Abstract
Here, we examined the chronic effects of two cannabinoid receptor-1 (CB1) inverse agonists, rimonabant and ibipinabant, in hyperinsulinemic Zucker rats to determine their chronic effects on insulinemia. Rimonabant and ibipinabant (10 mg·kg−1·day−1) elicited body weight-independent improvements in insulinemia and glycemia during 10 wk of chronic treatment. To elucidate the mechanism of insulin lowering, acute in vivo and in vitro studies were then performed. Surprisingly, chronic treatment was not required for insulin lowering. In acute in vivo and in vitro studies, the CB1 inverse agonists exhibited acute K channel opener (KCO; e.g., diazoxide and NN414)-like effects on glucose tolerance and glucose-stimulated insulin secretion (GSIS) with approximately fivefold better potency than diazoxide. Followup studies implied that these effects were inconsistent with a CB1-mediated mechanism. Thus effects of several CB1 agonists, inverse agonists, and distomers during GTTs or GSIS studies using perifused rat islets were unpredictable from their known CB1 activities. In vivo rimonabant and ibipinabant caused glucose intolerance in CB1 but not SUR1-KO mice. Electrophysiological studies indicated that, compared with diazoxide, 3 μM rimonabant and ibipinabant are partial agonists for K channel opening. Partial agonism was consistent with data from radioligand binding assays designed to detect SUR1 KATP KCOs where rimonabant and ibipinabant allosterically regulated 3H-glibenclamide-specific binding in the presence of MgATP, as did diazoxide and NN414. Our findings indicate that some CB1 ligands may directly bind and allosterically regulate Kir6.2/SUR1 KATP channels like other KCOs. This mechanism appears to be compatible with and may contribute to their acute and chronic effects on GSIS and insulinemia.
Keywords: ATP-sensitive potassium channels, sulfonylurea receptor 1, diazoxide, glibenclamide, Zucker rat, oral glucose tolerance, insulin, glucose-stimulated insulin secretion
within the last decade, cannabinoid receptor-1 (CB1) inverse agonists (e.g., rimonabant) appeared as a promising new class of therapeutics for obesity. In addition to its effects on food intake, rimonabant exhibited a surprising array of food intake- or body weight-independent peripheral effects. These included improvements in insulinemia, glycemic control, adiponectin, homeostatic model assessment method to quantify insulin resistance (HOMA-IR), atherogenic profile, and cardiovascular risk factors, along with markers of improved β-cell function and rest (21, 25, 61, 75, 78). Given this potential, in 2006, it was even approved in several countries for, among other indications, obesity and diabetes prevention. Unfortunately, due to serious neuropsychiatric effects, both rimonabant and its clinical trials were abruptly terminated (41). This was a bellwether for other emerging CB1 inverse agonists that were experiencing their own problems with central nervous system side effects (76).
More recently, interest in CB1 inverse agonists has been reemerging on the heels of two studies suggesting that the body weight-independent improvements in glucose homeostasis and metabolic profile might be mediated by peripheral mechanisms (50, 75). This implies that peripherally acting compounds could help prevent obesity and its comorbidities, such as cardiovascular disease, while at the same time avoiding any adverse central side effects. However, such compounds would not be expected to affect hunger or satiety.
Rimonabant treatment produced improvements in insulinemia and glucose homeostasis in rodent models and man. In rodents this was associated with a histomorphological phenomenon termed β-cell rest, which is associated with prevention of progression from obesity to diabetes conversion (25). The mechanism by which rimonabant led to improvements in insulinemia and β-cell rest has not been determined. One possibility is that CB1 inverse agonists primarily improve peripheral insulin sensitivity either directly (50) or in part through central mechanisms (51). This should secondarily lessen insulin demand and insulinemia over time, thereby promoting β-cell rest. Alternatively, another class of drugs exerts β-cell rest and insulin lowering through a different mechanism. ATP-sensitive potassium (KATP) channel openers (KCOs) such as diazoxide and NN414 directly inhibit glucose-stimulated insulin secretion (GSIS), facilitating β-cell rest, lowering plasma insulin, and reducing insulin hypersecretion in obesity (79).
Here, the ability of another CB1 inverse agonist, ibipinabant, to improve insulinemia was examined and found to be comparable with rimonabant in this regard. Surprisingly, in vivo and in vitro mechanistic studies revealed an acute insulin-lowering diazoxide/NN414-like effect of these drugs. Pharmacological and CB1-knockout (KO) studies indicated that certain cannabinoid agonists, inverse agonists, and their distomers may affect KATP-dependent insulin secretion in a CB1-independent fashion. Studies in sulfonylurea receptor 1 (SUR1)-KO mice suggest the involvement of SUR1 KATP channels. Consistently, rimonabant and ibipinabant opened K channels in cells overexpressing hamster Kir6.2/SUR1 KATP channels and tested positive in a modulator titration assay for KCOs, indicating that they can bring about these effects through a direct allosteric regulation of human Kir6.2/SUR1 KATP channels. Our finding that CB1 distomers also inhibit insulin secretion has important implications for the development of new insulin-lowering therapeutics from existing chemical structures developed initially for CB1 applications, thereby avoiding both the adverse neuropsychiatric effects of CB1 blockers and the toxicities or side effects of 1,2,4-thiadiazine 1,1-dioxide (diazoxide-based) derivatives developed for SUR1. Such compounds might be helpful to preserve β-cell mass in type 1 diabetes for long-term treatment of congenital hyperinsulinism or persistent hyperinsulinemic hypoglycemia of infancy and/or to prevent the conversion of obesity diabetes by eliciting β-cell rest and decreasing the contribution of hyperinsulinemia to otherwise worsening insulin resistance (for review, see Refs. 17 and 79).
RESEARCH DESIGN AND METHODS
Animals.
The Pennsylvania State College of Medicine Institutional Animal Care and Use Committee approved the animal protocols in this study. Animals were maintained on a 12:12-h light-dark cycle (7 AM to 7 PM) and provided Teklad 2018 diet. Male Sprague-Dawley or obese Zucker rats from Charles River Laboratories were allowed to acclimatize for 7–14 days before experimental handling. Male C57BL/6J, obese ob/ob, and db/db C57BL/6J genetic background and KKAy mice were obtained from JAX (Bangor, ME).
SUR1 and CB1 receptor-KO mice on the C57BL/6 background were generously provided by Dr. Joseph Bryan (Pacific Northwest Diabetes Research Institute, Seattle, WA) (67) and Dr. James Pickel (NIMH Transgenic Core, National Institutes of Health, Bethesda, MD) (83), respectively. Mice were maintained by heterozygote breeding, with occasional back-crossing to new C57BL/6J mice from Jackson Laboratories. Genotypes were determined using protocols from the donating laboratories in multiplex PCR assays, followed by size analysis of the electrophoretically separated bands of amplified DNA.
In one experiment, mice were maintained for ≥11 wk on a 60% fat diet from Research Diets (no. D12492; Research Diets, New Brunswick, NJ) to induce obesity (DIO mice).
Chronic study.
Zucker rats were allocated to three treatment groups: vehicle control, rimonabant (10 mg·kg−1·day−1), and ibipinabant [a.k.a., SLV-319, BMS-646256 (10 mg·kg−1·day−1)]. Rats were individually caged with a Nyda chew bone with ad libitum water and food, except for the evening before measurements that required food deprivation. During the study, body weight, food provided, and refusal were monitored daily between 8:30 and 9:30 AM, when rats received their oral dose of vehicle or drug by gavage. The two drugs were suspended at a concentration of 10 mg/ml in vehicle containing 2% polyethylene glycol (MW = 400) and 98% diluted carboxymethyl cellulose (1% suspension). Vehicle controls received an equivalent volume per kilogram body weight of vehicle only. Study duration was 10 wk.
Acute actions in vivo on glucose tolerance test.
For acute mechanistic studies, we initially examined the effect of 24-h treatments with various compounds, thinking that some acute effects might require some time to manifest themselves. We used several dosing schemes due to the half-life of some of the drugs, such as once/day dosing or twice/day dosing. In the latter case, the total daily dose was split either equally (½ dose in the morning and ½ dose in the afternoon) or as one-third in the morning and two-thirds in the afternoon. This means that over 24 h some animals in some experiments received two drug or vehicle gavages before the glucose tolerance test (GTT), whereas in other experiments the animals received three gavages. Thus, CB1 inverse agonists were dosed once daily, i.e., a full indicated daily dose in the morning on day 0 and another full dose on day 1, with the second dose being 45 min before the GTT. NN414 was 5 mg·kg−1·day−1 bid, with a half-daily dose in the morning on day 0, another half that evening, and the last gavage being a half-dose on the morning of day 1, again 45 min before the GTT. Diazoxide's daily dose was 100 mg·kg−1·day−1 with a one-third daily dose in the morning, two-thirds in the afternoon, and one-third on day 1 in the morning, also 45 min prior to the GTT.
Subsequently, it was found that 24 h was not necessary to observe early effects on GTT. Therefore, in other studies described here, only a single dose of drug was provided 30–45 min before a GTT.
GTT and plasma insulin assays.
Oral (rats) or intraperitoneal (ip; mice) GTTs were performed in overnight-fasted animals; plasma insulin was determined by enzyme-linked immunosorbant assay (ELISA) and the HOMA-IR, as described previously (68).
Islet perifusion/concentration-dependent screening.
Pancreatic islets were isolated using a method based on the ductal injection of collagenase, as described previously (22). Oxygenated (95% O2/5% CO2) Gey & Gey buffer (28) was used throughout with the following constituents (in mM): NaCl (111), NaHCO3 (27), KCl (4.96), CaCl2 (2.0), MgCl2.6H2O (0.28), Na2HPO4 (0.63), KH2PO4 (0.22), 1 mg/ml bovine serum albumin, and 4 mM glucose, unless stated otherwise. Rats were euthanized by CO2 asphyxiation, which was followed by cervical dislocation. The peritoneal cavity was opened, and the branch of the bile duct leading to the liver and the duodenal end of the duct in the pancreas were clamped. The pancreas was distended by the injection of ∼10 ml of ice-cold 0.9 mg/ml collagenase (Sigma XI Lot 79F-6827) solution into the bile duct using a syringe and a 23-gauge needle. The pancreas was then removed and incubated statically in a Universal container for 10–12 min at 37°C. Following the incubation, 10 ml of ice-cold buffer was added, and the suspension was shaken vigorously by hand for 1 min and finally allowed to stand on ice for 5 min. Islets separated from the digested pancreas by settling as a loose pellet at the bottom of the container. The aspirated islet pellet was then washed three times using (∼20 ml) ice-cold buffer. Well-formed and good-sized islets were hand-picked (under a low-power microscope) and pooled for loading into the perifusion equipment.
On each day, two experiments were performed in parallel using two identical, independent sets of perifusion apparatus, as described previously (22). Each perifusion apparatus consisted of six perifusion chambers. Chambers (25 mm of Nalgene, 0.8-μm cellulose acetate syringe filters) were loaded with 20 islets from the same pooled batch and perifused with Gey & Gey buffer at 1 ml/min for 30 min. Fractions from the perifusate were collected at 2-min intervals. Perifusate was collected for an initial 10-min period (to obtain baseline insulin values), and then the buffer to each chamber was changed to one containing the relevant vehicle, drug, or glucose. The perifusate was then collected for an additional 60 min. Perifusate fractions were stored at −75°C until required for insulin assay.
Insulin assays on perifusate samples were performed using 96-well ELISA kits from Mercodia (Rat Ultrasensitive, cat. no. 10-1137-10). Insulin is unstable when diluted in physiological buffers; therefore, perifusate fractions were not subjected to repeated freeze-thaw cycles. Baseline perifusate fractions were assayed undiluted, and all other fractions were diluted prior to assay in Gey & Gey buffer as required (≤10 times). Optical density at 450 nm was determined using a Molecular Devices VERSAmax tunable microplate reader, and readings were transferred to GraphPad Prism. A cubic spline curve was fitted to the calibration data, and unknown values were extrapolated from this curve fit. The results were then expressed as picograms of insulin released per islet per minute for each sample. Initially, every third fraction was assayed for insulin as a single replicate. If necessary, repeat insulin determinations were performed on the next unfrozen fraction.
Diazoxide was purchased from Sigma (D9035 Lot 117H47074). Ibipinabant and rimonabant were supplied by Solvay Pharmaceuticals. All drug solutions were made up fresh in dimethyl sulfoxide (DMSO; Fisher specified grade) for each experiment. Diazoxide remained in solution when diluted in assay buffer at the highest (100 μM) tested concentration (1,000-fold dilution of DMSO stock). Both SLV319 and rimonabant formed a slight, milky precipitate when diluted (1,000-fold) to the highest tested concentration of 10 μM in assay buffer; however, a clear solution was formed after vortexing. All perifusion solutions (vehicle and drug) had a final DMSO concentration of 0.1%.
For the concentration dependency experiments comparing the effect of ibipinabant rimonabant, diazoxide, and WIN 55212-1, data for pooled fractions were expressed as a simple mean of the three experiments. Mean insulin secretion was also calculated for each experiment as an average of fractions 16–36, and statistical comparisons were made by multiple two-tailed t-tests (P < 0.05 was defined as significantly different). IC50 or EC50 values were calculated using a sigmoidal dose-response nonlinear curve fit (GraphPad Prism).
Islet perifusion/simplified screening protocol.
The simple screening design was repeated three to four times using islets isolated from three separate islet preparations. All chambers (20 islets/chamber) were perifused with 4 mM glucose for 1 h prior to the start of the experiment. The first perifusate sample (sample 1) was taken from the last 10 min of this wash phase. Islets were then perifused with the test solutions containing vehicle or test compounds on a blind basis, and two perifusate samples were collected from each chamber from 0 to 30 min (sample 2) and from 30 to 60 min (sample 3). These experiments were performed at 11 mM glucose, which is approximately the EC50 for GSIS, so this screening test should be able to identify both agonists and antagonists of insulin secretion. Insulin was then assayed in aliquots from samples 1 (last 10 min of the 4-mm-glucose wash phase), 2, and 3, and the rise in insulin secretion in samples 2 and 3 over that in sample 1 was calculated and set to 100%. Such responses were measured in at least three different islet preparations. Two separate perifusion rigs were used. GSIS responses from rigs 1 and 2 in the final 30 min of the two experiments reported led to insulin concentrations of 123 and 142 ng/ml of insulin (Fig. 3H) and 146 and 135 ng/ml (Fig. 3I), respectively, indicating excellent reproducibility between the two rigs used.
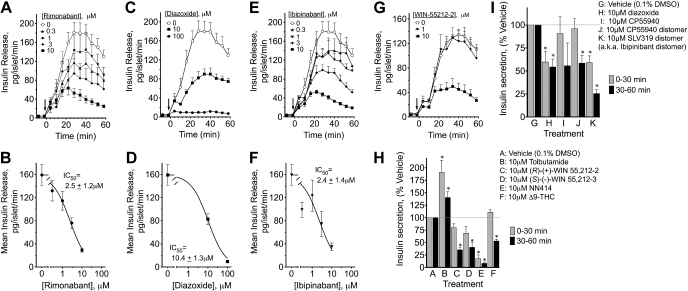
Fig. 3.
Time- and concentration-dependent effects of rimonabant, diazoxide, ibipinabant, and WIN-5512-2 and responses to a single 10-μM concentration of several other CB1-related ligands and distomers on insulin secretion from perifused rat islets. Islets were perifused with vehicle or the indicated concentrations of the indicated drug before the switch from low to 11 mM glucose to measure glucose-stimulated insulin secretion. The arrow indicates the simultaneous switch to high glucose and indicated drug or vehicle. The pattern of insulin release in response to vehicle or indicated concentrations of rimonabant (A), diazoxide (C), ibipinabant (E), or WIN-55212-2 (G) is shown. For concentration dependency analysis, the mean ± SE insulin release was determined in fractions 22–36 from 3 to 6 separate perifusions. Concentration-response data were analyzed using nonlinearizing curve-fitting software (Graphpad Prism), using single-component models. IC50s ± SE are shown in the graphs for rimonabant (B), diazoxide (D), and ibipinabant (F). Using a simplified protocol, samples were pooled during perifusion with 0.1% DMSO and low (4 mM) glucose and then for the first 30 (0–30 min) and next 30 min (30–60 min) after the switch to 11 mM glucose in the presence of either vehicle or indicated drug (H and I). The response to high glucose above the low glucose response was calculated for the vehicle samples, and that was set to 100%. Actual values are reported in the text. Results are means ± SE from at least 3–4 different islet preparations. *P < 0.05.
Test drugs [except for Δ9-tetrahydrocannabinol (Δ9-THC)] were dissolved initially at 10 mM in DMSO, and all were soluble at this concentration. Drugs were then diluted to a final assay concentration of 10 μM in buffer (0.1% final DMSO), and all drugs appeared soluble at this concentration. Δ9-THC was supplied as a 95-mM solution in ethanol, and this was added directly to the flask at a final concentration of 10 μM in assay buffer (0.01% final ethanol) along with a separate addition of DMSO to a final concentration of 0.1%. Most test drugs were supplied and coded by Solvay Pharmaceuticals, whereas diazoxide, tolbutamide, and Δ9-THC were purchased from Sigma. Dronabinol for in vivo use was obtained from Solvay Pharmaceuticals.
Patch clamp recordings.
COSm6 cells were cotransfected with pECE-SUR1 (hamster SUR1) and pCDNA1-Kir6.2 (rat Kir6.2) as well as a plasmid encoding the green fluorescent protein to facilitate identification of transfected cells. Cells were plated onto coverslips 12–24 h later, and patch clamp recordings were performed 36–72 h posttransfection at room temperature. Patch clamp electrodes were fabricated from soda lime glass microhematocrit tubes (Kimble 73813) on a horizontal puller (Sutter Instruments, Novato, CA). Electrode resistance was typically ∼2.0 MΩ when filled with pipette solution. Cell-attached patches were voltage-clamped with an Axopatch 1D amplifier (Axon, Foster City, CA). The bath and pipette solutions (K-INT) had the same composition (in mM): 140 KCl, 10 K-HEPES, and 1 K-EGTA, pH 7.3. Rimonabant, ibipinabant, and glibenclamide stocks were made in DMSO and diluted in prewarmed K-INT solution (37°C) at 1:1,000 ratios such that the final DMSO concentration was 0.1%. All currents were measured at a membrane potential of −50 mV and filtered at 2 kHz.
Patch clamp data analysis.
For recordings in which single-channel events could be resolved, digitized records of 35-s duration under drug application were analyzed with Clampfit 9.2 using 50% threshold-crossing criterion. Amplitude histograms were constructed and fitted with Gaussian function. The sums of the areas under all peaks with amplitudes ≥3.5 pA (corresponding to single-channel opening) were normalized to the corresponding controls (without drug) obtained in individual patches to yield fold of changes in normalized open probability (NPo; i.e., relative channel activity). For patches where multiple-channel currents were observed and single-channel events could not be resolved, integrated currents were used to calculate NPo. After currents had reached the maximum in response to drug applications, the integrated current of a 35-s segment was normalized to the corresponding control prior to drug application from the same patch.Z
3H-glibenclamide-specific binding and KCO allosteric modulator titration assay.
Membranes were prepared from a human embryonic kidney-293 cell line that stably overexpresses human Kir6.2/SUR1 (ChanTest, Cleveland, OH) and stored at −84°C (5 mg protein/ml) in buffer containing 10 mM Tris·HCl (pH 7.4), 0.1 mM EGTA, and 10% sucrose. 3H-glibenclamide (a.k.a. 3H-glyburide; PerkinElmer, Boston, MA) saturation binding was performed in the presence of MgATP as described (62). Nonspecific binding was determined in the presence of 20 μM glipizide. MgATP-dependent allosteric modulation of high-affinity 3H-glibenclamide-specific binding was measured as described (18).
Statistical analysis.
All of the measurements are reported as means ± SE. A one- or two-way ANOVA or repeated-measures ANOVAs and curve fitting were performed as appropriate for the kind of comparisons using Prism and Instat programs from Graphpad software. When significant differences (P < 0.05) were detected in one-way ANOVA, Dunnett's multiple-comparison posttests were used. Bonferroni posttests were used for two-way ANOVAs.
RESULTS
Chronic administration of rimonabant and ibipinabant lead to body weight-independent improvements in fed and fasted insulinemia and plasma insulin during a GTT.
Zucker rats were used because they are less affected by the food intake effects of CB1-inverse agonists due to their disrupted leptin axis. Thirty-three male Zucker rats were randomized to one of three groups: vehicle (control), rimonabant (10 mg·kg−1·day−1), and ibipinabant (10 mg·kg−1·day−1). Starting body weights between the groups were not significantly different (vehicle: 262 ± 5 g; rimonabant: 250 ± 4 g; ibipinabant: 248 ± 4 g). After 10 wk of treatment, body weights were not significantly different between the vehicle and rimonabant groups, although ibipinabant caused a 13% decrease in body weight compared with controls (Fig. 1A). Compared with vehicle control, ibipinabant decreased food intake significantly during 5 of the 10 wk, whereas rimonabant only decreased food intake significantly in weeks 1 and 3.
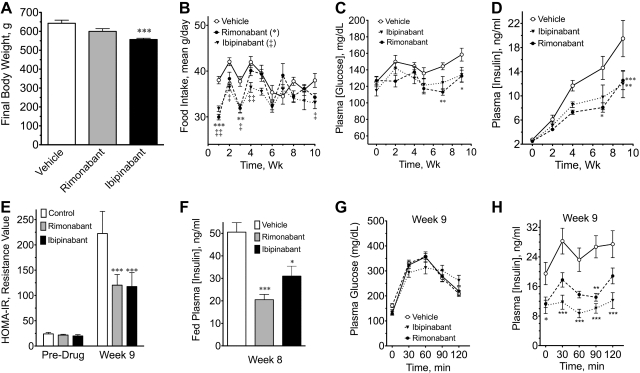
Fig. 1.
Cannibinoid receptor-1 (CB1) inverse agonists prevent age-related rise in plasma insulin concentration in male Zucker rats. Zucker rats were administered vehicle, rimonabant (10 mg/kg), or ibipinabant (10 mg/kg) daily for 10 wk. Starting body weights are described in the text. A: final body weights. B: daily food intake was averaged from 3 consecutive days for each week of the study. C: fasting plasma glucose during the study. D: fasting plasma insulin during the study. E: week 9 homeostatic model assessment method to quantify insulin resistance (HOMA-IR) values. F: fed plasma insulin was measured during week 8. G: results of an oral glucose tolerance test (GTT) during week 9 (glucose dose 1.25 g/kg). H: plasma insulin concentrations from week 9 GTT. * and ‡P < 0.05; ** and ‡‡P < 0.01; ***P < 0.001.
Over the course of the study, only small rises in fasting plasma glucose were observed (Fig. 1C) despite large changes in body weight. Notably, Zuckers resist body weight-dependent hyperglycemia and the progression from obesity to diabetes through compensatory increases in insulin secretion and β-cell mass (40). Thus, as expected, plasma insulin concentrations rose about ninefold in the control group over the 10 wk of the study (Fig. 1D). Both rimonabant and ibipinabant attenuated this rise in plasma insulin (Fig. 1D) and either significantly lowered or had no significant effect, respectively, on fasting plasma glucose over time (Fig. 1C). Consistently, insulin resistance values rose during the study in the control group; however, the increase in HOMA-IR was blunted ∼50% by rimonabant and ibipinabant (Fig. 1E).
During week 8, random, fed plasma glucose, and insulin concentrations were determined. The fed plasma glucose was not significantly different between the groups (not shown); however, fed insulin concentrations were 40–60% significantly lower after chronic CB1 blocker treatment (Fig. 1F). Figure 1G shows a GTT performed during week 9. No significant difference was observed in the glucose areas under the curve (AUCs); however, the concentration of insulin during the GTT was lower (Fig. 1H). Consistently, insulin AUCs were 37–53% lower after rimonabant and ibipinabant (vehicle: 3,052 ± 369 ng·ml−1·min−1; rimonabant: 1,927 ± 174 ng·ml−1·min−1, P < 0.05 compared with vehicle; ibipinabant: 1,432 ± 235 ng·ml−1·min−1, P < 0.001).
Rimonabant and ibipinabant have an acute diazoxide action in vivo.
Chronic treatment with either KCOs (1, 5, 8, 15, 16, 19, 34, 35, 49, 59, 60, 69, 81, 82) or rimonabant (25) led to similar improvements in insulinemia, glucose tolerance, and β-cell rest. KCOs possess a distinct but transient signature effect in the GTT after acute exposure. However, since we did not know whether any acute effects of CB1 inverse agonists might involve changes in gene expression, we initially opted to examine the effects of the CB1 inverse agonists after 24 h of drug or vehicle exposure. Therefore, in separate cohorts of male Zucker rats the acute effects of rimonabant, ibipinabant, and the prototypical KATP channel openers diazoxide and NN414 were examined during a GTT (Fig. 2). Consistent with a KCO-like mechanism, rimonabant acutely increased the plasma glucose concentration after a glucose challenge (Fig. 2A). This effect was dose dependent, with an EC50 of 6.0 ± 1.4 mg/kg, leading to a nearly threefold increase in glucose AUC at the highest dose (Fig. 2I). Ibipinabant similarly increased glucose AUC from 14,000 ± 1,022 to 24,440 ± 1,738 mg·dl−1·min at 10 mg/kg (P < 0.001). These effects during GTTs were similar to those of diazoxide (Fig. 2C) and its SUR1-specific analog NN414 (Fig. 2D).
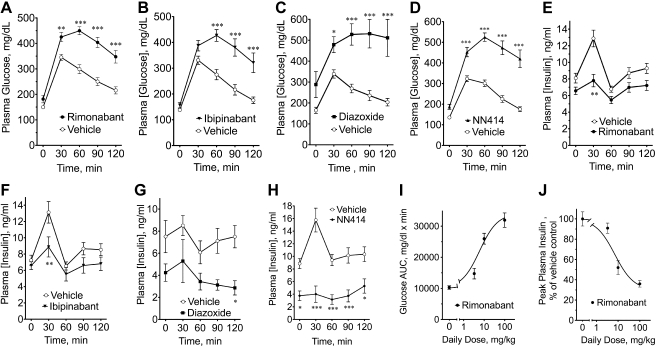
Fig. 2.
The CB1 inverse agonists rimonabant and ibipinabant and the sulfonylurea receptor 1 (SUR1)-specific ATP-sensitive potassium (KATP) channel opener NN414 exhibit acute diazoxide/NN414-like effects on plasma glucose and insulin during an oral GTT in male Zucker rats. Different cohorts of male Zucker rats received 2 daily doses of either vehicle or rimonabant (A and E) or ibipinabant (B and F; 10 mg·kg−1·day−1) 45 min before an oral GTT. C and G: diazoxide (100 mg·kg−1·day−1) or vehicle was administered with divided daily dosing: daily dose in the morning, in the afternoon, and the next morning before the GTT. D and H: NN414 (5 mg·kg−1·day−1) or vehicle was administered with divided daily dosing: ½ daily dose in the morning, ½ in the afternoon, and ½ on the morning before the GTT. Blood glucose (A–D) and plasma insulin (E–H) during the GTT are shown. Dose dependency of rimonabant on glucose area under the curve (AUC; I) and peak plasma insulin (J) during the GTTs. Data were analyzed using nonlinearizing curve-fitting software (Graphpad Prism), using single-component models. IC50s are described in the text. Body weights (g) were as follows: vehicle 457 ± 9.3, rimonabant 459 ± 19 (A and E); vehicle 466 ± 16, ibipinabant 462 ± 7.3 (B and F); vehicle 354 ± 11, diazoxide 324 ± 13 (C and G); vehicle 484 ± 12, NN414 492 ± 10 (D and H). *P < 0.05; **P < 0.01; ***P < 0.001.
Consistently, rimonabant dose-dependently lowered insulin AUCs during GTTs, leading to 60–70% decreases in plasma insulin at the highest doses, with an IC50 of 7.1 ± 1.4 mg/kg, i.e., not statistically different from EC50 of raising glucose AUC (Figs. 2, E and J). Acute treatment with 10 mg·kg−1·day−1 ibipinabant also led to significant reductions in insulin AUCs (Fig. 2F and AUCs; vehicle: 1,113 ± 70.83 ng·ml−1·min vs. ibipinabant: 830.8 ± 94.13 ng·ml−1·min, P < 0.05). As expected, diazoxide caused a 48% reduction in the insulin AUC (Fig. 2G and insulin AUCs; vehicle: 876.4 ± 117.8 ng·ml−1·min vs. 100 mg diazoxide: 461.7 ± 92.01 ng·ml−1·min, P < 0.05. The apparently lesser effect of diazoxide on plasma insulin may be explainable by the smaller increase in vehicle-treated rats, which in turn may be related to the smaller n = 6 vs. n = 10–11 for other experiments). NN414 also lowered plasma insulin during a GTT, leading to an ∼66% decrease in the mean insulin AUC (Fig. 2H and insulin AUCs; vehicle: 1,348 ± 136.5 ng·ml−1·min vs. NN414: 462.9 ± 114.7 ng·ml−1·min, P < 0.001). Variability in the time 0 plasma insulins for the various vehicle controls in Figs. 1 and 2 generally correlated to differences in body weights as expected (see above text, Fig. 1A, and Fig. 2 legend for body weights), with higher body weights being associated with higher plasma insulin concentrations.
Similar KCO-like effects on glucose AUC were seen 30–45 min after a single dose of rimonabant in Zucker rats, male Sprague-Dawley rats, and C57BL6 mice as well as obese KKAy and C57BL/6B6.v-Lpt ob/ob mice (data not shown).
The diazoxide/NN414-like effects of CB1 ligands tested are mediated at the level of pancreatic islets, but in vitro and in vivo responses could not be predicted from their known activity at CB1.
In vitro studies were conducted to determine whether the diazoxide/NN414-like effects of rimonabant and ibipinabant were mediated at the level of pancreatic islets by examining GSIS in perifused islets. At perifusate concentrations of 10 μM, rimonabant and ibipinabant reduced insulin secretion by ∼74–75% (Fig. 3, A and E) with IC50s of 2.4–2.5 μM (Fig. 3, B and F). Diazoxide had a smaller effect at the same concentration; it exhibited less potency compared with the CB1 receptor inverse agonists (IC50 of 10.4 μM; Fig. 3, C and D) but abolished insulin secretion at the highest (100 μM) dose tested. A CB1 agonist, WIN 55212-2, significantly inhibited insulin secretion to 33.9% of control at the highest (10 μM) tested concentration (Fig. 3G). These findings suggest that the acute effects of rimonabant and ibipinabant observed in Zucker rats are mediated at the level of pancreatic islets. The finding that the CB1 agonist WIN 55212-2 and the two CB1 inverse agonists exerted similar diazoxide/NN414-like effects prompted an investigation of several other compounds on insulin secretion.
Other perifusate studies were performed at one concentration (10 μM), using the simplified screening protocol in two separate rigs as described in research design and methods. GSIS responses in these studies represented 30- to 40-fold increase in insulin secretion (Fig. 3, H and I). As expected for a sulfonylurea, tolbutamide increased insulin secretion after the addition of 11 mM glucose 191% above vehicle in the first 30 min and 140.4% in the second 30 min. Both the R eutomer and S distomer of the CB1 agonist WIN 55,212 had a trend toward lowering insulin secretion during the first 30 min, but for both compounds this was significant in the second 30 min (WIN 55,212-2, 35 ± 4.4% of control; and WIN 55,212-3, 40.3 ± 9.3% of control). The switch to glucose and drug or vehicle occurred at the same time. Thus differences in access to the channel between the different compounds used might explain the temporal pattern (e.g., delays), of some responses and could potentially influence the earlier concentration-response curves. The SUR1-selective KATP channel opener NN414 decreased insulin secretion as expected in the first and second sample periods (Fig. 3H). The cannabinoid agonist Δ9-THC decreased insulin secretion to 53 ± 3.4% of control, but only in the second 30 min. Diazoxide lowered insulin secretion as expected in the first 30 and last 30 min of the perifusion (Fig. 3I). CP55940 eutomer, a CB1 agonist, had a trend toward lowering insulin secretion, but only in the last 30 min, whereas its distomer caused a similar but statistically significant reduction in the last 30 min (58.5 ± 8.5% of control). The CB1-inactive distomer of ibipinabant (SLV319 distomer) caused a significant reduction of insulin secretion in the first (59 ± 7.2% of control) and second 30-min (25 ± 4.7% of control) sample (Fig. 3I). These findings show that several CB1 agonists, antagonists, and their distomers have similar inhibitory effects on insulin secretion. That is inconsistent with these responses being mediated by CB1 receptors and suggests a direct effect at the level of pancreatic islets.
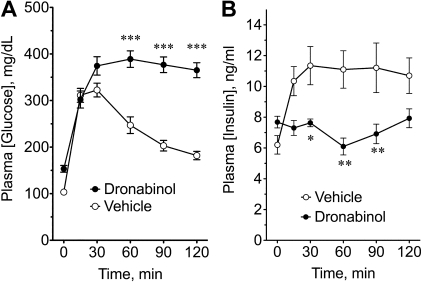
Dronabinol (a.k.a., marinol), a sesame oil dissolved preparation of the phytocannabinoid agonist Δ9-THC from Cannabis sp, was examined for its acute effects on plasma glucose or insulin during a GTT. Figure 4, A and B, shows that a single dose of dronabinol significantly worsened glucose tolerance and lowered plasma insulin during a GTT in Zucker rats. This finding is consistent with the effects of Δ9-THC in the islet perfusion studies. Taken together, the results of the studies in Figs. 3 and 4 strongly suggest that the inhibition of GSIS and glucose tolerance by these cannabinoid-related compounds is not mediated by CB1.
Fig. 4.
Immediate glycemic and insulinemic responses to the CB agonist dronabinol during a GTT. Changes in blood glucose (A) and plasma insulin (B) during a GTT after a single oral dose of sesame oil vehicle or 30 mg/kg of the CB agonist dronabinol (also known as Δ9-tetrahydrocannabinol and marinol) in male Zucker rats. Results are means ± SE. *P < 0.05; **P < 0.01; ***P < 0.001.
The diazoxide/NN414-like effects of ibipinabant and rimonabant are maintained in CB1-KO mice but abolished in SUR1-KO mice.
GTTs were performed in 14–16 male CB1-KO, SUR1-KO, and their respective sibling controls (age 15–18 wk; Fig. 5) ∼24 h after the initial exposure to drug and 45 min after the second daily dose (10 mg/kg). CB1-KO mice had ∼8% lower body weights compared with wild-type mice (+/+: 28.2 ± 0.37 g, n = 15; −/−: 26.3 ± 0.37 g, n = 18; P < 0.01). No significant effect on body weight was observed in SUR1-KO mice (data not shown).
Fig. 5.
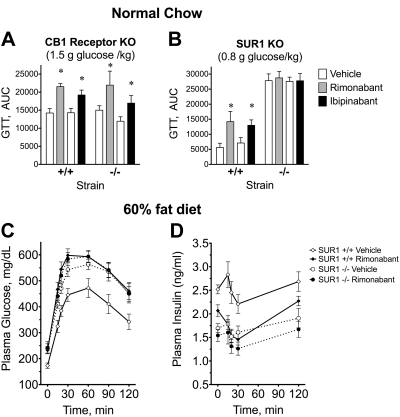
The acute effects of rimonabant and ibipinabant on glucose tolerance AUC are observed in CB1-knockout (KO) mice but not in SUR1-KO mice. Each 2 bars represent the results of separate GTTs performed at different times that compared either the acute effects of vehicle and 10 mg/kg rimonabant or vehicle and 10 mg/kg ibipinabant in mice with different genotypes. Mean ± SE of the glucose tolerance AUC is shown. A: wild-type sibling controls and CB1-KO mice. B: wild-type sibling controls and SUR1-KO mice. A lower glucose dose was used for the glucose challenge in these experiments (0.8 g glucose/kg). C and D: a separate cohort of SUR1 mice was fed a 60% fat diet from Research Diets (D12492) for 11 wk. Mice were separated into groups of 10–13 for GTT [SUR1+/+ vehicle (body weight 45 ± 3.0 g), SUR1+/+ rimonabant (44 ± 3.3 g), SUR1−/− vehicle (43 ± 2.7 g), SUR1−/− rimonabant (43 ± 3.4 g)]. Vehicle group received oral 5 ml/kg of 2% polyethylene glycol, with the balance being a 1% carboxymethyl cellulose solution, and the rimonabant animals received 10 mg·kg−1·day−1 of rimonabant the day before and another dose 45 min prior to an intraperitoneal GTT (glucose 0.8 g/kg mouse).
Rimonabant increased glucose AUCs 51% in wild-type and 46% in CB1-KO mice (Fig. 5A). Ibipinabant elevated glucose AUCs 34% in wild-type mice and 42% in CB1-KOs (Fig. 5A). These responses were not significantly different across genotypes. These findings are incompatible with a role for CB1 in these peripheral actions.
SUR1 disruption in mice paradoxically results in lower basal and GSIS despite elevated resting membrane potentials in β-cells (67). Thus, as reported previously, GTT AUCs were elevated in SUR1-KOs compared with their wild-type sibling controls. Consequently, a lower glucose dose (0.8 g/kg) was chosen for these GTTs to insure that the glucose concentrations remained within the range of the glucometer and so that further elevations in glucose could be observed if they occurred. In wild-type mice, rimonabant and ibipinabant elevated the glucose AUC by 152 and 83%, respectively; however, no effect of either was observed in SUR1-KO mice (Fig. 5B).
We attempted to measure plasma insulin concentrations in the above studies, but the concentrations were low and not in a reliable area of the standard curves for the insulin assay kits we use. We were reminded of the findings of Getty-Kaushik et al. (27) showing that inhibition of GSIS was more readily observed in obese than in lean animals, which had also been our observation in preliminary studies. Therefore, it was decided that an additional GTT study would be performed after we induced obesity in a separate cohort of SUR1-KO mice and their wild-type siblings by feeding them a high-fat diet for 11 wk (60% kcal from fat, D12492; Research Diets). These SUR1-DIO mice showed a significant increase in body weight that was not significantly different between the groups tested (Fig. 5, C and D). It was decided that this approach would not be appropriate for the CB1-KO mice, which have a well-described food intake phenotype that we expected would lead to significant differences in body weight that could confound the GTTs. In the SUR1+/+ DIO mice, rimonabant worsened glucose tolerance and significantly lowered insulin during the GTT, consistent with acute effects on Zucker rats. In the SUR1−/− DIO vehicle-treated mice, insulin concentrations during the GTT were similar to those for rimonabant treated SUR1+/+ mice. Rimonabant did not produce a significant further lowering of plasma insulin in the SUR1-KO mice (Fig. 5D).
Rimonabant and ibipinabant have diazoxide/NN414-like effects on KATP channels.
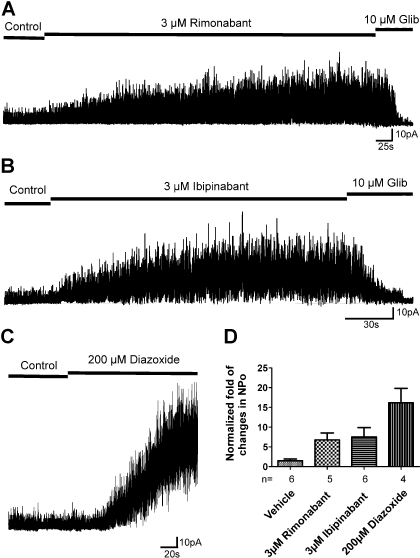
To determine whether rimonabant and ibipinabant activate KATP channels, cell-attached patches containing reconstituted Kir6.2/SUR1 (β-cell KATP) channels were recorded. Figure 6 shows that KATP channel activity was increased in the presence of 3 μM CB1 reverse agonists compared with that observed before drug applications (changes in NPo for rimonabant and ibipinabant are 6.76 ± 1.76- and 7.50 ± 2.36-fold, respectively), and these increases were statistically significant (P < 0.05, 2-tailed t-test) compared with the vehicle control (0.1% DMSO, NPo changes 1.48 ± 0.48-fold). For comparison, we examined the effects of diazoxide, a known KCO, on channel activity. At 200 μM, diazoxide stimulated channel activity 16.13 ± 3.70-fold, much greater than that seen for rimonabant and ibipinabant at 3 μM. Higher concentrations of rimonabant and ibipinabant (10 μM) did not lead to further stimulation of channel activity. The results demonstrate that rimonabant and ibipinabant, like diazoxide, can activate the KATP channels but have reduced efficacy.
Fig. 6.
CB1 inverse agonists stimulate Kir6.2/SUR1 channels. Recombinant Kir6.2/SUR1 channels were expressed in COSm6 cells by transient transfection. Recordings were performed in symmetrical high-potassium solutions at room temperature, and the membrane potential was clamped at −50mV. A–C: representative cell-attached patch clamp recordings. The segment 1 min after the patch formation was used as control; 10 μM glibenclamide was applied at the end of the recordings to show that the current is from KATP channels. D: average fold of changes of normalized open probability (NPo) after drug applications in cell-attached patches. Channel activity of 35-s segments during CB1 inverse agonist application (after response had reached the maximum) was normalized to the corresponding control (without drugs) obtained in the same patch. Data are presented as means ± SE of 4–6 patches. The NPo values of rimonabant-, ibipinabant-, or diazoxide-treated groups are all statistically significantly different from the vehicle control group (P < 0.05), using unpaired Student's t-test between each drug-treated and the respective control.
Allosterically mediated inhibition of high-affinity sulfonylurea-specific binding to human KIR6.2/SUR.
KCOs and closers bind to distinct sites on KATP channels. Unfortunately, no suitable radioligands that bind directly to the opener site on SUR1 exist (66). However, KCOs can allosterically regulate high-affinity sulfonylurea-specific binding in the presence, but not in the absence, of MgATP (62–64, 66). MgATP lowers the binding affinity for 3H-glibenclamide at KATP channels but is also required to indirectly observe the binding and activity of KCOs (64, 66). This finding forms the basis for a radioligand-binding assay to detect KCOs.
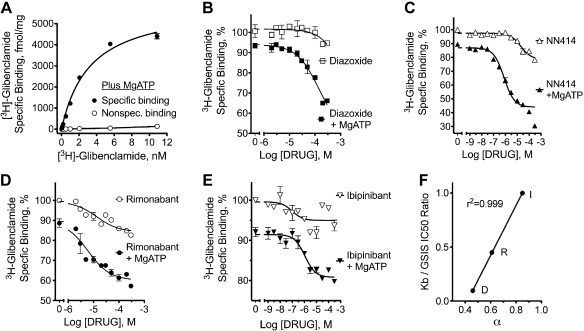
3H-glibenclamide bound specifically to membranes from a cell line stably expressing human KIR6.2/SUR1 (Fig. 7A). The Kd was 3.2 ± 0.3 nM in the presence of MgATP, which compares well with ∼2 nM reported for binding to murine pancreatic islet membranes under the same conditions (64). The Bmax was 5,977 ± 232 fmol/mg, demonstrating excellent KATP channel expression in these cells. Consistent with previous reports, diazoxide had minimal effects on specific binding in the absence of MgATP (64, 66); in its presence binding was reduced ∼10% at 1 nM 3H-glibenclamide, and under those conditions diazoxide titration led to displacement of 3H-glibenclamide (Fig. 7B). The diazoxide analog NN414 exhibited similar MgATP-dependent effects on specifically bound 3H-glibenclamide, but with a better equilibrium dissociation constant (Kb; Fig. 7C and Table 1).
Fig. 7.
3H-glibenclamide saturation binding and negative allosteric modulator tritration by KATP channel openers and CB1 inverse agonists. Binding studies were performed using membranes from a human embryonic kidney-293 cell line that stably expresses Kir6.2/SUR1. A: saturable 3H-glibenclamide-specific binding (closed symbols) in the presence of MgATP was determined by subtracting nonspecific binding (open symbols) determined In the presence of 20 μM glipizide from the total binding (not shown). B–E: radioligand-binding assay to detect presence of ligands at the SUR1 KATP opener site (66). Allosterical displacement of 3H-glibenclamide-specific (1 nM) binding to Kir6.2/SUR1-containing membranes by diazoxide (B), NN414 (C), rimonabant (D) or ibipinabant (E) was measured at 37°C in the presence or absence of the indicated concentrations of KCOs or CB1 inverse agonists in the presence of either 100 mM MgATP [closed symbols, with free Mg2+ adjusted to ∼1 mM, as described previously (66)] or 2 mM EDTA with no added ATP or Mg (open symbols). A nonlinearizing approach for curve fitting using the allosteric modulator titration routine in Graphpad Prism was used to analyze the data. F: relationship between the ternary complex constant (Table 1) for rimonabant (R), ibipinabant (I), and diazoxide (D) to their equilibrium dissociation constant (Kb; Table 1)/IC50 [i.e., for glucose-stimulated insulin secretion (GSIS); Fig. 3] ratio. Assuming that the Kb/IC50 ratios should not exceed 1, the ratio for ibipinabant was set to 1. The correlation coefficient without this correction was 0.98.
Table 1.
Parameters for allosteric regulation of 3H-glibenclamide-specific binding
| Modulator | α | Kb, μM |
|---|---|---|
| Rimonabant | 0.61 ± 0.02 | 5.5 ± 1.9 |
| Ibipinabant | 0.85 ± 0.01 | 1.1 ± 0.43 |
| NN414 | 0.39 ± 0.01 | 0.7 ± 0.09 |
| Diazoxide | 0.46 ± 0.08 | 120 ± 36 |
Values are means ± SE from studies shown in Fig. 6, where α is the ternary complex constant and Kb is the equilibrium dissociation constant of modulator binding at 37°C in the presence of 100 μM MgATP and ∼1 mM MgCl2. An α <1 indicates negative allosteric modulation of binding.
Rimonabant and ibipinabant exhibited similar MgATP-dependent effects on 3H-glibenclamide binding to human Kir6.2/SUR1 membranes. Ibipinabant and NN414 had equivalent Kb values, whereas that for rimonabant was about fivefold lower (Table 1 and Fig. 7). The ternary complex constant-α was also calculated. This constant is inversely related to the efficacy of the allosteric effect; values range from 1 (no effect) to 0 (strongest effect). The allosteric effects for rimonabant were greater than those for ibipinabant; however, the efficacy of diazoxide and NN414 was greater than rimonabant and ibipinabant. (Fig. 7 and Table 1). Although the α for rimonabant and ibipinabant suggest a weaker effect than diazoxide and NN414, even the smallest MgATP-dependent displacement with ibipinabant represents ∼8,000 disintegrations/min in this assay, with standard errors typically ranging from 1 to 3%.
Although differences in α (efficacy) were detected between rimonabant and ibipinabant in the binding studies (Table 1), this was not the case in the electrophysiological studies (Fig. 6). This may represent a difference between human and hamster KATP channels. Nevertheless, both the electrophysiological and binding studies suggest that rimonabant and ibipinabant are partial agonists for KCO compared with the full agonists diazoxide and NN414. However, because only a fraction of the KATP channels in islets are needed to exhibit a maximal effect on GSIS, the differences in the α are more likely to affect the IC50 rather than the efficacy of these compounds on GSIS. In agreement with this idea, rimonabant, ibipinabant, and diazoxide produced equivalent inhibition of GSIS (Fig. 3). However, because diazoxide had a lower value for α (stronger allosteric effects) compared with the two CB1 inverse agonists, there were larger differences between its IC50 for GSIS (Fig. 4D) and Kb (Fig. 7B). Supporting this idea and the concept of spare KATP channels in insulin secretion, a linear relationship was found between the IC50 for GSIS/Kb ratios and the ternary complex constants for rimonabant, ibipinabant, and diazoxide (Fig. 7F).
DISCUSSION
Rimonabant has been reported to improve insulinemia and glycemia in obesity and type 2 diabetes. Here, we showed that chronic administration of another CB1 inverse agonist, ibipinabant, also improves fed and fasting insulinemia, plasma insulin during a GTT, and glycemia during a 10-wk study. To begin to investigate the mechanism underlying these chronic effects, we performed acute in vivo and in vitro studies. Those studies indicated that rimonabant and ibipinibant can lower insulin secretion acutely in vivo, and this effect appears to be working at the level of pancreatic islets. The inhibitory effects of rimonabant on GSIS are in agreement with the findings of Getty-Kaushik et al. (27). Evidence began to mount during our acute studies that the diazoxide/NN414-like effects of the CB1 inverse agonists were not mediated by CB1. First, we found that a CB agonist, dronabinol, caused glucose intolerance, and its active ingredient, Δ9-THC, decreased the plasma insulin in response to a glucose challenge, as did the two CB1 inverse agonists, rimonabant and ibipinabant. Consistent with these findings, previous studies have found glucose intolerance after phytocannabinoid exposure in man, rabbits, and rats (33, 37). However, if mediated by CB1 receptors, opposite responses to CB1 agonists and inverse agonists were expected. Second, both of the CB1 agonists and antagonists we tested here inhibited GSIS. Third, distomers and eutomers of CB1-related ligands we tested were equally efficacious at preventing GSIS. The inability to predict the diazoxide/NN414-like effects of these compounds based on their activities at CB1 receptors along with the similar effects of the CB1 receptor eutomers and distomers makes it difficult to accept that these responses are mediated by CB1 receptors. A fourth point is that rimonabant and ibipinabant caused this glucose intolerance in CB1-KO mice. Taken together, these findings argue against a role of CB1 in these diazoxide/NN414-like effects.
In contrast to CB1-KO mice, effects of rimonabant and ibipinibant on glucose tolerance were absent in SUR1-KO mice, and rimonabant failed to lower insulin in SUR1-KO mice on a high-fat diet. Thus, SUR1 appears to be required to observe these effects. Consistently, rimonabant and ibipinibant were found to be partial agonists for SUR1 KATP channel opening in cells overexpressing hamster SUR1 KATP channels. Both rimonabant and ibipinibant also tested positive as partial agonists in an allosteric modulator titration binding assay designed to detect direct MgATP-dependent binding of ligands to the KCO site on human KIR6.2/SUR1. Compared with diazoxide and NN414, rimonabant and ibipinibant appear to be potent SUR1 KCOs.
Our initial goal was to help understand how rimonabant improves insulinemia in human and animal models and elicits β-cell rest in Zucker rats. Our data suggest a potential lead in this regard; however, we ackowlege that additional work is needed to determine the extent to which a KCO mechanism contributes to chronic improvements in insulinemia with rimonabant and ibipinibant. Although rimonabant, diazoxide, and NN414 treatments have led to similar improvements in insulinemia, pancreatic function, and/or morphology in Zucker rats (1, 3, 4, 6, 8, 16, 25), caution is warranted in interpreting all of the chronic positive effects of rimonabant and ibipinabant on insulinemia as being mediated through direct KATP channel opening at this time.
One caveat relates to the fact that obese rodents have higher levels of endocannabinoids in organs involved in setting insulin sensitivity (39, 46, 47, 53, 73). In contrast, many of the mechanistic studies, such as the KO studies we conducted, were in lean animals where a lower endocannabinoid tone might unmask off-targets. So it could be argued that we carried out some experiments, such as those using islets or KO animals, under conditions that favor nonselective actions of the two drugs. However, this argument may not really be relevant for the special case of SUR1. As shown by Getty-Kaushik et al. (27), the GSIS effects of rimonabant were more prevalent in obese as opposed to lean animals. We also found that the acute effects of diazoxide, rimonabant, and ibipinabant on glucose tolerance were more robust in obese vs. lean animals (not shown). Getty-Kaushik et al. (27) also found that rimonabant was beneficial in obese Zucker islets or islets exposed to high fat and high glucose to lower basal hypersecretion without diminishing the fold secretion induced by glucose, consistent with our chronic studies.
Another caveat relates to the relatively higher affinity of rimonabant and ibipinabant for CB1 compared with SUR1. For example, the steady-state plasma concentration after chronic administration of rimonabant in humans has been calculated to be around 100 nM, higher than the potencies we observed for SUR1 actions in vitro. In favor of a potential involvement of SUR1, concentrations of ingested lipophilic drugs can be elevated substantially in the portal circulation (20), and it is not possible to tell from the peripheral plasma concentrations whether lipophilic CB1 ligands become concentrated in the membrane or the cytoplasm, where the opener and closer sites face (55, 65, 74). Although the KCO effects of the compounds we investigated are not as potent as many G protein-coupled receptor blockers, they are quite potent for SUR1 KATP openers, as exemplified by diazoxide. Nevertheless, as indicated earlier, further studies are needed to determine the relative contribution of K channel opening to the long-term effects of CB1 inverse agonists.
Although CB1 and CB2 receptors have been identified by at least two groups in human islet β-cells (11, 44), the effects reported for various cannabinoid-related compounds have been inconsistent (23). For example, Bermúdez-Siva and colleagues (10, 12) found that endocannabinoid analogs caused glucose intolerance that was blocked by AM251, suggesting a CB1-related mechanism and conversely activation of CB2 receptors was linked to improved glucose intolerance. On the other hand, Li et al. (44) found that CB1 and CB2 endocannabinoid analogs and several antagonists not used here stimulated insulin secretion from human islets. They concluded that CB1 and CB2 agonists and blockers possess stimulatory effects on human islet insulin secretion and that this effect is cannabinoid receptor independent. However, for CB1 blockers, these findings with perifused human islets are not consistent with studies in humans and animals where rimonabant has been consistently found to lower and not raise insulin even at the earliest time point measured in clinical trials, consistent with our in vitro and in vivo findings and those of Duvivier et al. (25), Getty-Kaushik et al. (27), and Van Gaal et al. (77). Oz et al. (54), studying cromakalim-activated K currents in frog oocytes, found that anandamide acted to close SUR2 and SUR1 KATP channels in an AM251-independent fashion and bound directly to the SUR2 closer site. Consistently, Spivak and Doyle (71) have reported that the endocannabinoid 2-AG inhibits KATP channels independent of CB1 in murine insulinoma cells, although these effects are glucose dependent. They also reported effects on a number of islet channels, including sodium and calcium channels, in addition to SUR1 KATP channels. These effects of 2-AG would be predicted to lead to opposing effects on insulin secretion. Thus some reports are consistent with endocannabinoid lipids acting directly on the closer site of KATP channels in addition to CB receptors. Taken together, these findings raise a caution flag about generalizing the insulin secretion or channel effects of a limited set of compounds to all other CB-related compounds. Rather, CB-related compounds and their inactive analogs or distomers should be evaluated individually for their potential effects on therapeutically relevant ion channels and GSIS. Further studies with human as opposed to rodent models are also warranted based on the report of Li et al. (44). Here we used human KATP channels for our radioligand-binding studies.
Our findings raise the possibilty that new SUR1 KCOs might be found by examining chemical structures related to cannabinoid ligands and/or their distomers. Diazoxide is approved for management of hypoglycemia due to hyperinsulinism associated with some conditions. Other potential uses have also been posited. For example, some (13, 14, 30, 31, 52, 56, 57, 60, 70, 81, 82) but not all (24, 58) clinical studies have suggested benefits of KCOs associated with improvements in glucose tolerance, glycemia, and/or insulinemia in diabetes. Nevertheless, this is not an approved use. SUR1-acting KCOs also appear to promote β-cell rest in animal studies (1–9, 15, 16, 29, 32, 35, 36, 38, 42, 43, 45, 48, 49, 59, 69, 72, 80), and this has led to a proposal to begin to explore their use for prevention of obesity to diabetes conversion (17). Although additional human studies would be needed to examine the safety and efficacy of diazoxide for other uses, the potential for any future use is limited by diazoxide possessing a number of side effects. Furthermore, it has been difficult to identify new SUR1-specific KCOs like NN414 that have improved toxicity and side effect profiles. Other structure classes of KCOs, such as those related to cromakalim, have frequently been more SUR2 specific. Thus the identification of new structure classes of SUR1-acting KCOs could be welcomed. Here, we found that distomers of some CB1 agonists and antagonists exhibited the diazoxide effects of their eutomers on GSIS. By examining stereoisomers or other CB1-inactive modifications of existing CB1 ligands, it may be possible to identify new ion channel-acting compounds that exhibit KATP but not CB1 effects, thereby facilitating the development of new therapeutics for diazoxide-approved indications lacking both the adverse central effects of CB1 blockade as well as the hepatotoxicity that has been associated with some diazoxide analogs.
GRANTS
This project was supported by funds provided by Solvay Pharmaceuticals (C. J. Lynch, D. J. Heal) and National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-084428 (to C. J. Lynch) and DK-57699 (to S. L. Shyng).
DISCLOSURES
The authors declare no conflicts of interest, financial or otherwise.
AUTHOR CONTRIBUTIONS
C.J.L., Q.Z., S.-L.S., D.J.H., S.C.C., P.-C.G., M.F., U.N., D.R., and J.A. did the conception and design of the research; C.J.L., Q.Z., S.-L.S., S.C.C., K.D., and P.-C.G. analyzed the data; C.J.L., Q.Z., S.-L.S., D.J.H., S.C.C., K.D., P.-C.G., M.F., U.N., D.R., L.T., and J.A. interpreted the results of the experiments; C.J.L., S.-L.S., S.C.C., and K.D. prepared the figures; C.J.L. and D.J.H. drafted the manuscript; C.J.L., Q.Z., S.-L.S., D.J.H., P.-C.G., S.D.G., D.R., and J.A. edited and revised the manuscript; C.J.L., Q.Z., S.-L.S., D.J.H., S.C.C., K.D., P.-C.G., M.F., U.N., S.D.G., D.R., L.T., and J.A. approved the final version of the manuscript; Q.Z., S.C.C., K.D., and S.D.G. performed the experiments.
ACKNOWLEDGMENTS
We thank Jamie Spicer, Heng Lui, and Yuping Xu at Pennsylvania State College of Medicine for exceptional technical assistance. We thank Abbott Laboratories (Marrietta, GA) for providing dronabinol and its vehicle.
REFERENCES
- 1. Alemzadeh R, Fledelius C, Bodvarsdottir T, Sturis J. Attenuation of hyperinsulinemia by NN414, a SUR1/Kir6.2 selective K-adenosine triphosphate channel opener, improves glucose tolerance and lipid profile in obese Zucker rats. Metabolism 53: 441– 447, 2004 [DOI] [PubMed] [Google Scholar]
- 2. Alemzadeh R, Holshouser S. Effect of diazoxide on brain capillary insulin receptor binding and food intake in hyperphagic obese Zucker rats. Endocrinology 140: 3197– 3202, 1999 [DOI] [PubMed] [Google Scholar]
- 3. Alemzadeh R, Holshouser S, Massey P, Koontz J. Chronic suppression of insulin by diazoxide alters the activities of key enzymes regulating hepatic gluconeogenesis in Zucker rats. Eur J Endocrinol 146: 871– 879, 2002 [DOI] [PubMed] [Google Scholar]
- 4. Alemzadeh R, Jacobs W, Pitukcheewanont P. Antiobesity effect of diazoxide in obese Zucker rats. Metabolism 45: 334– 341, 1996 [DOI] [PubMed] [Google Scholar]
- 5. Alemzadeh R, Karlstad MD, Tushaus K, Buchholz M. Diazoxide enhances basal metabolic rate and fat oxidation in obese Zucker rats. Metabolism 57: 1597– 1607, 2008 [DOI] [PubMed] [Google Scholar]
- 6. Alemzadeh R, Slonim AE, Zdanowicz MM, Maturo J. Modification of insulin resistance by diazoxide in obese Zucker rats. Endocrinology 133: 705– 712, 1993 [DOI] [PubMed] [Google Scholar]
- 7. Alemzadeh R, Tushaus K. Diazoxide attenuates insulin secretion and hepatic lipogenesis in zucker diabetic fatty rats. Med Sci Monit 11: BR439– BR448, 2005 [PubMed] [Google Scholar]
- 8. Alemzadeh R, Tushaus KM. Modulation of adipoinsular axis in prediabetic zucker diabetic fatty rats by diazoxide. Endocrinology 145: 5476– 5484, 2004 [DOI] [PubMed] [Google Scholar]
- 9. Alemzadeh R, Zhang J, Tushaus K, Koontz J. Diazoxide enhances adipose tissue protein kinase B activation and glucose transporter-4 expression in obese Zucker rats. Med Sci Monit 10: BR53– BR60, 2004 [PubMed] [Google Scholar]
- 10. Bermudez-Silva FJ, Sanchez-Vera I, Suárez J, Serrano A, Fuentes E, Juan-Pico P, Nadal A, Rodríguez de Fonseca F. Role of cannabinoid CB2 receptors in glucose homeostasis in rats. Eur J Pharmacol 565: 207– 211, 2007 [DOI] [PubMed] [Google Scholar]
- 11. Bermúdez-Silva FJ, Suárez J, Baixeras E, Cobo N, Bautista D, Cuesta-Muñoz AL, Fuentes E, Juan-Pico P, Castro MJ, Milman G, Mechoulam R, Nadal A, Rodríguez de Fonseca F. Presence of functional cannabinoid receptors in human endocrine pancreas. Diabetologia 51: 476– 487, 2008 [DOI] [PubMed] [Google Scholar]
- 12. Bermúdez-Siva FJ, Serrano A, Diaz-Molina FJ, Sánchez Vera I, Juan-Pico P, Nadal A, Fuentes E, Rodríguez de Fonseca F. Activation of cannabinoid CB1 receptors induces glucose intolerance in rats. Eur J Pharmacol 531: 282– 284, 2006 [DOI] [PubMed] [Google Scholar]
- 13. Björk E, Berne C, Kämpe O, Wibell L, Oskarsson P, Karlsson FA. Diazoxide treatment at onset preserves residual insulin secretion in adults with autoimmune diabetes. Diabetes 45: 1427– 1430, 1996 [DOI] [PubMed] [Google Scholar]
- 14. Björk E, Berne C, Karlsson FA. Induction of beta-cell rest in type 1 diabetes. Studies on the effects of octreotide and diazoxide. Diabetes Care 21: 427– 430, 1998 [DOI] [PubMed] [Google Scholar]
- 15. Björklund A, Bondo Hansen J, Falkmer S, Grill V. Openers of ATP-dependent K+-channels protect against a signal-transduction-linked and not freely reversible defect of insulin secretion in a rat islet transplantation model of Type 2 diabetes. Diabetologia 47: 885– 891, 2004 [DOI] [PubMed] [Google Scholar]
- 16. Carr RD, Brand CL, Bodvarsdottir TB, Hansen JB, Sturis J. NN414, a SUR1/Kir6.2-selective potassium channel opener, reduces blood glucose and improves glucose tolerance in the VDF Zucker rat. Diabetes 52: 2513– 2518, 2003 [DOI] [PubMed] [Google Scholar]
- 17. Corkey BE. Banting lecture 2011: hyperinsulinemia: cause or consequence? Diabetes 61: 4– 13, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dabrowski M, Ashcroft FM, Ashfield R, Lebrun P, Pirotte B, Egebjerg J, Bondo Hansen J, Wahl P. The novel diazoxide analog 3-isopropylamino-7-methoxy-4H-1,2,4-benzothiadiazine 1,1-dioxide is a selective Kir6.2/SUR1 channel opener. Diabetes 51: 1896– 1906, 2002 [DOI] [PubMed] [Google Scholar]
- 19. Dabrowski M, Larsen T, Ashcroft FM, Bondo Hansen J, Wahl P. Potent and selective activation of the pancreatic beta-cell type K(ATP) channel by two novel diazoxide analogues. Diabetologia 46: 1375– 1382, 2003 [DOI] [PubMed] [Google Scholar]
- 20. Desai NM, Goss JA, Deng S, Wolf BA, Markmann E, Palanjian M, Shock AP, Feliciano S, Brunicardi FC, Barker CF, Naji A, Markmann JF. Elevated portal vein drug levels of sirolimus and tacrolimus in islet transplant recipients: local immunosuppression or islet toxicity? Transplantation 76: 1623– 1625, 2003 [DOI] [PubMed] [Google Scholar]
- 21. Di Marzo V, Despres JP. CB1 antagonists for obesity—what lessons have we learned from rimonabant? Nat Rev Endocrinol 5: 633– 638, 2009 [DOI] [PubMed] [Google Scholar]
- 22. Dickinson K, North TJ, Sills S, Anthony DM, Lock JI, Vowles DT, Jones RB. BTS 67 582 stimulates insulin secretion from perifused rat pancreatic islets. Eur J Pharmacol 339: 69– 76, 1997 [DOI] [PubMed] [Google Scholar]
- 23. Doyle ME. The role of the endocannabinoid system in islet biology. Curr Opin Endocrinol Diabetes Obes 18: 153– 158, 2011 [DOI] [PubMed] [Google Scholar]
- 24. Due A, Flint A, Eriksen G, Møller B, Raben A, Hansen JB, Astrup A. No effect of inhibition of insulin secretion by diazoxide on weight loss in hyperinsulinaemic obese subjects during an 8-week weight-loss diet. Diabetes Obes Metab 9: 566– 574, 2007 [DOI] [PubMed] [Google Scholar]
- 25. Duvivier VF, Delafoy-Plasse L, Delion V, Lechevalier P, Le Bail JC, Guillot E, Pruniaux MP, Galzin AM. Beneficial effect of a chronic treatment with rimonabant on pancreatic function and beta-cell morphology in Zucker Fatty rats. Eur J Pharmacol 616: 314– 320, 2009 [DOI] [PubMed] [Google Scholar]
- 27. Getty-Kaushik L, Richard AM, Deeney JT, Krawczyk S, Shirihai O, Corkey BE. The CB1 antagonist rimonabant decreases insulin hypersecretion in rat pancreatic islets. Obesity (Silver Spring) 17: 1856– 1860, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gey GO, Gey MK. The maintenance of human normal cells and tumor cells in continuous culture. I. Preliminary report: cultivation of mesoblastic tumors and normal tissue and notes on methods of cultivation. Am J Cancer 27: 45– 76, 1936 [Google Scholar]
- 29. Grill V, Björklund A. Impact of metabolic abnormalities for beta cell function: clinical significance and underlying mechanisms. Mol Cell Endocrinol 297: 86– 92, 2009 [DOI] [PubMed] [Google Scholar]
- 30. Grill V, Radtke M, Qvigstad E, Kollind M, Björklund A. Beneficial effects of K-ATP channel openers in diabetes: an update on mechanisms and clinical experiences. Diabetes Obes Metab 11, Suppl 4: 143– 148, 2009 [DOI] [PubMed] [Google Scholar]
- 31. Guldstrand M, Grill V, Björklund A, Lins PE, Adamson U. Improved beta cell function after short-term treatment with diazoxide in obese subjects with type 2 diabetes. Diabetes Metab 28: 448– 456, 2002 [PubMed] [Google Scholar]
- 32. Guo Z, Bu S, Yu Y, Ghatnekar G, Wang M, Chen L, Bu M, Yang L, Zhu B, Feng Z, Huang Q. Diazoxide prevents abdominal adiposity and fatty liver in obese OLETF rats at prediabetic stage. J Diabetes Complications 22: 46– 55, 2008 [DOI] [PubMed] [Google Scholar]
- 33. Ham MT, De Jong Y. Effects of delta-9-tetrahydrocannabinol and cannabidiol on blood glucose concentrations in rabbits and rat. Pharm Weekbl 110: 1157– 1161, 1975 [Google Scholar]
- 34. Hansen JB. Towards selective Kir6.2/SUR1 potassium channel openers, medicinal chemistry and therapeutic perspectives. Curr Med Chem 13: 361– 376, 2006 [DOI] [PubMed] [Google Scholar]
- 35. Hansen JB, Arkhammar PO, Bodvarsdottir TB, Wahl P. Inhibition of insulin secretion as a new drug target in the treatment of metabolic disorders. Curr Med Chem 11: 1595– 1615, 2004 [DOI] [PubMed] [Google Scholar]
- 36. Hensley IE, Lawler JE, Alemzadeh R, Holshouser SJ. Diazoxide effects on hypothalamic and extra-hypothalamic NPY content in Zucker rats. Peptides 22: 899– 908, 2001 [DOI] [PubMed] [Google Scholar]
- 37. Hollister LE, Reaven GM. Delta-9-tetrahydrocannabinol and glucose tolerance. Clin Pharmacol Ther 16: 297– 302, 1974 [DOI] [PubMed] [Google Scholar]
- 38. Huang Q, Bu S, Yu Y, Guo Z, Ghatnekar G, Bu M, Yang L, Lu B, Feng Z, Liu S, Wang F. Diazoxide prevents diabetes through inhibiting pancreatic beta-cells from apoptosis via Bcl-2/Bax rate and p38-beta mitogen-activated protein kinase. Endocrinology 148: 81– 91, 2007 [DOI] [PubMed] [Google Scholar]
- 39. Izzo AA, Piscitelli F, Capasso R, Aviello G, Romano B, Borrelli F, Petrosino S, Di Marzo V. Peripheral endocannabinoid dysregulation in obesity: relation to intestinal motility and energy processing induced by food deprivation and re-feeding. Br J Pharmacol 158: 451– 461, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jetton TL, Lausier J, LaRock K, Trotman WE, Larmie B, Habibovic A, Peshavaria M, Leahy JL. Mechanisms of compensatory beta-cell growth in insulin-resistant rats: roles of Akt kinase. Diabetes 54: 2294– 2304, 2005 [DOI] [PubMed] [Google Scholar]
- 41. Jones D. End of the line for cannabinoid receptor 1 as an anti-obesity target? Nat Rev Drug Discov 7: 961– 962, 2008 [DOI] [PubMed] [Google Scholar]
- 42. Kullin M, Li Z, Hansen JB, Björk E, Sandler S, Karlsson FA. K(ATP) channel openers protect rat islets against the toxic effect of streptozotocin. Diabetes 49: 1131– 1136, 2000 [DOI] [PubMed] [Google Scholar]
- 43. Leahy JL, Bumbalo LM, Chen C. Diazoxide causes recovery of beta-cell glucose responsiveness in 90% pancreatectomized diabetic rats. Diabetes 43: 173– 179, 1994 [DOI] [PubMed] [Google Scholar]
- 44. Li C, Bowe JE, Huang GC, Amiel SA, Jones PM, Persaud SJ. Cannabinoid receptor agonists and antagonists stimulate insulin secretion from isolated human islets of Langerhans. Diabetes Obes Metab 13: 903– 910, 2011 [DOI] [PubMed] [Google Scholar]
- 45. Ma Z, Portwood N, Brodin D, Grill V, Björklund A. Effects of diazoxide on gene expression in rat pancreatic islets are largely linked to elevated glucose and potentially serve to enhance beta-cell sensitivity. Diabetes 56: 1095– 1106, 2007 [DOI] [PubMed] [Google Scholar]
- 46. Matias I, Cristino L, Di Marzo V. Endocannabinoids: some like it fat (and sweet too). J Neuroendocrinol 20, Suppl 1: 100– 109, 2008 [DOI] [PubMed] [Google Scholar]
- 47. Matias I, Gonthier MP, Orlando P, Martiadis V, De Petrocellis L, Cervino C, Petrosino S, Hoareau L, Festy F, Pasquali R, Roche R, Maj M, Pagotto U, Monteleone P, Di Marzo V. Regulation, function, and dysregulation of endocannabinoids in models of adipose and beta-pancreatic cells and in obesity and hyperglycemia. J Clin Endocrinol Metab 91: 3171– 3180, 2006 [DOI] [PubMed] [Google Scholar]
- 48. Matsuda M, Kawasaki F, Mikami Y, Takeuchi Y, Saito M, Eto M, Kaku K. Rescue of beta-cell exhaustion by diazoxide after the development of diabetes mellitus in rats with streptozotocin-induced diabetes. Eur J Pharmacol 453: 141– 148, 2002 [DOI] [PubMed] [Google Scholar]
- 49. Nielsen FE, Ebdrup S, Jensen AF, Ynddal L, Bodvarsdottir TB, Stidsen C, Worsaae A, Boonen HC, Arkhammar PO, Fremming T, Wahl P, Kornø HT, Hansen JB. New 3-alkylamino-4H-thieno-1,2,4-thiadiazine 1,1-dioxide derivatives activate ATP-sensitive potassium channels of pancreatic beta cells. J Med Chem 49: 4127– 4139, 2006 [DOI] [PubMed] [Google Scholar]
- 50. Nogueiras R, Veyrat-Durebex C, Suchanek PM, Klein M, Tschöp J, Caldwell C, Woods SC, Wittmann G, Watanabe M, Liposits Z, Fekete C, Reizes O, Rohner-Jeanrenaud F, Tschöp MH. Peripheral, but not central, CB1 antagonism provides food intake-independent metabolic benefits in diet-induced obese rats. Diabetes 57: 2977– 2991, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. O'Hare JD, Zielinski E, Cheng B, Scherer T, Buettner C. Central endocannabinoid signaling regulates hepatic glucose production and systemic lipolysis. Diabetes 60: 1055– 1062, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ortqvist E, Björk E, Wallensteen M, Ludvigsson J, Aman J, Johansson C, Forsander G, Lindgren F, Berglund L, Bengtsson M, Berne C, Persson B, Karlsson FA. Temporary preservation of beta-cell function by diazoxide treatment in childhood type 1 diabetes. Diabetes Care 27: 2191– 2197, 2004 [DOI] [PubMed] [Google Scholar]
- 53. Osei-Hyiaman D, DePetrillo M, Pacher P, Liu J, Radaeva S, Bátkai S, Harvey-White J, Mackie K, Offertáler L, Wang L, Kunos G. Endocannabinoid activation at hepatic CB1 receptors stimulates fatty acid synthesis and contributes to diet-induced obesity. J Clin Invest 115: 1298– 1305, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Oz M, Yang KH, Dinc M, Shippenberg TS. The endogenous cannabinoid anandamide inhibits cromakalim-activated K+ currents in follicle-enclosed Xenopus oocytes. J Pharmacol Exp Ther 323: 547– 554, 2007 [DOI] [PubMed] [Google Scholar]
- 55. Panten U, Schwanstecher M, Schwanstecher C. Sulfonylurea receptors and mechanism of sulfonylurea action. Exp Clin Endocrinol Diabetes 104: 1– 9, 1996 [DOI] [PubMed] [Google Scholar]
- 56. Qvigstad E, Kollind M, Grill V. Nine weeks of bedtime diazoxide is well tolerated and improves beta-cell function in subjects with Type 2 diabetes. Diabet Med 21: 73– 76, 2004 [DOI] [PubMed] [Google Scholar]
- 57. Radtke M, Kollind M, Qvigstad E, Grill V. Twelve weeks' treatment with diazoxide without insulin supplementation in Type 2 diabetes is feasible but does not improve insulin secretion. Diabet Med 24: 172– 177, 2007 [DOI] [PubMed] [Google Scholar]
- 58. Radtke MA, Nermoen I, Kollind M, Skeie S, Sørheim JI, Svartberg J, Hals I, Moen T, Dørflinger GH, Grill V. Six months of diazoxide treatment at bedtime in newly diagnosed subjects with type 1 diabetes does not influence parameters of {beta}-cell function and autoimmunity but improves glycemic control. Diabetes Care 33: 589– 594, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Rasmussen SB, Sørensen TS, Hansen JB, Mandrup-Poulsen T, Hornum L, Markholst H. Functional rest through intensive treatment with insulin and potassium channel openers preserves residual beta-cell function and mass in acutely diabetic BB rats. Horm Metab Res 32: 294– 300, 2000 [DOI] [PubMed] [Google Scholar]
- 60. Ritzel RA, Hansen JB, Veldhuis JD, Butler PC. Induction of beta-cell rest by a Kir6.2/SUR1-selective K(ATP)-channel opener preserves beta-cell insulin stores and insulin secretion in human islets cultured at high (11 mM) glucose. J Clin Endocrinol Metab 89: 795– 805, 2004 [DOI] [PubMed] [Google Scholar]
- 61. Scheen AJ, Van Gaal LG, Després JP, Pi-Sunyer X, Golay A, Hanotin C. [Rimonabant improves cardiometabolic risk profile in obese or overweight subjects: overview of RIO studies]. Rev Med Suisse 2: 1916– 1923, 2006 [PubMed] [Google Scholar]
- 62. Schwanstecher M, Brandt C, Behrends S, Schaupp U, Panten U. Effect of MgATP on pinacidil-induced displacement of glibenclamide from the sulphonylurea receptor in a pancreatic beta-cell line and rat cerebral cortex. Br J Pharmacol 106: 295– 301, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Schwanstecher M, Löser S, Brandt C, Scheffer K, Rosenberger F, Panten U. Adenine nucleotide-induced inhibition of binding of sulphonylureas to their receptor in pancreatic islets. Br J Pharmacol 105: 531– 534, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Schwanstecher M, Löser S, Rietze I, Panten U. Phosphate and thiophosphate group donating adenine and guanine nucleotides inhibit glibenclamide binding to membranes from pancreatic islets. Naunyn Schmiedebergs Arch Pharmacol 343: 83– 89, 1991 [DOI] [PubMed] [Google Scholar]
- 65. Schwanstecher M, Schwanstecher C, Dickel C, Chudziak F, Moshiri A, Panten U. Location of the sulphonylurea receptor at the cytoplasmic face of the beta-cell membrane. Br J Pharmacol 113: 903– 911, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Schwanstecher M, Sieverding C, Dörschner H, Gross I, Aguilar-Bryan L, Schwanstecher C, Bryan J. Potassium channel openers require ATP to bind to and act through sulfonylurea receptors. EMBO J 17: 5529– 5535, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Seghers V, Nakazaki M, DeMayo F, Aguilar-Bryan L, Bryan J. Sur1 knockout mice. A model for K(ATP) channel-independent regulation of insulin secretion. J Biol Chem 275: 9270– 9277, 2000 [DOI] [PubMed] [Google Scholar]
- 68. She P, Reid TM, Bronson SK, Vary TC, Hajnal A, Lynch CJ, Hutson SM. Disruption of BCATm in mice leads to increased energy expenditure associated with the activation of a futile protein turnover cycle. Cell Metab 6: 181– 194, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Skak K, Gotfredsen CF, Lundsgaard D, Hansen JB, Sturis J, Markholst H. Improved beta-cell survival and reduced insulitis in a type 1 diabetic rat model after treatment with a beta-cell-selective K(ATP) channel opener. Diabetes 53: 1089– 1095, 2004 [DOI] [PubMed] [Google Scholar]
- 70. Song SH, Rhodes CJ, Veldhuis JD, Butler PC. Diazoxide attenuates glucose-induced defects in first-phase insulin release and pulsatile insulin secretion in human islets. Endocrinology 144: 3399– 3405, 2003 [DOI] [PubMed] [Google Scholar]
- 71. Spivak CE, Doyle ME. Endocannabinoid Inhibition of Ion Channels of Pancreatic Beta Cells (Abstract). Biophys J 100: 92a, 2011 [Google Scholar]
- 72. Standridge M, Alemzadeh R, Zemel M, Koontz J, Moustaid-Moussa N. Diazoxide down-regulates leptin and lipid metabolizing enzymes in adipose tissue of Zucker rats. FASEB J 14: 455– 460, 2000 [DOI] [PubMed] [Google Scholar]
- 73. Starowicz KM, Cristino L, Matias I, Capasso R, Racioppi A, Izzo AA, Di Marzo V. Endocannabinoid dysregulation in the pancreas and adipose tissue of mice fed with a high-fat diet. Obesity (Silver Spring) 16: 553– 565, 2008 [DOI] [PubMed] [Google Scholar]
- 74. Stephan D, Salamon E, Weber H, Russ U, Lemoine H, Quast U. KATP channel openers of the benzopyran type reach their binding site via the cytosol. Br J Pharmacol 149: 199– 205, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Tam J, Vemuri VK, Liu J, Batkai S, Mukhopadhyay B, Godlewski G, Osei-Hyiaman D, Ohnuma S, Ambudkar SV, Pickel J, Makriyannis A, Kunos G. Peripheral CB1 cannabinoid receptor blockade improves cardiometabolic risk in mouse models of obesity. J Clin Invest 120: 2953– 2966, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Topol EJ, Bousser MG, Fox KA, Creager MA, Despres JP, Easton JD, Hamm CW, Montalescot G, Steg PG, Pearson TA, Cohen E, Gaudin C, Job B, Murphy JH, Bhatt DL; CRESCENDO Investigators Rimonabant for prevention of cardiovascular events (CRESCENDO): a randomised, multicentre, placebo-controlled trial. Lancet 376: 517– 523, 2010 [DOI] [PubMed] [Google Scholar]
- 77. Van Gaal L, Pi-Sunyer X, Després JP, McCarthy C, Scheen A. Efficacy and safety of rimonabant for improvement of multiple cardiometabolic risk factors in overweight/obese patients: pooled 1-year data from the Rimonabant in Obesity (RIO) program. Diabetes Care 31, Suppl 2: S229– S240, 2008 [DOI] [PubMed] [Google Scholar]
- 78. Van Gaal LF, Rissanen AM, Scheen AJ, Ziegler O, Rossner S. Effects of the cannabinoid-1 receptor blocker rimonabant on weight reduction and cardiovascular risk factors in overweight patients: 1-year experience from the RIO-Europe study. Lancet 365: 1389– 1397, 2005 [DOI] [PubMed] [Google Scholar]
- 79. Wajchenberg BL. beta-cell failure in diabetes and preservation by clinical treatment. Endocr Rev 28: 187– 218, 2007 [DOI] [PubMed] [Google Scholar]
- 80. Yoshikawa H, Ma Z, Björklund A, Grill V. Short-term intermittent exposure to diazoxide improves functional performance of β-cells in a high-glucose environment. Am J Physiol Endocrinol Metab 287: E1202– E1208, 2004 [DOI] [PubMed] [Google Scholar]
- 81. Zdravkovic M, Kruse M, Rost KL, Møss J, Kecskes A. The effects of NN414, a SUR1/Kir6.2 selective potassium channel opener in subjects with type 2 diabetes. Exp Clin Endocrinol Diabetes 115: 405– 406, 2007 [DOI] [PubMed] [Google Scholar]
- 82. Zdravkovic M, Kruse M, Rost KL, Møss J, Kecskes A, Dyrberg T. The effects of NN414, a SUR1/Kir6.2 selective potassium channel opener, in healthy male subjects. J Clin Pharmacol 45: 763– 772, 2005 [DOI] [PubMed] [Google Scholar]
- 83. Zimmer A, Zimmer AM, Hohmann AG, Herkenham M, Bonner TI. Increased mortality, hypoactivity, and hypoalgesia in cannabinoid CB1 receptor knockout mice. Proc Natl Acad Sci USA 96: 5780– 5785, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]