Abstract
DRA (downregulated in adenoma) or SLC26A3 is the major apical anion exchanger mediating Cl− absorption in intestinal epithelial cells. Disturbances in DRA function and expression have been implicated in diarrheal conditions such as congenital chloride diarrhea and inflammatory bowel diseases. Previous studies have shown that DRA is subject to regulation by short-term and transcriptional mechanisms. In this regard, we have recently shown that short-term treatment by lysophosphatidic acid (LPA), an important bioactive phospholipid, stimulates Cl−/HCO3−(OH−) exchange activity via an increase in DRA surface levels in human intestinal epithelial cells. However, the long-term effects of LPA on DRA at the level of gene transcription have not been examined. The present studies were aimed at investigating the effects of LPA on DRA function and expression as well as elucidating the mechanisms underlying its transcriptional regulation. Long-term LPA treatment increased the Cl−/HCO3− exchange activity in Caco-2 cells. LPA treatment (50–100 μM) of Caco-2 cells significantly stimulated DRA mRNA levels and DRA promoter activity (−1183/+114). This increase in DRA promoter activity involved the LPA2 receptor and phosphatidylinositol 3-kinase (PI3K)/AKT pathways. Progressive deletions from −1183/+114 to −790/+114 abrogated the stimulatory effects of LPA, indicating that the −1183/−790 promoter region harbors LPA response elements. Utilizing EMSA and mutational studies, our results showed that LPA induced the DRA promoter activity in a c-Fos-dependent manner. LPA also increased the protein expression of c-Fos and c-Jun in Caco-2 cells. Furthermore, overexpression of c-Fos but not c-Jun enhanced the DRA promoter activity. This increase in DRA transcription in response to LPA indicates that LPA may act as an antidiarrheal agent and could be exploited for the treatment of diarrhea associated with inflammatory or infectious diseases of the gut.
Keywords: SLC26A3, chloride absorption, human intestine, c-Fos, phosphatidylinositol 3-kinase
NaCl absorption is an electroneutral process that involves the coupled operation of Na+/H+ and Cl−/HCO3− exchangers (8, 10). Disturbances in NaCl absorption lead to diarrhea associated with various bacterial infections and inflammatory bowel diseases (8, 10). Cl−/HCO3− exchangers are the key intestinal transporters that play an important role in transepithelial Cl− absorption and HCO3− secretion (8). DRA (downregulated in adenoma) and PAT-1 (putative anion transporter-1) have been characterized as the apical membrane Cl−/HCO3− exchangers of intestinal epithelial cells that belong to the family of SLC26 anion exchangers (21). Previous studies have demonstrated that DRA and PAT-1 mRNA are expressed along the entire length of the human intestine (10). However, their expression exhibits regional and surface-crypt axis differences. Studies have shown DRA expression to be higher in the mouse colon compared with small intestine, whereas PAT-1 expression was higher in the mouse small intestine compared with colon (10).
DRA has been shown to be the major apical Cl− absorbing isoform in the colon and ileum as mutations in DRA gene have been implicated in congenital chloride diarrhea, which is characterized by high-volume watery diarrhea with a massive loss of chloride (1, 18). Additionally, studies have shown that DRA-knockout mice show reduction in apical Cl−/HCO3− exchange activity and exhibit diarrheal phenotype with increased chloride and water content in stools (28). PAT-1-knockout mice also show reduction in Cl−/HCO3− exchange activity but do not exhibit diarrheal phenotype (32). It appears that, although PAT-1 is involved in transporting Cl−, however, unlike DRA it is not directly coupled to the water movement.
Since impairment of intestinal epithelial cell luminal membrane Cl−/HCO3− exchange process has been shown to result in diarrhea, it is therefore critical to understand the molecular mechanisms underlying the expression and regulation of apical Cl−/HCO3− exchangers. Previous studies from our laboratory and others have shown that apical Cl−/HCO3− exchangers are subject to extensive regulation via both transcriptional and posttranslational mechanisms (2, 24, 27, 30). In this regard, we have recently shown that lysophosphatidic acid (LPA), a naturally occurring glycerophospholipid, acts as a proabsorptive agent by stimulating apical Cl−/HCO3− exchange activity in Caco-2 cells via LPA receptor 2 and phosphatidylinositol 3 (PI3)-kinase (PI3K)/AKT-dependent pathways (30). However, nothing is known about the long-term effects of LPA on DRA and PAT-1 gene expression. In this regard, LPA has been shown previously to play a critical role in the transcriptional regulation of various genes in different cell types via activation of signaling events downstream of LPA receptors that further activate transcription factors (7, 11, 25).
Therefore, studies were undertaken to examine the long-term effects of LPA on apical Cl−/HCO3− exchangers and to elucidate the molecular mechanisms involved in regulating DRA and PAT-1 gene expression. Our results suggest that LPA stimulates DRA expression and function in Caco-2 cells via LPA2 receptor- and PI3K/AKT-dependent pathways. Our data also showed that LPA stimulates DRA promoter activity in a c-Fos-dependent manner.
MATERIALS AND METHODS
Materials.
1-Oleoyl-sn-glycerol 3-phosphate sodium salt (LPA) was purchased from Sigma-Aldrich or Avanti Polar Lipids (Alabaster, AL) and was prepared in PBS containing 0.1% BSA (vol/vol). Caco-2 cells and minimum essential medium (MEM) were obtained from American Type Culture Collection (ATCC, Manassas, VA). Radionuclide 36Cl was obtained from American Radiolabeled Chemicals (St. Louis, MO). 4,4′-Diisothiocyanostilbene-2,2′ disulfonic acid (DIDS) and niflumic acid (NFA) were obtained from Sigma-Aldrich. Pharmacological inhibitor LY294002 was purchased from Biomol (Plymouth Meeting, PA) and triciribine was procured from Calbiochem (San Diego, CA). Luciferase assay system was procured from Promega (Madison, WI), and a β-galactosidase assay kit was obtained from Clontech (Palo Alto, CA). c-Fos and c-Jun antibodies were procured from Santa Cruz Biotechnology (Santa Cruz, CA). The c-Fos and c-Jun expression vectors were the generous gift from Dr. Nancy Colburn (National Cancer Institute). All other chemicals were of at least reagent grade and were purchased from Sigma or Fisher Scientific (Pittsburgh, PA).
Cell culture.
Caco-2 cells were grown at 37°C in a 5% CO2 environment in T-75-cm2 plastic flasks. Cells were cultured in MEM with high glucose, 50 units/ml penicillin, 50 μg/ml streptomycin, 2 mg/l gentamycin, and 20% fetal bovine serum. Caco-2 cells between passages 25 and 45 were plated on Transwell inserts (Costar, Corning, NY) at a density of 1 × 104 cells/Transwell, and uptake studies were performed using fully differentiated cells at days 10-14 postplating. For the promoter studies, Caco-2 cells were plated at a density of 130 × 105 cells on a 24-well plate and were transfected by utilizing Amaxa nucleofactor system while still in suspension. At 24 h posttransfection, cells were treated with different doses of LPA for different time points in 1% FBS-containing media.
Assessment of Cl−/HCO3− exchange activity.
Cl−/HCO3− exchange activity was measured as described previously by us (30). Caco-2 cells were incubated with loading buffer pH 8.5 for 30 min at room temperature and were rapidly washed with 1 ml tracer-free uptake mannitol buffer containing 260 mM mannitol, 20 mM Tris/2-(N-morpholino)ethanesulfonic acid, pH 7.0. Cells were then incubated with the uptake buffer containing 1.4 μCi of 36Cl−(2.9 mM) of hydrochloric acid (specific activity: 17.12 mCi/g) for 5 min in the absence or presence of 600 μM DIDS or 200 μM NFA. The 5-min time period was chosen because it falls within the linear range of Cl− uptake in this system. The uptake was stopped by washing the cells rapidly two times with 1 ml of ice-cold phosphate-buffered saline, pH 7.2. The cells were then solubilized by incubation with 0.5 N NaOH for 4 h. The protein concentration was measured by the method of Bradford (3) and the radioactivity was counted by a Packard Liquid Scintillation Analyzer, TRI-CARB 1600-TR (Packard Instruments; PerkinElmer, Downers Grove, IL). The Cl−/HCO3− exchange activity was assessed as DIDS-sensitive 36Cl− uptake, and the values were expressed as nanomoles per milligram protein per 5 min.
Western blotting.
Caco-2 cells were treated with 50 μM LPA for 24 h. After treatment, cells were washed with ice-cold 1× PBS and lysed in 20 mM Tris·HCl, pH 7.5, 150 mM NaCl, 1% Triton X-100, 1 mM EDTA, 1 mM EGTA, and 1× protease cocktail inhibitor mixture. The cells were lysed by sonication and the lysate was centrifuged at 7,000 rpm for 7 min at 4°C. Protein concentration was determined by the Bradford assay.
To examine the expression levels of DRA, c-Fos, c-Jun, and β-actin, 75–100 μg of cell lysates were loaded on SDS-polyacrylamide gels and transblotted to nitrocellulose membranes; 1× PBS and 5% nonfat dry milk were used as a blocking buffer for 1 h. The membranes were then probed with human DRA (1:100 dilution) or human c-Fos (1:100 dilution) or human c-Jun (1:100 dilution) or human β-actin antibody (1:1,000 dilution) in 1× PBS and 2.5% nonfat dry milk overnight at 4°C for DRA and 1 h for c-Fos, c-Jun, and β-actin at room temperature. The membranes were washed four times with the wash buffer containing 1× PBS and 0.1% Tween-20 for 5 min. Finally, the membranes were probed with horseradish peroxidase-conjugated goat anti-rabbit IgG antibody (1:2,000 dilution) for 1 h, and the bands were visualized with enhanced chemiluminescence detection reagents.
RNA extraction and quantitative real-time PCR.
Qiagen RNeasy kits were utilized to extract the RNA from control or LPA-treated Caco-2 cells. Extracted RNA was amplified by Brilliant SYBR Green QRT-PCR Master Mix Kit (Stratagene, Santa Clara, CA) utilizing gene specific primers as shown in Table 1. Relative levels of DRA and PAT-1 mRNA were expressed as fold changes and were normalized to internal control gene.
Table 1.
Primers used for real-time PCR
| Gene | Species | Accession No. | Primer Sequence |
|---|---|---|---|
| SLC26A3 or DRA | Human | BC025671 | (F) 5′-TTCAGTTGCCAGCGTCTATTC-3′ |
| (R) 5′-GTGTTTTGCCTCCTGTGCTCT-3′ | |||
| SLC26A6 or PAT-1 | Human | NM_022911 | (F) 5′-AGATGCCCCACTACTCTGTCCT-3′ |
| (R) 5′-ATCCACACCACACCTCTGCTT-3′ | |||
| β-Actin | Human | NM_001101.3 | (F) 5′-CATGTTTGAGACCTTCAACAC-3′ |
| (R) 5′-CCAGGAAGGAAGGCTGGAA-3′ | |||
| Mutant primer sequences: | |||
| 5′-AGATGGTTCCCAGGTCTCTGACACTTGTAAAGGGGAATATTCTTTACTG-3′ | |||
| 5′-TCTACCAAGGGTCCAGAGACTGTGAACATTTCCCCTTATAAGAAATGAC-3′ | |||
DRA, downregulated in adenoma; PAT-1, putative anion transporter-1.
Transient transfection studies.
Caco-2 cells were transiently transfected with different DRA promoter fragments cloned upstream of the luciferase reporter gene utilizing Amaxa nucleofactor system as described previously by us (27). At 24 h posttransfection, cells were treated with LPA or LPA receptor agonists or signaling inhibitors for different time points and were lysed in reporter lysis buffer. Luciferase and β-galactosidase were measured utilizing kits from Promega and Clontech according to the manufacturer's instructions. Promoter activity was calculated as a ratio of luciferase value to β-galactosidase value and is expressed as % of control. For c-Fos and c-Jun overexpression studies, transient transfections were performed via Amaxa electroporation as per manufacturer's instructions. At 24 h posttransfection, luciferase and β-galactosidase were measured as described above.
Nuclear extracts and EMSA.
Nuclear extracts were prepared from the control and LPA-treated cells by utilizing the nuclear extraction kit from Pierce Biotechnology according to the manufacturer's instructions. Oligonucleotides for the gel shift assay were synthesized by Invitrogen. The sequences for the oligonucleotides of the DRA promoter are (−1155/−1133): 5′-TCTCTGACATGAGTAAAGGGGAAT-3′ and 5′-ATTCCCCTTTACTCATGTCAGAGA-3′; (−846/−825): 5′-GCAAAAAGGATGAGGTGAAAAACA-3′ and 5′-TGTTTTTCACCTCATCCTTTTTGC-3′; (−888/−865): 5′-GAATCTTAGGAAATGCCAAAGAAA-3′ and 5′-TTTCTTTGGCATTTCCTAAGATTC-3′; (−850/−827): 5′-ATTGGCAAAAAGGATGAGGTGAAA-3′ and 5′-TTTCACCTCATCCTTTTTGCCAAT-3′. The sequences for the mutant (−1155/−1133 region) oligonucleotide are 5′-TCTCTGACACTTGTAAAGGGGAAT-3′ and 5′-ATTCCCCTTTACAAGTGTCAGAGA-3′. Double-stranded oligonucleotides were end labeled with γ32P-ATP (Amersham, Arlington Heights, IL), and the DNA/protein binding reactions were performed as previously described by us (26). In the competition assays, 50- or 100-fold molar excess of unlabeled oligonucleotide or unlabeled mutant oligonucleotide was added at the start of the reaction.
Site-directed mutagenesis.
Site-directed mutations were carried out in the (−1155/−1133) region of DRA promoter by using the QuickChange Site-directed mutagenesis kit (Stratagene, La Jolla, CA) according to the manufacturer's instructions. Desired mutations were confirmed by sequencing.
Statistical analysis.
Results are expressed as means ± SE and represent the data from three to five independent experiments. One-way ANOVA with Tukey's multiple-comparison test or unpaired t-test was used for statistical analysis. Differences between control and treated groups were considered significant at P < 0.05.
RESULTS
Long-term treatment with LPA stimulates Cl−/HCO3− exchange activity.
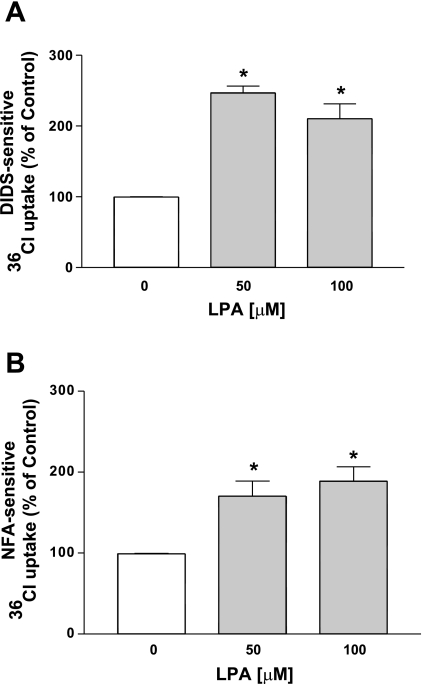
Our recent studies showed that the acute treatment of Caco-2 cells with LPA (100 μM, 30 min) increased the Cl−/HCO3− exchange activity (30). To determine the long-term effects of LPA on Cl−/HCO3− exchange activity, Caco-2 cells were treated with different doses of LPA (50–100 μM) for 24 h in a medium containing 1% FBS, and Cl−/HCO3− exchange activity was measured as DIDS- or NFA-sensitive 36Cl− uptake after base loading the cells. At 24 h LPA treatment increased the Cl−/HCO3− exchange activity (∼2.5-fold) with maximal stimulation at 50 μM LPA (Fig. 1, A and B). Therefore, 50 μM LPA was used for the subsequent experiments. These data suggest that, similar to the acute effects of LPA on Cl−/HCO3− exchangers, the 24-h LPA treatment also stimulates Cl−/HCO3− exchange activity in Caco-2 cells.
Fig. 1.
Lysophosphatidic acid (LPA; 24 h) stimulates apical Cl−/HCO3− exchange activity in Caco-2 cells. Caco-2 cells grown on Transwell inserts were treated with different doses of LPA (50–100 μM) for 24 h in a cell culture medium containing 1% FBS. Cl−/HCO3− exchange activity was measured as DIDS-sensitive (600 μM) 36Cl− uptake (A) or niflumic acid (NFA)-sensitive (200 μM) 36Cl− uptake (B) for 5 min. Results are expressed as % of control and represent means ± SE of 3 separate experiments performed in triplicate. *P < 0.05 compared with control.
LPA increases DRA but not PAT-1 mRNA expression in Caco-2 cells.
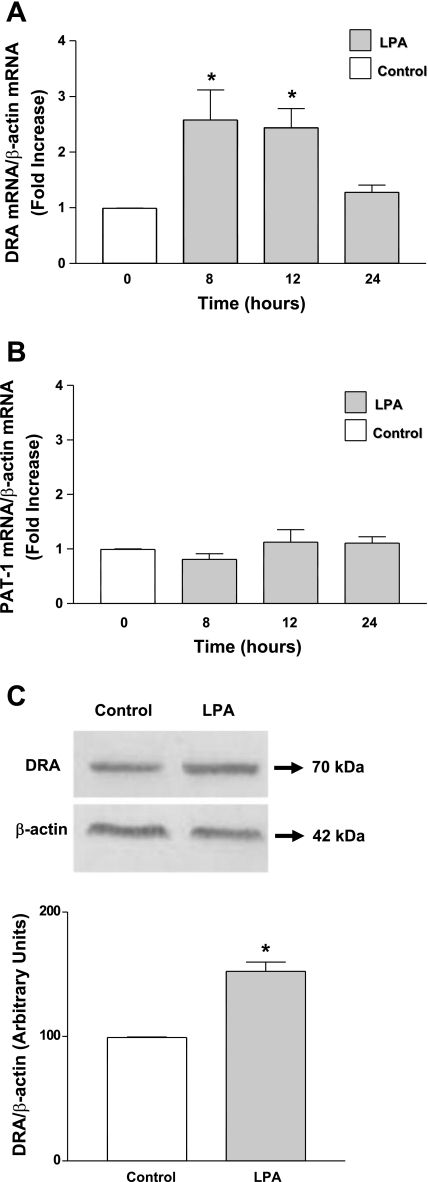
Since chronic LPA treatment increased the Cl−/HCO3− exchange activity, we next examined whether the long-term LPA treatment alters DRA and PAT-1 mRNA and protein expression. As shown in Fig. 2A, LPA treatment increased DRA mRNA expression (2-fold) at 8 h that persisted until 12 h. However, PAT-1 mRNA levels remained unaltered in response to LPA treatment (Fig. 2B). These results suggest that long-term effects of LPA are specific to DRA. We further examined the effect of LPA on DRA protein expression. Consistent with the increase with mRNA results, LPA treatment significantly increased DRA protein expression (Fig. 2C). Densitometric analysis of the protein bands showed that LPA treatment increased DRA protein levels by ∼50% compared with control (Fig. 2C). Our results clearly indicate that the long-term LPA treatment specifically increases the DRA mRNA and protein expression but not PAT-1 mRNA.
Fig. 2.
LPA stimulates DRA (downregulated in adenoma) mRNA and protein expression levels in Caco-2 cells. Caco-2 cells were treated with 50 μM LPA in a cell culture medium containing 1% FBS. RNA was amplified utilizing DRA (A) or PAT-1 (B) gene-specific primers for real-time PCR quantification. Data represent the relative expression of DRA or PAT-1 normalized to the respective actin mRNA (internal control) levels. Results are expressed as fold changes in mRNA levels compared with control mRNAs considered as 1.0. C: cell lysates were subjected to 10% SDS-polyacrylamide gel and transferred to nitrocellulose membrane. The blot was immunostained with rabbit anti-DRA antibody. A representative blot of 3 separate experiments is shown. Results of densitometric analysis are expressed as DRA/actin levels. Values represent means ± SE of 3 different experiments. *P < 0.05 compared with control.
LPA activates DRA promoter activity in Caco-2 cells.
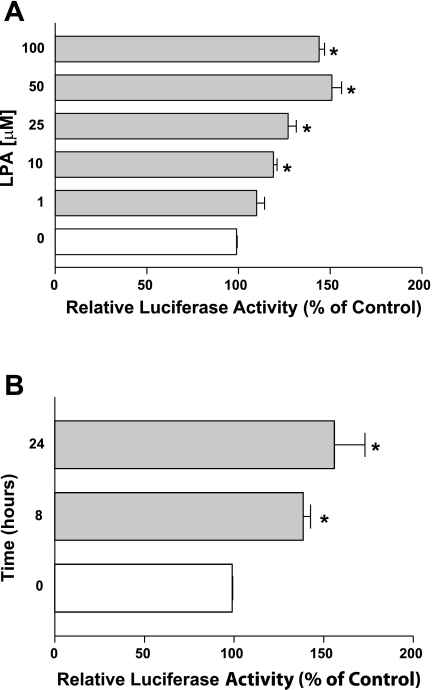
DRA has been shown to be regulated via transcriptional mechanisms (24, 27). Since LPA increased DRA mRNA levels, we next investigated whether LPA alters the DRA gene at the transcriptional level. We have previously characterized the activity of DNA fragments representing the promoter region of the DRA gene and its 5′ progressive deletions (27). Utilizing these DRA promoter constructs, we examined the effects of LPA on DRA promoter activity in Caco-2 cells. Caco-2 cells were transiently cotransfected with DRA promoter constructs along with pCMVβgal and were then treated with LPA. As shown in Fig. 3A, LPA treatment for 8 h significantly increased the activity of DRA promoter comprising the region p-1183/+114 in a dose-dependent manner with the maximal increase at 50 μM. A time course for LPA treatment on DRA promoter activity was also assessed for different time points in Caco-2 cells. LPA-mediated increase in DRA promoter activity occurred at 8 h and persisted until 24 h (Fig. 3B). These data suggest that the LPA-mediated increase in DRA gene expression is via an increase in DRA promoter activity.
Fig. 3.
LPA stimulates DRA promoter activity in a dose-dependent manner. Caco-2 cells were transiently transfected with DRA luciferase promoter construct (p-1183/+114) along with the mammalian expression vector for β-galactosidase (pCMVβgal). At 24 h posttransfection, Caco-2 cells were treated with different doses of LPA ranging from 1 to 100 μM for 8 h (A) or with 50 μM LPA for different time points (B). Promoter activity was measured by luciferase assay and luciferase values were normalized to β-galactosidase activity. Results are expressed as % of control and represent means ± SE of 4 separate experiments. *P < 0.05 compared with control.
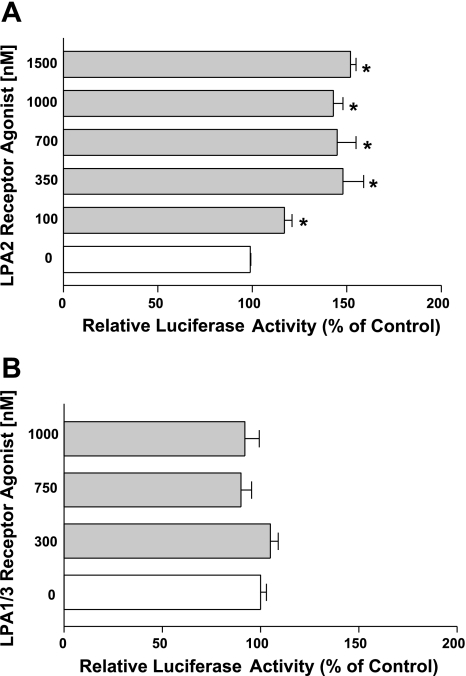
LPA2 receptor is involved in the induction of DRA promoter activity.
LPA mediates its effects through seven G protein-coupled receptors: LPA receptors 1–7 (4). Previously we have shown the involvement of LPA2 receptor in mediating the acute effects of LPA on Cl−/HCO3− exchange activity (30). To identify which receptor subtype is involved in stimulating DRA promoter activity, we used specific LPA2 and LPA1/3 receptor agonists. Cells were treated with different doses of LPA2 receptor agonist (Dodecyl phosphate) or LPA1/3 agonist (VPC 31143) for 8 h. As shown in Fig. 4A, treatment with LPA2 receptor agonist (100–1500 nM) significantly increased the DRA promoter activity whereas LPA1/3 receptor agonist treatment (300–1,000 nM) did not show any effect (Fig. 4B). These results indicate that LPA2 but not LPA1 and LPA3 receptors are involved in stimulating DRA promoter activity.
Fig. 4.
LPA2 receptor but not LPA1/3 receptor is involved. Caco-2 cells transfected with DRA luciferase promoter construct were treated with different doses of the LPA2 receptor agonist dodecyl phosphate (A) or with the LPA1/3 receptor agonist VPC31143 for 8 h (B). Luciferase values were normalized to β-galactosidase activity. Results are expressed as % of control and represent means ± SE of 3 separate experiments. *P < 0.05 compared with control.
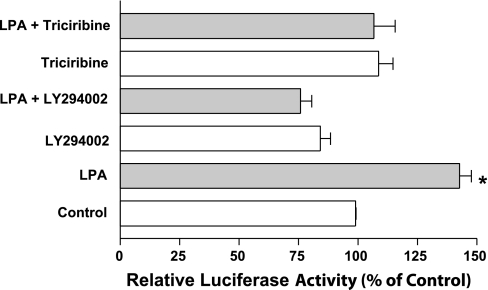
LPA induced DRA promoter activity is PI3K/AKT dependent.
Previous studies have shown that LPA2 receptor signals through PI3K/AKT pathway in Caco-2 cells (33). Our studies also showed that acute LPA treatment increased Cl−/HCO3− exchange activity via PI3K/AKT-dependent pathways (30). We thus examined the involvement of PI3K/AKT pathway in induction of DRA promoter activity utilizing PI3K- and AKT-specific pharmacological inhibitors. Caco-2 cells were pretreated with PI3K inhibitor (LY294002, 50 μM) or AKT inhibitor (triciribine, 1 μM) for 1 h followed by coincubation with LPA for another 8 h. LPA significantly increased the DRA promoter activity as shown in Fig. 5. This increase in DRA promoter activity was completely abrogated in the presence of PI3K- and AKT-specific inhibitors, indicating that PI3K and AKT are involved in LPA-mediated induction of DRA promoter activity. Consistent with the results of our promoter studies, LPA-mediated increase in DRA mRNA level (1.6 ± 0.09-fold) was attenuated in the presence of LY294002 (0.4 ± 0.08-fold) and triciribine (1.2 ± 0.01-fold) compared with the control.
Fig. 5.
LPA-mediated stimulation of DRA promoter activity is phosphatidylinositol-3 kinase (PI3K)/AKT dependent. Caco-2 cells transfected with DRA luciferase promoter construct were pretreated with specific PI3K inhibitor LY294002 (50 μM) or with the AKT inhibitor triciribine (1 μM) for 60 min followed by coincubation with 50 μM LPA for 8 h in media containing 1% FBS. Results are expressed as % of control and represent means ± SE of 3 separate experiments. *P < 0.05 compared with control.
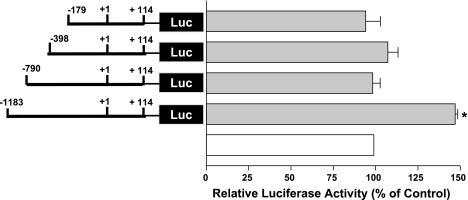
LPA response elements are located in the p-1183/−790 region of DRA promoter.
To determine the region involved in LPA-mediated stimulation of DRA promoter activity, we utilized different constructs of DNA representing progressive 5′ deletions of DRA promoter and examined the effect of LPA on the activity of each promoter construct. As shown in Fig. 6, LPA treatment significantly increased the DRA promoter activity of the full-length promoter (p-1183/+114). However, deletion of the DRA promoter from p-1183/+114 to p-790/+114 blocked the LPA-mediated activation of DRA promoter activity. Further deletions from p-790/+114 to p-398/+114 and to p-179/+114 were also not affected by LPA. These data showed that LPA response elements are located in −1183 to −790 region of the DRA promoter.
Fig. 6.
The region between −1183 and −790 of DRA promoter harbors the LPA response element. Caco-2 cells were transiently transfected with full-length DRA promoter and its progressive 5′ deletion constructs and were treated with 50 μM LPA for 8 h. Luciferase values were normalized to β-galactosidase activity. Results are expressed as % of respective control for each promoter construct and represent means ± SE of 3 separate experiments. *P < 0.05 compared with control. Luc, luciferase reporter construct.
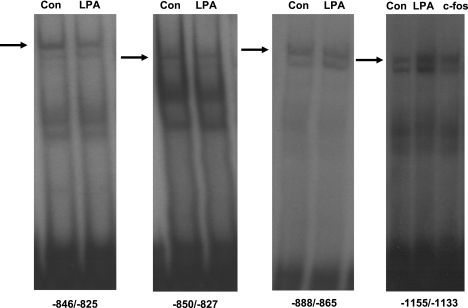
LPA increases the binding of putative transcription factor to the −1155/−1133 region.
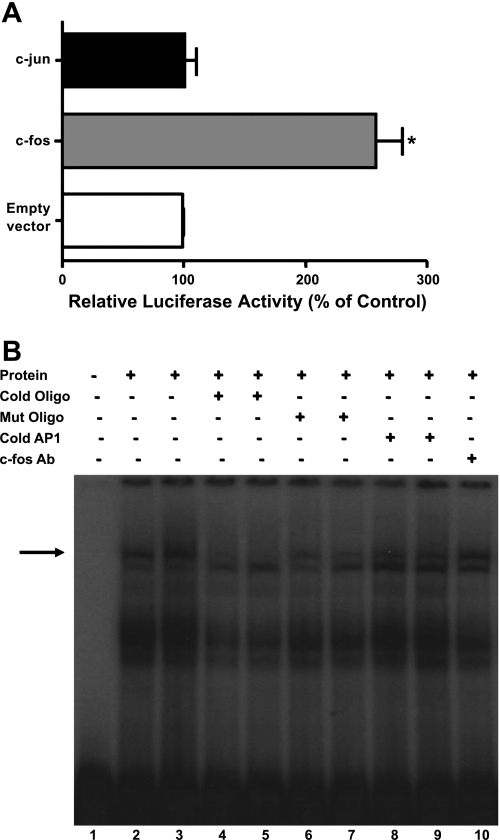
To further identify the cis-elements mediating the effects of LPA on DRA promoter activity, EMSA was performed utilizing different end-labeled probes representing potential binding sites in the region of DRA promoter exhibiting the LPA response. As shown in Fig. 7, the nuclear extracts from the LPA-treated cells did not alter the DNA protein binding to fragments representing the regions −846/−825, −850/−827, and −888/−865 of DRA promoter. Interestingly, the region −1155/−1133 showed significantly increased DNA protein binding in response to LPA compared with control (Fig. 7). Sequence analysis of the −1155/−1133 region revealed a potential binding site for activator protein 1, which is a heterodimer of c-Fos and c-Jun proteins. Noticeably, DNA protein binding was also increased in the cells overexpressing c-Fos compared with control cells (Fig. 7).
Fig. 7.
The LPA response cis-element is located in the −1155/−1133 region of DRA promoter. EMSA was performed by using double-stranded oligonucleotides (−846/−825, −850/−827, −888/−865 and −1155/−1133) as end-labeled probes; 10 μg of nuclear proteins from control or LPA-treated Caco-2 cells were combined with the probe and incubated at 37°C for 30 min. Samples were resolved on 7% nondenaturing polyacrylamide gels. LPA treatment and c-Fos overexpression increased the DNA protein binding in −1155/−1133 region compared with control (Con). Gels are shown as a representative of 3 separate experiments.
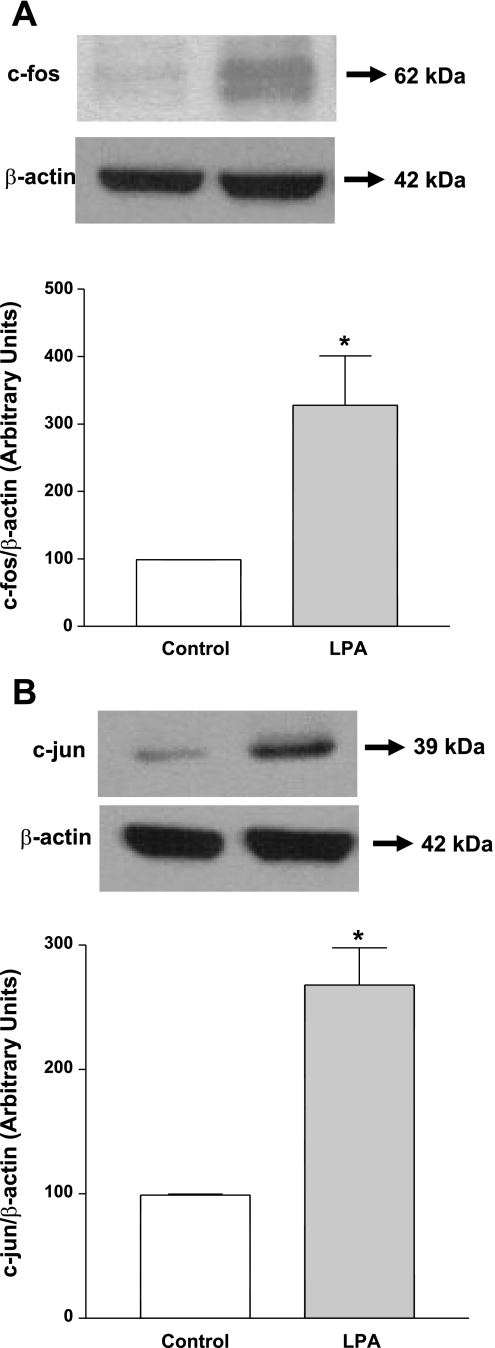
LPA increases c-Fos and c-Jun protein expression in Caco-2 cells.
To examine the involvement of AP1, we first examined the protein expression of c-Fos and c-Jun in Caco-2 cells. LPA (50 μM, 24 h) treatment of the Caco-2 cells significantly increased the c-Fos and c-Jun protein expression as shown in Fig. 8, A and B. Densitometric analysis showed ∼3-fold and 2.5-fold increase in c-Fos and c-Jun protein expression levels, respectively. Furthermore, c-Fos but not c-Jun overexpression significantly increased the DRA promoter activity in Caco-2 cells (Fig. 9A). These studies implicate a role of c-Fos in LPA-mediated increase in DRA promoter activity and rule out the role of c-Jun in transactivating DRA promoter. To further examine the potential involvement of c-Fos, competition assays were performed utilizing EMSA. As shown in Fig. 9B, DNA protein binding was increased in response to LPA (lane 3) compared with control (lane 2). As shown before, DNA protein binding was also increased in the cells overexpressing c-Fos compared with control cells (Fig. 7). This DNA protein binding was abolished in the presence of increasing amounts of excess of cold unlabeled oligo (50× in lane 4 and 100× in lane 5), but not completely in the presence of cold unlabeled mutated oligo (50× in lane 6 and 100× in lane 7), indicating the specificity of binding and that the mutations introduced in to the cold unlabeled oligo attenuated the protein binding. However, DNA protein complex was not eliminated in the presence of cold unlabeled consensus to the site AP1 (50× in lane 8 and 100× in lane 9), and no supershift band was observed in the presence of c-Fos antibodies (lane 10). These results suggest that LPA may increase the binding of an as-yet-unidentified transcription factor to the DRA promoter in a c-Fos-dependent manner rather than a direct binding of c-Fos itself.
Fig. 8.
LPA increases c-Fos and c-Jun protein expression in Caco-2 cells. Cells were treated with LPA (50 μM) for 24 h in cell culture medium. Cell lysate was subjected to 10% SDS-polyacrylamide gel and transferred to nitrocellulose membrane. The blot was immunostained with anti-c-Fos (A) or anti-c-Jun (B) antibody. A representative blot of 3 separate experiments is shown. Results of densitometric analysis are expressed as % of control of c-Fos or c-Jun/β-actin levels. Values represent means ± SE of 3 different experiments. *P < 0.05 compared with control.
Fig. 9.
c-Fos overexpression transactivates DRA promoter activity but does not bind directly to LPA response element. A: Caco-2 cells were cotransfected with DRA promoter and c-Fos or c-Jun overexpression vector or empty vector and the promoter activity was measured by luciferase assay. Results are expressed as % of control and represent means ± SE of 3 separate experiments. *P < 0.05 compared with control. B: EMSA was performed by using a double-stranded oligonucleotide (−1155/−1133) as end-labeled probe. Lane 1 depicts free probe. LPA increased the DNA protein binding (lane 3) compared with control (lane 2). Competition experiments were performed in the presence of 50- or 100-fold molar excess of unlabeled cold oligo (lanes 4 and 5), cold mutant (Mut) oligo (lanes 6 and 7), cold consensus AP1 (lanes 8 and 9), and c-Fos antibody (lane 10). Gels are shown as a representative of 3 separate experiments.
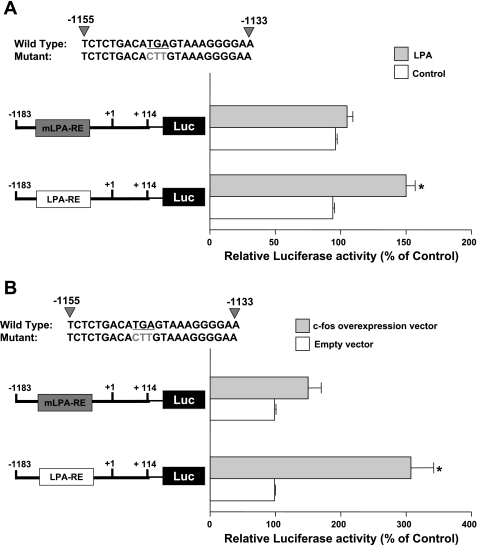
Potential cis-element −1155/−1133 is essential for LPA-mediated increase in DRA promoter activity.
To further confirm the role of −1155/−1133 region in LPA-mediated stimulation of DRA promoter activity in Caco-2 cells, we introduced the mutations utilized in EMSA analysis to the same region in DRA promoter. Mutations in the −1155/−1133 region blocked the LPA-mediated stimulation of DRA activity in Caco-2 cells (Fig. 10A). Furthermore, c-Fos overexpression induced increase in DRA promoter activity was abrogated with the mutations in the −1155/−1133 region (Fig. 10B). These results suggest that −1155/−1133 is essential for the LPA-mediated effects.
Fig. 10.
LPA response cis-element (−1155/−1133) is involved. A: Caco-2 cells were transiently transfected with full-length DRA promoter and a construct with mutated −1155/−1133 region (mutations are shown in gray color mLPA-RE). At 24 h posttransfection, cells were treated with 50 μM LPA for 8 h. B: Caco-2 cells were cotransfected with c-Fos overexpression vector or empty vector and full-length DRA promoter or mutated construct (gray color LPA-RE). Results are expressed as % of control and represent means ± SE of 3 separate experiments. *P < 0.05 compared with control.
DISCUSSION
Mammalian intestine is involved in the absorption of solutes and electrolytes such as Na+ and Cl−. Electroneutral NaCl absorption is known to occur via the parallel functioning of dual cationic and anionic exchangers, namely Na+/H+ exchangers (NHEs) and Cl−/HCO3− exchangers (DRA and PAT-1) (10). Decreased absorption or increased secretion of water and electrolytes can lead to diarrheal conditions associated with various gastrointestinal disorders such as inflammatory bowel diseases and bacterial infections (10). Various studies have shown the regulation of Na+/H+ and Cl−/HCO3− exchangers via both posttranslational and transcriptional mechanisms (2, 8, 10, 24, 26, 27, 30). In this regard, we have shown that a bioactive phospholipid, LPA, acts as a proabsorptive agent by stimulating the Cl−/HCO3− exchange activity in Caco-2 cells via posttranslational mechanism by increasing the surface levels of DRA on the apical membranes (30). LPA has also been shown to modulate the expression of various genes such as interleukin-13, early growth response gene (EGR-1), and cox-2 (7, 11, 25). However, there are no studies regarding the regulation of DRA gene expression and promoter activity by LPA. In the present study, our data demonstrated that LPA stimulates Cl−/HCO3− exchange activity via increasing the DRA gene expression and its promoter activity.
LPA is a biologically active phospholipid that mediates a number of physiological and pathophysiological effects (6, 19, 20). It is known to inhibit chloride secretion by inhibiting CFTR channels and to stimulate sodium absorption by increasing NHE3 activity in intestinal epithelia (15, 16). With regard to the long-term effects of LPA on Cl−/HCO3− exchangers, we found that LPA stimulated the Cl−/HCO3− exchange activity at 24 h, indicating that it might be due to the transcriptional regulation of Cl−/HCO3− exchangers. LPA treatment increased the DRA mRNA and protein levels with no change in PAT-1 mRNA levels, indicating that the effects of LPA are specific to DRA. The specificity of these long-term effects of LPA on DRA expression is parallel to our previous short-term study, which showed that LPA treatment enhanced Cl−/HCO3− exchange activity via increased surface levels of DRA but not PAT-1 (30). Also, earlier studies from our group showed that incubating Caco-2 cells with the culture supernatant of Lactobacillus acidophilus increased the expression of DRA and not PAT-1 in Caco-2 cells (24). In contrast, both PAT-1 and DRA have been shown to be regulated via transcriptional mechanisms by interferon-γ (IFN-γ) (26, 27). Our results suggest that LPA may act as an antidiarrheal agent by upregulating the major Cl−/HCO3− exchanger DRA via transcriptional mechanisms.
LPA treatment increased the DRA promoter activity at 8 h, and this increase persisted till 24 h. LPA is known to mediate its effects via interacting with G protein-coupled receptors (4). About seven isoforms of LPA receptors have been identified so far that belong either to the family of endothelial differentiation gene subfamily or to the purinergic receptor family (4). Utilizing the LPA receptor-specific agonists, LPA2 receptor agonist mimicked the effects of LPA on DRA promoter activity, indicating the involvement of LPA2 receptors; however, LPA1/3 receptor agonist did not increase the DRA promoter activity, ruling out their involvement in LPA-mediated effects. This lack of effect of LPA1/3 receptor agonist could also be partly due to lower expression levels of these receptors in Caco-2 cells. However, our LPA2-specific agonist clearly shows that these effects are LPA2 mediated. LPA2 receptor agonist has been demonstrated as receptor subtype-specific agonist for LPA2 both experimentally as well as by computational and docking studies (31). These data are in agreement with previous study, which showed the involvement of LPA2 receptors in inhibiting the chloride secretion (15). Additionally, our short-term study showed the involvement of LPA2 receptors in stimulating the Cl−/HCO3− exchange activity (30).
LPA2 receptors are known to be expressed in Caco-2 cells and signal through Gi protein-dependent PI3K/AKT pathways (33). Activation of the PI3K/AKT pathway and a coordinated increase in AKT phosphorylation has also been documented in a transgenic mice model overexpressing all the three LPA (LPA1/2/3) receptors (17). We found that the LPA increased DRA promoter activity was dependent on PI3K/AKT pathways. PI3K/AKT signaling is known to be involved in alterations in the expression of various genes. For example, it is required for the growth factor-induced expression of genes involved in cell cycle progression such as cyclin D1 in intestinal epithelial cells (29). It is also known to be involved in the expression of MadCAM-1 gene in response to inflammatory cytokines in human intestinal microvascular endothelial cells (22).
Progressive 5′-deletions of the DRA promoter from the −1183/+114 to −790/+114 region completely abrogated the stimulatory effects of LPA, suggesting that the −1183 to −790 region harbors LPA response element of DRA promoter. In fact, our previous studies have also shown the −1183 to −790 region to be responsive to IFN-γ, and the detailed analysis of the region utilizing EMSA and mutation studies showed the involvement of GAS element (via STAT1) in mediating the inhibitory effects of IFN-γ on DRA promoter activity (27). Our EMSA results demonstrated the increased binding of DNA (−1155/−1133) to Caco-2 nuclear proteins in response to LPA with no change in the DNA protein binding in other regions such as −846/−825, −850/−827, and −888/−865, suggesting −1155/−1133 to be the LPA response cis-element. Sequence analysis of the LPA response cis-element region (−1155/−1133) revealed the presence of potential AP1 binding sites. In this regard, previous studies have shown the involvement of AP1 transcription factor in regulating various genes involved in cell survival and cell proliferation, such as VEGF, IL-8, and plasminogen activator receptor by LPA (9, 13, 14). Therefore, the potential role of AP1 in LPA-mediated upregulation of DRA expression was further investigated. AP1 is a heterodimeric protein composed of c-Fos and c-Jun proteins. Our results showed the increased DNA protein binding in c-Fos overexpressed cells; however, DNA protein binding was not eliminated in the presence of an excess of cold unlabeled consensus AP1 binding sequence, and no supershift was seen with c-Fos antibody. These data suggest that LPA increases the DRA promoter activity in a c-Fos-dependent manner, possibly via binding to another unidentified transcription factor and not to AP1. Also, our results showed that PI3K/Akt pathway is involved in LPA-mediated effects on DRA. Both PI3K and AKT kinase have been previously shown to regulate the c-Fos gene expression (12). For example, in the glomerular mesangial cells AKT positively modulates the expression of c-Fos via transactivation of another transcription factor, Elk-1 (5). Earlier studies have shown the induction of AP1 proteins in response to LPA in ovarian and other cancer cell lines (23). Similarly, our studies utilizing Caco-2 cells showed an increase in c-Fos and c-Jun protein expression in response to long-term LPA treatment. Therefore we can speculate that there is a downstream activation of AP-1 (c-Fos) by LPA via AKT. This signaling further activates an unidentified transcription factor that binds directly to the DRA promoter to enhance DRA expression. Additionally, the mutations in the −1155/−1133 region blocked the stimulatory effects of LPA on DRA promoter activity. These results were further substantiated by overexpression of c-Fos in Caco-2 cells. DRA promoter activity was transactivated in cells overexpressing c-Fos compared with cells transfected with empty vector. Mutations in the −1155/−1133 abrogated the c-Fos-mediated increase in DRA promoter activity as the difference between empty vector and c-Fos overexpressed cells was not significant. These data clearly demonstrate the essential role of the identified LPA responsive cis-elements (−1155/−1133) in the activation of DRA promoter.
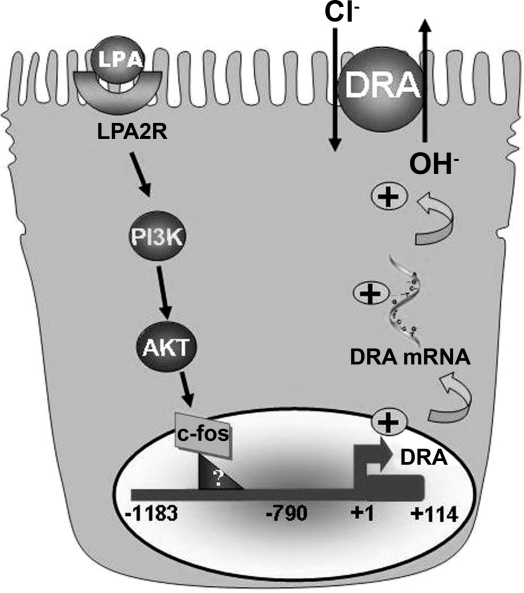
In summary, our results for the first time demonstrate that LPA regulates DRA function and expression via transcriptional mechanisms. We propose a model (Fig. 11) that LPA stimulates DRA function and expression with the involvement of LPA2 receptor and PI3K/AKT signaling pathways in a c-Fos-dependent manner. The resulting increase in Cl−/HCO3− exchange activity may underlie the potential proabsorptive effects of LPA.
Fig. 11.
Proposed model for the effects of LPA on DRA promoter.
GRANTS
These studies were supported by the department of Veteran Affairs and the National Institute of Diabetes and Digestive and Kidney Diseases Grants DK 54016 and DK 81858 (P. K. Dudeja), DK 71596 (W. Alrefai), PO1 DK 067887 (P. K. Dudeja), DK74458 (R. Gill), and Crohn's and Colitis Foundation of America grant ref. no. 1942 (S. Saksena).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Bieberdorf FA, Gorden P, Fordtran JS. Pathogenesis of congenital alkalosis with diarrhea. Implications for the physiology of normal ileal electrolyte absorption and secretion. J Clin Invest 51: 1958–1968, 1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Borthakur A, Gill RK, Tyagi S, Koutsouris A, Alrefai WA, Hecht GA, Ramaswamy K, Dudeja PK. The probiotic Lactobacillus acidophilus stimulates chloride/hydroxyl exchange activity in human intestinal epithelial cells. J Nutr 138: 1355–1359, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254, 1976 [DOI] [PubMed] [Google Scholar]
- 4. Choi JW, Herr DR, Noguchi K, Yung YC, Lee CW, Mutoh T, Lin ME, Teo ST, Park KE, Mosley AN, Chun J. LPA receptors: subtypes and biological actions. Annu Rev Pharmacol Toxicol 50: 157–186, 2010 [DOI] [PubMed] [Google Scholar]
- 5. Choudhury GG. Akt serine threonine kinase regulates platelet-derived growth factor-induced DNA synthesis in glomerular mesangial cells: regulation of c-fos AND p27(kip1) gene expression. J Biol Chem 276: 35636–35643, 2001 [DOI] [PubMed] [Google Scholar]
- 6. Contos JJ, Ishii I, Chun J. Lysophosphatidic acid receptors. Mol Pharmacol 58: 1188–1196, 2000 [DOI] [PubMed] [Google Scholar]
- 7. Cui MZ, Laag E, Sun L, Tan M, Zhao G, Xu X. Lysophosphatidic acid induces early growth response gene 1 expression in vascular smooth muscle cells: CRE and SRE mediate the transcription. Arterioscler Thromb Vasc Biol 26: 1029–1035, 2006 [DOI] [PubMed] [Google Scholar]
- 8. Dudeja PK, Gill RK, Ramaswamy K. Absorption-secretion and epithelial cell function. In: Colonic Diseases, edited by Koch TR. Totowa, NJ: Humana, 2003, p. 23–24 [Google Scholar]
- 9. Ediger TL, Schulte NA, Murphy TJ, Toews ML. Transcription factor activation and mitogenic synergism in airway smooth muscle cells. Eur Respir J 21: 759–769, 2003 [DOI] [PubMed] [Google Scholar]
- 10. Gill RK, Alrefai WA, Ramaswamy K, Dudeja PK. Mechanisms and regulation of NaCl absorption in the human intestine. In: Recent Research Development in Physiology (vol. 1). Trivandrum, India: Research Signpost, 2003, p. 643–677 [Google Scholar]
- 11. He D, Natarajan V, Stern R, Gorshkova IA, Solway J, Spannhake EW, Zhao Y. Lysophosphatidic acid-induced transactivation of epidermal growth factor receptor regulates cyclo-oxygenase-2 expression and prostaglandin E2 release via C/EBPβ in human bronchial epithelial cells. Biochem J 412: 153–162, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hu Q, Klippel A, Muslin AJ, Fantl WJ, Williams LT. Ras-dependent induction of cellular responses by constitutively active phosphatidylinositol-3 kinase. Science 268: 100–102, 1995 [DOI] [PubMed] [Google Scholar]
- 13. Hu YL, Tee MK, Goetzl EJ, Auersperg N, Mills GB, Ferrara N, Jaffe RB. Lysophosphatidic acid induction of vascular endothelial growth factor expression in human ovarian cancer cells. J Natl Cancer Inst 93: 762–768, 2001 [DOI] [PubMed] [Google Scholar]
- 14. Kim MH, Park JS, Chang HJ, Baek MK, Kim HR, Shin BA, Ahn BW, Jung YD. Lysophosphatidic acid promotes cell invasion by up-regulating the urokinase-type plasminogen activator receptor in human gastric cancer cells. J Cell Biochem 104: 1102–1112, 2008 [DOI] [PubMed] [Google Scholar]
- 15. Li C, Dandridge KS, Di A, Marrs KL, Harris EL, Roy K, Jackson JS, Makarova NV, Fujiwara Y, Farrar PL, Nelson DJ, Tigyi GJ, Naren AP. Lysophosphatidic acid inhibits cholera toxin-induced secretory diarrhea through CFTR-dependent protein interactions. J Exp Med 202: 975–986, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lin S, Yeruva S, He P, Singh AK, Zhang H, Chen M, Lamprecht G, de Jonge HR, Tse M, Donowitz M, Hogema BM, Chun J, Seidler U, Yun CC. Lysophosphatidic acid stimulates the intestinal brush border Na+/H+ exchanger 3 and fluid absorption via LPA5 and NHERF2. Gastroenterology 138: 649–658, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liu S, Umezu-Goto M, Murph M, Lu Y, Liu W, Zhang F, Yu S, Stephens LC, Cui X, Murrow G, Coombes K, Muller W, Hung MC, Perou CM, Lee AV, Fang X, Mills GB. Expression of autotaxin and lysophosphatidic acid receptors increases mammary tumorigenesis, invasion, and metastases. Cancer Cell 15: 539–550, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Makela S, Kere J, Holmberg C, Hoglund P. SLC26A3 mutations in congenital chloride diarrhea. Hum Mutat 20: 425–438, 2002 [DOI] [PubMed] [Google Scholar]
- 19. Mills GB, Moolenaar WH. The emerging role of lysophosphatidic acid in cancer. Nat Rev Cancer 3: 582–591, 2003 [DOI] [PubMed] [Google Scholar]
- 20. Moolenaar WH. Bioactive lysophospholipids and their G protein-coupled receptors. Exp Cell Res 253: 230–238, 1999 [DOI] [PubMed] [Google Scholar]
- 21. Mount DB, Romero MF. The SLC26 gene family of multifunctional anion exchangers. Pflügers Arch 447: 710–721, 2004 [DOI] [PubMed] [Google Scholar]
- 22. Ogawa H, Binion DG, Heidemann J, Theriot M, Fisher PJ, Johnson NA, Otterson MF, Rafiee P. Mechanisms of MAdCAM-1 gene expression in human intestinal microvascular endothelial cells. Am J Physiol Cell Physiol 288: C272–C281, 2005 [DOI] [PubMed] [Google Scholar]
- 23. Oyesanya RA, Greenbaum S, Dang D, Lee Z, Mukherjee A, Wu J, Dent P, Fang X. Differential requirement of the epidermal growth factor receptor for G protein-mediated activation of transcription factors by lysophosphatidic acid. Mol Cancer 9: 8, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Raheja G, Singh V, Ma K, Boumendjel R, Borthakur A, Gill RK, Saksena S, Alrefai WA, Ramaswamy K, Dudeja PK. Lactobacillus acidophilus stimulates the expression of SLC26A3 via a transcriptional mechanism. Am J Physiol Gastrointest Liver Physiol 298: G395–G401, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rubenfeld J, Guo J, Sookrung N, Chen R, Chaicumpa W, Casolaro V, Zhao Y, Natarajan V, Georas S. Lysophosphatidic acid enhances interleukin-13 gene expression and promoter activity in T cells. Am J Physiol Lung Cell Mol Physiol 290: L66–L74, 2006 [DOI] [PubMed] [Google Scholar]
- 26. Saksena S, Dwivedi A, Singla A, Gill RK, Tyagi S, Borthakur A, Alrefai WA, Ramaswamy K, Dudeja PK. Characterization of the 5′-flanking region and regulation of expression of human anion exchanger SLC26A6. J Cell Biochem 105: 454–466, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Saksena S, Singla A, Goyal S, Katyal S, Bansal N, Gill RK, Alrefai WA, Ramaswamy K, Dudeja PK. Mechanisms of transcriptional modulation of the human anion exchanger SLC26A3 gene expression by IFN-γ. Am J Physiol Gastrointest Liver Physiol 298: G159–G166, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schweinfest CW, Spyropoulos DD, Henderson KW, Kim JH, Chapman JM, Barone S, Worrell RT, Wang Z, Soleimani M. slc26a3 (dra)-deficient mice display chloride-losing diarrhea, enhanced colonic proliferation, and distinct up-regulation of ion transporters in the colon. J Biol Chem 281: 37962–37971, 2006 [DOI] [PubMed] [Google Scholar]
- 29. Sheng H, Shao J, Townsend CM, Jr, Evers BM. Phosphatidylinositol 3-kinase mediates proliferative signals in intestinal epithelial cells. Gut 52: 1472–1478, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Singla A, Dwivedi A, Saksena S, Gill RK, Alrefai WA, Ramaswamy K, Dudeja PK. Mechanisms of lysophosphatidic acid (LPA) mediated stimulation of intestinal apical Cl−/OH− exchange. Am J Physiol Gastrointest Liver Physiol 298: G182–G189, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Virag T, Elrod DB, Liliom K, Sardar VM, Parrill AL, Yokoyama K, Durgam G, Deng W, Miller DD, Tigyi G. Fatty alcohol phosphates are subtype-selective agonists and antagonists of lysophosphatidic acid receptors. Mol Pharmacol 63: 1032–1042, 2003 [DOI] [PubMed] [Google Scholar]
- 32. Wang Z, Wang T, Petrovic S, Tuo B, Riederer B, Barone S, Lorenz JN, Seidler U, Aronson PS, Soleimani M. Renal and intestinal transport defects in Slc26a6-null mice. Am J Physiol Cell Physiol 288: C957–C965, 2005 [DOI] [PubMed] [Google Scholar]
- 33. Yun CC, Sun H, Wang D, Rusovici R, Castleberry A, Hall RA, Shim H. LPA2 receptor mediates mitogenic signals in human colon cancer cells. Am J Physiol Cell Physiol 289: C2–C11, 2005 [DOI] [PubMed] [Google Scholar]