Abstract
Men and women with hyperandrogenemia have a more proatherogenic plasma lipid profile [e.g., greater triglyceride (TG) and total and low-density lipoprotein-cholesterol and lower high-density lipoprotein-cholesterol concentrations] than healthy premenopausal women. Furthermore, castration of male rats markedly reduces testosterone availability below normal and decreases plasma TG concentration, and testosterone replacement reverses this effect. Testosterone is, therefore, thought to be an important regulator of plasma lipid homeostasis. However, little is known about the effect of testosterone on plasma TG concentration and kinetics. Furthermore, testosterone is a potent skeletal muscle protein anabolic agent in men, but its effect on muscle protein turnover in women is unknown. We measured plasma lipid concentrations, hepatic very low density lipoprotein (VLDL)-TG and VLDL-apolipoprotein B-100 secretion rates, and the muscle protein fractional synthesis rate in 10 obese women before and after trandermal testosterone (1.25 g of 1% AndroGel daily) treatment for 3 wk. Serum total and free testosterone concentrations increased (P < 0.05) by approximately sevenfold in response to testosterone treatment, reaching concentrations that are comparable to those in women with hyperandrogenemia, but lower than the normal range for eugonadal men. Except for a small (∼10%) decrease in plasma high-density lipoprotein particle and cholesterol concentrations (P < 0.04), testosterone therapy had no effect on plasma lipid concentrations, lipoprotein particle sizes, and hepatic VLDL-TG and VLDL-apolipoprotein B-100 secretion rates (all P > 0.05); the muscle protein fractional synthesis rate, however, increased by ∼45% (P < 0.001). We conclude that testosterone is a potent skeletal muscle protein anabolic agent, but not an important regulator of plasma lipid homeostasis in obese women.
Keywords: triglyceride, apolipoprotein B, muscle protein synthesis
men have a more proatherogenic plasma lipid profile, including greater plasma triglyceride (TG) and total and low-density lipoprotein (LDL)-cholesterol concentrations, lower high-density lipoprotein (HDL)-cholesterol concentration, and smaller HDL particles, than women (24, 25). Sex differences in the plasma lipid profile have traditionally been attributed to differences in the sex hormone milieu; particularly the availability of estradiol, but also progesterone. However, we and others have shown that changes in ovarian steroid concentration during the normal menstrual cycle (26) and after menopause (6, 23, 50) are not associated with changes in very-low-density lipoprotein (VLDL)-TG and VLDL-apolipoprotein B-100 (apoB-100) kinetics and concentrations that are consistent with the observed sexual dimorphism in VLDL metabolism (53). Furthermore, oral estrogen preparations given to women and men increase total plasma TG and VLDL-TG concentrations due to increased hepatic VLDL-TG secretion, and transdermal administration of 17β-estradiol, which better mimics normal endogenous estrogen delivery, does not affect or only modestly decreases (by <10%) plasma TG concentration (53). Thus female sex steroids alone cannot explain the differences in the plasma lipid profile between men and women, and testosterone may be an important regulator of plasma TG homeostasis and VLDL metabolism. However, few studies have evaluated the effect of testosterone on plasma lipid metabolism and concentrations, and the results are equivocal (1, 7, 12, 18, 30, 44, 55), most likely because many studies used synthetic androgen receptor agonists rather than testosterone per se, or used supraphysiological doses of testosterone (i.e., such as those given to female-to-male transsexuals), or evaluated the effects of testosterone therapy in men who already have high baseline testosterone availability. On the other hand, castration of male rats decreases plasma TG concentration, and testosterone replacement reverses this effect (47). Furthermore, women with hyperandrogenemia due to, e.g., the polycystic ovarian syndrome (PCOS) also have a more proatherogenic plasma lipid profile and increased plasma TG concentrations than healthy women; although PCOS is typically associated with body fat redistribution and central obesity and insulin resistance, i.e., factors that could affect plasma lipid concentrations, independent of testosterone availability (49, 56).
The purpose of our study, therefore, was to evaluate the effects of hyperandrogenemia on hepatic VLDL-TG and VLDL-apoB-100 secretion rates, the plasma lipid profile, and skeletal muscle protein synthesis rates in obese, premenopausal women. Our goal was to increase plasma testosterone availability, through transdermal testosterone delivery, to within the range observed in women with hyperandrogenemia due to PCOS (41, 49). Obese women were chosen as study subjects because, in clinical practice, hypertestosteronemia (i.e., PCOS) affects predominantly obese women (4, 42), and the metabolic abnormalities associated with hypertestosteronemia are more severe in obese than lean women (20, 42). Because testosterone is a potent skeletal muscle protein anabolic agent in men, but its effect on muscle protein turnover in women is unknown, we also measured the muscle protein fractional synthesis rate (FSR) before and after testosterone treatment in our women.
METHODS
Subjects.
Ten obese, premenopausal women (age: 34.1 ± 2.7 yr; body mass index: 36.4 ± 0.9 kg/m2; values are means ± SE) volunteered for the study. All subjects completed a comprehensive medical examination, including a detailed history and physical examination, a resting electrocardiogram, standard blood tests, and an oral glucose tolerance test. None of the subjects had evidence of chronic illness or were taking medications known to affect lipid or protein metabolism. All subjects were sedentary (<1.5 h of exercise/wk) and had regular menses. Written, informed consent was obtained from all subjects before participation in the study, which was approved by the Institutional Review Board and the Center for Applied Research Sciences Scientific Advisory Committee of Washington University School of Medicine in St. Louis.
Experimental design.
All subjects completed two lipid/protein metabolism studies: one before and one after testosterone administration for 3 wk (1.25 g of 1% AndroGel was applied transdermally after showering in the evening, which was expected to deliver ∼1,250 μg of testosterone daily, assuming an absorption rate of 10%). Before and on the last day of treatment, subjects were admitted to the Clinical Research Unit in the late afternoon, after having been instructed to abstain from exercise and alcohol consumption for 3 days. At 1900, they consumed a standard meal and then rested in bed and fasted (except water) until completion of the study the next day.
The following morning, at ∼0530, a catheter was inserted into a forearm vein to administer stable isotope labeled tracers, and a second catheter was inserted into a contralateral hand vein, which was heated to 55°C by using a thermostatically controlled box, to obtain arterialized blood samples. Blood samples were obtained immediately before the administration of stable isotope labeled tracers (all purchased from Cambridge Isotope Laboratories, Andover, MA) to determine plasma substrate and insulin concentrations, serum testosterone concentration, and background glycerol, palmitate, and leucine tracer-to-tracee ratios (TTR) in plasma and in VLDL-TG and VLDL-apoB-100, and α-ketoisocaproate (KIC) TTR in plasma. At 0700, a bolus of [1,1,2,3,3-2H5]glycerol (50 μmol/kg body wt), dissolved in 0.9% NaCl solution, was administered through the catheter in the forearm vein, followed by constant infusions of [2,2-2H2]palmitate (0.035 μmol·kg body wt−1·min−1), dissolved in 25% human albumin solution, and [5,5,5-2H3]leucine (0.12 μmol·kg body wt−1·min−1; prime: 7.2 μmol/kg body wt) dissolved in 0.9% NaCl solution, for 12 h. Blood samples were obtained at 5, 15, 30, 60, 90, and 120 min, and then every hour for 10 h to determine glycerol, palmitate, and leucine TTRs in plasma and in VLDL-TG and VLDL-apoB-100 and α-KIC TTR in plasma. Muscle biopsies were obtained from the vastus lateralis, during local anesthesia (2% lidocaine), at 60 min and 240 min after the start of the tracer infusion to determine leucine TTR in muscle protein and the muscle free leucine pool.
Abdominal subcutaneous and visceral adipose tissue volumes were determined by using magnetic resonance imaging, and intrahepatic TG content was determined by using magnetic resonance spectroscopy (3T Siemens Magnetom Trio scanner; Siemens, Erlanger, Germany) before and at the end of the treatment period.
Sample collection and storage.
Blood samples to determine serum testosterone concentration were collected in vaccutainers without additives. Blood samples to determine lipid concentrations and α-KIC, palmitate, glycerol, and leucine TTRs in plasma were collected in chilled tubes containing EDTA. To determine insulin and glucose concentrations in plasma, blood samples were collected in chilled tubes containing heparin. Blood samples were placed in an ice bath, and plasma was separated by centrifugation within 30 min of collection. Aliquots of plasma were kept in the refrigerator for immediate isolation of VLDL and measurement of plasma apoB-100 concentration; the remaining plasma samples were stored at −80°C until analyses were performed.
VLDL was isolated from plasma by ultracentrifugation (27, 34). Approximately 1.5 ml of plasma were transferred into OptiSeal polyallomer tubes (Beckman Instruments, Palo Alto, CA), overlaid with a NaCl/EDTA solution (1.006 g/ml), and centrifuged at 100,000 g for 16 h at 10°C in an Optima LE-80K preparative ultracentrifuge, equipped with a type 50.4 Ti rotor (Beckman Instruments). The top layer, containing VLDL, was removed by tube slicing (CentriTube slicer; Beckman Instruments). Aliquots of the VLDL fraction were used for measuring VLDL-apoB-100 concentration immediately after collection; the remaining samples were stored at −80°C until further processing and analyses.
Muscle tissue was rinsed in ice-cold saline immediately after collection, blotted dry, cleared of visible fat and connective tissue, transferred to sample storage tubes and submerged in liquid nitrogen, and then stored at −80°C until further processing and analyses.
Sample processing and analyses.
Plasma concentrations of total TG, total cholesterol, HDL-cholesterol, and LDL-cholesterol were measured by standard hospital laboratory methods using a Hitachi 917 autoanalyzer (Hitachi, Tokyo, Japan). Plasma lipoprotein particle concentrations (VLDL and LDL in nmol/l and HDL in μmol/l) and average lipoprotein sizes (particle diameter in nm) were determined by using proton nuclear magnetic resonance spectroscopy (LipoScience, Raleigh, NC), as previously described (25, 36, 37).
Plasma glucose concentration was determined on an automated glucose analyzer (YSI 2300 STAT plus; Yellow Springs Instrument, Yellow Springs, OH). Plasma insulin concentration was measured by using an automated chemoluminescent immunoanalyzer (IMMULITE, Siemens Healthcare Diagnostic, Los Angeles, CA). Plasma free fatty acid (FFA) concentrations were quantified by gas chromatography (Hewlett-Packard 5890-II, Palo Alto, CA) after adding heptadecanoic acid to plasma as an internal standard (40). Plasma apoB-100 and VLDL-apoB-100 concentrations were measured by using a turbidimetric immunoassay (Kamiya Biomedical, Seattle, WA). VLDL-TG concentration was measured by using a colorimetric enzymatic kit (Sigma-Aldrich, St. Louis, MO). Serum total and free testosterone concentrations were determined by liquid chromatography-tandem mass spectrometry at the Mayo Clinic (Rochester, MN). Plasma estradiol and progesterone concentrations were measured by using commercially available ELISA kits (Immuno-Biological Laboratories, Minneapolis, MN).
Plasma free glycerol, palmitate, leucine, and α-KIC TTRs, the TTRs of glycerol and palmitate in VLDL-TG, the TTRs of leucine in VLDL-apoB-100 and muscle proteins, and the TTR of leucine in the muscle free leucine pool were determined by using gas chromatography-mass spectrometry (MSD 5973 System, Hewlett-Packard), as previously described (19, 26–28, 31, 34, 38–40, 43). Plasma free palmitate and palmitate in VLDL-TG were analyzed as methylesters. The heptafluorobutyryl derivative was formed for the analysis of glycerol in plasma and VLDL-TG, the N-heptafluorobytyryl n-propyl ester derivative was used for leucine in VLDL-apoB-100 and muscle protein, the t-butyldimethylsilyl derivative was used for free leucine in plasma and muscle, and the O-t-butyldimethylsilyl quinoxalinol derivative was used for α-KIC in plasma. The concentration of leucine in plasma was determined by adding a known amount of norleucine to each sample.
Calculations.
The fractional turnover rates of VLDL-TG and VLDL-apoB-100 were determined by fitting the glycerol and leucine TTR time courses in plasma and in VLDL-TG and VLDL-apoB-100, respectively, to a multicompartmental model, as previously described (27, 34, 38). The hepatic secretion rates of VLDL-TG (μmol per liter of plasma per minute) and VLDL-apoB-100 (nmol per liter of plasma per minute) into plasma, which represent the amounts of VLDL-TG and VLDL-apoB-100 (i.e., VLDL particles) secreted by the liver per unit of plasma, were calculated by multiplying the fractional turnover rates of VLDL-TG and VLDL-apoB-100 (pools/min) by the concentrations of VLDL-TG (μmol/l) and VLDL-apoB-100 (nmol/l) in plasma, respectively (27, 34, 38). Palmitate and leucine rates of appearance (Ra) in plasma were calculated by dividing the palmitate and leucine tracer infusion rates by the average plasma palmitate and α-KIC TTR values between 60 and 240 min, respectively. Total FFA Ra in plasma was calculated by dividing palmitate Ra by the proportional contribution of palmitate to total plasma FFA concentration (32). The relative contribution of systemic plasma FFA and nonsystemic fatty acids to total VLDL-TG production was calculated on the basis of isotopic dilution by fitting the palmitate TTR in plasma and in VLDL-TG to a compartmental model (23, 28, 34). A dilution factor was derived, indicating the extent to which the plasma palmitate enrichment was diluted by unlabeled sources of palmitate before being incorporated into VLDL-TG. These unlabeled nonsystemic fatty acids in VLDL-TG are derived from pools of fatty acids that are not labeled with tracer during the infusion period, and include the following: 1) fatty acids released from preexisting, slowly turning over lipid stores in the liver and tissues draining directly into the portal vein; 2) fatty acids resulting from lipolysis of plasma lipoproteins that are taken up by the liver; and 3) fatty acids derived from hepatic de novo lipogenesis (21). The remaining fatty acids in VLDL-TG (systemic plasma FFA) represent FFA from the systemic circulation that are taken up by the liver and directly incorporated into VLDL-TG or temporarily incorporated into rapidly turning over intrahepatic and intraperitoneal TG stores before incorporation into VLDL-TG.
The FSR of muscle proteins was calculated based on the incorporation rate of [5,5,5-2H3]leucine into muscle proteins by using a standard precursor-product model as follows: FSR (%·h−1) = ΔEp/Eic × 1/t × 100, where ΔEp is the change in enrichment (TTR) of protein-bound leucine between two subsequent biopsies, Eic is the mean enrichment over time of the precursor used for protein synthesis (i.e., the mean muscle free leucine enrichment from the two muscle biopsies), and t is the time interval between biopsies (43, 54).
The homeostasis model assessment of insulin resistance score, a global index of whole body insulin resistance, was calculated by dividing the product of the plasma concentrations of insulin (in μIU/ml) and glucose (in mmol/l) by 22.5 (29).
Statistical analysis.
Statistical analyses were performed using SAS software, version 9.1 (SAS Institute, Cary, NC). For normally distributed variables, data are presented as means ± SE, and Student's paired t-test was used to compare results before and after testosterone treatment. For nonnormally distributed variables, data are presented as median and quartiles, and Wilcoxon's signed-rank test was used to compare results before and after testosterone treatment. A P value of ≤0.05 was considered statistically significant.
RESULTS
Body weight and body composition.
Body weight did not change during testosterone treatment (103.2 ± 3.6 and 103.1 ± 3.6 kg before and after treatment, respectively, P = 0.91). Likewise, there were no significant effects of testosterone treatment on intrahepatic TG content [1.7 (0.6, 5.4) and 1.3 (0.7, 3.7)% (median and lower and upper quartiles), before and after treatment, respectively; P = 0.25], abdominal visceral adipose tissue volume (1,261 ± 292 and 1,232 ± 285 cm3, respectively; P = 0.54), or abdominal subcutaneous adipose tissue volume (4,923 ± 250 and 4,922 ± 289 cm3, respectively; P = 0.99).
Hormone, glucose, and lipid concentrations and kinetics.
After 3 wk of treatment, serum total and free testosterone concentrations increased (P < 0.05) by approximately sevenfold (Table 1), toward the high end of the range for women with hyperandrogenemia/PCOS (41, 49), but still below the normal range for eugonadal men (13). Plasma estradiol, progesterone, insulin and glucose concentrations, and the homeostasis model assessment of insulin resistance score were not affected by testosterone treatment (Table 1). Likewise, there were no changes in the plasma concentrations of FFA, total and LDL-cholesterol, total TG and apoB-100, and VLDL and LDL particles (Tables 1 and 2), and the average sizes of VLDL and LDL particles (Table 2). There was a small (∼10%) but significant decrease in both total HDL particle and cholesterol concentrations (P < 0.04) after treatment (Tables 1 and 2) due to small and statistically not significant decreases in all three (large, medium, and small) HDL particle fractions so that the average size of HDL particles in the circulation was not affected by testosterone treatment (Table 2).
Table 1.
Plasma hormone, glucose, and lipid concentrations before and after 3 wk of transdermal testosterone treatment in obese, premenopausal women
| Before | After | P Value | |
|---|---|---|---|
| Free testosterone, pmol/l | 20.8 (17.4, 26.9) | 138.8 (100.6, 242.9) | 0.002 |
| Total testosterone, nmol/l | 0.83 (0.56, 1.10) | 5.43 (3.78, 8.14) | 0.002 |
| Progesterone, nmol/l | 2.23 (0.89, 15.65) | 1.30 (0.80, 1.90) | 0.38 |
| Estradiol, pmol/l | 239 ± 32 | 286 ± 57 | 0.44 |
| Glucose, mmol/l | 5.05 ± 0.09 | 5.03 ± 0.11 | 0.69 |
| Insulin, pmol/l | 67.8 ± 13.5 | 66.8 ± 5.4 | 0.91 |
| HOMA-IR score | 2.2 (1.9, 2.8) | 2.4 (2.0, 2.7) | 0.49 |
| Free fatty acids, μmol/l | 483 ± 45 | 537 ± 31 | 0.41 |
| Total cholesterol, mmol/l | 3.96 ± 0.23 | 3.47 ± 0.42 | 0.16 |
| HDL-cholesterol, mmol/l | 1.33 ± 0.07 | 1.20 ± 0.08 | 0.034 |
| LDL-cholesterol, mmol/l | 2.26 ± 0.20 | 2.15 ± 0.19 | 0.11 |
| Total TG, mmol/l | 1.01 ± 0.16 | 1.01 ± 0.24 | 0.99 |
| Total apoB-100, μmol/l | 1.10 ± 0.08 | 1.13 ± 0.14 | 0.78 |
Values are means ± SE or median (quartiles). HOMA-IR, homeostasis model assessment of insulin resistance; HDL, high-density lipoprotein; LDL, low-density lipoprotein; TG, triglyceride; apoB-100, apolipoprotein B-100.
Table 2.
Plasma lipoprotein particle concentrations and sizes before and after 3 wk of transdermal testosterone treatment in obese, premenopausal women
| Before | After | P Value | |
|---|---|---|---|
| VLDL particles, total, nmol/l | 52.2 ± 8.5 | 52.5 ± 11.5 | 0.96 |
| Large | 0.52 (0.10, 1.27) | 0.37 (0.22, 2.33) | 0.77 |
| Medium | 21.4 ± 3.9 | 20.0 ± 4.7 | 0.65 |
| Small | 28.9 ± 5.3 | 30.2 ± 6.3 | 0.69 |
| Average VLDL size, nm | 47.6 ± 2.2 | 49.6 ± 2.3 | 0.25 |
| LDL particles, nmol/l | 902 (745, 918) | 875 (830, 940) | 0.43 |
| Large | 430 ± 43 | 391 ± 43 | 0.17 |
| Small | 407 ± 71 | 446 ± 58 | 0.33 |
| Average LDL size, nm | 21.6 ± 0.2 | 21.4 ± 0.2 | 0.13 |
| HDL particles, μmol/l | 32.7 ± 1.3 | 30.1 ± 1.0 | 0.03 |
| Large | 7.5 ± 1.2 | 6.7 ± 1.1 | 0.19 |
| Medium | 4.7 ± 1.3 | 4.4 ± 1.1 | 0.80 |
| Small | 20.1 (16.5, 23.3) | 18.0 (16.9, 19.0) | 0.23 |
| Average HDL size, nm | 9.0 ± 0.1 | 9.0 ± 0.1 | 0.37 |
Values are means ± SE or median (quartiles). VLDL, very low-density lipoprotein.
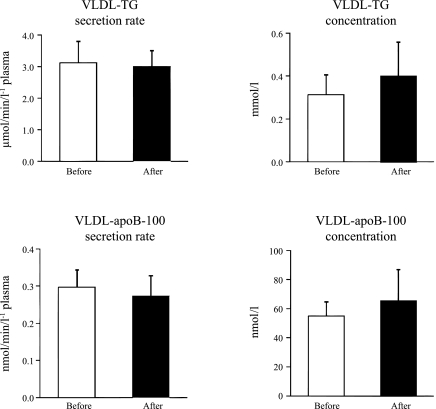
There were no significant changes in hepatic VLDL-TG and VLDL-apoB-100 secretion rates and VLDL-TG and VLDL-apoB-100 concentrations after testosterone treatment (Fig. 1). The relative contribution of systemic plasma FFA and nonsystemic fatty acid sources to total VLDL-TG production was also not affected by testosterone: systemic plasma FFA contributed 66 ± 5% before and 69 ± 4% after testosterone treatment (P = 0.57). Total FFA Ra in plasma was also not significantly different before and after testosterone treatment (436 ± 34 and 456 ± 34 μmol/min, respectively; P = 0.65).
Fig. 1.
Very-low-density lipoprotein (VLDL)-triglyceride (TG; top) and VLDL-apolipoprotein B-100 (apoB-100; bottom) secretion rates in plasma (left) and concentrations in plasma (right) before and after 3 wk of transdermal testosterone treatment in obese, premenopausal women. Values are means ± SE.
Plasma leucine concentration and muscle protein FSR.
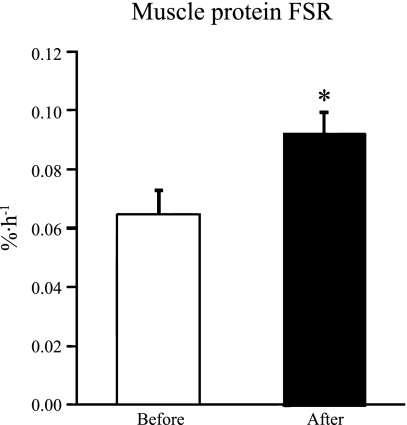
Testosterone treatment had no effect on plasma leucine concentration (from 115 ± 6 to 115 ± 6 μmol/l, P = 0.83) or leucine Ra (from 146 ± 6 to 149 ± 8 μmol/min, P = 0.71), but increased the muscle protein FSR by ∼45% (P < 0.001, Fig. 2).
Fig. 2.
Skeletal muscle protein fractional synthesis rate (FSR) during basal, postabsorptive conditions before and after 3 wk of transdermal testosterone treatment in obese, premenopausal women. Values are means ± SE. *Significantly different from value before testosterone treatment (P < 0.001).
DISCUSSION
We evaluated the effects of transdermal testosterone administration for 3 wk on plasma FFA and VLDL-TG and VLDL-apoB-100 release into plasma, plasma lipid concentrations, and muscle protein synthesis in obese, premenopausal women. Testosterone treatment resulted in plasma testosterone concentrations that were approximately seven times greater than before treatment, but comparable to those in women with hyperandrogenemia due to PCOS (41, 49), although lower than the normal range for eugonadal men (13). The approximately sevenfold increase in testosterone availability did not affect plasma FFA availability, hepatic VLDL-TG and VLDL-apoB-100 secretion rates, or plasma lipoprotein particle concentrations and particle sizes, except for a small (∼10%) decrease in HDL particle and cholesterol concentrations, but robustly increased the muscle protein FSR. These findings indicate that testosterone is a potent skeletal muscle protein anabolic agent, but not an important regulator of plasma lipid kinetics; hyperandrogenemia is, therefore, most likely not responsible for the greater plasma TG concentration in men (24) and women with hyperandrogenemia/PCOS (49, 56) compared with healthy premenopausal women.
Our findings are consistent with the results from studies conducted by other investigators who demonstrated that testosterone treatment in older and hypogonadal men has no effect on hepatic VLDL-apoB-100 secretion (12) and plasma TG concentrations (1, 12, 44, 55). Likewise, no changes or only small decreases (∼5 mg/dl) in plasma HDL-cholesterol and small increases in LDL-cholesterol concentrations have been observed after testosterone treatment in these studies (1, 12, 17, 44, 55). On the other hand, our results are at odds with those from studies that administered the synthetic androgen receptor agonist methandrostenolone orally to premenopausal women (18) and studies in which testosterone was given intramuscularly in female-to-male transsexuals (7, 30), which resulted in a marked increase in hepatic TG secretion and plasma TG concentration and a marked (∼20%) reduction in plasma HDL-cholesterol concentration. However, these findings are likely confounded by the changes in body weight and body composition and insulin sensitivity that accompany long-term, high-dose androgen therapy (7, 30), or could potentially be due to unknown side effects of methandrostenolone or due to a hepatic “first-pass” phenomenon, similar to that seen with oral vs. transdermal estrogen preparations (46, 51, 53). Furthermore, orally administered androgens are known to be hepatotoxic and may promote hepatic steatosis (5), which is known to be associated with dyslipidemia and increased VLDL-TG secretion (8). The absence of changes in plasma lipid concentrations and kinetics in response to increased plasma testosterone availability within the normal physiological range in obese, premenopausal women (present study) and older and hypogonadal men (1, 12, 44, 55) suggests that testosterone is not a major regulator of plasma lipid kinetics and homeostasis.
Our study has several limitations. First, we studied our women before and after only 3 wk of testosterone treatment to avoid potential adverse side effects (e.g., masculinization). It is possible that more prolonged treatment would have led to different results. However, these would be difficult to interpret, because long-term testosterone replacement therapy is accompanied by changes in body composition, body fat distribution, and insulin sensitivity (2), which can independently affect lipid metabolism and kinetics (3, 34, 35). Furthermore, we did not include a control group receiving placebo in our study. However, it is unlikely that this affected the conclusions from our study, because our laboratory has recently demonstrated that VLDL-TG and VLDL-apoB-100 metabolism and concentrations in no-intervention control subjects do not change significantly over a 1- to 3-mo time period (22, 27).
It is also unlikely that we failed to observe a significant effect of testosterone treatment in our study due to a statistical type 2 error. Based on the reproducibility of VLDL-TG and VLDL-apoB-100 kinetic and concentration measurements in our laboratory (22, 27), our study was adequately powered (80%) to detect significant changes in VLDL-apoB-100 and VLDL-TG secretion rates in the order of 15–20%, if they existed. Moreover, the robust increase in muscle protein synthesis we observed in this study strongly suggests that failure to observe significant testosterone-induced alterations in plasma lipid homeostasis is not an artifact related to our experimental design. In fact, it indicates that women are very sensitive to the effects of testosterone. Testosterone-induced increases in muscle protein FSR have previously been described in healthy or hypogondal young and old men or hypogonadal men with myotonic dystrophy (11, 14, 15, 48) during the active muscle growth phase, which occurs during the first 6 mo of treatment; continued testosterone therapy helps maintain the newly gained muscle mass, but does not result in a further increase in muscle mass (45, 52, 57). Accordingly, studies that measured the muscle protein FSR after 6 or 12 mo of testosterone therapy found that it was not different compared with baseline in healthy or hypogonadal older men, but the absolute rate of muscle protein synthesis (FSR × muscle protein pool size) was greater due to the greater muscle mass (9, 10, 16). However, the doses used in these studies were much greater (∼100–300 mg/wk intramuscularly or 5 mg/day transdermally) than the dose given to our women.
In summary, we found that testosterone administration for 3 wk in obese but otherwise healthy premenopausal women increased the skeletal muscle protein synthesis rate, but did not affect plasma lipid kinetics and concentrations. It is, therefore, unlikely that sexual dimorphism in the plasma lipid profile (23, 25, 33, 53) and the alterations in plasma TG concentration associated with hyperandrogenemia (49, 56) are mediated by testosterone.
GRANTS
This publication was made possible by National Institutes of Health Grants HD 57796, AG 31297, DK 56341 (Nutrition and Obesity Research Center), RR024992 (Washington University School of Medicine Clinical Translational Science Award), and RR-00954 (Biomedical Mass Spectrometry Resource).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
X.W., G.I.S., and J.K. performed experiments; X.W., B.W.P., and F.M. analyzed data; X.W., F.M., and B.M. interpreted results of experiments; X.W. prepared figures; X.W. drafted manuscript; X.W., G.I.S., B.W.P., D.N.R., J.K., F.M., and B.M. approved final version of manuscript; G.I.S., B.W.P., D.N.R., J.K., F.M., and B.M. edited and revised manuscript; B.M. conception and design of research.
ACKNOWLEDGMENTS
The authors thank Freida Custodio, Jennifer Shew, and Dr. Adewole Okunade for technical assistance, the staff of the Clinical Research Unit for help in performing the studies, and the study subjects for participation.
REFERENCES
- 1. Agledahl I, Hansen JB, Svartberg J. Impact of testosterone treatment on postprandial triglyceride metabolism in elderly men with subnormal testosterone levels. Scand J Clin Lab Invest 68: 641–648, 2008 [DOI] [PubMed] [Google Scholar]
- 2. Bhasin S. Effects of testosterone administration on fat distribution, insulin sensitivity, and atherosclerosis progression. Clin Infect Dis 37, Suppl 2: S142–S149, 2003 [DOI] [PubMed] [Google Scholar]
- 3. Bioletto S, Golay A, Munger R, Kalix B, James RW. Acute hyperinsulinemia and very-low-density and low-density lipoprotein subfractions in obese subjects1. Am J Clin Nutr 71: 443–449, 2000 [DOI] [PubMed] [Google Scholar]
- 4. Ciampelli M, Lanzone A. Insulin and polycystic ovary syndrome: a new look at an old subject. Gynecol Endocrinol 12: 277–292, 1998 [DOI] [PubMed] [Google Scholar]
- 5. Conway AJ, Handelsman DJ, Lording DW, Stuckey B, Zajac JD. Use, misuse and abuse of androgens. The Endocrine Society of Australia consensus guidelines for androgen prescribing. Med J Aust 172: 220–224, 2000 [DOI] [PubMed] [Google Scholar]
- 6. Derby CA, Crawford SL, Pasternak RC, Sowers M, Sternfeld B, Matthews KA. Lipid changes during the menopause transition in relation to age and weight: the Study of Women's Health Across the Nation. Am J Epidemiol 169: 1352–1361, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Elbers JM, Giltay EJ, Teerlink T, Scheffer PG, Asscheman H, Seidell JC, Gooren LJ. Effects of sex steroids on components of the insulin resistance syndrome in transsexual subjects. Clin Endocrinol (Oxf) 58: 562–571, 2003 [DOI] [PubMed] [Google Scholar]
- 8. Fabbrini E, Magkos F, Mohammed BS, Pietka T, Abumrad NA, Patterson BW, Okunade A, Klein S. Intrahepatic fat, not visceral fat, is linked with metabolic complications of obesity. Proc Natl Acad Sci U S A 106: 15430–15435, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ferrando AA, Sheffield-Moore M, Paddon-Jones D, Wolfe RR, Urban RJ. Differential anabolic effects of testosterone and amino acid feeding in older men. J Clin Endocrinol Metab 88: 358–362, 2003 [DOI] [PubMed] [Google Scholar]
- 10. Ferrando AA, Sheffield-Moore M, Yeckel CW, Gilkison C, Jiang J, Achacosa A, Lieberman SA, Tipton K, Wolfe RR, Urban RJ. Testosterone administration to older men improves muscle function: molecular and physiological mechanisms. Am J Physiol Endocrinol Metab 282: E601–E607, 2002 [DOI] [PubMed] [Google Scholar]
- 11. Ferrando AA, Tipton KD, Doyle D, Phillips SM, Cortiella J, Wolfe RR. Testosterone injection stimulates net protein synthesis but not tissue amino acid transport. Am J Physiol Endocrinol Metab 275: E864–E871, 1998 [DOI] [PubMed] [Google Scholar]
- 12. Giannoulis MG, Jackson N, Shojaee-Moradie F, Sonksen PH, Martin FC, Umpleby AM. Effects of growth hormone and/or testosterone on very low density lipoprotein apolipoprotein B100 kinetics and plasma lipids in healthy elderly men: a randomised controlled trial. Growth Horm IGF Res 16: 308–317, 2006 [DOI] [PubMed] [Google Scholar]
- 13. Goncharov NP, Katsya GV, Chagina NA, Gooren LJ. Testosterone and obesity in men under the age of 40 years. Andrologia 41: 76–83, 2009 [DOI] [PubMed] [Google Scholar]
- 14. Griggs RC, Halliday D, Kingston W, Moxley RT., 3rd Effect of testosterone on muscle protein synthesis in myotonic dystrophy. Ann Neurol 20: 590–596, 1986 [DOI] [PubMed] [Google Scholar]
- 15. Griggs RC, Kingston W, Jozefowicz RF, Herr BE, Forbes G, Halliday D. Effect of testosterone on muscle mass and muscle protein synthesis. J Appl Physiol 66: 498–503, 1989 [DOI] [PubMed] [Google Scholar]
- 16. Henderson GC, Dhatariya K, Ford GC, Klaus KA, Basu R, Rizza RA, Jensen MD, Khosla S, O'Brien P, Nair KS. Higher muscle protein synthesis in women than men across the lifespan, and failure of androgen administration to amend age-related decrements. FASEB J 23: 631–641, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Isidori AM, Giannetta E, Greco EA, Gianfrilli D, Bonifacio V, Isidori A, Lenzi A, Fabbri A. Effects of testosterone on body composition, bone metabolism and serum lipid profile in middle-aged men: a meta-analysis. Clin Endocrinol (Oxf) 63: 280–293, 2005 [DOI] [PubMed] [Google Scholar]
- 18. Kissebah AH, Harrigan P, Adams PW, Wynn V. Effect of methandienone on free fatty acid and triglyceride turnover in normal females. Horm Metab Res 5: 275–279, 1973 [DOI] [PubMed] [Google Scholar]
- 19. Langenbeck U, Luthe H, Schaper G. Keto acids in tissues and biological fluids: O-t-butyldimethylsilyl quinoxalinols as derivatives for sensitive gas chromatographic/mass spectrometric determination. Biomed Mass Spectrom 12: 507–509, 1985 [DOI] [PubMed] [Google Scholar]
- 20. Legro RS, Kunselman AR, Dunaif A. Prevalence and predictors of dyslipidemia in women with polycystic ovary syndrome. Am J Med 111: 607–613, 2001 [DOI] [PubMed] [Google Scholar]
- 21. Lewis GF. Fatty acid regulation of very low density lipoprotein production. Curr Opin Lipidol 8: 146–153, 1997 [DOI] [PubMed] [Google Scholar]
- 22. Magkos F, Fabbrini E, Korenblat K, Okunade AL, Patterson BW, Klein S. Reproducibility of glucose, fatty acid and VLDL kinetics and multi-organ insulin sensitivity in obese subjects with non-alcoholic fatty liver disease. Int J Obes (Lond) 35: 1233–1240, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Magkos F, Fabbrini E, Mohammed BS, Patterson BW, Klein S, Mittendorfer B. Estrogen deficiency after menopause does not result in male very-low-density lipoprotein metabolism phenotype. J Clin Endocrinol Metab 95: 3377–3384, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Magkos F, Mittendorfer B. Gender differences in lipid metabolism and the effect of obesity. Obstet Gynecol Clin North Am 36: 245–265, 2009 [DOI] [PubMed] [Google Scholar]
- 25. Magkos F, Mohammed BS, Mittendorfer B. Effect of obesity on the plasma lipoprotein subclass profile in normoglycemic and normolipidemic men and women. Int J Obes 32: 1655–1664, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Magkos F, Patterson BW, Mittendorfer B. No effect of menstrual cycle phase on basal very-low-density lipoprotein triglyceride and apolipoprotein B-100 kinetics. Am J Physiol Endocrinol Metab 291: E1243–E1249, 2006 [DOI] [PubMed] [Google Scholar]
- 27. Magkos F, Patterson BW, Mittendorfer B. Reproducibility of stable isotope-labeled tracer measures of VLDL-triglyceride and VLDL-apolipoprotein B-100 kinetics. J Lipid Res 48: 1204–1211, 2007 [DOI] [PubMed] [Google Scholar]
- 28. Magkos F, Patterson BW, Mohammed BS, Klein S, Mittendorfer B. Women produce fewer but triglyceride-richer very low-density lipoproteins than men. J Clin Endocrinol Metab 92: 1311–1318, 2007 [DOI] [PubMed] [Google Scholar]
- 29. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28: 412–419, 1985 [DOI] [PubMed] [Google Scholar]
- 30. Meyer WJ, 3rd, Webb A, Stuart CA, Finkelstein JW, Lawrence B, Walker PA. Physical and hormonal evaluation of transsexual patients: a longitudinal study. Arch Sex Behav 15: 121–138, 1986 [DOI] [PubMed] [Google Scholar]
- 31. Mittendorfer B, Andersen JL, Plomgaard P, Saltin B, Babraj JA, Smith K, Rennie MJ. Protein synthesis rates in human muscles: neither anatomical location nor fibre-type composition are major determinants. J Physiol 563: 203–211, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mittendorfer B, Liem O, Patterson BW, Miles JM, Klein S. What does the measurement of whole-body fatty acid rate of appearance in plasma by using a fatty acid tracer really mean? Diabetes 52: 1641–1648, 2003 [DOI] [PubMed] [Google Scholar]
- 33. Mittendorfer B, Patterson BW, Klein S. Effect of sex and obesity on basal VLDL-triacylglycerol kinetics. Am J Clin Nutr 77: 573–579, 2003 [DOI] [PubMed] [Google Scholar]
- 34. Mittendorfer B, Patterson BW, Klein S. Effect of weight loss on VLDL-triglyceride and apoB-100 kinetics in women with abdominal obesity. Am J Physiol Endocrinol Metab 284: E549–E556, 2003 [DOI] [PubMed] [Google Scholar]
- 35. Ng TWK, Watts GF, Barrett PHR, Rye KA, Chan DC. Effect of weight loss on LDL and HDL kinetics in the metabolic syndrome. Diabetes Care 30: 2945–2950, 2007 [DOI] [PubMed] [Google Scholar]
- 36. Otvos J, Jeyarajah E, Bennett D, Krauss R. Development of a proton nuclear magnetic resonance spectroscopic method for determining plasma lipoprotein concentrations and subspecies distributions from a single, rapid measurement. Clin Chem 38: 1632–1638, 1992 [PubMed] [Google Scholar]
- 37. Otvos JD, Jeyarajah EJ, Bennett DW. Quantification of plasma lipoproteins by proton nuclear magnetic resonance spectroscopy. Clin Chem 37: 377–386, 1991 [PubMed] [Google Scholar]
- 38. Patterson BW, Mittendorfer B, Elias N, Satyanarayana R, Klein S. Use of stable isotopically labeled tracers to measure very low density lipoprotein-triglyceride turnover. J Lipid Res 43: 223–233, 2002 [PubMed] [Google Scholar]
- 39. Patterson BW, Zhang XJ, Chen Y, Klein S, Wolfe RR. Measurement of very low stable isotope enrichments by gas chromatography/mass spectrometry: application to measurement of muscle protein synthesis. Metabolism 46: 943–948, 1997 [DOI] [PubMed] [Google Scholar]
- 40. Patterson BW, Zhao G, Elias N, Hachey DL, Klein S. Validation of a new procedure to determine plasma fatty acid concentration and isotopic enrichment. J Lipid Res 40: 2118–2124, 1999 [PubMed] [Google Scholar]
- 41. Phelan N, O'Connor A, Kyaw-Tun T, Correia N, Boran G, Roche HM, Gibney J. Lipoprotein subclass patterns in women with polycystic ovary syndrome (PCOS) compared with equally insulin-resistant women without PCOS. J Clin Endocrinol Metab 95: 3933–3939, 2010 [DOI] [PubMed] [Google Scholar]
- 42. Salehi M, Bravo-Vera R, Sheikh A, Gouller A, Poretsky L. Pathogenesis of polycystic ovary syndrome: what is the role of obesity? Metabolism 53: 358–376, 2004 [DOI] [PubMed] [Google Scholar]
- 43. Smith GI, Villareal DT, Mittendorfer B. Measurement of human mixed muscle protein fractional synthesis rate depends on the choice of amino acid tracer. Am J Physiol Endocrinol Metab 293: E666–E671, 2007 [DOI] [PubMed] [Google Scholar]
- 44. Snyder PJ, Peachey H, Berlin JA, Hannoush P, Haddad G, Dlewati A, Santanna J, Loh L, Lenrow DA, Holmes JH, Kapoor SC, Atkinson LE, Strom BL. Effects of testosterone replacement in hypogonadal men. J Clin Endocrinol Metab 85: 2670–2677, 2000 [DOI] [PubMed] [Google Scholar]
- 45. Snyder PJ, Peachey H, Berlin JA, Hannoush P, Haddad G, Dlewati A, Santanna J, Loh L, Lenrow DA, Holmes JH, Kapoor SC, Atkinson LE, Strom BL. Effects of testosterone replacement in hypogonadal men. J Clin Endocrinol Metab 85: 2670–2677, 2000 [DOI] [PubMed] [Google Scholar]
- 46. Stevenson JC. Type and route of estrogen administration. Climacteric 12, Suppl 1: 86–90, 2009 [DOI] [PubMed] [Google Scholar]
- 47. Takeuchi N, Go S, Murase M, Nomura Y, Takase H, Uchida K. Effects of castration and testosterone administration on serum lipoproteins and their apoproteins in male spontaneously hypertensive rat. Endocrinology 118: 1787–1794, 1986 [DOI] [PubMed] [Google Scholar]
- 48. Urban RJ, Bodenburg YH, Gilkison C, Foxworth J, Coggan AR, Wolfe RR, Ferrando A. Testosterone administration to elderly men increases skeletal muscle strength and protein synthesis. Am J Physiol Endocrinol Metab 269: E820–E826, 1995 [DOI] [PubMed] [Google Scholar]
- 49. Valkenburg O, Steegers-Theunissen RPM, Smedts HPM, Dallinga-Thie GM, Fauser BCJM, Westerveld EH, Laven JSE. A more atherogenic serum lipoprotein profile is present in women with polycystic ovary syndrome: a case-control study. J Clin Endocrinol Metab 93: 470–476, 2008 [DOI] [PubMed] [Google Scholar]
- 50. Verhoeven MO, van der Mooren MJ, Teerlink T, Verheijen RHM, Scheffer PG, Kenemans P. The influence of physiological and surgical menopause on coronary heart disease risk markers. Menopause 16: 37–49, 2009 [DOI] [PubMed] [Google Scholar]
- 51. Vongpatanasin W, Tuncel M, Wang Z, Arbique D, Mehrad B, Jialal I. Differential effects of oral versus transdermal estrogen replacement therapy on C-reactive protein in postmenopausal women. J Am Coll Cardiol 41: 1358–1363, 2003 [DOI] [PubMed] [Google Scholar]
- 52. Wang C, Cunningham G, Dobs A, Iranmanesh A, Matsumoto AM, Snyder PJ, Weber T, Berman N, Hull L, Swerdloff RS. Long-term testosterone gel (AndroGel) treatment maintains beneficial effects on sexual function and mood, lean and fat mass, and bone mineral density in hypogonadal men. J Clin Endocrinol Metab 89: 2085–2098, 2004 [DOI] [PubMed] [Google Scholar]
- 53. Wang X, Magkos F, Mittendorfer B. Sex differences in lipid and lipoprotein metabolism: it's not just about sex hormones. J Clin Endocrinol Metab 96: 885–893, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Watt PW, Lindsay Y, Scrimgeour CM, Chien PA, Gibson JN, Taylor DJ, Rennie MJ. Isolation of aminoacyl-tRNA and its labeling with stable-isotope tracers: use in studies of human tissue protein synthesis. Proc Natl Acad Sci U S A 88: 5892–5896, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Whitsel EA, Boyko EJ, Matsumoto AM, Anawalt BD, Siscovick DS. Intramuscular testosterone esters and plasma lipids in hypogonadal men: a meta-analysis. Am J Med 111: 261–269, 2001 [DOI] [PubMed] [Google Scholar]
- 56. Wild RA, Rizzo M, Clifton S, Carmina E. Lipid levels in polycystic ovary syndrome: systematic review and meta-analysis. Fertil Steril 95: 1073–1079, e1071–e1011, 2011 [DOI] [PubMed] [Google Scholar]
- 57. Wittert GA, Chapman IM, Haren MT, Mackintosh S, Coates P, Morley JE. Oral testosterone supplementation increases muscle and decreases fat mass in healthy elderly males with low-normal gonadal status. J Gerontol A Biol Sci Med Sci 58: 618–625, 2003 [DOI] [PubMed] [Google Scholar]