Abstract
Prenatal insults have been shown to lead to elevated blood pressure in offspring when they are studied as adults. Prenatal administration of dexamethasone and dietary protein deprivation have demonstrated that there is an increase in transporter abundance for a number of nephron segments but not the subunits of the epithelial sodium channel (ENaC) in the cortical collecting duct. Recent studies have shown that aldosterone is elevated in offspring of protein-deprived mothers when studied as adults, but the physiological importance of the increase in serum aldosterone is unknown. As an indirect measure of ENaC activity, we compared the natriuretic response to benzamil in offspring of mothers who ate a low-protein diet (6%) with those who ate a normal diet (20%) for the last half of pregnancy. The natriuretic response to benzamil was greater in the 6% group (821.1 ± 161.0 μmol/24 h) compared with the 20% group (279.1 ± 137.0 μmol/24 h), consistent with greater ENaC activity in vivo (P < 0.05). In this study, we also directly studied cortical collecting tubule function from adult rats using in vitro microperfusion. There was no difference in basal or vasopressin-stimulated osmotic water permeability. However, while cortical collecting ducts of adult offspring whose mothers ate a 20% protein diet had no sodium transport (−1.9 ± 3.1 pmol·mm−1·min−1), the offspring of rats that ate a 6% protein diet during the last half of pregnancy had a net sodium flux of 10.7 ± 2.6 pmol·mm−1·min−1 (P = 0.01) in tubules perfused in vitro. Sodium transport was measured using ion-selective electrodes, a novel technique allowing measurement of sodium in nanoliter quantities of fluid. Thus we directly demonstrate that there is prenatal programming of cortical collecting duct sodium transport.
Keywords: hypertension, microperfusion
prenatal insults to the fetus have been shown to be a risk factor for hypertension and cardiovascular disease in humans (4–7). Animal models have been used to study prenatal programming of adult disease including maternal dietary protein deprivation, uteroplacental insufficiency, and prenatal administration of dexamethasone. By and large these insults result in offspring that are small for their gestational age and develop hypertension as adults. There are a number of potential causes for the increase in blood pressure including a reduction in nephron number, increased tubular sodium transport, and increased vascular tone that have been implicated as the cause for the increase in blood pressure (1, 2, 8–11, 15, 20, 22, 23, 25, 26). Indeed, the etiology of the increase in blood pressure may be multifactorial or may be dependent on the severity and type of insult as well as when the insult occurs during gestation.
Adult offspring of rats whose mothers ate a low-protein diet had higher serum aldosterone levels than rats whose mothers ate a normal-protein diet (25). However, there is no increase in renal epithelial sodium channel (ENaC) subunit protein expression in hypertensive rats that received prenatal dexamethasone or a low-protein diet (10, 17). The above studies examined the total cellular abundance of ENaC subunits but not whether there is a difference in ENaC activity in the cortical collecting duct that could affect sodium transport. The purpose of this study was to assess directly whether a prenatal insult using maternal dietary protein deprivation programs the cortical collecting duct to increase sodium transport and to determine whether prenatal programming affected osmotic water permeability in the cortical collecting duct.
METHODS
Animals.
Pregnant Sprague-Dawley rats were fed either a 20% protein diet or an isocaloric diet containing 6% protein from gestation day 12 until birth as previously described to examine the effect of prenatal dietary protein deprivation on cortical collecting duct water and sodium transport (9, 17, 18). After birth, the mother was fed the 20% protein diet as were the offspring when weaned. The rats were studied as adults that were 2–4 mo of age and weighed >200 g. There were a total of eight dams used in the low-protein and eight dams used in the control diet group. Offspring of at least two dams were used in the assessment of each parameter in each group. Previous studies, including from our laboratory, using this protocol have demonstrated that rats whose mothers ate a low-protein diet are hypertensive at this age (9, 25). Only males were studied to lessen the variability; however, other studies using this protocol found comparable changes in blood pressure and serum aldosterone levels in male and female rats (25). These studies were approved by the Institutional Animal Care and Use Committee at the University of Texas Southwestern Medical Center.
Serum aldosterone levels.
Blood was taken from rats at the time of death. An aldosterone enzyme immunoassay (Cayman Chemical, Ann Arbor MI) was used to determine the plasma aldosterone concentration per the manufacturer's instructions.
Effect of benzamil on urinary sodium excretion.
Adult rats whose mothers ate a control (20% protein) diet or a low-protein diet (6% protein) during the last half of pregnancy were acclimatized to metabolic cages for at least 24 h. They were then administered a single intraperitoneal injection of vehicle (equivalent volume to that of benzamil), and urine was collected over the next 24 h. The rats were then given one intraperitoneal injection of benzamil, an inhibitor of ENaC (0.7 mg/kg body wt), and urine was collected for 24 h. The difference in sodium excretion between the benzamil and vehicle group was an indirect measure of ENaC in vivo as previously described (14).
In vitro microperfusion flux studies.
After the rats were euthanized, the kidneys were removed quickly, sliced in thin coronal sections, and placed in Hanks' solution containing (in mM) 137 NaCl, 5 KCl, 0.8 MgSO4, 0.33 Na2HPO4, 0.44 KH2PO4, 1 MgCl2 10 Tris, 0.25 CaCl2, 2 glutamine, and 2 l-lactate at 4°C. Cortical collecting tubules were then dissected free hand without the aid of collagenase and transferred to a 1-ml temperature-controlled bathing chamber. Tubules were perfused in vitro as previously described (9, 24).
Measurement of osmotic water permeability.
Briefly, cortical collecting tubules were perfused with a solution containing (in mM) 30 NaCl, 25 NaHCO3, 2.3 Na2HPO4, 10 Na acetate, 1.8 CaCl2, 1 MgSO4, 5 KCl, 8.3 glucose and 5 alanine that had an osmolality of 150 mosmol/kgH2O. The bathing solution contained (in mM) 115 NaCl, 25 NaHCO3, 2.3 Na2HPO4, 10 Na acetate, 1.8 CaCl2, 1 MgSO4, 5 KCl, 8.3 glucose, and 5 alanine and also 6 gm/dl of albumin with a final osmolality of 300 mosmol/kgH2O. All solutions in this study were gassed with 95% O2-5% CO2 at 37°C and had a pH of 7.4. The bathing solution was exchanged at a rate of 0.5 ml/min to keep the osmolality constant. The temperature of the bath was maintained at 37°C. Net volume absorption (JV; in nl·min−1·mm−1) was measured as the difference between the perfusion and collection rates and normalized per millimeter of tubule length. Tubules were perfused for at least 30 min before the first measurement. In experiments where osmotic water permeability (Pf) was determined, the perfusion rate was 10–15 nl/min. The collection rate was determined by timed collections using a 40-nl constant-volume pipette. [methoxy-3H]inulin (New England Nuclear) was added to the perfusate at a concentration of 50 μCi/ml so that the perfusion rate could be calculated. The length (L) of the tubule was measured using an eyepiece micrometer.
Pf was calculated using the equation (3, 24)
where V̇0 is the perfusion rate, V̇L is the collection rate and C0, Cb, and CL represent the osmolality of the perfusate, bath, and collected fluid respectively; A is the surface area calculated from the inner radius; and V̄w is the molar volume of water. The collected fluid osmolality was calculated from the relationship
There were three measurements in each period, and the average was used as the measurement for that period in the experiment.
Measurement of sodium transport and transepithelial potential difference.
In experiments where sodium transport and transepithelial potential difference were measured, tubules were perfused at 1–2 nl/min to be able to detect a small change in luminal fluid composition. The perfusate in these experiments contained (in mM) 115 NaCl, 25 NaHCO3, 2.3 Na2HPO4, 10 Na acetate, 1.8 CaCl2, 1 MgSO4, 5 KCl, 8.3 glucose. and 5 alanine and had an osmolality equal to that of the bathing solution which contained 6 gm/dl albumin. The collection pipette had a volume of 24 nl in these experiments. There were at least three measurements of the perfusion and the collected tubular fluid in each experiment. Sodium transport JNa was calculated as
where L is the tubular length. The transepithelial potential difference was determined using the perfusion pipette as the bridge into the tubular and referenced to the bathing solution using a Keithley 617 programmable electrometer (Cleveland, OH).
The sodium concentration of the perfusion and collected fluid was determined using a method modified from that of Maddrell (16). At least three 24-nl samples of the perfusate and collected fluid on Rinzle plastic microslides (Hatfield, PA) that were coated with a equal mixture of poly-l-lysine 0.1% (Sigma, St. Louis, MO) and water were used. The slide was then air dried. The slide was then placed under water-equilibrated mineral oil, and the perfusate and collected fluid samples were deposited on the slide. The sodium concentration of fluid was measured with a sodium-selective electrode. The protocol was modified from a previous study (16). Fire-polished unfilamented borosilicate glass capillary tubes (GC120–10, Harvard Apparatus) were soaked in 70% nitric acid for 5 min, rinsed three to five times in deionized water, and dried for a minimum of 20 min at 200°C on a hot ceramic plate. Micropipettes were pulled using a vertical micropipette puller (Narishige PP-830, Tokyo, Japan) with the tip diameter of ∼3 μm. The pulled pipettes were replaced on the hot plate for 10 min before silanization, which makes the glass surface hydrophobic and helps retain the hydrophobic sodium resin (Sodium Ionophore I Cocktail A, Sigma-Aldrich). A drop (∼4 μl) of dimethyldichlorosilane (Sigma-Aldrich) was pipetted onto the inside of a 150-mm-diameter pyrex petri dish, which was then inverted to cover the micropipettes and placed on the hot plate. After a minimum of 20-min exposure, these micropipettes were removed from hot plate and stored in a jar filled with silica gel before use. Using a 34-gauge Microfil (World Precision Instruments, Sarasota, FL), half-molar NaCl was backfilled into the microelectrode. Negative pressure created by a closed narrow tubing system (a narrow tube attached to the back of the microelectrode at one end and to the 1-ml syringe at the other end) sucked up a small amount of sodium resin into the microelectrodes. Reference electrodes were pulled from a 1.2-mm-outer diameter filamented glass capillary (GC120F-10, Harvard Apparatus) and backfilled with 1 M lithium chloride. Microelectrodes were connected through chloride silver wire to an electrometer of high-input impedance (>1015 Ω; FD223A, World Precision Instruments) and calibrated in solutions containing the following concentrations of NaCl: 10, 20, 100, and 200 mM. With the assistance of stereomicroscope and micromanipulators, both sodium-selective and reference electrodes were positioned in the fluid samples. Activities of sodium in fluid were recorded (in mV). Data were sampled at 10 kHz with a 0.01-kHz low-pass filter. Clamp X 8.2 software (Molecular Devices, Sunnyvale, CA) was used for data acquisition. The potential measured by sodium-selective electrodes was converted to concentration using the equation
where [Na]c is the sodium concentration of the collected drop, [Na]s is the concentration of the standard solution (20 mM), V is the change in potential between the collected and standard solution (20 mM), and s is the slope for a 10-fold change (20–200 mM) in sodium concentration. All measurements were performed at room temperature.
Statistics.
There were at least six experiments in each group except for in vitro microperfusion data examining the effect of vasopressin, where there were five experiments in each group. Differences between groups were assessed using Student's t-test, and a P value of <0.05 was considered statistically significant.
RESULTS
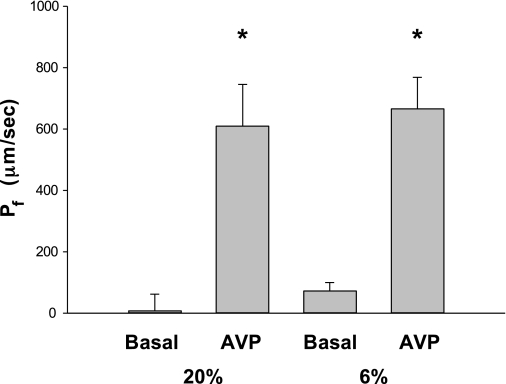
To determine whether there was a defect in urinary concentrating ability, we first examined whether there was a difference in urine osmolality in rats that were the product of mothers fed a 6% protein diet during the last half of pregnancy (6% group) compared with rats that were the product of mothers fed a 20% protein diet throughout pregnancy (20% group). The urine osmolality in the 20% group was 1,852 ± 145 compared with 1,698 ± 158 mosmol/kgH2O (P = not significant). We also placed rats in metabolic cages to determine their urine output. The 24-h urine volume was 10.3 ± 1.0 ml in the 20% group, which was greater than 6.1 ± 0.7 ml in the 6% group. The cause for the lower urine output in the low-protein group is not clear. To more rigorously determine whether there was a defect in the cortical collecting duct, we measured Pf under the basal state and after the addition of vasopressin. As shown in Fig. 1, basal Pf was not different from zero in the 20% protein group as well as the 6% group. With addition of 10−10 M vasopressin to the bath, Pf increased significantly but there was no difference in the vasopressin-stimulated Pf between the two groups.
Fig. 1.
Effect of prenatal programming on basal cortical collecting duct osmotic water permeability (Pf) and the Pf in response to vasopressin. Adult rat cortical collecting ducts were perfused in vitro for at least 30 min before Pf was measured, which was not different from zero in male rats born from mothers who ate a 20% control diet or a 6% low-protein diet during the last half of gestation. Addition of 10−10 M vasopressin resulted in an increase in Pf that was not different between the low-protein and control group. The increase in Pf was significant in both groups (*P < 0.001).
Aldosterone levels and serum electrolytes (Table 1) were obtained from blood at the time of euthanasia. The serum aldosterone levels in the offspring of the mothers in the 6% group were significantly higher than the offspring of the 20% group measured at 4 mo of age (388.9 ± 79.1 vs. 762.8 ± 146.2 pg/ml, P < 0.05). These results are comparable to those previously published using the same prenatal diets in rats at 1 and 2 mo of age (25).
Table 1.
Serum electrolytes
| 20% Group | 6% Group | P Value | |
|---|---|---|---|
| Sodium, meq/l | 142.0 ± 0.5 | 141.7 ± 0.7 | NS |
| Potassium, meq/l | 4.1 ± 0.2 | 4.2 ± 0.2 | NS |
| Chloride, meq/l | 102.4 ± 0.5 | 104.5 ± 0.8 | P = 0.05 |
| Bicarbonate, meq/l | 31.1 ± 0.5 | 29.7 ± 0.3 | P < 0.05 |
Values are means ± SE. NS, not significant.
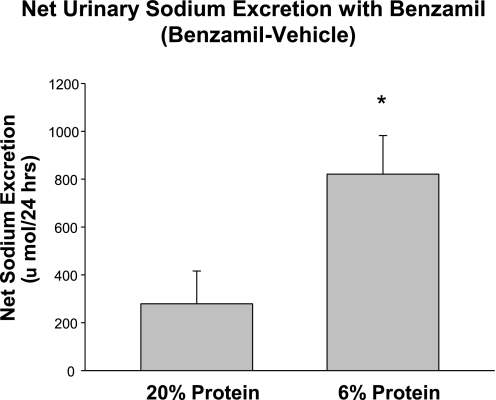
To examine whether prenatal insults led to an increase in ENaC activity, we examined the effect of benzamil on 24-h urinary sodium excretion. In both groups, urinary sodium excretion was measured in response to vehicle and compared with that after benzamil. The difference between these values was an indirect assessment of ENaC activity. As shown in Fig. 2, there was an increase in net sodium excretion in the offspring of mothers that were fed a 6% protein diet compared with those that were fed a 20% protein diet during the last half of pregnancy, consistent with an increase in basal ENaC activity in the low-protein group. In the control rats studied for this experiment, the 24-h urine output was 11.5 ± 1.3 and 14.1 ± 0.8 ml after benzamil. In the low-protein group, the urine output was 4.7 ± 0.5 ml in the control group and 7.1 ± 1.1 ml after benzamil. The increase in urine output was not significant in either group.
Fig. 2.
Effect of prenatal programming on urinary sodium excretion in response to benzamil. The effect of benzamil, an inhibitor of the epithelial sodium channel, was examined in adult rats that were the offspring of mothers fed a low-protein diet and 20% protein diet. The result shown is the difference in sodium excretion postbenzamil minus that with vehicle. The rats in the low-protein group had a more significant natriuresis in response to benzamil (*P < 0.05).
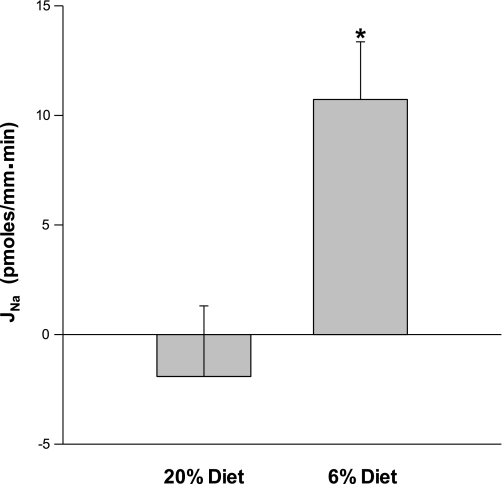
The transepithelial potential difference was 0.08 ± 0.35 in the 20% group and −0.72 ± 0.32 in the 6% group, which were not statistically different (P = 0.13). To determine directly whether there was a difference in the sodium transport by the cortical collecting duct, we measured net sodium transport using in vitro microperfusion. The concentration of sodium was determined using ion-selective electrodes. As shown in Fig. 3, there was no net sodium transport in the 20% control group; however, there was a significant net sodium flux in the 6% group. Thus offspring of rats that were fed a low-protein diet during the last half of pregnancy have an increase in sodium transport in the cortical collecting duct.
Fig. 3.
Effect of prenatal programming on cortical collecting duct sodium reabsorption. Cortical collecting ducts were perfused in vitro, and sodium transport was measured using ion-selective electrodes. The rate of sodium transport (JNa) was not different from zero in rats whose mothers ate the control diet, while sodium transport was significantly higher in rats whose mothers were fed a low-protein diet during the last half of pregnancy (*P = 0.01).
DISCUSSION
In the present study, we examined whether there was a difference in cortical collecting duct function by prenatal programming. The role of cortical collecting ducts in prenatal programming is equivocal until this study. We used two different approaches to examine whether prenatal programming affected sodium transport in the distal nephron. First, we demonstrated that the natriuretic response to benzamil, an inhibitor of ENaC, was greater in the prenatal low-protein group compared with the control group in vivo. We then used a direct approach by measuring sodium transport in adult offspring of rats that had a prenatal insult. We directly demonstrate that prenatal administration of a low-protein diet results in increased cortical collecting duct sodium transport when the rats are studied as adults.
We found no evidence for a defect in urinary concentrating ability in rats that were the product of mothers fed a 20% diet compared with those fed a 6% diet. While we did not water deprive the rats, their basal urine osmolality measured in a 24-h urine collection was comparable between the two groups. Most importantly, their basal and vasopressin-stimulated Pf were not different, showing that there is no defect in urinary concentrating ability between the two groups. However, we did not water deprive the rats nor did we administer vasopressin, so we cannot rule out a subtle concentrating defect.
Previous studies have shown that compared with controls, prenatal dietary protein deprivation resulted in offspring with a nearly twofold increase in serum aldosterone level at 8 wk of age (25), while there was no difference in ENaC subunit protein expression in offspring of mothers with dietary protein deprivation compared with controls (17). These studies measured total ENaC subunit expression and not what is present on the apical membrane. In addition, there is not a good correlation between ENaC subunit protein expression and serum aldosterone levels (19). In studies where rats were either placed on a low-salt diet with a 4- to 5-fold increase in aldosterone or where aldosterone was infused over 10 days resulting in a 40-fold increase in serum aldosterone levels, the increase in α-ENaC was very modest and there was no effect on the β- or γ- subunits (19). Thus it is not surprising that there is no increase in ENaC expression in this study, yet direct measurement of sodium transport shows that there is an increase in transport in the cortical collecting duct.
In both prenatal administration of dexamethasone and dietary protein deprivation, there is evidence for an increase in sodium transport in a number of nephron segments. In all nephron segments, the driving force for sodium transport is the low intracellular sodium concentration mediated by the Na+-K+-ATPase on the basolateral membrane. Prenatal administration of dexamethasone during the last half of pregnancy resulted in an increase in mRNA and protein levels of the α1-subunit of the Na+-K+-ATPase in male offspring when they were studied as adults (27). Both prenatal dexamethasone and dietary protein deprivation resulted in an increase in the Na-K-2Cl cotransporter and the thiazide-sensitive cotransporter (9, 10, 17). Direct measurement of chloride transport showed that both prenatal administration of dexamethasone and dietary protein deprivation resulted in an increase in thick ascending limb transport in adult male offspring (9). Similar to this study, while there was no effect of prenatal dietary protein deprivation on postnatal Na+/H+ exchanger protein abundance (17), prenatal administration of dexamethasone resulted in an increase in proximal tubule volume absorption and Na+/H+ exchanger activity in adult offspring (8).
This study extends the above-noted studies where an increase in transport has been shown. We find that there was an increase in serum aldosterone in adult rats similar to what others have shown (25). This was likely the cause for the increase in sodium transport found in the offspring of rats that had dietary protein deprivation in our study. We also found that there was a slightly lower serum bicarbonate concentration in the 6% group compared with the 20% group. The cause for this is unclear at present, and it may or may not be of renal origin. Whether maternal dietary protein deprivation affects intestinal transport has not as yet been determined.
It should be noted that we did not measure blood pressure in this study; however, we have measured the effect of a prenatal low-protein diet on blood pressure previously using the tail cuff method. Blood pressure in male offspring of mothers fed a 6% diet rats at 2 mo of age was higher than the 20% control offspring (12, 13). Vehaskari et al. (25) found that males were hypertensive using tail cuff measurements from 1 mo to 10 mo of age (25). Studies examining the effect of prenatal dexamethasone on blood pressure found that blood pressures were only elevated when the rats whose mothers received prenatal dexamethasone were stressed (21). The effect of a maternal low-protein diet on blood pressure measured using telemetry has not as yet been performed.
In summary, this study used a unique approach to determine directly that there was an increase in sodium transport in the cortical collecting duct of adults. We were able to dissect and perfuse cortical collecting ducts from adult rats despite the renal fibrosis and difficulty of dissection. We demonstrate that there is no sodium transport in cortical collecting ducts of control rats; however, prenatal dietary protein deprivation resulted in an augmentation of sodium transport in perfused cortical collecting ducts. Finally, we showed the utility of using ion-selective electrodes to measure sodium in nanoliter quantities of fluid from perfused tubules.
GRANTS
This work was supported by National Institutes of Health Grants DK41612 (M. Baum) and DK078596 (M. Baum) and the O'Brien Center (P30DK079328 to Peter Igarashi).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: C.-J.C. and M.B. provided conception and design of research; C.-J.C., G.L., and M.B. performed experiments; C.-J.C., G.L., and M.B. analyzed data; C.-J.C., G.L., and M.B. interpreted results of experiments; C.-J.C. and M.B. drafted manuscript; C.-J.C. and M.B. edited and revised manuscript; C.-J.C., G.L., and M.B. approved final version of manuscript; M.B. prepared figures.
REFERENCES
- 1. Alexander BT. Placental insufficiency leads to development of hypertension in growth-restricted offspring. Hypertension 41: 457–462, 2003 [DOI] [PubMed] [Google Scholar]
- 2. Alexander BT. Fetal programming of hypertension. Am J Physiol Regul Integr Comp Physiol 290: R1–R10, 2006 [DOI] [PubMed] [Google Scholar]
- 3. Al-Zahid G, Schafer JA, Troutman SL, Andreoli TE. Effect of antidiuretic hormone on water and solute permeation, and the activation energies for these processes, in mammalian cortical collecting tubules. J Membr Biol 31: 103–129, 1977 [DOI] [PubMed] [Google Scholar]
- 4. Barker DJ. The fetal origins of adult hypertension. J Hypertens Suppl 10: S39–S44, 1992 [PubMed] [Google Scholar]
- 5. Barker DJ, Bull AR, Osmond C, Simmonds SJ. Fetal and placental size and risk of hypertension in adult life. BMJ 301: 259–262, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Barker DJ, Godfrey KM, Osmond C, Bull A. The relation of fetal length, ponderal index and head circumference to blood pressure and the risk of hypertension in adult life. Paediatr Perinat Epidemiol 6: 35–44, 1992 [DOI] [PubMed] [Google Scholar]
- 7. Barker DJ, Osmond C. Low birth weight and hypertension. BMJ 297: 134–135, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dagan A, Gattineni J, Cook V, Baum M. Prenatal programming of rat proximal tubule Na+/H+ exchanger by dexamethasone. Am J Physiol Regul Integr Comp Physiol 292: R1230–R1235, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dagan A, Habbib S, Gattineni J, Dwarakanath V, Baum M. Prenatal programming of rat thick ascending limb chloride transport by low protein diet and dexamethasone. Am J Physiol Regul Integr Comp Physiol 297: R93–R99, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dagan A, Kwon HM, Dwarakanath V, Baum M. Effect of renal denervation on prenatal programming of hypertension and renal tubular transporter abundance. Am J Physiol Renal Physiol 295: F29–F34, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Figueroa JP, Rose JC, Massmann GA, Zhang J, Acuna G. Alterations in fetal kidney development and elevations in arterial blood pressure in young adult sheep after clinical doses of antenatal glucocorticoids. Pediatr Res 58: 510–515, 2005 [DOI] [PubMed] [Google Scholar]
- 12. Habib S, Gattineni J, Twombley K, Baum M. Evidence that prenatal programming of hypertension by dietary protein deprivation is mediated by fetal glucocorticoid exposure. Am J Hypertens 24: 96–101, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Habib S, Zhang Q, Baum M. Prenatal programming of hypertension in the rat: effect of postnatal rearing. Nephron Extra 1: 157–165 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Halagappa VK, Tiwari S, Riazi S, Hu X, Ecelbarger CM. Chronic candesartan alters expression and activity of NKCC2, NCC, and ENaC in the obese Zucker rat. Am J Physiol Renal Physiol 294: F1222–F1231, 2008 [DOI] [PubMed] [Google Scholar]
- 15. Kantorowicz L, Valego NK, Tang L, Figueroa JP, Chappell MC, Carey LC, Rose JC. Plasma and renal renin concentrations in adult sheep after prenatal betamethasone exposure. Reprod Sci 15: 831–838, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Maddrell SH, O'Donnell MJ, Caffrey R. The regulation of haemolymph potassium activity during initiation and maintenance of diuresis in fed Rhodnius prolixus. J Exp Biol 177: 273–285, 1993 [DOI] [PubMed] [Google Scholar]
- 17. Manning J, Beutler K, Knepper MA, Vehaskari VM. Upregulation of renal BSC1 and TSC in prenatally programmed hypertension. Am J Physiol Renal Physiol 283: F202–F206, 2002 [DOI] [PubMed] [Google Scholar]
- 18. Manning J, Vehaskari VM. Low birth weight-associated adult hypertension in the rat. Pediatr Nephrol 16: 417–422, 2001 [DOI] [PubMed] [Google Scholar]
- 19. Masilamani S, Kim GH, Mitchell C, Wade JB, Knepper MA. Aldosterone-mediated regulation of ENaC alpha, beta, and gamma subunit proteins in rat kidney. J Clin Invest 104: R19–R23, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Massmann GA, Zhang J, Rose JC, Figueroa JP. Acute and long-term effects of clinical doses of antenatal glucocorticoids in the developing fetal sheep kidney. J Soc Gynecol Investig 13: 174–180, 2006 [DOI] [PubMed] [Google Scholar]
- 21. O'Regan D, Kenyon CJ, Seckl JR, Holmes MC. Prenatal dexamethasone ‘programmes’ hypotension, but stress-induced hypertension in adult offspring. J Endocrinol 196: 343–352, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ortiz LA, Quan A, Weinberg A, Baum M. Effect of prenatal dexamethasone on rat renal development. Kidney Int 59: 1663–1669, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ortiz LA, Quan A, Zarzar F, Weinberg A, Baum M. Prenatal dexamethasone programs hypertension and renal injury in the rat. Hypertension 41: 328–334, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Quigley R, Chakravarty S, Baum M. Antidiuretic hormone resistance in the neonatal cortical collecting tubule is mediated in part by elevated phosphodiesterase activity. Am J Physiol Renal Physiol 286: F317–F322, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vehaskari VM, Aviles DH, Manning J. Prenatal programming of adult hypertension in the rat. Kidney Int 59: 238–245, 2001 [DOI] [PubMed] [Google Scholar]
- 26. Vehaskari VM, Woods LL. Prenatal programming of hypertension: lessons from experimental models. J Am Soc Nephrol 16: 2545–2556, 2005 [DOI] [PubMed] [Google Scholar]
- 27. Wyrwoll CS, Mark PJ, Waddell BJ. Developmental programming of renal glucocorticoid sensitivity and the renin-angiotensin system. Hypertension 50: 579–584, 2007 [DOI] [PubMed] [Google Scholar]