Abstract
Orogastric tube feeding is indicated for neonates with impaired ability to ingest and can be administered by intermittent bolus or continuous schedule. Our aim was to determine whether feeding modalities affect muscle protein deposition and to identify mechanisms involved. Neonatal pigs were overnight fasted (FAS) or fed the same amount of food continuously (CON) or intermittently (INT; 7 × 4 h meals) for 29 h. For 8 h, between hours 20 and 28, pigs were infused with [2H5]phenylalanine and [2H2]tyrosine, and amino acid (AA) net balances were measured across the hindquarters. Insulin, branched-chain AA, phenylalanine, and tyrosine arterial concentrations and whole body phenylalanine and tyrosine fluxes were greater for INT after the meal than for CON or FAS. The activation of signaling proteins leading to initiation of mRNA translation, including eukaryotic initiation factor (eIF)4E·eIF4G complex formation in muscle, was enhanced by INT compared with CON feeding or FAS. Signaling proteins of protein degradation were not affected by feeding modalities except for microtubule-associated protein light chain 3-II, which was highest in the FAS. Across the hindquarters, AA net removal increased for INT but not for CON or FAS, with protein deposition greater for INT. This was because protein synthesis increased following feeding for INT but remained unchanged for CON and FAS, whereas there was no change in protein degradation across any dietary treatment. These results suggest that muscle protein accretion in neonates is enhanced with intermittent bolus to a greater extent than continuous feeding, mainly by increased protein synthesis.
Keywords: protein degradation, amino acid, protein turnover, stable isotope, translation initiation
orogastric tube feeding is indicated in neonates with impaired ability for normal ingestion and can be administered by an intermittent bolus or continuous schedule. Evidence suggests that feeding frequency can affect feeding outcome, but whether intermittent or continuous feeding is more beneficial remains inconclusive (48). Intermittent feeding is considered more physiological on the basis of a more natural feed-fast cycle and promotes gastrointestinal hormone secretions (3, 41). Intermittent feeding also stimulates intestinal growth and development in newborn piglets by increasing mucosal and intestinal protein mass compared with continuous feeding (54). Most comparisons between intermittent and continuous feeding have focused on clinical outcomes, including days to full feed, time to regain birth weight, time to discharge, feeding intolerance, and weight gain (22, 53), but with little attention given to macronutrient retention (55). Although intermittent feeding may increase diet-induced thermogenesis compared with continuous feeding (30), it remains unclear whether this also involves greater amino acid oxidation.
High rates of protein turnover and deposition are characteristics of growth during the neonatal period (17, 19, 29, 38). Studies in pigs and rats have shown that neonates are efficient at depositing ingested amino acids into proteins, although this efficiency decreases with age (7, 8, 10, 15, 16). Such responses to feeding are common in all growing animals, including humans (21). Accordingly, we have used the piglet as a model for the human neonate to study early postnatal muscle growth and metabolism.
It is well recognized that insulin plays an important role in protein accretion in skeletal muscle of the neonate (11). Although the insulin-specific effect on muscle continues to play a role in protein deposition during growth, the sensitivity greatly diminishes with age (68). In contrast, amino acids exert a positive effect on muscle protein synthesis throughout life (11, 21, 64). The signaling pathways for both insulin and amino acids are upregulated in muscle during early life. Although the postprandial rise in insulin and the subsequent activation of the insulin signaling cascade is well defined, amino acid signaling upstream of the translation initiation pathway is less well understood.
Insulin, through protein kinase B (PKB), and amino acids independently activate mammalian target of rapamycin (mTOR), which induces an increase in phosphorylation of 4E-binding protein 1 (4E-BP1) and ribosomal protein S6K1 (S6K1) (39, 56). The phosphorylation of 4E-BP1 results in dissociation from eukaryotic initiation factor 4E (eIF4E) and subsequent formation of the eIF4E·eIF4G complex, a key component in the regulation of mRNA binding to the 43S preinitiation complex (39, 56). We have shown previously that the fractional rate of protein synthesis increases concomitantly with the rise in insulin and amino acids following a complete meal in the neonate (67). Consequently, protein synthesis has both a short latency and a transient response, returning to fasting levels between 2 and 4 h after a meal.
The ubiquitin-proteasome and autophagy-lysosome systems are thought to be the two most important proteolytic pathways in skeletal muscle (52, 63). High rates of proteolysis are necessary to provide amino acids for ongoing tissue modeling and rapid growth (29, 38, 47) as well as to provide amino acids to other organs when dietary supplies are insufficient (63). Acute parenteral amino acid infusion reduces whole body proteolysis in neonates, but this suppression is lost after prolonged (>24 h) treatment (32, 45). Although pharmacological levels of insulin decrease whole body proteolysis in neonates (1, 47), concentrations within the physiological range appear to exert little effect on protein catabolism (20, 31).
Recently, we showed that protein synthesis, measured 90 min following a meal, was enhanced by intermittent bolus compared with continuous feeding (28). However, whether feeding frequency affects protein degradation and protein deposition was not determined. This study examined how feeding frequency affects amino acid and protein metabolism in both muscle and the whole body of neonates over a 4-h complete feeding cycle 24 h after initiation of intermittent bolus or continuous feeding. The objectives of this study were to 1) compare the effect of continuous vs. intermittent bolus feeding on amino acid net balance across the hindquarters of the neonatal pig, 2) determine whether any difference in protein deposition between continuous and intermittent bolus feeding is due to altered protein synthesis and/or degradation, and 3) identify the signaling mechanisms by which feeding frequency modulates protein deposition. We hypothesized that intermittent feeding increases muscle protein synthesis, whereas it decreases protein degradation compared with continuous feeding in neonates.
MATERIALS AND METHODS
Animals and surgeries.
Pigs (n = 6/group) 2–3 days of age and weighing ∼2 kg were surgically fitted under general anesthesia using sterile techniques, with indwelling catheters in the carotid artery, jugular vein, and inferior vena cava, and a blood flow probe was placed around the caudal aorta. Pigs were allowed to recover for 3 days postsurgery and studied at 9 days of age weighing ∼2.5 kg. The Animal Care and Use Committee of Baylor College of Medicine approved all experimental procedures. This study was conducted in accordance with the National Research Council's Guide for the Care and Use of Laboratory Animals.
Diet.
Throughout the preexperimental and recovery periods, pigs were allowed free access to sow milk replacer (Soweena Litter Life; Merrik's, Middleton, WI). Following recovery, pigs were divided into three groups; two were fed the same amount of sow milk replacer (240 ml·kg−1·day−1; 10 g CP·kg−1·day−1 and 175 kcal·kg−1·day−1) either continuously (CON; 10 ml·kg−1·h−1) or intermittently (INT; 7 equal meals given over 15 min at 4-h intervals, 40 ml·kg−1·bolus−1) for 29 h (Table 1). The milk replacer was supplied via an orogastric feeding tube. The third group was fasted overnight (FAS).
Table 1.
Ingredients and nutrient composition of the experimental diet
| Ingredient | g/kg As Fed |
|---|---|
| Whey protein concentrate (80% CP)* | 50.3 |
| Lactose | 9.0 |
| FatPak 80† | 65.4 |
| Water | 862.4 |
| Xanthan gum | 2.0 |
| Vitamin premix‡ | 2.0 |
| Mineral premix‡ | 9.0 |
Hilmar Ingredients, Hilmar, CA.
Milk Specialties Global Animal Nutrition, Carpentersville, IL.
Dyets, Bethlehem, PA. Vitamin premix provided (in g/kg): thiamine HCl, 0.1; riboflavin, 0.375; pyridoxine HCl, 0.1; niacin, 1; calcium pantothenate, 1.2; folic acid, 0.13; biotin, 0.02; cobalamin B12, 1.5; retinyl palmitate, 0.8; cholecalciferol, 0.05; tocopheryl acetate, 8.8; menadione sodium bisulfite, 0.08. Trace mineral premix provided (in g/kg): calcium phosphate dibasic, 187; calcium carbonate, 279; sodium chloride, 85; potassium phosphate monobasic, 155; magnesium sulfate anhydrous, 44; manganous carbonate, 0.93; ferric citrate, 10; zinc carbonate, 1.84; cupric carbonate, 0.193; potassium iodate, 0.005; sodium selenite, 0.007.
Tracer infusion and blood sampling.
To assess amino acid kinetics, primed continuous infusions of [2H5]phenylalanine and [2H2]tyrosine (Cambridge Isotope Laboratories, Andover, MA) were given (each primed at 10 ml/kg followed by infusion at 10 μmol·kg−1·h−1) into the jugular vein for 8 h between 20 and 28 h of the 29-h feeding period (CON and INT) or the last 8 h of the fasting period (FAS). During the final 4 h of the tracer infusion, blood samples were taken every 15 min from 0 to 90 min and every 30 min thereafter (Fig. 1). Blood flow was measured using ultrasonic flow probes (Transonic Systems, Ithaca, NY). The concentration and enrichment of amino acids were determined gravimetrically using [U-13C]- and [15N]amino acids added to plasma samples. Plasma samples were stored at −20°C until analysis. N-heptafluorobutyryl derivatives of amino acids were prepared by esterification with n-propanol, followed by derivatization with heptafluorobutyric anhydride (43). Amino acid enrichment and concentration were measured by gas chromatography-mass spectrometry (GC 6890 with 5973 mass selective detector; Agilent Technologies, Wilmington, DE) under negative chemical ionization.
Fig. 1.
Schematic representation of tracer infusions and blood sampling in 10-day-old pigs. Feeding was initiated after overnight fasting and continued for 29 h for intermittently and continuously fed pigs. Tracer infusion was initiated after overnight fasting for the fasted overnight group. At 20 h, primed continuous infusions of [2H5]phenylalanine ([2H5]Phe; 10 μmol/kg at 10 μmol·kg−1·h−1) and [2H2]tyrosine ([2H2]Tyr; 10 μmol/kg at 10 μmol·kg−1·h−1) were given (8 h) in the jugular vein and blood sampled during the last 4 h of the tracer infusion. Arrows indicate collection of blood samples.
Hindquarters mass balance.
All calculations assume that there is no change in the size of the muscle free phenylalanine pool during treatments. Because phenylalanine is neither catabolized nor synthesized in muscle, then phenylalanine net removal by the hindquarters occurs when protein synthesis exceeds protein degradation, with the converse true if there is net release. Thus
where [A] is arterial and [V] is venous amino acid concentration (μmol/ml) and PF is plasma flow (ml/h). Similarly, phenylalanine tracer kinetics were calculated using the following equations:
 |
where [A[2H5]Phe] and [V[2H5]Phe] are the arterial and venous [2H5]phenylalanine tracer concentrations and [Aphe] is the phenylalanine tracee arterial concentration.
Systemic phenylalanine flux.
Whole body flux of phenylalanine and tyrosine (μmol·kg−1·ml−1) was calculated according to the Steele equation (57), as modified by Proietto et al. (49) for non-steady-state conditions (5, 6):
where IR is the infusion rate of the [2H5]phenylalanine or [2H2]tyrosine tracers (μmol·kg−1·h−1), p is the pool fraction for instant mixing (p = 0.25), V is the volume of distribution (V = 0.5 l/kg body wt), C(t) is the mean arterial phenylalanine or tyrosine concentration (μmol/ml), dE/dt is the variation in phenylalanine or tyrosine enrichment (atom% excess), and E(t) is the mean arterial phenylalanine or tyrosine enrichment between two time points. When steady-state conditions were achieved, whole body flux was calculated using the following equation:
where EA and EI are the arterial and infusate phenylalanine or tyrosine enrichments, and IR is the [2H5]phenylalanine or [2H2]tyrosine tracer infusion rate (μmol·kg−1·h−1).
Hindquarters protein synthesis and degradation.
Synthesis and degradation (μmol/h) were calculated using the following equations, with venous phenylalanine enrichments assumed similar to the true precursor pool (27):
and
where [Aphe] and EA are arterial and [Vphe] and EV are venous phenylalanine concentrations (μmol/ml) and enrichments, and PF is plasma flow (ml/h).
Whole body phenylalanine hydroxylation to tyrosine.
Phenylalanine oxidation was estimated from the rate of phenylalanine hydroxylation to tyrosine according to the following equation (9):
where Qtyrosine is the whole body flux of tyrosine (μmol·kg−1·h−1), and Ed4 tyrosine and Ed5 phenylalanine are the enrichments of [ring-d4]tyrosine and [ring-d5]phenylalanine, respectively, in plasma.
Glucose and insulin.
Plasma samples were analyzed for glucose (model 2300; Yellow Springs Instruments, Yellow Springs, OH). Plasma radioimmunoreactive insulin concentrations were measured using porcine insulin radioimmunoassay kits (Millipore, St. Charles, MO).
Protein immunoblot analysis.
Pigs were euthanized with pentobarbital sodium 1 h following the last meal for the INT group and at the same time for CON (i.e., 29 h after the initiation of feeding for both groups) and FAS groups. Muscle samples were removed and frozen at −70°C until analysis. Proteins from longissimus dorsi and gastrocnemius muscle homogenates were separated on polyacrylamide gels (PAGE). For each assay, all samples were run at the same time on triple-wide gels (CBS Scientific C, Del Mar, CA) to eliminate interassay variation. Proteins were transferred electrophoretically to polyvinlidene difluoride transfer membranes (Pall, Pensicola, FL), incubated with appropriate primary antibodies, washed, and exposed to an appropriate secondary antibody, as described previously (61). Immunoblotting was performed using the following primary antibodies: PKB (Ser473; Cell Signaling Technology, Beverly, MA), S6K1 (Thr389; Millipore, Bedford, MA), 4E-BP1 (Thr46; Invitrogen, Carlsbad, CA), AMP-activated protein kinase-α (AMPKα; Thr172; Cell Signaling Technology), eIF2α (Ser51; Cell Signaling Technology), eukaryotic elongation factor 2 (eEF2; Thr56; Cell Signaling Technology), microtubule-associated protein 1 light chain 3 (LC3; Cell Signaling Technology), muscle RING finger-1 (MuRF1; R & D Systems, Minneapolis, MN), F-box protein atrogin-1/MAFbx (atrogin-1; ECM Biosciences, Versailles, KY), forkhead transcription factor-1 (Fox01; Ser256; Cell Signaling Technology), and forkhead transcription factor-4 (Fox04; Ser262; Cell Signaling Technology) and β-actin (Cell Signaling Technology). Blots were developed using an enhanced chemiluminescence kit (GE Health Sciences, Buckinghamshire, UK), visualized, and analyzed using a ChemiDoc-It Imaging System (UVP, Upland, CA).
Quantification of the eIF4E·eIF4G complex.
The complex was immunoprecipitated using an anti-eIF4E monoclonal antibody (gift of Dr. Leonard Jefferson, Penn State University College of Medicine, Hershey, PA) from aliquots of fresh tissue homogenates (24). Immunoprecipates were recovered with goat anti-mouse IgG magnetic beads (Polysciences, Warrington, PA), washed, resuspended in sample buffer as described elsewhere (33), and immediately subjected to protein immunoblot analysis using rabbit anti-eIF4G (Novus Biologicals, Littleton, CO). Amounts of eIF4G were corrected by the eIF4E (Cell Signaling Technology) recovered from the immunoprecipitate.
Statistical analysis.
Treatments were assigned to experimental units using Complete Randomized Design. To investigate temporal effects, all samples were considered in a repeated-measures analysis. Various autocorrelation structures were investigated based on Bayesian Information Criterion. Compound Symmetry gave the best fit, and this structure was then used for all repeated-measures analyses. When a significant effect was determined by ANOVA, means were compared using Fisher's protected least significant differences post hoc test. Data are presented as least square means ± SE and differences considered significant at P ≤ 0.05.
RESULTS
Glucose and insulin.
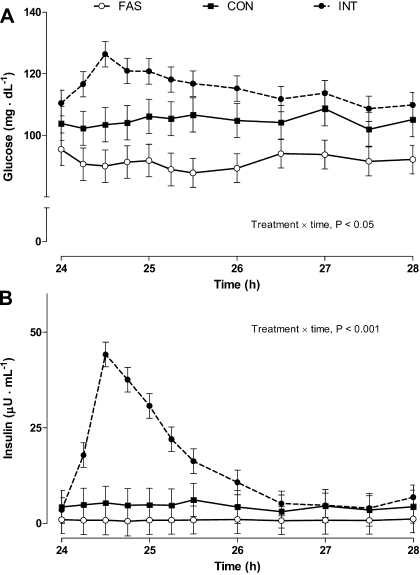
There was a modest increase (P < 0.05) in plasma glucose concentration (Fig. 2) following the meal for INT compared with CON or FAS pigs, but plasma glucose concentrations returned to baseline by 27.5 h (210 min after the bolus meal). Plasma insulin concentration was highest at 24.5 h (30 min following the meal) for the INT fed pigs (P < 0.001), whereas plasma insulin levels for the CON and FAS groups remained unchanged throughout the sampling period.
Fig. 2.
Arterial plasma glucose (A) and insulin concentrations (B) in pigs either fasted overnight (FAS) or fed continuously (CON) or intermittently (INT; 7 × 4 h meals) for 29 h. Values are means ± SE; n = 6.
Amino acid concentration.
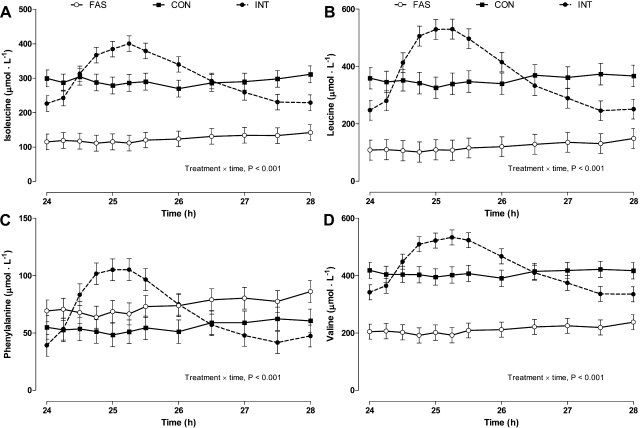
Level and frequency of feeding impacted plasma amino acid concentrations (Fig. 3). Plasma branched-chain amino acids (i.e., isoleucine, leucine, and valine), phenylalanine, and tyrosine (data not shown) increased after the bolus meal (P < 0.001) and reached the highest concentrations between 24.75 and 25.5 h (45 and 90 min after the meal) but returned to baseline by 26.5 h (150 min after the meal). The temporal concentrations of all these amino acids were unaffected during either continuous feeding or fasting.
Fig. 3.
Arterial plasma isoleucine (A), leucine (B), phenylalanine (C), and valine (D) concentrations in pigs either FAS, CON, or INT (7 × 4 h meals) for 29 h. Values are means ± SE; n = 6.
Whole body flux of phenylalanine and tyrosine and phenylalanine hydroxylation.
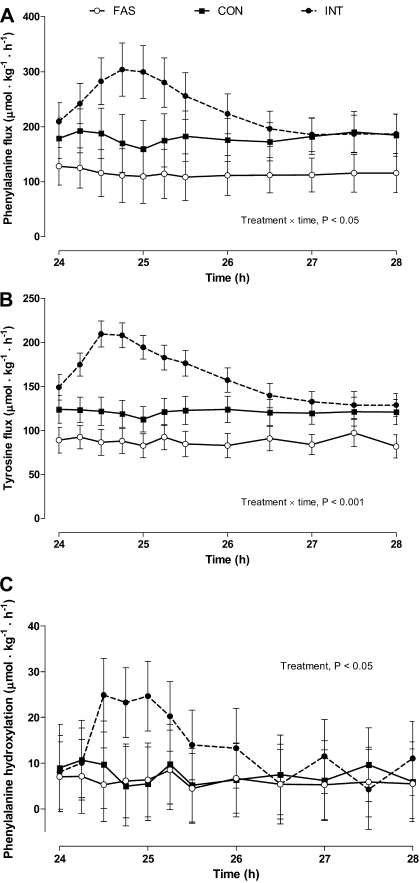
Feeding pigs intermittently increased whole body flux of phenylalanine and tyrosine (Fig. 4). During this period there was also a numerical increase in phenylalanine hydroxylation, a measurement of oxidation, which over the 4-h sampling period was higher for the INT compared with the CON and FAS groups (P < 0.05).
Fig. 4.
Whole body phenylalanine (A) and tyrosine flux (B) and phenylalanine hydroxylation to tyrosine (C) in pigs either FAS, CON, or INT (7 × 4 h meals) for 29 h. Values are means ± SE; n = 6.
Hindquarters amino acid net balance.
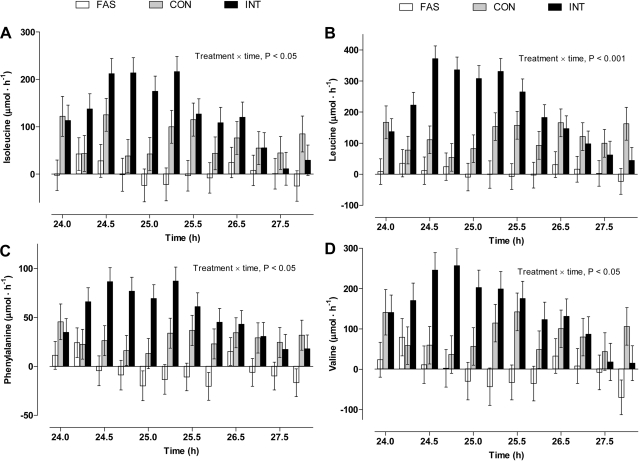
The net balance of the branched-chain amino acids phenylalanine (Fig. 5) and tyrosine (data not shown) increased (P ≤ 0.05) in the INT group between 24.5 and 25.25 h (30–75 min after the bolus meal). There were no temporal changes in the net removal of these amino acids by the hindquarters for either the CON or FAS groups.
Fig. 5.
Hindquarters net balance of isoleucine (A), leucine (B), phenylalanine (C), and valine (D) in pigs either FAS, CON, or INT (7 × 4 h meals) for 29 h. Positive values denote net removal and negative values net release. Values are means ± SE; n = 6.
Hindquarters protein turnover.
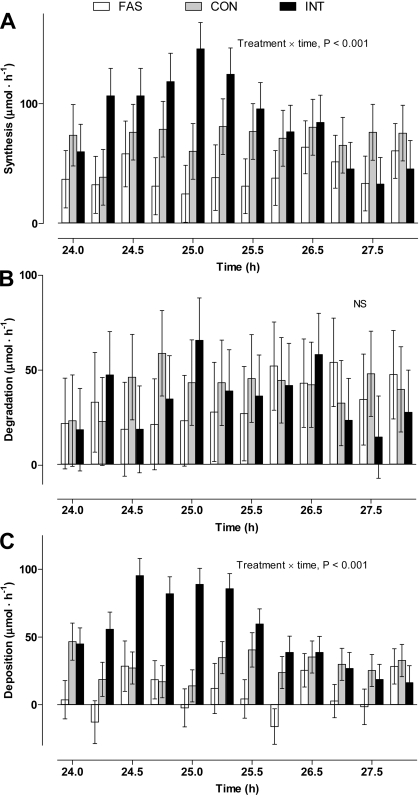
Protein synthesis (Fig. 6), estimated on the basis of venous phenylalanine as indicative of the true precursor enrichment, increased (P < 0.001) in the INT group between 24.25 and 26.5 h (15 and 150 min following the meal). In contrast, there were no temporal changes in protein synthesis for either the CON or FAS pigs. Furthermore, protein degradation also remained constant across all groups. However, these differences in protein turnover did result in increased protein deposition in the INT pigs compared with CON (P < 0.001), with both INT and CON greater than for the FAS pigs. This pattern of net retention was similar to that determined from the mass balance.
Fig. 6.
Hindquarters protein synthesis (A), degradation (B), and deposition (C) in pigs either FAS, CON, or INT (7 × 4 h meals) for 29 h. Values are means ± SE; n = 6.
Translation initiation and degradation signaling.
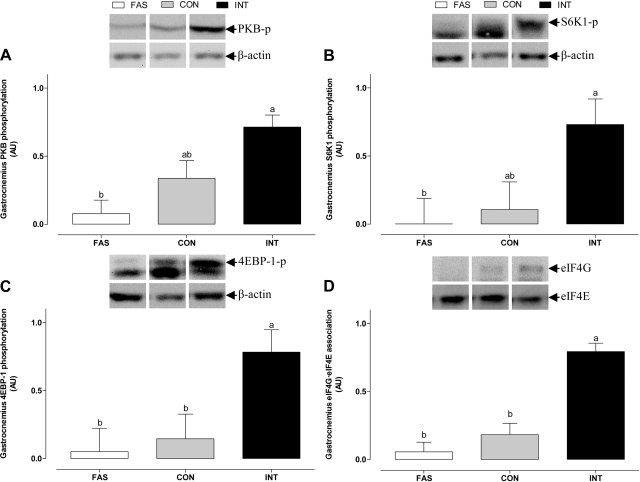
In the gastrocnemius muscle from the hindquarters (Fig. 7), INT feeding stimulated PKB phosphorylation compared with FAS (P < 0.05) but was intermediate for the CON group. Phosphorylation of S6K1 and 4E-BP1 and formation of the active eIF4E·eIF4G complex in the hindquarters was also enhanced in response to INT feeding compared with FAS (P < 0.05). For the CON group, whereas S6K1 phosphorylation was intermediate (NS from either INT or FAS), formation of eIF4E·eIF4G was lower than that of the INT group (P < 0.05).
Fig. 7.
Protein kinase B (PKB; A), ribosomal protein S6 kinase 1 (S6K1; B), and eukaryotic initiation factor (eIF)4B-binding protein-1 (4E-BP1; C) phosphorylation and association of the eIF4E·eIF4G complex (D) in the gastrocnemius muscle of pigs either FAS, CON, or INT (7 × 4 h meals) for 29 h. Values are means ± SE; n = 6. Values with different letters differ significantly (P < 0.05).
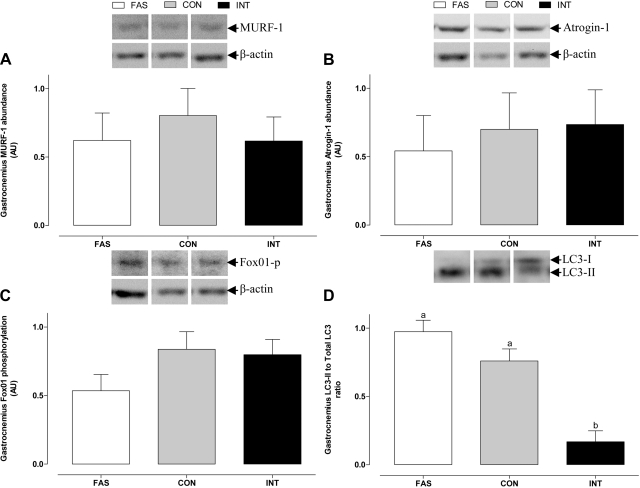
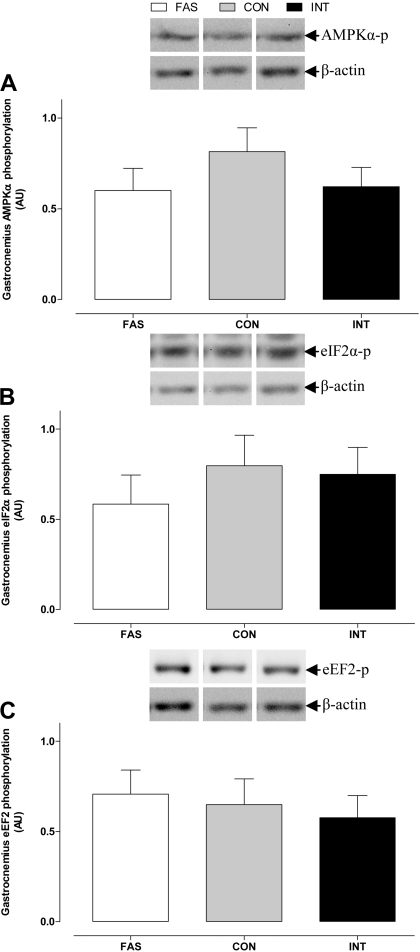
In the gastrocnemius muscle, MuRF1 and atrogin-1 abundance and Fox01 phosphorylation (Fig. 8) were unaffected by treatment. Nonetheless, the ratio of LC3-II to total LC3 was lower (P < 0.05) in the INT than in either the FAS or CON groups. eEF2, eIF2α, and AMPKα phosphorylation did not differ between the three groups (Fig. 9).
Fig. 8.
Muscle RING finger protein-1 (MuRF1; A) and atrophy F-box (atrogin-1; B) abundance, forkhead box-containing protein-01 (Fox01; C) phosphorylation, and microtubule-associated protein light chain 3 (LC3)-II to total LC3 ratio (D) in the gastrocnemius muscle of pigs either FAS, CON, or INT (7 × 4 h meals) for 29 h. Values are means ± SE; n = 6. Values with different letters differ significantly (P < 0.05).
Fig. 9.
AMP-activated protein kinase-α (AMPKα; A), eIF2α (B), and elongation factor 2 (eEF2; C) phosphorylation in the gastrocnemius muscle of pigs either FAS, CON, or INT (7 × 4 h meals) for 29 h. Values are means ± SE; n = 6. Control blots in this figure also appear in Fig. 8.
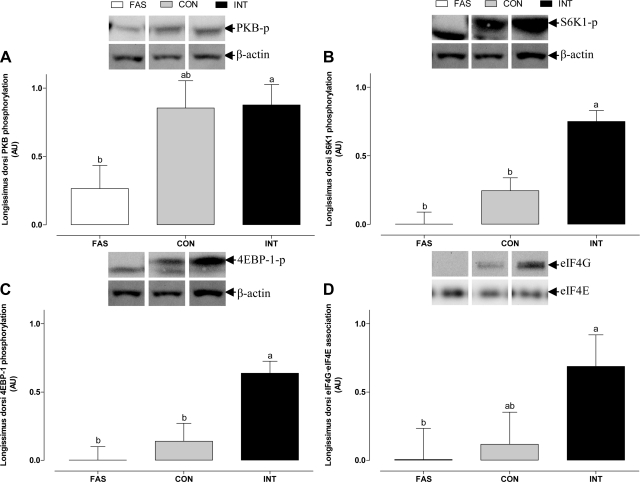
Protein synthesis and degradation pathways in the longissimus dorsi were also examined to check the representativeness of the results from the gastrocnemius muscle. In longissimus dorsi muscle, PKB, S6K1, and 4E-BP1 phosphorylation (Fig. 10) were all stimulated in the INT group compared with both the CON and FAS groups (P < 0.05). INT feeding also increased the formation of the active eIF4E·eIF4G complex compared with FAS (P < 0.05), with the CON group intermediate. The abundance of MuRF1 and atrogin-1 and the phosphorylation of Fox01 in the longissimus dorsi (Fig. 11) were not affected by either feeding status or frequency. In contrast, the ratio of microtubule-associated protein LC3-II to total LC3 (sum of LC3-II and LC3-I) in the longissimus dorsi was greater in the FAS (P < 0.001) compared with both the CON and INT groups.
Fig. 10.
PKB (A), S6K1 (B), and 4E-BP1 phosphorylation (C) and association of the eIF4E·eIF4G complex (D) in the longissimus dorsi muscle of pigs either FAS, CON, or INT (7 × 4 h meals) for 29 h. Values are means ± SE; n = 6. Values with different superscripts differ significantly (P < 0.05). Control blots in this figure appear in more than 1 part of the figure.
Fig. 11.
MuRF1 (A) and atrogin-1 abundance (B), Fox01 phosphorylation (C), and microtubule-associated protein LC3-II to total LC3 ratio (D) in the longissimus dorsi muscle of pigs either FAS, CON, or INT (7 × 4 h meals) for 29 h. Values are means ± SE; n = 6. Values with different letters differ significantly (P < 0.05). Control blots in this figure also appear in Fig. 10.
DISCUSSION
Experimental aspects.
In the current study, a combination of protein expression and stable isotope dilution methods in a multicatheterized piglet model was used to assess how feeding modalities affect protein accretion in skeletal muscle of the neonate. Because of their invasive nature, these techniques preclude using human neonates, but the pig has already been proven as an excellent surrogate (50). Although it is well established that the simultaneous postprandial increases in insulin and amino acids activate a cascade of intracellular signaling events leading to increased protein synthesis (11, 13, 14), much less attention has been focused on the regulation of protein degradation. The approaches adopted here allow dynamic measurement of both protein synthesis and breakdown coupled with state-of-the-art analysis of appropriate signaling cascades. This provides integrated information, both metabolic and molecular, and helps address key issues related to how best to feed the neonate under clinical conditions.
Feeding modalities and protein deposition.
Whether intermittent or continuous feeding is more beneficial remains contentious. This stems in part from limited data and confounding factors, including selection bias, protocol violations, pathological and clinical complications, and the provision of nutrient parenterally for different periods of time (48). In addition, the type of food and/or the mode of nutrient delivery can have adverse metabolic consequences for the neonate (58). Most comparative studies have focused mainly on clinical outcomes, and the effect of intermittent and continuous feeding on muscle protein accretion has not been elucidated. We demonstrated recently that protein synthesis measured 90 min after a meal was enhanced by intermittent bolus feeding compared with continuous feeding (28); however, the effects of feeding frequency on protein degradation and deposition were not determined. In the current study, protein deposition was greater in pigs fed intermittently compared with those fed continuously. This contrasts with one study where human neonates fed continuously showed faster linear growth (based on lower leg length) compared with those fed intermittently, although weight gains were similar (23). However, most studies show that intermittent feeding results in equal (2, 40, 55) or better (53) weight gain than continuous feeding. Although muscle growth is fastest in the neonatal period, weight gain may not necessarily be a good indicator of muscle protein deposition. Indeed, data from a study where macronutrient retention was determined by net balance suggest that nitrogen retention is similar between infants fed either continuously or intermittently (55). In practice, nitrogen balance may not be ideal to monitor small changes in retention due to measurement errors associated with both intake and losses (4, 51, 62). A limitation of the current study is that pigs were fed for only 29 h, which was short compared with long periods of hospitalization. Thus, further studies are needed to evaluate whether the enhanced protein deposition observed in this study persists over longer treatment periods.
Protein synthesis.
In the current study, skeletal muscle protein synthesis was greater in intermittent compared with continuous feeding. We have shown previously, using pancreatic substrate clamps, that insulin and amino acids can independently stimulate protein synthesis in a dose-dependent manner in neonates (44), and this explains the responses observed in response to the bolus meal. In addition, earlier studies showed that meal feeding stimulates signals of translation initiation, with changes in mTOR activation leading to increased fractional protein synthesis rates in muscle (25, 26, 67). This rapid process is sustained for at least 2 h following a meal, mirroring that of insulin and amino acids, in particular leucine (66), and this is supported by the current findings.
Changes in the energy status of the cell can influence the mTOR pathway; for example, an increase in AMP levels activates AMPK with resultant activation of the tuberous sclerosis complex 1/2, an allosteric inhibitor of mTOR (46). Previously, we have shown that insulin and amino acids have no effect on AMPK phosphorylation (28, 59, 61, 66). Similarly, in the current study, AMPK phosphorylation was not affected by changes in energy supply through either fasting or feeding. Together, these data suggest that, under such normal physiological conditions, AMPK activity has little impact on the mTOR signaling pathway.
Muscle PKB phosphorylation did increase in response to feeding, and this was greatest for the INT group. We have shown previously that PKB phosphorylation increases after a meal, with this being due to insulin and not amino acids (60), and returns to prefeeding values by 4 h after the meal (28, 67). PKB phosphorylation inactivates tuberous sclerosis complex 1/2, thereby activating mTOR (12, 28) with subsequent phosphorylation of the downstream target proteins S6K1 and 4E-BP1, activation of ribosomal subunit 6, the dissociation of eIF4E·4E-BP1, and the formation of eIF4E·eIF4G, leading to enhanced translation initiation (39).
Translation initiation is controlled by two regulatory processes. The first involves binding of methionyl-tRNA to the 40S ribosomal subunit forming the 43S preinitiation complex, a step mediated by eIF2α (39, 56). The second is through binding of the active mRNA to the 43S preinitiation complex, facilitated by the eIF4E·eIF4G complex. In this study, eIF2α phosphorylation was not affected by either feeding status or frequency and supports previous findings that the response to feeding is independent of changes in eIF2α phosphorylation (28, 61). Both insulin and amino acids (44) can activate mTOR with effects on the downstream regulators of protein synthesis. Although it is not possible in this study to separate the effects of insulin and amino acids when both change in response to a bolus feed (as in INT), the mTOR pathway is stimulated above the value observed for an equivalent amount of food supplied over a longer period. Therefore, over a relatively short duration (29 h), regulatory mechanisms do not involve integration of total intake but rather are designed to follow the metabolic patterns created by meal feeding.
The regulation of signaling components by amino acids upstream of mTOR is not completely understood but has been shown to be independent of PKB (60). Amino acids activate mTOR, which leads to the phosphorylation of S6K1 and 4E-BP1 and the formation of the active eIF4E·eIF4G complex (44). The current study shows that activation of downstream effectors of mTOR was enhanced with intermittent feeding compared with continuous feeding. It also showed that the enhanced activation of the intracellular signaling proteins that regulate mTOR-dependant translation initiation resulted in an increased protein deposition when measured by stable isotope balance despite a slight rise in amino acid oxidation. Thus, the increased activation of downstream effectors of mTOR is likely due to the simultaneous rise in insulin and amino acids.
Although studies in vitro have shown that insulin accelerates peptide elongation through inactivation of eEF2 kinase, a regulator of eEF2 activity (65), studies in vivo have indicated that neither amino acids nor insulin affect eEF2 phosphorylation (28, 61). Combined, these data suggest that binding of the 43S ribosomal subunit responds to feeding status and frequency and that peptide elongation is not a rate-limiting step in protein synthesis under normal physiological conditions.
Protein degradation.
Skeletal muscle is an important source of amino acids under conditions where dietary supply is insufficient to meet critical organ requirements or where extra glucose precursors are required (63). Although a number of proteolytic pathways exist, only two are thought to play important roles in skeletal muscle, the ubiquitin-proteasome (63) and autophagy-lysosome (52) systems. In mammals, polyubiquitination by ubiquitin ligases is the rate-limiting step of the ubiquitin-proteasome system (52). The two most important muscle-specific ubiquitin ligases are atrogin-1 and MuRF1 (37, 63). Reduced activity of PKB decreases phosphorylation of the forkhead family of transcription factors (Fox01, -3, and -4), causing enhanced expression of atrogin-1 and MuRF1 and a resultant increase in protein degradation (37). In addition, Fox01 induces muscle atrophy in vitro through increased expression of ubiquitin ligases atrogin-1 and MuRF1 (42). In the current study, although PKB phosphorylation increased in muscle of both continuously and intermittently fed pigs compared with those that were fasted, there was no effect on Fox01 activation or on the expression of atrogin-1 and MuRF1, nor were there observed differences in protein degradation. These findings agree with data from human studies suggesting that, unlike catabolic and cachectic diseased states, 40-h fasting was not long enough to induce changes in the expression of atrogin-1 and MuRF1 genes (36). Similarly, in rodents, only long (51 h) fasting periods (18) or severe amino acid starvation (35) led to an increase in atrogin-1 and MuRF1 expression.
A reduction in PKB phosphorylation has been proposed as a mechanism to induce autophagy (52). Autophagy-lysosomal protein degradation requires activation of the ubiquitin-like molecule LC3 (LC3-II), followed by the transfer and conjugation to phosphatidylethanolamine and the formation of autophagosomes (34, 52). Autophagosome fusion with lysosomes to form the mature autolysosomes is the final and rate-limiting step leading to autophagy. Efforts to relate autophagy markers to protein degradation are confounded because accumulation of autophagosomes may reflect increased degradation or decreased autophagosome turnover (i.e., failure to fuse with lysosomes) (34). The increased LC3-II lipidation observed here suggests that accumulation of autophagosomes is the probable cause because no increase in protein degradation was detected by isotope dilution. Alternatively, the upregulation of the autophagy-lysosome pathway may precede muscle loss (52), and although the piglets were fasted overnight, the duration may have been insufficient to fully induce the pathway. Nonetheless, under the current experimental conditions, protein degradation signaling appeared to be more sensitive to dietary changes via the autophagy-lysosome rather than the ubiquitin-proteasome system, but further investigation is required.
In conclusion, the feeding-induced stimulation of protein synthesis is greater in intermittently than continuously fed neonatal pigs, and this was the driving force for the improved protein deposition because protein degradation was insensitive to feeding frequency. Moreover, the results suggest that the increased protein synthesis during intermittent feeding was via elevated initiation of mTOR-dependent translation. Furthermore, under the current experimental condition, neither the ubiquitin-proteasome nor autophagy-lysosomal system was activated despite a rise in LC3-II levels.
GRANTS
This work is a publication of the US Department of Agriculture/Agricultural Research Service (USDA/ARS) Children's Nutrition Research Center, Department of Pediatrics, Baylor College of Medicine. This project has been funded in part by National Institute of Arthritis and Musculoskeletal and Skin Diseases Grant AR-044474 (T. A. Davis) and by the USDA/ARS under Cooperative Agreement no. 6250-510000-055 (T. A. Davis). The contents of this publication do not necessarily reflect the views or policies of the US Department of Agriculture, nor does the mention of trade names, commercial products, or organizations imply endorsement by the US Government.
DISCLOSURES
The authors have no conflicts of interest to disclose.
AUTHOR CONTRIBUTIONS
S.W.E.-K., A.S., G.E.L., and T.A.D. did the conception and design of the research; S.W.E.-K., A.S., M.C.G., N.S., R.A.O., and H.V.N. performed the experiments; S.W.E.-K., A.S., H.V.N., G.E.L., and T.A.D. analyzed the data; S.W.E.-K., A.S., G.E.L., and T.A.D. interpreted the results of the experiments; S.W.E.-K. prepared the figures; S.W.E.-K. drafted the manuscript; S.W.E.-K., A.S., N.S., R.A.O., H.V.N., G.E.L., and T.A.D. edited and revised the manuscript; all authors approved the final version of the manuscript.
AKNOWLEDGMENTS
We thank M. L. Fiorotto, D. G. Burrin, and B. Stoll for helpful discussions, R. D. Almonaci and S. J. Koo for technical assistance, J. C. Stubblefield for animal care, E. O'Brian Smith for statistical assistance, A. Gillum for graphics, and L. F. Kemper for secretarial assistance.
REFERENCES
- 1. Agus MS, Javid PJ, Ryan DP, Jaksic T. Intravenous insulin decreases protein breakdown in infants on extracorporeal membrane oxygenation. J Pediatr Surg 39: 839–844, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Akintorin SM, Kamat M, Pildes RS, Kling P, Andes S, Hill J, Pyati S. A prospective randomized trial of feeding methods in very low birth weight infants. Pediatrics 100: E4, 1997 [DOI] [PubMed] [Google Scholar]
- 3. Aynsley-Green A. The endocrinology of feeding in the newborn. Baillieres Clin Endocrinol Metab 3: 837–868, 1989 [DOI] [PubMed] [Google Scholar]
- 4. Bier DM. Intrinsically difficult problems: the kinetics of body proteins and amino acids in man. Diabetes Metab Rev 5: 111–132, 1989 [DOI] [PubMed] [Google Scholar]
- 5. Boirie Y, Gachon P, Corny S, Fauquant J, Maubois JL, Beaufrere B. Acute postprandial changes in leucine metabolism as assessed with an intrinsically labeled milk protein. Am J Physiol Endocrinol Metab 271: E1083–E1091, 1996 [DOI] [PubMed] [Google Scholar]
- 6. Bos C, Stoll B, Fouillet H, Gaudichon C, Guan X, Grusak MA, Reeds PJ, Tome D, Burrin DG. Intestinal lysine metabolism is driven by the enteral availability of dietary lysine in piglets fed a bolus meal. Am J Physiol Endocrinol Metab 285: E1246–E1257, 2003 [DOI] [PubMed] [Google Scholar]
- 7. Burrin DG, Davis TA, Fiorotto ML, Reeds PJ. Hepatic protein synthesis in suckling rats: effects of stage of development and fasting. Pediatr Res 31: 247–252, 1992 [DOI] [PubMed] [Google Scholar]
- 8. Burrin DG, Davis TA, Fiorotto ML, Reeds PJ. Stage of development and fasting affect protein synthetic activity in the gastrointestinal tissues of suckling rats. J Nutr 121: 1099–1108, 1991 [DOI] [PubMed] [Google Scholar]
- 9. Clarke JT, Bier DM. The conversion of phenylalanine to tyrosine in man. Direct measurement by continuous intravenous tracer infusions of l-[ring-2H5]phenylalanine and l-[1–13C] tyrosine in the postabsorptive state. Metabolism 31: 999–1005, 1982 [DOI] [PubMed] [Google Scholar]
- 10. Davis TA, Burrin DG, Fiorotto ML, Nguyen HV. Protein synthesis in skeletal muscle and jejunum is more responsive to feeding in 7- than in 26-day-old pigs. Am J Physiol Endocrinol Metab 270: E802–E809, 1996 [DOI] [PubMed] [Google Scholar]
- 11. Davis TA, Burrin DG, Fiorotto ML, Reeds PJ, Jahoor F. Roles of insulin and amino acids in the regulation of protein synthesis in the neonate. J Nutr 128: 347S–350S, 1998 [DOI] [PubMed] [Google Scholar]
- 12. Davis TA, Fiorotto ML. Regulation of muscle growth in neonates. Curr Opin Clin Nutr Metab Care 12: 78–85, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Davis TA, Fiorotto ML, Beckett PR, Burrin DG, Reeds PJ, Wray-Cahen D, Nguyen HV. Differential effects of insulin on peripheral and visceral tissue protein synthesis in neonatal pigs. Am J Physiol Endocrinol Metab 280: E770–E779, 2001 [DOI] [PubMed] [Google Scholar]
- 14. Davis TA, Fiorotto ML, Burrin DG, Reeds PJ, Nguyen HV, Beckett PR, Vann RC, O'Connor PM. Stimulation of protein synthesis by both insulin and amino acids is unique to skeletal muscle in neonatal pigs. Am J Physiol Endocrinol Metab 282: E880–E890, 2002 [DOI] [PubMed] [Google Scholar]
- 15. Davis TA, Fiorotto ML, Nguyen HV, Burrin DG, Reeds PJ. Response of muscle protein synthesis to fasting in suckling and weaned rats. Am J Physiol Regul Integr Comp Physiol 261: R1373–R1380, 1991 [DOI] [PubMed] [Google Scholar]
- 16. Davis TA, Fiorotto ML, Nguyen HV, Reeds PJ. Enhanced response of muscle protein synthesis and plasma insulin to food intake in suckled rats. Am J Physiol Regul Integr Comp Physiol 265: R334–R340, 1993 [DOI] [PubMed] [Google Scholar]
- 17. Davis TA, Fiorotto ML, Nguyen HV, Reeds PJ. Protein turnover in skeletal muscle of suckling rats. Am J Physiol Regul Integr Comp Physiol 257: R1141–R1146, 1989 [DOI] [PubMed] [Google Scholar]
- 18. Dehoux M, Van Beneden R, Pasko N, Lause P, Verniers J, Underwood L, Ketelslegers JM, Thissen JP. Role of the insulin-like growth factor I decline in the induction of atrogin-1/MAFbx during fasting and diabetes. Endocrinology 145: 4806–4812, 2004 [DOI] [PubMed] [Google Scholar]
- 19. Denne SC, Kalhan SC. Leucine metabolism in human newborns. Am J Physiol Endocrinol Metab 253: E608–E615, 1987 [DOI] [PubMed] [Google Scholar]
- 20. Denne SC, Karn CA, Wang J, Liechty EA. Effect of intravenous glucose and lipid on proteolysis and glucose production in normal newborns. Am J Physiol Endocrinol Metab 269: E361–E367, 1995 [DOI] [PubMed] [Google Scholar]
- 21. Denne SC, Rossi EM, Kalhan SC. Leucine kinetics during feeding in normal newborns. Pediatr Res 30: 23–27, 1991 [DOI] [PubMed] [Google Scholar]
- 22. Dollberg S, Kuint J, Mazkereth R, Mimouni FB. Feeding tolerance in preterm infants: randomized trial of bolus and continuous feeding. J Am Coll Nutr 19: 797–800, 2000 [DOI] [PubMed] [Google Scholar]
- 23. Dsilna A, Christensson K, Alfredsson L, Lagercrantz H, Blennow M. Continuous feeding promotes gastrointestinal tolerance and growth in very low birth weight infants. J Pediatr 147: 43–49, 2005 [DOI] [PubMed] [Google Scholar]
- 24. Escobar J, Frank JW, Suryawan A, Nguyen HV, Kimball SR, Jefferson LS, Davis TA. Regulation of cardiac and skeletal muscle protein synthesis by individual branched-chain amino acids in neonatal pigs. Am J Physiol Endocrinol Metab 290: E612–E621, 2006 [DOI] [PubMed] [Google Scholar]
- 25. Frank JW, Escobar J, Suryawan A, Kimball SR, Nguyen HV, Jefferson LS, Davis TA. Protein synthesis and translation initiation factor activation in neonatal pigs fed increasing levels of dietary protein. J Nutr 135: 1374–1381, 2005 [DOI] [PubMed] [Google Scholar]
- 26. Frank JW, Escobar J, Suryawan A, Nguyen HV, Kimball SR, Jefferson LS, Davis TA. Dietary protein and lactose increase translation initiation factor activation and tissue protein synthesis in neonatal pigs. Am J Physiol Endocrinol Metab 290: E225–E233, 2006 [DOI] [PubMed] [Google Scholar]
- 27. Fujita S, Rasmussen BB, Cadenas JG, Grady JJ, Volpi E. Effect of insulin on human skeletal muscle protein synthesis is modulated by insulin-induced changes in muscle blood flow and amino acid availability. Am J Physiol Endocrinol Metab 291: E745–E754, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gazzaneo MC, Suryawan A, Orellana RA, Torrazza RM, El-Kadi SW, Wilson FA, Kimball SR, Srivastava N, Nguyen HV, Fiorotto ML, Davis TA. Intermittent bolus feeding has a greater stimulatory effect on protein synthesis in skeletal muscle than continuous feeding in neonatal pigs. J Nutr 141: 2152–2158, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Goldspink DF, Kelly FJ. Protein turnover and growth in the whole body, liver and kidney of the rat from the foetus to senility. Biochem J 217: 507–516, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Grant J, Denne SC. Effect of intermittent versus continuous enteral feeding on energy expenditure in premature infants. J Pediatr 118: 928–932, 1991 [DOI] [PubMed] [Google Scholar]
- 31. Hertz DE, Karn CA, Liu YM, Liechty EA, Denne SC. Intravenous glucose suppresses glucose production but not proteolysis in extremely premature newborns. J Clin Invest 92: 1752–1758, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kadrofske MM, Parimi PS, Gruca LL, Kalhan SC. Effect of intravenous amino acids on glutamine and protein kinetics in low-birth-weight preterm infants during the immediate neonatal period. Am J Physiol Endocrinol Metab 290: E622–E630, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kimball SR, Horetsky RL, Jefferson LS. Signal transduction pathways involved in the regulation of protein synthesis by insulin in L6 myoblasts. Am J Physiol Cell Physiol 274: C221–C228, 1998 [DOI] [PubMed] [Google Scholar]
- 34. Klionsky DJ, Abeliovich H, Agostinis P, Agrawal DK, Aliev G, Askew DS, Baba M, Baehrecke EH, Bahr BA, Ballabio A, Bamber BA, Bassham DC, Bergamini E, Bi X, Biard-Piechaczyk M, Blum JS, Bredesen DE, Brodsky JL, Brumell JH, Brunk UT, Bursch W, Camougrand N, Cebollero E, Cecconi F, Chen Y, Chin LS, Choi A, Chu CT, Chung J, Clarke PG, Clark RS, Clarke SG, Clavé C, Cleveland JL, Codogno P, Colombo MI, Coto-Montes A, Cregg JM, Cuervo AM, Debnath J, Demarchi F, Dennis PB, Dennis PA, Deretic V, Devenish RJ, Di Sano F, Dice JF, Difiglia M, Dinesh-Kumar S, Distelhorst CW, Djavaheri-Mergny M, Dorsey FC, Dröge W, Dron M, Dunn WA, Jr, Duszenko M, Eissa NT, Elazar Z, Esclatine A, Eskelinen EL, Fésüs L, Finley KD, Fuentes JM, Fueyo J, Fujisaki K, Galliot B, Gao FB, Gewirtz DA, Gibson SB, Gohla A, Goldberg AL, Gonzalez R, González-Estévez C, Gorski S, Gottlieb RA, Häussinger D, He YW, Heidenreich K, Hill JA, Høyer-Hansen M, Hu X, Huang WP, Iwasaki A, Jäättelä M, Jackson WT, Jiang X, Jin S, Johansen T, Jung JU, Kadowaki M, Kang C, Kelekar A, Kessel DH, Kiel JA, Kim HP, Kimchi A, Kinsella TJ, Kiselyov K, Kitamoto K, Knecht E, Komatsu M, Kominami E, Kondo S, Kovács AL, Kroemer G, Kuan CY, Kumar R, Kundu M, Landry J, Laporte M, Le W, Lei HY, Lenardo MJ, Levine B, Lieberman A, Lim KL, Lin FC, Liou W, Liu LF, Lopez-Berestein G, López-Otín C, Lu B, Macleod KF, Malorni W, Martinet W, Matsuoka K, Mautner J, Meijer AJ, Meléndez A, Michels P, Miotto G, Mistiaen WP, Mizushima N, Mograbi B, Monastyrska I, Moore MN, Moreira PI, Moriyasu Y, Motyl T, Münz C, Murphy LO, Naqvi NI, Neufeld TP, Nishino I, Nixon RA, Noda T, Nürnberg B, Ogawa M, Oleinick NL, Olsen LJ, Ozpolat B, Paglin S, Palmer GE, Papassideri I, Parkes M, Perlmutter DH, Perry G, Piacentini M, Pinkas-Kramarski R, Prescott M, Proikas-Cezanne T, Raben N, Rami A, Reggiori F, Rohrer B, Rubinsztein DC, Ryan KM, Sadoshima J, Sakagami H, Sakai Y, Sandri M, Sasakawa C, Sass M, Schneider C, Seglen PO, Seleverstov O, Settleman J, Shacka JJ, Shapiro IM, Sibirny A, Silva-Zacarin EC, Simon HU, Simone C, Simonsen A, Smith MA, Spanel-Borowski K, Srinivas V, Steeves M, Stenmark H, Stromhaug PE, Subauste CS, Sugimoto S, Sulzer D, Suzuki T, Swanson MS, Tabas I, Takeshita F, Talbot NJ, Tallóczy Z, Tanaka K, Tanaka K, Tanida I, Taylor GS, Taylor JP, Terman A, Tettamanti G, Thompson CB, Thumm M, Tolkovsky AM, Tooze SA, Truant R, Tumanovska LV, Uchiyama Y, Ueno T, Uzcátegui NL, van der Klei I, Vaquero EC, Vellai T, Vogel MW, Wang HG, Webster P, Wiley JW, Xi Z, Xiao G, Yahalom J, Yang JM, Yap G, Yin XM, Yoshimori T, Yu L, Yue Z, Yuzaki M, Zabirnyk O, Zheng X, Zhu X, Deter RL. Guidelines for the use and interpretation of assays for monitoring autophagy in higher eukaryotes. Autophagy 4: 151–175, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Koyama S, Hata S, Witt CC, Ono Y, Lerche S, Ojima K, Chiba T, Doi N, Kitamura F, Tanaka K, Abe K, Witt SH, Rybin V, Gasch A, Franz T, Labeit S, Sorimachi H. Muscle RING-finger protein-1 (MuRF1) as a connector of muscle energy metabolism and protein synthesis. J Mol Biol 376: 1224–1236, 2008 [DOI] [PubMed] [Google Scholar]
- 36. Larsen AE, Tunstall RJ, Carey KA, Nicholas G, Kambadur R, Crowe TC, Cameron-Smith D. Actions of short-term fasting on human skeletal muscle myogenic and atrogenic gene expression. Ann Nutr Metab 50: 476–481, 2006 [DOI] [PubMed] [Google Scholar]
- 37. Lecker SH, Goldberg AL, Mitch WE. Protein degradation by the ubiquitin-proteasome pathway in normal and disease states. J Am Soc Nephrol 17: 1807–1819, 2006 [DOI] [PubMed] [Google Scholar]
- 38. Lewis SE, Kelly FJ, Goldspink DF. Pre- and post-natal growth and protein turnover in smooth muscle, heart and slow- and fast-twitch skeletal muscles of the rat. Biochem J 217: 517–526, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ma XM, Blenis J. Molecular mechanisms of mTOR-mediated translational control. Nat Rev Mol Cell Biol 10: 307–318, 2009 [DOI] [PubMed] [Google Scholar]
- 40. Macdonald PD, Skeoch CH, Carse H, Dryburgh F, Alroomi LG, Galea P, Gettinby G. Randomised trial of continuous nasogastric, bolus nasogastric, and transpyloric feeding in infants of birth weight under 1400 g. Arch Dis Child 67: 429–431, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mashako MN, Bernard C, Cezard JP, Chayvialle JA, Navarro J. Effect of total parenteral nutrition, constant rate enteral nutrition, and discontinuous oral feeding on plasma cholecystokinin immunoreactivity in children. J Pediatr Gastroenterol Nutr 6: 948–952, 1987 [DOI] [PubMed] [Google Scholar]
- 42. McLoughlin TJ, Smith SM, DeLong AD, Wang H, Unterman TG, Esser KA. FoxO1 induces apoptosis in skeletal myotubes in a DNA-binding-dependent manner. Am J Physiol Cell Physiol 297: C548–C555, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Motil KJ, Opekun AR, Montandon CM, Berthold HK, Davis TA, Klein PD, Reeds PJ. Leucine oxidation changes rapidly after dietary protein intake is altered in adult women but lysine flux is unchanged as is lysine incorporation into VLDL-apolipoprotein B-100. J Nutr 124: 41–51, 1994 [DOI] [PubMed] [Google Scholar]
- 44. O'Connor PM, Kimball SR, Suryawan A, Bush JA, Nguyen HV, Jefferson LS, Davis TA. Regulation of translation initiation by insulin and amino acids in skeletal muscle of neonatal pigs. Am J Physiol Endocrinol Metab 285: E40–E53, 2003 [DOI] [PubMed] [Google Scholar]
- 45. Parimi PS, Kadrofske MM, Gruca LL, Hanson RW, Kalhan SC. Amino acids, glutamine, and protein metabolism in very low birth weight infants. Pediatr Res 58: 1259–1264, 2005 [DOI] [PubMed] [Google Scholar]
- 46. Petroulakis E, Mamane Y, Le Bacquer O, Shahbazian D, Sonenberg N. mTOR signaling: implications for cancer and anticancer therapy. Br J Cancer 94: 195–199, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Poindexter BB, Karn CA, Denne SC. Exogenous insulin reduces proteolysis and protein synthesis in extremely low birth weight infants. J Pediatr 132: 948–953, 1998 [DOI] [PubMed] [Google Scholar]
- 48. Premji S, Chessell L. Continuous nasogastric milk feeding versus intermittent bolus milk feeding for premature infants less than 1500 grams. Cochrane Database Syst Rev 1: CD001819, 2003 [DOI] [PubMed] [Google Scholar]
- 49. Proietto J, Rohner-Jeanrenaud F, Ionescu E, Terrettaz J, Sauter JF, Jeanrenaud B. Non-steady-state measurement of glucose turnover in rats by using a one-compartment model. Am J Physiol Endocrinol Metab 252: E77–E84, 1987 [DOI] [PubMed] [Google Scholar]
- 50. Puiman P, Stoll B. Animal models to study neonatal nutrition in humans. Curr Opin Clin Nutr Metab Care 11: 601–606, 2008 [DOI] [PubMed] [Google Scholar]
- 51. Rand WM, Young VR. Statistical analysis of nitrogen balance data with reference to the lysine requirement in adults. J Nutr 129: 1920–1926, 1999 [DOI] [PubMed] [Google Scholar]
- 52. Sandri M. Autophagy in health and disease. 3. Involvement of autophagy in muscle atrophy. Am J Physiol Cell Physiol 298: C1291–C1297, 2010 [DOI] [PubMed] [Google Scholar]
- 53. Schanler RJ, Shulman RJ, Lau C, Smith EO, Heitkemper MM. Feeding strategies for premature infants: randomized trial of gastrointestinal priming and tube-feeding method. Pediatrics 103: 434–439, 1999 [DOI] [PubMed] [Google Scholar]
- 54. Shulman RJ, Redel CA, Stathos TH. Bolus versus continuous feedings stimulate small-intestinal growth and development in the newborn pig. J Pediatr Gastroenterol Nutr 18: 350–354, 1994 [DOI] [PubMed] [Google Scholar]
- 55. Silvestre MA, Morbach CA, Brans YW, Shankaran S. A prospective randomized trial comparing continuous versus intermittent feeding methods in very low birth weight neonates. J Pediatr 128: 748–752, 1996 [DOI] [PubMed] [Google Scholar]
- 56. Sonenberg N, Hinnebusch AG. Regulation of translation initiation in eukaryotes: mechanisms and biological targets. Cell 136: 731–745, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Steele R. Influences of glucose loading and of injected insulin on hepatic glucose output. Ann NY Acad Sci 82: 420–430, 1959 [DOI] [PubMed] [Google Scholar]
- 58. Stoll B, Horst DA, Cui L, Chang X, Ellis KJ, Hadsell DL, Suryawan A, Kurundkar A, Maheshwari A, Davis TA, Burrin DG. Chronic parenteral nutrition induces hepatic inflammation, steatosis, and insulin resistance in neonatal pigs. J Nutr 140: 2193–2200, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Suryawan A, Davis TA. Developmental regulation of protein kinase B activation is isoform specific in skeletal muscle of neonatal pigs. Pediatr Res 58: 719–724, 2005 [DOI] [PubMed] [Google Scholar]
- 60. Suryawan A, O'Connor PM, Kimball SR, Bush JA, Nguyen HV, Jefferson LS, Davis TA. Amino acids do not alter the insulin-induced activation of the insulin signaling pathway in neonatal pigs. J Nutr 134: 24–30, 2004 [DOI] [PubMed] [Google Scholar]
- 61. Suryawan A, Orellana RA, Nguyen HV, Jeyapalan AS, Fleming JR, Davis TA. Activation by insulin and amino acids of signaling components leading to translation initiation in skeletal muscle of neonatal pigs is developmentally regulated. Am J Physiol Endocrinol Metab 293: E1597–E1605, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Tomé D, Bos C. Dietary protein and nitrogen utilization. J Nutr 130: 1868S–1873S, 2000 [DOI] [PubMed] [Google Scholar]
- 63. Ventadour S, Attaix D. Mechanisms of skeletal muscle atrophy. Curr Opin Rheumatol 18: 631–635, 2006 [DOI] [PubMed] [Google Scholar]
- 64. Volpi E, Ferrando AA, Yeckel CW, Tipton KD, Wolfe RR. Exogenous amino acids stimulate net muscle protein synthesis in the elderly. J Clin Invest 101: 2000–2007, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Wang X, Li W, Williams M, Terada N, Alessi DR, Proud CG. Regulation of elongation factor 2 kinase by p90(RSK1) and p70 S6 kinase. EMBO J 20: 4370–4379, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Wilson FA, Suryawan A, Gazzaneo MC, Orellana RA, Nguyen HV, Davis TA. Stimulation of muscle protein synthesis by prolonged parenteral infusion of leucine is dependent on amino acid availability in neonatal pigs. J Nutr 140: 264–270, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Wilson FA, Suryawan A, Orellana RA, Kimball SR, Gazzaneo MC, Nguyen HV, Fiorotto ML, Davis TA. Feeding rapidly stimulates protein synthesis in skeletal muscle of neonatal pigs by enhancing translation initiation. J Nutr 139: 1873–1880, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Wray-Cahen D, Beckett PR, Nguyen HV, Davis TA. Insulin-stimulated amino acid utilization during glucose and amino acid clamps decreases with development. Am J Physiol Endocrinol Metab 273: E305–E314, 1997 [DOI] [PubMed] [Google Scholar]