Abstract
Metabolism of arachidonic acid by cytochrome P450 (CYP) to biologically active eicosanoids has been recognized increasingly as an integral mediator in the pathogenesis of cardiovascular and metabolic disease. CYP epoxygenase-derived epoxyeicosatrienoic and dihydroxyeicosatrienoic acids (EET + DHET) and CYP ω-hydroxylase-derived 20-hydroxyeicosatetraenoic acid (20-HETE) exhibit divergent effects in the regulation of vascular tone and inflammation; thus, alterations in the functional balance between these parallel pathways in liver and kidney may contribute to the pathogenesis and progression of metabolic syndrome. However, the impact of metabolic dysfunction on CYP-mediated formation of endogenous eicosanoids has not been well characterized. Therefore, we evaluated CYP epoxygenase (EET + DHET) and ω-hydroxylase (20-HETE) metabolic activity in liver and kidney in apoE−/− and wild-type mice fed a high-fat diet, which promoted weight gain and increased plasma insulin levels significantly. Hepatic CYP epoxygenase metabolic activity was significantly suppressed, whereas renal CYP ω-hydroxylase metabolic activity was induced significantly in high-fat diet-fed mice regardless of genotype, resulting in a significantly higher 20-HETE/EET + DHET formation rate ratio in both tissues. Treatment with enalapril, but not metformin or losartan, reversed the suppression of hepatic CYP epoxygenase metabolic activity and induction of renal CYP ω-hydroxylase metabolic activity, thereby restoring the functional balance between the pathways. Collectively, these findings suggest that the kinin-kallikrein system and angiotensin II type 2 receptor are key regulators of hepatic and renal CYP-mediated eicosanoid metabolism in the presence of metabolic syndrome. Future studies delineating the underlying mechanisms and evaluating the therapeutic potential of modulating CYP-derived EETs and 20-HETE in metabolic diseases are warranted.
Keywords: epoxyeicosatrienoic acid, dihydroxyeicosatrienoic acid, 20-hydroxyeicosatetraenoic acid, angiotensin II, bradykinin, metabolic syndrome
cytochrome p450 enzymes (CYPs) metabolize arachidonic acid to various biologically active eicosanoids. Olefin epoxidation by CYP2C and CYP2J isoforms produces four epoxyeicosatrienoic acid (EET) regioisomers (5,6-, 8,9-, 11,12-, and 14,15-EET), which possess potent vasodilatory and anti-inflammatory properties (12, 45). The EETs are rapidly hydrolyzed by soluble epoxide hydrolase (sEH, Ephx2) to the corresponding dihydroxyeicosatrienoic acids (DHETs), which are generally less biologically active (12, 45). In contrast, CYP4A and CYP4F isoforms catalyze the ω-hydroxylation of arachidonic acid to 20-hydroxyeicosatetraenoic acid (20-HETE), a vasoconstrictive and proinflammatory eicosanoid (35). Numerous preclinical studies have demonstrated that the CYP epoxygenase and ω-hydroxylase pathways maintain cardiovascular homeostasis following pathological insult (12, 35, 45), and modulating CYP-derived EETs and 20-HETE offers enormous potential as a therapeutic strategy for cardiovascular disease.
The pathophysiology of cardiovascular disease is complex, and multiple risk factors contribute to its development and progression. One risk factor that is becoming increasing prevalent is metabolic syndrome, a prothrombotic, proinflammatory state characterized by the presence of dyslipidemia, insulin resistance, and hypertension (14). The liver and kidney are integrally involved in the pathophysiology of metabolic syndrome via their roles in the regulation of inflammation, insulin sensitivity, and blood pressure. In addition, CYP epoxygenases and ω-hydroxylases are expressed most abundantly, and consequently EETs and 20-HETE are produced at the highest levels in the liver and kidney (38). Because of the divergent effects of the CYP epoxygenase-derived EETs and CYP ω-hydroxylase-derived 20-HETE in the regulation of vascular tone and inflammation, alterations in the functional balance between these parallel pathways in liver and kidney may contribute to the pathogenesis and progression of metabolic syndrome and cardiovascular disease.
Preclinical studies suggest that CYP expression and metabolic activity are altered in models of metabolic syndrome. Alterations in hepatic and renal CYP expression and metabolic activity have been observed in rodents genetically predisposed to obesity, including obese Zucker rats (47, 49), ob/ob mice (13, 40), and db/db mice (22). Although these models exhibit many of the characteristics of human metabolic syndrome, other metabolic derangements may also be present (3). For example, these obese rodent models appear to have lower renin-angiotensin system activity compared with lean controls (2, 15), whereas the renin-angiotensin system is activated in obese humans (10). Consequently, the alterations in CYP expression and metabolic activity observed in rodents genetically predisposed to obesity may not accurately model the pathophysiology underlying human metabolic syndrome. In contrast, the phenotype induced by high-fat diet feeding in rodents more closely mimics human metabolic syndrome (4).
Importantly, the effects of a high-fat diet on hepatic and renal CYP epoxygenase and ω-hydroxylase expression and metabolic activity and the functional balance between the pathways have not been rigorously evaluated to date. Moreover, the mechanisms underlying the observed alterations in CYP expression and metabolic activity in response to high-fat diet feeding have not been elucidated. Therefore, we sought to 1) characterize the effect of high-fat diet feeding on the functional balance between CYP epoxygenase and ω-hydroxylase metabolism in liver and kidney and 2) investigate the role of hypercholesterolemia, insulin resistance, and renin-angiotensin system activation in mediating alterations in CYP-mediated eicosanoid metabolism in high-fat diet-fed mice.
MATERIALS AND METHODS
Reagents.
All reagents were purchased from Fisher Scientific (Pittsburgh, PA) unless otherwise noted.
Experimental protocol.
All experiments were performed in 8- to 10-wk-old male mice (Taconic, Hudson, NY). Mice randomized to high-fat diet received the RD Western Diet (21% fat, 0.21% cholesterol; Research Diets, New Brunswick, NJ). Standard diet-fed mice received ProLab RMH 3000 rodent chow (5% fat; PMI Nutrition International, Brentwood, MO). Mice had access to food and water ad libitum. The treatment schemes for the effect of high-fat diet over time (experiment 1) and the effects of enalapril, metformin, and losartan treatment (experiments 2–4) are depicted in Fig. 1.
Fig. 1.
Treatment schemes to assess the effect of high-fat diet on cytochrome P450 enzyme (CYP) epoxygenase and ω-hydroxylase metabolic activity over time (A) and the effect of enalapril, metformin, and losartan treatment on high-fat diet-induced alterations in CYP epoxygenase and ω-hydroxylase metabolic activity (B).
In experiment 1, wild-type (WT) C57BL/6 and apolipoprotein E (apoE)−/− mice were randomized to receive high-fat or standard diet for 2, 4, or 8 wk (n = 4/group), as shown in Fig. 1A. In experiment 2, WT and apoE−/− mice were randomized to high-fat (n = 10/genotype) or standard diet (n = 4/genotype). After 2 wk of the assigned diet, a subset of high-fat diet-fed mice were treated with the angiotensin-converting enzyme (ACE) inhibitor enalapril (30 mg·kg−1·day−1; Sigma-Aldrich, St. Louis, MO) (9) administered in the drinking water for 2 wk (n = 6/genotype), as shown in Fig. 1B. In experiment 3, WT mice were randomized to high-fat (n = 18) or standard diet (n = 23). After 2 wk of the assigned diet, a subset of mice were treated with the insulin sensitizer metformin (300 mg·kg−1·day−1, n = 6/diet; Sigma-Aldrich) (28) or enalapril (30 mg·kg−1·day−1, n = 6/diet) administered in the drinking water for 2 wk, as shown in Fig. 1B. In experiment 4, WT mice were randomized to high fat (n = 12) or standard diet (n = 12). After 2 wk of the assigned diet, a subset of mice were treated with the angiotensin II type 1 (AT1) receptor blocker losartan (25 mg·kg−1·day−1, n = 6/diet; Cayman Chemical, Ann Arbor, MI) (20) administered in the drinking water for 2 wk, as shown in Fig. 1B.
Mice were euthanized by CO2 inhalation, and liver and kidney tissue were harvested and flash-frozen in liquid nitrogen. Blood was collected via cardiac puncture, and plasma was separated by centrifugation. Tissue and plasma were stored at −80°C pending analysis. Nonfasting plasma insulin concentrations were measured with the Rat/Mouse Insulin ELISA kit (Millipore, Billerica, MA) per the manufacturer's instructions. Nonfasting plasma glucose and total cholesterol levels were measured by the Animal Clinical Laboratory Core Facility at University of North Carolina at Chapel Hill using a Vitros 350 automated chemical analyzer (Ortho-Clinical Diagnostics, Rochester, NY). All studies were conducted in accordance with the principles outlined in the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee at the University of North Carolina at Chapel Hill.
RNA isolation, reverse transcription, and quantitative RT-PCR.
Total RNA was isolated from whole tissue homogenates using the RNeasy Miniprep Kit (Qiagen, Valencia, CA) per the manufacturer's instructions, as described previously (38). Total RNA was reverse transcribed to cDNA using the ABI High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA) with a reaction temperature of 25°C for 10 min and then 37°C for 120 min. Expression of murine Cyp2c29, Cyp2c44, Cyp2j5, Cyp4a12a, Ephx2, Ccl2, and GAPDH were quantified by quantitative RT-PCR using commercially available Taqman Assays on Demand (Applied Biosystems). CYP mRNA levels were normalized to GAPDH and expressed relative to the WT/standard diet controls using the 2−ΔΔCT method (26).
Microsome isolation.
Hepatic and renal microsomal fractions were isolated as described previously (38). Briefly, frozen tissue was homogenized in 0.25 M sucrose-10 mM Tris·HCl buffer (pH 7.5) containing protease inhibitors. Homogenates were centrifuged at 4°C at 2,570 g for 20 min and then at 10,300 g for 20 min to remove cellular debris. The supernatants were then centrifuged at 100,000 g at 4°C for 90 min. The resulting microsomal pellets were resuspended in 50 mM Tris-1 mM DTT-1 mM EDTA buffer (pH 7.5) containing 20% glycerol. Protein concentrations were quantified using the Bio-Rad protein assay (Bio-Rad, Hercules, CA) per the manufacturer's instructions.
Microsomal incubations.
Incubations contained 300 μg of microsomal protein and 50 μM arachidonic acid in a 1-ml volume of 0.12 M potassium phosphate incubation buffer containing 5 mM magnesium chloride, as described previously (33, 38). Reactions were initiated by the addition of 1 mM NADPH and carried out at 37°C for 20 min. Incubations were carried out at saturating concentrations of substrate, and metabolite formation was linear with respect to incubation time and microsomal protein, as determined from preliminary incubations. In the presence of these saturating substrate concentrations, formation rates reflect the amount of metabolically active protein (33) and are significantly correlated with CYP mRNA and protein levels (38). The reactions were stopped by placing the samples on ice, and 12.5 ng of 20-HETE-d6 was added as an internal standard. Because of high metabolite formation, liver incubations were diluted 10-fold in incubation buffer prior to the addition of internal standard. Metabolites were extracted with diethyl ether, evaporated to dryness under nitrogen gas, and reconstituted in 80% methanol in deionized water for analysis.
Tissue extraction.
Tissue concentrations of EETs, DHETs, and 20-HETE in liver and kidney were quantified as described previously (33). Briefly, frozen tissue was homogenized in 0.12 M potassium phosphate buffer containing 0.113 mM butylated hydroxytoluene and centrifuged at 4°C at 10,000 g for 30 min to remove cellular debris. The supernatant was retained, and 12.5 ng of 20-HETE-d6 was added as an internal standard. Samples were loaded onto Oasis HLB (30 mg) SPE cartridges (Waters, Milford, MA) that were conditioned and equilibrated with 1 ml of methanol and 1 ml of water, respectively. Columns were washed with three 1-ml volumes of 5% methanol and were eluted with 100% methanol. Extracts were evaporated to dryness under nitrogen gas and reconstituted in 80% methanol in deionized water for analysis.
Ultraperformance liquid chromatography-tandem mass spectrometry.
Arachidonic acid metabolites (14,15-EET, 11,12-EET, 8,9-EET, 14,15-DHET, 11,12-DHET, 8,9-DHET, 5,6-DHET, and 20-HETE) in microsomal incubations and tissue extracts were quantified by ultraperformance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS), as described previously (30, 38). Briefly, analytes were separated on a UPLC BEH C-18, 1.7 μm (2.1 × 100 mm) reversed-phase column (Waters). Mass spectrometric analysis was performed with a TSQ Quantum Ultra (Thermo Fisher Scientific, San Jose, CA) triple-quadrupole mass spectrometer coupled with heated electrospray ionization operated in negative selective reaction monitoring mode. Analytical data was acquired and analyzed using Xcaliber software version 2.0.6 (ThermoFinnigan, San Jose, CA). Metabolite concentrations were calculated from a standard curve and expressed as formation rates (pmol·mg protein−1·min−1) or relative to tissue weight (pmol/g tissue).
Statistical analysis.
All data are expressed as means ± SE. The sum formation rate of all EET and DHET regioisomers was calculated and used as an index of total CYP epoxygenase metabolic activity. The functional balance between the CYP epoxygenase and ω-hydroxylase pathways was assessed by calculating the ratio of 20-HETE formation to EET + DHET formation (20-HETE/EET + DHET). Because the data were not normally distributed, mRNA levels were transformed to ranks, and metabolite formation rates, clinical chemistry values, and metabolite concentrations were log-transformed prior to statistical analysis. To evaluate the effect of high-fat diet on CYP expression and metabolic activity over time, data from WT/standard diet-fed mice at each time point were pooled to create a single control group, and data were analyzed by one-way ANOVA followed by post hoc Dunnett's test for comparison with the pooled control group. To determine the effect of enalapril, metformin, and losartan treatment, data were analyzed by one-way ANOVA followed by post hoc Tukey's test. The relationship between CYP mRNA levels and EET + DHET or 20-HETE formation was evaluated by Spearman rank correlation. Statistical analysis was performed using SAS software (version 9.1.3; SAS Institute, Cary, NC). P < 0.05 was considered statistically significant.
RESULTS
Experiment 1: effect of high-fat diet on CYP epoxygenase and ω-hydroxylase metabolic activity over time.
To characterize the effect of high-fat diet feeding on hepatic and renal CYP epoxygenase and ω-hydroxylase metabolic activity over time, we randomized WT and apoE−/− mice to high-fat or standard diet for 2, 4, or 8 wk. Baseline body weight was significantly greater in apoE−/− mice (25.5 ± 0.5 g) compared with WT mice (21.4 ± 0.2 g, P < 0.001). High-fat diet promoted weight gain and altered clinical chemistry values in both WT and apoE−/− mice (Table 1). apoE−/− mice had significantly higher plasma total cholesterol levels compared with WT mice fed the same diet. Within each genotype, high-fat diet-fed mice had significantly higher plasma total cholesterol levels than standard diet-fed mice. Hepatic Ccl2 mRNA levels were significantly higher in apoE−/− mice (data not shown) and significantly correlated with plasma total cholesterol levels (rs = 0.68, P < 0.001). No significant differences in renal Ccl2 mRNA levels were observed across genotype or diet. In contrast, plasma insulin levels were not significantly different between WT and apoE−/− mice fed a standard diet, but high-fat diet feeding resulted in significantly higher plasma insulin levels in both genotypes. No significant differences in plasma glucose levels were observed among the diet and genotype groups.
Table 1.
Weight and plasma clinical chemistry in WT and apoE−/− mice fed a standard or high-fat diet
| 2 wk | 4 wk | 8 wk | |
|---|---|---|---|
| Weight (change from baseline), g | |||
| WT/standard diet | 2.1 ± 0.6 | 3.6 ± 0.2 | 5.0 ± 0.5 |
| WT/high-fat diet | 3.5 ± 0.9 | 10.3 ± 0.2* | 11.0 ± 1.5* |
| apoE−/−/standard diet | 3.7 ± 0.1 | 4.3 ± 0.2 | 4.2 ± 0.2 |
| apoE−/−/high-fat diet | 3.5 ± 0.3 | 4.7 ± 0.2 | 10.0 ± 0.9* |
| Total cholesterol, mg/dl | |||
| WT/standard diet | <45† | 86 ± 2 | 81 ± 8 |
| WT/high-fat diet | 104 ± 16 | 248 ± 14* | 194 ± 23* |
| apoE−/−/standard diet | 614 ± 84* | 512 ± 49* | 918 ± 70* |
| apoE−/−/high-fat diet | 902 ± 207* | 1,099 ± 130* | 1,398 ± 125* |
| Insulin, ng/ml | |||
| WT/standard diet | 0.72 ± 0.08 | 0.95 ± 0.14 | 0.68 ± 0.14 |
| WT/high-fat diet | 2.23 ± 0.61* | 2.67 ± 0.54* | 4.55 ± 0.82* |
| apoE−/−/standard diet | 0.83 ± 0.08 | 0.56 ± 0.04 | 1.06 ± 0.17 |
| apoE−/−/high-fat diet | 1.90 ± 0.54* | 1.72 ± 0.31* | 4.09 ± 0.87* |
| Glucose, mg/dl | |||
| WT/standard diet | 331 ± 19 | 399 ± 52 | 335 ± 15 |
| WT/high-fat diet | 446 ± 57 | 386 ± 26 | 382 ± 32 |
| apoE−/−/standard diet | 359 ± 16 | 325 ± 19 | 306 ± 20 |
| apoE−/−/high-fat diet | 361 ± 34 | 382 ± 19 | 418 ± 34 |
Values are means ± SE.
WT, wild-type; apoE, apolipoprotein E.
P < 0.05 compared with WT/standard diet.
All values were below the limit of quantitation.
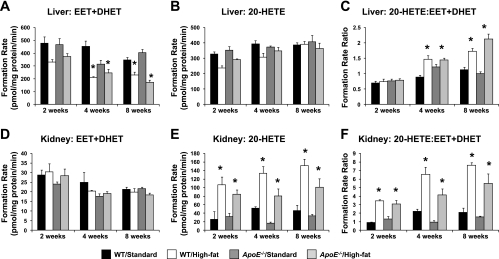
High-fat diet feeding differentially altered CYP epoxygenase and ω-hydroxylase expression and metabolic activity in liver and kidney in both WT and apoE−/− mice (Fig. 2). However, no significant differences were observed between WT and apoE−/− mice fed a standard diet.
Fig. 2.
Effect of high-fat diet on hepatic total CYP epoxygenase [epoxyeicosatrienoic and dihydroxyeicosatrienoic acid (EET + DHET)] metabolic activity (A), hepatic CYP ω-hydroxylase [20-hydroxyeicosatetraenoic acid (20-HETE)] metabolic activity (B), hepatic 20-HETE/EET + DHET formation rate ratio (C), renal total CYP epoxygenase (EET + DHET) metabolic activity (D), renal CYP ω-hydroxylase (20-HETE) metabolic activity (E), and renal 20-HETE/EET + DHET formation rate ratio in wild-type (WT) and apolipoprotein E (apoE)−/− mice (F) (n = 4/group). *P < 0.05 vs. WT/standard diet control group.
In liver, high-fat diet significantly suppressed EET + DHET formation at 4 and 8 wk in both WT and apoE−/− mice relative to the WT/standard diet control group (Fig. 2A). At 2 wk, EET + DHET formation appeared to be lower in WT mice, but this difference was not statistically significant (P = 0.204). Hepatic Cyp2c29 and Cyp2c44 mRNA levels were significantly lower in high-fat diet-fed mice (data not shown), and mRNA levels for each CYP epoxygenase examined significantly correlated with EET + DHET formation (Cyp2c29: rS = 0.68; Cyp2c44: rS = 0.56; Cyp2j5: rS = 0.53; P < 0.001 for all). No significant differences in hepatic Ephx2 mRNA levels were observed across either diet or genotype (data not shown). In contrast, no significant differences in hepatic 20-HETE formation (Fig. 2B) or Cyp4a12a mRNA levels (data not shown) were observed in response to high-fat diet. The 20-HETE/EET + DHET formation rate ratio was significantly greater in high-fat diet-fed mice at 4 and 8 wk, suggesting that the functional balance was shifted in favor of the CYP ω-hydroxylase pathway secondary to suppression of EET + DHET formation (Fig. 2C).
In contrast to what was observed in liver, no significant differences in renal EET + DHET formation (Fig. 2D) or Cyp2c44 and Cyp2j5 mRNA levels (data not shown) were observed in high-fat diet-fed mice. Similarly, no significant differences in renal Ephx2 mRNA levels were observed (data not shown). However, renal 20-HETE formation was markedly induced after 2, 4, and 8 wk of high-fat diet in both WT and apoE−/− mice, relative to the WT/standard diet group (Fig. 2E), and was significantly correlated with Cyp4a12a mRNA levels (rS = 0.60, P < 0.001). This induction of renal CYP ω-hydroxylase metabolic activity resulted in a significantly higher renal 20-HETE/EET + DHET formation rate ratio in high-fat diet-fed mice in both genotype groups (Fig. 2F).
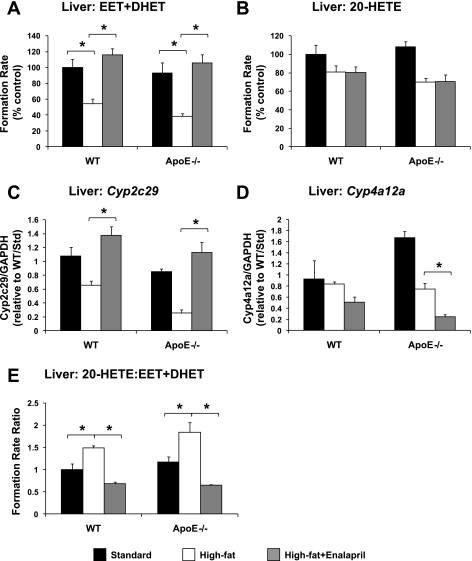
Experiment 2: effect of enalapril administration on high-fat diet-induced alterations in CYP epoxygenase and ω-hydroxylase metabolic activity.
Consistent with the results of experiment 1, high-fat diet significantly suppressed CYP epoxygenase metabolic activity in liver (Fig. 3A). Hepatic Cyp2c29 mRNA levels also tended to be lower in high-fat diet-fed mice, but this difference was not statistically significant (WT: P = 0.129 vs. standard diet; apoE−/−: P = 0.139 vs. standard diet; Fig. 3C). Enalapril administration reversed these effects in both WT and apoE−/− mice. Similarly, the high-fat diet-induced elevation in the 20-HETE/EET + DHET formation rate ratio in liver was reversed by enalapril treatment (Fig. 3E). No significant differences in hepatic CYP ω-hydroxylase metabolic activity (Fig. 3B) or Cyp4a12a mRNA levels (Fig. 3D) were observed in high-fat diet- compared with standard diet-fed mice. Enalapril decreased Cyp4a12a mRNA levels significantly (P = 0.014 vs. high-fat diet) in apoE−/− but not WT mice.
Fig. 3.
Effect of high-fat diet and enalapril treatment on hepatic total CYP epoxygenase (EET + DHET) metabolic activity (A), CYP ω-hydroxylase (20-HETE) metabolic activity (B), Cyp2c29 mRNA levels (C), Cyp4a12a mRNA levels (D), and 20-HETE/EET + DHET formation rate ratio in WT and apoE−/− mice (E) (standard diet: n = 4; high-fat diet: n = 4; high-fat diet + enalapril: n = 6). *P < 0.05 vs. high-fat diet group.
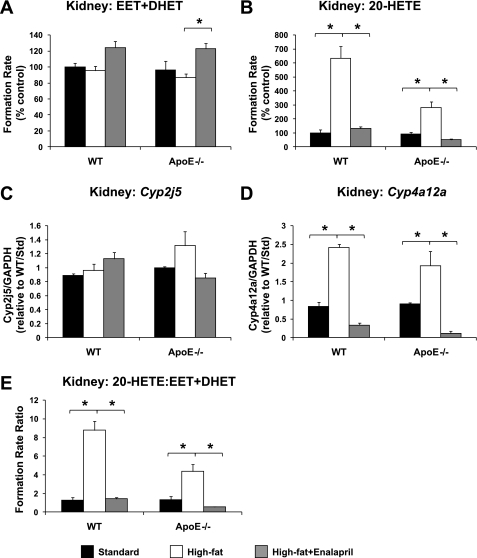
In kidney, 20-HETE formation (Fig. 4B), Cyp4a12a mRNA levels (Fig. 4D), and the 20-HETE/EET + DHET formation rate ratio (Fig. 4E) were significantly higher in high-fat diet- compared with standard diet-fed mice, regardless of genotype, and these effects were reversed by enalapril treatment. No significant differences in renal EET + DHET formation (Fig. 4A) or Cyp2j5 mRNA levels (Fig. 4C) were observed in high-fat diet-fed mice. Enalapril appeared to modestly increase renal EET + DHET formation in WT (P = 0.072 vs. high-fat diet) and apoE−/− (P = 0.009 vs. high-fat diet) mice; however, no significant differences in Cyp2j5 mRNA levels were observed.
Fig. 4.
Effect of high-fat diet and enalapril treatment on renal total CYP epoxygenase (EET + DHET) metabolic activity (A), CYP ω-hydroxylase (20-HETE) metabolic activity (B), Cyp2j5 mRNA levels (C), Cyp4a12a mRNA levels (D), and 20-HETE/EET + DHET formation rate ratio in WT and apoE−/− mice (E) (standard diet: n = 4; high-fat diet: n = 4; high-fat diet + enalapril: n = 6). *P < 0.05 vs. high-fat diet group.
Consistent with prior studies demonstrating that ACE inhibitors have insulin-sensitizing effects (34), enalapril significantly lowered plasma insulin levels in high-fat diet-fed WT mice (high-fat diet/no treatment: 2.95 ± 0.82 ng/ml; high-fat diet/enalapril: 0.72 ± 0.08 ng/ml; P = 0.022). A similar trend was observed in apoE−/− mice, but this difference was not statistically significant (high-fat diet/no treatment: 1.85 ± 0.27 ng/ml; high-fat diet/enalapril: 0.90 ± 0.07 ng/ml, P = 0.260).
Experiment 3: effect of enalapril and metformin administration on high-fat diet-induced alterations in CYP epoxygenase and ω-hydroxylase metabolic activity.
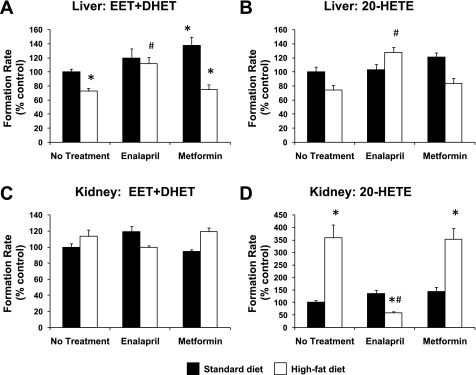
To discern whether the effect of enalapril on high-fat diet-induced alterations in CYP epoxygenase and ω-hydroxylase metabolic activity was related to its insulin-sensitizing effects, high-fat diet-fed mice were administered metformin, an insulin-sensitizing agent with a mechanism of action independent of the renin-angiotensin system. Compared with untreated high-fat diet-fed mice (3.14 ± 0.60 ng/ml), both enalapril (1.53 ± 0.30 ng/ml, P = 0.036) and metformin (1.40 ± 0.16 ng/ml, P = 0.028) lowered plasma insulin levels significantly. As observed in the two previous experiments, high-fat diet suppressed hepatic CYP epoxygenase (Fig. 5A) and induced renal CYP ω-hydroxylase metabolic activity significantly (Fig. 5D), and enalapril administration reversed these effects. In contrast, metformin treatment had no effect on high-fat diet-induced changes in hepatic EET + DHET or renal 20-HETE formation. Hepatic CYP epoxygenase activity was modestly higher in standard diet-fed mice treated with metformin compared with untreated mice (Fig. 5A). Enalapril modestly increased hepatic 20-HETE formation in high-fat diet-fed mice (Fig. 5B), but no significant differences in renal EET + DHET formation (Fig. 5C) were observed across diet or treatment groups.
Fig. 5.
Effect of enalapril and metformin treatment on hepatic total CYP epoxygenase (EET + DHET) metabolic activity (A), hepatic CYP ω-hydroxylase (20-HETE) metabolic activity (B), renal total CYP epoxygenase (EET + DHET) metabolic activity (C), and renal CYP ω-hydroxylase (20-HETE) metabolic activity (D) (standard diet: no treatment, n = 11; metformin: n = 6; enalapril: n = 6; high-fat diet: n = 6/group). *P < 0.05 vs. no treatment/standard diet; #P < 0.05 vs. no treatment/high-fat diet.
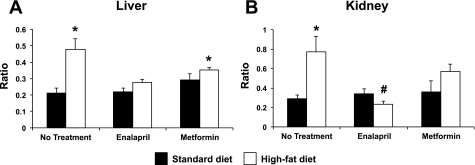
To determine whether these alterations in CYP epoxygenase and ω-hydroxylase metabolic activity reflected CYP-derived eicosanoid levels in vivo, we quantified EET + DHET and 20-HETE tissue concentrations in liver and kidney. Consistent with the microsomal incubation studies, renal 20-HETE concentrations were significantly higher in high-fat diet (21.2 ± 3.9 pmol/g tissue) compared with standard diet-fed mice (7.1 ± 0.8 pmol/g tissue, P < 0.001). Enalapril reversed the high-fat diet-induced increase in renal 20-HETE concentrations (4.3 ± 0.4 pmol/g tissue, P < 0.001 vs. high-fat diet/no treatment), but metformin treatment did not (16.2 ± 2.5 pmol/g tissue, P = 0.868 vs. high-fat diet/no treatment). Furthermore, high-fat diet increased the 20-HETE/EET + DHET concentration ratio significantly in both liver and kidney compared with standard diet-fed mice (Fig. 6). Enalapril treatment reversed the high-fat diet-induced increase in the renal 20-HETE/EET + DHET concentration ratio. A similar trend was observed in liver, but this result was not statistically significant (P = 0.103 vs. high-fat diet/no treatment). Metformin treatment did not significantly alter the 20-HETE/EET + DHET concentration ratio in either liver (P = 0.632 vs. high-fat diet/no treatment) or kidney (P = 0.956 vs. high-fat diet/no treatment).
Fig. 6.
Effect of enalapril and metformin treatment on the 20-HETE/EET + DHET concentration ratio in liver (A) and kidney (B) (n = 4–6/group). *P < 0.05 vs. no treatment/standard diet; #P < 0.05 vs. no treatment/high-fat diet.
Experiment 4: effect of losartan administration on high-fat diet-induced alterations in CYP epoxygenase and ω-hydroxylase metabolic activity.
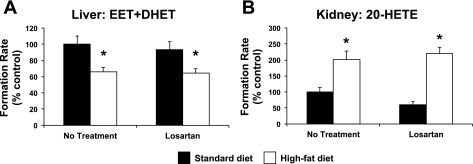
To determine whether the effects of enalapril on high-fat diet-induced alterations in CYP-mediated eicosanoid metabolism were mediated by suppression of angiotensin II signaling through the AT1 receptor, high-fat diet-fed mice were administered losartan, an AT1 receptor blocker. Consistent with experiments 1–3, high-fat diet significantly suppressed hepatic CYP epoxygenase and induced renal CYP ω-hydroxylase metabolic activity (Fig. 7). However, treatment with losartan did not reverse these high-fat diet-induced alterations in CYP-mediated eicosanoid metabolism.
Fig. 7.
Effect of losartan treatment on hepatic total CYP epoxygenase (EET + DHET) metabolic activity (A) and renal CYP ω-hydroxylase (20-HETE) metabolic activity (B) (n = 6/group). *P < 0.05 vs. no treatment/standard diet.
DISCUSSION
CYP expression and metabolic activity is altered in rodent models of metabolic syndrome, but the underlying mechanisms remain poorly understood. To our knowledge, this is the first study to demonstrate that high-fat diet feeding shifts the functional balance between the CYP epoxygenase and ω-hydroxylase pathways in liver and kidney in favor of proinflammatory, vasoconstrictive 20-HETE formation. Moreover, enalapril, but not metformin or losartan, reverses high-fat diet-induced suppression of hepatic CYP epoxygenase metabolic activity and induction of renal CYP ω-hydroxylase metabolic activity and restores the functional balance between the pathways. Collectively, these findings suggest that the kinin-kallikrein system and AT2 receptor are key regulators of CYP-mediated eicosanoid metabolism in the presence of metabolic syndrome.
High-fat diet feeding lowered hepatic CYP epoxygenase activity and Cyp2c29 and Cyp2c44 expression relative to standard diet controls, consistent with a prior study showing that hepatic Cyp2c protein was suppressed by high-fat diet (44). In kidney, we observed a marked induction of 20-HETE formation in microsomal incubations and tissue concentrations in vivo but no differences in EET + DHET formation or tissue concentrations in high-fat diet-fed mice. In contrast, suppression of renal tubular CYP2C and CYP4A expression and metabolic activity has been observed in rats fed a high-fat diet relative to lean controls (39). These conflicting results may be due to species differences in the regulation of renal CYP expression. In both liver and kidney, the 20-HETE/EET + DHET ratios in both the microsomal incubations and direct tissue extractions were significantly greater in high-fat diet-fed mice relative to controls, suggesting that the functional balance between the pathways had been shifted toward the CYP ω-hydroxylase pathway secondary to changes in CYP expression.
High-fat diet feeding induces multiple metabolic derangements, including dyslipidemia, insulin resistance, activation of the renin-angiotensin system, and suppression of the kinin-kallikrein system (4, 10, 24), which may contribute to the alterations in CYP-mediated eicosanoid metabolism that we observed. To investigate the effect of dyslipidemia and inflammation, we evaluated CYP epoxygenase and ω-hydroxylase expression and metabolic activity in WT and apoE−/− mice. apoE−/− mice have impaired cholesterol clearance and develop profound hyperlipidemia, which promotes the development of atherosclerotic lesions and vascular inflammation similar to what is observed in humans with atherosclerotic disease (29). Although plasma total cholesterol and hepatic inflammatory gene expression was substantially higher in apoE−/− mice, no significant differences in CYP epoxygenase or ω-hydroxylase activity in liver or kidney were observed between WT and apoE−/− mice, and suppression of hepatic EET + DHET formation and induction of renal 20-HETE formation was observed in both genotypes in response to high-fat diet feeding. Thus, the observed changes in CYP epoxygenase and ω-hydroxylase metabolic activity were driven by the high-fat diet rather than by genotype and were independent of plasma cholesterol levels.
Interestingly, enalapril treatment reversed the effects of high-fat diet feeding on CYP-mediated eicosanoid metabolism in both apoE−/− and WT mice. In addition to inhibiting the formation of angiotensin II and the degradation of bradykinin, ACE inhibitors have also improved insulin sensitivity in animal models (34) and prevented the development of diabetes in humans (1). Consistent with these reports, enalapril lowered plasma insulin levels in high-fat diet-fed mice. Insulin modulates CYP expression in vitro (42), and CYP expression and metabolic activity are altered in models of diabetes (22, 37). Consequently, we administered metformin, an insulin-sensitizing agent, to determine whether insulin resistance was driving the changes in CYP-mediated eicosanoid metabolism observed in high-fat diet-fed mice. Although metformin treatment normalized plasma insulin levels, it did not reverse the alterations in CYP expression and metabolic activity, suggesting that the effect of enalapril was mediated by inhibition of ACE rather than by improving insulin sensitivity. However, administration of the AT1 receptor antagonist losartan also did not reverse the observed suppression of hepatic CYP epoxygenase or induction of renal CYP ω-hydroxylase metabolic activity. Collectively, these data demonstrate that high-fat diet-induced alterations in renal and hepatic CYP-mediated eicosanoid metabolism are not mediated via angiotensin II signaling through the AT1 receptor and suggest that enalapril reverses these effects via inhibition of bradykinin degradation and suppression of angiotensin II signaling through the AT2 receptor.
Several prior studies have demonstrated that angiotensin II alters CYP-mediated eicosanoid metabolism. For example, renal and vascular CYP2C and EET formation are suppressed in angiotensin II-infused rats (48) and double transgenic rats that overexpress the human renin and angiotensinogen genes (19). In contrast, angiotensin II stimulates 20-HETE release in isolated perfused kidneys (5), and Ren-2 transgenic rats, which have elevated renal and plasma angiotensin II levels, exhibit significantly higher renal 20-HETE formation relative to wild-type controls (7). Consistent with these preclinical studies, humans with renovascular disease, a condition characterized by activation of the renin-angiotensin system, tended to have lower plasma EET levels and had significantly higher plasma 20-HETE levels compared with healthy controls (31). Although suppression of EET and induction of 20-HETE biosynthesis have been ascribed primarily to angiotensin II signaling through the AT1 receptor, AT2 receptor activation has been shown to promote 20-HETE biosynthesis in rat renal microvessels (8) and EET biosynthesis in rabbit afferent arterioles and human dermal fibroblasts (21, 36). Several studies have also demonstrated that bradykinin stimulates the production and release of EETs in multiple vascular beds (16, 35, 41). In addition, chronic treatment with ACE inhibitors, but not an angiotensin AT1 receptor blocker, directly altered renal CYP-mediated eicosanoid metabolism via a bradykinin B2 receptor-dependent mechanism (18). Although these and our studies collectively demonstrate that the kinin-kallikrein system and renin-angiotensin system are key regulators of CYP-mediated eicosanoid metabolism, further studies remain necessary to delineate the relative contribution of B2 and AT2 receptor signaling to the regulation of renal and hepatic CYP-mediated eicosanoid metabolism in the presence of metabolic syndrome.
Potentiation of the CYP epoxygenase pathway via enhanced EET biosynthesis or inhibition of EET hydrolysis has been shown to lower blood pressure in angiotensin II-dependent models of hypertension (23) and attenuate acute and chronic inflammation (11, 46). A series of studies have demonstrated that EETs also activate the phosphatidylinositol 3-kinase/Akt pathway (12), a key component of insulin signaling, and potentiation of EETs enhances insulin signaling and improves insulin sensitivity (27, 43). Thus, prolonged suppression of hepatic CYP epoxygenase metabolic activity in the presence of the metabolic syndrome would be hypothesized to promote inflammation and hepatic insulin resistance. In contrast, 20-HETE is a potent vasoconstrictor (35) and promotes inflammation via activation of nuclear factor-κB (17); although renal inflammatory gene expression was not upregulated in this model, prolonged induction of renal CYP ω-hydroxylase metabolic activity would be hypothesized to promote hypertension and renal injury. Indeed, prior studies have demonstrated that inhibition of 20-HETE formation has antihypertensive effects and attenuates renal inflammation and injury (6, 7). A recent study demonstrated that elevations in plasma 20-HETE levels were associated with shortened bleeding time in mice (25), suggesting that induction of 20-HETE formation may also contribute to the prothrombotic state observed in metabolic syndrome patients. Collectively, our findings suggest that a shift in the functional balance between the CYP epoxygenase and ω-hydroxylase pathways in favor of 20-HETE formation may be a key contributor to the pathological consequences of a high-fat diet and the metabolic syndrome and that increasing EETs and/or decreasing 20-HETE may have therapeutic utility to abrogate these effects. Future studies are necessary to test this hypothesis. It also is important to note that our experiments were performed exclusively in male mice. Renal Cyp4a12a expression is regulated by androgens, and male mice exhibit markedly higher renal Cyp4a12a expression than female mice (32). Thus, future studies are necessary to evaluate the effects of sex hormones on high-fat diet-induced alterations in CYP epoxygenase and ω-hydroxylase metabolic activity.
In conclusion, induction of the metabolic syndrome by high-fat diet feeding suppressed hepatic CYP epoxygenase and induced renal CYP ω-hydroxylase expression and metabolic activity, thereby shifting the functional balance between the pathways in favor of 20-HETE formation. Treatment with enalapril, but not metformin or losartan, reversed this effect, suggesting that the kinin-kallikrein system and AT2 receptor are key mediators of high-fat diet-induced alterations in CYP-mediated eicosanoid metabolism. Further studies are necessary to delineate the mechanisms underlying these alterations, elucidate the pathophysiological significance, and ultimately determine the therapeutic potential of modulating the CYP epoxygenase and ω-hydroxylase pathways in metabolic diseases.
GRANTS
This work was supported by a predoctoral fellowship from the American Foundation for Pharmaceutical Education (K. N. Theken), a predoctoral training program in Integrative Vascular Biology supported by the National Heart, Lung, and Blood Institute (NHLBI; T32-HL-069768; R. N. Schuck), grants from the National Institute of Neurological Disorders and Stroke (NINDS; Grant R01-NS-052315) and National Center of Research Resources (Grant S10-RR-023461; S. M. Poloyac), and a grant from the National Institute of General Medical Sciences (NIGMS; Grant R01-GM-088199; C. R. Lee). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NHLBI, NIGMS, NINDS, or National Institutes of Health.
DISCLOSURES
The authors have no conflicts of interest, financial or otherwise, related to the work in this article.
AUTHOR CONTRIBUTIONS
K.N.T., S.M.P., and C.R.L. did the conception and design of the research; K.N.T., Y.D., R.N.S., A.O.-O., T.M.M., and M.A.K. performed the experiments; K.N.T. analyzed the data; K.N.T., Y.D., R.N.S., and C.R.L. interpreted the results of the experiments; K.N.T. prepared the figures; K.N.T. drafted the manuscript; K.N.T., S.M.P., and C.R.L. edited and revised the manuscript; all authors approved the final version of the manuscript.
ACKNOWLEDGMENTS
We thank Kimberly C. Molnar and Dr. Bhavani P. Thampatty for their technical assistance.
REFERENCES
- 1. Abuissa H, Jones PG, Marso SP, O'Keefe JH., Jr Angiotensin-converting enzyme inhibitors or angiotensin receptor blockers for prevention of type 2 diabetes: a meta-analysis of randomized clinical trials. J Am Coll Cardiol 46: 821– 826, 2005. [DOI] [PubMed] [Google Scholar]
- 2. Alonso-Galicia M, Brands MW, Zappe DH, Hall JE. Hypertension in obese Zucker rats. Role of angiotensin II and adrenergic activity. Hypertension 28: 1047– 1054, 1996. [DOI] [PubMed] [Google Scholar]
- 3. Argiles JM. The obese Zucker rat: a choice for fat metabolism 1968–1988: twenty years of research on the insights of the Zucker mutation. Prog Lipid Res 28: 53– 66, 1989. [DOI] [PubMed] [Google Scholar]
- 4. Buettner R, Schölmerich J, Bollheimer LC. High-fat diets: modeling the metabolic disorders of human obesity in rodents. Obesity (Silver Spring) 15: 798– 808, 2007. [DOI] [PubMed] [Google Scholar]
- 5. Carroll MA, Balazy M, Huang DD, Rybalova S, Falck JR, McGiff JC. Cytochrome P450-derived renal HETEs: storage and release. Kidney Int 51: 1696– 1702, 1997. [DOI] [PubMed] [Google Scholar]
- 6. Certikova Chabova V, Walkowska A, Kompanowska-Jezierska E, Sadowski J, Kujal P, Vernerova Z, Vanourkova Z, Kopkan L, Kramer HJ, Falck JR, Imig JD, Hammock BD, Vaneckova I, Cervenka L. Combined inhibition of 20-hydroxyeicosatetraenoic acid formation and of epoxyeicosatrienoic acids degradation attenuates hypertension and hypertension-induced end-organ damage in Ren-2 transgenic rats. Clin Sci (Lond) 118: 617– 632, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chabova VC, Kramer HJ, Vaneckova I, Vernerova Z, Eis V, Tesar V, Skaroupkova P, Thumova M, Schejbalova S, Huskova Z, Vanourkova Z, Kolsky A, Imig JD, Cervenka L. Effects of chronic cytochrome P-450 inhibition on the course of hypertension and end-organ damage in Ren-2 transgenic rats. Vascul Pharmacol 47: 145– 159, 2007. [DOI] [PubMed] [Google Scholar]
- 8. Croft KD, McGiff JC, Sanchez-Mendoza A, Carroll MA. Angiotensin II releases 20-HETE from rat renal microvessels. Am J Physiol Renal Physiol 279: F544– F551, 2000. [DOI] [PubMed] [Google Scholar]
- 9. da Cunha V, Tham DM, Martin-McNulty B, Deng G, Ho JJ, Wilson DW, Rutledge JC, Vergona R, Sullivan ME, Wang YX. Enalapril attenuates angiotensin II-induced atherosclerosis and vascular inflammation. Atherosclerosis 178: 9– 17, 2005. [DOI] [PubMed] [Google Scholar]
- 10. de Kloet AD, Krause EG, Woods SC. The renin angiotensin system and the metabolic syndrome. Physiol Behav 100: 525– 534, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Deng Y, Edin ML, Theken KN, Schuck RN, Flake GP, Kannon MA, DeGraff LM, Lih FB, Foley J, Bradbury JA, Graves JP, Tomer KB, Falck JR, Zeldin DC, Lee CR. Endothelial CYP epoxygenase overexpression and soluble epoxide hydrolase disruption attenuate acute vascular inflammatory responses in mice. FASEB J 25: 703– 713, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Deng Y, Theken KN, Lee CR. Cytochrome P450 epoxygenases, soluble epoxide hydrolase, and the regulation of cardiovascular inflammation. J Mol Cell Cardiol 48: 331– 341, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Enriquez A, Leclercq I, Farrell GC, Robertson G. Altered expression of hepatic CYP2E1 and CYP4A in obese, diabetic ob/ob mice, and fa/fa Zucker rats. Biochem Biophys Res Commun 255: 300– 306, 1999. [DOI] [PubMed] [Google Scholar]
- 14. Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC, Jr, Spertus JA, Costa F. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 112: 2735– 2752, 2005. [DOI] [PubMed] [Google Scholar]
- 15. Hilzendeger AM, Morais RL, Todiras M, Plehm R, da Costa Goncalves A, Qadri F, Araujo RC, Gross V, Nakaie CR, Casarini DE, Carmona AK, Bader M, Pesquero JB. Leptin regulates ACE activity in mice. J Mol Med (Berl) 88: 899– 907, 2010. [DOI] [PubMed] [Google Scholar]
- 16. Imig JD, Falck JR, Wei S, Capdevila JH. Epoxygenase metabolites contribute to nitric oxide-independent afferent arteriolar vasodilation in response to bradykinin. J Vasc Res 38: 247– 255, 2001. [DOI] [PubMed] [Google Scholar]
- 17. Ishizuka T, Cheng J, Singh H, Vitto MD, Manthati VL, Falck JR, Laniado-Schwartzman M. 20-Hydroxyeicosatetraenoic acid stimulates nuclear factor-kappaB activation and the production of inflammatory cytokines in human endothelial cells. J Pharmacol Exp Ther 324: 103– 110, 2008. [DOI] [PubMed] [Google Scholar]
- 18. Ito O, Omata K, Ito S, Hoagland KM, Roman RJ. Effects of converting enzyme inhibitors on renal P-450 metabolism of arachidonic acid. Am J Physiol Regul Integr Comp Physiol 280: R822– R830, 2001. [DOI] [PubMed] [Google Scholar]
- 19. Kaergel E, Muller DN, Honeck H, Theuer J, Shagdarsuren E, Mullally A, Luft FC, Schunck WH. P450-dependent arachidonic acid metabolism and angiotensin II-induced renal damage. Hypertension 40: 273– 279, 2002. [DOI] [PubMed] [Google Scholar]
- 20. Keidar S, Attias J, Smith J, Breslow JL, Hayek T. The angiotensin-II receptor antagonist, losartan, inhibits LDL lipid peroxidation and atherosclerosis in apolipoprotein E-deficient mice. Biochem Biophys Res Commun 236: 622– 625, 1997. [DOI] [PubMed] [Google Scholar]
- 21. Kohagura K, Arima S, Endo Y, Chiba Y, Ito O, Abe M, Omata K, Ito S. Involvement of cytochrome P450 metabolites in the vascular action of angiotensin II on the afferent arterioles. Hypertens Res 24: 551– 557, 2001. [DOI] [PubMed] [Google Scholar]
- 22. Lam JL, Jiang Y, Zhang T, Zhang EY, Smith BJ. Expression and functional analysis of hepatic cytochromes P450, nuclear receptors, and membrane transporters in 10- and 25-week-old db/db mice. Drug Metab Dispos 38: 2252– 2258, 2010. [DOI] [PubMed] [Google Scholar]
- 23. Lee CR, Imig JD, Edin ML, Foley J, DeGraff LM, Bradbury JA, Graves JP, Lih FB, Clark J, Myers P, Perrow AL, Lepp AN, Kannon MA, Ronnekleiv OK, Alkayed NJ, Falck JR, Tomer KB, Zeldin DC. Endothelial expression of human cytochrome P450 epoxygenases lowers blood pressure and attenuates hypertension-induced renal injury in mice. FASEB J 24: 3770– 3781, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li LO, Hu YF, Wang L, Mitchell M, Berger A, Coleman RA. Early hepatic insulin resistance in mice: a metabolomics analysis. Mol Endocrinol 24: 657– 666, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Liu JY, Li N, Yang J, Qiu H, Ai D, Chiamvimonvat N, Zhu Y, Hammock BD. Metabolic profiling of murine plasma reveals an unexpected biomarker in rofecoxib-mediated cardiovascular events. Proc Natl Acad Sci USA 107: 17017– 17022, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2[−Delta Delta C(T)] Method. Methods 25: 402– 408, 2001. [DOI] [PubMed] [Google Scholar]
- 27. Luria A, Bettaieb A, Xi Y, Shieh GJ, Liu HC, Inoue H, Tsai HJ, Imig JD, Haj FG, Hammock BD. Soluble epoxide hydrolase deficiency alters pancreatic islet size and improves glucose homeostasis in a model of insulin resistance. Proc Natl Acad Sci USA 108: 9038– 9043, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Matsui Y, Hirasawa Y, Sugiura T, Toyoshi T, Kyuki K, Ito M. Metformin reduces body weight gain and improves glucose intolerance in high-fat diet-fed C57BL/6J mice. Biol Pharm Bull 33: 963– 970, 2010. [DOI] [PubMed] [Google Scholar]
- 29. Meir KS, Leitersdorf E. Atherosclerosis in the apolipoprotein-E-deficient mouse: a decade of progress. Arterioscler Thromb Vasc Biol 24: 1006– 1014, 2004. [DOI] [PubMed] [Google Scholar]
- 30. Miller TM, Donnelly MK, Crago EA, Roman DM, Sherwood PR, Horowitz MB, Poloyac SM. Rapid, simultaneous quantitation of mono and dioxygenated metabolites of arachidonic acid in human CSF and rat brain. J Chromatogr B Analyt Technol Biomed Life Sci 877: 3991– 4000, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Minuz P, Jiang H, Fava C, Turolo L, Tacconelli S, Ricci M, Patrignani P, Morganti A, Lechi A, McGiff JC. Altered release of cytochrome p450 metabolites of arachidonic acid in renovascular disease. Hypertension 51: 1379– 1385, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Muller DN, Schmidt C, Barbosa-Sicard E, Wellner M, Gross V, Hercule H, Markovic M, Honeck H, Luft FC, Schunck WH. Mouse Cyp4a isoforms: enzymatic properties, gender- and strain-specific expression, and role in renal 20-hydroxyeicosatetraenoic acid formation. Biochem J 403: 109– 118, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Poloyac SM, Tortorici MA, Przychodzin DI, Reynolds RB, Xie W, Frye RF, Zemaitis MA. The effect of isoniazid on CYP2E1- and CYP4A-mediated hydroxylation of arachidonic acid in the rat liver and kidney. Drug Metab Dispos 32: 727– 733, 2004. [DOI] [PubMed] [Google Scholar]
- 34. Premaratna SD, Manickam E, Begg DP, Rayment DJ, Hafandi A, Jois M, Cameron-Smith D, Weisinger RS. Angiotensin-converting enzyme inhibition reverses diet-induced obesity, insulin resistance and inflammation in C57BL/6J mice. Int J Obes (Lond). In press [DOI] [PubMed] [Google Scholar]
- 35. Roman RJ. P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiol Rev 82: 131– 185, 2002. [DOI] [PubMed] [Google Scholar]
- 36. Rompe F, Artuc M, Hallberg A, Alterman M, Ströder K, Thöne-Reineke C, Reichenbach A, Schacherl J, Dahlöf B, Bader M, Alenina N, Schwaninger M, Zuberbier T, Funke-Kaiser H, Schmidt C, Schunck WH, Unger T, Steckelings UM. Direct angiotensin II type 2 receptor stimulation acts anti-inflammatory through epoxyeicosatrienoic acid and inhibition of nuclear factor kappaB. Hypertension 55: 924– 931, 2010. [DOI] [PubMed] [Google Scholar]
- 37. Shimojo N, Ishizaki T, Imaoka S, Funae Y, Fujii S, Okuda K. Changes in amounts of cytochrome P450 isozymes and levels of catalytic activities in hepatic and renal microsomes of rats with streptozocin-induced diabetes. Biochem Pharmacol 46: 621– 627, 1993. [DOI] [PubMed] [Google Scholar]
- 38. Theken KN, Deng Y, Kannon MA, Miller TM, Poloyac SM, Lee CR. Activation of the acute inflammatory response alters cytochrome P450 expression and eicosanoid metabolism. Drug Metab Dispos 39: 22– 29, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wang MH, Smith A, Zhou Y, Chang HH, Lin S, Zhao X, Imig JD, Dorrance AM. Downregulation of renal CYP-derived eicosanoid synthesis in rats with diet-induced hypertension. Hypertension 42: 594– 599, 2003. [DOI] [PubMed] [Google Scholar]
- 40. Watson AM, Poloyac SM, Howard G, Blouin RA. Effect of leptin on cytochrome P-450, conjugation, and antioxidant enzymes in the ob/ob mouse. Drug Metab Dispos 27: 695– 700, 1999. [PubMed] [Google Scholar]
- 41. Weintraub NL, Fang X, Kaduce TL, VanRollins M, Chatterjee P, Spector AA. Potentiation of endothelium-dependent relaxation by epoxyeicosatrienoic acids. Circ Res 81: 258– 267, 1997. [DOI] [PubMed] [Google Scholar]
- 42. Woodcroft KJ, Novak RF. Insulin differentially affects xenobiotic-enhanced, cytochrome P-450 (CYP)2E1, CYP2B, CYP3A, and CYP4A expression in primary cultured rat hepatocytes. J Pharmacol Exp Ther 289: 1121– 1127, 1999. [PubMed] [Google Scholar]
- 43. Xu X, Zhao CX, Wang L, Tu L, Fang X, Zheng C, Edin ML, Zeldin DC, Wang DW. Increased CYP2J3 expression reduces insulin resistance in fructose-treated rats and db/db mice. Diabetes 59: 997– 1005, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yoshinari K, Takagi S, Yoshimasa T, Sugatani J, Miwa M. Hepatic CYP3A expression is attenuated in obese mice fed a high-fat diet. Pharm Res 23: 1188– 1200, 2006. [DOI] [PubMed] [Google Scholar]
- 45. Zeldin DC. Epoxygenase pathways of arachidonic acid metabolism. J Biol Chem 276: 36059– 36062, 2001. [DOI] [PubMed] [Google Scholar]
- 46. Zhang LN, Vincelette J, Cheng Y, Mehra U, Chen D, Anandan SK, Gless R, Webb HK, Wang YX. Inhibition of soluble epoxide hydrolase attenuated atherosclerosis, abdominal aortic aneurysm formation, and dyslipidemia. Arterioscler Thromb Vasc Biol 29: 1265– 1270, 2009. [DOI] [PubMed] [Google Scholar]
- 47. Zhao X, Dey A, Romanko OP, Stepp DW, Wang MH, Zhou Y, Jin L, Pollock JS, Webb RC, Imig JD. Decreased epoxygenase and increased epoxide hydrolase expression in the mesenteric artery of obese Zucker rats. Am J Physiol Regul Integr Comp Physiol 288: R188– R196, 2005. [DOI] [PubMed] [Google Scholar]
- 48. Zhao X, Pollock DM, Inscho EW, Zeldin DC, Imig JD. Decreased renal cytochrome P450 2C enzymes and impaired vasodilation are associated with angiotensin salt-sensitive hypertension. Hypertension 41: 709– 714, 2003. [DOI] [PubMed] [Google Scholar]
- 49. Zhao X, Quigley JE, Yuan J, Wang MH, Zhou Y, Imig JD. PPAR-α activator fenofibrate increases renal CYP-derived eicosanoid synthesis and improves endothelial dilator function in obese Zucker rats. Am J Physiol Heart Circ Physiol 290: H2187– H2195, 2006. [DOI] [PubMed] [Google Scholar]