Abstract
Central obesity is associated with chronic inflammation, insulin resistance, β-cell dysfunction, and endoplasmic reticulum (ER) stress. The 12/15-lipoxygenase enzyme (12/15-LO) promotes inflammation and insulin resistance in adipose and peripheral tissues. Given that obesity is associated with ER stress and 12/15-LO is expressed in adipose tissue, we determined whether 12/15-LO could mediate ER stress signals. Addition of 12/15-LO lipid products 12(S)-HETE and 12(S)-HPETE to differentiated 3T3-L1 adipocytes induced expression and activation of ER stress markers, including BiP, XBP-1, p-PERK, and p-IRE1α. The ER stress inducer, tunicamycin, upregulated ER stress markers in adipocytes with concomitant 12/15-LO activation. Addition of a 12/15-LO inhibitor, CDC, to tunicamycin-treated adipocytes attenuated the ER stress response. Furthermore, 12/15-LO-deficient adipocytes exhibited significantly decreased tunicamycin-induced ER stress. 12/15-LO action involves upregulation of interleukin-12 (IL-12) expression. Tunicamycin significantly upregulated IL-12p40 expression in adipocytes, and IL-12 addition increased ER stress gene expression; conversely, LSF, an IL-12 signaling inhibitor, and an IL-12p40-neutralizing antibody attenuated tunicamycin-induced ER stress. Isolated adipocytes and liver from 12/15-LO-deficient mice fed a high-fat diet revealed a decrease in spliced XBP-1 expression compared with wild-type C57BL/6 mice on a high-fat diet. Furthermore, pancreatic islets from 12/15-LO-deficient mice showed reduced high-fat diet-induced ER stress genes compared with wild-type mice. These data suggest that 12/15-LO activity participates in ER stress in adipocytes, pancreatic islets, and liver. Therefore, reduction of 12/15-LO activity or expression could provide a new therapeutic target to reduce ER stress and downstream inflammation linked to obesity.
Keywords: obesity, inflammation, insulin resistance
obesity is becoming a worldwide epidemic due to lifestyle and dietary changes and can lead to a decline in life expectancy (26, 30). Obesity is associated with increased intracellular lipid storage in adipocytes, causing expansion of adipose tissue and ensuing adipocyte dysfunction (9, 12). Adipocyte dysfunction is associated with numerous pathologies, including chronic low-grade inflammation, insulin resistance, type 2 diabetes, and cardiovascular disease (9, 12).
Increasing evidence suggests that a contributing factor leading to adipocyte dysfunction is endoplasmic reticulum (ER) stress. The ER is a highly specialized organelle responsible for protein and lipid biosynthesis. However, when nutrients are in excess, the ER becomes overwhelmed and an accumulation of misfolded proteins occurs, leading to activation of the unfolded protein response (UPR) (9, 12). The UPR signals through three sensors: RNA-dependent protein kinase-like ER-regulated kinase (PERK), inositol-requiring enzyme 1α (IRE1α), and the activating transcription factor (ATF)6. Activated PERK phosphorylates the eukaryotic translation initiation factor 2α (eIF2α) to attenuate protein synthesis and increases ATF4 translation to increase ER-dependent gene transcription. Activated IRE1α and ATF6 upregulate expression of protein chaperones important for protein folding [i.e., binding immunoglobulin protein (BiP)]. ATF6 also upregulates X-box protein-1 (XBP-1) mRNA, which is then cleaved by the IRE1α response arm to further increase protein chaperone content and increase transcription of genes for ER biogenesis. Additionally, ATF6 upregulates genes important for the ER-associated degradation response that promotes clearance of excess misfolded proteins. Furthermore, Wolfram syndrome 1 (WFS1) is often upregulated by XBP-1 to mitigate the ER stress response in part through downregulation of ATF6 (10, 11, 17). If ER stress is prolonged, the UPR can induce apoptosis via several mechanisms [i.e., ATF4-induced upregulation of CCAAT/enhancer-binding protein homologous protein (CHOP) and ATF3].
Obesity-associated ER stress can lead to inflammation, loss of pancreatic β-cell function, and insulin resistance (2, 9). Major inflammatory pathways are activated by ER stress in part via phosphorylation and activation of c-Jun NH2-terminal kinase 1 (JNK1), activation and nuclear translocation of nuclear factor-κB (NF-κB), and accumulation of reactive oxygen species. Thus the ER is a proximal site that may activate critical pathways important for obesity-induced inflammation and insulin resistance. In support of this concept, increased ER stress responses have been observed in adipose tissue from insulin-resistant obese human patients and mice (3, 13, 27, 28, 34), and caloric restriction of both obese patients and mice can attenuate this response and restore insulin sensitivity (13, 39).
12/15-Lipoxygenase (12/15-LO) is an enzyme that converts arachidonic acid by oxygenation to form lipid proinflammatory products, including 12-hydroxyeicosatetranoic acid [12(S)-HETE] (8). 12/15-LO expression is upregulated in adipocytes from mice fed a high-fat diet and from insulin-resistant Zucker obese rats in vivo (6, 7). 12/15-LO also induces inflammation, insulin resistance, and β-cell damage in vitro and in vivo, leading to whole body metabolic dysfunction (6–8, 25, 33). Deletion of 12/15-LO in C57BL/6 mice reduces inflammation in adipose tissue, prevents insulin resistance, and preserves β-cell function in mice fed a high-fat diet (25, 33).
Given that obesity is associated with ER stress and 12/15-LO is expressed in adipocytes, we sought to investigate whether one mechanism by which 12/15-LO promotes inflammation is by mediating ER stress signals in adipocytes. We utilized the in vitro model of 3T3-L1 adipocytes and 12/15-LO-deficient mice to demonstrate that products of 12/15-LO can induce ER stress and that inhibition of 12/15-LO activity can abrogate the ER stress response.
MATERIALS AND METHODS
Reagents.
3T3-L1 cells were kindly provided by the late Dr. John C. Lawrence, Jr. (University of Virginia, Charlottesville, VA). Dulbecco's modified Eagle's medium (DMEM), penicillin-streptomycin, and trypsin were purchased from Invitrogen (Carlsbad, CA). CMRL medium was purchased from Mediatech (Manassas, VA). FBS was purchased from Zen-Bio (Research Triangle Park, NC). Dexamethasone, isobutylmethylxanthine, protease inhibitors, tunicamycin, and 4-phenylbutyric acid (PBA) were purchased from Sigma-Aldrich (St. Louis, MO). 12(S)-HETE, 12-hydroperoxyeicosatetraenoic acid [12(S)-HPETE], 12(R)-HETE, 15(S)-HETE, and cinnamyl 1–3,4-dihydroxy-α-cyanocinnamate (CDC) were purchased from Enzo Life Sciences (Plymouth Meeting, PA). Lisofylline (LSF) was provided by DiaKine Therapeutics (Norfolk, VA). The PCR oligonucleotides and Taqman probes were purchased from Operon Biotechnologies (Huntsville, AL) and Applied Biosystems (Carlsbad, CA), respectively. Antibodies were obtained as follows: actin, adiponectin, ATF6 (Abcam, Cambridge, MA); ATF4 (Abnova, Taipei City, Taiwan); BiP, CCAAT/enhancer-binding protein-α (C/EBPα), phospho-Akt (Ser473), phospho-insulin receptor (IR; Tyr1146), phospho-PERK (Tyr980), phospho-eIF2α (Ser51), phospho-IRE1α (Ser724) (Cell Signaling Technology, Danvers, MA); IL-12p40 (eBioscience, San Diego, CA); phospho-insulin receptor substrate-1 (IRS-1; Tyr896) (Invitrogen); α-tubulin (Sigma-Aldrich). Mouse IL-12p40 cytokine was purchased from R & D Systems (Minneapolis, MN). A primary antibody to 12/15-LO was raised to a peptide derived from the sequence of 12/15-LO in our laboratory (18).
3T3-L1 cell culture and differentiation.
3T3-L1 preadipocytes were fully differentiated into adipocytes, as described previously (6). Briefly, 3T3-L1 preadipocytes were grown to confluence, and at 2 days postconfluence (day 0) differentiation was induced with dexamethasone (0.25 μM), isobutylmethylxanthine (0.5 mM), and insulin (1 μg/ml) for 2 days. After this, insulin was maintained for 2 additional days. All experiments were performed on day 10–12 differentiated 3T3-L1 adipocytes, and cells were kept overnight in DMEM containing 1% FBS prior to experimental treatment.
In vitro cell treatments.
For H(P)ETE and tunicamycin experiments, cells were washed and incubated with DMEM (3T3-L1 cells or isolated mouse adipocytes) or CMRL medium (isolated mouse pancreatic islets) containing 0.2% BSA for 2 h before treatment with 10 nM HETE or 1 nM 12(S)-HPETE products (or ethanol solvent control), 5 μM tunicamycin (or DMSO solvent control), or 2.5 ng/ml IL-12 cytokine (or water control) for 4 or 24 h. H(P)ETEs were added every 8 h because of their labile nature. Cells were incubated with 0.1 μM CDC, 10 μM LSF, or 10 mM PBA 2 h prior to and during tunicamycin treatment. For IL-12-neutralizing experiments, 2 μg/ml IL-12p40 antibody was added to cells.
Animals and treatments.
Male C57BL/6J mice were purchased from The Jackson Laboratory (Bar Harbor, ME), and 12/15-lipoxygenase-deficient (12-LO KO) mice were originally a generous gift from Dr. Colin Funk (1, 36) and maintained as a colony in our laboratory. The 12-LO KO mice have been backcrossed to the C57BL/6 background for at least six generations prior to inbreeding for homozygosity in the experimental mice (25). Mice were housed in a pathogen-free facility at Eastern Virginia Medical School (EVMS), and all experiments were performed in accordance with an animal study protocol approved by the EVMS Institutional Animal Care and Use Committee. Mice were placed on a normal chow or high-fat diet (D12331, consisting of 58% of calories from fat, 16.4% of calories from protein, and 25.5% of calories from carbohydrate, primarily sucrose; Research Diets, New Brunswick, NJ) beginning at 8 wk of age for 16 wk (5 mice/experimental group).
Isolation of white epididymal adipocytes.
Isolation of white epididymal adipocytes was based on a previously described protocol (42). Briefly, mice were euthanized by CO2 asphyxiation. Epididymal fat pads were removed and minced into fine pieces in Krebs-Ringer-HEPES-BSA buffer (3 mmol/l glucose, 20 nmol/l adenosine, and 10 mg/ml bovine serum) with 1 mg/ml collagenase. Collagenase digestion was performed at 37°C for 1 h in a shaking water bath. Once digestion of adipose tissue was complete, the cell suspension was filtered through a 0.4-mm Nitex nylon mesh (Sefar America, Kansas City, MO) and washed several times in Krebs-Ringer-HEPES-BSA buffer, and the adipocytes were allowed to float and processed for RNA extraction.
Islet isolation.
Mouse pancreatic islets were isolated following euthanization, as described previously (4, 25). Briefly, the pancreas was perfused through the common bile duct with 5 ml of 1.4 mg/ml collagenase P and then removed and incubated at 37°C for 8–11 min in 1 ml of Hanks' buffered salt solution. Following incubation, pancreatic tissue was centrifuged, resuspended in Histopaque 1077 (Sigma-Aldrich), and centrifuged again to separate islets from acinar tissue. Islets were then processed for RNA extraction.
RNA extraction and real-time PCR.
RNA from adipocytes was prepared using the RiboPure kit (Ambion, Austin, TX), and RNA from islets and liver was prepared using the RNeasy kit (Qiagen, Valencia, CA) according to the manufacturer's instructions. cDNA was made from 5 μg of total RNA using MMLV reverse transcriptase (Invitrogen) in a 20-μl reaction volume using random hexamers (Invitrogen). For quantitative measurement of PCR products, 3 μl of the cDNA reaction (5-fold diluted) was used as template for PCR with Jump Start Taq Polymerase (Sigma-Aldrich) in a reaction volume of 25 μl for PCR. Primer oligonucleotides (see Table 1) with SYBR Green I (Molecular Probes, Carlsbad, CA) or Taqman probes were used for PCR. All thermal cycling was performed using the CFX96 Thermal Cycler (Bio-Rad, Hercules, CA). All reactions were performed in triplicate, and the data were normalized to the actin housekeeping gene and evaluated using the 2−ΔΔCT method. Expression levels are presented as fold induction/downregulation of transcripts of respective genes relative to control.
Table 1.
Oligonucleotide primer sequences used in RT-PCR
| Mouse Genes | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|
| 12/15-LO* | ctctcaaggcctgttcagga | gtccattgtccccagaacct |
| Actin | aggtcatcactattggcaacga | ccctccatgatggaattgaatgtagtt |
| BiP | ttcagccaattatcagcaaactct | ttttctgatgtatcctcttcaccagt |
| CHOP | ccaccacacctgaaagcagaa | aggtgaaaggcagggactca |
| WFS1 | ccatcaacatgctcccgttc | gggtaggcctcgccataca |
| XBP-1 total | tggccgggtctgctgagtccg | gtccatgggaagatgttctgg |
| XBP-1 spliced | ctgagtccgaatcaggtgcag | gtccatgggaagatgttctgg |
12/15-LO, 12/15-lipoxygenase; BiP, binding immunoglobulin protein; CHOP, CCAAT/enhancer-binding protein homologous protein; WFS1, Wolfram syndrome 1; XBP-1, X-box protein-1.
For amplification of 12/15-LO, the cycling conditions were 95°C for 30 s, 60°C for 30 s, and 72°C for 30 s, followed by 81°C for 15 s.
Protein extraction and Western blot analysis.
Adipocytes were harvested in SDS gel loading buffer containing leupeptin (5 μg/ml), aprotinin (5 μg/ml), PMSF (1 mM), sodium orthovanadate (1 mM), benzamidine (1 mM), sodium fluoride (50 mM), and DTT (25 mM). The lysates were sheared by passing through a 20-gauge needle. Equal quantities of protein were separated by SDS-PAGE, transferred to PVDF membrane, and probed at 4°C overnight in primary antibody. Detection was performed with secondary HRP-conjugated antibodies and ECL plus (GE Healthcare, Piscataway, NJ) according to the manufacturer's instructions. Western blot quantitation was performed by measuring protein band intensities using ImageJ software. Protein expression levels were normalized to actin or tubulin and presented as fold induction/downregulation of band intensities relative to control. Most bands represented for Western blots in the figures come from the same gel; however, samples run on more than one gel are indicated in the figures and figure legends. In cases where multiple gels were run, an identical set of experimental replicates was run on each gel, and intensities normalized to this sample so that samples could be compared across gels and thus compensated for transfer efficiency. Moreover, exposure levels are the same for each antibody compared across gels since multiple gels were processed at the same time and exposed on the same film. Finally, each band is presented singly in the figures since multiple samples were run on a gel and thus were not contiguous.
Measurement of 12/15-LO activity and IL-12p40 secretion.
12/15-LO activity was determined by measuring 12(S)-HETE secretion by 3T3-L1 adipocytes into culture media. 12(S)-HETE was extracted from culture media, and levels were measured using the 12(S)-HETE Enzyme Immunoassay Kit (Enzo Life Sciences) according to the manufacturer's instructions. Total IL-12p40 (IL-12p40 subunit and IL-12p70) was measured in media from cultured 3T3-L1 adipocytes or mouse serum using the IL-12 Platinum ELISA kit (eBioscience) according to the manufacturer's instructions.
Data analysis.
Data are presented as means ± SE. Student's t-test was used to establish statistically significant differences between samples. A P value of <0.05 was considered to indicate statistically significant differences.
RESULTS
12/15-LO modulates the ER stress response.
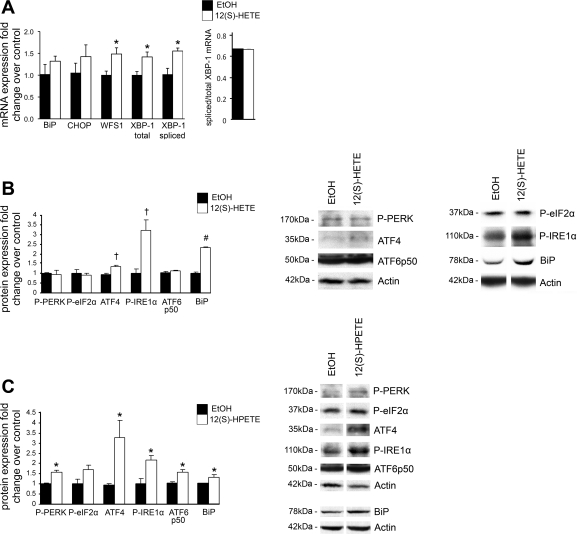
To determine whether 12/15-LO may play a direct role in ER stress in adipose tissue, we added the major enzymatic product of 12/15-LO, 12(S)-HETE, to the well-established in vitro model of fully differentiated 3T3-L1 adipocytes. Our previous study demonstrated that addition of 10 nM 12(S)-HETE is able to induce inflammation and insulin resistance in 3T3-L1 adipocytes (6). Given this, we assessed the ER stress response when 3T3-L1 adipocytes were treated with 10 nM 12(S)-HETE compared with ethanol solvent control for 24 h. Significant upregulation was observed for WFS1 (1.5-fold), total (1.4-fold) and spliced XBP-1 (1.6-fold) mRNA and ATF4 (1.3-fold), phosphorylated-IRE1α (3.2-fold), and BiP (2.3-fold) protein (Fig. 1, A and B). Apoptosis was not induced under these conditions (data not shown). We also added 1 nM 12(S)-HPETE, which is the transient but more potent precursor lipid to 12(S)-HETE, for 24 h and observed similar results (Fig. 1C). In addition, 12(S)-HPETE was able to significantly increase phosphorylated PERK (1.6-fold) and ATF6p50 (1.6-fold; the cleaved active form of ATF6) and also showed a trend to increase phosphorylated eIF2α (1.7-fold) (Fig. 1C). However, treatment of 3T3-L1 adipocytes with the inactive 12(R)-HETE analog or the minor 15(S)-HETE lipid product generated by 12/15-LO did not induce noticeable changes in ER stress gene expression (data not shown). Thus products of 12/15-LO, 12(S)-HETE and 12(S)-HPETE, can lead to induction of several key ER stress markers particularly important in all UPR branches of the ER stress response.
Fig. 1.
12-Hydroxyeicosatetranoic acid [12(S)-HETE] and 12-hydroperoxyeicosatetraenoic acid [12(S)-HPETE] induce expression of endoplasmic reticulum (ER) stress markers. 3T3-L1 adipocytes were treated with 10 nM 12(S)-HETE, 1 nM 12(S)-HPETE, or ethanol (EtOH) solvent control for 24 h, and mRNA (A) and protein measurements (B and C) of ER stress markers were examined. The mRNA measurements were done by RT-PCR and protein measurements done by Western blot analysis. Representative Western blots are shown, and separate panels for each antibody represent the same exposure from the same gel (B and C). All data were normalized to total actin, and the fold changes in expression were calculated relative to EtOH control. All data represent means ± SE; n = 3. *P < 0.05, †P < 0.02, and #P < 0.001 vs. control. BiP, binding immunoglobulin protein; CHOP, CCAAT/enhancer-binding protein homologous protein; WFS1, Wolfram syndrome 1; XBP-1, X-box protein-1; P, phosphorylated; PERK, RNA-dependent protein kinase-like ER-regulated kinase; eIF2α, eukaryotic translation initiation factor 2α; ATF4 and -6, activating transcription factor 4 and 6, respectively; IRE1α, inositol-requiring enzyme 1α.
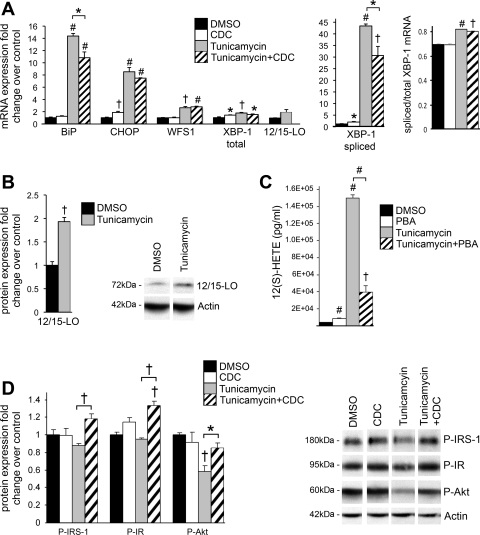
To further dissect the role of 12/15-LO in mediating ER stress, we added the well-known ER stress inducer tunicamycin, an N-glycosylation inhibitor of de novo protein synthesis, to 3T3-L1 adipocytes. These cells were pretreated for 2 h and incubated with the 12/15-LO inhibitor CDC (0.1 μM). As expected, addition of 5 μM tunicamycin for 24 h led to significant upregulation of mRNA for many ER stress markers, such as BiP, CHOP, WFS1, total XBP-1, and spliced XBP-1 (Fig. 2A). Pretreatment with CDC significantly ameliorated ER stress induction of BiP and spliced XBP-1 (Fig. 2A). Most interestingly, tunicamycin upregulated 12/15-LO mRNA (1.9-fold) and significantly increased 12/15-LO protein (1.9-fold) expression (Fig. 2, A and B). In addition, tunicamycin significantly increased secretion of 12(S)-HETE from 3T3-L1 adipocytes into the culture medium, indicating that 12/15-LO activity is upregulated (Fig. 2C); this effect was significantly prevented by addition of the chemical chaperone, PBA (10 mM), that is known to alleviate ER stress (Fig. 2C) (28). These data indicate that 12/15-LO activity is upregulated upon ER stress and that inhibiting 12/15-LO activity can partially abrogate many aspects of the tunicamycin-induced ER stress response.
Fig. 2.
Inhibition of 12/15-lipoxygenase (12/15-LO) activity attenuates tunicamycin-induced ER stress and insulin resistance. 3T3-L1 adipocytes were treated with 5 μM tunicamycin or DMSO solvent control for 24 h (A–C) with or without a 2-h pretreatment and coincubation with 0.1 μM of the 12/15-LO inhibitor cynnamyl 1–3,4-dihydroxy-α-cyanocinnamate (CDC) or 10 mM of the chemical chaperone 4-phenylbutyric acid (PBA). mRNA (A) and protein measurements (B) of ER stress markers and 12/15-LO were examined. C: 12(S)-HETE was measured in medium cultured with treated 3T3-L1 adipocytes by ELISA. D: 3T3-L1 adipocytes were treated with 5 μM tunicamycin or DMSO solvent control for 4 h and stimulated with 10 nM insulin for 10 min, and protein measurements of key insulin signaling markers were examined. The mRNA measurements were done by RT-PCR and protein measurements done by Western blot analysis; the data were normalized to total actin, and the fold changes in expression were calculated relative to DMSO control. Western blots are shown, and separate panels for each antibody represent the same exposure from the same gel (B and D). All data represent means ± SE; n = 3. Statistics were performed comparing each treatment with control as well as tunicamycin with tunicamycin + CDC/PBA; only statistically significant results are shown. *P < 0.05, †P < 0.02, and #P < 0.001 vs. control unless otherwise indicated.
Furthermore, ER stress can lead to insulin resistance, and we have shown that addition of 12(S)-HETE to 3T3-L1 adipocytes leads to impaired insulin signaling by decreasing IRS-1 and protein kinase B (Akt) phosphorylation (6). We tested whether inhibiting 12/15-LO activity could decrease insulin resistance induced by tunicamycin. Indeed, after 3T3-L1 adipocytes were treated with 5 μM tunicamycin for 4 h, phosphorylation of IR, IRS-1, and Akt were decreased, and addition of CDC was able to significantly improve insulin signaling in tunicamycin-treated 3T3-L1 adipocytes (Fig. 2D). However, after a 24-h incubation with tunicamycin, phosphorylation of IR, IRS-1, and Akt was reduced dramatically, and CDC pretreatment was not able to significantly improve insulin signaling (data not shown). Therefore, these data suggest that 12/15-LO may play a role in the early stages of ER stress-mediated insulin resistance, but clearly, other factors are involved as ER stress progresses.
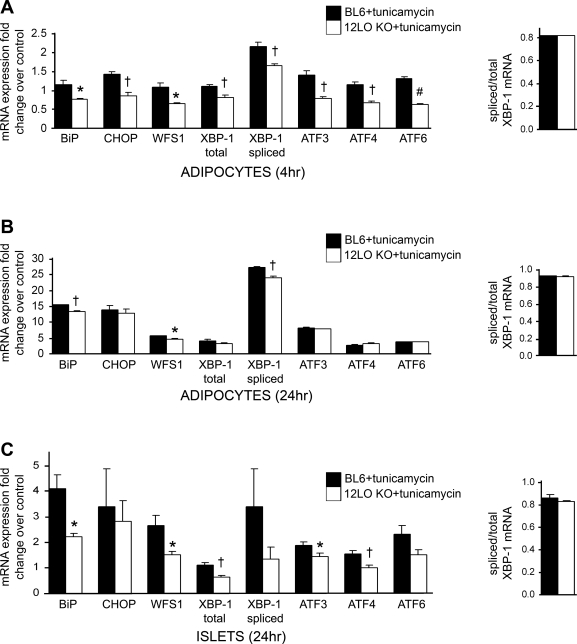
To confirm a role of 12/15-LO in mediating the ER stress response, we isolated epididymal adipocytes from 8-wk-old male wild-type C57BL/6 (BL6) and 12-LO KO mice, incubated the adipocytes with 5 μM tunicamycin for 4 or 24 h, and analyzed mRNA expression of key ER stress genes (Fig. 6, A and B). A more pronounced effect was seen after the 4-h treatment in which 12/15-LO deficiency significantly decreased tunicamycin-induced expression of all ER stress genes examined (Fig. 6A).
Fig. 6.
12/15-LO deficiency attenuates tunicamycin-induced ER stress in isolated adipocytes and pancreatic islets. Adipocytes and pancreatic islets were isolated from 8-wk-old BL6 and 12-LO KO male mice and treated with 5 μM tunicamycin or DMSO solvent control for 4 or 24 h. A–C: mRNA measurements of ER stress markers were examined and done by RT-PCR; the data were normalized to total actin. Each bar indicates the tunicamycin-induced fold change in expression relative to DMSO control of the same genotype. All data represent means ± SE; n = 4 mice/group. *P < 0.05, †P < 0.02, and #P < 0.001 vs. BL6 + tunicamycin control.
Data generated from the tunicamycin experiments suggest that 12/15-LO activation may be a downstream event of ER stress induction. However, given that 12/15-LO products can increase ER stress gene expression and that loss or inhibition of 12/15-LO leads to an attenuated ER stress response, 12/15-LO may also be an initiator of ER stress. Therefore, 12/15-LO may play a role in inducing ER stress and is further upregulated in a feedforward manner by ER stress.
IL-12 signaling is a component of the ER stress response.
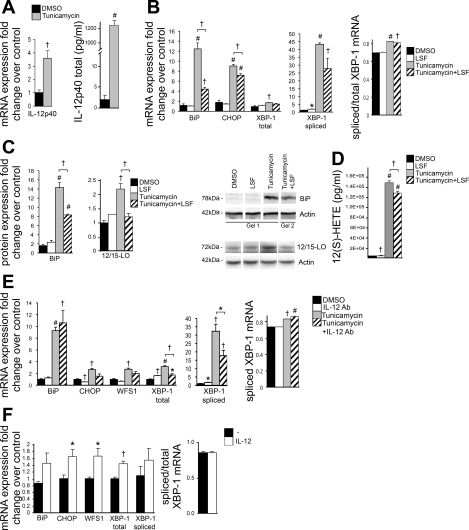
We and others have shown previously that 12(S)-HETE induces IL-12 expression in 3T3-L1 adipocytes and that 12/15-LO-deficient macrophages are defective in IL-12 production (6, 7, 44). Given that 12/15-LO mediates ER stress and acts through the IL-12 pathway, we investigated whether the IL-12 pathway is upregulated by ER stress. Indeed, tunicamycin significantly upregulated IL-12p40 mRNA (3.6-fold) and IL-12p40 secretion by 3T3-L1 adipocytes (Fig. 3A). Furthermore, we also confirmed that the IL-12 receptor (composed of IL-12Rβ1 and IL-12Rβ2 subunits) is expressed in 3T3-L1 adipocytes by RT-PCR (CT values: actin = 18.0, IL-12Rβ1 = 32.1, IL-12Rβ2 = 34.3). LSF is an anti-inflammatory agent that has been demonstrated to decrease 12/15-LO expression in adipocytes from Zucker obese rats (7). LSF reduces IL-12 signaling and consequent STAT4 activation, thereby decreasing inflammation and improving insulin sensitivity (7, 29, 43). When 3T3-L1 adipocytes were pretreated and incubated with 10 μM LSF, tunicamycin-induced 12/15-LO protein expression and 12(S)-HETE secretion was decreased significantly, with concomitant significant reduction in BiP and CHOP expression as well as reduced spliced XBP-1 expression (Fig. 3, B–D). Furthermore, addition of an IL-12p40-neutralizing antibody significantly attenuated tunicamycin-induced total and spliced XBP-1 expression and reduced CHOP and WFS1 expression (Fig. 3E), consistent with a role of IL-12 in inducing ER stress.
Fig. 3.
ER stress signals through IL-12 and is attenuated by the anti-inflammatory agent lysofylline (LSF). A–E: 3T3-L1 adipocytes were treated with 5 μM tunicamycin or DMSO solvent control for 24 h, pretreated for 2 h, and coincubated with the anti-inflammatory compound LSF (10 μM) or with 2 μg/ml IL-12p40 antibody (Ab). F: 3T3-L1 adipocytes were treated with 2.5 ng/ml mouse IL-12 cytokine for 24 h. mRNA (A, B, E, and F) and protein measurements (C) of IL-12p40, ER stress markers, and 12/15-LO were examined. IL-12p40 (A) and 12(S)-HETE (D) were measured in media cultured with treated 3T3-L1 adipocytes by ELISA. The mRNA measurements were done by RT-PCR and protein measurements done by Western blot analysis; the data were normalized to total actin, and the fold changes in expression were calculated relative to DMSO control. Representative Western blots are shown, and separate panels for each antibody represent the same exposure; however, samples for BiP and corresponding actin were run on different gels and clearly demarcated (C) (see materials and methods for quantitation). All data represent means ± SE; n = 3. Statistics were performed, comparing each treatment with control as well as tunicamycin with tunicamycin + LSF/Ab; only statistically significant results are shown. *P < 0.05, †P < 0.02, and #P < 0.001 vs. control unless otherwise indicated.
Given that IL-12 signaling appears to be a component of the ER stress response, we added 2.5 ng/ml IL-12 cytokine to 3T3-L1 adipocytes for 24 h and measured ER stress gene expression. Similarly to the 12(S)-HETE and 12(S)-HPETE experiments, we observed significantly increased expression of CHOP (1.7-fold), WFS1 (1.7-fold), and total XBP-1 (1.4-fold) and a trend for increased expression of BiP (1.5-fold) and spliced XBP-1 (1.6-fold) (Fig. 3F).
ER stress and 12/15-LO impair adiponectin, C/EBPα, and ATF3 expression.
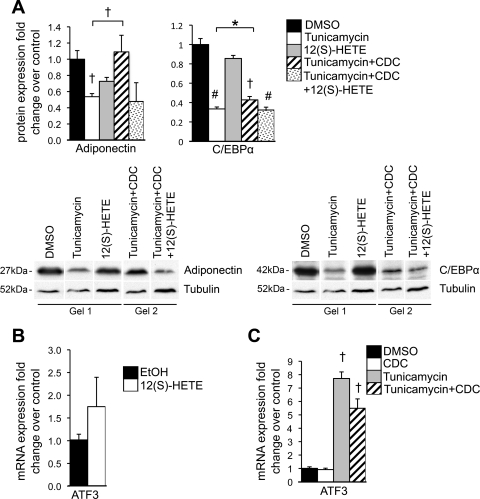
ER stress is accompanied by insulin resistance in part through its downregulation of the insulin-sensitizing adipokine adiponectin in 3T3-L1 adipocytes (41). We have also reported previously that 12/15-LO induces insulin resistance and decreases expression of adiponectin (6, 25). Twenty-four-hour treatment with 5 μM tunicamycin or 10 nM 12(S)-HETE reduced adiponectin protein expression (Fig. 4A). CDC pretreatment prevented the tunicamycin-induced downregulation of adiponectin, and this effect could be reversed by the addition of 12(S)-HETE (Fig. 4A). This provides evidence that 12/15-LO is one of the key players in ER stress-mediated downregulation of adiponectin expression.
Fig. 4.
Effect of 12/15-LO and ER stress on adiponectin, CCAAT/enhancer-binding protein-α (C/EBPα), and ATF3 expression. 3T3-L1 adipocytes were treated with 5 μM tunicamycin (or DMSO solvent control) and/or 10 nM 12(S)-HETE (or EtOH solvent control) for 24 h with or without a 2-h pretreatment and coincubation with the 12/15-LO inhibitor CDC (0.1 μM). Protein (A) and mRNA measurements (B and C) were performed for genes indicated by Western blot analysis and RT-PCR, respectively. All data were normalized to tubulin (A) or actin (B and C), and fold changes in expression were calculated relative to indicated solvent control. Representative Western blots are shown, and separate panels for each antibody represent the same exposure; however, samples were run on different gels and clearly demarcated (see materials and methods for quantitation). Furthermore, tubulin samples are shown for both adiponectin and C/EBPα because the same sample is not displayed for all experimental conditions (A). All data represent means ± SE; n = 3. Statistics were performed, comparing each treatment with control as well as tunicamycin with tunicamycin + CDC and tunicamycin + CDC with tunicamycin + CDC + 12(S)-HETE; only statistically significant results are shown. *P < 0.05, †P < 0.02, and #P < 0.001 vs. control unless otherwise indicated.
Given that adiponectin expression is reduced by ER stress and 12/15-LO, we sought to determine whether adiponectin expression was modulated through a common pathway. The transcription factor C/EBPα is important during adipogenesis to promote insulin sensitivity by inducing adiponectin expression (5, 32), and C/EBPα expression is inhibited by CHOP (23). Thus we investigated whether 12/15-LO modulates C/EBPα during the ER stress response to regulate adiponectin levels. 12(S)-HETE alone was able to slightly reduce C/EBPα protein levels (Fig. 4A). Furthermore, pretreatment with CDC partially prevented tunicamycin-induced downregulation of C/EBPα, and importantly, addition with 12(S)-HETE restored this tunicamycin effect (Fig. 4A).
ATF3 may also provide another link explaining the mechanism behind ER stress and 12/15-LO regulation of adiponectin expression. ATF3 is induced by the PERK UPR arm of the ER stress pathway and has been shown to negatively regulate adiponectin expression in adipocytes (16, 19, 31). We saw a 1.8-fold enhancement of ATF3 mRNA expression in 3T3-L1 adipocytes treated with 10 nM 12(S)-HETE for 24 h (Fig. 4B). Furthermore, tunicamycin-induced upregulation of ATF3 was attenuated with CDC pretreatment (Fig. 4C). These results support the hypothesis that 12/15-LO participates in ATF3-induced metabolic changes.
In summary, these results demonstrate that ER stress and 12/15-LO modulate adiponectin expression through several common pathways, such as C/EBPα and ATF3.
12/15-LO-deficient mice fed a high-fat diet display reduced ER stress in adipocytes, pancreatic islets, and liver.
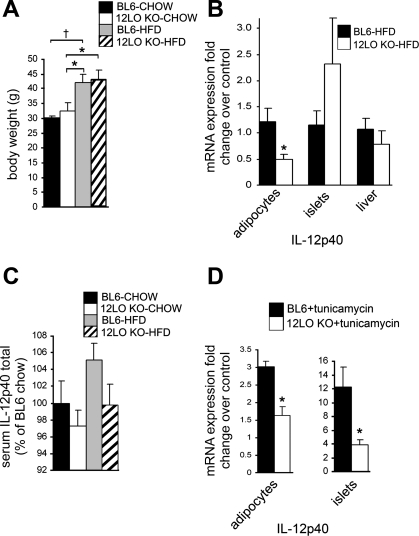
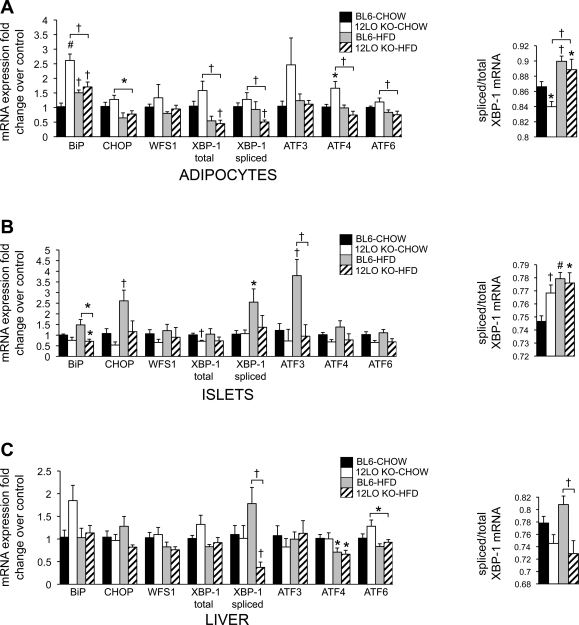
To determine whether 12/15-LO is important in modulating ER stress in an in vivo setting, we placed wild-type BL6 and 12/15-LO-deficient (12-LO KO) mice on a normal chow or high-fat diet for 16 wk and measured ER stress gene expression. Both BL6 and 12-LO KO exhibited similar weight gain at the end of the 16-wk diet regimen (Fig. 7A), thus ruling out the effect of differences in body weight on gene expression. In chow-fed mice, there were variable changes in ER stress markers in adipocytes from BL6 and 12-LO KO mice (Fig. 5A). Whereas BiP mRNA expression was significantly higher in adipocytes from the chow-fed 12-LO KO mice compared with the chow-fed BL6 mice, BiP expression was significantly decreased in adipocytes from high-fat diet-fed compared with chow-fed 12-LO KO mice (Fig. 5A). In addition, BiP expression increased significantly in adipocytes from the high-fat diet-fed compared with chow-fed BL6 mice, indicating UPR activation in adipocytes from BL6 mice fed a high-fat diet (Fig. 5A). Of note, significant decreases in mRNA expression for CHOP, total XBP-1, spliced XBP-1, ATF4, and ATF6 were observed in adipocytes from HFD-fed vs. chow-fed 12-LO KO mice but not high-fat diet- vs. chow-fed BL6 mice (Fig. 5A).
Fig. 7.
12/15-LO deficiency leads to decreased IL-12p40 expression. A: body weights of BL6 and 12-LO KO male mice fed a chow or HFD for 16 wk are shown. IL-12p40 mRNA expression was measured in isolated adipocytes and pancreatic islets from BL6 and 12-LO KO mice fed a HFD for 16 wk (B) or from isolated adipocytes and pancreatic islets from normal 8-wk-old BL6 and 12-LO KO mice that were treated with 5 μM tunicamycin or DMSO solvent control for 24 h (D); mRNA measurements were performed by RT-PCR, and the data were normalized to total actin. In B, fold changes in expression are calculated relative to BL6-HFD; in D, each bar indicates the tunicamycin-induced fold change in expression relative to DMSO control of the same genotype. C: IL-12p40 protein level was measured in serum from BL6 and 12-LO KO mice fed a chow diet or HFD for 16 wk by ELISA. All data represent means ± SE; n = 3–5. *P < 0.05 and †P < 0.02 vs. control unless otherwise indicated.
Fig. 5.
12/15-LO deficiency protects mouse adipocytes, pancreatic islets, and liver from several aspects of the high-fat diet-induced ER stress response. Eight-week-old C57BL/6 (BL6) and 12/15-LO-deficient (12-LO KO) male mice were placed on a chow or high-fat diet (HFD) for 16 wk. Epididymal adipocytes (A), pancreatic islets (B), and liver (C) were isolated, and mRNA measurements by RT-PCR were performed for ER stress genes. All data were normalized to actin, and fold changes in expression were calculated relative to BL6-CHOW control. All data represent means ± SE; n = 5 mice/group. Statistics were performed, comparing each group with BL6-CHOW control as well as 12-LO KO-CHOW with 12-LO KO-HFD and BL6-HFD with 12-LO KO-HFD; only statistically significant results are shown. *P < 0.05, †P < 0.02, and #P < 0.001 vs. control unless otherwise indicated.
Mori et al. (24) reported that inflammation in the adipose tissue precedes the development of inflammation elsewhere and thus can act as an early indicator for development and progression of disease in other tissues, such as β-cell failure and liver dysfunction. Therefore, we evaluated pancreatic islets and liver from BL6 and 12-LO KO mice fed a chow or high-fat diet to ascertain whether 12/15-LO deficiency could protect peripheral tissues from the ER stress response known to be induced by a high-fat diet (9). Pancreatic islets exhibited upregulation of BiP, CHOP, spliced XBP-1, and ATF3 in high-fat diet-fed wild-type BL6 mice compared with chow-fed BL6 mice (Fig. 5B). In contrast, these genes were downregulated in pancreatic islets from both chow- and high-fat diet-fed 12-LO KO mice (Fig. 5B). Additionally, in a similar manner, pancreatic islets isolated from 8-wk-old male BL6 and 12-LO KO mice revealed that 12/15-LO deficiency significantly reduced tunicamycin-induced expression of BiP, WFS1, total XBP-1, ATF3, and ATF4 (Fig. 6C). Finally, spliced XBP-1 was decreased significantly in the liver from high-fat diet-fed 12-LO KO mice (Fig. 5C). Thus 12/15-LO deficiency in mice prevents high-fat diet-induced upregulation of some key ER stress response genes in peripheral tissues such as pancreatic islets and liver.
Finally, to evaluate an in vivo role of IL-12 in the ER stress response, we measured IL-12p40 mRNA expression in adipocytes, pancreatic islets, and liver and secreted IL-12p40 protein in serum from BL6 and 12-LO KO mice on a chow or high-fat diet. IL-12p40 mRNA expression was reduced significantly in adipocytes, but no significant changes were observed in pancreatic islets or liver (IL-12p40 mRNA was below the level of detection in chow groups; Fig. 7B). In addition, IL-12p40 in serum showed a trend toward increasing in high-fat diet-fed BL6 mice, but interestingly, IL-12p40 expression remained low in high-fat diet-fed 12-LO KO mice (Fig. 7C). Finally, analysis of IL-12p40 expression in isolated adipocytes and pancreatic islets from normal 8-wk-old mice revealed that 12/15-LO deficiency significantly decreased tunicamycin-induced expression of IL-12p40 (Fig. 7D).
DISCUSSION
Visceral adiposity is of serious concern since it is associated with chronic low-grade inflammation, which promotes adipocyte dysfunction, insulin resistance, and β-cell damage. Inflammation also plays a key role in modulating the ER stress response, which is associated with the above pathologies. We demonstrate here for the first time that 12/15-LO is a novel inflammatory pathway regulating the ER stress response in key tissues, including adipocytes. The 12(S)-HETE and 12(S)-HPETE products of 12/15-LO can upregulate ER stress markers associated with each branch of the UPR in a manner consistent with reported in vivo ER stress induction in obese patient or mouse adipose tissue (3, 13, 34, 39).
12/15-LO exerts proinflammatory responses in part by upregulating the expression of IL-12 in activated inflammatory cells (6, 22, 38, 40). Previous studies demonstrate that 12/15-LO and its products can also induce IL-12 expression, STAT4 phosphorylation, and JNK1 activation in adipocytes (6, 7). Prolonged UPR activation is accompanied by activation of the JNK1 and NF-κB pathways, which upregulate expression of inflammatory genes, including IL-12, and promote insulin resistance (12, 14, 38). Although the role of IL-12 in ER stress-induced inflammation remains to be determined, we show here that IL-12 induced expression of ER stress genes, was upregulated by tunicamycin, and was decreased significantly by 12/15-LO deficiency. The anti-inflammatory small-molecule drug LSF, which exerts its role primarily by decreasing IL-12-induced activation of STAT4 (43), reduced 12/15-LO expression and activity along with many aspects of the ER stress response in tunicamycin-treated 3T3-L1 adipocytes. Additionally, IL-12-neutralizing antibodies also attenuated tunicamycin-induced ER stress. These results suggest that ER stress may involve 12/15-LO-mediated JNK1 and NF-κB activation and subsequent inflammatory consequences in part through IL-12 and STAT4 signaling. However, it remains to be determined the extent to which JNK1 activates ER stress and vice versa (15).
12/15-LO activation plays a role in impairing insulin sensitivity. Mice deficient in 12/15-LO are protected from systemic insulin resistance and reductions in adiponectin expression induced by a high-fat diet in wild-type mice, whereas weight gain is not affected (25, 33). Addition of 12/15-LO products to 3T3-L1 adipocytes led to reduced adiponectin expression and insulin resistance (6). In this study, we demonstrate further that ER stress and 12/15-LO products can modulate C/EBPα and ATF3 expression, leading to decreased adiponectin expression (5, 16, 19, 23, 31, 32). This area is far less understood, and there are likely additional mechanisms by which ER stress and 12/15-LO can decrease insulin sensitivity.
The in vitro 3T3-L1 data provide support for a role of 12/15-LO in mediating ER stress responses. To determine the in vivo significance of these data, we measured ER stress induction in BL6 and 12-LO KO mice fed a high-fat diet. In contrast to reports describing upregulation of ER stress genes in adipose tissue under obese conditions (3, 13, 27, 28, 34), most of the ER stress markers were unchanged in BL6 mice fed a chow or high-fat diet. A likely explanation accounting for these differences is that our study examined isolated epididymal adipocytes instead of whole adipose tissue. Whole fat is a complex heterogeneous tissue comprising adipocytes and the stromal vascular compartment containing preadipocytes, leukocytes, macrophages, fibroblasts, and endothelial cells. Further study of whole adipose tissue from these mice will likely begin to reveal significant changes in ER stress gene expression. In fact, the study published by Sharma et al. (34) compared ER stress gene expression of subcutaneous adipose tissue and isolated adipocytes from patients and observed that significant positive correlations between high ER stress gene expression and increasing body mass index or percent fat were lost in the adipocyte fraction. Thus the complex milieu and interactions that make up the adipose tissue suggest that analysis of isolated adipocytes from an in vivo setting requires analysis of each of its various cell types. In addition, we observed that 12-LO KO mice exhibit a trend for elevated ER stress gene expression in epididymal adipocytes from mice fed a normal chow diet compared with high-fat diet-fed mice. Mouse 12/15-LO generates proresolving anti-inflammatory lipoxins in mice fed a normal chow diet (21). However, hyperlipidemia induced by high-fat feeding leads to disruption of this protective function of 12/15-LO, leading to generation of proinflammatory lipid products (21). Thus, in the chow-fed 12-LO KO mice, loss of lipoxin production may further explain the increased ER stress response in the adipocytes. However, of significance, we were able to observe that 12/15-LO deficiency led to significant decreases of spliced XBP-1 mRNA in adipocytes from high-fat diet-fed 12-LO KO mice compared with other groups. This trend was also observed in the liver and is important because hyperlipidemia from an excessive diet can lead to fatty liver and ER stress in the liver, both of which are associated with insulin resistance and ensuing type 2 diabetes (27, 37, 39). Indeed, XBP-1 regulates lipid metabolism. Mice with a specific deletion of XBP-1 in the liver exhibited decreased serum cholesterol and triglyceride levels and protection from hepatic steatosis (20). Therefore, reduced spliced XBP-1 may be sufficient to protect 12-LO KO mice from the deleterious consequences of high-fat diet-induced ER stress since activation of XBP-1 is a key downstream event of the UPR in modulating ER stress.
In addition, we examined ER stress induction in pancreatic islets, another key target tissue of visceral obesity. 12/15-LO deficiency significantly protected the pancreatic islets from ER stress induction when the mice were fed a high-fat diet. A marked inhibition of high-fat diet-induced CHOP and spliced XBP-1 mRNA expression was observed in the 12-LO KO pancreatic islets compared with BL6 controls. This is consistent with a report demonstrating that CHOP deficiency in db/db mice promotes β-cell survival (35) and the notion that 12/15-LO signals through XBP-1. This is a very interesting finding in the context of diabetes because the insulin-producing β-cells found in pancreatic islets are under increased demand to produce more insulin to compensate for insulin resistance, leading to disruption of ER homeostasis. In preliminary findings, we also found that 12(S)-HETE was able to upregulate ER stress genes in the mouse βTC3 and Min6 pancreatic β-cell lines as well as isolated human pancreatic islets (data not shown).
Chronic dysfunction of adipose tissue induced by obesity may lead to ER stress in peripheral tissues. New studies reveal that an early inflammatory response in adipose tissue precedes the development of insulin resistance and thus disease progression in peripheral organs (24). This is interesting given that the 4-h tunicamycin treatment of isolated BL6 and 12-LO KO adipocytes showed significant differences in ER stress activation compared with the 24-h time point. This early protection in adipocytes may account for protection seen at the systemic level in pancreatic islets. In addition, circulating IL-12p40 levels mimicked IL-12p40 mRNA levels seen in adipocytes. Given that the volume of adipose tissue is much larger than certain peripheral tissues, such as pancreatic islets, changes in adipose tissue gene expression may more significantly impact circulating systemic cytokine levels. Thus serum IL-12p40 level may be more influenced by IL-12p40 secretion from adipose tissue. Future studies examining 12/15-LO deletion in adipose tissue will reveal the local and systemic role of fat 12/15-LO.
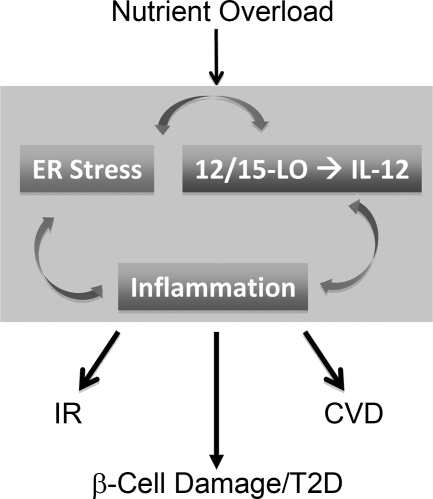
Since 12/15-LO deficiency and a LO inhibitor do not completely prevent tunicamycin-induced ER stress in adipocytes, there are likely other mechanisms in addition to induction of 12/15-LO that mediate ER stress by this agent. Our results demonstrate that LSF is able to attenuate tunicamycin-induced ER stress more efficiently that CDC, suggesting that 12/15-LO-independent regulation of IL-12 may also be important for ER stress responses. In addition, further studies will be needed to determine which is the initiating event, 12/15-LO activation, inflammation, ER stress, or all of the above (see Fig. 8).
Fig. 8.
12/15-LO and ER stress. A schematic describing the proposed hypothetical complex dynamic interplay between arms of 12/15-LO activation, inflammation, and ER stress induction when cells are exposed to a pathological excess of nutrients. ER stress can activate 12/15-LO to generate 12(S)-HETE, and 12(S)-HETE can induce ER stress in an IL-12-dependent manner. Activation of each arm creates a vicious cycle that predisposes individuals to the development of insulin resistance (IR), type 2 diabetes (T2D), and cardiovascular disease (CVD).
In summary, we provide evidence for the first time that activation of 12/15-LO can lead to the ER stress response. Chronic 12/15-LO activation associated with obesity can lead to excessive ER stress and its deleterious inflammatory consequences in several key metabolic tissues. Therefore, blockade of 12/15-LO activation or downstream IL-12 signaling may provide a new therapeutic strategy in alleviating inflammation, β-cell dysfunction, and insulin resistance associated with ER stress, thereby reducing metabolic complications associated with visceral adiposity.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants P01-HL-55798 and R01-HL-112605 and a National Institutes of Health National Research Service Award Postdoctoral Research Fellowship (1-F32-DK-085716-01).
DISCLOSURES
Jerry L. Nadler is a stockholder and founder of Diakine Therapeutics.
AUTHOR CONTRIBUTIONS
B.K.C. and S.K.C. did the conception and design of the research; B.K.C., N.S.K., S.M.G.-M., K.A.L., R.M.R., and S.K.C. performed the experiments; B.K.C. and S.K.C. analyzed the data; B.K.C., J.L.N., and S.K.C. interpreted the results of the experiments; B.K.C. prepared the figures; B.K.C. drafted the manuscript; B.K.C., J.L.N., and S.K.C. edited and revised the manuscript; B.K.C., J.L.N., and S.K.C. approved the final version of the manuscript.
ACKNOWLEDGMENTS
We gratefully acknowledge Kaiwen Ma (EVMS) for the βTC3 and Min6 studies, Susanna R. Keller (University of Virginia) for assistance in critically reviewing the manuscript, and Lindsey M. Grier (EVMS) for mouse husbandry issues related to mouse experiments. Also, human islets were obtained from the Islet Cell Resource Consortium and the Juvenile Diabetes Research Foundation Basic Science Human Islet Distribution Program.
REFERENCES
- 1. Bleich D, Chen S, Zipser B, Sun D, Funk CD, Nadler JL. Resistance to type 1 diabetes induction in 12-lipoxygenase knockout mice. J Clin Invest 103: 1431–1436, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Boden G. Endoplasmic reticulum stress: another link between obesity and insulin resistance/inflammation? Diabetes 58: 518–519, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Boden G, Duan X, Homko C, Molina EJ, Song W, Perez O, Cheung P, Merali S. Increase in endoplasmic reticulum stress-related proteins and genes in adipose tissue of obese, insulin-resistant individuals. Diabetes 57: 2438–2444, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Carter JD, Dula SB, Corbin KL, Wu R, Nunemaker CS. A practical guide to rodent islet isolation and assessment. Biol Proced Online 11: 3–31, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cha HC, Oak NR, Kang S, Tran TA, Kobayashi S, Chiang SH, Tenen DG, MacDougald OA. Phosphorylation of CCAAT/enhancer-binding protein alpha regulates GLUT4 expression and glucose transport in adipocytes. J Biol Chem 283: 18002–18011, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chakrabarti SK, Cole BK, Wen Y, Keller SR, Nadler JL. 12/15-Lipoxygenase products induce inflammation and impair insulin signaling in 3T3-L1 adipocytes. Obesity (Silver Spring) 17: 1657–1663, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chakrabarti SK, Wen Y, Dobrian AD, Cole BK, Ma Q, Pei H, Williams MD, Bevard MH, Vandenhoff GE, Keller SR, Gu J, Nadler JL. Evidence for activation of inflammatory lipoxygenase pathways in visceral adipose tissue of obese Zucker rats. Am J Physiol Endocrinol Metab 300: E175–E187, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dobrian AD, Lieb DC, Cole BK, Taylor-Fishwick DA, Chakrabarti SK, Nadler JL. Functional and pathological roles of the 12- and 15-lipoxygenases. Prog Lipid Res 50: 115–131, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Eizirik DL, Cardozo AK, Cnop M. The role for endoplasmic reticulum stress in diabetes mellitus. Endocr Rev 29: 42–61, 2008 [DOI] [PubMed] [Google Scholar]
- 10. Fonseca SG, Fukuma M, Lipson KL, Nguyen LX, Allen JR, Oka Y, Urano F. WFS1 is a novel component of the unfolded protein response and maintains homeostasis of the endoplasmic reticulum in pancreatic beta-cells. J Biol Chem 280: 39609–39615, 2005 [DOI] [PubMed] [Google Scholar]
- 11. Fonseca SG, Ishigaki S, Oslowski CM, Lu S, Lipson KL, Ghosh R, Hayashi E, Ishihara H, Oka Y, Permutt MA, Urano F. Wolfram syndrome 1 gene negatively regulates ER stress signaling in rodent and human cells. J Clin Invest 120: 744–755, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gregor MF, Hotamisligil GS. Thematic review series: Adipocyte Biology. Adipocyte stress: the endoplasmic reticulum and metabolic disease. J Lipid Res 48: 1905–1914, 2007 [DOI] [PubMed] [Google Scholar]
- 13. Gregor MF, Yang L, Fabbrini E, Mohammed BS, Eagon JC, Hotamisligil GS, Klein S. Endoplasmic reticulum stress is reduced in tissues of obese subjects after weight loss. Diabetes 58: 693–700, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hirosumi J, Tuncman G, Chang L, Görgün CZ, Uysal KT, Maeda K, Karin M, Hotamisligil GS. A central role for JNK in obesity and insulin resistance. Nature 420: 333–336, 2002 [DOI] [PubMed] [Google Scholar]
- 15. Hotamisligil GS. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell 140: 900–917, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jiang HY, Wek SA, McGrath BC, Lu D, Hai T, Harding HP, Wang X, Ron D, Cavener DR, Wek RC. Activating transcription factor 3 is integral to the eukaryotic initiation factor 2 kinase stress response. Mol Cell Biol 24: 1365–1377, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kakiuchi C, Ishiwata M, Hayashi A, Kato T. XBP1 induces WFS1 through an endoplasmic reticulum stress response element-like motif in SH-SY5Y cells. J Neurochem 97: 545–555, 2006 [DOI] [PubMed] [Google Scholar]
- 18. Kang SW, Adler SG, Nast CC, LaPage J, Gu JL, Nadler JL, Natarajan R. 12-lipoxygenase is increased in glucose-stimulated mesangial cells and in experimental diabetic nephropathy. Kidney Int 59: 1354–1362, 2001 [DOI] [PubMed] [Google Scholar]
- 19. Kim HB, Kong M, Kim TM, Suh YH, Kim WH, Lim JH, Song JH, Jung MH. NFATc4 and ATF3 negatively regulate adiponectin gene expression in 3T3-L1 adipocytes. Diabetes 55: 1342–1352, 2006 [DOI] [PubMed] [Google Scholar]
- 20. Lee AH, Scapa EF, Cohen DE, Glimcher LH. Regulation of hepatic lipogenesis by the transcription factor XBP1. Science 320: 1492–1496, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Merched AJ, Serhan CN, Chan L. Nutrigenetic disruption of inflammation-resolution homeostasis and atherogenesis. J Nutrigenet Nutrigenomics 4: 12–24, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Middleton MK, Rubinstein T, Puré E. Cellular and molecular mechanisms of the selective regulation of IL-12 production by 12/15-lipoxygenase. J Immunol 176: 265–274, 2006 [DOI] [PubMed] [Google Scholar]
- 23. Miller RS, Diaczok D, Cooke DW. Repression of GLUT4 expression by the endoplasmic reticulum stress response in 3T3-L1 adipocytes. Biochem Biophys Res Commun 362: 188–192, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mori MA, Liu M, Bezy O, Almind K, Shapiro H, Kasif S, Kahn CR. A systems biology approach identifies inflammatory abnormalities between mouse strains prior to development of metabolic disease. Diabetes 59: 2960–2971, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nunemaker CS, Chen M, Pei H, Kimble SD, Keller SR, Carter JD, Yang Z, Smith KM, Wu R, Bevard MH, Garmey JC, Nadler JL. 12-Lipoxygenase-knockout mice are resistant to inflammatory effects of obesity induced by western diet. Am J Physiol Endocrinol Metab 295: E1065–E1075, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Olshansky SJ, Passaro DJ, Hershow RC, Layden J, Carnes BA, Brody J, Hayflick L, Butler RN, Allison DB, Ludwig DS. A potential decline in life expectancy in the United States in the 21st century. N Engl J Med 352: 1138–1145, 2005 [DOI] [PubMed] [Google Scholar]
- 27. Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E, Tuncman G, Görgün C, Glimcher LH, Hotamisligil GS. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science 306: 457–461, 2004 [DOI] [PubMed] [Google Scholar]
- 28. Ozcan U, Yilmaz E, Ozcan L, Furuhashi M, Vaillancourt E, Smith RO, Görgün CZ, Hotamisligil GS. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science 313: 1137–1140, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pei H, Gu J, Thimmalapura PR, Mison A, Nadler JL. Activation of the 12-lipoxygenase and signal transducer and activator of transcription pathway during neointima formation in a model of the metabolic syndrome. Am J Physiol Endocrinol Metab 290: E92–E102, 2006 [DOI] [PubMed] [Google Scholar]
- 30. Poirier P, Giles TD, Bray GA, Hong Y, Stern JS, Pi-Sunyer FX, Eckel RH. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss. Arterioscler Thromb Vasc Biol 26: 968–976, 2006 [DOI] [PubMed] [Google Scholar]
- 31. Qi L, Saberi M, Zmuda E, Wang Y, Altarejos J, Zhang X, Dentin R, Hedrick S, Bandyopadhyay G, Hai T, Olefsky J, Montminy M. Adipocyte CREB promotes insulin resistance in obesity. Cell Metab 9: 277–286, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Qiao L, Maclean PS, Schaack J, Orlicky DJ, Darimont C, Pagliassotti M, Friedman JE, Shao J. C/EBPalpha regulates human adiponectin gene transcription through an intronic enhancer. Diabetes 54: 1744–1754, 2005 [DOI] [PubMed] [Google Scholar]
- 33. Sears DD, Miles PD, Chapman J, Ofrecio JM, Almazan F, Thapar D, Miller YI. 12/15-lipoxygenase is required for the early onset of high fat diet-induced adipose tissue inflammation and insulin resistance in mice. PLoS One 4: e7250, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sharma NK, Das SK, Mondal AK, Hackney OG, Chu WS, Kern PA, Rasouli N, Spencer HJ, Yao-Borengasser A, Elbein SC. Endoplasmic reticulum stress markers are associated with obesity in nondiabetic subjects. J Clin Endocrinol Metab 93: 4532–4541, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Song B, Scheuner D, Ron D, Pennathur S, Kaufman RJ. Chop deletion reduces oxidative stress, improves beta cell function, and promotes cell survival in multiple mouse models of diabetes. J Clin Invest 118: 3378–3389, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sun D, Funk CD. Disruption of 12/15-lipoxygenase expression in peritoneal macrophages. Enhanced utilization of the 5-lipoxygenase pathway and diminished oxidation of low density lipoprotein. J Biol Chem 271: 24055–24062, 1996 [PubMed] [Google Scholar]
- 37. Sung KC, Kim SH. Interrelationship between fatty liver and insulin resistance in the development of type 2 diabetes. J Clin Endocrinol Metab 96: 1093–1097, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol 3: 133–146, 2003 [DOI] [PubMed] [Google Scholar]
- 39. Tsutsumi A, Motoshima H, Kondo T, Kawasaki S, Matsumura T, Hanatani S, Igata M, Ishii N, Kinoshita H, Kawashima J, Taketa K, Furukawa N, Tsuruzoe K, Nishikawa T, Araki E. Caloric restriction decreases ER stress in liver and adipose tissue in ob/ob mice. Biochem Biophys Res Commun 404: 339–344, 2011 [DOI] [PubMed] [Google Scholar]
- 40. Wen Y, Gu J, Chakrabarti SK, Aylor K, Marshall J, Takahashi Y, Yoshimoto T, Nadler JL. The role of 12/15-lipoxygenase in the expression of interleukin-6 and tumor necrosis factor-alpha in macrophages. Endocrinology 148: 1313–1322, 2007 [DOI] [PubMed] [Google Scholar]
- 41. Xu L, Spinas GA, Niessen M. ER stress in adipocytes inhibits insulin signaling, represses lipolysis, and alters the secretion of adipokines without inhibiting glucose transport. Horm Metab Res 42: 643–651, 2010 [DOI] [PubMed] [Google Scholar]
- 42. Yang Z, Chen M, Ellett JD, Fialkow LB, Carter JD, McDuffie M, Nadler JL. Autoimmune diabetes is blocked in Stat4-deficient mice. J Autoimmun 22: 191–200, 2004 [DOI] [PubMed] [Google Scholar]
- 43. Yang Z, Chen M, Nadler JL. Lisofylline: a potential lead for the treatment of diabetes. Biochem Pharmacol 69: 1–5, 2005 [DOI] [PubMed] [Google Scholar]
- 44. Zhao L, Cuff CA, Moss E, Wille U, Cyrus T, Klein EA, Praticò D, Rader DJ, Hunter CA, Puré E, Funk CD. Selective interleukin-12 synthesis defect in 12/15-lipoxygenase-deficient macrophages associated with reduced atherosclerosis in a mouse model of familial hypercholesterolemia. J Biol Chem 277: 35350–35356, 2002 [DOI] [PubMed] [Google Scholar]