptor cells without being in close proximity to them. This effect was presumably due to diffusible growth factors. These results, indicating a rescue phenomenon by WJCs, fit a model for the positive effects of MSC therapy in stroke [68] or myocardial infarction [69].
WJCs, like neural stem cells and mesenchymal stem cells [70–72], appear to migrate to areas of tumor growth. Human breast carcinoma (MDA 231) cells were intravenously injected into SCID mice, followed by i.v. transplantation of fluorescently labeled WJCs. One week after transplant WJCs were found near or within lung tumors and not in other tissues. WJCs were engineered to express human interferon beta and were administered intravenously into SCID mice bearing MDA 231 tumors. This treatment significantly reduced the tumor burden [73].
Recent work by Cho et al., evaluated the ability of allogeneic WJCs to stimulate the immune system in a swine model [63]. The WJCs were non-immunogenic on the first injection into allogeneic recipients. However, repeated injection of WJCs produced an immunogenic response. Furthermore, when WJCs were injected into inflamed skin or when WJCs were exposed to interferon prior to injection, they were immunogenic. This work is the first to demonstrate immunogenicity of WJCs in vivo and has implications for their allogeneic use in disease tissues.
The migration or, homing, ability of umbilical cord and other MSCs is thought to be due to the expression of chemokine or other surface receptors. For example, a subpopulation of BMSCs was shown to express CXCR4, the receptor for stem cell-derived factor one (SDF-1) and CXCR3, the receptor for fractaline. WJCs also express CXCR4 [40]. Tumors secrete factors that recruit cells from surrounding tissue as well as from the bone marrow to provide support and nutrition.
WJCs as Primitive Stromal Cells
Wharton’s jelly cells represent a unique, easily accessible, and non-controversial source of early stem cells that can be readily manipulated. In one species examined, the pig, WJCs resemble pluripotent cells, since they express Oct4, Nanog, and Sox-2, and are alkaline phophatase positive [64]. In agricultural species this is important, as there are few economically viable sources of primitive stem cells available for future transgenic or biotechnology applications. Our contention that umbilical matrix cells are primitive stromal cells is based on plastic adherence, immunophenotyping, real-time PCR, immunohistochemistry, ELISA, multipotency, and other results mentioned above. Since the cells are isolated at birth, there is less of a temporal separation from fetal cells than those isolated from adult tissues, and the isolates are consistent in terms of age at collection. Other authors also surmised that WJCs are earlier-stage cells than MSCs derived from adult fat or bone marrow [56, 62]. This argument is based upon population doubling times and more extensive expansion prior to senescence. Another indication that WJCs are a primitive population is that an unusually high percentage of them express the ABCG2 transporter and efflux Hoechst dye [40, 64], since these are markers of other primitive stem cells. When we used flow sorting to enrich for this population, the enriched cells appeared morphologically smaller than the Hoechst-bright population [40].
The umbilical cord vessels and surrounding mesenchyme (including the connective tissue matrix that becomes Wharton’s jelly) are derived from extra-embryonic mesoderm and/or embryonic mesoderm. The yolk sac component is the embryologic source of both primordial germ cells and the first hematopoietic stem cells [27]. It is possible that some of these stem cell populations remain behind and co-exist with the mesenchymal stromal cells of Wharton’s jelly [74, 75]. It is also possible that, during development, cells in the Wharton’s jelly migrate into the fetus along with the primordial germ cells and hematopoietic cells to the AGM region.
Since the WJCs express some genes characteristic of primitive stem cells including embryonic stem cells (ESCs) and since ESCs sometimes form tumors after transplantation [76, 77], we transplanted large numbers of WJCs into SCID mice. When the mice were examined 50 days later, there was no evidence of tumor formation or long-term engraftment [73]. The fact that the engraftment was not detected could be due to failure of the WJCs to engraft or lack of sensitivity in the detection method. Rat WJCs did not form tumors when transplanted into rats with retinal degeneration [33]. WJCs are karyotypically stable over many passages [26, 33, 40] and do not lose anchorage dependence, contact inhibition, or serum dependence [26, 40], as cancer cells do. A more definitive test of engraftment potential has not yet been tested. For example, transplantation following irradiation injury, is needed to evaluate whether WJCs, like MSCs, can engraft in vivo and thus be true stem cells.
As mentioned above, like mouse embryonic fibroblasts, WJCs can support the growth of other primitive stem cells. For example, Saito et al. reported that when a feeder layer derived from Wharton’s jelly fibroblasts was used for equine embryonic stem cell-like cells, they could be expanded successfully without leukemia inhibitor factor for more than 350 divisions [43]. Like BMSCs, human WJC feeder layers facilitate the ex vivo expansion of human umbilical cord blood cells [28]. Currently, it is unclear whether WJCs are different from BMSCs in terms of their ability to expand hematopoietic cells in vitro.
Summary
Wharton’s jelly cells, like bone marrow stromal cells and other mesenchymal cells, are plastic adherent, stained positively for markers of the mesenchymal cells such as CD10, CD13, CD29, CD44, CD90, and CD105 and negatively for markers of the hematopoietic lineage. Moreover, WJCs morphologically resemble MSCs and can be expanded more then bone-marrow-derived MSCs in culture. Human WJCs express precursor cell markers such as nestin. WJCs can be induced to form adipose tissue, bone, cartilage, skeletal muscle cells, cardiomyocyte-like cells, and neural cells and are amenable to biomedical engineering applications. Therefore, these cells fit into the category of primitive stromal cells; and, because Wharton’s jelly is a plentiful and inexpensive source of cells, it appears to potentially impact fields such as regenerative medicine, biotechnology, and agriculture. Further work is needed to determine whether WJCs engraft long-term and display self-renewal and multipotency in vivo and, as such, demonstrate that WJCs are a true stem cell population.
Acknowledgments
The authors acknowledge support from the National Institute of Health (MLW NS34160), The Midwest Institute for Comparative Stem Cell Biology, Kansas Idea Network of Biomedical Research Excellence, Terry C. Johnson Center for Basic Cancer Research, the Kansas State Uuniversity Developing Scholars Program, the Kansas State Uuniversity Targeted Excellence Program, and the State of Kansas. We thank Drs. D. Davis, F. Blecha, F. Marini, S. Medicetty, K. Mitchell, R. Rachakatla, C. Ganta, K. Seshareddy, and B. Lutjemeier for their assistance with this work. We thank Drs. J. Auerbach and A. Toujmadje of GlobalStem for sharing their unpublished observations.
Footnotes
Disclosure of Potential Conflicts of Interest
M. Weiss has acted as a consultant to Regenerative Medicine Institute (Las Vegas, NV), Nacelle Therapeutics (Manhattan, KS), and KPL (Rockville, MD); has performed contract work for Toucan Capital II (Bethesda, MD) and Nacelle Therapeutics; and has a financial interest in Regenerative Medicine Institute.
References
- 1.Spraycar M, editor. Stedman’s Medical Dictionary. 27. Baltimore: Williams & Wilkins; [Google Scholar]
- 2.Baksh D, Song L, Tuan RS. Adult mesenchymal stem cells: characterization, differentiation, and application in cell and gene therapy. J Cell Mol Med. 2004;8:301–316. doi: 10.1111/j.1582-4934.2004.tb00320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Friedenstein AJ. Osteogenetic activity of transplanted transitional epithelium. Acta Anat (Basel) 1961;45:31–59. doi: 10.1159/000141739. [DOI] [PubMed] [Google Scholar]
- 4.Martinez C, Hofmann TJ, Marino R, et al. Human bone marrow mesenchymal stromal cells express the neural ganglioside GD2: a novel surface marker for the identification of MSCs. Blood. 2007;109:4245–4248. doi: 10.1182/blood-2006-08-039347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gang EJ, Bosnakovski D, Figueiredo CA, et al. SSEA-4 identifies mesenchymal stem cells from bone marrow. Blood. 2007;109:1743–1751. doi: 10.1182/blood-2005-11-010504. [DOI] [PubMed] [Google Scholar]
- 6.Battula VL, Treml S, Abele H, et al. Prospective isolation and characterization of mesenchymal stem cells from human placenta using a frizzled-9-specific monoclonal antibody. Differentiation. 2007;75:279–291. doi: 10.1111/j.1432-0436.2007.00225.x. [DOI] [PubMed] [Google Scholar]
- 7.Bühring HJ, Battula VL, Treml S, et al. Novel markers for the prospective isolation of human MSC. Ann N Y Acad Sci. 2007;1106:262–271. doi: 10.1196/annals.1392.000. [DOI] [PubMed] [Google Scholar]
- 8.Kafienah W, Mistry S, Williams C, et al. Nucleostemin is a marker of proliferating stromal stem cells in adult human bone marrow. Stem Cells. 2006;24:1113–1120. doi: 10.1634/stemcells.2005-0416. [DOI] [PubMed] [Google Scholar]
- 9.Dominici M, Le BK, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular: Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 10.Bruder SP, Jaiswal N, Haynesworth SE. Growth kinetics, self-renewal, and the osteogenic potential of purified human mesenchymal stem cells during extensive subcultivation and following cryopreservation. J Cell Biochem. 1997;64:278–294. doi: 10.1002/(sici)1097-4644(199702)64:2<278::aid-jcb11>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 11.Jaiswal N, Haynesworth SE, Caplan AI, et al. Osteogenic differentiation of purified, culture-expanded human mesenchymal stem cells in vitro. J Cell Biochem. 1997;64:295–312. [PubMed] [Google Scholar]
- 12.Kadiyala S, Young RG, Thiede MA, et al. Culture expanded canine mesenchymal stem cells possess osteochondrogenic potential in vivo and in vitro. Cell Transplant. 1997;6:125–134. doi: 10.1177/096368979700600206. [DOI] [PubMed] [Google Scholar]
- 13.Ferrari G, Cusella-De AG, Coletta M, et al. Muscle regeneration by bone marrow-derived myogenic progenitors. Science. 1998;279:1528–1530. doi: 10.1126/science.279.5356.1528. [DOI] [PubMed] [Google Scholar]
- 14.Dennis JE, Merriam A, Awadallah A, et al. A quadripotential mesenchymal progenitor cell isolated from the marrow of an adult mouse. J Bone Miner Res. 1999;14:700–709. doi: 10.1359/jbmr.1999.14.5.700. [DOI] [PubMed] [Google Scholar]
- 15.Young RG, Butler DL, Weber W, et al. Use of mesenchymal stem cells in a collagen matrix for Achilles tendon repair. J Orthop Res. 1998;16:406–413. doi: 10.1002/jor.1100160403. [DOI] [PubMed] [Google Scholar]
- 16.Hermann A, Gastl R, Liebau S, et al. Efficient generation of neural stem cell-like cells from adult human bone marrow stromal cells. J Cell Sci. 2004;117:4411–4422. doi: 10.1242/jcs.01307. [DOI] [PubMed] [Google Scholar]
- 17.Ito Y, Hasauda H, Kitajima T, et al. Ex vivo expansion of human cord blood hematopoietic progenitor cells using glutaraldehyde-fixed human bone marrow stromal cells. J Biosci Bioeng. 2006;102:467–469. doi: 10.1263/jbb.102.467. [DOI] [PubMed] [Google Scholar]
- 18.da Silva CL, Goncalves R, Crapnell KB, et al. A human stromal-based serum-free culture system supports the ex vivo expansion/maintenance of bone marrow and cord blood hematopoietic stem/progenitor cells. Exp Hematol. 2005;33:828–835. doi: 10.1016/j.exphem.2005.03.017. [DOI] [PubMed] [Google Scholar]
- 19.Almeida-Porada G, Flake AW, Glimp HA, et al. Cotransplantation of stroma results in enhancement of engraftment and early expression of donor hematopoietic stem cells in utero. Exp Hematol. 1999;27:1569–1575. doi: 10.1016/s0301-472x(99)00090-9. [DOI] [PubMed] [Google Scholar]
- 20.Anklesaria P, Kase K, Glowacki J, et al. Engraftment of a clonal bone marrow stromal cell line in vivo stimulates hematopoietic recovery from total body irradiation. Proc Natl Acad Sci U S A. 1987;84:7681–7685. doi: 10.1073/pnas.84.21.7681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nilsson SK, Haylock DN, Johnston HM, et al. Hyaluronan is synthesized by primitive hemopoietic cells, participates in their lodgment at the endosteum following transplantation, and is involved in the regulation of their proliferation and differentiation in vitro. Blood. 2003;101:856–862. doi: 10.1182/blood-2002-05-1344. [DOI] [PubMed] [Google Scholar]
- 22.Campagnoli C, Roberts IA, Kumar S, et al. Identification of mesenchymal stem/progenitor cells in human first-trimester fetal blood, liver, and bone marrow. Blood. 2001;98:2396–2402. doi: 10.1182/blood.v98.8.2396. [DOI] [PubMed] [Google Scholar]
- 23.Guillot PV, Gotherstrom C, Chan J, et al. Human first-trimester fetal MSC express pluripotency markers and grow faster and have longer telomeres than adult MSC. Stem Cells. 2007;25:646–654. doi: 10.1634/stemcells.2006-0208. [DOI] [PubMed] [Google Scholar]
- 24.Götherström C, Ringden O, Westgren M, et al. Immunomodulatory effects of human foetal liver-derived mesenchymal stem cells. Bone Marrow Transplant. 2003;32:265–272. doi: 10.1038/sj.bmt.1704111. [DOI] [PubMed] [Google Scholar]
- 25.Götherström C, West A, Liden J, et al. Difference in gene expression between human fetal liver and adult bone marrow mesenchymal stem cells. Haematologica. 2005;90:1017–1026. [PubMed] [Google Scholar]
- 26.Karahuseyinoglu S, Cinar O, Kilic E, et al. Biology of the stem cells in human umbilical cord stroma: in situ and in vitro surveys. Stem Cells. 2007;25:319–331. doi: 10.1634/stemcells.2006-0286. [DOI] [PubMed] [Google Scholar]
- 27.Sadler TW. Langmann’s Medical Embryology. Philadelphia: Williams & Wilkins; 2004. Second week of development: Bilaminar germ disc; pp. 51–64. [Google Scholar]
- 28.Lu LL, Liu YJ, Yang SG, et al. Isolation and characterization of human umbilical cord mesenchymal stem cells with hematopoiesis-supportive function and other potentials. Haematologica. 2006;91:1017–1026. [PubMed] [Google Scholar]
- 29.Friedman R, Betancur M, Boissel L, et al. Umbilical cord mesenchymal stem cells: Adjuvants for human cell transplantation. Biol Blood Marrow Transplant. 2007;13:1477–1486. doi: 10.1016/j.bbmt.2007.08.048. [DOI] [PubMed] [Google Scholar]
- 30.Bieback K, Kern S, Kluter H, et al. Critical parameters for the isolation of mesenchymal stem cells from umbilical cord blood. Stem Cells. 2004;22:625–634. doi: 10.1634/stemcells.22-4-625. [DOI] [PubMed] [Google Scholar]
- 31.Kern S, Eichler H, Stoeve J, et al. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24:1294–1301. doi: 10.1634/stemcells.2005-0342. [DOI] [PubMed] [Google Scholar]
- 32.Baksh D, Yao R, Tuan RS. Comparison of proliferative and multilineage differentiation potential of human mesenchymal stem cells derived from umbilical cord and bone marrow. Stem Cells. 2007;25:1384–1392. doi: 10.1634/stemcells.2006-0709. [DOI] [PubMed] [Google Scholar]
- 33.Lund RD, Wang S, Lu B, et al. Cells isolated from umbilical cord tissue rescue photoreceptors and visual functions in a rodent model of retinal disease. Stem Cells. 2007;25:602–611. doi: 10.1634/stemcells.2006-0308. [DOI] [PubMed] [Google Scholar]
- 34.Gao L, Chen X, Zhang X, et al. Human umbilical cord blood-derived stromal cell, a new resource of feeder layer to expand human umbilical cord blood CD34+ cells in vitro. Blood Cells Mol Dis. 2006;36:322–328. doi: 10.1016/j.bcmd.2005.12.036. [DOI] [PubMed] [Google Scholar]
- 35.Wang JF, Wang LJ, Wu YF, et al. Mesenchymal stem/progenitor cells in human umbilical cord blood as support for ex vivo expansion of CD34(+) hematopoietic stem cells and for chondrogenic differentiation. Haematologica. 2004;89:837–844. [PubMed] [Google Scholar]
- 36.Ye ZQ, Burkholder JK, Qiu P, et al. Establishment of an adherent cell feeder layer from human umbilical cord blood for support of long-term hematopoietic progenitor cell growth. Proc Natl Acad Sci U S A. 1994;91:12140–12144. doi: 10.1073/pnas.91.25.12140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jang YK, Jung DH, Jung MH, et al. Mesenchymal stem cells feeder layer from human umbilical cord blood for ex vivo expanded growth and proliferation of hematopoietic progenitor cells. Ann Hematol. 2006;85:212–225. doi: 10.1007/s00277-005-0047-3. [DOI] [PubMed] [Google Scholar]
- 38.Goodwin HS, Bicknese AR, Chien SN, et al. Multilineage differentiation activity by cells isolated from umbilical cord blood: expression of bone, fat, and neural markers. Biol Blood Marrow Transplant. 2001;7:581–588. doi: 10.1053/bbmt.2001.v7.pm11760145. [DOI] [PubMed] [Google Scholar]
- 39.Sarugaser R, Lickorish D, Baksh D, et al. Human umbilical cord perivascular (HUCPV) cells: a source of mesenchymal progenitors. Stem Cells. 2005;23:220–229. doi: 10.1634/stemcells.2004-0166. [DOI] [PubMed] [Google Scholar]
- 40.Weiss ML, Medicetty S, Bledsoe AR, et al. Human umbilical cord matrix stem cells: preliminary characterization and effect of transplantation in a rodent model of Parkinson’s disease. Stem Cells. 2006;24:781–792. doi: 10.1634/stemcells.2005-0330. [DOI] [PubMed] [Google Scholar]
- 41.Nilsson SK, Johnston HM, Whitty GA, et al. Osteopontin, a key component of the hematopoietic stem cell niche and regulator of primitive hematopoietic progenitor cells. Blood. 2005;106:1232–1239. doi: 10.1182/blood-2004-11-4422. [DOI] [PubMed] [Google Scholar]
- 42.Raio L, Cromi A, Ghezzi F, et al. Hyaluronan content of Wharton’s jelly in healthy and Down syndrome fetuses. Matrix Biol. 2005;24:166–174. doi: 10.1016/j.matbio.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 43.Saito S, Ugai H, Sawai K, et al. Isolation of embryonic stem-like cells from equine blastocysts and their differentiation in vitro. FEBS Lett. 2002;531:389–396. doi: 10.1016/s0014-5793(02)03550-0. [DOI] [PubMed] [Google Scholar]
- 44.Hiroyama T, Sudo K, Aoki N, et al. Human umbilical cord-derived cells can often serve as feeder cells to maintain primate embryonic stem cells in a state capable of producing hematopoietic cells. Cell Biol Int. 2008;32:1–7. doi: 10.1016/j.cellbi.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 45.Kubota H, Avarbock MR, Brinster RL. Growth factors essential for self-renewal and expansion of mouse spermatogonial stem cells. Proc Natl Acad Sci U S A. 2004;101:16489–16494. doi: 10.1073/pnas.0407063101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mitchell KE, Weiss ML, Mitchell BM, et al. Matrix cells from Wharton’s jelly form neurons and glia. Stem Cells. 2003;21:50–60. doi: 10.1634/stemcells.21-1-50. [DOI] [PubMed] [Google Scholar]
- 47.Boquest AC, Noer A, Collas P. Epigenetic programming of mesenchymal stem cells from human adipose tissue. Stem Cell Rev. 2006;2:319–329. doi: 10.1007/BF02698059. [DOI] [PubMed] [Google Scholar]
- 48.Noer A, Boquest AC, Collas P. Dynamics of adipogenic promoter DNA methylation during clonal culture of human adipose stem cells to senescence BMC. Cell Biol. 2007;8:18–29. doi: 10.1186/1471-2121-8-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Romanov YA, Svintsitskaya VA, Smirnov VN. Searching for alternative sources of postnatal human mesenchymal stem cells: candidate MSC-like cells from umbilical cord. Stem Cells. 2003;21:105–110. doi: 10.1634/stemcells.21-1-105. [DOI] [PubMed]
- 50.Covas DT, Siufi JL, Silva AR, et al. Isolation and culture of umbilical vein mesenchymal stem cells. Braz J Med Biol Res. 2003;36:1179–1183. doi: 10.1590/s0100-879x2003000900006. [DOI] [PubMed] [Google Scholar]
- 51.Kim JW, Kim SY, Park SY, et al. Mesenchymal progenitor cells in the human umbilical cord. Ann Hematol. 2004;83:733–738. doi: 10.1007/s00277-004-0918-z. [DOI] [PubMed] [Google Scholar]
- 52.Panepucci RA, Siufi JL, Silva WA, Jr, et al. Comparison of gene expression of umbilical cord vein and bone marrow-derived mesenchymal stem cells. Stem Cells. 2004;22:1263–1278. doi: 10.1634/stemcells.2004-0024. [DOI] [PubMed] [Google Scholar]
- 53.Fu YS, Shih YT, Cheng YC, et al. Transformation of human umbilical mesenchymal cells into neurons in vitro. J Biomed Sci. 2004;11:652– 660. doi: 10.1007/BF02256131. [DOI] [PubMed] [Google Scholar]
- 54.Yarygin KN, Suzdal’tseva YG, Burunova VV, et al. Comparative study of adult human skin fibroblasts and umbilical fibroblast-like cells. Bull Exp Biol Med. 2006;141:161–166. doi: 10.1007/s10517-006-0117-0. [DOI] [PubMed] [Google Scholar]
- 55.Bailey MM, Wang L, Bode CJ, et al. A comparison of human umbilical cord matrix stem cells and temporomandibular joint condylar chondrocytes for tissue engineering temporomandibular joint condylar cartilage. Tissue Eng. 2007;13:2003–2010. doi: 10.1089/ten.2006.0150. [DOI] [PubMed] [Google Scholar]
- 56.Wang HS, Hung SC, Peng ST, et al. Mesenchymal stem cells in the Wharton’s jelly of the human umbilical cord. Stem Cells. 2004;22:1330–1337. doi: 10.1634/stemcells.2004-0013. [DOI] [PubMed] [Google Scholar]
- 57.Ma L, Feng XY, Cui BL, et al. Human umbilical cord Wharton’s Jelly-derived mesenchymal stem cells differentiation into nerve-like cells. Chin Med J. 2005;118:1987–1993. [PubMed] [Google Scholar]
- 58.Conconi MT, Burra P, Di LR, et al. CD105(+) cells from Wharton’s jelly show in vitro and in vivo myogenic differentiative potential. Int J Mol Med. 2006;18:1089–1096. [PubMed] [Google Scholar]
- 59.Breymann C, Schmidt D, Hoerstrup SP. Umbilical cord cells as a source of cardiovascular tissue engineering. Stem Cell Rev. 2006;2:87–92. doi: 10.1007/s12015-006-0014-y. [DOI] [PubMed] [Google Scholar]
- 60.Hoerstrup SP, Kadner A, Breymann C, et al. Living, autologous pulmonary artery conduits tissue engineered from human umbilical cord cells. Ann Thorac Surg. 2002;74:46–52. doi: 10.1016/s0003-4975(02)03649-4. [DOI] [PubMed] [Google Scholar]
- 61.Schmidt D, Mol A, Odermatt B, et al. Engineering of biologically active living heart valve leaflets using human umbilical cord-derived progenitor cells. Tissue Eng. 2006;12:3223–3232. doi: 10.1089/ten.2006.12.3223. [DOI] [PubMed] [Google Scholar]
- 62.Wu KH, Zhou B, Lu SH, et al. In vitro and in vivo differentiation of human umbilical cord derived stem cells into endothelial cells. J Cell Biochem. 2007;100:608–616. doi: 10.1002/jcb.21078. [DOI] [PubMed] [Google Scholar]
- 63.Cho PS, Messina DJ, Hirsh EL, et al. Immunogenicity of umbilical cord tissue derived cells. Blood. 2007;111:430–438. doi: 10.1182/blood-2007-03-078774. [DOI] [PubMed] [Google Scholar]
- 64.Carlin R, Davis D, Weiss M, et al. Expression of early transcription factors Oct4, Sox2 and Nanog by porcine umbilical cord (PUC) matrix cells. Reprod Biol Endocrinol. 2006;4:8–21. doi: 10.1186/1477-7827-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hoynowski SM, Fry MM, Gardner BM, et al. Characterization and differentiation of equine umbilical cord-derived matrix cells. Biochem Biophys Res Commun. 2007;362:347–353. doi: 10.1016/j.bbrc.2007.07.182. [DOI] [PubMed] [Google Scholar]
- 66.Fu YS, Cheng YC, Lin MY, et al. Conversion of human umbilical cord mesenchymal stem cells in Wharton’s jelly to dopaminergic neurons in vitro: potential therapeutic application for Parkinsonism. Stem Cells. 2006;24:115–124. doi: 10.1634/stemcells.2005-0053. [DOI] [PubMed] [Google Scholar]
- 67.Jomura S, Uy M, Mitchell K, et al. Potential treatment of cerebral global ischemia with Oct-4+ umbilical cord matrix cells. Stem Cells. 2007;25:98–106. doi: 10.1634/stemcells.2006-0055. [DOI] [PubMed] [Google Scholar]
- 68.Borlongan CV, Hadman M, Sanberg CD, et al. Central nervous system entry of peripherally injected umbilical cord blood cells is not required for neuroprotection in stroke. Stroke. 2004;35:2385–2389. doi: 10.1161/01.STR.0000141680.49960.d7. [DOI] [PubMed] [Google Scholar]
- 69.Grinnemo KH, Mansson A, Dellgren G, et al. Xenoreactivity and engraftment of human mesenchymal stem cells transplanted into infarcted rat myocardium. J Thorac Cardiovasc Surg. 2004;127:1293–1300. doi: 10.1016/j.jtcvs.2003.07.037. [DOI] [PubMed] [Google Scholar]
- 70.Aboody KS, Brown A, Rainov NG, et al. Neural stem cells display extensive tropism for pathology in adult brain: evidence from intracranial gliomas. Proc Natl Acad Sci U S A. 2000;97:12846–12851. doi: 10.1073/pnas.97.23.12846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Studeny M, Marini FC, Champlin RE, et al. Bone marrow-derived mesenchymal stem cells as vehicles for interferon-beta delivery into tumors. Cancer Res. 2002;62:3603–3608. [PubMed] [Google Scholar]
- 72.Studeny M, Marini FC, Dembinski JL, et al. Mesenchymal stem cells: potential precursors for tumor stroma and targeted-delivery vehicles for anticancer agents. J Natl Cancer Inst. 2004;96:1593–1603. doi: 10.1093/jnci/djh299. [DOI] [PubMed] [Google Scholar]
- 73.Rachakatla RS, Marini F, Weiss ML, et al. Development of human umbilical cord matrix stem cell-based gene therapy for experimental lung tumors. Cancer Gene Ther. 2007;14:828–835. doi: 10.1038/sj.cgt.7701077. [DOI] [PubMed] [Google Scholar]
- 74.Cetrulo CL, Cetrulo KJ. Placental and pregnancy stem cells: everyone is, or should be, interested in the placenta. Stem Cell Rev. 2006;2:79–80. doi: 10.1385/scr:2:2:79. [DOI] [PubMed] [Google Scholar]
- 75.Weiss ML, Troyer DL. Stem cells in the umbilical cord. Stem Cell Rev. 2006;2:155–162. doi: 10.1007/s12015-006-0022-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Arnhold S, Klein H, Semkova I, et al. Neurally selected embryonic stem cells induce tumor formation after long-term survival following engraftment into the subretinal space. Invest Ophthalmol Vis Sci. 2004;45:4251– 4255. doi: 10.1167/iovs.03-1108. [DOI] [PubMed] [Google Scholar]
- 77.Wakitani S, Takaoka K, Hattori T, et al. Embryonic stem cells injected into the mouse knee joint form teratomas and subsequently destroy the joint. Rheumatology. 2003;42:162–165. doi: 10.1093/rheumatology/keg024. [DOI] [PubMed] [Google Scholar]