Abstract
Although bortezomib is clinically approved for the treatment of mantle cell lymphoma (MCL), only limited effects of this treatment have been demonstrated. To improve survival for bortezomib-resistant patients, it is necessary to develop new therapeutic strategies. In the present study, we used biochemical and molecular methodologies to demonstrate that tissue transglutaminase (TG) activates downstream NF-κB signaling pathways. The signaling axis from TG to NF-κB could be a new therapeutic target to overcome bortezomib resistance in MCL. TG2 is a calcium-dependent protein cross-linking enzyme reported to be overexpressed in various cancer cells. We found that MCL cells expressed elevated levels of TG2 and that the modification of TG2 activities altered NF-κB expression and downstream signaling in MCL cells. When TG2 signaling was inhibited by calcium blockers, the combination of a calcium blocker (perillyl alcohol) with bortezomib suppressed NF-κB expression and improved the cytotoxicity of bortezomib in MCL cells. Our study is the first to show the expression of TG2 and the contribution of TG2 to NF-κB signaling in MCL. TG2 inhibition may be used as an alternative target anti-MCL therapy, and calcium blockers may be combined with bortezomib to overcome the bortezomib resistance in MCL.
Introduction
Mantle cell lymphoma (MCL) is an aggressive subtype of B-cell lymphoma that accounts for 5%-7% of cases of non-Hodgkin lymphoma. Despite good responses with first-line treatments for newly diagnosed, untreated MCL patients,1–3 MCL patients often relapse and demonstrate highly refractory responses to common antilymphoma chemotherapy, which results in inevitable chemoresistance and poor clinical outcomes.4–7
Bortezomib (Velcade), a reversible inhibitor of the 26S proteasome, first gained United States Food and Drug Administration approval as a single-agent treatment in patients with relapsed or refractory MCL.8 Bortezomib inhibits the ubiquitin-proteasome pathway and alters multiple cellular signaling cascades, including those regulating cell growth, differentiation, and survival.9–11 For example, proteasome inhibition prevents the degradation of pro-apoptotic factors, which facilitates the activation of programmed cell death in neoplastic cells; however, the precise mechanisms of action are controversial.
One of the known bortezomib targets for inhibition is NF-κB and its related pathway. Constitutive NF-κB expression has been reported in MCL cell lines and primary cells.12 However, therapies such as bortezomib targeting NF-κB have shown limited effects in MCL.13–15 Bortezomib was also reported to elicit the unfolded protein response, which is activated when the physiologic environment of the endoplasmic reticulum is altered.16–18 The induction of endoplasmic reticulum stress induces reactive oxygen species, which affects treatment responses to bortezomib in MCL18 and multiple myeloma.19 In addition, some studies have suggested that bortezomib could increase NF-κB activity20,21 or the presence of bortezomib-resistant NF-κB activity in MCL.13
The resistance to drugs such as bortezomib in MCL suggest the presence of drug-resistant populations in MCL. In a previous study, we prospectively identified stem-like cells in MCL, which we have termed MCL-initiating cells (MCL-ICs).22 The stem-like MCL cells (CD45+CD19−CD34−CD3−) were highly tumorigenic and display self-renewal capacities in NOD/SCID mice. In contrast, the majority of the tumor population contains CD45+CD19+ MCL cells, which show no self-renewal capacity and have greatly reduced tumorigenicity.22 We also demonstrated that these CD45+CD19− MCL-ICs confer drug resistance properties to MCL. MCL-ICs were highly resistant in vitro to clinically relevant anti-MCL chemotherapeutic regimens compared with bulk CD45+CD19+ MCL cells.23 Moreover, CD45+CD19− MCL-ICs were resistant to bortezomib and bortezomib-based chemotherapeutic regimens despite constitutive NF-κB expression.24 Bortezomib-based regimens targeted CD45+CD19− MCL-ICs less efficiently compared with CD45+CD19+ bulk MCL cells. Based on these findings, a new strategy is required to overcome bortezomib resistance in MCL.
Recent studies have demonstrated that perillyl alcohol (POH), a naturally occurring monoterpene that inhibits L-type calcium channels, inhibits cancer cell growth and enhances the pro-apoptotic effects of combined chemotherapeutic drugs such as bortezomib or cisplatin in several malignant tumors including MCL.13,25,26 Another study indicated that the L-type calcium-channel blocker verapamil enhanced the cytotoxic effects of bortezomib.27 Therefore, in the present study, we investigated whether combination treatment with bortezomib plus calcium-channel blockers such as POH decreases the bortezomib-resistant properties of MCL-ICs.
POH treatments with bortezomib largely enhanced cytotoxicity of MCL-ICs in vitro. Interestingly, the bortezomib-resistant and calcium-dependent NF-κB expression of MCL-ICs was modulated by tissue transglutaminase (TG2) activities. TG2 is an 80-kDa enzyme that cross-links proteins between an ϵ-amino group of a lysine residue and a γ-carboxamide group of glutamine residue, creating an inter- or intramolecular bond that is highly resistant to proteolysis (protein degradation). TG2 has multiple physiologic functions and is associated with cancer cell survival and drug resistance.28–30 TG2 shows anti-apoptotic effects by promoting interactions between cell-surface integrins31 by interacting with the retinoblastoma (Rb) protein29 or by down-regulation of caspase 3.32 TG2 is also highly expressed in drug-resistant cancer cells.30,33,34
Chemotherapy-resistant cancer cells express higher levels of TG2 than parental drug-sensitive cell lines.30,33,35,36 Some studies have suggested that TG2 is associated with constitutive NF-κB expression in cancer cells by modifying the inhibitory α-subunit of NF-κB (IκBα) or by the association of TG2 with NF-κB components, resulting in interference with the binding of IκBα to the NF-κB complex.33,35,37,38 In the present study, we have demonstrated that CD45+CD19− MCL-ICs and MCL cell lines express TG2 and that modifications of TG2 activities alter NF-κB expression in MCL cell lines and MCL-ICs. This study is the first to show the link between calcium-dependent TG2 and NF-κB in bortezomib-resistant MCL populations, and our data suggest that the combination of bortezomib with a calcium-channel blocker may improve the efficacy of bortezomib-based chemotherapeutic regimens in MCL.
Methods
Cells and cell culture
The well-characterized EBV-negative human MCL cell lines SP-53, Jeko-1, Mino, and REC-1 were obtained from the American Type Culture Collection. All patients were diagnosed with MCL at the time of collection based on t(11;14) translocation and cyclin D1 reactivity and were in the leukemic phase at the time of aphaeresis. PBMCs were isolated from aphaeresis blood by standard Ficoll gradient methods. All patients were previously treated, although the course of therapy differed somewhat between patients. Blood specimens from MCL patients were obtained after informed consent in accordance with the Declaration of Helsinki and as approved by the MD Anderson Cancer Center and University of Texas Health Science Center Institutional Review Boards.
MCL-ICs were isolated as described previously.22 SP-53, Jeko-1, and primary MCL cells were cultured in complete RPMI 1640 medium (Cellgro; Mediatech) containing 10% heat-inactivated FBS, 2mM glutamine, 100 μg/mL of streptomycin, and 100 μg/mL of penicillin. Mino and REC-1 cells were cultured in RPMI 1640 medium containing 20% heat-inactivated FBS, 100 μg/mL of streptomycin, and 100 μg/mL of penicillin.
Reagents
The following commercially available Abs were used: anti-CD45 (HI30, IgG1, κ), anti-CD19 (HIB19, IgG1, κ), anti-CD3 (HIT3a, IgG2a, κ), anti-CD34 (581, IgG1, κ), anti-p50 (H-119), anti-p65 (C-20), anti-TG2 (CUB 7402 + TG100, IgG1), and anti-IκBα (C-21). All Abs were purified or conjugated with the appropriate fluorochromes based on the combinations of Abs used in each experiment. Abs were purchased from BD Biosciences, BioLegend, eBioscience, or NeoMarkers. Bortezomib was obtained from the pharmacy at the MD Anderson Cancer Center. A23187 (calcimycin, calcium ionophore) was purchased from Acros Organics.
We used the same drug concentrations used in our previous study,23 which were based on our preliminary data using MCL patient samples and on the concentrations reported in various studies on human hematologic malignancies.
Xenotransplantation assay
Immunodeficient NOD/SCID mice were purchased from The Jackson Laboratory and bred and maintained in a pathogen-free facility at the University of Texas Health Science Center. The appropriate cell numbers and types, as described previously,22 were injected intraperitoneally into NOD/SCID mice given a dose of 2.25 G using γ rays from a cesium irradiator. Mice were kept until they showed obvious signs of distress or discomfort in accordance with approved guidelines established by the Animal Care Committee at the University of Texas Health Science Center.
Immunohistochemical analysis
Paraffin sections were made from xenograft tumors or spleens and slide sections were heated at 60°C for 20 minutes. Sections were deparaffinized by standard methods (ie, 100% xylene, 100% ethanol, 95% ethanol, and 80% ethanol) and Ags were retrieved by incubating slides in 0.1M citrate buffer in a vegetable steamer for 30 minutes. After cooling slides to room temperature, 0.3% H2O2 was added, and the slides were incubated for 10 minutes. After washing, slides were blocked in 10% swine serum for 30 minutes, followed by incubation with primary Abs for 2 hours. Slides were washed and incubated with biotinylated secondary Abs (Dako). Signals were detected after incubating slides with the avidin-biotin complex (Vector Laboratories), followed by incubation with diaminobenzidine (Vector Laboratories). Counterstaining was done with Mayer hematoxylin (Sigma-Aldrich) for 10 minutes at room temperature. Slides were dehydrated before photographs were taken.
Immunocytochemical analysis
The localization of TG2 and the translocation of the NF-κB components p50 and p65 were visualized by immunostaining and confocal microscopy. Confocal scanning analysis of the cells was done with a TCS SP5 laser scanning confocal microscope (Leica) in accordance with established methods, using sequential laser excitation to minimize the fluorescent emission bleed-through. Each section was examined for presence of each stain at 561, 488, or 633 nm excitations, and the data were compared pixel by pixel. Each image represented z-sections at the same cellular level and magnification. Merging red and blue showed the localization of the protein in the nuclei, producing a violet color.
Fluorometric cytotoxicity assay
Cytotoxicity was assessed with a fluorometric cell-viability assay using CellTiter-Blue (Promega). Briefly, cells were incubated for the incubated times at 37°C with determined doses of drugs. After washing treated cells, CellTiter-Blue reagents (20 μL) were added to suspended cells with new complete RPMI 1640 medium (80 μL) and incubated in 96-well plates for 4 hours at 37°C. The fluorescence signal was measured at 560Ex/590Em using a fluorescence plate reader equipped with SoftMax Pro Version 5 software (Molecular Devices). Dose-response curves were calculated based on the cell-viability assay of cells treated with each chemotherapeutic drug. Cell viability was assessed based on the value of fluorescence signal of live cells with no drug treatments. The viabilities of drug-treated cells were calculated based on a ratio of the fluorescence signal, as described previously.23,24
IC50
The IC50 (the concentration of a drug that is required for 50% inhibition in vitro) was used to indicate the quantitative measure of the different cell killing effects of the drugs. The Hill-Slope logistical model was used to calculate IC50 using CompuSyn software (ComboSyn).
Drug combination assay
The synergic cytotoxic effects of bortezomib and conventional combination chemotherapeutic regimens were determined by the combination index (CI) method based on the Chou and Talalay equation,39 and analyzed with CompuSyn software, as described previously.39–41
Flow cytometry
CD3+ cells, CD34+ cells, and CD19+ cells were deleted using the magnetic beads selection method. After culture with bortezomib or the combination of bortezomib and POH, CD3−CD34−CD19− MCL-ICs were stained by 7-amino-actinomycin (BD Pharmingen) and then analyzed with FACS LSRII flow cytometer (BD Biosciences). All assays were performed in duplicate.
TG2 enzymatic activity assay
The levels of TG2 enzymatic activity were determined using the TG2-CovTest TG2-specific colorimetric assay kit (Novus Biologicals). In this assay, the color intensity is directly proportional to the TG2 activity in the sample. All test steps were performed according to the manufacturer's instructions.
Proteasome activity assay
The 26S proteasome, an ATP-dependent proteolytic complex, is formed by the association of the barrel-shaped 20S proteasome and two 19S regulatory complexes.42 This catalytic core of the proteasome complex is responsible for the breakdown of key proteins involved with apoptosis, DNA repair, endocytosis, and cell-cycle control.43 We tested proteasome activity using a proteasome activity assay kit (Chemicon).
Statistical analysis
All assays were performed in duplicate or triplicate, and data are expressed as means ± SD. Statistical analyses were performed using SPSS Version 12.0 software for Windows. Statistical significance of differences between the cell groups was evaluated by the Student t test. P < .05 was considered statistically significant.
Results
Expression of TG2 in MCL cells
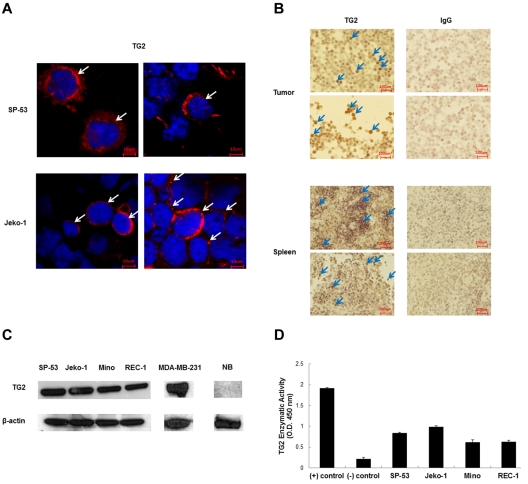
We first examined the expression of TG2 in different human MCL cell lines (Figure 1A). The confocal microscopic images showed the localization of TG2 in the cytoplasm in MCL cells. TG2 is mainly localized in the cytoplasm in the MCL cell lines SP-53 and Jeko-1. We then used xenograft tumors and spleen tissue blocks generated from human MCL cells and evaluated TG2 expression using immunohistochemistry under identical standardized conditions for all of the samples with consecutive tissue sections. Figure 1B shows the examples of TG2 and control IgG staining. The MCL cells in xenograft tumor and spleen sections from xenograft mice expressed comparable levels of human TG2.
Figure 1.
Tissue TG2 is expressed in MCL. (A) Immunocytochemistry analyses showed that the MCL cell lines SP-53 and Jeko-1 were positive for TG2 expression. Confocal microscopic images showed the localization of TG2 in the cytoplasm in MCL cells. Arrows in each panel indicate representative signals. (B) Xenograft tumor and spleen sections generated from sorted CD45+CD19− MCL-ICs were immunostained for TG2 protein expression. Representative sections with positive immunostaining for the anti–human TG2 Ab and the anti–human IgG Ab as a control are shown. The xenograft tumors and spleens expressed high levels of human TG2 (arrows). At least 5 sections from each xenograft tumor were analyzed. (C) Immunoblot analyses of TG2 expression were performed using cell lysate proteins of 4 different MCL cell lines, SP-53, Jeko-1, Mino, and REC-1. The breast cancer cell line MDA-MB-231 was used as a positive control and normal B cells were used as negative controls. β-actin was also used as a control. TG2 was expressed in MCL cells. (D) TG2 enzymatic activity was measured using TG2-CovTest TG2-specific colorimetric assay kit (Novus Biologicals) using extracts from SP-53, Jeko-1, Mino, and REC-1 cells. Recombinant human TG2 was used as a positive control, and normal B cells were used as negative controls. Comparable levels of enzymatic activities were noted between the different MCL cell lines.
To further confirm the expression of TG2 in MCL, we determined TG2 protein expression via immunoblots using 4 different MCL cell lines: SP-53, Jeko-1, Mino, and REC-1. Immunoblots revealed that the TG2 protein was constitutively expressed in different MCL cell lines (Figure 1C). Although TG2 expression in MCL cell lines were detectable, these levels were lower than that of a breast cancer line MDA-MB-231, which expresses robust levels of TG2 and has been used to study breast cancer metastasis.
Because TG2 is an enzyme with catalytic function, we also measured the functional enzymatic activities of TG2 in MCL cell lines using an ELISA-based colorimetric assay kit. The result indicated that there are functional TG2 enzymatic activities in MCL (Figure 1D). Recombinant human TG2 was used in the assay to measure TG2 enzymatic activity.
These results show that TG2 is expressed and has enzymatic activity in different MCL cells.
TG2 expression is associated with NF-κB activation in MCL
The link between TG2 and NF-κB has been reported in stressful cellular conditions such as infection, inflammation, and cancer. The biologic action of TG2 could be mediated by TG2-induced nonphosphorylated IκBα modifications such as polymerization or the change of the binding of IκBα to the NF-κB complex.35,37,44,45 TG2 not only forms a complex with NF-κB/IκBα, but also associates with the p50/p65 complex, interfering with the binding of IκBα to NF-κB complex.35,37 Conversely, TG2 and calcium can catalyze the IκBα protein into the polymeric forms of IκBα, which have a lower binding affinity for the p65/p50 complex.38 Some studies have suggested that TG2 forms a ternary complex with NF-κB/IκBα and translocates to the nucleus in a complex with p65/p50.35
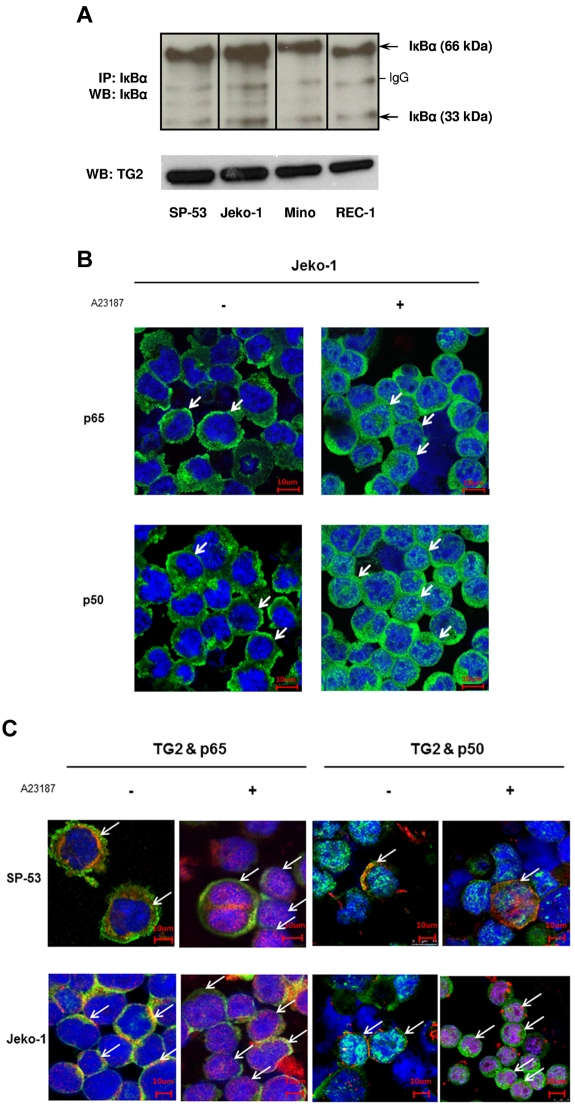
To determine the association of TG2 expression with NF-κB, we first analyzed the forms of IκBα in TG2-expressing MCL cells. Immunoprecipitation followed by Western blotting of cell extracts from MCL cell lines with an anti-IκBα Ab indicated the presence of dimeric and monomeric bands of IκBα (Figure 2A). Interestingly, the intensities of the dimeric bands were higher than those of the monomeric bands, suggesting that TG2-expressing MCL cells constitutively display IκBα polymerization and that TG2 may play an important role in constitutive activation of NF-κB.
Figure 2.
TG2 is associated with NF-κB expression in MCL. (A) Cell lysates from 4 different MCL cell lines, SP-53, Jeko-1, Mino, and REC-1, were immunoprecipitated and immunoblotted with an anti-IκBα Ab. Analysis indicated the presence of strong dimeric forms (66 kDa) and monomeric forms (33 kDa) of IκBα. The polymeric forms of IκBα persisted in TG2-expressed MCL cells. IP indicates immunoprecipitation; WB, Western blot. (B) TG2 activation induced NF-κB activation after translocation to the nucleus and increased the expression of NF-κB components in MCL cells. SP-53 and Jeko-1 cells were treated with A23187 (2μM for 24 hours), followed by immunochemical staining using anti-p65 and anti-p50 Abs. Confocal microscopic images revealed the translocation of NF-κB components p65 and p50 into the nucleus (arrows) in response to A23187 treatment. A further increase in the cytoplasmic intensity of p65 and p50 was clearly observed in Jeko-1 cells after A23187 treatment. (C) TG2 and NF-κB components were colocalized in MCL cells. Confocal microscopic images showed the colocalization of TG2 and NF-κB components p65 and p50 (arrows). Before treatment with A23187, TG2 (red) and the NF-κB components p65 or p50 (green) were located mainly in the cytoplasm. After A23187 treatment (2μM for 24 hours), simultaneous translocations of TG2 and the NF-κB components p65 or p50 were observed.
We also investigated whether TG2 formed a complex with NF-κB components and mediated translocation of NF-κB to the nucleus. Figure 2B shows the effects of TG2 on NF-κB components in MCL. A23187-induced TG2 activation increased p65 and p50 translocation to the nucleus from the cytoplasm. Further confocal microscope staining supported the colocalization of TG2 and p65 in MCL cells (Figure 2C). TG2 and p65 mainly localized in the cytoplasm without treatment. However, the translocation of the TG2/p65 complex into the nucleus was observed in A23187-treated MCL cells. Immunoprecipitation of Jeko-1 cell lysates with an anti-TG2 Ab readily pulled down the NF-κB component p65 (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Furthermore, the intensity of TG2-conjugated p65 bands was increased in response to treatment with the TG2 activator A23187, but decreased in response to a specific TG2 inhibitor (monodansylcadaverine, MDC), and a calcium blocker (POH; supplemental Figure 1). These findings show that TG2 forms a complex with NF-κB and IκBα and further regulates the affinity between TG2 and p65 in MCL cells. These results show that TG2 is associated with a complex of IκBα and NF-κB components and has effects on NF-κB expression in MCL, similar to previous reports in other cancer cells.33,35,37
TG2 expression is correlated with NF-κB activation in MCL
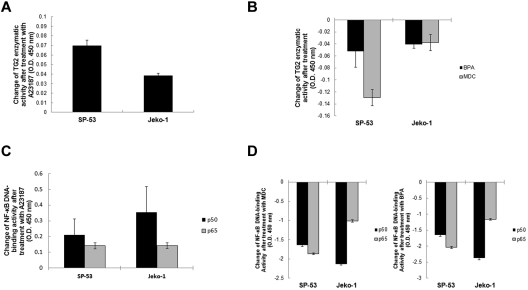
To determine the effects of TG2 on NF-κB expression, we investigated whether A23187-mediated TG2 activation increased NF-κB activity. We treated SP-53 and Jeko-1 cells with A23187 and measured the enzymatic activities of TG2 in MCL cells before and after A23187 treatment using colorimetric assay kits. TG2 enzymatic activities of SP-53 and Jeko-1 cells increased in response to A23187 treatments (Figure 3A). We examined the DNA-binding activities of NF-κB transcription factors in A23187-treated MCL cells using nuclear extracts of each cell type in an ELISA-based assay. NF-κB p65 and p50 DNA-binding activities were dramatically increased after A23187-mediated TG2 activation in SP-53 and Jeko-1 cells (Figure 3B). These results clearly show that TG2 activation resulted in NF-κB activation.
Figure 3.
TG2 activity is correlated with NF-κB expression in MCL. (A) SP-53 and Jeko-1 cells were treated with A23187 (2μM for 24 hours) to activate TG2, and TG2 enzymatic activity was measured using TG2-CovTest TG2-specific colorimetric assay kit (Novus Biologicals) with cell extracts from untreated and treated SP-53 and Jeko-1 cells. Changes in TG2 enzymatic levels before and after treatment with A23187 were measured. The colorimetric values (optical density, 450 nm) in untreated samples were subtracted from the values measured in treated samples. Results are shown as the means ± SD. Treatment with A23187 clearly induced the activation of TG2. (B) Nuclear extracts from SP-53 and Jeko-1 cells that were untreated or treated with A23187 (2μM for 24 hours) were analyzed using ELISA assays to evaluate p50 and p65 DNA-binding activities. Changes in p50 and p65 DNA-binding activity levels before and after treatment with A23187 were measured. The colorimetric values (optical density, 450 nm) in untreated samples were subtracted from the values measured in treated samples. Results are shown as the means ± SD. TG2 activation increased NF-κB DNA-binding activities in MCL cells. (C) SP-53 and Jeko-1 cells were treated with MDC (50μM for 24 hours) or BPA (1mM for 24 hours) for TG2 inhibition. TG2 enzymatic activities were measured using the TG2-specific colorimetric assay with cell extracts from untreated and treated SP-53 and Jeko-1 cells. Changes in TG2 enzymatic levels before and after treatment with MDC or BPA were measured. The colorimetric values in untreated samples were subtracted from those measured in treated samples. Results are shown as the means ± SD. Treatment with the TG2-specific inhibitors MDC and BPA inhibited TG2 expression. (D) Nuclear extracts from SP-53 and Jeko-1 cells with or without the TG2-specific inhibitors MDC (50μM for 24 hours) and BPA (1mM for 24 hours) were subjected to ELISA assays to evaluate p50 and p65 DNA-binding activities. Changes in p50 and p65 DNA-binding activity levels before and after treatment with MDC or BPA were measured. The colorimetric values in untreated samples were subtracted from those measured in treated samples. Results are shown as the means ± SD. TG2 inhibition suppressed NF-κB expression in MCL cells.
We also investigated whether inhibition of TG2 activity decreased NF-κB activity. We treated SP-53 and Jeko-1 cells with specific TG2 inhibitors, MDC and BPA, and measured the enzymatic activities of TG2 in MCL cells before and after treatment. TG2 enzymatic activities of SP-53 and Jeko-1 cells were decreased after treatment with MDC or BPA (Figure 3C). Treatment with MDC and BPA for 4 hours (supplemental Figure 2A) or 12 hours (Figure 3D) further inhibited the NF-κB DNA-binding activities of p65 and p50 in SP-53 and Jeko-1 cells. Treatment with MDC and BPA did not significantly affect cell viability (supplemental Figure 2B). These results demonstrate that TG2 enzymatic activities are closely linked to NF-κB expression status in MCL cells.
Calcium blockers inhibit NF-κB activation and TG2 activity in MCL
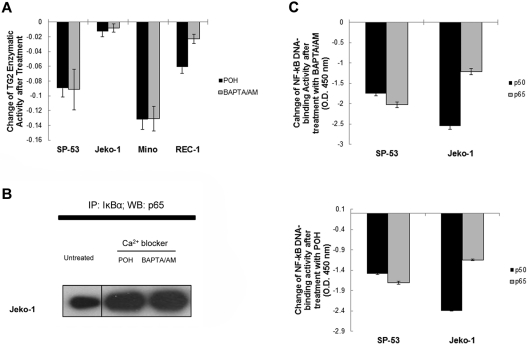
Calcium-channel antagonists are generally used for various cardiovascular diseases such as hypertension, arrhythmia, or cluster headaches, and have been implicated recently in combination-drug regimens to improve the function of anticancer agents such as bortezomib.13,25,26 TG2 is a calcium-dependent cross-linking enzyme and, in addition to calcium, catalyzes the polymerization of IκBα.37,38 We hypothesized that calcium blockers inhibit NF-κB activation by inhibiting TG2 function. We determined that calcium blockers inhibited TG2 enzymatic activities using POH, a monoterpene that inhibits L-type calcium channels, and a calcium chelator, 1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid-acetoxymethyl ester (BAPTA/AM). Figure 4A shows that POH and BAPTA/AM inhibited the TG2 enzymatic activities in SP-53, Jeko-1, Mino, and Rec-1 cells. To confirm the effects of calcium blockers on the affinity between IκBα and p65 after TG2 inhibition, cell extracts from untreated, POH-treated, and BAPTA/AM-treated Jeko-1 cells were immunoprecipitated using an anti-IκBα Ab and probed with an anti-p65 Ab (Figure 4B). The intensity of IκBα-conjugated p65 bands was increased in response to POH or BAPTA/AM treatments, supporting the effects of calcium blockers on the TG2-IκBα-p65 complex. We examined the DNA-binding activities of NF-κB transcription factors in calcium blocker–treated MCL cells using nuclear extracts of each cell type. NF-κB p65 and p50 DNA-binding activities were readily decreased after BAPTA/AM or POH treatment in SP-53 and Jeko-1 cells (Figure 4C). Four-hour treatment of BAPTA/AM or POH also reduced the DNA-binding activities of NF-κB components (supplemental Figure 2C). These results confirm that calcium blockers inhibited NF-κB expression in MCL through the inhibition of TG2 activities.
Figure 4.
Calcium blockers suppressed TG2 and NF-κB activity in MCL. (A) SP-53, Jeko-1, Mino, and REC1 cells were treated with the intracellular Ca2+ chelator BAPTA/AM (60μM for 24 hours) or the L-type calcium-channel blocker POH (1mM for 24 hours). TG2 enzymatic activity was subsequently measured using a TG2-CovTest TG2-specific colorimetric assay kit (Novus Biologicals) with cell extracts from untreated and treated SP-53 and Jeko-1 cells. Changes in TG2 enzymatic levels before and after treatment with BAPTA/AM or POH were measured. The colorimetric values in untreated samples were subtracted from the values measured in treated samples. Results are shown as the means ± SD. Treatment with the calcium blockers BAPTA/AM or POH suppressed TG2 expression in MCL cell lines. (B) Calcium blockers affected the affinity between IκBα and p65 in MCL cells. Jeko-1 cells were treated with BAPTA/AM (60μM) or POH (1mM) for 24 hours. Cell lysates were prepared from untreated and treated SP-53 and Jeko-1 cells, immunoprecipitated using an anti-IκBα Ab, and probed with an anti-p65 Ab. Equal amounts of proteins were used. The amount of p65 proteins that interacted with IκBα was increased in response to BAPTA/AM or POH treatment. IP indicates immunoprecipitation; and WB, Western blot. (C) Calcium blocker–induced TG2 inhibition suppressed NF-κB activity in MCL cells. Nuclear extracts from SP-53 and Jeko-1 cells that were untreated or treated with BAPTA/AM (60μM for 24 hours) or POH (1mM for 24 hours) were analyzed for p50 and p65 DNA-binding activities using ELISA assays. Changes in p50 and p65 DNA-binding activity levels before and after treatment with BAPTA/AM or POH were measured. The colorimetric values in untreated samples were subtracted from the values measured in treated samples. Results are shown as the means ± SD.
Calcium blockers affect TG2 activity and NF-κB activation in CD45+CD19− MCL-ICs
We investigated whether CD45+CD19− MCL-ICs expressed high levels of TG2 and whether calcium blockers reduced NF-κB expression by inhibiting TG2 activities in CD45+CD19− MCL-ICs, as was shown in MCL cell lines. We analyzed the expression of TG2 protein in 5 different CD45+CD19− MCL-ICs using Western blots (Figure 5A). Western blot analyses and ELISA revealed that all primary CD45+CD19− MCL-ICs expressed constitutive NF-κB components and DNA-binding activities (supplemental Figure 3A-B). We measured the enzymatic activities of TG2 in CD45+CD19− MCL-ICs using colorimetric assay kits. The levels of TG2 enzymatic activity expression in CD45+CD19− MCL-ICs were comparable to the positive control recombinant human TG2 (Figure 5B). These findings indicate that both CD45+CD19− MCL-ICs and MCL cell lines express functional TG2. To confirm the association of TG2 expression with NF-κB in CD45+CD19− MCL-ICs, we investigated the forms of IκBα in CD45+CD19− MCL-ICs. Immunoprecipitation and Western blotting of cell extracts from 5 different CD45+CD19− MCL-ICs with an anti-IκBα Ab showed the presence of dimeric bands of IκBα (supplemental Figure 4A).
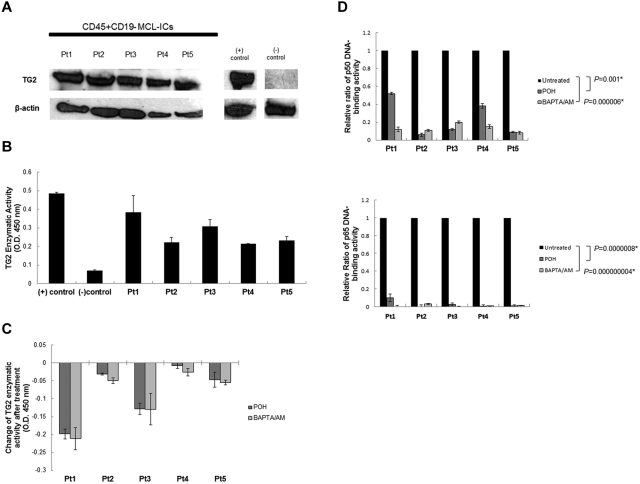
Figure 5.
Calcium blockers affect TG2 expression and NF-κB activity in CD45+CD19− MCL-ICs. (A) Stem like MCL cells (CD45+CD19− MCL-ICs) expressed TG2 protein. Western blot analysis of TG2 expression was performed using cell lysates from 5 different primary CD45+CD19− MCL-ICs. The breast cancer cell line MDA-MB-231 was used as a positive control, and normal B cells were used as negative controls. β-actin was used as a loading control. Pt indicates patient. (B) Stem-like MCL cells expressed TG2 enzymatic activities comparable to positive controls. TG2 enzymatic activity was measured using the TG2-CovTest TG2-specific colorimetric assay kit (Novus Biologicals) with cell extracts from 5 different primary CD45+CD19− MCL-ICs. Recombinant human TG2 was used as a positive control, and normal B cells were used as negative controls. (C) Treatment with BAPTA/AM or POH suppressed TG2 enzymatic activity in CD45+CD19− MCL-ICs. Five different CD45+CD19− MCL-ICs were treated with BAPTA/AM (60μM) or POH (1mM) for 16 hours. TG2 enzymatic activity was measured using a TG2-specific colorimetric assay kit with cell extracts from untreated and treated CD45+CD19− MCL-ICs. Changes in TG2 enzymatic levels before and after treatment with BAPTA/AM or POH were measured. The colorimetric values in untreated samples were subtracted from the values measured in treated samples. Results are shown as the means ± SD. (D) Calcium blockers inhibited NF-κB DNA-binding activities in primary CD45+CD19− MCL-ICs. Nuclear extracts from CD45+CD19− MCL-ICs that were untreated or treated with BAPTA/AM (60μM for 16 hours) or POH (1mM for 16 hours) were analyzed using ELISA assays to evaluate p50 and p65 DNA-binding activities. The relative ratio values of NF-κB DNA-binding activities after drug treatment are shown as the means ± SD. *P < .05 by unpaired t test.
Because calcium blockers inhibited NF-κB expression and TG2 activities in MCL cell lines, we further investigated the effects of calcium blockers on TG2 activities and NF-κB expression in primary CD45+CD19− MCL-ICs. We treated 5 different CD45+CD19− MCL-ICs with POH or BAPTA/AM and measured the enzymatic activities of TG2 in untreated and treated cells using ELISA-based colorimetric assays. In all of the tested MCL-ICs, TG2 enzymatic activities were decreased after treatment with calcium blockers (Figure 5C). The DNA-binding activities of NF-κB transcription factors in CD45+CD19− MCL-ICs were measured before and after treatment with POH or BAPTA/AM (Figure 5D). After treatment with calcium blockers, the NF-κB DNA-binding activities of p65 and p50 were decreased by more than 90% in CD45+CD19− MCL-ICs, with the exception of patient 1. In patient 1, BAPTA/AM effectively suppressed p50 DNA-binding activities, although POH only decreased p50 DNA-binding activities by 50%. This could have been because of patient-to-patient variability in drug response. To confirm the effects of calcium blockers in NF-κB DNA-binding activities, we treated cells for shorter period of time. NF-κB binding activities were also decreased at 4 hours, revealing effects of calcium blockers in NF-κB DNA-binding activities (supplemental Figure 4B).
These results show that stem-like MCL cells express TG2 protein and that calcium blockers inhibit TG2 activities to block constitutive NF-κB expression in MCL-ICs.
Calcium blockers synergistically improve the cytotoxicity of bortezomib on CD45+CD19− MCL-ICs
We demonstrated previously that CD45+CD19− MCL-ICs are resistant to bortezomib.24 The IC50 values of bortezomib on CD45+CD19− MCL-ICs were much higher than CD45+CD19+ MCL cells that were isolated from the same patients and MCL cell lines (supplemental Figure 5A). We hypothesized that calcium blockers may be used as a combinatorial therapy with bortezomib by overcoming the bortezomib resistance in CD45+CD19− MCL-ICs. To assess the effects of calcium blockers on NF-κB expression using a proteasome-independent pathway, we compared the proteasome activities of CD45+CD19− MCL-ICs after treatment with bortezomib and the calcium blockers POH and BAPTA/AM using colorimetric substrate-based assay kits (supplemental Figure 5B). In contrast to bortezomib, sustainable proteasome activities were noted after treatment with POH or BAPTA/AM.
To evaluate the synergy between bortezomib and calcium blockers, we decided to use POH as a calcium blocker after considering clinical availability because it has been used previously as a chemotherapeutic and chemopreventive drug in multiple clinical trials.25,46,47 We compared the effects on NF-κB suppression between bortezomib alone and the combination of bortezomib and POH. The DNA-binding activities of NF-κB transcription factors in CD45+CD19− MCL-ICs were measured before and after treatment with bortezomib alone or with the combination of bortezomib and POH (Figure 6A). The combination of bortezomib and POH significantly decreased the NF-κB DNA-binding activities of p65 and p50 compared with the bortezomib alone treatment (P < .05).
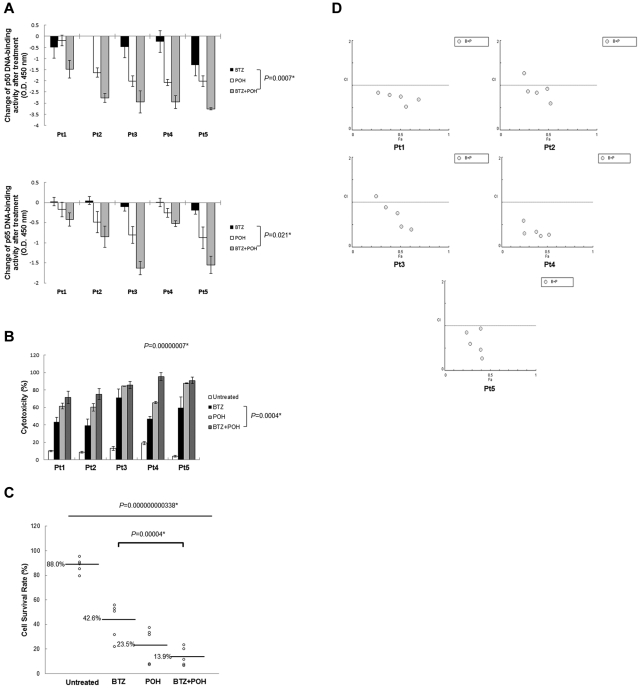
Figure 6.
POH improves the cytotoxic effects of bortezomib on CD45+19− MCL-ICs. (A) The combination of bortezomib and POH inhibited NF-κB DNA-binding activities in CD45+CD19− MCL-ICs compared with bortezomib. Stem-like cells (CD45+CD19−) from 5 different patients were treated with bortezomib (100nM), POH (1mM), or the combination of bortezomib (100nM) and POH (1mM) for 16 hours. Nuclear extracts from untreated, bortezomib-treated, POH-treated, and combination-treated CD45+CD19− MCL-ICs were analyzed using ELISA assays to assess p50 and p65 DNA-binding activities. Changes in NF-κB DNA-binding activity levels before and after treatment were measured. The colorimetric values in untreated samples were subtracted from the values measured in treated samples. Results are shown as the means ± SD. *P < .05 by unpaired t test. BTZ indicates bortezomib. (B) The addition of POH to bortezomib significantly increased the cytotoxicity in CD45+CD19− MCL-ICs. Cell viability of CD45+CD19− primary MCL-ICs were measured after 16 hours of incubation with bortezomib (100nM), POH (1mM), or the combination of bortezomib (100nM) and POH (1mM) using 7-amino-actinomycin–stained flow cytometry. The columns represent the percentages of dead cells, and the results are shown as the means ± SD. P values were calculated using the ANOVA test and the unpaired t test. *P < .05. BTZ indicates bortezomib. (C) Live cell proportions were analyzed using 7-amino-actinomycin–stained flow cytometry with untreated, bortezomib-treated, POH-treated, and combination-treated CD45+CD19− MCL-ICs. The combination treatement inhibited the survival of CD45+CD19− MCL-ICs compared with bortezomib alone, POH alone, or no treatment. P values were calculated using the ANOVA test and the unpaired t test. *P < .05. BTZ indicates bortezomib. (D) The synergic cytotoxic effects of bortezomib and POH were determined using the CI based on the data from cell viability assays. CI plots were generated using CompuSyn software according to the Chou-Talalay method. The combination is synergic when CI < 1.0, additive when CI = 1, and antagonistic when CI > 1.0. POH synergized with bortezomib to induce cytotoxicity in CD45+CD19− MCL-ICs.
To compare the cytotoxic effects between bortezomib alone treatment and combination treatment of bortezomib and POH in CD45+CD19− MCL-ICs, we analyzed whether the dead and live cell proportions of CD45+CD19− MCL-ICs were changed after treatment with bortezomib alone or the combination of bortezomib and POH using 7-amino-actinomycin–stained flow cytometry (supplemental Figure 6A). CD45+CD19− MCL-ICs were treated with bortezomib (100nM) alone or the combination of bortezomib (100nM) and POH (1mM) for 16 hours, followed by flow cytometric analysis. The combination of bortezomib and POH significantly induced the cytotoxicity of CD45+CD19− MCL-ICs compared with bortezomib alone (P < .05; Figure 6B). Cell survival rates were also significantly decreased in response to the combined treatment of bortezomib and POH (P < .05; Figure 6C). The more effective cytotoxic effects of the combined treatment of bortezomib and POH were confirmed using cell viability tests. CD45+CD19− MCL-ICs from 5 different patient samples were treated for 16 hours with bortezomib alone or the combination of bortezomib and POH. Cell viability was measured using a fluorometric assay, and the percentage of cell survival was calculated using the ratio between treated and untreated cells. Compared with treatment with bortezomib alone, the combination of POH with bortezomib improved the cytotoxic effects of bortezomib in CD45+CD19− MCL-ICs (supplemental Figure 6B).
The synergistic cytotoxic effects of bortezomib and POH were analyzed using CI plots based on the Chou and Talalay method. When CD45+CD19− MCL cells were treated with the combination of bortezomib and POH, the majority of CI values were less than 1.0, suggesting synergism (Figure 6D).
These findings indicate that the combination treatment of calcium-channel blockers such as POH with bortezomib induced the suppression of NF-κB expression in CD45+CD19− MCL-ICs and decreased the bortezomib-resistant properties of MCL-ICs.
Discussion
MCL is a particularly deadly subtype of B-cell lymphoma and is highly refractory to most antitumor therapies.4–7 Although various new drugs such as bortezomib have improved the treatment options for MCL patients, the effects of these treatments are not sufficient to cure MCL. MCL patients commonly became resistant or refractory to bortezomib.13–15 To overcome this bortezomib resistance in MCL, a new combinatorial treatment with bortezomib for MCL must be developed.
TG2 is a unique member of the TG family that has multiple normal cellular processes such as apoptosis, wound healing, cell migration, and cell survival.28,29 TG2 is also associated with certain pathologic conditions such as inflammatory diseases and other malignancies.28,35,44 Many studies have determined that TG2 is expressed in various types of cancer, such as cancers of the breast, pancreas, and brain.35,36,48 Some studies have shown that high levels of TG2 expression are related to chemotherapy resistance in cancer cells, and TG2 overexpression is linked to constitutive activation of NF-κB. These studies have also suggested that TG2 overexpression and subsequent NF-κB activation contribute to chemotherapy resistance in the malignant cells.33,35,37,49
Because MCL is a representative chemotherapy-resistant subtype of lymphoma, we hypothesized that MCL expresses TG2 and that the modification of TG2 expression alters NF-κB activation in MCL cells. In addition, we rationalized that the inhibition of TG2 expression may improve the sensitivity of MCL cells to bortezomib, a proteosome inhibitor that inhibits NF-κB to induce apoptosis in cancer cells. In the present study, we demonstrated that TG2 was expressed in MCL cells (Figure 1), including CD45+CD19− MCL-ICs (Figure 5A-B), which are highly resistant to conventional anti-MCL regimens23 and bortezomib,24 and that the modification of TG2 activities altered NF-κB expression in MCL cells (Figure 3).
Because TG2 is a calcium-dependent enzyme,28,29 we hypothesized that calcium blockers may be a promising combination regimen with bortezomib to improve the sensitivity of MCL to bortezomib by modifying TG enzymatic activities. In the present study, we investigated whether POH, which is currently used clinically, augments the antitumor properties of bortezomib in MCL. POH is a naturally occurring monoterpene and blocks L-type calcium channels. This drug has been reported to inhibit cancer cell growth and to enhance the pro-apoptotic effects of combined chemotherapeutic drugs such as bortezomib or cisplatin in several malignant tumors including MCL.13,25,26 We have shown herein that the combination of POH with bortezomib suppressed NF-κB expression and enhanced the cytotoxicity of bortezomib in CD45+CD19−MCL-ICs (Figure 6).
In conclusion, this study is the first to show the expression of TG2 and the contribution of TG2 to NF-κB in the hematologic malignancy MCL. Our findings provide evidence for a new combination regimen of bortezomib and calcium blockers to overcome the bortezomib resistance of MCL. Moreover, this study raises the possibility that TG2 inhibition may be an attractive new target that could be used to improve the cure rates of MCL patients.
Supplementary Material
Acknowledgments
Tissue samples were provided by the University of Texas MD Anderson Cancer Center Satellite Lymphoma Tissue Bank, which is supported by institutional core grant CA16672 and by MD Anderson Cancer Center Lymphoma Specialized Programs of Research Excellence (SPORE) grant P50CA136411 from the National Cancer Institute/National Institutes of Health. This work was funded by a Conquer Cancer Now award (CONCERN Foundation), a National Cancer Institute/National Institutes of Health grant, and an American Cancer Society Scholar Award (all to N.M.).
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: H.J.J. performed and analyzed the experiments and wrote the manuscript; Z.C. performed and analyzed the experiments; M.W., L.F., J.R., and L.W.K. contributed the samples; and N.M. supervised and planned the experiments and wrote the manuscript.
Conflict-of-interest disclosure: J.R. is a principal investigator for clinical research protocols funded by Millennium Pharmaceuticals. The remaining authors declare no competing financial interests.
Correspondence: Nami McCarty, University of Texas Health Science Center at Houston, 1835 Pressler St, IMM-630F, Houston, TX 77030; e-mail: nami.mccarty@uth.tmc.edu.
References
- 1.Romaguera JE, Fayad LE, Feng L, et al. Ten-year follow-up after intense chemoimmunotherapy with Rituximab-HyperCVAD alternating with Rituximab-high dose methotrexate/cytarabine (R-MA) and without stem cell transplantation in patients with untreated aggressive mantle cell lymphoma. Br J Haematol. 2010;150(2):200–208. doi: 10.1111/j.1365-2141.2010.08228.x. [DOI] [PubMed] [Google Scholar]
- 2.Goy A, Kahl B. Mantle cell lymphoma: The promise of new treatment options. Crit Rev Oncol Hematol. 2011;80(1):69–86. doi: 10.1016/j.critrevonc.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 3.Romaguera JE, Fayad LE, McLaughlin P, et al. Phase I trial of bortezomib in combination with rituximab-HyperCVAD alternating with rituximab, methotrexate and cytarabine for untreated aggressive mantle cell lymphoma. Br J Haematol. 2010;151(1):47–53. doi: 10.1111/j.1365-2141.2010.08315.x. [DOI] [PubMed] [Google Scholar]
- 4.Campo E, Raffeld M, Jaffe ES. Mantle-cell lymphoma. Semin Hematol. 1999;36(2):115–127. [PubMed] [Google Scholar]
- 5.Mounter PJ, Lennard AL. Management of non-Hodgkin's lymphomas. Postgrad Med J. 1999;75(879):2–6. doi: 10.1136/pgmj.75.879.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salaverria I, Perez-Galan P, Colomer D, Campo E. Mantle cell lymphoma: from pathology and molecular pathogenesis to new therapeutic perspectives. Haematologica. 2006;91(1):11–16. [PubMed] [Google Scholar]
- 7.Pileri SA, Falini B. Mantle cell lymphoma. Haematologica. 2009;94(11):1488–1492. doi: 10.3324/haematol.2009.013359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kane RC, Dagher R, Farrell A, et al. Bortezomib for the treatment of mantle cell lymphoma. Clin Cancer Res. 2007;13(18 Pt 1):5291–5294. doi: 10.1158/1078-0432.CCR-07-0871. [DOI] [PubMed] [Google Scholar]
- 9.Rajkumar SV, Richardson PG, Hideshima T, Anderson KC. Proteasome inhibition as a novel therapeutic target in human cancer. J Clin Oncol. 2005;23(3):630–639. doi: 10.1200/JCO.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 10.Voorhees PM, Dees EC, O'Neil B, Orlowski RZ. The proteasome as a target for cancer therapy. Clin Cancer Res. 2003;9(17):6316–6325. [PubMed] [Google Scholar]
- 11.Adams J. The proteasome: a suitable antineoplastic target. Nat Rev Cancer. 2004;4(5):349–360. doi: 10.1038/nrc1361. [DOI] [PubMed] [Google Scholar]
- 12.Pham LV, Tamayo AT, Yoshimura LC, Lo P, Ford RJ. Inhibition of constitutive NF-kappa B activation in mantle cell lymphoma B cells leads to induction of cell cycle arrest and apoptosis. J Immunol. 2003;171(1):88–95. doi: 10.4049/jimmunol.171.1.88. [DOI] [PubMed] [Google Scholar]
- 13.Yang DT, Young KH, Kahl BS, Markovina S, Miyamoto S. Prevalence of bortezomib-resistant constitutive NF-kappaB activity in mantle cell lymphoma. Mol Cancer. 2008;7:40. doi: 10.1186/1476-4598-7-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O'Connor OA, Wright J, Moskowitz C, et al. Phase II clinical experience with the novel proteasome inhibitor bortezomib in patients with indolent non-Hodgkin's lymphoma and mantle cell lymphoma. J Clin Oncol. 2005;23(4):676–684. doi: 10.1200/JCO.2005.02.050. [DOI] [PubMed] [Google Scholar]
- 15.Goy A, Younes A, McLaughlin P, et al. Phase II study of proteasome inhibitor bortezomib in relapsed or refractory B-cell non-Hodgkin's lymphoma. J Clin Oncol. 2005;23(4):667–675. doi: 10.1200/JCO.2005.03.108. [DOI] [PubMed] [Google Scholar]
- 16.Fribley A, Zeng Q, Wang C.-Y. Proteasome inhibitor PS341 induces apoptosis through induction of endoplasmic reticulum stress-reactive oxygen species in head and neck squamous cell carcinoma cells. Mol Cell Biol. 2004;24(22):9695–9704. doi: 10.1128/MCB.24.22.9695-9704.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Obeng EA, Carlson LM, Gutman DM, Harrington WJ, Lee KP, Boise LH. Proteasome inhibitors induce a terminal unfolded protein response in multiple myeloma cells. Blood. 2006;107(12):4907–4916. doi: 10.1182/blood-2005-08-3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weniger MA, Rizzatti EG, Perez-Galan P, et al. Treatment-induced oxidative stress and cellular antioxidant capacity determine response to bortezomib in mantle cell lymphoma. Clin Cancer Res. 2011;17(15):5101–5112. doi: 10.1158/1078-0432.CCR-10-3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ri M, Iida S, Nakashima T, et al. Bortezomib-resistant myeloma cell lines: a role for mutated PSMB5 in preventing the accumulation of unfolded proteins and fatal ER stress. Leukemia. 2010;24(8):1506–1512. doi: 10.1038/leu.2010.137. [DOI] [PubMed] [Google Scholar]
- 20.Hideshima T, Ikeda H, Chauhan D, Okawa Y, Raje N, et al. Bortezomib induces canonical nuclear factor-kappaB activation in multiple myeloma cells. Blood. 2009;114(5):1046–1052. doi: 10.1182/blood-2009-01-199604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dolcet X, Llobet D, Encinas M, et al. Proteasome inhibitors induce death but activate NF-kappaB on endometrial carcinoma cell lines and primary culture explants. J Biol Chem. 2006;281(31):22118–22130. doi: 10.1074/jbc.M601350200. [DOI] [PubMed] [Google Scholar]
- 22.Chen Z, Ayala P, Wang M, et al. Prospective isolation of clonogenic mantle cell lymphoma-initiating cells. Stem Cell Res. 2010;5(3):212–225. doi: 10.1016/j.scr.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jung HJ, Chen Z, McCarty N. Stem-like tumor cells confer drug resistant properties to mantle cell lymphoma. Leuk Lymphoma. 2011;52(6):1066–79. doi: 10.3109/10428194.2011.562570. [DOI] [PubMed] [Google Scholar]
- 24.Jung HJ, Chen Z, Fayad L, et al. Bortezomib-resistant nuclear factor kappa B expression in stem like cells in mantle cell lymphoma (MCL). Exp Hematol. 2012;40(2):107–118. doi: 10.1016/j.exphem.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yeruva L, Hall C, Elegbede JA, Carper SW. Perillyl alcohol and methyl jasmonate sensitize cancer cells to cisplatin. Anticancer Drugs. 2010;21(1):1–9. doi: 10.1097/CAD.0b013e32832a68ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berchtold CM, Chen KS, Miyamoto S, Gould MN. Perillyl alcohol inhibits a calcium-dependent constitutive nuclear factor-kappaB pathway. Cancer Res. 2005;65(18):8558–8566. doi: 10.1158/0008-5472.CAN-04-4072. [DOI] [PubMed] [Google Scholar]
- 27.Meister S, Frey B, Lang VR, et al. Calcium channel blocker verapamil enhances endoplasmic reticulum stress and cell death induced by proteasome inhibition in myeloma cells. Neoplasia. 2010;12(7):550–561. doi: 10.1593/neo.10228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Griffin M, Casadio R, Bergamini CM. Transglutaminases: nature's biological glues. Biochem J. 2002;368(pt 2):377–396. doi: 10.1042/BJ20021234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boehm JE, Singh U, Combs C, Antonyak MA, Cerione RA. Tissue transglutaminase protects against apoptosis by modifying the tumor suppressor protein p110 Rb. J Biol Chem. 2002;277(23):20127–20130. doi: 10.1074/jbc.C200147200. [DOI] [PubMed] [Google Scholar]
- 30.Mehta K, Fok J, Miller FR, Koul D, Sahin AA. Prognostic significance of tissue transglutaminase in drug resistant and metastatic breast cancer. Clin Cancer Res. 2004;10(23):8068–76. doi: 10.1158/1078-0432.CCR-04-1107. [DOI] [PubMed] [Google Scholar]
- 31.Akimov SS, Belkin AM. Cell-surface transglutaminase promotes fibronectin assembly via interaction with the gelatin-binding domain of fibronectin: a role in TGFbeta-dependent matrix deposition. J Cell Sci. 2001;114(Pt 16):2989–3000. doi: 10.1242/jcs.114.16.2989. [DOI] [PubMed] [Google Scholar]
- 32.Yamaguchi H, Wang HG. Tissue transglutaminase serves as an inhibitor of apoptosis by cross-linking caspase 3 in thapsigargin-treated cells. Mol Cell Biol. 2006;26(2):569–579. doi: 10.1128/MCB.26.2.569-579.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim DS, Park SS, Nam BH, Kim IH, Kim SY. Reversal of drug resistance in breast cancer cells by transglutaminase 2 inhibition and nuclear factor-kappaB inactivation. Cancer Res. 2006;66(22):10936–10943. doi: 10.1158/0008-5472.CAN-06-1521. [DOI] [PubMed] [Google Scholar]
- 34.Mehta K. High levels of transglutaminase expression in doxorubicin-resistant human breast carcinoma cells. Int J Cancer. 1994;58(3):400–406. doi: 10.1002/ijc.2910580316. [DOI] [PubMed] [Google Scholar]
- 35.Mann AP, Verma A, Sethi G, et al. Overexpression of tissue transglutaminase leads to constitutive activation of nuclear factor-kappaB in cancer cells: delineation of a novel pathway. Cancer Res. 2006;66(17):8788–8795. doi: 10.1158/0008-5472.CAN-06-1457. [DOI] [PubMed] [Google Scholar]
- 36.Yuan L, Choi K, Khosla C, et al. Tissue transglutaminase 2 inhibition promotes cell death and chemosensitivity in glioblastomas. Mol Cancer Ther. 2005;4(9):1293–1302. doi: 10.1158/1535-7163.MCT-04-0328. [DOI] [PubMed] [Google Scholar]
- 37.Verma A, Mehta K. Transglutaminase-mediated activation of nuclear transcription factor-kappaB in cancer cells: a new therapeutic opportunity. Curr Cancer Drug Targets. 2007;7(6):559–565. doi: 10.2174/156800907781662275. [DOI] [PubMed] [Google Scholar]
- 38.Lee J, Kim YS, Choi DH, et al. Transglutaminase 2 induces nuclear factor-kappaB activation via a novel pathway in BV-2 microglia. J Biol Chem. 2004;279(51):53725–53735. doi: 10.1074/jbc.M407627200. [DOI] [PubMed] [Google Scholar]
- 39.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 40.Chou TC, Talalay P. Generalized equations for the analysis of inhibitions of Michaelis-Menten and higher-order kinetic systems with two or more mutually exclusive and nonexclusive inhibitors. Eur J Biochem. 1981;115(1):207–216. doi: 10.1111/j.1432-1033.1981.tb06218.x. [DOI] [PubMed] [Google Scholar]
- 41.Chou TC. Derivation and properties of Michaelis-Menten type and Hill type equations for reference ligands. J Theor Biol. 1976;59(2):253–276. doi: 10.1016/0022-5193(76)90169-7. [DOI] [PubMed] [Google Scholar]
- 42.Baumeister W, Walz J, Zuhl F, Seemuller E. The proteasome: paradigm of a self-compartmentalizing protease. Cell. 1998;92(3):367–380. doi: 10.1016/s0092-8674(00)80929-0. [DOI] [PubMed] [Google Scholar]
- 43.Coux O, Tanaka K, Goldberg AL. Structure and functions of the 20S and 26S proteasomes. Annu Rev Biochem. 1996;65:801–847. doi: 10.1146/annurev.bi.65.070196.004101. [DOI] [PubMed] [Google Scholar]
- 44.Kim SY. Transglutaminase 2 in inflammation. Front Biosci. 2006;11:3026–3035. doi: 10.2741/2030. [DOI] [PubMed] [Google Scholar]
- 45.Ientile R, Caccamo D, Griffin M. Tissue transglutaminase and the stress response. Amino Acids. 2007;33(2):385–394. doi: 10.1007/s00726-007-0517-0. [DOI] [PubMed] [Google Scholar]
- 46.Stratton SP, Alberts DS, Einspahr JG, et al. A phase 2a study of topical perillyl alcohol cream for chemoprevention of skin cancer. Cancer Prev Res (Phila) 2010;3(2):160–169. doi: 10.1158/1940-6207.CAPR-09-0183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.da Fonseca CO, Simao M, Lins IR, Caetano RO, Futuro D, Quirico-Santos T. Efficacy of monoterpene perillyl alcohol upon survival rate of patients with recurrent glioblastoma. J Cancer Res Clin Oncol. 2011;137(2):287–293. doi: 10.1007/s00432-010-0873-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Elsässer HP, MacDonald R, Dienst M, Kern HF. Characterization of a transglutaminase expressed in human pancreatic adenocarcinoma cells. Eur J Cell Biol. 1993;61(2):321–328. [PubMed] [Google Scholar]
- 49.Verma A, Mehta K. Tissue transglutaminase-mediated chemoresistance in cancer cells. Drug Resist Updat. 2007;10(4-5):144–151. doi: 10.1016/j.drup.2007.06.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.