Abstract
Necrotizing enterocolitis (NEC) is the leading gastrointestinal cause of mortality and morbidity in the premature infant. Premature infants have a delay in intestinal colonization by commensal bacteria and colonization with potentially pathogenic organisms. Lactobacillus reuteri is a probiotic that inhibits enteric infections, modulates the immune system, and may be beneficial to prevent NEC. In previous studies, L. reuteri strains DSM 17938 and ATCC PTA 4659 differentially modulated inflammation in vitro; however, the strains had equivalent anti-inflammatory responses in LPS feeding-induced ileitis in neonatal rats in vivo. The impact of these two strains in the prevention of NEC has not been previously investigated. NEC was induced in newborn rats by orogastric formula feeding and exposure to hypoxia. L. reuteri was added to the formula to prevent NEC. NEC score, Toll-like receptor (TLR)-signaling genes, phospho-IκB activity, and cytokine levels in the intestine were examined. Both strains significantly increased survival rate and decreased the incidence and severity of NEC, with optimal effects from DSM 17938. In response to probiotic, mRNA expression of IL-6, TNF-α, TLR4, and NF-κB was significantly downregulated, while mRNA levels of anti-inflammatory cytokine IL-10 were significantly upregulated. In parallel, L. reuteri treatment led to decrease intestinal protein levels of TLR4 and cytokine levels of TNF-α and IL-1β in newborn rats with NEC. Both strains significantly inhibited not only intestinal LPS-induced phospho-IκB activity in an ex vivo study but also decreased the levels of phospho-IκB in the intestines of NEC rat model. Cow milk formula feeding produced a similar but milder proinflammatory profile in the intestine that was also ameliorated by 17938. Our studies demonstrate that each of the two L. reuteri strains has potential therapeutic value in our NEC model and in enteritis associated with cow milk feeding. These results support the concept that L. reuteri may represent a valuable treatment to prevent NEC.
Keywords: probiotic, cytokine, formula, hypoxia, Toll-like receptor-4, nuclear factor-κB
necrotizing enterocolitis (NEC) is the most common gastrointestinal disease in premature infants. In NEC, injury to the small or large intestine results in accumulation of intramural air and may progress to necrosis with perforation, peritonitis, sepsis, and death (19; 40). Prematurity remains the most important and consistent risk factor for NEC (40). The premature infant has been shown to have incompletely developed gastrointestinal motility and digestion (4), circulatory regulation (41, 43), and barrier function (35, 39). Additionally, the premature infant's intestinal defense mechanisms may be poorly regulated (7, 10). Preterm infants are colonized by low numbers of beneficial bacteria, such as bifidobacteria and lactobacilli, but have high numbers of potentially pathogenic bacteria due to treatment with antibiotics, artificial enteral feeding, and the introduction of hospital-associated microbiota (34). This microbiota, which includes enterobacteriaciae and Staphylococci, may contribute to an increase in inflammation.
The gut microbiota is a major regulator of the immune system. Late acquisition of gut bacteria or a reduced complexity of the microbiota may delay immune maturation (2). Probiotics (live microorganisms that, when ingested, produce a therapeutic or preventative health benefit; Ref. 57) may have the opposite effect. There is emerging clinical evidence for the beneficial effect of probiotics in preventing and/or treating gastrointestinal diseases, including necrotizing enterocolitis (12).
Lactobacillus reuteri is a promising therapy for many different conditions, including diarrheal disease (47), infantile colic (49), eczema (1), and Helicobacter pylori infection (21). L. reuteri was originally isolated from a Peruvian woman's breast milk and may be present in normal humans on the mucosa of the gastric corpus and antrum, duodenum, and ileum (44, 56). L. reuteri produces a potent antibacterial compound, reuterin, which is capable of inhibiting the growth of a wide spectrum of microorganisms (53). L. reuteri also modulates TNF-α production from bacterial lipopolysaccharide (LPS)-activated monocytoid cells in a strain-dependent manner (22, 28). L. reuteri strain ATCC 55730 and CF48–3A did not suppress TNF-α production, whereas ATCC PTA 6475- and ATCC PTA 5289 suppressed TNF-α production by LPS-activated monocytoid cells. In our studies, L. reuteri strains DSM 17938 and ATCC PTA4659 both significantly reduced intestinal mucosal levels of KC/GRO (∼IL-8) when newborn rats were fed cow milk formula plus Escherichia coli LPS. In addition, both strains improved LPS-induced intestinal morphological damage, including villus length and density (29). The impact of these two specific L. reuteri strains in the prevention of NEC, a more severe condition, has not yet been investigated.
In this study, we show evidence that L. reuteri stains reduce intestinal inflammation in an experimental model of NEC via inhibiting a Toll-like receptor-4 (TLR4) signaling pathway that leads to cytokine expression.
MATERIALS AND METHODS
Bacterial strains and preparation.
Human-derived L. reuteri strains DSM 17938 and ATCC PTA 4659 were provided by Biogaia (Stockholm, Sweden). L. reuteri DSM 17938 was derived from L. reuteri ATCC 55730 from a Peruvian mother's milk by removal of the two plasmids harboring antibiotic penicillin resistance genes (46). ATCC PTA 4659 was isolated from the breast milk of healthy Finnish women (52). Lactobacillus acidophilus DDS (La DDS; kindly provided by Dr. David R. Mack, Children's Hospital of Eastern Ontario, Ontario, Canada) and non-lactobacillus strain E. coli DH5α (purchased from GIBCO, Bethesda Research Laboratory) were used as control bacteria. L. reuteri was anaerobic cultured in deMan-Rogosa-Sharpe (MRS; Difco, Detroit, MI) medium at 37°C for 24 h and then plated in MRS agar at specific serial dilutions and grown at 37 ° C for 48–72 h. Quantitative analysis of bacteria in culture media was performed by comparing absorbance (at 600 nm) of cultures to known concentrations, using a standard curve of bacterial colony-forming units (CFU) per milliliter grown on MRS agar (using an Eppendorf Photometer; Eppendorf, Hamburg, Germany). Bacteria in culture media were harvested by centrifugation at 1,500 g for 15 min and were resuspended in formula before feeding.
Animal model and experimental design.
We studied newborn Sprague-Dawley rat pups (Harlan Laboratories, Indianapolis, IN) weighing 5–6 g. Studies were approved by the Animal Welfare Committee of the University of Texas Health Science Center at House (HSC-AWC-07–124). Litters of newborn rats were separated from their dams after they suckled for 12 h. Then rat pups were housed in an incubator and were then starved for 12 h before the initiation of formula feeding (day 1) with 100–200 μl, four times daily for 3 days. The formula consisted of 15 g Similac 60/40 (Ross Pediatrics, Columbus, OH) in 75 ml of Esbilac canine milk replacement (Pet-Ag, Hampshire, IL; Ref. 30). This technique was developed by Caplan and Jilling (6) and Nadler et al. (36). NEC were induced by formula feeding by gavage and exposure to 10 min of hypoxia (5% oxygen-95% nitrogen) three times daily in a Modular Incubator Chamber (Billups-Rothenberg, Del Mar, CA) for 3 days. Experiments included seven groups: 1) dam fed, in which the pups were left with their mothers (n = 22); 2) formula fed (n = 31); 3) NEC (n = 46); 4) NEC + 17938, in which rat pups were fed with formula containing DSM 17938 (106 CFU·g body wt−1·day−1) followed by hypoxia (n = 38); 5) NEC + 4659, in which rat pups were fed with formula containing ATCC PTA 4659 (106 CFU·g body wt−1·day−1) followed by hypoxia (n = 36); 6) formula + 17938 (n = 22); and 7) formula + 4659 (n = 17). Pups were euthanized at day 4 after live animal numbers were counted. In some cases, pups in the NEC group were euthanized on day 3, when severe signs of NEC were observed.
In ex vivo experiments, the terminal ileum from each newborn rat was transiently cultured in an antibiotic-free complete DMEM (Invitrogen, Carlsbad, CA) at 37°C, 5% CO2, and 100% humidity. Tissues were treated with probiotics (strain17938 or strain 4659) or control bacteria (La. DDS or E. coli DH5α; 3–4 × 107 CFU) for 4 h and washed with PBS, pH 7.4; subsequently, we added LPS from E. coli subtype 0111:B4 (Sigma, St. Louis, MO) at 100 μg/ml for 30 min. These LPS-exposed tissues were compared with matched tissues that were not exposed to LPS or probiotic and were subsequently studied for evidence of phospho-IκB (activation of the NF-κB pathway; see below). In another set of ex vivo experiments, tissues were fixed in formalin after 4 h to evaluate for histological changes.
Tissue harvest and NEC evaluation.
Following incision of the abdomen, the gastrointestinal tract was carefully removed. The small intestine was evaluated visually for typical gross signs of NEC, such as intestinal distension, wall hemorrhage, or necrosis. The last 4 cm of terminal ileum was excised. Part of ileum for each animal was washed with PBS and fresh frozen immediately in liquid nitrogen for RNA and protein isolation. Part of each sample was formalin fixed and processed by the Cellular and Molecular Morphology Core Laboratory (the Texas Medical Center Digestive Disease Center, Houston, TX) and stained with hematoxylin and eosin for histological evaluation.
Pathological changes in intestinal architecture were quantified using a modified NEC scoring system (15, 36). Histological changes in the ileum were scored by a blinded evaluator on a scale of 0 (normal), 1 (mild, separation of the villous core, without other abnormalities), 2 (moderate, villous core separation, submucosal edema, and epithelial sloughing), and 3 (severe, denudation of epithelium with loss of villi, full-thickness necrosis). Animals with histological scores ≥2 were defined as having NEC.
Tissue preparation for Western blot, cytokines, and phospho-IκB assays.
Ileal tissues were homogenized in 0.4 ml of lysis buffer containing protease inhibitors with 20 mmol/l Tris·HCl (pH 7.5), 150 mmol/l NaCl, 1 mmol/l Na2EDTA, 1 mmol/l EGTA, 1% NP-40, 1% sodium deoxycholate, 2.5 mmol/l sodium pyrophosphate, 1 mmol/l β-glycerophosphate, 1 mmol/l sodium orthovandadate (Na3VO4), 1 μg/ml leupeptin, 1 μg/ml aprotinin, and 1 mmol/l PMSF. The homogenates were centrifuged at 14,000 g for 10 min at 4°C after incubation on ice for 30 min. The collected supernatants were used to measure the levels of total protein using Bio-Rad Dc Protein Assay (Bio-Rad Laboratories, Hercules, CA). To detect the expression levels of TLR4, phospho-IκB, and total IκB by Western immunoblot analysis, proteins (50 μg/lane) were immunoblotted with mouse anti-TLR4 IgG2b monoclonal antibody (Imgenex; IMG-5031A, 2 μg/ml), rabbit anti phospho-IκB-α (Ser 32;14D4) antibody (Cell Signaling, 1:1000), rabbit anti-IκB-α (C-21) antibody (Santa Cruz Biotechnology; 2 μg/ml), and goat anti-actin (I-19) antibody (Santa Cruz Biotechnology; 0.2 μg/ml) for 12 h at 4°C. After incubation with goat anti-mouse (or rabbit) or bovine anti-goat IgG conjugated to horseradish peroxidase (Bio-Rad; 1: 5,000) for 1 h at room temperature, immunoreactive bands were visualized by chemiluminescence (ECL+; GE Bioscience) on X-ray film and were densitometrically analyzed with Kodak 1D Image (Eastman Kodak, Rochester, NY). Cytokines were assessed by using MSD rat multiplex kit (Meso Scale Discovery, Gaithersburg, MD). Cytokine levels were reported as picograms of cytokine per milligram total protein. Phospho-IκB levels were determined, in ex vivo experiments by using an antibody to phospho-IκB (Ser32) STAR ELISA kit (Millipore, Temecula, CA). The level of phospho-IκB in tissue lysates was expressed as units of phospho-IκB per milligram total protein (U/mg). All assays were performed using manufacturers' protocols.
Analysis of mRNA expression by quantitative real-time RT-PCR.
RNA was isolated from frozen tissue samples using Trizol (Invitrogen, Carlsbad, CA), followed by RNA purification and On-Column DNase digestion (Qiagen, Valencia, CA) according to the manufacturer's protocols. Quantitative (q)RT-PCR was performed with the rat TLR signaling pathway RT2 profiler PCR and SYBR green/ROY qPCR master mix (Qiagen-SABiosciences, Valencia, CA). All qRT-PCR reactions were run in the Quantitative Genomics Core Laboratory (UTHSC-Houston Medical School, Houston, TX) utilizing a 7700 Detector (Applied Biosystems, Foster City, CA). The threshold cycle (Ct) value for each well was obtained using the instrument's software. Data analysis by the ΔΔCt method was automated (PCR Array Data Analysis Web Portal provided by Qiagen-SABiosciences). To determine changes in gene expression, normalized expression of each gene of interest (GOI) in the experimental sample was divided by the normalized expression of the same GOI in the control sample and expressed as fold increase or decrease. Eighty-four GOIs in TLR signal pathway were assessed. Three housekeeping genes (HKG) were used: ribosomal protein large P1 (Rplp1, NM_001007604); ribosomal protein L13A (Rpl13a, NM_173340); and actin (Actb, NM_031144). We used the average Ct value of HKGs that were not influenced by our experimental conditions for normalization with the ΔΔCt method. The calculation was as follows: 2−ΔΔCt = 2−[Ct(GOI) − Ct(HKG)] experiment/2−[Ct(GOI) − Ct(HKG)] control. In our study, the fold changes of transcripts in the intestine of groups of NEC + 17938 (n = 7) or NEC + 4659 (n = 6) were compared with the changes in NEC group (n = 6). To monitor the quality of tested samples, the controls for genomic DNA, reverse transcription, and a positive PCR were examined simultaneously. All samples passed the tests for quality control.
Statistics.
Experimental results are expressed as means ± SE. Statistical analyses were performed using one-way ANOVA (GraphPad Prism 4.0; GraphPad Software, San Diego, CA). Dunnett's and Tukey's multiple-comparison tests were used for comparison of multiple groups with a control group. A P value of < 0.05 was considered statistically significant.
RESULTS
L. reuteri increases the survival rate of rat pups with NEC.
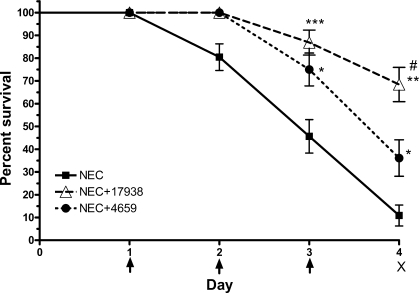
Feeding L. reuteri DSM 17938 or ATCC PTA 4659 to neonatal rats with NEC significantly increased survival rate compared with rats exposed to the NEC protocol without probiotic; improved survival was seen on days 3 and 4. Survival on day 3 was 86.8% in rats with NEC that were fed 17938 (P < 0.001), 75% in rats with NEC that were fed 4659 (P < 0.05), and 45.6% in rats with NEC without probiotics. Survival on day 4 was 68.4% in rats with NEC that were fed 17938 (P < 0.01), 36.1% in rats with NEC that were fed 4659 (P < 0.05), and 10.8% in rats with NEC without probiotics (Fig. 1). Survival was greater with strain 17938 than with 4659 on day 4 (P < 0.05).
Fig. 1.
Survival rate of rat pups with necrotizing enterocolitis (NEC) compared with NEC pups that were supplemented with Lactobacillus reuteri strains. Newborn rats were separated from their mothers and housed in an incubator. NEC was induced by formula feeding plus exposure to hypoxia (5% oxygen-95% nitrogen for 10 min, 3 times per day) from days 1 to 3 (indicated as arrow). Rat pups were euthanized on day 4 (indicated by “X”), and tissues were collected. L. reuteri strain DSM 17938 or ATCC PTA 4659 (106 CFU·g body wt−1·day−1, where CFU is colony-forming unit) was added in formula for treatment. Groups studied were as follows: 1): NEC + 17938 (n = 38); 2) NEC + 4659 (n = 36); and 3) NEC + no probiotic (n = 46). Both strains reduced mortality rate on days 3 and 4 compared with NEC without probiotics. *P < 0.05, **P < 0.01, ***P < 0.001, NEC + 17938 or NEC + 4659 vs. NEC without probiotic; #P < 0.05, NEC + 17938 vs. NEC + 4659.
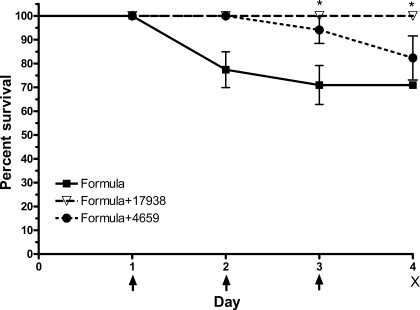
In separate studies of rat pups fed with formula with or without L. reuteri strains without hypoxia, we found that mortality in formula-fed rats was also reduced by strain 17938 at both days 3 and 4 (100% survival in formula-fed rats receiving strain 17938 vs. 70.9% in formula-fed rats receiving no probiotic; P < 0.05; Fig. 2).
Fig. 2.
Survival rate of rat pups with cow milk-based formula feeding compared with formula plus L. reuteri. Newborn rats were separated from their mothers and fed with formula (without hypoxia exposure) beginning on day 1 for 3 days (indicated with arrow). Rat pups were euthanized on day 4 (indicated by “X”). L. reuteri strain DSM 17938 or strain ATCC PTA 4659 (106 CFU·g body wt−1·day−1) was added in formula for treatment without hypoxia. Groups studied were as follows: 1) formula + 17938 (n = 22); 2) formula + 4659 (n = 17); and 3) formula alone (n = 31). *P < 0.05.
L. reuteri reduces the incidence and severity of NEC.
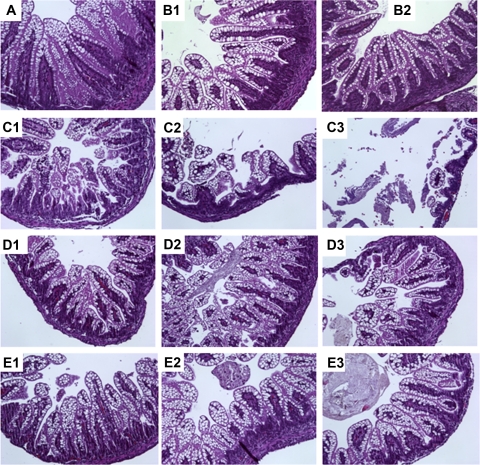
Intestines for evaluation of histological changes were obtained only from living animals on day 4. However, tissues were collected on day 3 for the purpose of comparison in some groups with severe NEC. Morphologic analysis of ileal segments from formula-fed animals with/without hypoxia revealed various degrees of inflammatory changes ranging from villus core separation and submucosal edema to epithelial sloughing and necrosis (Fig. 3). In contrast, dam-fed pups had normal intestinal architecture and rarely showed any inflammatory changes. The severity of NEC was evaluated by histological NEC score in the ileum and graded as 0 to 3. Animals with histological scores of ≥2 were defined as having NEC. The incidence of histological NEC was significantly higher in NEC (formula + hypoxia) group (11/14, 78%) compared with either dam-fed (0/22, 0%) or formula-fed (4/22, 18%) rat pups (P < 0.01).
Fig. 3.
Intestinal morphology of neonatal rat ileum. Ileal tissues of rat pups were collected after 3 days of induction of NEC, with or without L. reuteri treatment. Tissue sections were stained with hematoxylin and eosin with magnification = ×100. Groups studied were as follows: 1) dam fed (A); 2) formula fed (B1–B2); 3) NEC (C1–C3); 4) NEC + 17938 (D1–D3); and 5) NEC + 4659 (E1–E3).
Feeding L. reuteri to neonatal rats exposed to experimental NEC significantly reduced the incidence of histological NEC to 34% (9/26) in NEC-exposed pups fed L. reuteri strain 17938 and 36% (7/19) in NEC-exposed pups fed L. reuteri strain 4659, compared with NEC without probiotic (78%, 11/14; P < 0.05; Table 1). In addition, the severity of NEC also decreased with administration of either strain 17938 or 4659, demonstrating that probiotic administration shifted intestinal damage from severe to mild, as indicated by NEC score changing from 3 to 1. In the NEC group, 42.9% (6/14) had a NEC score of 3 compared with 11.5% (3/26) in the NEC + L. reuteri strain 17938 group or 10.5% (2/19) in the NEC + L. reuteri strain 4659 group. Conversely, a score of 1 was seen in ∼46% of animals in NEC + L. reuteri strain 17938 (12/26) or NEC + L. reuteri strain 4659 (9/19), compared with 7.1% (1/14) in pups with NEC that were not given L. reuteri (Table 1). The average NEC score was 2.1, which was decreased to 1.2 by treatment with strain 17938 and 1.3 by treatment with strain 4659 (Table 1).
Table 1.
Effect of formula feeding and hypoxia on intestinal morphology
| Dam (n = 22) | Formula (n = 22) | NEC (n = 14) | NEC + 17938 (n = 26) | NEC + 4659 (n = 19) | |
|---|---|---|---|---|---|
| Treatment scores | |||||
| Score = 0 (normal) | 20 (90.9%) | 7 (31.8%) | 2 (14.3%) | 5 (19.2%) | 3 (15.8%) |
| Score = 1 (mild) | 2 (10%) | 11 (50%) | 1 (7.1%) | 12 (46.2%) | 9 (47.4%) |
| Score = 2 (moderate) | 0 | 3 (13.6%) | 5 (35.7%) | 6 (23.1%) | 5 (26.3%) |
| Score = 3 (severe) | 0 | 1 (4.5%) | 6 (42.9%) | 3 (11.5%) | 2 (10.5%) |
| Incidence of NEC | 0 (0/22) | 18% (4/22) | 78%* (11/14) | 34%† (9/26) | 36%† (7/19) |
| Average NEC score | 0.1 | 1.0 | 2.1 | 1.2 | 1.3 |
Newborn rats were randomized into 1 of the 5 groups as indicated above; n = numbers of live animals from which tissue samples could be collected. Terminal ileum of each rat was harvested at day 4 [or day 3 for some of samples in necrotizing enterocolitis NEC group], fixed in formalin, and stained with hematoxylin and eosin. Morphological changes graded (0-3) were blinded to the treatment groups (see materials and methods). 17938 and 4659, Lactobacillus reuteri strains DSM 17938 and ATCC PTA 4659.
P < 0.01 vs. dam or formula group;
P < 0.05 vs. NEC group.
Interestingly, we noted that formula feeding alone also produced mild to severe inflammatory histological changes. Of the formula-fed animals (without hypoxia) 31.8% (7/22) had normal ileal histology (score = 0), 50% (11/22) had mild inflammation (score = 1), 13.6% (3/22) had moderate inflammation (score = 2), and 4.5% (1/22) had severe inflammation (score = 3) (Table 1).
L. reuteri downregulates expression of TLR-signal genes induced by NEC.
In various models of inflammation, the activation of epithelial and leukocyte TLRs, combined with subsequent signaling events, leads to the pivotal event of NF-κB activation, resulting in secretion of downstream products, especially inflammatory cytokines (26). We examined the expression of 84 TLR-signal genes using qRT-PCR. Fold regulation (FR) of genes in the intestine of pups in the group (NEC + probiotic) were compared with the group (NEC + no probiotic). A cutoff value was defined as FR less than −2 indicating downregulation, and FR greater than 2 indicating upregulation.
We found out that five genes, including TLRs (TLR1 and TLR4), proinflammatory cytokines (IL-6 and TNF-α), and nuclear factor related to κB-binding protein (nfrκb) were significantly downregulated by both strains in the intestines of rat pups of NEC model. However, the anti-inflammatory cytokine IL-10 was significantly increased by both 17938 and 4659 in the intestine of rats with NEC (Table 2). In addition, L. reuteri DSM17938 significantly inhibited mitogen-activated protein kinase 8 interacting protein 3 (Mapk8ip3; XM_220232; −2.5-fold) and upregulated NF-κB inhibitor-β (Nfκbib; NM_030867; 2.3-fold; P < 0.05), while ATCC PTA 4659 significantly inhibited myelin and lymphocyte protein (Mal; NM_012798; −2.5-fold; P < 0.05), which are all TLR-signal-interacting proteins. In summary, both genes that were upregulated by L. reuteri strains (IL-10 and Nfκbib) are known to be associated with downregulation of inflammation (13).
Table 2.
mRNAs are up- or downregulated by L. reuteri strains both DSM 17938 and ATCC PTA 4659 in the intestines of NEC rat model by quantitative RT-PCR
| Fold Regulation* | GenBank | NEC + 17938/NEC† | NEC + 4659/NEC† |
|---|---|---|---|
| IL-6 | NM_012589 | −40.6 (0.01) | −27.3 (0.01) |
| TNF-α | NM_012675 | −35.3 (0.005) | −24.1 (0.009) |
| TLR4 | NM_019178 | −5.3 (0.02) | −7.1 (0.03) |
| TLR1 | XM_223421 | −2.8 (0.03) | −3.3 (0.03) |
| nfrκb | XM_236009 | −8.1 (0.0006) | −2.1 (0.02) |
| IL-10 | NM_012854 | 3.1 (0.01) | 4.6 (0.002) |
TLR, Toll-like receptor; nfrκb, nuclear factor related to κB-binding protein.
Fold regulation: <−2 downregulated, >2 upregulated.
NEC + 17938: n = 7; NEC + 4659: n = 6; NEC: n = 9. P values are in parenthesis and are statistically significant at <0.05.
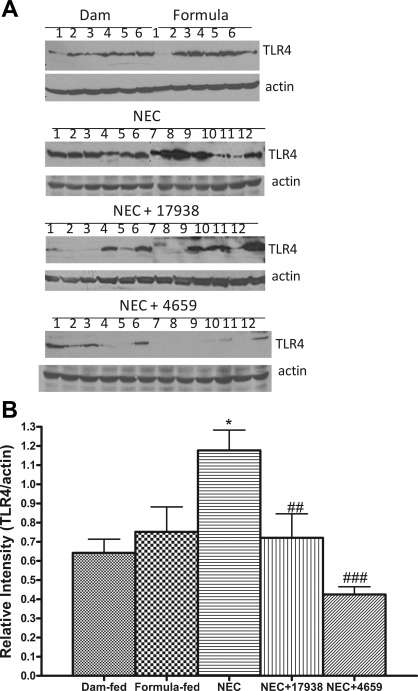
L. reuteri decreases the expression of TLR4 in the intestines of rat pups with NEC.
To validate the effect of L. reuteri strains on the expression of TLR4 in the intestines of rat pups with NEC, protein levels of TLR4 were examined by Western immunoblot analysis (Fig. 4A). We found increased relative intensities of TLR4 protein in rat pups with NEC compared with the dam-fed or formula-fed controls (P < 0.05; Fig. 4, A and B). The expression levels of TLR4 in pups with NEC were significantly decreased by feeding the rat pups with strain 17938 (P < 0.01) or 4659 (P < 0.001; Fig. 4, A and B).
Fig. 4.
Expression of Toll-like receptor-4 (TLR4) protein in the ileum. Tissue lysates were prepared for Western blot analysis, and 50 μg of total protein were loaded and immunoblotted with anti-TLR4 antibody with β-actin as a loading control. A: typical blot. Numbers represent number of animals examined in each group. B: relative densitometric values, means ± SE. Groups studied were as follows: 1) dam fed (n = 6); 2) formula fed (n = 6); 3) NEC (n = 12); 4) NEC + 17938 (n = 12); and 5) NEC + 4659 (n = 12). *P < 0.05, NEC vs. dam fed or formula fed; ##P < 0.01, ###P < 0.001, NEC + 17938 or NEC + 4659 vs. NEC.
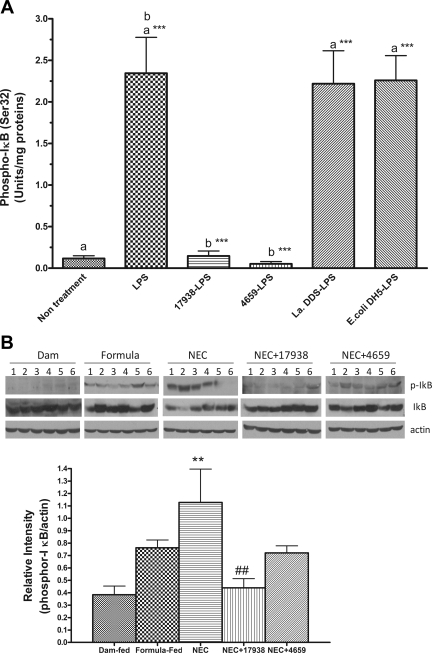
L. reuteri inhibits IκB phosphorylation in the intestine during LPS exposure of ex vivo experiments and in experimental NEC.
NF-κB transcription complexes are present in an inactive form in the cytoplasm, bound to an inhibitor IκB. The phosporylation, ubiquitination, and subsequent degradation of IκB enables translocation of free NF-κB into the nucleus, where it subsequently actives target genes and produce inflammatory responses (27). Phosporylation of IκB induced by LPS in IEC6 cells generally occurs as early as 15–30 min after treatment (17). To confirm the inhibitory activity of probiotics on NF-κB pathway, we treated small intestinal explants from newborn rats (day of life = 0) with probiotic or control bacteria that included lactobacillus strain (La DDS) and a non-lactobacillus strain (E. coli DH5α) followed by LPS stimulation in vitro. La DDS has been shown to be lacking the properties of adherence to epithelial cells, induction of mucin expression by intestinal epithelial cells, and inhibition of enteropathogenic E. coli epithelial cell adherence (31). Gram-negative E. coli DH5α, which expresses very low levels of LPS, is also a nonpathogenic strain (3, 45). Phosphorylated IκB in the tissue lysates was then determined by using phospho-IκB (Ser32) STAR ELISA kit. LPS, a component of Gram-negative bacteria, triggers inflammation by binding its cognate receptor (TLR4) on the surface of innate immune system sensory cells (macrophages) and/or enterocytes and stimulates production of proinflammatory molecules (37).
We pretreated intact ileum from newborn rat was with probiotic DSM 17938 or ATCC PTA 4659 or one of the two control bacteria (3–4×107 CFU) for 3–4 h, washed the tissues, and subsequently exposed them to LPS for 30 min. The tissues were homogenized, and phospho-IκB in the tissue lysate was assessed. Pretreatment with both strains of L. reuteri significantly inhibited LPS-induced IκB activation (phosphorylation) in the intestine in newborn rats (P < 0.001), whereas pretreatment with control bacteria (La DDS or E. coli DH5) did not affect LPS-induced NFκB activation (Fig. 5A).
Fig. 5.
Phospho-IκB activation in the intestines of newborn rats in an ex vivo experiment (A) and in the NEC model (B). A: ileal tissues from each newborn rat (day of life 0) were collected in an antibiotic-free DMEM complete medium and immediately treated with probiotics (strain17938 or strain 4659) or control bacteria [Lactobacillus acidophilus DDS (La DDS) or Escherichia coli DH5α; 3–4 × 107 CFU] for 4 h, washed, and subsequently treated with LPS (E. coli subtype 0111:B4), 100 μg/ml for 30 min. Tissues were washed with PBS and homogenized in a lysis buffer containing protease inhibitors. Tissue lysates were used to determine phospho-IκB activity by ELISA. Activity of phospho-IκB (Ser32) was expressed as units/ mg total protein in tissue lysates; n = 6 rats for each group. aGroups compared with no treatment; b17938-LPS or 4659-LPS compared with LPS. Strains 17938 and 4659 both inhibited IκB phosphorylation by > 90%: ***P < 0.001. B: phospho-IκB analysis in the ileum of rat pup with NEC as examined by Western blot analysis. Fifty micrograms of total protein were loaded and immunoblotted with anti-phospho-IκB and IκB antibodies, with β-actin as a loading control. Top: a typical blot; numbers represent number of animals examined in each group. Bottom: relative densitometric values are means ± SE; n = 6 for each groups. **P < 0.01, NEC vs. dam fed; ##P < 0.01, NEC + 17938 vs. NEC.
In the NEC model, expression of IκB and phospho-IκB in the intestines of rap pups were examined. We found that intestinal level of phospho-IκB significantly increased in NEC. However, feeding rat pups either 17938 or 4659 significantly decreased the levels of phospho-IκB in the intestines of rat pups with NEC (Fig. 5B).
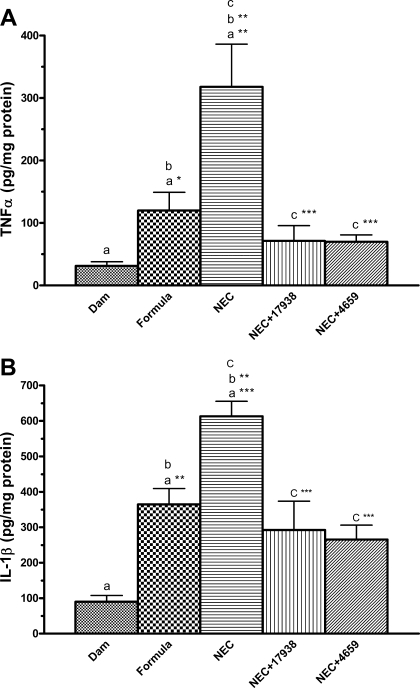
L. reuteri decreases inflammatory cytokine protein levels in the intestine of rats with NEC.
Cytokines are key mediators in inflammation, and several cytokines are dysregulated in this disease (30, 51). Gene expression of selected inflammatory cytokines in the terminal ileum was determined by RT-PCR. Proinflammatory IL-1β, IL-6, IL-12, IL-18, and TNF-α are major cytokines associated with NEC pathogenesis and neonatal sepsis (18, 51). Expression levels of IL-6 and TNF-α mRNAs in the intestines were significantly decreased in NEC treated with L. reuteri DSM 17938 or ATCC PTA 4659 compared with NEC without probiotic (Table 2).
Analysis of intestinal protein levels of TNF-α (Fig. 6A) and IL-1β (Fig. 6B) indicated that 1) these proinflammatory cytokines significantly increased in the intestines of rat pups fed with formula compared with dam feeding (P < 0.01); 2) the levels of those cytokines further increased in rat pups exposed to NEC (P < 0.001 compared with dam-fed controls; P < 0.01 compared with formula-fed controls); and 3) feeding L. reuteri to rat pups with NEC significantly decreased cytokine levels compared with NEC without probiotics (P < 0.001 compared with NEC).
Fig. 6.
TNF-α (A) and IL-1β (B) levels in the intestines of rats with NEC compared with rats with NEC fed with supplemental L. reuteri. Tissue lysates were examined cytokine levels by using MSD multiplex cytokine assay; n = 6–7 rats per group. aGroups were compared with dam fed; bNEC group was compared with formula-fed group; cgroups NEC + 17938 or NEC + 4659 were compared with NEC without probiotic. Increased cytokine levels in NEC were both decreased by strains 17938 and 4659. *P < 0.05, **P < 0.01, ***P < 0.001.
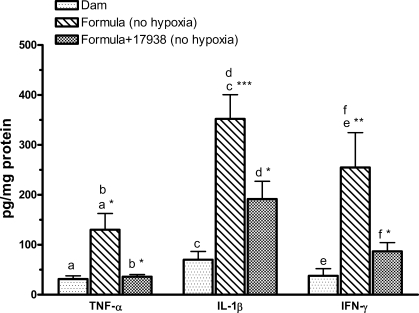
L. reuteri strain DSM 17938 decreases TNF-α, IL-1β and IFN-γ protein levels in the intestine of formula-fed rats.
We (30) previously found out that formula feeding without hypoxia increases the protein levels of several inflammatory cytokines in the intestines of newborn rats. In this study, we consistently observed increased cytokine levels in the intestinal tissue lysates of formula-fed rats compared with dam-fed rats, including TNF-α (P < 0.05), IL-1β (P < 0.001), and IFN-γ (P < 0.01). We asked if L. reuteri treatment would reduce these levels in the formula-fed rat pups without hypoxia. We found that formula-associated (high) levels of proinflammatory cytokines (TNF-α, IL-1β, and IFN-γ) in the intestines of newborn rats could be suppressed by feeding L. reuteri strain DSM 17938 (all P < 0.05 compared with formula) but not consistently with L. reuteri strain ATCC PTA 4659 (Fig. 7).
Fig. 7.
Cytokine levels of TNF-α, IL-1β, and IFN-γ in the intestines of rats with formula ± 17938 feeding (no hypoxia). Newborn rats were left with their mothers (dam fed) or separated from their mothers and housed in an incubator for formula feeding only without hypoxic exposure. L. reuteri strain DSM 17938 (106 CFU·g body wt−1·day−1) was added to formula. Ileal tissues were homogenized in a lysis buffer containing protease inhibitors. Tissue lysates were examined cytokine levels by using MSD multiplex cytokine assay; n = 6–9 rats per group. Comparisons were as follows: a,c,eformula fed vs. dam fed; b,d,fL. reuteri strain 17938-supplemented vs. formula without 17938. Strain 17938 significantly decreased formula-induced cytokine levels in the intestines of newborn rats. *P < 0.05, **P < 0.01, ***P < 0.001.
DISCUSSION
We examined the effects of human-derived probiotic L. reuteri strains DSM 17938 and ATCC PTA 4659 on experimental NEC. Our hypothesis was that different strains would differ in their anti-inflammatory effects in this disease. Previous studies from our laboratory (29) indicated that L. reuteri strains differentially modulate LPS-induced inflammation in vitro and in vivo. For example, even though L. reuteri strain DSM 17938 did not inhibit LPS-induced IL-8 production in cultured intestinal cells, all strains including DSM 17938 and ATCC PTA 4659 significantly reduced the intestinal levels of KC/GRO (∼IL-8) when newborn rats fed with LPS and/or L. reuteri. Moreover, intestinal histological damage produced by LPS addition to the cow milk formula was also significantly reduced by those strains. Thus we went on to ask which of these strains could affect the development of NEC in newborn rats. In the present study, the important findings are the following: L. reuteri strains 1) significantly increased survival rate and reduced the incidence of severity of NEC; 2) decreased proinflammatory cytokine levels in parallel with inhibition of TLR4-signaling via the NF-κB pathway; and 3) reduced mortality and intestinal inflammation due to cow milk formula feeding (strain 17938). In all studies, both L. reuteri strains DSM 17938 and ATCC PTA 4659 were effective in vivo.
NEC as an acute inflammatory disease.
NEC is a devastating disease of neonates associated with high morbidity and mortality (19). NEC generally occurs after the introduction of oral feedings in conjunction with initial bacterial colonization of the gut. Factors conferring a predisposition to NEC include an altered microbiota, inadequate intestinal barrier function, and intestinal hypoxia/ischemia (40). Clinical observations suggest excessive inflammation in response to intestinal stimuli including higher serum levels of several cytokines and chemokines in patients with NEC (33, 37, 51). Currently, observations in animal models of NEC and in human fetal cell cultures have suggested that the fetus and preterm infant have an excessive inflammatory response to luminal microbial stimuli. In support of this concept, the expression of TLR4 appears to be increased in a fetal intestinal cell line compared with an adult cell line (16), and an important factor IκB, the inhibitory regulator of NF-κB, which regulates inflammation, is developmentally underexpressed (8).
Our animal model was derived from the extensive work of Ford and colleagues (19, 36) and included three key components: maternal separation stress, deprivation of optimal enteral nutrition, artificial feeding with cow milk formula, and hypoxic insult. Both our previous and current studies underscore the intensity of the inflammatory response in this NEC model, with a heightened intestinal tissue expression of TLRs and increased proinflammatory cytokine (TNF-α and IL-1β) production, which precede evidence of histological injury (30).
Role of probiotics in the prevention of NEC.
The commensal intestinal microbiota represents a major modulator of intestinal homeostasis. Inappropriate initial microbial colonization in preterm infants is an important risk factor for NEC (9). A delay in intestinal colonization by commensally nonpathogenic bacteria, particularly bifidobacteria and lactobacilli, and colonization with potentially pathogenic organisms often occurs in preterm infants due to a unique environmental exposure. Contributing factors include artificial enteral feeding, treatment with broad-spectrum antibiotics, and the introduction of hospital-associated microbiota (2). Recent studies have demonstrated that the microbial community structure in NEC patients (during the time of NEC and antibiotic use) is distinct, characterized by a significant decrease in diversity of microbial species with increased Proteobacteria dominance (59) and possibly by increased gamma-Proteobacteria (32).
Probiotic bacteria have been studied in other countries for prevention of neonatal NEC (5). In the recent review of Caplan and Frost (5), there are nine published randomized, placebo-controlled trials and one historical cohort trial designed specifically to measure the outcome of reducing the risk for NEC in premature infants. Taken together, these studies showed significant a benefit for probiotics such as Lactobacillus rhamnosus GG, Lactobacillus acidophilus LA5 and NFCM, Bifidobacterium infantis, and Bifidobacterium bifidum to reduce the risk of NEC (5). A second recent meta-analysis also suggested efficacy (12).
Several probiotics have been studied in the animal models of NEC (20, 24, 25). For example, Bifidobacterium bifidum reduces apoptosis in the intestinal epithelium and improves intestinal integrity in the rat NEC model (24, 25). Lactobacillus bulgaricus prevents intestinal epithelial cell injury in a similar rat NEC model (20).
L. reuteri is a promising therapy for many different conditions, including diarrheal disease (47), infant colic (50), and Helicobacter pylori infection (21). However, the impact of L. reuteri on NEC in animal and human has not been investigated. In our current study, we first found out that both L. reuteri strains DSM 17938 and ATCC PTA 4659 could increase the survival rate and decrease the incidence and severity of NEC. In particular, strain 17938 was better than 4659 in that it showed greater survival rate and also reduced formula-associated inflammation and death.
Differential modulation of inflammation by L. reuteri strains.
L. reuteri has antimicrobial actions conferred by its ability to convert glycerol into a potent broad-spectrum antimicrobial compound, reuterin (52). In addition, the immunomodulatory properties of L. reuteri strains have been studied in vitro, and strain-specific differences have been observed (22, 28, 54). Some L. reuteri strains (ATCC PTA 4659, 5289, and 6475; Refs. 22, 23, 28) have the ability to produce small (<3 kDa), secreted factors that suppress LPS-induced TNF production in primary monocytes, myeloid cell lines and macrophages, whereas others (ATCC 55730, DSM 17938, and CF48–3A) are incapable of reducing TNF production (23). Genomic analysis from four L. reuteri strains suggested that these strains can be divided into two different groups (strains 55730 and CF48–3A in one group, and strains 4659 and 6475 in a separate group; Ref. 38). L. reuteri strains from these two different groups differed according to their immunomodulatory properties (22, 23, 28), ability to produce reuterin, and tendency to form biofilms in vitro.
However, in both our LPS-feeding inflammation model described previously (29) and in the NEC model in our current study, we noted that oral administration of several different L. reuteri strains including DSM 17938, ATCC PTA 4659, 5289, and 6475 significantly reduced intestinal inflammation. Both strains 17938 and 4659 increased NEC survival and decreased the incidence and severity of NEC. In an ex vivo experiment for analyzing phospho-IκB activity, we found that both live strains significantly inhibited the activity of phospho-IκB induced by LPS in the intestines. Moreover, the phospho-IκB levels were reduced by L. reuteri stains in the intestine in the rat NEC model. This inhibition, combined with downregulation of TLR4-related gene and TLR4 protein expression in NEC intestine by both strains, indicates that TLR signaling was modulated by direct interaction of live probiotics and the intestinal epithelium (including epithelial and/or immune cells). Thus, at least in part, the mucosal immune system plays a mechanistic role of inflammation reduction in response to L. reuteri.
Previous studies (11) from an animal model of NEC demonstrated that inhibition of NF-κB by a NF-κB essential modulator (NEMO)-binding domain (NBD) peptide that selectively inhibits the critical upstream IκB kinase (IKK) decreased mortality and bowel injury (11).
The mechanism of immune regulation by the different L. reuteri strains is still incompletely understood. Recently, metabolic pathway reconstruction and genome-wide expression profiling from each of two groups of L. reuteri (ATCC 55730 and ATCC PTA 6475) have been explored using metabolic modeling and transcriptomics (48). These results demonstrate that both strains contain candidate genes involved in bacterial survival and persistence in the gut, such as genes encoding mucus-binding proteins and enzymes capable of scavenging reactive oxygen species. Both strains were predicted to produce health-promoting factors, including antimicrobial agents and vitamins (folate and vitamin B12). Additionally, the complete pathways for thiamine biosynthesis and an exopolysaccharide synthesis were uniquely identified and predicted in strain 55730, the parent strain of DSM 17938.
The differential effects of L. reuteri strains on inflammation in vitro and in vitro implicate an interaction between probiotics and immune cells (most likely dendritic cells and T cells) in the intestinal mucosa. Recent studies (55) have shown that Bifidobacterium infantis 35624 induces Foxp3+ regulatory T cells (Tregs) in mucosa, which are known to provide key anti-inflammatory signals. Importantly, adoptive transfer of the Tregs transferred the NF-κB inhibitory activity (42). Similarly, the combination probiotic VSL#3 ameliorates recurrent Th1-mediated murine colitis by an early increase in numbers of IL-10-dependent TGF-β-bearing Tregs (14), associated with an alteration in the distribution and phenotypes of dendritic cells in the intestinal mucosa (58). Our study shows that both L. reuteri strains significantly increase the expression of IL-10 in NEC. Future work will be aimed at determining if L. reuteri modulates Treg numbers in the prevention of NEC.
In conclusion, our study demonstrates that human-derived L. reuteri strains DSM 17938 and ATCC PTA 4659 reduce intestinal inflammation in experimental NEC. The mechanism of action involves (at least in part) reduced signaling via TLR4, leading to reduced NF-κB gene transcription. Our results support the concept that L. reuteri may represent a useful treatment to prevent NEC. They also support suggest that cow milk-based formula-associated inflammation in the newborn intestine is ameliorated by L. reuteri.
GRANTS
This study was supported by the Department of Pediatrics of the University of Texas Health Science Center at Houston Medical School and a pilot feasibility apart from Public Health Service Grant DK-56338, which funds the Texas Medical Center Digestive Diseases Center.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: Y.L. and J.M.R. conception and design of research; Y.L., N.Y.F., and N.M. performed experiments; Y.L. and N.Y.F. analyzed data; Y.L. and J.M.R. interpreted results of experiments; Y.L. prepared figures; Y.L. drafted manuscript; Y.L. and J.M.R. edited and revised manuscript; J.M.R. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Gregory L. Shipley from the Quantitative Genomics Core Laboratory, University of Texas Medical School at Houston for assisting in qRT-PCR technique and analysis of transcript profiling results; Cellular and Molecular Morphology Core, the Texas Medical Center Digestive Disease Center at Houston for performing histological preparations; Dr. Eamon Connolly in Biogaia (Stockholm, Sweden) for providing the different strains of probiotic L. reuteri, and Dr. David Mack for providing La DDS.
REFERENCES
- 1. Abrahamsson TR, Jakobsson T, Bottcher MF, Fredrikson M, Jenmalm MC, Bjorksten B, Oldaeus G. Probiotics in prevention of IgE-associated eczema: a double-blind, randomized, placebo-controlled trial. J Allergy Clin Immunol 119: 1174–1180, 2007 [DOI] [PubMed] [Google Scholar]
- 2. Adlerberth I, Wold AE. Establishment of the gut microbiota in Western infants. Acta Paediatr 98: 229–238, 2009 [DOI] [PubMed] [Google Scholar]
- 3. Amor PA, Mutharia LM. Cloning and expression of rfb genes from Vibrio anguillarum serotype O2 in Escherichia coli: evidence for cross-reactive epitopes. Infect Immun 63: 3537–3542, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Berseth CL. Gut motility and the pathogenesis of necrotizing enterocolitis. Clin Perinatol 21: 263–270, 1994 [PubMed] [Google Scholar]
- 5. Caplan M, Frost B. Myth: necrotizing enterocolitis: Probiotics will end the disease, and surgical intervention improves the outcome. Semin Fetal Neonatal Med 16: 264–268, 2011 [DOI] [PubMed] [Google Scholar]
- 6. Caplan MS, Jilling T. The role of polyunsaturated fatty acid supplementation in intestinal inflammation and neonatal necrotizing enterocolitis. Lipids 36: 1053–1057, 2001 [DOI] [PubMed] [Google Scholar]
- 7. Caplan MS, Simon D, Jilling T. The role of PAF, TLR, and the inflammatory response in neonatal necrotizing enterocolitis. Semin Pediatr Surg 14: 145–151, 2005 [DOI] [PubMed] [Google Scholar]
- 8. Claud EC, Lu L, Anton PM, Savidge T, Walker WA, Cherayil BJ. Developmentally regulated IkappaB expression in intestinal epithelium and susceptibility to flagellin-induced inflammation. Proc Natl Acad Sci USA 101: 7404–7408, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Claud EC, Walker WA. Bacterial colonization, probiotics, and necrotizing enterocolitis. J Clin Gastroenterol 42, Suppl 2: S46–S52, 2008 [DOI] [PubMed] [Google Scholar]
- 10. Corfield AP, Myerscough N, Longman R, Sylvester P, Arul S, Pignatelli M. Mucins and mucosal protection in the gastrointestinal tract: new prospects for mucins in the pathology of gastrointestinal disease. Gut 47: 589–594, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. De P, I, Liu SX, Tian R, Neequaye I, May MJ, Han XB, Hsueh W, Jilling T, Lu J, Caplan MS. Inhibition of nuclear factor-kappaB ameliorates bowel injury and prolongs survival in a neonatal rat model of necrotizing enterocolitis. Pediatr Res 61: 716–721, 2007 [DOI] [PubMed] [Google Scholar]
- 12. Deshpande G, Rao S, Patole S, Bulsara M. Updated meta-analysis of probiotics for preventing necrotizing enterocolitis in preterm neonates. Pediatrics 125: 921–930, 2010 [DOI] [PubMed] [Google Scholar]
- 13. Dharmani P, Chadee K. Biologic therapies against inflammatory bowel disease: a dysregulated immune system and the cross talk with gastrointestinal mucosa hold the key. Curr Mol Pharmacol 1: 195–212, 2008 [DOI] [PubMed] [Google Scholar]
- 14. Di GC, Marinaro M, Sanchez M, Strober W, Boirivant M. Probiotics ameliorate recurrent Th1-mediated murine colitis by inducing IL-10 and IL-10-dependent TGF-beta-bearing regulatory cells. J Immunol 174: 3237–3246, 2005 [DOI] [PubMed] [Google Scholar]
- 15. Dvorak B, Khailova L, Clark JA, Hosseini DM, Arganbright KM, Reynolds CA, Halpern MD. Comparison of epidermal growth factor and heparin-binding epidermal growth factor-like growth factor for prevention of experimental necrotizing enterocolitis. J Pediatr Gastroenterol Nutr 47: 11–18, 2008 [DOI] [PubMed] [Google Scholar]
- 16. Fusunyan RD, Nanthakumar NN, Baldeon ME, Walker WA. Evidence for an innate immune response in the immature human intestine: toll-like receptors on fetal enterocytes. Pediatr Res 49: 589–593, 2001 [DOI] [PubMed] [Google Scholar]
- 17. Grishin AV, Wang J, Potoka DA, Hackam DJ, Upperman JS, Boyle P, Zamora R, Ford HR. Lipopolysaccharide induces cyclooxygenase-2 in intestinal epithelium via a noncanonical p38 MAPK pathway. J Immunol 176: 580–588, 2006 [DOI] [PubMed] [Google Scholar]
- 18. Halpern MD, Holubec H, Dominguez JA, Williams CS, Meza YG, McWilliam DL, Payne CM, McCuskey RS, Besselsen DG, Dvorak B. Upregulation of IL-18 and IL-12 in the ileum of neonatal rats with necrotizing enterocolitis. Pediatr Res 51: 733–739, 2002 [DOI] [PubMed] [Google Scholar]
- 19. Hunter CJ, Upperman JS, Ford HR, Camerini V. Understanding the susceptibility of the premature infant to necrotizing enterocolitis (NEC). Pediatr Res 63: 117–123, 2008 [DOI] [PubMed] [Google Scholar]
- 20. Hunter CJ, Williams M, Petrosyan M, Guner Y, Mittal R, Mock D, Upperman JS, Ford HR, Prasadarao NV. Lactobacillus bulgaricus prevents intestinal epithelial cell injury caused by Enterobacter sakazakii-induced nitric oxide both in vitro and in the newborn rat model of necrotizing enterocolitis. Infect Immun 77: 1031–1043, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 21. Imase K, Tanaka A, Tokunaga K, Sugano H, Ishida H, Takahashi S. Lactobacillus reuteri tablets suppress Helicobacter pylori infection–a double-blind randomised placebo-controlled cross-over clinical study. Kansenshogaku Zasshi 81: 387–393, 2007 [DOI] [PubMed] [Google Scholar]
- 22. Jones SE, Versalovic J. Probiotic Lactobacillus reuteri biofilms produce antimicrobial and anti-inflammatory factors. BMC Microbiol 9: 35, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jones SE, Whitehead K, Saulnier D, Thomas CM, Versalovic J, Britton RA. Cyclopropane fatty acid synthase mutants of probiotic human-derived Lactobacillus reuteri are defective in TNF inhibition. Gut Microbes 2: 69–79, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Khailova L, Dvorak K, Arganbright KM, Halpern MD, Kinouchi T, Yajima M, Dvorak B. Bifidobacterium bifidum improves intestinal integrity in a rat model of necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol 297: G940–G949, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Khailova L, Mount Patrick SK, Arganbright KM, Halpern MD, Kinouchi T, Dvorak B. Bifidobacterium bifidum reduces apoptosis in the intestinal epithelium in necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol 299: G1118–G1127, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Krishnan J, Selvarajoo K, Tsuchiya M, Lee G, Choi S. Toll-like receptor signal transduction. Exp Mol Med 39: 421–438, 2007 [DOI] [PubMed] [Google Scholar]
- 27. Le Mandat SA, Bonnard A, Barreau F, Aigrain Y, Pierre-Louis C, Berrebi D, Peuchmaur M. Expression of TLR-2, TLR-4, NOD2 and pNF-kappaB in a neonatal rat model of necrotizing enterocolitis. PLos One 2: e1102, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lin YP, Thibodeaux CH, Pena JA, Ferry GD, Versalovic J. Probiotic Lactobacillus reuteri suppress proinflammatory cytokines via c-Jun. Inflamm Bowel Dis 14: 1068–1083, 2008 [DOI] [PubMed] [Google Scholar]
- 29. Liu Y, Fatheree NY, Mangalat N, Rhoads JM. Human-derived probiotic Lactobacillus reuteri strains differentially reduce intestinal inflammation. Am J Physiol Gastrointest Liver Physiol 299: G1087–G1096, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Liu Y, Zhu L, Fatheree NY, Liu X, Pacheco SE, Tatevian N, Rhoads JM. Changes in intestinal Toll-like receptors and cytokines precede histological injury in a rat model of necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol 297: G442–G450, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mack DR, Ahrne S, Hyde L, Wei S, Hollingsworth MA. Extracellular MUC3 mucin secretion follows adherence of Lactobacillus strains to intestinal epithelial cells in vitro. Gut 52: 827–833, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mai V, Young CM, Ukhanova M, Wang X, Sun Y, Casella G, Theriaque D, Li N, Sharma R, Hudak M, Neu J. Fecal microbiota in premature infants prior to necrotizing enterocolitis. PLos One 6: e20647, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Markel TA, Crisostomo PR, Wairiuko GM, Pitcher J, Tsai BM, Meldrum DR. Cytokines in necrotizing enterocolitis. Shock 25: 329–337, 2006 [DOI] [PubMed] [Google Scholar]
- 34. Mshvildadze M, Neu J, Mai V. Intestinal microbiota development in the premature neonate: establishment of a lasting commensal relationship? Nutr Rev 66: 658–663, 2008 [DOI] [PubMed] [Google Scholar]
- 35. Muller CA, Autenrieth IB, Peschel A. Innate defenses of the intestinal epithelial barrier. Cell Mol Life Sci 62: 1297–1307, 2005 [DOI] [PubMed] [Google Scholar]
- 36. Nadler EP, Dickinson E, Knisely A, Zhang XR, Boyle P, Beer-Stolz D, Watkins SC, Ford HR. Expression of inducible nitric oxide synthase and interleukin-12 in experimental necrotizing enterocolitis. J Surg Res 92: 71–77, 2000 [DOI] [PubMed] [Google Scholar]
- 37. Nanthakumar NN, Fusunyan RD, Sanderson I, Walker WA. Inflammation in the developing human intestine: A possible pathophysiologic contribution to necrotizing enterocolitis. Proc Natl Acad Sci USA 97: 6043–6048, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nelson KE, Weinstock GM, Highlander SK, Worley KC, Creasy HH, Wortman JR, Rusch DB, Mitreva M, Sodergren E, Chinwalla AT, Feldgarden M, Gevers D, Haas BJ, Madupu R, Ward DV, Birren BW, Gibbs RA, Methe B, Petrosino JF, Strausberg RL, Sutton GG, White OR, Wilson RK, Durkin S, Giglio MG, Gujja S, Howarth C, Kodira CD, Kyrpides N, Mehta T, Muzny DM, Pearson M, Pepin K, Pati A, Qin X, Yandava C, Zeng Q, Zhang L, Berlin AM, Chen L, Hepburn TA, Johnson J, McCorrison J, Miller J, Minx P, Nusbaum C, Russ C, Sykes SM, Tomlinson CM, Young S, Warren WC, Badger J, Crabtree J, Markowitz VM, Orvis J, Cree A, Ferriera S, Fulton LL, Fulton RS, Gillis M, Hemphill LD, Joshi V, Kovar C, Torralba M, Wetterstrand KA, Abouellleil A, Wollam AM, Buhay CJ, Ding Y, Dugan S, FitzGerald MG, Holder M, Hostetler J, Clifton SW, len-Vercoe E, Earl AM, Farmer CN, Liolios K, Surette MG, Xu Q, Pohl C, Wilczek-Boney K, Zhu D. A catalog of reference genomes from the human microbiome. Science 328: 994–999, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Neu J, Chen M, Beierle E. Intestinal innate immunity: how does it relate to the pathogenesis of necrotizing enterocolitis. Semin Pediatr Surg 14: 137–144, 2005 [DOI] [PubMed] [Google Scholar]
- 40. Neu J, Walker WA. Necrotizing enterocolitis. N Engl J Med 364: 255–264, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nowicki PT, Dunaway DJ, Nankervis CA, Giannone PJ, Reber KM, Hammond SB, Besner GE, Caniano DA. Endothelin-1 in human intestine resected for necrotizing enterocolitis. J Pediatr 146: 805–810, 2005 [DOI] [PubMed] [Google Scholar]
- 42. O'Mahony C, Scully P, O'Mahony D, Murphy S, O'Brien F, Lyons A, Sherlock G, MacSharry J, Kiely B, Shanahan F, O'Mahony L. Commensal-induced regulatory T cells mediate protection against pathogen-stimulated NF-kappaB activation. PLoS Pathog 4: e1000112, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Reber KM, Nankervis CA, Nowicki PT. Newborn intestinal circulation. Physiol Pathophysiol Clin Perinatol 29: 23–39, 2002 [DOI] [PubMed] [Google Scholar]
- 44. Reuter G. The Lactobacillus and Bifidobacterium microflora of the human intestine: composition and succession. Curr Issues Intest Microbiol 2: 43–53, 2001 [PubMed] [Google Scholar]
- 45. Ribes S, Ebert S, Czesnik D, Regen T, Zeug A, Bukowski S, Mildner A, Eiffert H, Hanisch UK, Hammerschmidt S, Nau R. Toll-like receptor prestimulation increases phagocytosis of Escherichia coli DH5alpha and Escherichia coli K1 strains by murine microglial cells. Infect Immun 77: 557–564, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Rosander A, Connolly E, Roos S. Removal of antibiotic resistance gene-carrying plasmids from Lactobacillus reuteri ATCC 55730 and characterization of the resulting daughter strain, L. reuteri DSM 17938. Appl Environ Microbiol 74: 6032–6040, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Saavedra J. Probiotics and infectious diarrhea. Am J Gastroenterol 95: S16–S18, 2000 [DOI] [PubMed] [Google Scholar]
- 48. Saulnier DM, Santos F, Roos S, Mistretta TA, Spinler JK, Molenaar D, Teusink B, Versalovic J. Exploring metabolic pathway reconstruction and genome-wide expression profiling in lactobacillus reuteri to define functional probiotic features. PLos One 6: e18783, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Savino F, Pelle E, Palumeri E, Oggero R, Miniero R. Lactobacillus reuteri (American Type Culture Collection Strain 55730) versus simethicone in the treatment of infantile colic: a prospective randomized study. Pediatrics 119: e124–e130, 2007 [DOI] [PubMed] [Google Scholar]
- 50. Savino F, Tarasco V. New treatments for infant colic. Curr Opin Pediatr 22: 791–797, 2010 [DOI] [PubMed] [Google Scholar]
- 51. Sharma R, Tepas JJ, III, Hudak ML, Mollitt DL, Wludyka PS, Teng RJ, Premachandra BR. Neonatal gut barrier and multiple organ failure: role of endotoxin and proinflammatory cytokines in sepsis and necrotizing enterocolitis. J Pediatr Surg 42: 454–461, 2007 [DOI] [PubMed] [Google Scholar]
- 52. Spinler JK, Taweechotipatr M, Rognerud CL, Ou CN, Tumwasorn S, Versalovic J. Human-derived probiotic Lactobacillus reuteri demonstrate antimicrobial activities targeting diverse enteric bacterial pathogens. Anaerobe 14: 166–171, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Talarico TL, Casas IA, Chung TC, Dobrogosz WJ. Production and isolation of reuterin, a growth inhibitor produced by Lactobacillus reuteri. Antimicrob Agents Chemother 32: 1854–1858, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Taweechotipatr M, Iyer C, Spinler JK, Versalovic J, Tumwasorn S. Lactobacillus saerimneri and Lactobacillus ruminis: novel human-derived probiotic strains with immunomodulatory activities. FEMS Microbiol Lett 293: 65–72, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Tran DQ, Ramsey H, Shevach EM. Induction of FOXP3 expression in naive human CD4+FOXP3 T cells by T-cell receptor stimulation is transforming growth factor-beta dependent but does not confer a regulatory phenotype. Blood 110: 2983–2990, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Valeur N, Engel P, Carbajal N, Connolly E, Ladefoged K. Colonization and immunomodulation by Lactobacillus reuteri ATCC 55730 in the human gastrointestinal tract. Appl Environ Microbiol 70: 1176–1181, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Vanderhoof JA, Young R. Probiotics in the United States. Clin Infect Dis 46, Suppl 2: S67–S72, 2008 [DOI] [PubMed] [Google Scholar]
- 58. Wang X, O'Gorman MR, Bu HF, Koti V, Zuo XL, Tan XD. Probiotic preparation VSL#3 alters the distribution and phenotypes of dendritic cells within the intestinal mucosa in C57BL/10J mice. J Nutr 139: 1595–1602, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wang Y, Hoenig JD, Malin KJ, Qamar S, Petrof EO, Sun J, Antonopoulos DA, Chang EB, Claud EC. 16S rRNA gene-based analysis of fecal microbiota from preterm infants with and without necrotizing enterocolitis. ISME J 3: 944–954, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]