Abstract
Overstimulation of endothelin type A (ETA) and nucleotide (P2Y) Gαq-coupled receptors in vascular smooth muscle causes vasoconstriction, hypertension, and, eventually, hypertrophy and vascular occlusion. G protein-coupled receptor kinases (GRKs) and arrestin proteins are sequentially recruited by agonist-occupied Gαq-coupled receptors to terminate phospholipase C signaling, preventing prolonged/inappropriate contractile signaling. However, these proteins also play roles in the regulation of several mitogen-activated protein kinase (MAPK) signaling cascades known to be essential for vascular remodeling. Here we investigated whether different arrestin isoforms regulate endothelin and nucleotide receptor MAPK signaling in rat aortic smooth muscle cells (ASMCs). When intracellular Ca2+ levels were assessed in isolated ASMCs loaded with Ca2+-sensitive dyes, P2Y2 and ETA receptor desensitization was attenuated by selective small-interfering (si)RNA-mediated depletion of G protein-coupled receptor kinase 2 (GRK2). Using similar siRNA techniques, knockdown of arrestin2 prevented P2Y2 receptor desensitization and enhanced and prolonged p38 and ERK MAPK signals, while arrestin3 depletion was ineffective. Conversely, arrestin3 knockdown prevented ETA receptor desensitization and attenuated ET1-stimulated p38 and ERK signals, while arrestin2 depletion had no effect. Using Transwell assays to assess agonist-stimulated ASMC migration, we found that UTP-stimulated migration was markedly attenuated following arrestin2 depletion, while ET1-stimulated migration was attenuated following knockdown of either arrestin. These data highlight a differential arrestin-dependent regulation of ETA and P2Y2 receptor-stimulated MAPK signaling. GRK2 and arrestin expression are essential for agonist-stimulated ASMC migration, which, as a key process in vascular remodeling, highlights the potential roles of GRK2 and arrestin proteins in the progression of vascular disease.
Keywords: G protein-coupled receptor kinase, arrestin, extracellular signal-regulated kinase, p38 mitogen-activated protein kinase, rat aortic smooth muscle, endothelin type A
increasing evidence suggests that overstimulation of Gαq-coupled receptor signaling pathways leads to vascular hypertrophy and hypertension (7), implying that understanding the mechanisms that regulate Gαq-mediated signaling is of potential importance in understanding and therapeutically treating vascular diseases. Continual or repeated agonist stimulation of G protein-coupled receptors (GPCRs) usually leads to reduced responsiveness to further agonist stimulation (36, 45), protecting cells from the adverse effects of overstimulation or inappropriate signaling. Phosphorylation at key serine or threonine residues within the third intracellular loop and/or COOH-terminal tail of the GPCR is thought to be the primary event initiating desensitization, and is mediated by a family of seven serine/threonine kinases, the G protein-coupled receptor kinases (GRKs) (36, 45). GRK-mediated receptor phosphorylation increases affinity for nonvisual arrestin2/arrestin3, sterically suppressing further receptor/G protein interactions (30). Arrestin binding also initiates clathrin-mediated endocytosis of phosphorylated receptors and, by acting as agonist-regulated adaptor scaffolds, can promote the activation of signaling pathways linked to the induction of hyperplasia (2) and hypertrophy (2, 15), such as the mitogen-activated protein kinases (14, 18). Indeed, data from arrestin knockout mice have highlighted roles for arrestins in the regulation of vascular smooth muscle migration and proliferation during atherosclerosis and neointimal hyperplasia (24). Furthermore, arrestins have also been shown to regulate angiotensin II-stimulated extracellular signal-regulated kinase (ERK)1/2-mediated vascular smooth muscle proliferation (23), highlighting the potential of arrestins as regulators of vasoconstrictor-mediated vascular pathophysiologies.1
Like angiotensin II, endothelins and extracellular nucleotides (ATP/UTP) are potent regulators of vascular tone, and are also known to induce hyperplasia (4, 15) and hypertrophy (2, 31), leading to hypertension and vascular remodeling (4, 15). Endothelin [acting through endothelin type A (ETA) receptors] and extracellular nucleotides (acting through P2Y receptors) cause vasoconstriction through Gαq-mediated activation of phospholipase C (PLC) and the production of inositol 1,4,5-trisphosphate (IP3) and diacylglycerol, leading to intracellular Ca2+ release and activation of protein kinase C, respectively. Recent findings in mesenteric arterial smooth muscle have identified GRK2 and arrestin proteins as negative regulators of ETA and P2Y2 receptor-mediated PLC signaling (33, 34). Considering the increasing number of physiological processes known to be regulated by arrestin proteins (14, 18), little is currently known regarding the regulation of ETA or P2Y2 receptor-stimulated signaling by arrestin proteins in vascular smooth muscle. To address this, fluorescence confocal imaging and biochemical techniques were combined with small interfering RNA (siRNA)-directed suppression of arrestin isoform expression to examine the specific interactions of arrestin2 and arrestin3 with endogenous ETA and P2Y2 receptor signaling pathways in isolated aortic smooth muscle cells. The effects of isoform-specific arrestin depletion on ETA and P2Y2 receptor-mediated ERK and p38 MAPK signaling, as well as migratory responses are reported.
MATERIALS AND METHODS
Isolation and Culture of Aortic Smooth Muscle Cells
Adult male Wistar rats (150–400 g) were killed by stunning and cervical dislocation. Care of animals was in accordance with the UK Animals (Scientific Procedures) Act 1986. The investigation also conforms to the Guide for the Care and Use of Laboratory Animals, published by the National Institutes of Health (NIH Publication No. 85-23, revised 1996). Dissection and enzymatic digestions were carried out as previously described for aortic smooth muscle cells (ASMCs) (13). ASMCs were cultured in Dulbecco's modified Eagle's medium (with glutaMAX) supplemented with fetal calf serum (10%), penicillin (100 IU/ml), streptomycin 100 μg/ml, and amphotericin B (2.5 μg/ml).
Knockdown of Endogenous P2Y Receptor, GRK2, and Arrestin Levels in ASMCs
For knockdown of endogenous P2Y, GRK2 and arrestin expression ASMCs (1 × 106) were transfected with 100 nM negative control (scrambled sequence), or our previously validated anti-P2Y2 (5′-GAACUGACACUGUGAGGAAtt-3′), or P2Y4 (5′-CGUCUACUUCAGUUCGGCAtt-3′) (33), anti-GRK2 (5′-GCAGGUACCUCCAGAUCUCtt-3′) (34), anti-arrestin2 (5′-GCCACUGACUCGGCUACAAtt-3′), or anti-arrestin3 (5′-GCCUUCUGUGCCAAAUCUAtt-3′) siRNAs (33). siRNAs were introduced using the Lonza nucleofection system (Lonza, Cologne, Germany) according to the manufacturer's instructions. Transfected cells were grown for a further 48 h before experimentation to allow for maximal suppression of the target protein expression.
Western Blot Analysis
Determination of GRK and arrestin expression.
GRK and arrestin protein expression were determined using standard immunoblotting protocols as described previously (46). Cell lysates (40 μg/lane) were separated by SDS-PAGE before Western transfer. GRK2 and arrestin protein expression were determined using standard immunoblotting techniques (33), using specific anti-GRK2 antibody (Santa Cruz Biotechnology, Santa Cruz, CA); arrestin2 and arrestin3 expression was determined using an anti-arrestin2 antibody (A1CT) (gift from Prof. R. J. Lefkowitz, Duke University, Durham, NC). The A1CT antibody was raised against arrestin2 (1) but also detects arrestin3 albeit with a lower affinity enabling detection of both proteins.
Determination of ERK1/2 and p38 MAPK activation.
Agonist-driven ERK1/2 or p38 MAPK activity was detected using standard Western blotting techniques as described previously (6). Briefly, ASMCs were transfected with anti-arrestin2, anti-arrestin3, or negative control siRNAs, as described above before seeding into six-well culture plates. After 24 h, cells were serum starved for a further 24 h before agonist addition. Next, cells were lysed, samples were separated using SDS-PAGE, and phospho-ERK/phospho-p38 levels were detected using specific anti-phospho-ERK1/2 or phospho-p38 antibodies (Promega, Madison, WI), and standard Western blotting techniques. To ensure that all samples contained the same levels of protein, all membranes were stripped and reprobed for total ERK1 or total p38 levels, using anti-ERK1 (Santa Cruz), or anti-p38 antibodies (Promega). Protein expression was quantified using the GeneGnome image analysis system (Syngene, Cambridge, UK). Phospho-ERK1/2 absorbance levels for each treatment were corrected for differences in total ERK1/2 immunoreactivity before being expressed as a percent increase over basal phospho-ERK1/2 immunoreactivity.
Detection of epithelial growth factor receptor phosphorylation.
ASMCs were grown as described above for MAPK assay. After 24 h, cells were serum starved for a further 24 h before agonist addition. Cells were lysed and separated using SDS-PAGE. Phospho-epithelial growth factor receptor (EGFR) levels detected using a specific anti-phospho-EGFR antibody (Cell Signaling, Beverly, MA) that recognizes the Src phosphorylation site Tyr-845 (3). To confirm equal loading, membranes were stripped and reprobed for total EGFR using a specific anti-EGFR antibody (Cell Signaling). Protein expression was quantified using the GeneGnome image analysis system. Phospho-EGFR absorbance levels for each treatment were corrected for differences in total EGFR immunoreactivity before being expressed as a percent increase over basal phospho-EGFR immunoreactivity.
Relative Contributions of P2Y Receptors to UTP-Mediated Ca2+ Signaling in ASMCs
Relative contributions of the P2Y2, P2Y4, and P2Y6 receptor subpopulations in UTP-stimulated signaling were studied in cultured ASMCs. Cells were plated into 96-well multiplates, and at ∼90% confluency cells were loaded with the Ca2+-sensitive dye Fluo4-AM (3 μM) at room temperature for 60 min. Cells were stimulated with UTP (nonselective P2Y agonist), UTPγS (P2Y2 and P2Y4 agonist), 2-thio-UTP (P2Y2 agonist), or PSB0474 (P2Y6 agonist). Additionally, cells were preincubated with MRS2578 (P2Y6 antagonist, 10 μM for 15 min) before challenge with UTP (100 μM). Intracellular calcium concentration ([Ca2+]i) changes were determined as the relative change in fluorescence measured using a NovoStar imaging system (BMG Labtech, Aylesbury, UK). To further investigate the possible roles of P2Y2/P2Y4 receptor subpopulations, endogenous levels of P2Y2 and P2Y4 were reduced by siRNA transfection by nucleofection (see above). After 48 h, ASMCs were loaded with Fluo4-AM and stimulated with UTP (100 μM) and fluorescence measured on the NovoStar imaging system.
Single-Cell Ca2+ Imaging and Receptor Desensitization Studies
ASMCs were loaded with the Ca2+-sensitive dye Fura2-AM (3 μM, 60 min). Cells were maintained at 37°C using a Peltier unit and continually perfused with Krebs-Henseleit buffer (in mM: 134 NaCl, 6 KCl, 1 MgCl2, 1.2 KH2PO4, 10 glucose, 10 HEPES, 1.3 CaCl2, pH 7.4). Real-time images were taken using an epifluorescence Nikon Eclipse TE200 microscope (Nikon, Tokyo, Japan) (×40 objective) and RatioMastersoftware (Photon Technology International, London, ON, Canada). Cells were excited at 340 and 380 nm, and Fura2 emission was assessed at ≥520 nm. The 340/380 fluorescence ratio was measured from regions of interest within the cytosol. [Ca2+]i changes are displayed as the fluorescence emission relative to basal (F/F0). Agonists were applied via the perfusion line as indicated. Desensitization studies were undertaken using our previously verified protocols (33, 34). Briefly, to determine ETA receptor desensitization, a maximal ET1 concentration was applied for 30 s before (termed R1) and after (termed R2) a 5-min washout period. Reduced R2/R1 ratios are interpreted as an indication of receptor desensitization (34). Repeated addition of maximal concentrations of UTP (100 μM, 30 s) to ASMCs, interspersed by 5-min wash periods, produces reproducible [Ca2+]i increases with little evidence of tachyphylaxis (data not shown), which most likely indicates a significant P2Y2 receptor reserve with respect to the Ca2+ response (47). To unmask an agonist-induced desensitization of the P2Y2 receptor, an amended protocol was employed where ASMCs were challenged with approximate EC50 concentrations of UTP (10 μM) for 30 s before (R1) and after (R2) addition of a maximal UTP concentration (Rmax: 100 μM, for 30 s) with changes in R2 relative to R1 providing an indication of P2Y2 receptor desensitization caused by the Rmax UTP stimulus.
Cell Motility Assays
ASMCs were seeded onto the upper surfaces of Transwell inserts (8-μm pores). Transwells were placed into wells on a 24-well plate containing UTP (100 μM) or ET-1 (100 nM) in 500 μl of medium in the lower chambers. Cells were left at 37°C in 5% CO2 in humidified air for various time periods before cells were fixed and permeabilized in 100% methanol and nuclei were stained with propidium iodide. Membranes were removed and fixed to glass slides before the numbers of migrated cells on the lower surface of the membrane were counted using an Olympus FV500 laser scanning confocal IX70 inverted microscope (×10 objective). To assess the role of ERK or p38 MAPK in UTP- or ET1-stimulated cell migration, ASMCs were incubated with p38 MAPK inhibitor SB203580 (20 μM) or MEK (the upstream kinase from ERK1/2) inhibitor PD98059 (20 μM) for 30 min before agonist addition, and throughout the assay.
Data and Statistical Analysis
Data are presented for cells obtained from at least three separate ASMC preparations and are expressed as means ± SE. Data have been analyzed (GraphPad Prism, San Diego, CA) using one-way or two-way ANOVA as indicated, with appropriate post hoc testing. For all MAPK and EGRF studies, statistical analyses were conducted on the absorbance values after correction for variations in total ERK/p38/EGFR levels, to allow use of parametric testing, before normalization to basal protein expression.
RESULTS
Identification of P2Y Receptor Expression in ASMCs
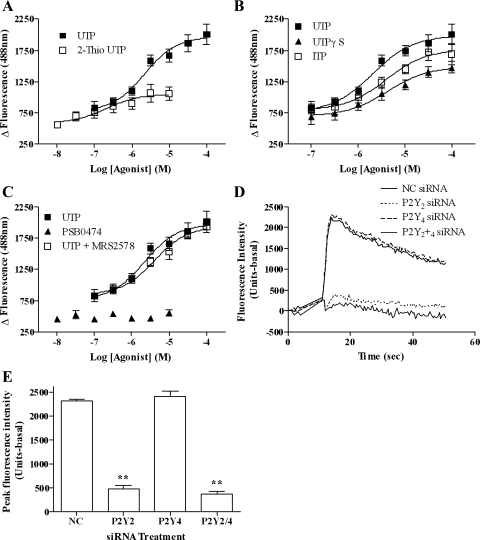
We have recently identified the P2Y2 receptor as the predominant mediator of UTP-stimulated PLC signaling in mesenteric arterial smooth muscle (33). However, since previous reports (15, 20) suggest that aortic smooth muscle expresses a mixed P2Y receptor population, we determined which P2Y receptor subtype(s) mediates UTP-signaling in ASMCs. Use of reportedly selective agonists and antagonists suggested the presence of a mixed P2Y2/4 receptor population and the absence of P2Y6 (Fig. 1, A–C). Analysis of UTP-stimulated signals, following application of previously validated siRNAs (34) to knock down P2Y2 (by >75%) or P2Y4 (by >90%) mRNA levels, showed that UTP-stimulated responses were virtually absent after P2Y2 knockdown, while P2Y4 depletion was without significant effect (Fig. 1, D and E).
Fig. 1.
Characterization of aortic smooth muscle cell (ASMC) P2Y receptor subtypes. ASMCs were loaded with Fluo4 (3 μM), and intracellular calcium concentration ([Ca2+]i)-response curves were generated after ASMC stimulation with increasing concentrations of UTP, the P2Y2 receptor agonist 2-thio-UTP (A), P2Y4 receptor agonists UTPγS and ITP (B), or P2Y6 receptor agonist PSB0474 and UTP ± MRS2578 (10 μM), a P2Y6 antagonist (C), using a NovoStar imaging system. Endogenous levels of P2Y2 and/or P2Y4 receptor expression in ASMCs were depleted by introducing P2Y2 or P2Y4 small interfering RNAs (siRNAs; 100 nM) using nucleofection (see materials and methods). After 48 h, ASMCs were loaded with Fluo4 and UTP-stimulated [Ca2+]i responses were recorded (see materials and methods). Representative traces (D) and cumulative data (E) show effects of siRNA-mediated P2Y2 and P2Y4 receptor depletion on UTP-stimulated (100 μM) [Ca2+]i signals. All data sets are means ± SE for 4 experiments using cell preparations from 4 different animals. Ca2+ signals were significantly attenuated in ASMCs transfected with P2Y2 compared with P2Y4 or negative control (NC) siRNAs (**P < 0.01; one-way ANOVA; Bonferroni's post hoc test).
Depletion of Arrestin2, But Not Arrestin3, Prolongs UTP-Stimulated PLC Signaling
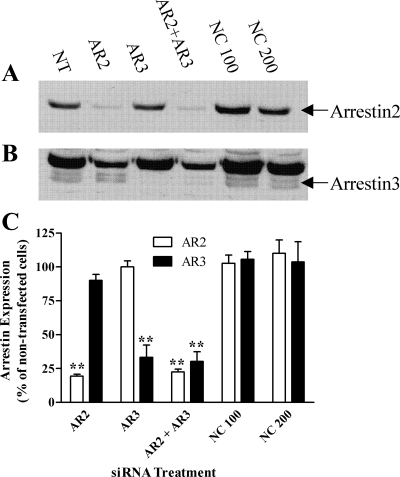
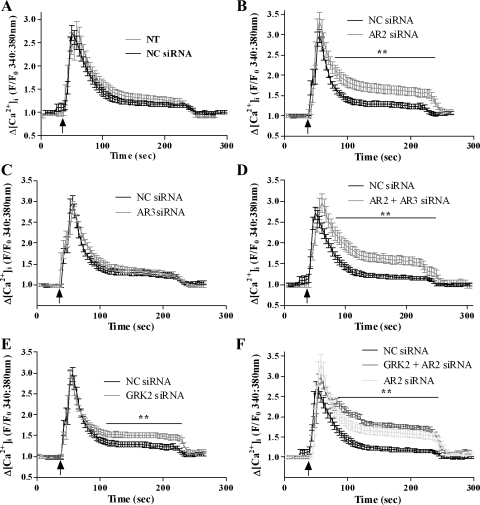
To assess whether arrestin proteins regulate UTP-stimulated PLC signaling in ASMCs, we used a siRNA approach to target endogenous arrestin expression. Optimal depletion of individual arrestins (>75% for arrestin2, >70% for arrestin3) was achieved 48 h postnucleofection with siRNA concentrations of 100 nM. Expression of the nontargeted arrestin isoform was unaffected (Fig. 2, A–C), and negative control siRNA was also without effect on arrestin2/3 expression (Fig. 2C). The effects of siRNA-mediated arrestin isoform depletion on UTP-stimulated Ca2+ signaling were examined in ASMCs loaded with the Ca2+-sensitive dye Fura2-AM. In the presence of the negative control siRNA, UTP-stimulated a similar robust peak-plateau rise in [Ca2+]i as that seen in nontransfected cells (Fig. 3A). Following arrestin2 depletion alone, or combined arrestin2/3 depletion, UTP-stimulated peak [Ca2+]i changes were unaltered; however, the plateau phases were elevated approximately twofold (Fig. 3, B and D). Contrastingly, UTP-stimulated [Ca2+]i responses were unaltered by arrestin3 knockdown (Fig. 3C). GRK-mediated GPCR phosphorylation normally precedes arrestin recruitment, an event specifically mediated by GRK2 for the P2Y2 receptor in mesenteric arterial smooth muscle (34). Depletion of endogenous GRK2 expression in ASMC using previously validated siRNAs (34) resulted in >80% knockdown (at 48 h after nucleofection of ASMCs with 10 nM anti-GRK2 siRNA; data not shown). UTP-stimulated peak [Ca2+]i changes were unaltered after GRK2 depletion, whereas the plateau phase was elevated (Fig. 3E). Combined GRK2/arrestin2 knockdown produced similar results to those obtained knocking down either protein alone (Fig. 3F).
Fig. 2.
siRNA-mediated arrestin knockdown in ASMCs. ASMCs were transfected with negative control (NC), anti-arrestin2 (AR2), or anti-arrestin3 (AR3) siRNAs using the nucleofection technique (see materials and methods). After 48 h, cells were lysed and arrestin expression levels were determined by Western blotting. A: representative immunoblot showing arrestin2 depletion. B: the same blot is shown after longer exposure to demonstrate arrestin3 (bottom band) depletion. Lane key: NT, nontransfected; AR2, anti-arrestin2 (100 nM); AR3, anti-arrestin3 (100 nM); AR2 + AR3, anti-arrestin2 (100 nM) and anti-arrestin3 (100 nM); NC100, negative control (100 nM); NC200, negative control (200 nM). C: cumulative data showing endogenous siRNA-targeted arrestin depletion. Data are shown as means ± SE for 4 separate transfections undertaken in ASMC preparations from 4 different animals. **P < 0.01 (one-way ANOVA; Dunnett's post hoc test) compared with arrestin expression in nontransfected or NC siRNA-transfected cells.
Fig. 3.
Arrestin knockdown enhances P2Y2 receptor-Ca2+ signaling. ASMCs were transfected with negative control, anti-arrestin2, or anti-arrestin3 siRNAs using the nucleofection technique (see materials and methods). After 48 h, ASMCs were loaded with the Ca2+-sensitive dye Fura2-AM for 1 h before UTP challenge (3 min; 100 μM). Application of UTP is indicated by the arrows. Mean traces (± SE; 32–52 cells prepared from 5 different animals) are shown after depletion of arrestin2 (B), arrestin3 (C), arrestin2 + arrestin3 (D), G protein-coupled protein kinase 2 (GRK2) alone (E), or arrestin2 + GRK2 knockdown (F). Comparisons of NC siRNA-transfected and nontransfected cell responses are shown in A. Arrestin2 and GRK2 depletion significantly enhanced UTP-stimulated [Ca2+]i signals when compared with negative control and arrestin3-transfected cells over the time points indicated by the bars (**P < 0.01; two-way ANOVA; Bonferroni's post hoc test).
Differential Effects of Arrestins and GRK2 on UTP- and ET1-Stimulated PLC Signaling
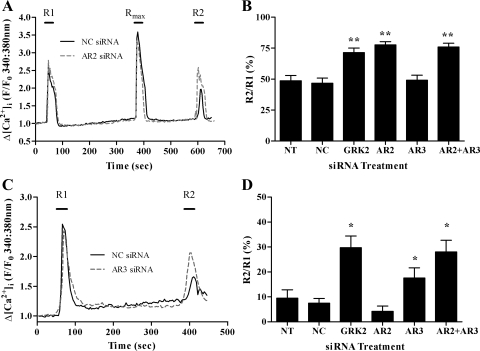
We have previously used both the IP3 biosensor eGFP-PH and the Ca2+-sensitive fluorescent dyes to measure agonist-stimulated desensitization of GPCR-mediated PLC responses, and using these techniques recently identified GRK2 and arrestin proteins as key regulators of P2Y2 and ETA receptor desensitization (33, 34). Here, using agonist-stimulated changes in [Ca2+]i, we have found that siRNA depletion of GRK2 attenuates both P2Y2 and ETA receptor desensitization in ASMCs (Fig. 4). Consistent with our previous findings, depletion of arrestin2, but not arrestin3, attenuated P2Y2-receptor desensitization (Fig. 4, A and B). Moreover, arrestin3, but not arrestin2 depletion (Fig. 4, C and D), partially attenuated ETA receptor desensitization though to a lesser extent than previously observed in arterial smooth muscle cells derived from mesentery (34).
Fig. 4.
Arrestin depletion differentially attenuates the desensitization of UTP- and ET1-stimulated Ca2+ signaling. ASMCs were either nontransfected or nucleofected with negative control, anti-GRK2, anti-arrestin2, anti-arrestin3, or anti-arrestin2 + anti-arrestin 3 siRNAs. After 48 h, ASMCs were loaded with the Ca2+-sensitive dye Fura2-AM for 1 h before application of standard desensitization protocols. For UTP, ASMCs were challenged with approximate EC50 concentrations of UTP (10 μM) for 30 s before (R1) and after (R2) addition of a maximal UTP concentration (Rmax: 100 μM, for 30 s). For R1/R2 for endothelin type A (ETA) receptor desensitization, a maximal ET1 concentration (50 nM) was applied for 30 s before (termed R1) and after (termed R2) a 5-min washout period. Reduced R2/R1 ratios are interpreted as an indication of receptor desensitization. Representative traces are shown for UTP (A) or ET1 (C)-treated cells transfected with NC or AR2 siRNAs (A) or NC or AR3 siRNAs (C). Desensitization of agonist-stimulated Ca2+ signaling was determined as the relative change in R2 response compared with R1. Cumulative data are shown for UTP (B) and ET1 (D) as means ± SE for % changes in R2 relative to R1; 20–38 cells for each treatment, from ≥4 experiments using cell preparations from ≥4 different animals. Statistically significant differences are indicated: *P < 0.05, **P < 0.01 vs. NC siRNA treatment (one-way ANOVA; Dunnett's post hoc test).
Contrasting Effects of Isoform-Specific Arrestin Knockdown on ET1- and UTP-Stimulated ERK1/2 and p38 MAPK Signaling
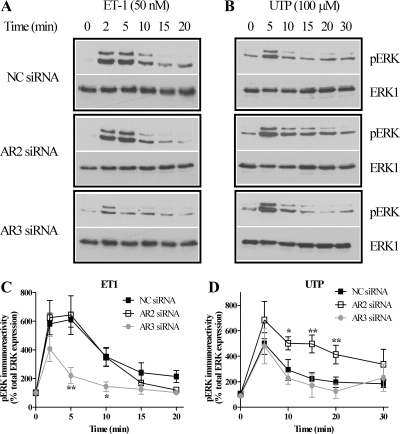
ET1 and UTP activate several MAPK pathways in ASMCs, including ERK1/2 and p38 signaling (2, 15). To assess the potential roles of arrestin proteins in regulating ET1- and UTP-activated ERK1/2 and p38 MAPK signaling, we selectively knocked down endogenous arrestin2 or arrestin3 in ASMCs. In cells nucleofected with negative control siRNA, ET1 (50 nM) or UTP (100 μM) challenge produced similar profiles of ERK1/2 phosphorylation (Fig. 5, A and B) as were observed in untransfected cells (data not shown). Following arrestin3 depletion, ET1-stimulated ERK1/2 phosphorylation was significantly decreased, whereas arrestin2 depletion was without effect (Fig. 5C). In contrast, reduced arrestin2 expression enhanced UTP-stimulated ERK1/2 phosphorylation, while suppression of arrestin3 was without effect (Fig. 5D).
Fig. 5.
Arrestins differentially regulate ET1- and UTP-stimulated ERK1/2 signaling. ASMCs were nucleofected with 100 nM negative control, anti-arrestin2, or anti-arrestin3 siRNAs. After 24 h, cells were serum starved for a further 24 h and then stimulated with ET1 (50 nM) or UTP (100 μM). A and B: ASMCs were lysed, and phospho-ERK1/2 (pERK) immunoreactivity was determined by standard immunoblotting techniques (top panels). To verify equal gel loading, all blots were stripped and reprobed with anti-ERK1 antibody (bottom panels). Representative immunoblots show the effects of isoform-specific arrestin depletion on ET1 (A)- or UTP (B)-stimulated phospho-ERK1/2 signals. C and D: cumulative densitometric analysis of ET1 (C)- or UTP (D)-stimulated ERK1/2 phosphorylation is shown: data are means ± SE for 4 experiments in cells prepared from 4 different animals. Arrestin3 depletion significantly attenuated ET1-stimulated phospho-ERK1/2 signals, while arrestin2 depletion significantly prolonged UTP-stimulated phospho-ERK1/2 signals when compared with cells transfected with NC siRNA (*P < 0.05, **P < 0.01; two-way ANOVA; Bonferroni's post hoc test).
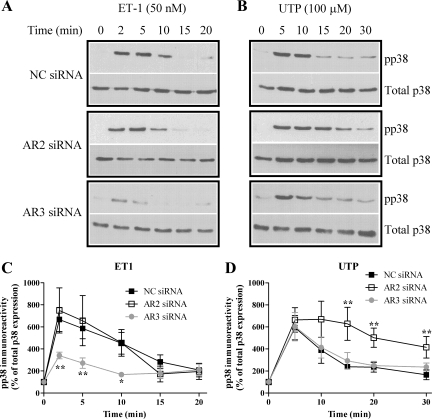
Time courses of ET1- or UTP-stimulated p38 MAPK phosphorylation were comparable following transfection of negative control siRNA (Fig. 6) to those observed in nontransfected cells (data not shown). Nucleofection with anti-arrestin3 siRNA suppressed ET1-stimulated p38 MAPK phosphorylation (at 2, 5, and 10 min; Fig. 6C), while anti-arrestin2 siRNA was without significant effect. Conversely, arrestin2 depletion caused a more sustained increase in p38 MAPK phosphorylation following UTP challenge (at 15, 20, and 30 min; Fig. 6D), while selective depletion of arrestin3 was without effect. Thus, while the ERK1/2 and p38 MAPK responses are similarly affected by manipulating the levels of endogenous arrestin isoforms for respective stimulations by ET1 or UTP, the data also provide striking contrasts in the arrestin2 and arrestin3 dependencies of MAPK responses stimulated by these two agents.
Fig. 6.
Arrestins differentially regulate ET1- and UTP-stimulated p38 MAPK signaling. ASMCs were nucleofected with 100 nM negative control, anti-arrestin2, or anti-arrestin3 siRNAs. After 24 h, cells were serum starved for a further 24 h and then stimulated with ET1 (50 nM) or UTP (100 μM). A and B: ASMCs were lysed, and phospho-p38 MAPK levels were determined by standard immunoblotting techniques (top panels). To verify equal gel loading, all blots were stripped and reprobed with anti-p38 antibody (bottom panels). Representative immunoblots show the effects of isoform-specific arrestin depletion on ET1 (A)- or UTP (B)-stimulated phospho-p38 MAPK signals. C and D: cumulative densitometric analysis of ET1 (C)- or UTP (D)-stimulated p38 phosphorylation is shown; data are means ± SE for 4 experiments in cells prepared from 4 different animals. Arrestin3 depletion significantly attenuated ET1-stimulated phospho-p38 MAPK signals, while arrestin2 depletion significantly prolonged UTP-stimulated phospho-p38 MAPK signals when compared with cells transfected with NC siRNA (*P < 0.05, **P < 0.01; two-way ANOVA; Bonferroni's post hoc test).
The fact that Ca2+ is known to activate many signaling pathways that ultimately lead to ERK phosphorylation, and that [Ca2+]i responses are significantly increased in the absence of arrestin2 (Fig. 3), could explain why ERK and p38 MAPK phosphorylation induced by UTP is enhanced following arrestin2 knockdown. Therefore, we examined the Ca2+ dependency of UTP-stimulated ERK1/2 and p38 MAPK phosphorylation. In the presence of a nominally Ca2+-free buffer, the profile of UTP-stimulated ERK1/2 or p38 MAPK phosphorylation was similar to that observed in normal Ca2+ (1.3 mM) Krebs-Henseleit buffer (data not shown). P2Y2 receptor activation can also recruit many PKC isoenymes (35); thus, their potential role in ERK1/2 phosphorylation was examined. Addition of the broad spectrum PKC inhibitor GF109203X (1 μM, 15 min preincubation) did not alter UTP-stimulated ERK phosphorylation (data not shown).
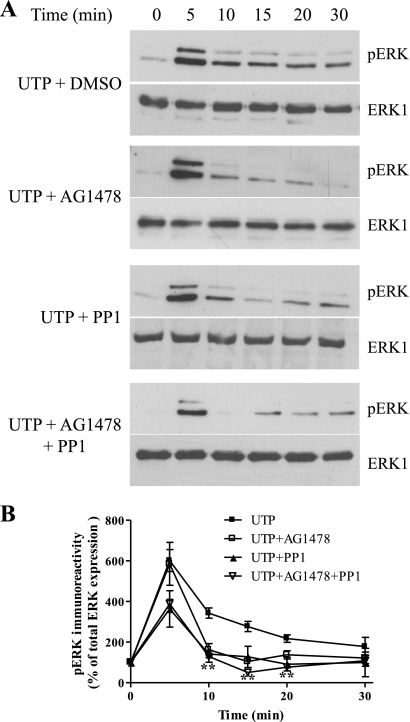
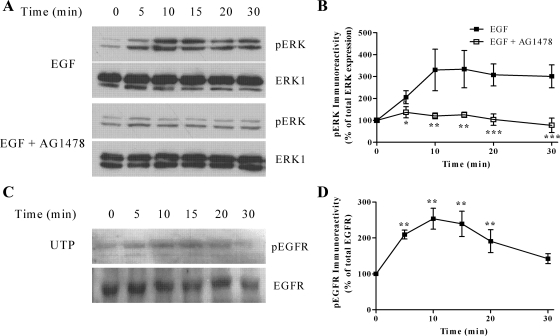
In other cell backgrounds the P2Y2 receptor is known to activate Src kinase to induce epithelial growth factor (EGF) receptor transactivation and ERK1/2 phosphorylation (39). Therefore, we examined Src and EGF receptor involvement in UTP-stimulated ERK1/2 phosphorylation. Our data show that inhibition of EGF receptor activity (AG1478, 250 nM, 30 min preincubation), a treatment that substantially inhibited EGF-stimulated ERK1/2 phosphorylation (see Fig. 7), leads to attenuation of the plateau phase of UTP-stimulated ERK phosphorylation, without affecting the initial peak phase (Fig. 8). Inclusion of the Src inhibitor PP1 (5 μM, 30 min preincubation) produced a similar attenuation of the plateau phase of UTP-stimulated ERK phosphorylation, but again had no effect on the initial peak phase (Fig. 8). Combined inhibition of the EGF receptor and Src did not suppress ERK1/2 signaling to a greater extent than either inhibitor alone (Fig. 8). Furthermore, UTP treatment induced phosphorylation of EGFR at Tyr-845, a known Src phosphorylation site (3) (Fig. 7). Our findings are consistent with the P2Y2 receptor recruiting Src to induce transactivation of the EGF receptor to support the sustained phase of UTP-stimulated ERK phosphorylation.
Fig. 7.
UTP-stimulated ERK1/2 phosphorylation is epithelial growth factor (EGF) receptor and Src dependent. A: confluent ASMCs were serum starved for 24 h before challenge with UTP (100 μM) for the indicated time periods. ASMCs were lysed, and phospho-ERK1/2 immunoreactivity was determined by standard immunoblotting techniques (top panels). To verify equal gel loading, all blots were stripped and reprobed with anti-ERK1 antibody (bottom panels). A: representative immunoblots show the effects of UTP alone or in the presence of the EGF receptor inhibitor AG1478 (250 nM), Src inhibitor PP1 (5 μM), or both inhibitors (added 30 min before UTP). B: cumulative densitometric analysis of UTP-stimulated ERK1/2 phosphorylation: data are shown as means ± SE for 4 experiments in cells prepared from 4 different animals. Inclusion of AG1478 or PP1 significantly attenuated UTP-stimulated ERK1/2 phosphorylation (**P < 0.01; two-way ANOVA, Bonferroni's post hoc test).
Fig. 8.
EGF-stimulated ERK1/2 phosphorylation is inhibited by preincubation with the EGF receptor (EGFR) inhibitor AG1478. A: confluent ASMCs were serum starved for 24 h before challenge with EGF (10 ng/ml) for the indicated time periods. ASMCs were lysed, and phospho-ERK1/2 immunoreactivity was determined by standard immunoblotting techniques (top panels). To verify equal gel loading, all blots were stripped and reprobed with anti-ERK1 antibody (bottom panels). A: representative immunoblots show EGF-stimulated ERK phosphorylation in the presence or absence of AG1478 (250 nM, preincubated for 30 min before EGF stimulation). B: cumulative densitometric analysis of EGF-stimulated ERK1/2 phosphorylation is shown in the presence or absence of AG1478; data are shown as means ± SE for 3 experiments in cells prepared from 3 different animals. EGF-stimulated ERK1/2 phosphorylation was significantly inhibited by AG1478 (*P < 0.05, **P < 0.01, ***P < 0.01; two-way ANOVA; Bonferroni's post hoc test). C: to determine whether UTP could stimulate EGFR phosphorylation, confluent ASMCs were serum starved for 24 h before challenge with UTP (100 μM) for the indicated time periods. ASMCs were lysed and phospho-EGFR levels determined by standard immunoblotting techniques (top). To verify equal gel loading, all blots were stripped and reprobed with anti-EGFR antibody (bottom). Representative immunoblots show the time dependency of EGF-stimulated EGFR phosphorylation. D: cumulative densitometric analysis of EGF-stimulated EGFR phosphorylation. Data are shown as means ± SE for 4 experiments in cells prepared from 4 different animals. EGF treatment significantly induced EGFR phosphorylation when compared with nontreated cells (**P < 0.01; one-way ANOVA; Dunnett's post hoc test).
GRK2 and Arrestin Dependencies of ET1- and UTP-Stimulated ASMC Migration
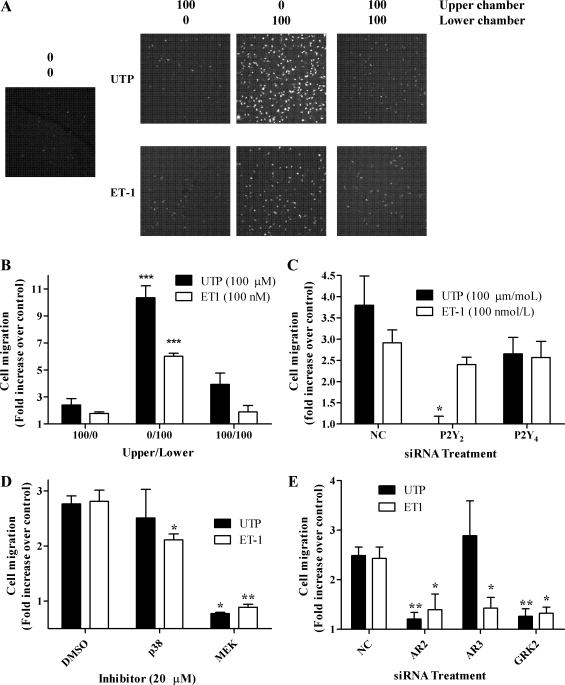
ET1 and UTP are known to promote smooth muscle cell migration through activation of signaling pathways downstream of ETA and P2Y receptor activation (15, 25). Furthermore, GRK2 and arrestin proteins have previously been reported to play a role in cell migration (12, 38). Therefore, the potential importance of arrestins and GRK2 in ET1- and UTP-stimulated cell migration was examined in ASMCs nucleofected with anti-arrestin2, anti-arrestin3, anti-GRK2, or negative control siRNAs. Cell migration induced by ET1 (100 nM) or UTP (100 μM) was time dependent, reaching a maximal effect (agonist-stimulated vs. control response) by 6 h (data not shown); therefore, this time point was used in all subsequent assays. A checkerboard analysis was undertaken to ensure that ASMC migration was due to UTP/ET1 stimulation, rather than random cell movement (11). Cell migration was 2.5-fold and 3-fold greater with UTP or ET1 present in the lower chambers, respectively, and only small numbers of cells migrated through to the lower chamber when agonists were present in the upper chamber alone (Fig. 9, A and B). Additionally, the receptor dependency of the migratory response to UTP was demonstrated by siRNA-dependent depletion of P2Y2 receptor expression (Fig. 9C). Moreover, both UTP- and ET1-stimulated ASMC migration was ablated following inclusion of the MEK inhibitor PD98059 (20 μM, Fig. 9D), a concentration previously shown to completely inhibit ERK1/2 phosphorylation in vascular smooth muscle cells (43). ET1-stimulated ASMC migration was also reduced, albeit to a lesser extent, in the presence of the p38 MAPK inhibitor SB203580 (20 μM, Fig. 9D). When compared with nontransfected cells, migratory behavior was unaltered in the presence of negative control siRNA (data not shown). Depletion of endogenous GRK2 or arrestin2 markedly decreased both ET1- and UTP-stimulated ASMC migration, while knockdown of arrestin3 attenuated ET1-, but not UTP-stimulated migration (Fig. 9E). Selective depletion of arrestin isoforms or GRK2 had no apparent effect on migratory responses to fetal calf serum (data not shown). These data strongly suggest that UTP and ET1 are promigratory agents for ASMCs and highlight a GRK2 and arrestin dependency of cell motility responses to these agonists.
Fig. 9.
ASMC migration is ERK, GRK2, and arrestin dependent. ASMCs were seeded onto the upper surfaces of Transwell inserts (8-μm pores). Transwells were placed into wells of a 24-well plate containing UTP (100 μM) or ET-1 (100 nM) in medium in the upper, lower, or both chambers. Cells were left at 37°C, 5% CO2 in humidified air for 6 h before processing as described in materials and methods. A and B: representative confocal images (A) and cumulative data (B) show that significant ASMC migration occurs only when UTP or ET1 are present in the lower chambers. C: endogenous levels of P2Y2 and or P2Y4 receptor expression were depleted by introducing P2Y2 or P2Y4 siRNAs (100 nM) using nucleofection (see materials and methods). After 48 h, ASMCs were seeded onto the upper surfaces of Transwell inserts and migration studies were undertaken with UTP (100 μM) in the lower chamber only. P2Y2 receptor knockdown significantly attenuated UTP-stimulated ASMC migration when compared with negative control and P2Y4 siRNA-transfected cells (*P < 0.05; one-way ANOVA; Newman-Keuls post hoc test). D: ASMCs were incubated with the p38 MAPK inhibitor SB203580 or the MEK inhibitor PD98059 (for 30 min before agonist addition, and throughout the assay). UTP- and ET1-stimulated cell migrations were ablated following MEK/ERK inhibition, while p38 MAPK inhibition caused a small but significant decrease in ET1-stimulated cell migration. E: endogenous levels of arrestin or GRK2 in ASMCs were depleted by introducing arrestin2, arrestin3, or GRK2 siRNAs (100 nM) by nucleofection (see materials and methods). After 48 h, AMSCs were subjected to migration assay as described above. Statistical significance is indicated for responses relative to vehicle or cells transfected with NC siRNA (*P < 0.05, **P < 0.01, ***P < 0.01; one-way ANOVA; Newman-Keuls post hoc test).
DISCUSSION
Previous data from recombinant cell systems have indicated that ETA (5) and P2Y2 (22) receptors may be equally adept at recruiting arrestins 2 and 3; however, such studies are not necessarily predictive of how or if these receptors are regulated by the nonvisual arrestins in native cell systems. We have recently identified more specific receptor-arrestin pairings in smooth muscle cells isolated from resistance arteries, with arrestin3 and arrestin2 being respective key negative regulators of ETA and P2Y2 receptor-PLC signaling (33, 34). Now, we have shown that siRNA-mediated depletions of endogenous GRK2 and/or arrestin3 in ASMC significantly attenuated ETA receptor desensitization, while arrestin2 knockdown was ineffective; conversely, endogenous GRK2 and/or arrestin2 depletion markedly attenuated P2Y2 receptor desensitization, while arrestin3 knockdown had no effect, indicating that similar receptor-arrestin selectivities occur in the different vascular preparations.
Further to their well-defined roles in GPCR desensitization and internalization (18, 30), arrestin proteins have been shown to act as agonist-regulated adaptor scaffolds promoting the activation of other signaling pathways, including the MAPKs (e.g., ERK1/2) (14, 26). Indeed, arrestin3, an arrestin isoform known to scaffold ERK1/2 (14, 26), is central to angiotensin II-mediated ERK1/2 signaling and proliferation in ASMCs (23). Because we identified a role for arrestin3 in the regulation of the ETA receptor, the ability of this isoform to mediate ET1-stimulated ERK1/2 signaling in ASMCs has been examined. Following arrestin3 knockdown, the ET1-stimulated ERK1/2 response was markedly reduced, whereas arrestin2 knockdown had no effect. Since arrestin3 does not appear to regulate P2Y2 receptor signaling, it was not surprising to find that its depletion had no effect on UTP-stimulated ERK1/2 signaling. These data extend the array of GPCRs that utilize arrestin3, at least in part, to link receptor activation to ERK signaling to include ETA receptors endogenously expressed in ASMC. Interestingly, arrestin2 depletion enhanced UTP-stimulated ERK1/2 signaling, confirming the role of this arrestin isoform in receptor desensitization, but providing no evidence for a scaffolding function in ERK activation.
ET1 and UTP are known also to activate other MAPK signaling pathways in arterial smooth muscle, including the p38 MAPK pathway (2, 15). As for ERK1/2, knockdown of arrestin3, but not arrestin2, markedly attenuated the p38 MAPK response across the ET1-stimulated time course assessed here. While our study provides the first report that ET1-stimulated p38 MAPK signaling is suppressed following selective depletion of endogenous arrestin3, a similar phenomenon has been observed previously for GPCRs in other cell backgrounds (8, 44). Although the mechanism linking arrestin3 and p38 MAPK signaling has not been investigated here, studies in astrocytes indicate that κ-opioid receptor-stimulated p38 MAPK signaling colocalizes with arrestin3 (8), suggesting that arrestin3 might act as a phospho-p38 MAPK scaffold. Additionally, apoptosis signal-regulating kinase 1 (ASK1), an upstream kinase responsible for activating p38 MAPK (via MKK4/7), has also been implicated in CXC chemokine receptor 4 (CXCR4)-mediated arrestin3-dependent regulation of p38 MAPK signaling (44), and arrestin/ASK1 complexes have been coimmunoprecipitated (48). Interestingly, the effects of arrestin3 on p38 MAPK signaling appear to be dependent on receptor-mediated recruitment of this arrestin isoform, since UTP-stimulated p38 MAPK phosphorylation was unaffected by depletion of endogenous arrestin3 in ASMC. The increased UTP-mediated ERK1/2 and p38 MAPK signaling following arrestin2 depletion could potentially be explained by an ability of arrestin2 to negatively regulate arrestin3 signaling (1). However, since arrestin3 does not appear to be important in P2Y2 receptor regulation, it is more likely that arrestin2 depletion attenuates P2Y2 receptor desensitization, prolonging MAPK signaling via a receptor-dependent and arrestin-independent mechanism. Indeed, our observation that P2Y2 receptor desensitization was attenuated in arrestin2-depleted cells supports this notion. It is also possible that elevated [Ca2+]i observed following arrestin2 knockdown drives enhanced ERK phosphorylation in ASMC; however, our data also suggest that UTP-stimulated ERK1/2 signaling is Ca2+ independent and PKC independent, which further supports the idea that enhanced MAPK signaling is a direct result of prolonged P2Y2 receptor activity in the absence of arrestin2.
Smooth muscle cell migration is thought to play a central role in the development of atherosclerotic plaques and re-stenosis lesions (4, 15). Although these pathophysiological changes are stimulated by a plethora of different inputs, UTP and ET1 are strong promoters of vascular remodeling (15, 25). Here we report an essential requirement for GRK2, arrestin2, and arrestin3 in ET1-stimulated migration and for GRK2 and arrestin2 in UTP-stimulated ASMC migration. These observations appear to be stimulus dependent, since fetal calf serum-stimulated migration was unaffected by arrestin2 or arrestin3 knockdown. This may at first seem surprising, since several components of serum are potent GPCR agonists, and thus might also utilize GRK/arrestin proteins to stimulate cell migration; for example, Lysophosphatidic acid receptor stimulation has been reported to recruit β-arrestin and activate Ral-dependent migration of human breast tumor cells (27). However, at present there is no evidence to suggest that GRK2 and arrestins are significantly involved in migratory responses downstream of GPCRs stimulated by serum components, and it should be noted that serum also contains high levels of non-GPCR-dependent growth factors, which may be major contributors to the promigratory effects of serum.
Since ETA and P2Y2 receptor activation each recruit GRK2 (33, 34), it is unsurprising that this GRK isoenzyme is involved in ET1- and UTP-stimulated cell migration. Indeed, previous studies in different cell backgrounds have shown that GRK2 can regulate cell migration through an interaction with GPCR-kinase interacting (GIT) proteins (37, 40), to activate the Rac/PAK/MEK/ERK pathway, increasing focal adhesion turnover and F-actin polymerization (37, 38). Alternatively, GRK2 might phosphorylate ezrin (10), promoting actin skeleton rearrangement, membrane ruffling, and filopodia, which are all necessary for cell motility. Therefore, it is conceivable that either process underlies ETA and P2Y2 receptor-GRK2-mediated ASMC migration. In addition, both arrestins 2 and 3 have been implicated in the regulation of GPCR-mediated cell migration through an array of mechanisms (12, 14). For arrestin3, these pathways are likely to involve activation of ERK1/2 and/or p38 MAPK, reflecting its role as a signaling scaffold (14, 30). Therefore, as expected, arrestin3 plays a critical role in ETA receptor-stimulated ERK1/2 and p38 MAPK signaling in ASMC. Moreover, depletion of endogenous arrestin3 and pharmacological inhibition of ERK1/2 (and to a lesser extent p38 MAPK) attenuated ET1-induced cell migration, suggesting a role for arrestin3-mediated PLC/ERK/p38 MAPK signaling in ETA receptor-driven cell migration. UTP can promote ASMC migration via ERK1/2 and Rho-dependent pathways (11), which, combined with our finding that arrestin2 depletion enhances UTP-stimulated ERK1/2 signaling, suggests that endogenous arrestin2 depletion should augment UTP-induced ASMC migration. Conversely, we find that arrestin2 depletion virtually eliminates ETA and P2Y2 receptor-induced migration, suggesting that arrestin2-dependent cell migration is ERK/p38 MAPK-independent. Interestingly, arrestin2 can induce Src-mediated EGF receptor transactivation to stimulate Akt-dependent signaling and cell migration (9). Together with our finding that the sustained phase of P2Y2 receptor-mediated ERK phosphorylation is dependent on Src and EGF receptor activity, it might be anticipated that, following arrestin2 knockout, UTP-stimulated ERK signals should be attenuated. However, we found that phosphorylated ERK1/2 levels in response to UTP were enhanced following arrestin2 depletion, suggesting that arrestin2-independent mechanisms mediate ERK signaling in response to UTP.
Similar findings were reported in mouse embryonic fibroblasts, where α2A-adrenergic receptor-induced ERK phosphorylation is arrestin/Src dependent. However, in the absence of arrestins, ERK signals are enhanced and prolonged via a Ras/Raf/MEK-dependent, Src-independent mechanism (16). Previous work in 1321N1 astrocytoma cells suggests that the agonist-bound P2Y2 receptors can recruit and activate Src, before EGFR recruitment and phosphorylation (29). Since P2Y2 receptors remain active for longer in the absence of arrestin2, it is possible that this mechanism is responsible for the enhanced and prolonged UTP-stimulated MAPK signals observed in ASMC. UTP-induced migration is also reported to occur following the synthesis of osteopontin, via an ERK- and Rho-dependent mechanism (11). Interestingly, arrestins have been shown to modulate Rho-dependent membrane blebbing, an actin-dependent process known to contribute to cell motility (19). However, since this process is mediated by arrestin3 and inhibited by arrestin2, it appears unlikely to be the mechanism by which arrestin2 regulates agonist-driven migration in ASMC. When expressed in 1321N1 astrocytoma cells, P2Y2 receptors are known to interact directly with αv/β3/β5-integrins via an Arg-Gly-Asp (RGD) domain in the first extracellular loop. These integrins subtypes are also known to be expressed in the cardiovascular system (32). The P2Y2/integrin interaction results in a Rho-dependent activation of LIM kinase and subsequent phosphorylation of cofilin (28), which is reported to control growth factor-mediated ASMC migration (42). Importantly, arrestins can regulate actin-assembly/disassembly through G protein/Ca2+-independent interactions with the actin filament-severing protein cofilin (49). The activity of cofilin is determined by its phosphorylation status, being active only when dephosphorylated. Cofilin phosphorylation is regulated by the relative activity of two proteins, cofilin-specific phosphatase (activated by arrestins) and LIM kinase (inhibited by arrestins) (49). Although it is not yet established whether an individual arrestin, or a combination of arrestins 2 and 3, undertakes these roles, evidence points towards arrestin2 as the dominant scaffold for the cofilin-specific phosphatase (49), which could explain why agonist-driven migration requires the presence of arrestin2. Finally, arrestins have also been reported to play a role in β-catenin stabilization and nuclear translocation, to promote gene expression and cell proliferation and/or invasion (17, 41). Arrestins are known to stabilize β-catenin, either through direct recruitment of Akt, or via interaction with axin to promote glycogen synthase kinase-3 (GSK3) phosphorylation, preventing GSK3-axin association (17). Therefore, it is possible that one or more of these pathways could be inhibited following arrestin depletion, preventing ASMC migration.
Finally, it is tempting to speculate that elevated levels of GRK2 found in hypertensive arterial smooth muscle (21) may promote smooth muscle cell migration, contributing to the atherosclerotic/re-stenosis changes reported to occur in hypertensive arteries (4, 15, 31). In the present study, we have demonstrated distinct vasoconstrictor-dependent profiles of arrestin-mediated regulation of MAPK signaling and ASMC migration, both of which are central to the progression of vascular remodeling. Although it is currently unclear whether expression of arrestin isoforms is altered during hypertension, our findings suggest that arrestins might undertake similar roles to GRK2 in regulating smooth muscle migration, implying that enhanced expression of these proteins, while initially useful to prevent excessive vasoconstriction, may not be beneficial in the longer term.
GRANTS
This work was supported by the British Heart Foundation (PG06/161/22136 and RG06/008/22062).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
G.E.M., C.P.N., P.J.B., and J.M.W. performed the experiments; G.E.M., C.P.N., P.J.B., and J.M.W. analyzed the data; G.E.M., C.P.N., R.A.J.C., and J.M.W. interpreted the results of the experiments; G.E.M. C.P.N., and J.M.W. prepared the figures; G.E.M. and J.M.W. drafted the manuscript; C.P.N., R.A.J.C., and J.M.W. edited and revised the manuscript; N.B.S., R.A.J.C., and J.M.W. conception and design of research; R.A.J.C., and J.M.W. approved the final version of the manuscript.
ACKNOWLEDGMENTS
We thank Robert J. Lefkowitz (Duke University, Durham, NC) for kindly providing the arrestin (A1CT) antibody.
Footnotes
This article is the topic of an Editorial Focus by Kathryn E. Meier (31a).
REFERENCES
- 1.Ahn S, Wei H, Garrison TR, Lefkowitz RJ. Reciprocal regulation of angiotensin receptor-activated extracellular signal-regulated kinases by β-arrestins 1 and 2. J Biol Chem 279: 7807–7811, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Berk BC. Vascular smooth muscle growth: autocrine growth mechanisms. Physiol Rev 81: 999–1030, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Biscardi JS, Maa MC, Tice DA, Cox ME, Leu TH, Parsons SJ. c-Src-mediated phosphorylation of the epidermal growth factor receptor on Tyr845 and Tyr1101 is associated with modulation of receptor function. J Biol Chem 274: 8335–8343, 1999 [DOI] [PubMed] [Google Scholar]
- 4.Bousette N, Giaid A. Endothelin-1 in atherosclerosis and other vasculopathies. Can J Physiol Pharmacol 81: 578–587, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Bremnes T, Paasche JD, Mehlum A, Sandberg C, Bremnes B, Attramadal H. Regulation and intracellular trafficking pathways of the endothelin receptors. J Biol Chem 275: 17596–17604, 2000 [DOI] [PubMed] [Google Scholar]
- 6.Brighton PJ, Rana S, Challiss RA, Konje JC, Willets JM. Arrestins differentially regulate histamine- and oxytocin-evoked phospholipase C and mitogen-activated protein kinase signalling in myometrial cells. Br J Pharmacol 162: 1603–1617, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brinks HL, Eckhart AD. Regulation of GPCR signaling in hypertension. Biochim Biophys Acta 1802: 1268–1275, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bruchas MR, Macey TA, Lowe JD, Chavkin C. κ opioid receptor activation of p38 MAPK is GRK3- and arrestin-dependent in neurons and astrocytes. J Biol Chem 281: 18081–18089, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buchanan FG, Gorden DL, Matta P, Shi Q, Matrisian LM, DuBois RN. Role of β-arrestin 1 in the metastatic progression of colorectal cancer. Proc Natl Acad Sci USA 103: 1492–1497, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cant SH, Pitcher JA. G protein-coupled receptor kinase 2-mediated phosphorylation of ezrin is required for G protein-coupled receptor-dependent reorganization of the actin cytoskeleton. Mol Biol Cell 16: 3088–3099, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chaulet H, Desgranges C, Renault MA, Dupuch F, Ezan G, Peiretti F, Loirand G, Pacaud P, Gadeau AP. Extracellular nucleotides induce arterial smooth muscle cell migration via osteopontin. Circ Res 89: 772–778, 2001 [DOI] [PubMed] [Google Scholar]
- 12.Cotton M, Claing A. G protein-coupled receptors stimulation and the control of cell migration. Cell Signal 21: 1045–1053, 2009 [DOI] [PubMed] [Google Scholar]
- 13.Davies LM, Purves GI, Barrett-Jolley R, Dart C. Interaction with caveolin-1 modulates vascular ATP-sensitive potassium (KATP) channel activity. J Physiol 588: 3255–3266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DeWire SM, Ahn S, Lefkowitz RJ, Shenoy SK. β-arrestins and cell signaling. Annu Rev Physiol 69: 483–510, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Erlinge D, Burnstock G. P2 receptors in cardiovascular regulation and disease. Purinergic Signal 4: 1–20, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feng YH, Wang L, Wang Q, Li X, Zeng R, Gorodeski GI. ATP stimulates GRK-3 phosphorylation and β-arrestin-2-dependent internalization of P2X7 receptor. Am J Physiol Cell Physiol 288: C1342–C1356, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Force T, Woulfe K, Koch WJ, Kerkela R. Molecular scaffolds regulate bidirectional crosstalk between Wnt and classical seven-transmembrane-domain receptor signaling pathways. Sci STKE 2007: pe41, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Gesty-Palmer D, Luttrell Heptahelical terpsichory LM. Who calls the tune? J Recept Signal Transduct Res 28: 39–58, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Godin CM, Ferguson SS. The angiotensin II type 1 receptor induces membrane blebbing by coupling to Rho A, Rho kinase, and myosin light chain kinase. Mol Pharmacol 77: 903–911 [DOI] [PubMed] [Google Scholar]
- 20.Govindan S, Taylor EJ, Taylor CW. Ca2+ signalling by P2Y receptors in cultured rat aortic smooth muscle cells. Br J Pharmacol 160: 1953–1962, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gros R, Chorazyczewski J, Meek MD, Benovic JL, Ferguson SS, Feldman RD. G-protein-coupled receptor kinase activity in hypertension: increased vascular and lymphocyte G-protein receptor kinase-2 protein expression. Hypertension 35: 38–42, 2000 [DOI] [PubMed] [Google Scholar]
- 22.Hoffmann C, Ziegler N, Reiner S, Krasel C, Lohse MJ. Agonist-selective, receptor-specific interaction of human P2Y receptors with β-arrestin-1 and -2. J Biol Chem 283: 30933–30941, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim J, Ahn S, Rajagopal K, Lefkowitz RJ. Independent β-arrestin2 and Gq/protein kinase Cξ pathways for ERK stimulated by angiotensin type 1A receptors in vascular smooth muscle cells converge on transactivation of the epidermal growth factor receptor. J Biol Chem 284: 11953–11962, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim J, Zhang L, Peppel K, Wu JH, Zidar DA, Brian L, DeWire SM, Exum ST, Lefkowitz RJ, Freedman NJ. β-arrestins regulate atherosclerosis and neointimal hyperplasia by controlling smooth muscle cell proliferation and migration. Circ Res 103: 70–79, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kirchengast M, Munter K. Endothelin and restenosis. Cardiovasc Res 39: 550–555, 1998 [DOI] [PubMed] [Google Scholar]
- 26.Lefkowitz RJ, Shenoy SK. Transduction of receptor signals by β-arrestins. Science 308: 512–517, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Li TT, Alemayehu M, Aziziyeh AI, Pape C, Pampillo M, Postovit LM, Mills GB, Babwah AV, Bhattacharya M. β-arrestin/Ral signaling regulates lysophosphatidic acid-mediated migration and invasion of human breast tumor cells. Mol Cancer Res 7: 1064–1077, 2009 [DOI] [PubMed] [Google Scholar]
- 28.Liao Z, Seye CI, Weisman GA, Erb L. The P2Y2 nucleotide receptor requires interaction with αv integrins to access and activate G12. J Cell Sci 120: 1654–1662, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu J, Liao Z, Camden J, Griffin KD, Garrad RC, Santiago-Perez LI, Gonzalez FA, Seye CI, Weisman GA, Erb L. Src homology 3 binding sites in the P2Y2 nucleotide receptor interact with Src and regulate activities of Src, proline-rich tyrosine kinase 2, and growth factor receptors. J Biol Chem 279: 8212–8218, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Luttrell LM, Lefkowitz RJ. The role of β-arrestins in the termination and transduction of G-protein-coupled receptor signals. J Cell Sci 115: 455–465, 2002 [DOI] [PubMed] [Google Scholar]
- 31.Mayet J, Hughes A. Cardiac and vascular pathophysiology in hypertension. Heart 89: 1104–1109, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31a.Meier KE. Arrestins as signaling modulators: the plot thickens. Focus on “Arrestins 2 and 3 differentially regulate ETA and P2Y2 receptor-mediated cell signaling and migration in arterial smooth muscle.” Am J Physiol Cell Physiol (December 21, 2011). doi:10.1152/ajpcell.00444.2011 [DOI] [PubMed]
- 32.Moiseeva EP. Adhesion receptors of vascular smooth muscle cells and their functions. Cardiovasc Res 52: 372–386, 2001 [DOI] [PubMed] [Google Scholar]
- 33.Morris GE, Nelson CP, Everitt D, Brighton PJ, Standen NB, Challiss RA, Willets JM. G protein-coupled receptor kinase 2 and arrestin2 regulate arterial smooth muscle P2Y-purinoceptor signalling. Cardiovasc Res 89: 193–203, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morris GE, Nelson CP, Standen NB, Challiss RA, Willets JM. Endothelin signalling in arterial smooth muscle is tightly regulated by G protein-coupled receptor kinase 2. Cardiovasc Res 85: 424–433, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nelson CP, Willets JM, Davies NW, Challiss RA, Standen NB. Visualizing the temporal effects of vasoconstrictors on PKC translocation and Ca2+ signaling in single resistance arterial smooth muscle cells. Am J Physiol Cell Physiol 295: C1590–C1601, 2008 [DOI] [PubMed] [Google Scholar]
- 36.Penela P, Murga C, Ribas C, Tutor AS, Peregrin S, Mayor F., Jr Mechanisms of regulation of G protein-coupled receptor kinases (GRKs) and cardiovascular disease. Cardiovasc Res 69: 46–56, 2006 [DOI] [PubMed] [Google Scholar]
- 37.Penela P, Ribas C, Aymerich I, Eijkelkamp N, Barreiro O, Heijnen CJ, Kavelaars A, Sanchez-Madrid F, Mayor F., Jr G protein-coupled receptor kinase 2 positively regulates epithelial cell migration. EMBO J 27: 1206–1218, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Penela P, Ribas C, Aymerich I, Mayor F., Jr New roles of G protein-coupled receptor kinase 2 (GRK2) in cell migration. Cell Adh Migr 3: 19–23, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peterson TS, Camden JM, Wang Y, Seye CI, Wood WG, Sun GY, Erb L, Petris MJ, Weisman GA. P2Y2 nucleotide receptor-mediated responses in brain cells. Mol Neurobiol 41: 356–366, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Premont RT, Claing A, Vitale N, Perry SJ, Lefkowitz RJ. The GIT family of ADP-ribosylation factor GTPase-activating proteins. Functional diversity of GIT2 through alternative splicing. J Biol Chem 275: 22373–22380, 2000 [DOI] [PubMed] [Google Scholar]
- 41.Rosano L, Cianfrocca R, Masi S, Spinella F, Di Castro V, Biroccio A, Salvati E, Nicotra MR, Natali PG, Bagnato A. β-arrestin links endothelin A receptor to beta-catenin signaling to induce ovarian cancer cell invasion and metastasis. Proc Natl Acad Sci USA 106: 2806–2811, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.San Martin A, Lee MY, Williams HC, Mizuno K, Lassegue B, Griendling KK. Dual regulation of cofilin activity by LIM kinase and Slingshot-1L phosphatase controls platelet-derived growth factor-induced migration of human aortic smooth muscle cells. Circ Res 102: 432–438, 2008 [DOI] [PubMed] [Google Scholar]
- 43.Schauwienold D, Plum C, Helbing T, Voigt P, Bobbert T, Hoffmann D, Paul M, Reusch HP. ERK1/2-dependent contractile protein expression in vascular smooth muscle cells. Hypertension 41: 546–552, 2003 [DOI] [PubMed] [Google Scholar]
- 44.Sun Y, Cheng Z, Ma L, Pei G. β-arrestin2 is critically involved in CXCR4-mediated chemotaxis, and this is mediated by its enhancement of p38 MAPK activation. J Biol Chem 277: 49212–49219, 2002 [DOI] [PubMed] [Google Scholar]
- 45.Willets JM, Challiss RA, Nahorski SR. Non-visual GRKs: are we seeing the whole picture? Trends Pharmacol Sci 24: 626–633, 2003 [DOI] [PubMed] [Google Scholar]
- 46.Willets JM, Mistry R, Nahorski SR, Challiss RA. Specificity of G protein-coupled receptor kinase 6-mediated phosphorylation and regulation of single-cell M3 muscarinic acetylcholine receptor signaling. Mol Pharmacol 64: 1059–1068, 2003 [DOI] [PubMed] [Google Scholar]
- 47.Willets JM, Taylor AH, Shaw H, Konje JC, Challiss RA. Selective regulation of H1 histamine receptor signaling by G protein-coupled receptor kinase 2 in uterine smooth muscle cells. Mol Endocrinol 22: 1893–1907, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang YM, Wang KQ, Zhou GM, Zuo J, Ge JB. Endothelin-1 promoted proliferation of vascular smooth muscle cell through pathway of extracellular signal-regulated kinase and cyclin D1. Acta Pharmacol Sin 24: 563–568, 2003 [PubMed] [Google Scholar]
- 49.Zoudilova M, Kumar P, Ge L, Wang P, Bokoch GM, DeFea KA. β-arrestin-dependent regulation of the cofilin pathway downstream of protease-activated receptor-2. J Biol Chem 282: 20634–20646, 2007 [DOI] [PubMed] [Google Scholar]