Abstract
Glucagon-like peptide-1 (GLP-1)-based incretin therapy is becoming central to the treatment of type 2 diabetes. Activation of incretin hormone receptors results in rapid elevation of cAMP followed by enhanced insulin secretion. However, the incretin effect may be significantly impaired in diabetes. The objective of this study is to investigate downregulation of GLP-1 signaling by small ubiquitin-related modifier protein (SUMO). Mouse islets exposed to high glucose showed increased expression of endogenous SUMO transcripts and its conjugating enzyme Ubc-9. Overexpression of SUMO-1 in mouse insulinoma 6 (MIN6) cells and primary mouse β-cells resulted in reduced static and real-time estimates of intracellular cAMP upon receptor stimulation with exendin-4, a GLP-1 receptor (GLP-1R) agonist. GLP1-R was covalently modified by SUMO. Overexpression of SUMO-1 attenuated cell surface trafficking of GLP-1R, which resulted in significantly reduced insulin secretion when stimulated by exendin-4. Partial knock down of SUMO-conjugating enzyme Ubc-9 resulted in enhanced exendin-4-stimulated insulin secretion in mouse islets exposed to high glucose. Thus, SUMO modification of the GLP-1R could be a contributing factor to reduced incretin responsiveness. Elucidating mechanisms of GLP-1R regulation by sumoylation will help improve our understanding of incretin biology and of GLP-1-based treatment of type 2 diabetes.
Keywords: glucagon-like peptide-1 receptor; sumoylation; adenosine 3′,5′-cyclic monophosphate; insulin secretion
glucagon like peptide -1 (GLP-1) is an incretin hormone secreted by intestinal L cells that is crucial to postprandial insulin secretion (9). GLP-1 exerts its insulinotropic action through its G protein-coupled receptor (GLP-1R) via various signaling pathways. Apart from its insulinotropic activity, GLP-1 signaling has been shown to increase insulin gene expression and promote β-cell proliferation, which makes it an ideal target for incretin-based therapy for type 2 diabetes (8). Like other G protein-coupled receptors, GLP-1R has been shown to undergo rapid desensitization under in vitro conditions (12). A reduced GLP-1 response was observed in animal models of hyperglycemia due to decreased gene expression of the receptor (37).
Here, we show that cAMP generation by agonist stimulation of GLP-1R is a target of small ubiquitin-related modifier protein (SUMO) and is downregulated by sumoylation in mouse pancreatic β-cell lines and primary mouse β-cells. Posttranslational modification by SUMO (sumoylation) has gained considerable attention during the last few years as an important regulatory mechanism of protein function (11). Besides nuclear proteins, SUMO proteins have recently been shown to regulate plasma membrane proteins (2, 22, 27, 30). Emerging evidence indicates that SUMO plays an important role in pancreatic β-cell function. Four β-cell-specific proteins have been shown to be sumoylated, including transcription factors and membrane proteins, as follows. Sumoylation of a transcription factor, MafA, negatively regulates its activity (32). Sumoylation inhibits activity of the granule membrane protein ICA512 by preventing its association with signal transducer and activator of transcription (STAT) 5, thereby regulating transcriptional activity of STAT5 in β-cells (24). SUMO modification of the voltage-gated potassium channel Kv2.1 inhibits its activity in rodent β-cells (5). Recently, sumoylation has been shown to potentially regulate insulin secretion by direct and reversible inhibition of insulin exocytosis downstream of granule docking (6).
Regulation of GLP-1R is clinically significant both because of its role in endogenous insulin secretion and its significance in incretin-based therapy for type 2 diabetes. We present evidence that sumoylation is likely to be one of the mechanisms that contribute to reduced β-cell incretin responsiveness.
MATERIALS AND METHODS
DNA Constructs, cDNA Preparation, and Quantitative RT-PCR
Human SUMO-1 with hemagglutinin (HA), 1d4, green fluorescent protein (GFP), and mCherry tags and human GLP-1R with HA and GFP tags were cloned into the expression vector pCDNA 3.1(+) (Invitrogen, La Jolla, CA). RNA from mouse insulinoma (MIN6) cells and mouse islets were prepared with an RNeasy Mini Kit (Qiagen, Valencia, CA), and cDNA was prepared from 500 ng RNA by Superscript III Reverse transcriptase (Invitrogen). Primer pairs for quantitative RT-PCR were purchased from Origene (Rockville, MD). Quantitative RT-PCR was performed using Fast SYBR Green reagent using a Step one instrument (Applied Biosystems, Carlsbad, CA).
The fold change in expression of SUMO transcripts in islets exposed to 16 mM glucose was calculated by normalizing to the levels obtained from islets maintained in 5 mM glucose. The significance of the normalized fold change after a step increase in glucose was calculated by Student's t-test using Graphpad prism software. The housekeeping gene, β-actin was used as an internal control.
Cell Culture, Islet Preparation, and Transfection
MIN6 cells were grown in DMEM supplemented with 15% FBS, 100 IU/ml penicillin, and 100 mg/ml streptomycin, and MIN6 cells stably expressing GFP-SUMO were grown in DMEM supplemented with 15% FBS and 500 ng/ml G418 (Invitrogen, Carlsbad, CA). Mouse islets were prepared following an approved protocol by The University of Chicago Institutional Animal Care and Use Committee. Islets were isolated from pancreata of 8- to 10-wk-old C57BL/6J wild-type mice (Jackson Laboratory, Bar Harbor, ME) using collagenase P (Roche Diagnostics, Basel, Switzerland) digestion and a Ficoll gradient. Islets were trypsinized, and the primary cells were cultured in RPMI 1640 medium supplemented with 10% FBS, 2 mM l-glutamine, 100 IU/ml penicillin, and 100 mg/ml streptomycin. MIN6 cells and isolated primary cells were transfected with Lipofectamine 2000 according to the manufacturer's instructions (Invitrogen).
Coimmunoprecipitation
Immunoprecipitation experiments were designed to detect noncovalent and covalent interactions between GLP-1R and SUMO-1. For the detection of noncovalent interaction with SUMO and GLP-1R, MIN6 cells stably expressing GFP-SUMO-1 and transiently expressing HA-tagged GLP-1 receptor (GLP-1R-HA) were lysed in RIPA buffer (Santa Cruz Biotechnology, Santa Cruz, CA) and immunoprecipitated with a rabbit monoclonal antibody raised against HA epitope, YPYDVPDYA from influenza hemagglutinin (Cell Signaling Technology, Boston, MA). GFP-SUMO-1 was detected by mouse monoclonal antibody raised against GFP (Roche Applied Science, Indianapolis, IN). To detect covalent interaction, MIN6 cells were cotransfected with 1d4 epitope-tagged GLP-1R and GFP-SUMO-1 or conjugation-deficient GFP-SUMO-ΔGG. The cells were lysed in RIPA lysis buffer containing 2% SDS. The lysate was diluted with PBS containing 0.5% Nonidet P-40 to get the final SDS concentration to 0.2%, and GLP-1R-1d4 was immunopurified with mouse monoclonal Rho 1d4 antibody raised against the COOH-terminal epitope −TETSQVAPA-(COOH) of bacterial rhodopsin (The University of British Columbia). GFP-SUMO-modified GLP-1R was detected by a rabbit polyclonal antibody raised against GFP (Invitrogen).
Fluorescent Resonance Energy Transfer Analysis
Cyan fluorescent protein-epac2-yellow fluorescent protein fluorescent resonance energy transfer analysis for cAMP generation.
Dynamic changes in cAMP were measured using the fluorescent resonance energy transfer (FRET) biosensor Epac-camps, following a previously described protocol (17). Briefly, for the detection of dynamic changes in cAMP on agonist stimulation, MIN6 cells and mouse islets were cotransfected with SUMO-1 fused to the red fluorescent protein mCherry and driven by rat insulin promoter 2 along with Epac-camps. Control cells were transfected with Epac-camps and mCherry vector alone. Live cells were imaged 48 h posttransfection in KR2 buffer on a Nikon inverted epifluorescence microscope with a charge-coupled device camera. About 12–15 cells were found to express Epac-camps, and 6–10 cells/35 mm glass bottom dish were found to express both fluorescent proteins, indicating the β-cells. Pancreatic β-cells from multiple 35-mm dishes were selected, and fluorescence was recorded before and during stimulation with 100 nM exendin-4. The FRET ratio was recorded every 5 s. Dynamic changes in cAMP estimated from the change in FRET ratio was acquired by MetaFluor software (Universal Imaging).
GLP-1R-cyan fluorescent protein and yellow fluorescent protein-SUMO-1 live cell FRET.
MIN6 cells were transfected with cyan fluorescent protein (CFP)-GLP-1R and yellow fluorescent protein (YFP)-SUMO-1 or conjugation-deficient YFP-SUMOΔGG construct where a stop codon was introduced at G96 to remove the last four amino acids essential for covalent interaction in SUMO-1. The cells were imaged on a live cell Olympus wide-field IX-50 fluorescent microscope. FRET was calculated by CFP (donor) bleaching, and the images were acquired every 200 ms. Fluorescence decay was calculated for the entire cell using ImageJ software (NIH). Time constants were calculated using Graphpad Prism software.
Cell-Surface Biotinylation
For cell-surface biotinylation, MIN6 cells cotransfected with untagged SUMO and GLP-1R-GFP were biotinylated on a 10-cm plate for 45 min and lysed in RIPA buffer. Biotinylated receptor was purified according to the manufacturer's instructions (Thermo Fisher Scientific, Rockford, IL). Biotinylated receptors at the membrane and nonbiotinylated receptors in the cytosol were detected by an anti-GFP monoclonal antibody (Roche Applied Science). Densitometric analysis was carried out using Image J software.
Immunofluorescence
MIN6 cells transfected with GLP-1R-HA and GFP-SUMO-1 were fixed in 4% PFA for 15 min, and permeabilization was not carried out to enable detection of only NH2-terminally HA epitope-tagged GLP-1R at the cell surface. The cells were incubated with rabbit HA antibody and stained with TRITC-conjugated secondary antibody (Jackson ImmunoResearch, West Grove, PA). Immunostained and live cell images were acquired on a Leica TCS SP2 laser scanning confocal microscope (Leica Microsystems, Wetzlar, Germany) with sequential scanning of GFP and mCherry signals.
cAMP Assay
MIN6 cells were transfected with GFP or GFP-SUMO and sorted according to GFP fluorescence 48 h posttransfection. Cells expressing GFP or GFP-SUMO were plated in 96-well plates and treated with 100 nM exendin-4 for 3 h with and without 100 μM IBMX. Quantitative analysis of cAMP was done using a sensitive chemiluminescence-based cAMP-specific enzyme-linked immunosorbent assay (ELISA), according to the manufacturer's instructions (Invitrogen).
Insulin ELISA and Partial Knock Down of Ubc-9
Insulin content was calculated from MIN6 cells stably expressing GFP-SUMO-1 and control cells expressing empty vector by a sensitive mouse insulin ELISA kit (ALPCO, Salem, NH). The cells were plated in 35-mm dishes and stimulated with 100 nM exendin-4 for 3 h. The supernatant was collected, and the cells were lysed in RIPA buffer. Insulin content in the supernatant and lysate was calculated according to the manufacturer's protocol.
Ubc-9 knock down was accomplished using a validated short-hairpin RNA (shRNA) vector (Origene). Retroviral particles were generated in HEK-292 cells according to the manufacturer's instructions (Clontech, Mountain View, CA). Four constructs were tested for efficiency in MIN6 cells, and one showing ∼50% knock-down efficiency was selected. Dispersed mouse islets were infected for 16 h and cultured in 16 or 5 mM glucose for 24 h. The islet cells were stimulated with 16 mM glucose and 50 nM exendin-4 for 2 h, and secreted and cytosolic insulin was quantified by ELISA (ALPCO).
Data Analysis
The statistical analysis was carried out by Graphpad Prism software.
RESULTS
Mouse Islets Exposed to High Glucose Show Increased Expression of SUMO and Ubc-9 Transcripts
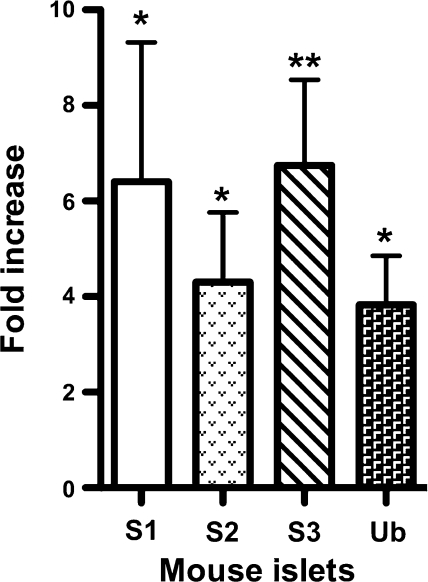
Expression of the components of the SUMO pathway is upregulated by various environmental stress conditions such as osmotic, hypoxic, heat, oxidative, and genotoxic stresses (36). Here, we investigated whether chronic exposure of islets to high glucose elicits a similar response. Mouse islets incubated in 16 mM glucose for 48 h showed increased expression of SUMO-1 (6.4-fold), SUMO-2 (4.3-fold), SUMO-3 (1.7-fold), and Ubc-9 (3.8-fold) compared with islets in 5 mM glucose (Fig. 1). This result shows that exposure of pancreatic islets to high glucose concentrations results in an increase in expression of SUMO and Ubc-9 transcripts.
Fig. 1.
Expression of small ubiquitin-related modifier protein (SUMO) transcripts in mouse islets. Quantitative real-time RT-PCR shows increased expression of SUMO-1 transcripts in mouse islets exposed to high glucose. The fold change in expression of SUMO transcripts and Ubc-9 from islets in 16 mM glucose and 5 mM glucose is shown; n = 3 independent experiments, and error bars indicate means ± SD. Student's t-test: P = 0.01–0.05 (*) and 0.001–0.01 (**). β-Actin was used as an internal control.
Enhanced Expression of SUMO-1 Downregulates GLP-1R
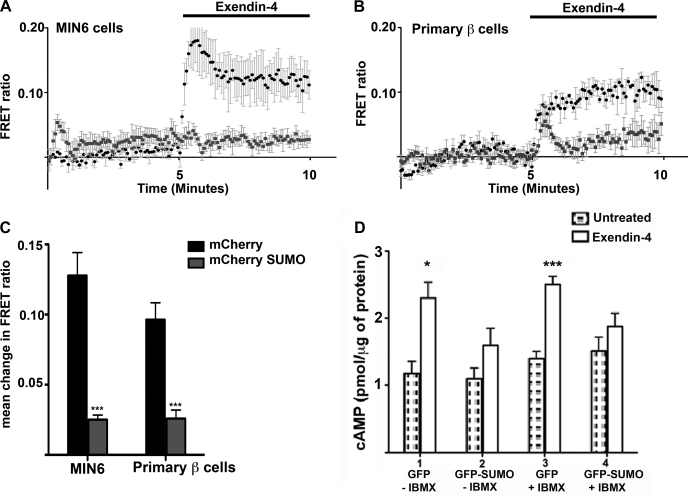
Activation of the GLP-1R signaling pathway stimulates adenylate cyclase, resulting in an increase in cytosolic cAMP. Therefore, the amount of free cAMP in the cell following agonist treatment should be a function of receptor activity assuming no change in PDE activity (10). The dynamic changes in cAMP concentration due to sumoylation were studied using a FRET-based biosensor. FRET between CFP and YFP fused to the cAMP-binding domain of the exchange protein Epac-2 (Epac-camps) was used to measure spatial and temporal changes in cAMP signaling (17). The Epac biosensor undergoes conformational changes upon cAMP binding that result in loss of FRET, measured as an increase in FRET ratio. MIN6 cells expressing endogenous GLP-1R were transiently cotransfected with Epac-camps and mCherry-tagged SUMO-1 or mCherry vector. Cells expressing both proteins were selected, and the FRET ratio was recorded after addition of 100 nM exendin-4. Similarly, dissociated primary mouse β-cells were transfected with Epac-camps and mCherry-SUMO-1 or mCherry alone as control, driven by rat insulin promoter-2 to ensure β-cell-specific expression. Addition of 100 nM exendin-4 caused a fivefold increase in the FRET ratio in MIN6 cells expressing mCherry compared with cells expressing mCherry-tagged SUMO-1 (Fig. 2, A and C). Mouse primary β-cells expressing mCherry showed a 3.7-fold increase in FRET ratio in response to exendin-4 compared with cells expressing mCherry-SUMO-1 (Fig. 2, B and C). This result shows that both, in insulinoma cells and primary β-cells overexpressing SUMO-1, stimulation of GLP-1 receptor by exendin-4 did not cause an increase in cAMP.
Fig. 2.
Overexpression of SUMO-1 decreases cAMP response following agonist stimulation of glucagon-like peptide-1 receptor (GLPP-1R). MIN6 cells and mouse primary islet cells were transfected with Epac-camps and mCherry-SUMO or mCherry vector. Dynamic changes in cAMP binding were measured as the change in the fluorescent resonance energy transfer (FRET) ratio. A: change in FRET ratio following exposure to 100 nM exendin-4 in MIN6 cells transfected with Epac-camps and mCherry (black) and Epac-camps and mCherry-SUMO (gray). B: change in FRET ratio in mouse primary β-cells transfected with Epac-camps and mCherry (black) or mCherry-SUMO (gray) when treated with 100 nM exendin-4. C: graph showing mean change in FRET ratio in MIN6 cells. Overexpression of SUMO-1 caused 5- and 3.7-fold reduction in FRET ratio compared with the control in MIN6 cells and mouse primary β-cells, respectively. Error bars indicate means ± SD. Student's t-test: ***P < 0.001; n = 6 cells from multiple dishes. D: total cAMP quantified by enzyme-linked immunosorbent assay (ELISA) after exendin-4 treatment of MIN6 cells transfected with green fluorescent protein (GFP) or GFP-SUMO. FAC-sorted GFP-SUMO cells showed only a 1.3-fold increase in total cAMP, whereas control GFP cells showed a 1.9-fold increase compared with untreated cells. A similar response of 1.8-fold increase was also seen in control cells when exendin-4 treatment was done in the presence of 100 μM IBMX, whereas SUMO-1-expressing cells showed only a 1.2-fold increase that is not statistically significant. Error bars indicate means ± SD; n = 6. Student's t-test: P = 0.01–0.05 (*) and <0.0001 (***) for exendin-4-treated cells without IBMX and with IBMX, respectively.
Next, we assessed total cAMP produced by exendin-4 treatment in MIN6 cells overexpressing SUMO-1 and compared it with control cells using a cAMP-specific ELISA. MIN-6 cells were transfected with GFP or GFP-tagged SUMO-1. The cells were sorted by FACS 48 h posttransfection and treated with 100 nM exendin-4. Control cells expressing GFP alone showed a 1.9-fold increase in total cAMP concentration, whereas cells expressing GFP-tagged SUMO showed only a 1.3-fold increase following exendin-4 treatment (Fig. 2D). A similar response was seen when exendin-4 treatment was done in the presence of phoshodiesterase inhibitor IBMX despite slight elevation in basal cAMP compared with cells not treated with IBMX. This assay confirmed that enhanced expression of SUMO-1 resulted in significantly reduced cAMP response following agonist treatment and that SUMO-mediated downregulation of GLP-1R signaling is independent of phoshodiesterase activity (Fig. 2D). We next investigated whether SUMO-1 directly modified the GLP-1 receptor.
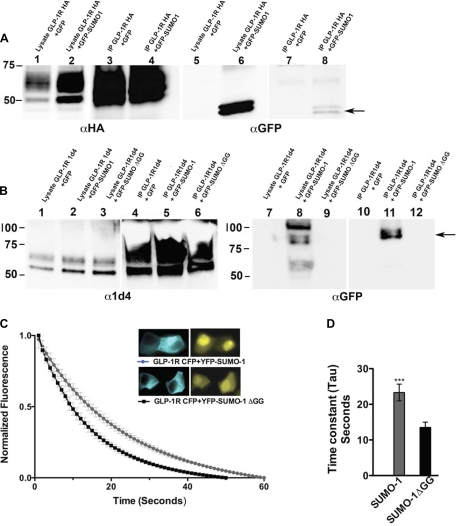
SUMO-1 Binds Covalently and Noncovalently to the GLP-1 Receptor
Similar to ubiquitin, SUMO covalently binds to its target proteins through a lysine residue. However, SUMO-1 is also known to interact noncovalently with some proteins, and noncovalent interaction was shown to be required for efficient E3 SUMO ligase activity (15, 23). To detect noncovalent interaction, COOH-terminally tagged GLP-1R-HA was expressed in MIN6 cells and coimmunoprecipitated with an anti-HA antibody. A double band corresponding to GFP-SUMO-1 coimmunoprecipitated with the GLP-1R-HA protein, demonstrating the presence of a noncovalent interaction between the two proteins (Fig. 3A). To investigate whether SUMO-1 covalently interacts with GLP-1R, we transfected MIN6 cells with 1d4 epitope-tagged GLP-1R and GFP-SUMO-1 or with a conjugation-deficient GFP-SUMOΔGG construct where the last four amino acids containing the diglycine motif were deleted. GLP-1R-1d4 was coimmunoprecipitated with 1d4 antibody under denaturing conditions as described in materials and methods. A higher-molecular-weight band that corresponds to GFP-SUMO-modified GLP-1R was detected with anti-GFP antibody in the presence of GFP-SUMO-1, but not GFP- or conjugation-deficient SUMO, indicating a likely covalent modification of GLP-1R by SUMO-1 (Fig. 3B).
Fig. 3.
SUMO-1 binds both noncovalently and covalently to GLP-1R. A: MIN6 cells stably expressing GFP-SUMO-1 or GFP were transfected with hemagglutinin (HA)-tagged GLP-1 receptor (GLP-1R HA) and immunoprecipitated to detect protein interactions. To detect noncovalent interaction, cells were lysed without N-ethylmaleimide, immunoprecipitated with an anti-HA antibody, and detected with mouse GFP antibody. The arrow indicates an ∼40-kDa double band representing GFP-SUMO-1. Lane 1, cell lysate GLP 1RHA + GFP; lane 2, cell lysate GLP-1R HA + GFP-SUMO-1; lane 3, immunoprecipitation (IP) from lane 1; lane 4, IP from lane 2; lanes 1–4, blotted with rabbit anti-HA antibody; lanes 5–8 are similar to lanes 1–4, blotted with mouse anti-GFP antibody. B: to detect covalent interaction, MIN6 cells stably expressing GFP-SUMO-1 or GFP or conjugation-deficient GFP-SUMO-1ΔGG were transfected with GLP-1R 1d4, lysed, and immunopurified under denaturing conditions with anti-1d4 antibody, and the eluates were blotted with rabbit GFP antibody. Arrow indicates a ∼90-kDa immunoprecipitated band that represents GLP-1R covalently linked to GFP-SUMO-1 present only in the lane expressing GFP-SUMO-1 and GLP-1R 1d4. Lane 1, cell lysate GLP-1R 1d4 + GFP; lane 2, cell lysate GLP-1R 1d4 + GFP-SUMO-1; lane 3, GLP-1R 1d4 + GFP-SUMO-1ΔGG; lane 4, IP from lane 1; lane 5, IP from lane 2; lane 6, IP from lane 3; lanes 1–6 are blotted with mouse anti-1d4; lanes 7–12 are the same as lanes 1–6 and blotted with rabbit anti-GFP antibody. C: FRET analysis by donor bleaching between GLP-1R-cyan fluorescent protein (CFP) and yellow fluorescent protein (YFP)-SUMO-1 shows interaction between GLP-1R and SUMO. Graph shows normalized fluorescent decay curves in cells expressing GLP-1R-CFP and YFP-SUMO-1 (gray) or GLP-1R-CFP and YFP-SUMO-1ΔGG (black). Inset: image of representative cells expressing GLP-1R-CFP and YFP-SUMO-1 or YFP-SUMO-1ΔGG; n = 14–17 cells from multiple experiments. D: graph showing time constants (tau) of the decay curves. Cells expressing GLP-1R-CFP and YFP-SUMO-1 showed 1.7-fold increase in time constant compared with cells expressing GLP-1R-CFP and YFP-SUMO-1ΔGG, indicating protein-protein interaction between GLP-1R and SUMO-1 but not with conjugation-deficient SUMO-1. Error bars indicate means ± SD; n = 6. Student's t-test: ***P < 0.0007.
The direct covalent modification of GLP-1R by SUMO in live cells was confirmed by FRET analysis by expressing GLP-1R-CFP and YFP-SUMO-1. Nonspecific interactions between CFP and YFP-SUMO were not observed, and this assay has extensively been used in several pervious studies (3, 27, 34, 35). MIN6 cells transfected with GLP-1R-CFP and YFP-SUMO-1 or YFP-SUMO-1ΔGG were imaged 24 h posttransfection. FRET was calculated by CFP (donor) bleaching as previously described (27, 28). The presence of a FRET interaction causes CFP to bleach more slowly, thus increasing the time constant (tau) compared with the non-FRET control. Analysis of the fluorescence decay curve shows a twofold increase in the time constant (tau) for CFP-GLP-1R only when YFP-SUMO-1 is present. Taken together, these two experiments support the conclusion that GLP-1R is directly modified by SUMO-1.
Enhanced Expression of SUMO-1 Impairs Cell Surface Trafficking of GLP-1R
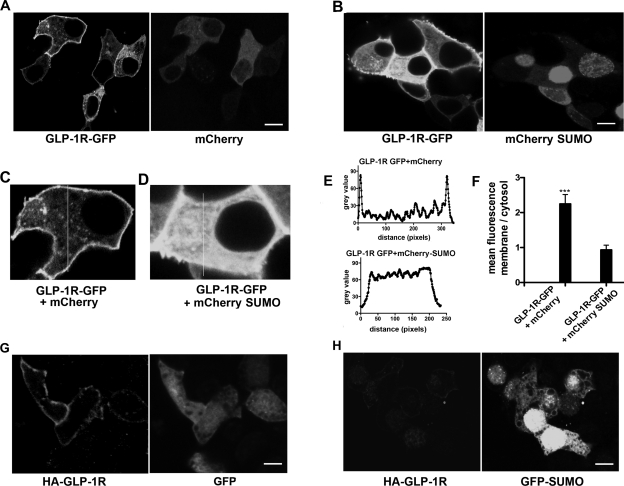
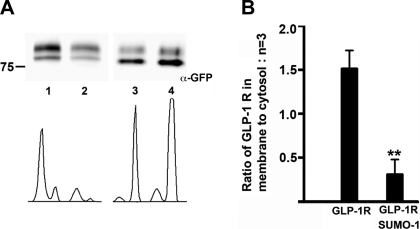
Because SUMO-1 expression attenuated GLP-1R signaling upon agonist stimulation, we investigated the mechanism that underlies SUMO-mediated loss of GLP-1R function. Three lines of evidence indicate that SUMO-1 interferes with the cell surface targeting of GLP-1R. First, we cotransfected GLP-1R fused with GFP at the COOH-terminus (GLP-1R-GFP) with mCherry-SUMO-1 in MIN6 cells. Confocal microscopy images showed apparent membrane localization of GLP-1R-GFP cotransfected with free mCherry vector (Fig. 4, A, C, E, and F). However, when GLP-1R-GFP was cotransfected with mCherry-SUMO, GFP fluorescence was found to be predominantly intracellular (Fig. 4, B, D, E, and F). To confirm these results, we used another GLP-1R construct with an HA epitope introduced after the signal peptide at the extracellular NH2-terminus (HA-GLP-1R) and cotransfected MIN6 cells with GFP or GFP-SUMO-1. The cells were fixed but not permeabilized and immunostained with anti-HA antibody to detect GLP-1R at the plasma membrane. Whereas HA antibody detected HA-GLP-1R at the membrane in cells transfected with GFP, very little or no HA-GLP-1R was observed in cells cotransfected with GFP-SUMO-1 (Fig. 4, G and H). These results were again confirmed by a cell surface biotinylation assay designed to detect the receptor at the cell surface. MIN6 cells were cotransfected with untagged SUMO or empty vector and the GLP-1R-GFP construct. Streptavidin-purified biotinylated membrane proteins were probed with an anti-GFP antibody to detect membrane-bound GLP-1R-GFP. The ratio of membrane vs. cytosol-associated GLP-1R was drastically reduced by fivefold in three independent experiments when the receptor was coexpressed with SUMO-1 (Fig. 5, A and B). Together, these results demonstrate that sumoylation interferes with the cell surface trafficking of the receptor, causing decreased receptor density at the membrane.
Fig. 4.
Intracellular retention of GLP-1R when coexpressed with SUMO-1. A: GLP-1R-GFP expressed with mCherry vector shows a predominant plasma membrane fluorescence of the GLP-1R-GFP. B: GLP-1R-GFP expressed with mCherry-SUMO-1 shows decreased plasma membrane localization consistent with enhanced intracellular retention. C: enlarged image of a representative cell expressing GLP-1R-GFP and mCherry. Vertical line represents the line chosen for fluorescence line scan. D: enlarged image of a cell expressing GLP-1R-GFP and mCherry SUMO-1 with the line chosen for the line scan. E: top, representative fluorescence line scan of GLP-1R-GFP with mCherry in cell C that shows distinct peaks. Bottom, fluorescence line scan of GLP-1R-GFP with mCherry-SUMO-1 that shows uniform distribution. F: mean fluorescence ratio of membrane to cytosol shows 2.2-fold increase, indicating enhanced membrane fluorescence when SUMO-1 is not present. Error bars indicate means ± SD; n = 6. Student's t-test: ***P < 0.0007; n = 12–15 cells from multiple dishes. Average length of the line scan at the membrane and cytosol for calculating the ratio is 0.4–0.5 μm. G: GLP-1R-HA coexpressed with GFP shows predominant plasma membrane fluorescence of the GLP-1R signal, detected with anti-HA antibody in nonpermeabilized cells. H: MIN6 cells transfected with the HA-GLP-1R construct coexpressed with mCherry-SUMO-1 and stained with anti-HA antibody shows diminished plasma membrane fluorescence of GLP-1R-HA in nonpermeabilized cells. Scale bar = 5 μm.
Fig. 5.
Impaired cell surface trafficking of GLP-1R coexpressed with SUMO-1. A: top, MIN6 cells transfected with GLP-1R-GFP and empty vector or untagged SUMO-1 and cell surface biotinylation was carried out to isolate plasma membrane-bound proteins, purified with streptavidin beads, and detected by mouse anti-GFP antibody. The upper bands in A are likely to be posttranslationally modified GLP-1R-GFP less abundant in the cytosolic fraction. Sumoylated GLP-1R is not observed since this assay was carried out under nondenaturing conditions. Lane 1, biotinylated GLP-1R-GFP with empty vector; lane 2, biotinylated GLP-1R-GFP with untagged SUMO-1; lane 3, lysate from lane 1; lane 4, lysate from lane 2. Bottom, intensity plot of the bands obtained by Image J software. B: GLP-1R-GFP when expressed with SUMO-1 shows an ∼5-fold reduction in plasma membrane-bound GLP-1R in three independent experiments. Error bars indicate means ± SD. Student's t-test: **P < 0.0014; n = 3.
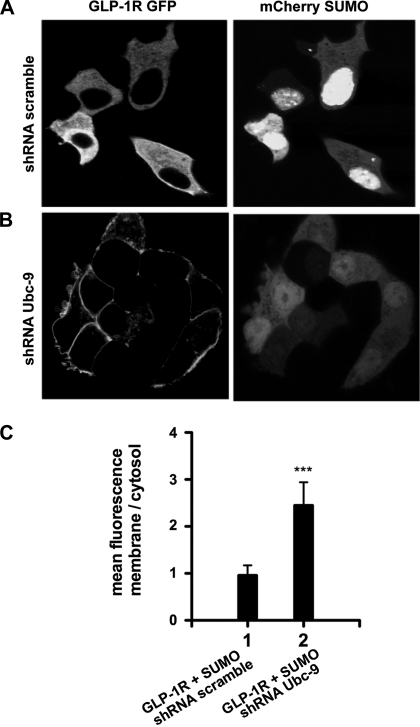
Next, we tested whether partial knock down of Ubc-9 is able to rescue SUMO-mediated intracellular retention of GLP-1R. MIN6 cells transfected with GLP-1R-GFP and mCherry SUMO-1 were transduced with retroviral particles expressing shRNA against Ubc-9. Reduced expression of Ubc-9 resulted in diminished nuclear mCherry-SUMO, and GLP-1R-GFP was predominantly localized at the plasma membrane (Fig. 6).
Fig. 6.
Partial knock down of Ubc-9 rescues SUMO-mediated impaired trafficking of GLP-1R. MIN6 cells transfected with GLP-1R-GFP and mCherry SUMO-1 were transduced with retroviral particles expressing scrambled or short-hairpin RNA (shRNA) against Ubc-9. A: MIN6 cells expressing GLP-1R-GFP and mCherry SUMO-1 transduced with shRNA scramble showing intracellularly retained GLP-1R. B: MIN6 cells expressing GLP-1R-GFP and mCherry SUMO-1 transduced with shRNA Ubc-9 shows plasma membrane localization of GLP-1R. C: graph showing the ratio of mean fluorescence at the membrane vs. cytosol from 10 randomly selected cells. Partial knock down of Ubc-9 results in a 2.8-fold increase in plasma membrane localization. Error bars indicate means ± SD. Student's t-test: ***P < 0.0007; n = 10 cells from multiple dishes. Scale bar = 5 μm.
Overexpression of SUMO-1 Results in Reduced Insulin Content and Agonist-Stimulated Insulin Secretion
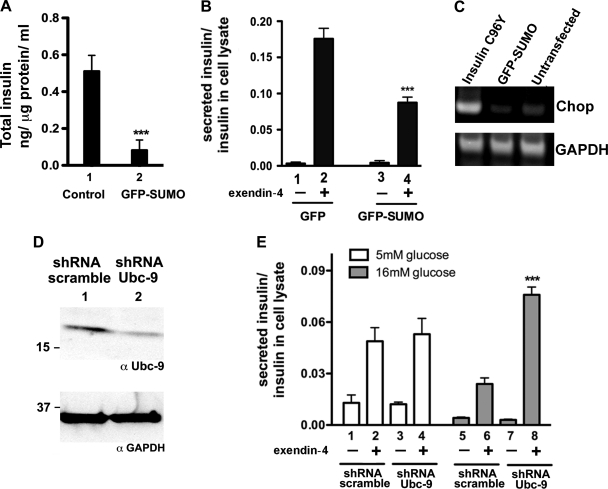
Transcription factors that are involved in insulin gene expression such as MafA and cleaved COOH-termini of ICA512 are targets of sumoylation (32) . SUMO-1 was not found to affect insulin content when overexpressed by transient transfection (6). However, MIN6 cells stably expressing GFP-SUMO-1 showed a 6.3-fold reduction in total insulin content compared with control cells that express empty vector. Similarly, GFP-SUMO-1 stable cells also showed a 2.3-fold reduction in secreted insulin when stimulated by exendin-4 compared with control cells (Fig. 7, A and B). These results indicate that prolonged expression of SUMO-1 reduces insulin content and GLP-1R agonist-stimulated insulin secretion.
Fig. 7.
Overexpression of SUMO-1 results in reduced insulin content and secretion. MIN6 cells, stably expressing GFP-SUMO-1 cells, and control cells were stimulated with 100 nM exendin-4 for 3 h. Insulin content in the supernatant and lysate was quantified by insulin-specific ELISA. A: total insulin content in control GFP- and GFP-SUMO-1-expressing cells. Overexpression of GFP-SUMO-1 results in a sixfold reduction in total insulin; n = 3. Error bars indicate means ± SD. Student's t-test: ***P < 0.0007. B: ratio of secreted insulin to cell lysate in control GFP and GFP-SUMO-1 stable cells. Bars 1 and 2, untreated and exendin-4-treated control GFP cells, respectively. Bars 3 and 4, untreated and exendin-4-treated GFP-SUMO cells, respectively. Student's t-test: ***P < 0.0007. Twofold reduction in exendin-4-stimulated insulin secretion was observed in GFP-SUMO-1 stable cells; n = 3. Error bars indicate means ± SD. C: endoplasmic reticulum (ER) stress marker Chop gene expression in GFP-SUMO-transfected MIN6 cells, analyzed by RT-PCR. Lane 1, Chop expression in MIN6 cells transfected by GFP-tagged insulin mutant C96Y (Akita); lane 2, GFP-SUMO-transfected cells; lane 3, untransfected cells. D: mouse islet cells transduced with retroviral shRNA against Ubc-9 shows partial knock down. Lane 1, cell lysate from cells transduced with shRNA Ubc-9; lane 2, lysate from cells transduced with shRNA scramble. Gels were blotted with rabbit anti-Ubc-9 antibody and GAPDH. E: exendin-4-stimulated insulin secretion in islet cells maintained in high glucose (16 mM), transduced with shRNA Ubc-9, shows improved insulin secretion compared with scrambled shRNA. Bars 1–4 represent islet cells maintained in 5 mM glucose; bars 1 and 2 represent cells transduced with shRNA scramble; bars 3 and 4 represent cells with shRNA Ubc-9; bars 5–8 represent cells in 16 mM glucose; bars 5 and 6 represent cells with shRNA scramble; and bars 7 and 8 represent cells with shRNA Ubc-9. Insulin secretion in sample 4 was compared with sample 2, and sample 8 was compared with sample 6 for statistical analysis. Error bars indicate means ± SD. ***P < 0.0007.
We tested the presence of an endoplasmic reticulum (ER) stress-induced gene “Chop” in GFP-SUMO-overexpressing and control GFP-expressing cells by RT-PCR. cDNA was prepared from MIN6 cells overexpressing GFP-SUMO-1 and untransfected cells. The presence of a 350-bp fragment of Chop cDNA was tested by RT-PCR. GFP-SUMO or untransfected MIN6 cells showed basal expression of Chop, whereas cells expressing GFP-tagged misfolded insulin mutant C96Y (Akita) showed enhanced Chop gene expression (29). This indicates that ER stress is unlikely the reason for attenuated insulin gene expression in MIN6 cells expressing GFP-SUMO.
Partial Knock Down of Ubc-9 Results in Improved Insulin Secretion
Next, we tested whether downregulation of the SUMO pathway by partial knock down of the SUMO-conjugating enzyme Ubc-9 improves agonist-induced insulin secretion in mouse islets. Islet cells cultured in 5 or 16 mM glucose were transduced with retroviral particles expressing shRNA against Ubc-9, and exendin-4-stimulated insulin secretion was quantified. No significant change in secretion between sh-RNA-Ubc-9 transduced and control cells was observed in cells maintained in low glucose, whereas cells in high glucose expressing Ubc-9 shRNA showed significant increase in insulin secretion compared with the scramble transduced control. Thus, partial inhibition of the SUMO pathway is shown to rescue glucose-induced reduction in exendin-4-stimulated insulin secretion.
DISCUSSION
The incretin pathway has important pancreatic and extrapancreatic effects but is impaired in type 2 diabetes. GLP-1R gene expression is downregulated in hyperglycemia, contributing to reduced β-cell incretin responses in a diabetic rodent model (37). In addition, islets from type 2 diabetic patients also showed diminished incretin responsiveness (13, 16, 25, 31, 33). Even though protein kinase C has been implicated in the reduced gene expression of incretin receptors in hyperglycemia (37), other mechanisms are likely to contribute to the lack of incretin responsiveness. In this report, we show elevated mRNA expression of three isoforms of SUMO and the SUMO-conjugating enzyme Ubc-9 on exposure of pancreatic islets to high-glucose conditions. Enhanced expression of SUMO-1 leads to downregulation of GLP-1 signaling when measured as a function of cAMP generation. In addition, elevated expression of SUMO-1 also causes reduction in total insulin content and GLP-1R agonist-stimulated insulin secretion.
The cellular conditions that cause an increase in sumoylation in pancreatic β-cells have not been well studied. The SUMO conjugation/deconjugation equilibrium is modified under various cellular stress conditions in other cell types (21, 36). SUMO-1 expression is upregulated in hypoxia, resulting in enhanced sumoylation of target proteins such as cAMP-response element-binding protein and hypoxia-inducible factor-1β (1, 4). Similarly, we found that RNA transcripts for SUMO and the SUMO-conjugating enzyme Ubc-9 are upregulated in mouse islets maintained in high glucose, indicating that expression of the SUMO pathway is upregulated when islets are exposed to high-glucose concentrations. Enhanced expression of the SUMO pathway is therefore likely to impair glucose- and incretin hormone-stimulated insulin secretion. Other proteins may also be regulated in this way in the β-cell. For example, SUMO protein was shown to inhibit the voltage-dependent K+ channel Kv2.1 that resulted in widening of β-cell action potentials and a decreased firing frequency, but inhibition of Kv2.1 might augment insulin secretion in mouse β-cells (5, 14). Similarly, SUMO-mediated upregulation of signaling pathways in cellular stress, such as the NF-κB transcription pathway, might be beneficial under certain circumstances (7). On the other hand, a recent report shows that SUMO-1 impairs glucose-stimulated insulin secretion by binding to synaptogamin VII and preventing exocytosis (6).
We found that overexpression of SUMO-1 resulted in intracellular retention of the GLP-1R that was associated with reduced receptor density at the cell membrane. Even though the role of SUMO in nucleocytoplasmic trafficking is well documented (11), how SUMO aids in forward trafficking to the plasma membrane is yet to be investigated. One possibility is that SUMO prevents GLP-1R oligomerization. Receptor oligomerization is essential for forward trafficking of the secretin family of class B G protein-coupled receptors, of which the GLP-1R is a member (20). SUMO modification was also shown to inhibit oligomerization of other proteins such as apoptosis signal-regulating kinase 1 and E26 leukemia (TEL) protein (18, 38). Furthermore, enhanced SUMO modification increases solubility of proteins as in the case of vaccinea virus protein (26). Thus SUMO modification may affect GLP-1R oligomerization, perhaps by a change in the solubility or binding ability of sumoylated proteins. The mechanism by which SUMO affects trafficking of GLP-1R could also be due to posttranslational modification of GLP-1R and concomitant effects on components of the trafficking machinery. Moreover, downregulation of GLP-1R signaling is likely due to a compound effect of direct SUMO modification of the GLP-1R and the possible modifications of the components of this pathway. For example, SUMO-mediated regulation of cAMP-specific phosphodiesterase-4D5 has been reported previously (19). Similarly, SUMO modification of transcription factors involved in insulin gene expression could also contribute to attenuation of exendin-4-stimulated insulin secretion (24, 32).
Incretin responses and its use in therapy are becoming central to the treatment of type 2 diabetes. Here, we describe sumoylation as a mechanism that regulates incretin responsiveness that could contribute to the reduction of the incretin effect in diabetes. A thorough understanding of the role of SUMO in incretin receptor regulation will help improve the efficacy of incretin-based therapy for diabetes.
GRANTS
This work was partially supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-48494 and DK-063493, DK-075706, P60 DK-020595, the Blum-Kovler Foundation, and the Merck IIS Grant 38186.
DISCLOSURES
The authors have no conflict of interest.
AUTHOR CONTRIBUTIONS
Author contributions: S. Rajan and L. H. Philipson conception and design of research; S. Rajan, J. Torres, and M. S. Thompson performed experiments; S. Rajan, M. S. Thompson, and L. H. Philipson analyzed data; S. Rajan, J. Torres, and L. H. Philipson interpreted results of experiments; S. Rajan and L. H. Philipson prepared figures; S. Rajan and L. H. Philipson drafted manuscript; S. Rajan and L. H. Philipson edited and revised manuscript; S. Rajan and L. H. Philipson approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Drs. G. Bell, C. Rhodes, and N. Tamarina for helpful discussions and E. Mathew and C. Labno for excellent technical assistance.
REFERENCES
- 1.Bae SH, Jeong JW, Park JA, Kim SH, Bae MK, Choi SJ, Kim KW. Sumoylation increases HIF-1alpha stability and its transcriptional activity. Biochem Biophys Res Commun 324: 394–400, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Benson MD, Li QJ, Kieckhafer K, Dudek D, Whorton MR, Sunahara RK, Iniguez-Lluhi JA, Martens JR. SUMO modification regulates inactivation of the voltage-gated potassium channel Kv1.5. Proc Natl Acad Sci USA 104: 1805–1810, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bossis G, Chmielarska K, Gartner U, Pichler A, Stieger E, Melchior F. A fluorescence resonance energy transfer-based assay to study SUMO modification in solution. Methods Enzymol 398: 20–32, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Comerford KM, Leonard MO, Cummins EP, Fitzgerald KT, Beullens M, Bollen M, Taylor CT. Regulation of protein phosphatase 1gamma activity in hypoxia through increased interaction with NIPP1: implications for cellular metabolism. J Cell Physiol 209: 211–218, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Dai XQ, Kolic J, Marchi P, Sipione S, Macdonald PE. SUMOylation regulates Kv2.1 and modulates pancreatic beta-cell excitability. J Cell Sci 122: 775–779, 2009 [DOI] [PubMed] [Google Scholar]
- 6.Dai XQ, Plummer G, Casimir M, Kang Y, Hajmrle C, Gaisano HY, Manning Fox JE, MacDonald PE. SUMOylation regulates insulin exocytosis downstream of secretory granule docking in rodents and humans. Diabetes 60: 838–847, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Desterro JM, Rodriguez MS, Hay RT. SUMO-1 modification of IkappaBalpha inhibits NF-kappaB activation. Mol Cell 2: 233–239, 1998 [DOI] [PubMed] [Google Scholar]
- 8.Drucker DJ. Biologic actions and therapeutic potential of the proglucagon-derived peptides. Nat Clin Pract Endocrinol Metab 1: 22–31, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Drucker DJ, Philippe J, Mojsov S, Chick WL, Habener JF. Glucagon-like peptide I stimulates insulin gene expression and increases cyclic AMP levels in a rat islet cell line. Proc Natl Acad Sci USA 84: 3434–3438, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fridlyand LE, Harbeck MC, Roe MW, Philipson LH. Regulation of cAMP dynamics by Ca2+ and G protein-coupled receptors in the pancreatic β-cell: a computational approach. Am J Physiol Cell Physiol 293: C1924–C1933, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Geiss-Friedlander R, Melchior F. Concepts in sumoylation: a decade on. Nat Rev Mol Cell Biol 8: 947–956, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Gromada J, Dissing S, Rorsman P. Desensitization of glucagon-like peptide 1 receptors in insulin-secreting beta TC3 cells: role of PKA-independent mechanisms. Br J Pharmacol 118: 769–775, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hansen KB, Vilsboll T, Bagger JI, Holst JJ, Knop FK. Reduced glucose tolerance and insulin resistance induced by steroid treatment, relative physical inactivity, and high-calorie diet impairs the incretin effect in healthy subjects. J Clin Endocrinol Metab 95: 3309–3317, 2010 [DOI] [PubMed] [Google Scholar]
- 14.Jacobson DA, Kuznetsov A, Lopez JP, Kash S, Ammala CE, Philipson LH. Kv2.1 ablation alters glucose-induced islet electrical activity, enhancing insulin secretion. Cell Metab 6: 229–235, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaukinen P, Vaheri A, Plyusnin A. Non-covalent interaction between nucleocapsid protein of Tula hantavirus and small ubiquitin-related modifier-1, SUMO-1. Virus Res 92: 37–45, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Knop FK, Vilsboll T, Hojberg PV, Larsen S, Madsbad S, Volund A, Holst JJ, Krarup T. Reduced incretin effect in type 2 diabetes: cause or consequence of the diabetic state? Diabetes 56: 1951–1959, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Landa LR, Jr, Harbeck M, Kaihara K, Chepurny O, Kitiphongspattana K, Graf O, Nikolaev VO, Lohse MJ, Holz GG, Roe MW. Interplay of Ca2+ and cAMP signaling in the insulin-secreting MIN6 beta-cell line. J Biol Chem 280: 31294–31302, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee YS, Jang MS, Lee JS, Choi EJ, Kim E. SUMO-1 represses apoptosis signal-regulating kinase 1 activation through physical interaction and not through covalent modification. EMBO Rep 6: 949–955, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li X, Vadrevu S, Dunlop A, Day J, Advant N, Troeger J, Klussmann E, Jaffrey E, Hay RT, Adams DR, Houslay MD, Baillie GS. Selective SUMO modification of cAMP-specific phosphodiesterase-4D5 (PDE4D5) regulates the functional consequences of phosphorylation by PKA and ERK. Biochem J 428: 55–65, 2010 [DOI] [PubMed] [Google Scholar]
- 20.Lisenbee CS, Miller LJ. Secretin receptor oligomers form intracellularly during maturation through receptor core domains. Biochemistry 45: 8216–8226, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manza LL, Codreanu SG, Stamer SL, Smith DL, Wells KS, Roberts RL, Liebler DC. Global shifts in protein sumoylation in response to electrophile and oxidative stress. Chem Res Toxicol 17: 1706–1715, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Martin S, Nishimune A, Mellor JR, Henley JM. SUMOylation regulates kainate-receptor-mediated synaptic transmission. Nature 447: 321–325, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Merrill JC, Melhuish TA, Kagey MH, Yang SH, Sharrocks AD, Wotton D. A role for non-covalent SUMO interaction motifs in Pc2/CBX4 E3 activity. PLoS One 5: e8794, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mziaut H, Kersting S, Knoch KP, Fan WH, Trajkovski M, Erdmann K, Bergert H, Ehehalt F, Saeger HD, Solimena M. ICA512 signaling enhances pancreatic beta-cell proliferation by regulating cyclins D through STATs. Proc Natl Acad Sci USA 105: 674–679, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nauck MA, Meier JJ. Individualised incretin-based treatment for type 2 diabetes. Lancet 376: 393–394, 2010 [DOI] [PubMed] [Google Scholar]
- 26.Palacios S, Perez LH, Welsch S, Schleich S, Chmielarska K, Melchior F, Locker JK. Quantitative SUMO-1 modification of a vaccinia virus protein is required for its specific localization and prevents its self-association. Mol Biol Cell 16: 2822–2835, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Plant LD, Dementieva IS, Kollewe A, Olikara S, Marks JD, Goldstein SA. One SUMO is sufficient to silence the dimeric potassium channel K2P1. Proc Natl Acad Sci USA 107: 10743–10748, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Plant LD, Dowdell EJ, Dementieva IS, Marks JD, Goldstein SA. SUMO modification of cell surface Kv2.1 potassium channels regulates the activity of rat hippocampal neurons. J Gen Physiol 137: 441–454, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rajan S, Eames SC, Park SY, Labno C, Bell GI, Prince VE, Philipson LH. In vitro processing and secretion of mutant insulin proteins that cause permanent neonatal diabetes. Am J Physiol Endocrinol Metab 298: E403–E410, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rajan S, Plant LD, Rabin ML, Butler MH, Goldstein SA. Sumoylation silences the plasma membrane leak K+ channel K2P1. Cell 121: 37–47, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Salehi M, Aulinger BA, D'Alessio DA. Targeting beta-cell mass in type 2 diabetes: promise and limitations of new drugs based on incretins. Endocr Rev 29: 367–379, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shao C, Cobb MH. Sumoylation regulates the transcriptional activity of MafA in pancreatic beta cells. J Biol Chem 284: 3117–3124, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shu L, Matveyenko AV, Kerr-Conte J, Cho JH, McIntosh CH, Maedler K. Decreased TCF7L2 protein levels in type 2 diabetes mellitus correlate with downregulation of GIP- and GLP-1 receptors and impaired beta-cell function. Hum Mol Genet 18: 2388–2399, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Song Y, Madahar V, Liao J. Development of FRET assay into quantitative and high-throughput screening technology platforms for protein-protein interactions. Ann Biomed Eng 39: 1224–1234, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tatham MH, Hay RT. FRET-based in vitro assays for the analysis of SUMO protease activities. Methods Mol Biol 497: 253–268, 2009 [DOI] [PubMed] [Google Scholar]
- 36.Tempe D, Piechaczyk M, Bossis G. SUMO under stress. Biochem Soc Trans 36: 874–878, 2008 [DOI] [PubMed] [Google Scholar]
- 37.Xu G, Kaneto H, Laybutt DR, Duvivier-Kali VF, Trivedi N, Suzuma K, King GL, Weir GC, Bonner-Weir S. Downregulation of GLP-1 and GIP receptor expression by hyperglycemia: possible contribution to impaired incretin effects in diabetes. Diabetes 56: 1551–1558, 2007 [DOI] [PubMed] [Google Scholar]
- 38.Zhang H, Smolen GA, Palmer R, Christoforou A, van den Heuvel S, Haber DA. SUMO modification is required for in vivo Hox gene regulation by the Caenorhabditis elegans Polycomb group protein SOP-2. Nat Genet 36: 507–511, 2004 [DOI] [PubMed] [Google Scholar]