Abstract
Previous data from our group have demonstrated (Arany I, Grifoni S, Clark JS, Csongradi, Maric C, Juncos LA. Am J Physiol Renal Physiol 301: F125–F133, 2011) that chronic nicotine (NIC) exposure exacerbates acute renal ischemic injury (AKI) in mice that could increase the risk for development and progression of chronic kidney disease (CKD). It has been shown that proximal tubules of the kidney can acquire characteristics that may compromise structural recovery and favor development of inflammation and fibrosis following injury. Chronic NIC exposure can amplify this epithelial process although the mechanism is not identified. Recently, the unphosphorylated form of signal transducer and activator of transcription-3 (U-STAT3) has emerged as a noncanonical mediator of inflammation and fibrosis that may be responsible for the effects of chronic NIC. We found that levels of transforming growth factor β-1 (TGF-β1), α-smooth muscle actin (α-SMA), fibronectin, monocyte chemotactic protein-1 (MCP-1), and expression of U-STAT3 were increased in the ischemic kidneys of NIC-exposed mice. Chronic NIC exposure also increased TGF-β1-dependent F-actin reorganization, vimentin, fibronectin, and α-SMA expression as well as promoter activity of α-SMA and MCP-1 without significant loss of epithelial characteristics (E-cadherin) in cultured renal proximal tubule cells. Importantly, transduction of cells with a U-STAT3 mimetic (Y705F-STAT3) augmented stress fiber formation and also amplified NIC+TGF-β1-induced expression of α-SMA, vimentin, fibronectin, as well as promoter activity of α-SMA and MCP-1. Our results reveal a novel, chronic NIC-exposure-related and U-STAT3-dependent mechanism as mediator of a sustained transcription of genes that are linked to remodeling and inflammation in the kidney during injury. This process may facilitate progression of AKI to CKD. The obtained data may lead to devising therapeutic methods to specifically enhance the protective and/or inhibit adverse effects of STAT3 in the kidney.
Keywords: fibrosis, inflammation, kidney
smoking significantly increases the rate of progression of chronic kidney disease (CKD) not only in the patient with established CKD but perhaps also in the healthy population (22, 23). Nicotine (NIC), a major tobacco alkaloid, is insinuated as a key culprit responsible for cigarette smoke-induced kidney injury (13). NIC aggravates renal fibrosis in rats and mice (12, 33) and induces reorganization of the endothelial actin cytoskeleton in vitro (9), thus causing sustained activation of profibrotic/-inflammatory responses in the proximal tubules, which may facilitate progression of CKD.
In addition to accelerating progression of CKD, we recently demonstrated that chronic exposure to NIC exacerbates ischemia-reperfusion-induced acute kidney injury (IR-AKI) in mice. It is now recognized that although much of the renal injury occurring during AKI resolves, some of it is irreversible and can progress to CKD, suggesting that AKI may initiate certain signaling cascades that change the tubular phenotype, thus impairing recovery and favoring the onset of inflammation and development of tubulointerstitial fibrosis (28). Indeed, analysis of human kidney biopsies with different injuries showed that some tubules possess both epithelial and mesenchymal markers (25) and a dedifferentiated phenotype (27) that may be a part of responses of the kidney to injury (10). However, the signals that are responsible for the conversion of AKI into CKD are incompletely understood.
One signaling pathway that may be important is through transforming growth factor-β1 (TGF-β1). TGF-β1 initiates sublethal cytoskeletal reorganization that can lead to phenotypic changes in the proximal tubules, resulting in imbalanced epithelial-mesenchymal communication and ultimately leading to fibrotic and inflammatory responses in the kidney (5, 28, 29). It is thought to exert these effects in part through the signal transducer and activator of transcription-3 (STAT3) pathway. STAT3 is a transcription factor with pleotropic functions (6) and is regulated by differential phosphorylation including tyrosine 705 phosphorylation of STAT3 which is responsible for canonical STAT3-dependent transcription (6). However, it has recently been recognized that unphosphorylated STAT3 (U-STAT3) can also drive expression of many proteins including several oncogenes, cytokines, and inflammatory agents (30, 31). Moreover, this U-STAT3-induced gene expression can be long lasting (7) in contrast to those activated through the canonical pathway (7). While we previously reported that renal IR injury increases levels of U-STAT3 (2), its role in determining the severity of AKI or its progression to CKD was not examined.
Because chronic exposure to NIC increases U-STAT3 expression (3), we hypothesize that chronic NIC exposure augments TGF-β1-induced profibrotic and proinflammatory signaling through U-STAT3, which in turn will exacerbate/accelerate progression of renal injury. In the current study, we used complementary in vivo and in vitro protocols to examine whether chronic NIC increases TGF-β1-induced cytoskeletal remodeling, E-cadherin (an epithelial marker), and expression/transcription of proinflammatory [monocyte chemotactic protein-1 (MCP-1)] and profibrotic [α-smooth muscle actin (SMA), vimentin, fibronectin] signals. We also tested the role of U-STAT3 in NIC-induced exacerbation of TGF-β1-mediated cell injury.
MATERIALS AND METHODS
Animals and surgery.
Ten-week-old male C57BL/6J mice were purchased from Jackson Laboratories (Bar Harbor, ME). Chronic NIC exposure was achieved with 200 μg/ml nicotine bitartrate (Sigma-Aldrich, St. Louis, MO) in 2% saccharine solution in drinking water for 4 wk as previously described (1). Control animals received 2% saccharine solution. Some animals underwent 18 min of warm renal ischemia followed by 24 h of reperfusion as detailed elsewhere (1). The control animals underwent sham surgery, where the kidneys were exposed but renal pedicles were not clamped. All these procedures were done in accordance with guidelines of the Institutional Animal Care and Use Committee of the University of Mississippi Medical Center and were performed in accordance with the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health.
In vitro experiments.
The porcine renal proximal tubule cell line (LLC-PK1) was purchased from ATCC (Manassas, VA) and maintained in high-glucose DMEM (Invitrogen, Grand Island, NY) supplemented with 10% fetal bovine serum (Invitrogen) and 1% antibiotic/antimycotic solution (Invitrogen) in a 5% CO2 atmosphere at 37°C. Semiconfluent cells were treated with 200 μM NIC (Sigma-Aldrich) for 24 h and/or with 10 ng/ml TGF-β1 (R&D Systems, Minneapolis, MN) for 3 days.
Western blotting.
Kidneys were homogenized in RIPA buffer supplemented with protease and phosphatase inhibitors (Santa Cruz Biotechnology, Santa Cruz, CA), and protein content was determined with a Bio-Rad protein assay (Bio-Rad, Richmond, CA). Monolayers of cultured cells were also lysed in RIPA. Twenty to fifty micrograms of kidney or cell lysates were separated on a 4–15% gradient NuPAGE Novex Bis-Tris mini gel (Invitrogen) and transferred to polyvinylidene difluoride membranes using iBlot (Invitrogen). Blots were hybridized with appropriate primary antibodies, visualized by Pierce ECL Western blotting substrate (Thermo Scientific, Rockford, IL), and exposed to X-ray film (Midwest Scientific, St. Louis, MO). Films were digitized and analyzed by Un-Scan-It Version 6.1 software (SilkScientific, Orem, UT). The source of antibodies is as follows: anti-α-SMA (Sigma-Aldrich), anti-E-cadherin (BD Biosciences, Franklin Lakes, NJ), anti-vimentin, anti-STAT3 (Cell Signaling Technology, Danvers, MA), and anti-actin (Millipore, Billerica, MA). Horseradish peroxidase-conjugated anti-mouse or anti-rabbit secondary antibodies were also purchased from Cell Signaling Technology.
TGF-β1 and MCP-1 content in kidney lysates.
Two hundred micrograms of total kidney lysates was used to determine renal TGF-β1 content by a mouse TGF-β1 or MCP-1 ELISA kit according to the manufacturer's (R&D Systems) recommendation.
Adenoviral infection.
The dnSTAT3 (Y705F mutant) adenovirus was a gift from Dr. B. D. Nelkin (24). LLC-PK1 cells grown in six-well-plates were infected with 25 MOI dnSTAT3 in regular medium for 3 h, which was replaced with complete medium for 24 h.
Reporter luciferase assay.
LLC-PK1 cells grown in 24-well-plates were transfected with the following luciferase reporter plasmids together with a Renilla luciferase plasmid (Promega, Madison, WI) using Xfect reagent (Clontech, Mountain View, CA): α-SMA promoter, a gift from Dr. R. A. Nemenoff (11), and MCP-1 promoter, a gift from Dr. J. L. Madrigal (20). Twenty-four hours after transfection, the cells were treated as described, and 24 h later the firefly and Renilla luciferase activities were determined by a Dual Luciferase assay kit (Promega).
Phalloidin staining and fluorescence microscopy.
Cells grown on chambered coverglass were washed with PBS and fixed in 4% paraformaldehyde for 10 min at room temperature followed by permeabilization with 0.1% Triton X-100 for 5 min. These cells were then washed with PBS again, labeled with Alexa Fluor 488-phalloidin (Invitrogen), and counterstained with 4′,6-diamidino-2-phenylindole (DAPI; Invitrogen) as recommended by the manufacturer. Fluorescence was observed with a Nikon Eclipse TS100F inverted microscope equipped with a DAPI, FITC, or CY3 filter at ×400 magnification. Images were captured by a Nikon DS cooled camera and analyzed with NIS Elements Basic Research 3.0 software.
Statistical analysis.
Continuous variables were expressed as means ± SD. Statistical differences between treated and control groups were determined by a one-way ANOVA with a post hoc test. Differences between means were considered significant if P < 0.05. All analyses were performed using the SigmaStat 3.5 (Systat, San Jose, CA) software package.
RESULTS
Chronic NIC exposure augments fibrosis and inflammation as well as U-STAT3 expression in the ischemic kidney.
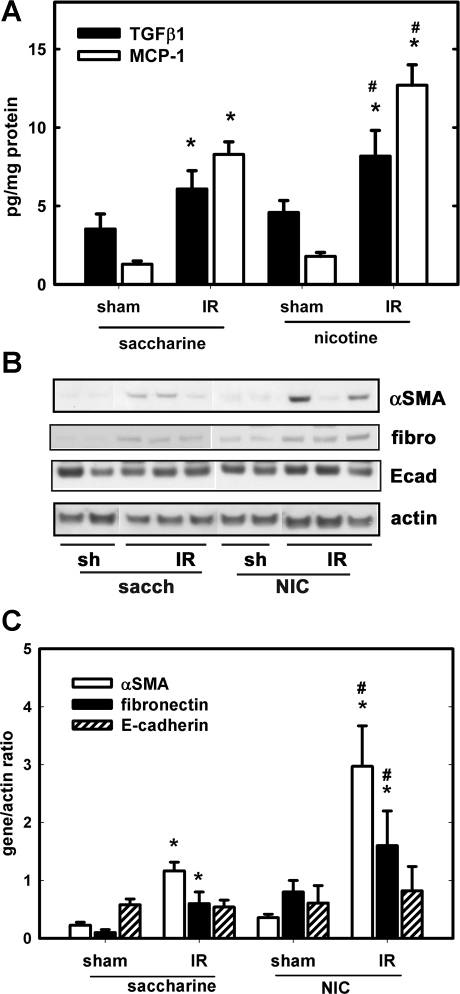
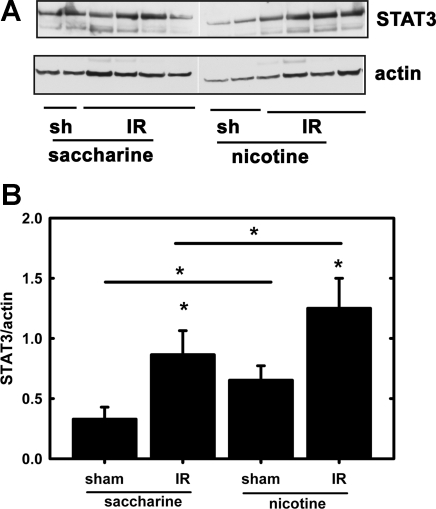
The levels of TGF-β1, MCP-1, α-SMA, fibronectin, and E-cadherin were determined in the postischemic (24 h) kidney by ELISA or Western blotting. Figure 1 shows that chronic NIC exposure augmented renal IR-induced TGF-β1 and MCP-1 (Fig. 1A), α-SMA, and fibronectin but not E-cadherin (Fig. 1B) expression. These results demonstrated the existence of a profibrotic and proinflammatory phenotype in the ischemic kidneys under chronic NIC exposure. Additionally, IR-induced induction of U-STAT3 was also increased by chronic NIC exposure. Interestingly, chronic NIC exposure by itself also managed to elevate U-STAT3 (Fig. 2).
Fig. 1.
Chronic nicotine (NIC) exposure augments mediators and markers of inflammation and fibrosis in the postischemic kidney. A group of mice received nicotine in their drinking water for 4 wk while another group received the vehicle (2% saccharine) for the same time as described in materials and methods. Some mice underwent 18-min warm renal ischemia while others were sham operated. Kidneys were collected 24 h postischemia and lysed in RIPA buffer. Transforming growth factor (TGF)-β1 and monocyte-chemotactic protein (MCP)-1 content was determined by ELISA (A) and α-smooth muscle actin (SMA), fibronectin (fibro), and E-cadherin (Ecad) expression along with actin by Western blotting (B). C: densitometry results of Western blots in B. Protein expression was normalized to actin; n = 4 (sham) and n = 8 [ischemia-reperfusion (IR)]. *P < 0.05 compared with sham. #P < 0.05 compared with the corresponding value in the saccharine group.
Fig. 2.
Chronic NIC exposure augments signal transducer and activator of transcription-3 (U-STAT3) expression in the postischemic kidney. A: kidney lysates, as described in Fig. 1, were separated by SDS-PAGE, and levels of U-STAT3 along with actin were determined by Western blotting. B: densitometry of Western blots described in A. Levels of U-STAT3 were expressed as the U-STAT3/actin ratio. *P < 0.05 compared with sham or as indicated.
Chronic NIC treatment augments TGF-β1-induced reorganization of the actin cytoskeleton in a U-STAT3-dependent fashion.
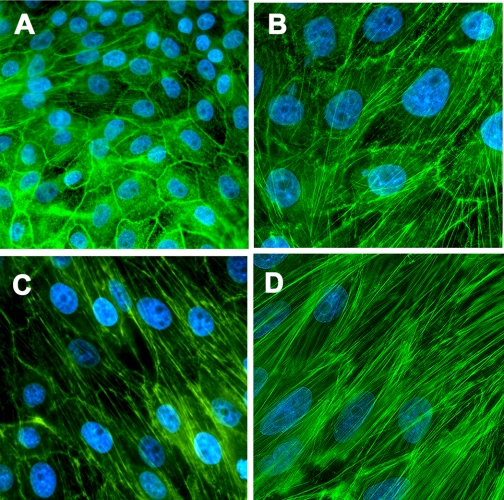
TGF-β1 increases reorganization of the actin cytoskeleton in renal proximal tubular cells (26). Thus chronic NIC exposure that augments renal TGF-β1 levels in the ischemic kidney (Fig. 1A) may further augment stress fiber formation. Accordingly, LLC-PK1 cells were treated with 200 μM NIC for 24 h followed by treatment with 10 ng/ml TGF-β1 for 3 days. Cells were then fixed, stained with Alexa Fluor 488-conjugated phalloidin, and counterstained with DAPI. Control cells exhibited strong peripheral F-actin staining (green fluorescence) with a rare occurrence of central stress fibers (Fig. 3A). In contrast, TGF-β1 treatment decreased marginal F-actin staining with an appearance of strong stress fibers (Fig. 3B). Pretreatment with NIC further enhanced formation of stress fibers in parallel with the long axis of the cells (Fig. 3C). Importantly, adenoviral transduction of the U-STAT3 mimetic Y705F-STAT3 further augmented NIC+TGF-β1-induced stress fiber formation (Fig. 3D).
Fig. 3.
Chronic NIC exposure aggravates TGF-β1-induced stress fiber formation in a U-STAT3-dependent fashion in cultured renal proximal tubule cells. LLC-PK1 cells were treated with 10 ng/ml TGF-β1 for 3 days (B–D). In a set of experiments, cells were pretreated with 200 μM NIC (C and D) for 24 h before treatment with TGF-β1. Cells in D were infected with a Y705F-STAT3 adenovirus for 24 h before treatment. Cells were fixed and stained with Alexa Fluor 488-labeled phalloidin plus 4′,6-diamidino-2-phenylindole (DAPI; magnification ×400). Results are representative of 3 separate experiments.
Chronic NIC treatment enhances TGF-β1-dependent induction of fibrotic markers at the level of transcription.
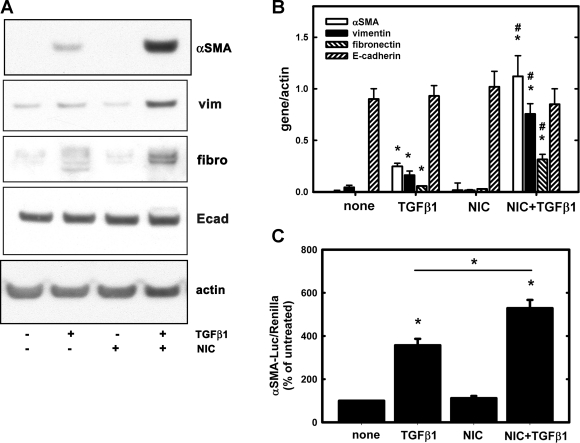
Previous studies have suggested that some tubules in the injured human kidneys maintain both epithelial and mesenchymal markers (25, 27). Thus we tested the hypothesis of whether chronic NIC treatment affects expression of epithelial (E-cadherin) and mesenchymal (α-SMA, vimentin, fibronectin) markers after treatment with TGF-β1. Accordingly, LLC-PK1 cells were treated with 200 μM NIC for 24 h followed by 10 ng/ml TGF-β1 for 3 days. Some cells were treated with NIC or TGF-β1 alone. Expression of E-cadherin, vimentin, fibronectin, and α-SMA were determined by Western blotting and normalized to actin. TGF-β1 treatment significantly increased expression of α-SMA, vimentin, and fibronectin (Fig. 4, A and B), which was further enhanced by chronic pretreatment with 200 μM NIC (Fig. 4, A and B). In contrast, expression of E-cadherin was unchanged (Fig. 4, A and B). These results imply that upon NIC+TGF-β1 treatment, proximal tubular cells retain their epithelial phenotype but may acquire mesenchymal characteristics similar to human biopsies (25, 27). In the ischemic kidneys, expression of fibronectin was increased while E-cadherin was virtually unchanged upon exposure to chronic NIC (Fig. 1, B and C).
Fig. 4.
Chronic NIC exposure augments TGF-β1-mediated induction of profibrotic genes as well as activation of α-SMA promoter in cultured renal proximal tubule cells. A: LLC-PK1 cells were treated with 10 ng/ml TGF-β1 for 3 days. In a set of experiments, cells were pretreated with 200 μM NIC for 24 h before treatment with TGF-β1. Expression of α-SMA, vimentin (vim), fibronectin, and E-cadherin was determined by Western blotting in cell lysates as described in materials and methods. B: densitometry results of blots shown in A; n = 3 independent experiments. Expression of those proteins were normalized to actin expression *P < 0.05 compared with none. #P < 0.05 compared with the TGF-β1 group. C: LLC-PK1 cells were transfected with an α-SMA promoter-luciferase reporter plasmid along with a Renilla luciferase plasmid for 24 h. Some cells were treated with 200 μM NIC for 24 h followed by treatment with 10 ng/ml TGF-β1 for 24 h while other cells were treated with TGF-β1 only. Firefly (α-SMA promoter) and Renilla luciferase activities were determined as described in materials and methods. Values represent relative luciferase activities (firefly/Renilla) expressed as a percentage of controls. Values are means ± SD; n = 3 independent experiments. *P < 0.05 compared with none or as indicated.
In the next set of experiments, LLC-PK1 cells were transfected with an α-SMA promoter-luciferase reporter along with a Renilla luciferase plasmid. After 24-h treatment with 200 μM NIC followed by treatment with 10 ng/ml TGF-β1 for 24 h, firefly luciferase (α-SMA) and Renilla luciferase activities were determined. We found that TGF-β1 but not NIC induced α-SMA promoter activity, which was further augmented by pretreatment with 200 μM NIC (Fig. 4C).
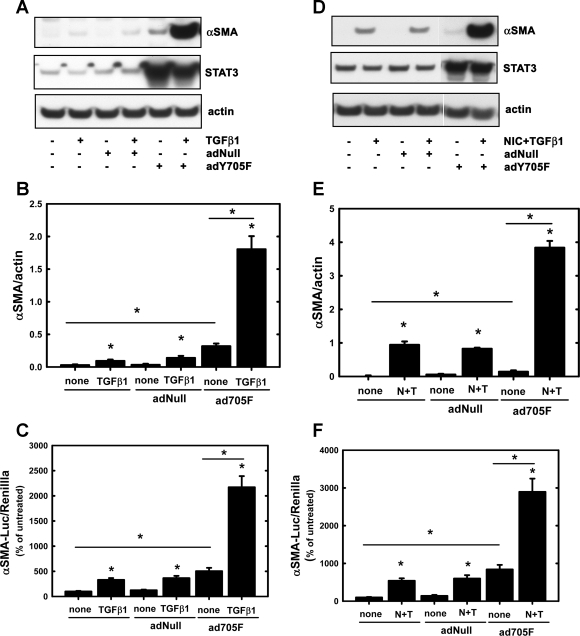
TGF-β1- or NIC+TGF-β1-induced α-SMA expression and promoter activation are U-STAT3 dependent.
Since levels of U-STAT3 are augmented in the kidney treated with NIC or NIC+IR (Fig. 2), we tested whether U-STAT3 would induce the α-SMA promoter either alone or after treatment with TGF-β1 or NIC+TGF-β1. LLC-PK1 cells were infected with a Y705F-STAT3 adenovirus (2) before treatment with TGF-β1 or NIC+TGF-β1. It has been shown by other groups that this Y705F mutant mimics U-STAT3 (31). An empty adenovirus (AdNull) served as a negative control. AdY705F-STAT3, but not AdNull, increased α-SMA expression but more dramatically when cells were treated with TGF-β1 (Fig. 5, A and B). Induction of the α-SMA promoter followed a similar pattern: adY705F-STAT3 induced the promoter, but drastic changes were seen in TGF-β1-treated cells (Fig. 5C). Similarly, adY705F-STAT3, but not AdNull, augmented NIC+TGF-β1-induced α-SMA expression and promoter activity (Fig. 5, D–F).
Fig. 5.
Overexpression of the Y705F-STAT3 mutant augments TGF-β1- and NIC+TGF-β1-induced α-SMA expression as well as activation of α-SMA promoter in cultured renal proximal tubule cells. LLC-PK1 cells were infected with either a negative control (AdNull) or with the Y705F-STAT3 adenovirus as described in materials and methods. Uninfected cells were also included. A: cells were treated with 10 ng/ml TGF-β1 for 3 days, and levels of α-SMA and STAT3 along with actin were determined by Western blotting. B: densitometry results of blots shown in A; n = 3 independent experiments. *P < 0.05 compared with none or as indicated. C: LLC-PK1 cells were transfected with an α-SMA promoter luciferase reporter plasmid along with a Renilla luciferase plasmid for 24 h and then treated with 10 ng/ml TGF-β1 for 24 h. Firefly (α-SMA promoter) and Renilla luciferase activities were determined as described in materials and methods. Values are means ± SD and represent relative luciferase activities (firefly/Renilla) expressed as a percentage of controls; n = 3 independent experiments. *P < 0.05 compared with none or as indicated. D: cells were treated with 200 μM NIC for 24 h followed by treatment with 10 ng/ml TGF-β1 for 3 days, and levels of α-SMA and STAT3 along with actin were determined by Western blotting. E: densitometry results of blots shown in D; n = 3 independent experiments. *P < 0.05 compared with none or as indicated. N+T, NIC+TGF-β1. F: LLC-PK1 cells were transfected with an α-SMA promoter luciferase reporter plasmid along with a Renilla luciferase plasmid for 24 h. Cells were treated with 200 μM NIC for 24 h followed by treatment with 10 ng/ml TGF-β1. Firefly (α-SMA promoter) and Renilla luciferase activities were determined as described in materials and methods. Values are means ± SD and represent relative luciferase activities (firefly/Renilla) expressed as a percentage of controls; n = 3 independent experiments. *P < 0.05 compared with none or as indicated.
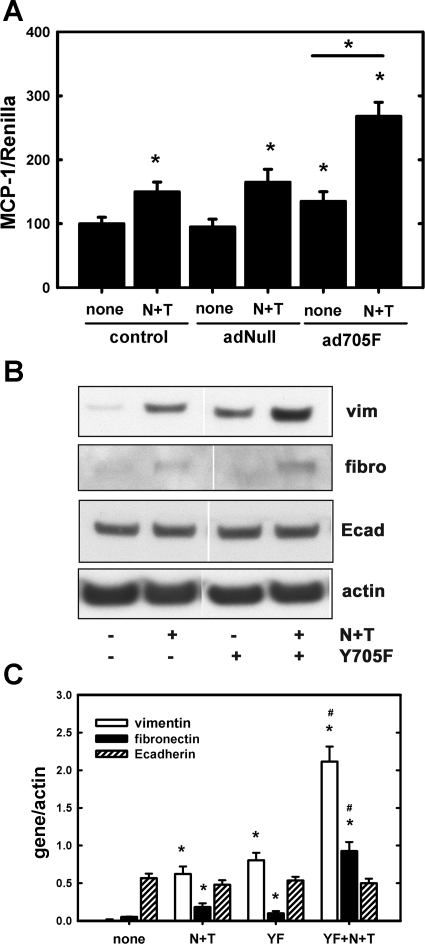
NIC+TGF-β1-induced activation of inflammatory and fibrotic markers is also mediated by U-STAT3.
Since MCP-1 levels are augmented in the ischemic kidney following chronic NIC exposure (Fig. 1), we tested the hypothesis of whether MCP-1 is transcriptionally activated by U-STAT3. Accordingly, LLC-PK1 cells were transfected with an MCP-1 promoter luciferase plasmid together with a Renilla luciferase plasmid. These transfected cells were treated with NIC for 24 h followed by treatment with TGF-β1 for an additional 24 h in the presence or absence of the U-STAT3 or the negative control adenovirus (AdNull) mimetic Y705F, as described above. Figure 6 also demonstrates that Y705F, but not AdNull, moderately but significantly augments NIC+TGF-β1-mediated induction of the MCP-1 promoter. In addition, NIC+TGF-β1-induced expression of fibronectin and vimentin but not E-cadherin was augmented by U-STAT3 (Fig. 6, B and C).
Fig. 6.
Overexpression of the U-STAT3-mimetic Y705F-STAT3 mutant augments NIC+TGF-β1-induced activation of MCP-1 promoter as well as expression of profibrotic genes in cultured renal proximal tubule cells. A: LLC-PK1 cells were transfected with an MCP-1 promoter luciferase reporter plasmid along with a Renilla luciferase plasmid for 24 h followed by infection with either an empty adenovirus (AdNull) or with the Y705F-STAT3 (ad705F) adenovirus as described in materials and methods. Cells were then treated with 200 μM NIC for 24 h followed by treatment with 10 ng/ml TGF-β1 for 24 h. Firefly (MCP-1) and Renilla luciferase activities were determined as described in materials and methods. Values are means ± SD and represent relative luciferase activities (firefly/Renilla) expressed as a percentage of controls; n = 3 independent experiments. *P < 0.05 compared with none or as indicated. B: LLC-PK1 cells were transfected with adY705F and then treated with NIC and TGF-β1 as described in materials and methods. Expression of vimentin, fibronectin, and E-cadherin along with actin was determined by Western blotting. Results shown are representatives of 3 independent experiments. C: densitometry of results in B; n = 3. YF, adY705F-STAT3. *P < 0.05 compared with none. #P < 0.05 compared with the corresponding N+T values.
DISCUSSION
STAT3 is important in embryogenesis, survival/proliferation, apoptosis, or inflammation depending on the target tissue (18). Canonical activation of STAT3 occurs through phosphorylation of its tyrosine 705 residue, which leads to STAT3 dimerization and translocation to the nucleus (15). However, it has lately been recognized that U-STAT3 can also drive expression of many proteins that may not be activated through the canonical pathway (7). Importantly, U-STAT3-induced gene expression is long lasting (7): U-STAT3-activated genes include several oncogenes or cytokines/inflammatory agents (30). Moreover, the significance of STAT3 in response to injury has also become much clearer of late (8). Earlier, we had reported that renal IR injury increases not only tyrosine phosphorylation of STAT3 but also expression of U-STAT3 (2).
Renal fibrosis and inflammation are important steps in progression of CKD (19). TGF-β1, especially that derived from infiltrating macrophages and/or epithelial cells in response to ischemia (17), induces phenotypic changes in the proximal tubules that can lead to imbalanced epithelial-mesenchymal communication and hence initiate a fibrotic and inflammatory response in the kidney (5). It has been shown that proximal tubules with an altered phenotype can compromise structural recovery and favor development of inflammation and fibrosis following injury (28). Smoking/chronic NIC exposure increases renal interstitial fibrosis in experimental animals (4, 16) and also enhances U-STAT3 expression in cultured oral keratinocytes (3). Thus chronic NIC exposure may amplify epithelial signaling that increases inflammation and fibrosis. Whether chronic NIC exposure affects STAT3 activation and proinflammatory and profibrotic signaling in the ischemic kidney is unknown.
Our earlier studies have revealed that chronic exposure of mice to NIC aggravated renal IR-injury (1). Since AKI by itself is a critical risk factor for development and progression of CKD (21), smoking/chronic NIC exposure may exacerbate AKI-induced tubulointerstitial injury and progression to chronic renal disease. Kidneys from NIC+IR mice contained significantly higher amount of TGF-β1, MCP-1, and also expressed more α-SMA than their IR-only counterparts (Fig. 1). Those kidneys are also characterized by elevated fibronectin but virtually unchanged E-cadherin levels (Fig. 1). These results imply that chronic NIC exposure may augment profibrotic/proinflammatory signaling in the ischemic kidney, which could accelerate progression of AKI to CKD.
We confirmed our in vivo observations with in vitro studies using renal proximal tubular cells: chronic NIC treatment markedly augments TGF-β1-induced fibronectin, vimentin, and α-SMA expression (Fig. 4, A and B) but not E-cadherin. NIC also augmented TGF-β1-induced actin cytoskeleton reorganization (Fig. 3, A–C). Importantly, α-SMA as well as MCP-1 induction are transcriptional events as both TGF-β1 and NIC+TGF-β1 increased activity of the α-SMA (Fig. 4C) as well as the MCP-1 promoter (Fig. 6).
Further studies also revealed that renal levels of IR-induced U-STAT3 were significantly augmented by chronic NIC exposure alone and even more so in the ischemic kidney (Fig. 2A). Recent data suggest that STAT3 itself is inducible by cytokines such as IL-6 (32). We found that IR-induced IL-6 accumulation is augmented by NIC exposure in the kidney (Arany et al., unpublished observations): whether IL-6 mediates U-STAT3 induction in the ischemic kidney needs further study.
The above-described results imply that U-STAT3 may mediate TGF-β1-dependent transcriptional events that result in reorganization of the actin cytoskeleton and α-SMA as well as MCP-1 transcription. Ectopic overexpression of a U-STAT3 mimetic (Y705F-STAT3 mutant) augmented actin stress fiber formation in cultured proximal tubule cells (Fig. 3D). U-STAT3 also increased α-SMA expression and augmented TGF-β1- or NIC+TGF-β1-induced α-SMA expression (Fig. 5, A, B, D, and E) through activation of its promoter (Fig. 5, C and F). Similarly, U-STAT3 enhanced promoter activity of MCP-1 (Fig. 6) and possibly also vimentin and fibronectin (Fig. 6, B and C). Hence, these data revealed a novel regulatory mechanism by which chronic NIC exposure, through noncanonical U-STAT3 signaling, regulates actin cytoskeleton integrity as well as TGF-β1-induced transcription of genes that participate in fibrosis and inflammation. Within this scenario, chronic NIC exposure may ensure a sustained activation of α-SMA, fibronectin, and vimentin as well as MCP-1, which can accelerate renal fibrosis and progression to CKD. Whether this process is an epithelial-mesenchymal transition (EMT) or other event needs further clarification. Recently, the existence of EMT in vivo has been seriously challenged (10, 14). The idea that renal proximal tubule cells can become producers of collagens, however, is highly plausible (10). Although the mechanism is unclear, proximal tubule cells with both epithelial and mesenchymal phenotypes may impair recovery from injury and promote development of interstitial fibrosis/inflammation through a complex network of cytokine/growth factor release and consequent signaling (28).
It is suggested that U-STAT3 forms a complex with unphosphorylated NF-κB and binds to the promoter of a set of genes that possesses a functional κB element (7). In addition, U-STAT3 can bind CEBP or c-jun to activate transcription in promoters that do not have a κB element (reviewed in Ref. 7). To determine the mechanism by which U-STAT3 activates transcription of α-SMA and MCP-1 needs further investigation.
In summary, chronic NIC exposure further upregulates IR-induced U-STAT3. The result is a novel and sustained activation of transcription of a set of genes that leads to actin cytoskeleton reorganization with consequent fibrosis and inflammation that may accelerate the progression of AKI to CKD.
GRANTS
These studies were supported by an American Heart Association Midwest Affiliate Grant-in-Aid (10GRNT3790019, I. Arany), an Intramural Research Support Program Award from the University of Mississippi Medical Center (I. Arany), and National Institutes of Health (NIH) Grants DK073401 (L. A. Juncos) and R01HL088101-04 and R01HL088101-02S1 (G. W. Booz).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: I.A., G.W.B., and L.A.J. provided conception and design of research; I.A., D.K.R., S.C.G., and K.C. performed experiments; I.A. analyzed data; I.A., G.W.B., and L.A.J. interpreted results of experiments; I.A. prepared figures; I.A. and L.A.J. drafted manuscript; I.A., K.C., G.W.B., and L.A.J. edited and revised manuscript; I.A. and L.A.J. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Dr. B. D. Nelkin for the dominant-negative (Y705F) STAT3 adenovirus, Dr. R. A. Nemenoff for the α-SMA promoter-luciferase, to Drs. J. L. Madrigal and D. L. Feinstein for the MCP-1 promoter-luciferase plasmid.
REFERENCES
- 1. Arany I, Grifoni S, Clark JS, Csongradi E, Maric C, Juncos LA. Chronic nicotine exposure exacerbates acute renal ischemic injury. Am J Physiol Renal Physiol 301: F125–F133, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Arany I, Megyesi J, Nelkin BD, Safirstein RL. STAT3 attenuates EGFR-mediated ERK activation and cell survival during oxidant stress in mouse proximal tubular cells. Kidney Int 70: 669–674, 2006 [DOI] [PubMed] [Google Scholar]
- 3. Arredondo J, Chernyavsky AI, Jolkovsky DL, Pinkerton KE, Grando SA. Receptor-mediated tobacco toxicity: cooperation of the Ras/Raf-1/MEK1/ERK and JAK-2/STAT-3 pathways downstream of alpha7 nicotinic receptor in oral keratinocytes. FASEB J 20: 2093–2101, 2006 [DOI] [PubMed] [Google Scholar]
- 4. Boor P, Casper S, Celec P, Hurbankova M, Beno M, Heidland A, Amann K, Sebekova K. Renal, vascular and cardiac fibrosis in rats exposed to passive smoking and industrial dust fibre amosite. J Cell Mol Med 13: 4484–4491, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bottinger EP. TGF-beta in renal injury and disease. Semin Nephrol 27: 309–320, 2007 [DOI] [PubMed] [Google Scholar]
- 6. Bromberg JF, Wrzeszczynska MH, Devgan G, Zhao Y, Pestell RG, Albanese C, Darnell JE., Jr Stat3 as an oncogene. Cell 98: 295–303, 1999 [DOI] [PubMed] [Google Scholar]
- 7. Cheon H, Yang J, Stark GR. The functions of signal transducers and activators of transcriptions 1 and 3 as cytokine-inducible proteins. J Interferon Cytokine Res 31: 33–30, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chuang PY, He JC. JAK/STAT signaling in renal diseases. Kidney Int 78: 231–234, 2010 [DOI] [PubMed] [Google Scholar]
- 9. Cucina A, Sapienza P, Borrelli V, Corvino V, Foresi G, Randone B, Cavallaro A, Santoro-D'Angelo L. Nicotine reorganizes cytoskeleton of vascular endothelial cell through platelet-derived growth factor BB. J Surg Res 92: 233–238, 2000 [DOI] [PubMed] [Google Scholar]
- 10. Fragiadaki M, Mason RM. Epithelial-mesenchymal transition in renal fibrosis - evidence for and against. Int J Exp Pathol 92: 143–150, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Garat C, Van Putten V, Refaat ZA, Dessev C, Han SY, Nemenoff RA. Induction of smooth muscle alpha-actin in vascular smooth muscle cells by arginine vasopressin is mediated by c-Jun amino-terminal kinases and p38 mitogen-activated protein kinase. J Biol Chem 275: 22537–22543, 2000 [DOI] [PubMed] [Google Scholar]
- 12. Iranloye BO, Bolarinwa AF. Effect of nicotine administration on weight and histology of some vital visceral organs in female albino rats. Niger J Physiol Sci 24: 7–12, 2009 [DOI] [PubMed] [Google Scholar]
- 13. Jaimes EA, Tian RX, Raij L. Nicotine: the link between cigarette smoking and the progression of renal injury? Am J Physiol Heart Circ Physiol 292: H76–H82, 2007 [DOI] [PubMed] [Google Scholar]
- 14. Kriz W, Kaissling B, Le Hir M. Epithelial-mesenchymal transition (EMT) in kidney fibrosis: fact or fantasy? J Clin Invest 121: 468–474, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kurdi M, Booz GW. Deciphering STAT3 signaling in the heart: plasticity and vascular inflammation. Congest Heart Fail 16: 234–238, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kurus M, Ugras M, Esrefoglu M. Effect of resveratrol on tubular damage and interstitial fibrosis in kidneys of rats exposed to cigarette smoke. Toxicol Ind Health 25: 539–544, 2009 [DOI] [PubMed] [Google Scholar]
- 17. Lee SB, Kalluri R. Mechanistic connection between inflammation and fibrosis. Kidney Int Suppl S22–S26, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Levy DE, Lee CK. What does Stat3 do? J Clin Invest 109: 1143–1148, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liu Y. Renal fibrosis: new insights into the pathogenesis and therapeutics. Kidney Int 69: 213–217, 2006 [DOI] [PubMed] [Google Scholar]
- 20. Madrigal JL, Leza JC, Polak P, Kalinin S, Feinstein DL. Astrocyte-derived MCP-1 mediates neuroprotective effects of noradrenaline. J Neurosci 29: 263–267, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Okusa MD, Chertow GM, Portilla D. The nexus of acute kidney injury, chronic kidney disease, and World Kidney Day 2009. Clin J Am Soc Nephrol 4: 520–522, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Orth SR. Smoking—a renal risk factor. Nephron 86: 12–26, 2000 [DOI] [PubMed] [Google Scholar]
- 23. Orth SR, Hallan SI. Smoking: a risk factor for progression of chronic kidney disease and for cardiovascular morbidity and mortality in renal patients–absence of evidence or evidence of absence? Clin J Am Soc Nephrol 3: 226–236, 2008 [DOI] [PubMed] [Google Scholar]
- 24. Park JI, Strock CJ, Ball DW, Nelkin BD. The Ras/Raf/MEK/extracellular signal-regulated kinase pathway induces autocrine-paracrine growth inhibition via the leukemia inhibitory factor/JAK/STAT pathway. Mol Cell Biol 23: 543–554, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rastaldi MP, Ferrario F, Giardino L, Dell'Antonio G, Grillo C, Grillo P, Strutz F, Muller GA, Colasanti G, D'Amico G. Epithelial-mesenchymal transition of tubular epithelial cells in human renal biopsies. Kidney Int 62: 137–146, 2002 [DOI] [PubMed] [Google Scholar]
- 26. Tian YC, Fraser D, Attisano L, Phillips AO. TGF-β1-mediated alterations of renal proximal tubular epithelial cell phenotype. Am J Physiol Renal Physiol 285: F130–F142, 2003 [DOI] [PubMed] [Google Scholar]
- 27. van Timmeren MM, van den Heuvel MC, Bailly V, Bakker SJ, van Goor H, Stegeman CA. Tubular kidney injury molecule-1 (KIM-1) in human renal disease. J Pathol 212: 209–217, 2007 [DOI] [PubMed] [Google Scholar]
- 28. Venkatachalam MA, Griffin KA, Lan R, Geng H, Saikumar P, Bidani AK. Acute kidney injury: a springboard for progression in chronic kidney disease. Am J Physiol Renal Physiol 298: F1078–F1094, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wen X, Murugan R, Peng Z, Kellum JA. Pathophysiology of acute kidney injury: a new perspective. Contrib Nephrol 165: 39–45, 2010 [DOI] [PubMed] [Google Scholar]
- 30. Yang J, Chatterjee-Kishore M, Staugaitis SM, Nguyen H, Schlessinger K, Levy DE, Stark GR. Novel roles of unphosphorylated STAT3 in oncogenesis and transcriptional regulation. Cancer Res 65: 939–947, 2005 [PubMed] [Google Scholar]
- 31. Yang J, Liao X, Agarwal MK, Barnes L, Auron PE, Stark GR. Unphosphorylated STAT3 accumulates in response to IL-6 and activates transcription by binding to NFkappaB. Genes Dev 21: 1396–1408, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yang J, Stark GR. Roles of unphosphorylated STATs in signaling. Cell Res 18: 443–451, 2008 [DOI] [PubMed] [Google Scholar]
- 33. Zhang G, Kernan KA, Thomas A, Collins S, Song Y, Li L, Zhu W, Leboeuf RC, Eddy AA. A novel signaling pathway: fibroblast nicotinic receptor alpha1 binds urokinase and promotes renal fibrosis. J Biol Chem 284: 29050–29064, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]