Abstract
It is well-recognized that excessive angiotensin II (ANG II) can mediate progressive renal injury. Previous studies by us and others have indicated that dopamine may modulate actions of ANG II in the kidney. The current studies investigated whether altering intrarenal dopamine levels affected ANG II-mediated renal fibrosis. We utilized a model of increased intrarenal dopamine, catechol-O-methyl-transferase knockout (COMT KO) mice, which have increased kidney dopamine levels due to deletion of a major intrarenal dopamine-metabolizing enzyme. In wild-type mice, chronic ANG II infusion increased renal expression of both of the major dopamine-metabolizing enzymes, COMT and monoamine oxidase. After 8 wk of ANG II infusion, there were no significant differences in blood pressure between wild-type and COMT KO mice. Compared with wild-type, COMT KO mice had decreased albuminuria and tubulointerstitial injury. In response to ANG II infusion, there was decreased expression of both glomerular and tubulointerstitial injury markers (fibronectin, connective tissue growth factor, fibroblast-specific protein-1, collagen I, podocyte vascular endothelial growth factor) in COMT KO mice. We recently reported that ANG II-mediated tubulointerstitial fibrosis is mediated by src-dependent epidermal growth factor receptor (EGFR) activation. In aromatic l-amino acid decarboxylase knockout (AADC KO) mice, a model of intrarenal dopamine deficiency due to selective proximal tubule AADC deletion, which inhibits intrarenal dopamine synthesis, ANG II infusion further increased expression of p-src and pTyr845-EGFR. In contrast, their expression was markedly attenuated in COMT KO mice. These results demonstrate a role for intrarenal dopamine to buffer the detrimental effects of ANG II upon the kidney.
Keywords: catechol-O-methyl-transferase, glomerulosclerosis, tubulointerstitial fibrosis, gludopa, epidermal growth factor receptor
although dopamine serves important functions in the central nervous system as a neurotransmitter, it is also an important endogenous modulator of kidney function. In mammalian kidney, dopamine is primarily produced in the proximal tubule. The dopamine precursor l-dihydroxyphenylalanine (l-DOPA) is filtered at the glomerulus and is taken up by the proximal tubule via luminal transporters and converted to dopamine by aromatic l-amino acid decarboxylase (AADC), which is highly expressed in the proximal tubule. In the kidney, dopamine is metabolized by catechol-O-methyl-transferase (COMT) and monoamine oxidase (MAO).
Dopamine's cellular actions are mediated by signaling through G protein-coupled seven transmembrane receptors. In mammals, there are five known renal dopamine receptors, which are divided into two subclasses: D1-like and D2-like receptors. D1-like receptors (D1 and D5) are coupled to Gs and stimulate adenylate cyclase. D2-like receptors (D2, D3, and D4) are coupled predominantly to Gi.
In the mammalian kidney, dopamine receptor activation inhibits both proximal and distal solute and water transport (1, 4). In addition to its role in regulation of salt and water excretion and blood pressure, dopamine may be protective to the kidney under pathological conditions. Both D1-like receptors and D2-like receptors have been reported to protect against acute kidney injury (23, 32). A recent randomized controlled trial indicates that donor pretreatment with dopamine limited cold-ischemia-induced kidney injury following transplantation (25). One mechanism underlying dopamine kidney protection may be its antioxidant effect (35).
Angiotensin II (ANG II), mediated through AT1 receptors, stimulates reabsorption in both proximal and distal nephrons. Thus, dopamine and ANG II appear to serve counterregulatory functions in the kidney (5, 15, 16). In this regard, dopamine inhibits renal renin expression (38) and inhibits ANG II-mediated tubule function and AT1 expression (9, 21, 28, 40). The goal of the present studies was to determine the potential role of intrarenal dopamine to modulate the effects of ANG II excess on renal function and development of progressive injury.
METHODS
Animals.
Animal experiments were performed in accordance with the guidelines of the Institutional Animal Care and Use Committee (IACUC) of Vanderbilt University. Animal protocol was reviewed and approved by IACUC of Vanderbilt University. All mice used in the studies were on a 129J/sv background. Wild-type and COMT knockout (KO) mice were obtained from Dr. Maria Karayiorgou at Rockefeller University (17). AADC floxed mice were generated in our laboratories, backcrossed onto the 129J/sv background for 10 generations, and then crossed with 129J/sv γ-GT Cre mice (40). All mice were genotyped before use. ANG II infusion induced more severe kidney injury in unilaterally nephrectomized (UNX) animals than in animals with two kidneys (22). Therefore, in our studies, the UNX mice were treated with ANG II (BACHEM) at a dose of 1.4 mg·kg−1·day−1 (COMT KO and corresponding wild-type mice) through subcutaneous osmotic minipumps (model 2004, Alzet) (20) and killed after 8 wk. Our preliminary experiment found that most of UNX AADC KO mice treated with 1.4 mg·kg−1·day−1 ANG II died within 4 wk after initiation of ANG II infusion. Therefore, a reduced ANG II dose (0.9 mg·kg−1·day−1) and shortened experiment period (4 wk) were employed for AADC KO and corresponding wild-type mice.
The renal selective dopamine precursor gludopa was synthesized in the Chemical Synthesis Core, Vanderbilt Institute of Chemical Biology. Gludopa at a dose of 5 or 10 mg·kg−1·day−1 was administered through subcutaneous osmotic minipumps (model 2001). Urine samples were collected by using the Credé technique and stored at −80°C until use. Urinary ANG II was measured by GC/electron capture/negative chemical ionization mass spectrometry assay as previously described (40).
Measurements of blood pressure and albuminuria.
Systolic blood pressure (SBP) was measured in conscious, trained mice at room temperature using a tail-cuff monitor (BP-2000 BP Analysis System, Visitech System). Twenty-four-hour urine was collected from individually caged mice using polycarbonate metabolic cages. Urinary albumin and creatinine excretion were determined using Albuwell-M kits (Exocell, Philadelphia, PA).
Measurement of kidney dopamine.
Dopamine was measured by HPLC coupled with electrochemical detection by the Neurochemistry Core Laboratory at Vanderbilt University's Center for Molecular Neuroscience Research.
Antibodies.
Rabbit antibodies against AADC (AB136) and collagen I were purchased from Millipore. Goat anti-COMT was from Novus. Rabbit anti-fibroblast-specific protein-1 (FSP-1) was a gift from Dr. Eric Neilson (Vanderbilt University), rabbit anti-human fibronectin was from Sigma, rabbit anti-Mas was from Alomone (AAR-013), monoclonal anti-angiotensinogen (AGT) was from ABBIOTEC (catalog no. 250551), and rabbit anti-p-Src was from Cell Signaling Technology. Rabbit anti-p-epidermal growth factor receptor (EGFR) (Tyr848) and EGFR, MAO A/B, goat anti-connective tissue growth factor (CTGF), and NADPH oxidase 1 (NOX-1) were from Santa Cruz Biotechnology.
Immunohistochemistry and Western analysis and quantitative image analysis.
Under deep anesthesia with Nembutal (70 mg/kg ip, Abbot Laboratories), mice were exsanguinated with ∼10 ml heparinized saline (0.9% NaCl, 2 U/ml heparin) through a transcardial aortic cannula. One kidney was removed for Western blot analysis. The other kidney was perfused as previously reported (37). The fixed kidneys were dehydrated through a graded series of ethanols, embedded in paraffin, sectioned at 4-μm thickness, and mounted on glass slides. The slides were deparaffinized, rehydrated, and stained with different antibodies, as previously described (18). Based on the distinctive density and color of immunostaining in video images, the number, size, and position of stained cells were quantified using the BIOQUANT true-color windows system (R & M Biometrics, Nashville, TN) as previously described (36, 37). Western blot analysis was carried out as described previously(7, 26).
Cell culture.
Mouse proximal tubule cells were generated based on our previously described methods (8). These cells were conditionally immortalized with a termperature-sensitive SV40 large T gene. The immortalized mouse proximal tubule cells were cultured at 37°C for experiment in the absence of interferon-γ. Cells were starved for 24 h before treatment with 100 nM dopamine.
RNA isolation and PCR.
Total RNA was isolated from cultured mouse proximal tubule cells using TRIzol reagents (Invitrogen) according to the manufacturer's instructions. Primers used for PCR were listed in Table 1.
Table 1.
Primers for dopamine and angiotensin systems in the cultured proximal tubule epithelial cells
| Primers | Forward | Reverse | Product |
|---|---|---|---|
| AADC | GTTTGTGCTACGCTTTGCTG | CGAAGACGGAGTGGTAGTTA | 200 bp |
| COMT | GCCAGGCTTCTCACCATG | TCGTACTTCTTCTTCAGCT | 143 bp |
| MAO-A | GCAACACAGTGGAGTGGCTA | GCCACAGAAGTGGAAAAACC | 224 bp |
| MAO-B | GTGGTATGTGAAGCAGTGTGG | TCAGTGCCTGCAAAGAAAATC | 723 bp |
| DAT | ATCATTGCCACATCCTCCAT | CATATGGCAAAAGGGGACTG | 295 bp |
| D1 | AACTGTATGGTGCCCTTCTGTGG | GCCCCGTTGTTGTTGATGCTTACA | 230 bp |
| D2 | CACTCCGCCACTTCTTGACATACA | AGCCCCATCCACAGCCTCCTCT | 208 bp |
| D3 | GTCCTGCCCTCTCCTCTTTGGTTT | CATTTGTCCCGTGGCATCTGA | 310 bp |
| D4 | TGCCCTCAACCCCATCATCTACAC | TCCCAACCCCCAGCCTTCATAA | 202 bp |
| D5 | GGGAGATCGCTGCTGCCTATGTC | ATTGGGGGTGAGTGGTGAGATTTT | 221 bp |
| AT1a | GACCAACTCAACCCAGAAAAGC | CGAAGCGATCTTACATAGGTG | 340 bp |
| AT1b | TGGGAATATTTGGAAACAGTTTGGT | CAGAGTATAGCTGGTGAGAATAAT | 530 bp |
| AT2 | TCCTGTTCTCTACTACATGATTTTTG | AGCCATAATACAAGCATTCACAC | 454 bp |
| Mas | ACTGCCGGGCGGTCATCATC | GGTGGAGAAAAGCAAGGAGA | 263 bp |
| AGT | CAAATGCACAGATCGGAGATGAC | CAGGTGCTCTTGTTGTGGTAAAGG | 143 bp |
| ACE | TAACTCGAGTGCCGAGGTG | CCAGCAGGTGGCAGTCTT | 340 bp |
| ACE2 | CTTCAGCACTCTCAGCAGACA | CAACTTCCTCCTCACATAGGC | 431 bp |
AADC, aromatic l-amino acid decarboxylase; COMT, catechol-O-methyl-transferase; MAO, monoamine oxidase; DAT, dopamine transporter; AT1a and AT1b, angiotensin II receptors type 1a and 1b; AT2, angiotensin II receptor type 2; Mas, Ang 1-7 receptor; AGT, angiotensinogen; ACE, angiotensin-converting enzyme.
Interstitial fibrotic analysis.
Interstitial fibrosis (Masson trichrome stain) was quantified using the BIOQUANT true-color windows system. Interstitial fibrosis in vehicle-treated animal was used as 100%.
Statistical analyses.
All values are presented as means ± SE. Bonferroni t-test corrected for multiple comparisons was used for statistical analysis, and differences were considered significant at P < 0.05.
RESULTS
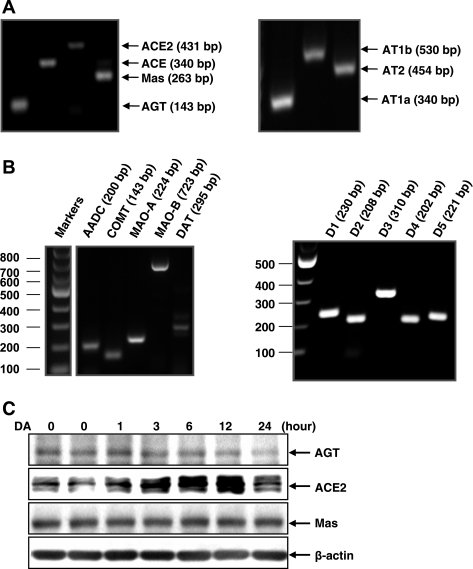
Previous studies have shown that intrarenal dopamine can modulate components of the intrarenal renin-angiotensin system. Cultured mouse proximal tubule cells expressed mRNA for the major components of the renin-angiotensin system (AGT, ACE1, ACE2, AT1a, AT1b, AT2, and MAS) as well as all components of the dopaminergic system (AADC, COMT, MAOs, DAT, D1, D2, D3, D4, and D5; Fig. 1, A and B). When these cells were cultured with dopamine (100 nM), there was a time-dependent decrease in AGT expression and an increase in ACE2. Mas expression was not affected by dopamine treatment (Fig. 1C).
Fig. 1.
Dopamine-mediated alterations in expression of the angiotensin system in cultured proximal tubule cells. A and B: PCR identified the existence of the components of angiotensin system (A) and dopamine system (B) in cultured mouse proximal tubule cells. C: dopamine inhibited angiotensinogen (AGT) expression and increased angiotensin-converting enzyme (ACE)2 expression. AADC, aromatic l-amino acid decarboxylase; COMT, catechol-O-methyl-transferase; MAO, monoamine oxidase.
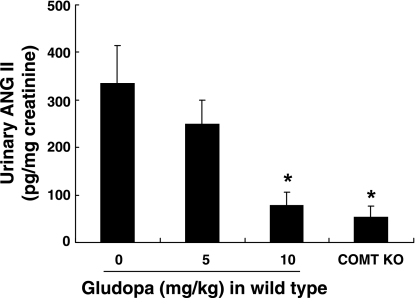
Gludopa is a renal selective dopamine precursor due to its sequential conversion in the proximal tubules of the kidney by proximal tubule-specific γ-glutamyl transpeptidase into l-DOPA, which, in turn, is converted to DA by AADC (3). Our preliminary data showed that renal dopamine levels increased >4- and >10-fold, respectively, after treatment with gludopa at doses of 5 and 10 mg·kg−1·day−1, while dopamine levels in the brain and blood were not altered (data not shown). Gludopa decreased urinary ANG II excretion in a concentration-dependent manner (Fig. 2). Of note, urinary ANG II excretion was markedly decreased in COMT KO mice, further indicating intrarenal effects of altering renal dopamine metabolism.
Fig. 2.
Urinary ANG II excretion was inhibited by activation of the intrarenal dopaminergic system. Gludopa, a renal specific dopamine precursor, inhibited urinary ANG II excretion in wild-type mice. Compared with wild-type mice, urinary ANG II excretion was reduced in COMT knockout (KO) mice. *P < 0.05 vs. control wild-type mice, n = 4 in each group.
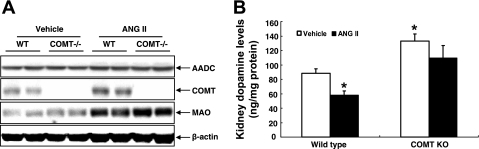
Intravenous infusion of ANG II in humans acutely reduces urinary dopamine levels (13), but the role of ANG II to modulate expression of components of intrarenal dopamine production has not been systematically examined. As indicated in Fig. 3A, chronic ANG II infusion did not alter renal expression of AADC, the enzyme that converts l-DOPA to active dopamine, in either wild-type or COMT KO mice. In contrast, it increased expression of COMT and MAO, the two major intrarenal dopamine-metabolizing enzymes in wild-type mice. In COMT KO mice, chronic ANG II infusion also increased expression of MAO. ANG II decreased renal dopamine in wild-type mice, but in COMT KO, intrarenal dopamine levels were not statistically different than nontreated mice. These results suggest that COMT, but not MAO, plays a major role in ANG II-mediated reduction of kidney dopamine production.
Fig. 3.
ANG II activated renal dopamine-metabolizing system and decreased renal dopamine levels. A: chronic ANG II infusion led to increases in expression of MAO and COMT. B: at baseline, renal dopamine levels were higher in COMT KO mice than in wild-type. ANG II infusion decreased renal dopamine levels in wild-type but not COMT KO mice (*P < 0.05 vs. control wild-type, n = 4 in each group).
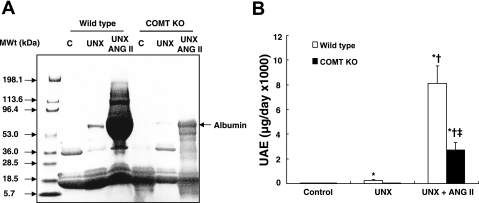
After 8 wk of ANG II infusion (1.4 mg·kg−1·day−1), there were no significant differences in blood pressure between wild-type and COMT KO mice (SBP: 185 ± 3 vs. 186 ± 2 mmHg of ANG II-infused wild-type mice, n = 8). As indicated in Fig. 4B, ANG II infusion led to markedly less proteinuria in UNX COMT KO mice than in UNX wild-type mice. At baseline, albuminuria was similar in wild-type and COMT KO mice (33 ± 3 vs. 35 ± 2 μg/day, n = 6). In wild-type mice, UNX for 8 wk led to increased albuminuria, which was further augmented by ANG II infusion (μg/day: UNX: 256 ± 79, P < 0.01 vs. control; UNX + ANG II: 8,110 ± 1,437, P < 0.01 vs. UNX, n = 6 in each group; Fig. 4). In contrast, COMT KO mice had minimal increases in albuminuria with UNX (45 ± 6) and significantly less albuminuria with ANG II than in wild-type (UNX + ANG II: 2,697 ± 632, P < 0.01, n = 6; Fig. 4).
Fig. 4.
ANG II-mediated albuminuria was significantly attenuated in COMT KO mice. A: SDS-PAGE gel electrophoresis indicates significantly less urinary protein excretion in ANG II-treated unilaterally nephrectomized (UNX) COMT KO mice than in the ANG II-treated UNX wild-type mice. Loading volume was determined using urinary creatinine as an internal control. B: 24-h urinary albumin excretion (UAE) was significantly lower in ANG II-treated UNX COMT KO mice than ANG II-treated UNX wild-type mice (*P < 0.01 vs. wild-type control; †P < 0.01 vs. corresponding UNX mice; ‡P < 0.01 vs. ANG II-treated UNX wild-type mice, n = 6 in each group).
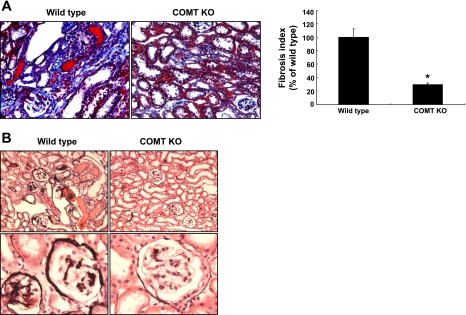
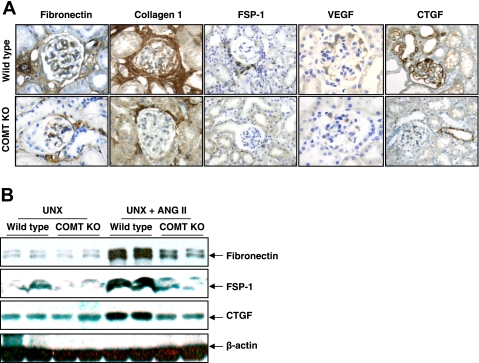
Histologic analysis indicated that ANG II infusion led to interstitial fibrosis, as indicated by Masson trichrome staining, and glomerular sclerosis as indicated by silver staining in wild-type mice, and both glomerular and tubulointerstitial fibrosis were significantly attenuated in COMT KO mice (Fig. 5). Immunohistochemistry showed that in COMT KO mice, there was attenuation of the ANG II-induced increases in interstitial fibronectin, collagen I, and the fibroblast marker FSP-1 observed in wild-type mice (Fig. 6A). Podocyte vascular endothelial growth factor expression was increased in response to ANG II in wild-type mice, but this increase was markedly attenuated in COMT−/− mice. Similarly, CTGF expression increased in both glomeruli and tubules in response to ANG II but there were minimal increases in COMT−/− mice. Immunoblotting confirmed these differences in renal expression of fibronectin, CTGF, and FSP-1 (Fig. 6B).
Fig. 5.
ANG II-mediated renal injury was markedly attenuated in COMT KO mice. ANG II infusion led to significantly less interstitial fibrosis (A) and glomerular sclerosis (B) in COMT KO mice than in wild-type mice (*P < 0.01 vs. wild-type, n = 4).
Fig. 6.
ANG II-mediated elevations of mediators and markers of fibrosis were attenuated in COMT KO mice. A: immunohistochemistry showed that ANG II-induced elevations of fibronectin, connective tissue growth factor (CTGF), fibroblast-specific protein-1 (FSP-1), collagen I, and vascular endothelial growth factor (VEGF) were significantly attenuated in COMT KO mice. B: immunoblotting indicated that ANG II-induced increases in fibronectin, FSP-1, and CTGF were attenuated in COMT KO mice.
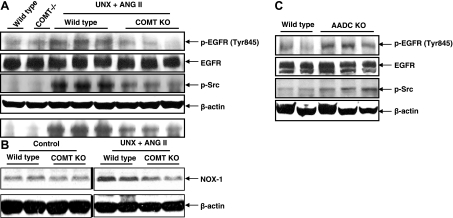
We and others reported previously that the tubulointerstitial injury induced by chronic ANG II infusion is predominantly mediated by EGFR transactivation (6, 20), and we previously found that this effect was mediated by reactive oxygen species activation of src, which activates EGFR by src-dependent phosphorylation of tyrosine residue 845 (6). In response to chronic ANG II infusion, both src activation and phospho-845YEGFR expression were inhibited in COMT KO mice compared with wild-type mice (Fig. 7A). Also of interest, NOX-1 expression increases in response to ANG II infusion were blunted in COMT KO mice (Fig. 7B). We previously described increased renal injury in response to ANG II in mice with selective intrarenal dopamine production due to genetic deletion of AADC (40). In these mice, expression of phospho-src and phospho-845YEGFR was increased in response to chronic ANG II administration compared with that in wild-type mice (Fig. 7C).
Fig. 7.
ANG II-mediated activation of Src-epidermal growth factor receptor (EGFR) signaling was attenuated by intrarenal dopamine. A: activation of Src and EGFR induced by ANG II was attenuated in COMT KO mice. B: ANG II-induced increases in NADPH oxidase 1 (NOX-1) expression were inhibited in COMT KO mice. Control and ANG II-infused samples were from the same gel. C: activation of Src and EGFR induced by ANG II was augmented in AADC KO mice.
DISCUSSION
In the mammalian kidney, dopamine and ANG II serve counterregulatory roles in the regulation of salt and water reabsorption, with ANG II stimulating and dopamine inhibiting both proximal and distal solute and water transport, mediated at least in part by regulation of specific tubule transporter activity (1, 4, 28, 33, 34). In addition to acute changes in nephron function, chronic ANG II excess leads to both initiation and progression of progressive glomerular and tubulointerstitial fibrotic injury. In association with our previous studies (40), the current studies provide compelling evidence that dopamine can also mitigate the profibrotic effects of ANG II in the kidney.
Increased renal dopamine in the COMT KO mice led to relative preservation of glomerular structure and function and marked decreases in tubulointerstitial fibrosis in response to chronic ANG II infusion. These studies complement our previous finding that tubulointerstitial fibrosis was markedly increased in mice with deficient intrarenal dopamine production (40). We (6) and others (20) reported that the tubulointerstitial injury induced by ANG II excess is in large part mediated by activation of the EGFR, and we found that this activation occurs by oxidant stress-mediated activation of src kinase, with activation of EGFR by phosphorylation of tyrosine 845 in the EGFR cytoplasmic domain, which is known to be a target of src phosphorylation (6). Therefore, it was noteworthy that in the current studies, src activation and 845YEGFR expression were significantly decreased in COMT KO mice and were increased in AADC KO mice. Whether this is secondary to the known effect of dopamine to decrease AT1 receptor expression in the proximal tubule (9, 27, 28) or to direct antioxidant effects of dopamine (2, 29–31) will require further studies.
COMT is a major intrarenal dopamine-metabolizing enzyme, and COMT KO mice have increased intrarenal and urinary dopamine levels due to the absence of COMT metabolism of dopamine. However, plasma dopamine concentrations are similar between wild-type and COMT KO mice (19, 38). Previous studies indicate that ANG II may regulate the intrarenal dopaminergic system at multiple levels. ANG II has been reported to inhibit dopamine uptake and AADC activity but to stimulate MAO activity in kidney cortex through activation of AT1 receptors (10–12). Nitecapone, a peripheral inhibitor of COMT, induces dopamine-dependent natriuresis (14). However, it has been suggested that entacapone, another inhibitor of COMT, protected from ANG II-induced inflammation and renal injury due to its anti-oxidant effect but not due to a change in renal dopaminergic tone (19). The possible explanation for the lack of effect of entacapone on renal dopaminergic tone is that MAO activity may be predominant in metabolism of dopamine in these transgenic rats. It is well-known that dopamine and ANG II can each antagonize the activity of the other hormone, but this observed increase in protein expression of the major intrarenal pathways of dopamine metabolism in response to ANG II represents a previously undescribed mechanism by which ANG II may decrease dopamine activity in the kidney.
We previously reported that dopamine may indirectly decrease ANG II production by inhibiting renal renin production (38–40). In the current studies, dopamine decreased AGT expression in cultured proximal tubule cells, and gludopa decreased urinary ANG II production. Administration of dopamine to proximal tubule cells also increased mRNA for ACE2, the mediator of production of Ang1–7, which serves as a counterregulatory hormone to AT1-mediated responses. In addition, previous studies demonstrated that dopamine will increase activity of AT2 receptors (24), which can also antagonize ANG II's actions via AT1 receptors. Therefore, in addition to direct inhibition of angiotensin expression and AT1 expression and activity, dopamine's antagonism of AT1 may also be mediated in part by upregulating angiotensin-mediated signaling pathways that antagonize AT1-mediated signaling.
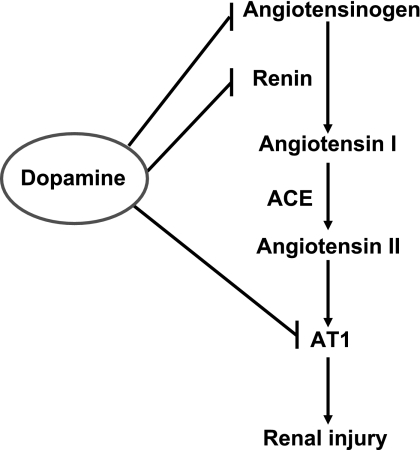
In summary, these studies provide evidence for the important counterregulatory role that the intrarenal dopaminergic system plays to counteract the effects of ANG II signaling through AT1 receptors (Fig. 8). In addition to previous studies indicating that dopamine can decrease renin production and decrease AT1 receptor expression in the kidney, the current studies indicate that dopamine decreases intrarenal angiotensin production. Furthermore, in the face of chronic ANG II excess, increased intrarenal dopamine prevents increases in expression of profibrotic signaling pathways and it decreases glomerular and tubulointerstitial injury.
Fig. 8.
Intrarenal dopamine antagonizes ANG II-induced renal injury at multiple levels. ANG II induces renal injury through activation of AT1 receptors. Intrarenal dopamine may antagonize ANG II-induced renal injury through inhibition of AT1 expression and decreases in intrarenal ANG II production due to inhibition of expression of both AGT and renin.
GRANTS
These studies were supported in part by grants from the National Institutes of Health (DK62794, DK51265, DK38226, CA122620, DK61018), by the Vanderbilt O'Brien Center (DK79341), and funds from the Veterans Administration.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: S.Y., B.Y., Y.Z., H.Y., and M.-Z.Z. performed experiments; S.Y., B.Y., M.-Z.Z., and R.C.H. analyzed data; S.Y., M.-Z.Z., and R.C.H. interpreted results of experiments; S.Y., M.-Z.Z., and R.C.H. prepared figures; M.-Z.Z. and R.C.H. conception and design of research; M.-Z.Z. and R.C.H. drafted manuscript; M.-Z.Z. and R.C.H. edited and revised manuscript; M.-Z.Z. and R.C.H. approved final version of manuscript.
REFERENCES
- 1. Aperia AC. Intrarenal dopamine: a key signal in the interactive regulation of sodium metabolism. Annu Rev Physiol 62: 621–647, 2000 [DOI] [PubMed] [Google Scholar]
- 2. Armando I, Wang X, Villar VA, Jones JE, Asico LD, Escano C, Jose PA. Reactive oxygen species-dependent hypertension in dopamine D2 receptor-deficient mice. Hypertension 49: 672–678, 2007 [DOI] [PubMed] [Google Scholar]
- 3. Berndt TJ, Liang M, Tyce GM, Knox FG. Intrarenal serotonin, dopamine, and phosphate handling in remnant kidneys. Kidney Int 59: 625–630, 2001 [DOI] [PubMed] [Google Scholar]
- 4. Carey RM. Theodore Cooper Lecture: renal dopamine system: paracrine regulator of sodium homeostasis and blood pressure. Hypertension 38: 297–302, 2001 [DOI] [PubMed] [Google Scholar]
- 5. Chen CJ, Apparsundaram S, Lokhandwala MF. Intrarenally produced angiotensin II opposes the natriuretic action of the dopamine-1 receptor agonist fenoldopam in rats. J Pharmacol Exp Ther 256: 486–491, 1991 [PubMed] [Google Scholar]
- 6. Chen JC, Chen JK, Nagai K, Plieth D, Tan M, Lee TC, Threadgill DW, Neilson EG, Harris RC. EGFR signaling promotes TGFβ-dependent renal fibrosis. JASN [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cheng H, Wang S, Jo YI, Hao CM, Zhang M, Fan X, Kennedy C, Breyer MD, Moeckel GW, Harris RC. Overexpression of cyclooxygenase-2 predisposes to podocyte injury. J Am Soc Nephrol 18: 551–559, 2007 [DOI] [PubMed] [Google Scholar]
- 8. Cheng HF, Becker BN, Burns KD, Harris RC. Angiotensin II upregulates type-1 angiotensin II receptors in renal proximal tubule. J Clin Invest 95: 2012–2019, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cheng HF, Becker BN, Harris RC. Dopamine decreases expression of type-1 angiotensin II receptors in renal proximal tubule. J Clin Invest 97: 2745–2752, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Choi MR, Lee BM, Medici C, Correa AH, Fernandez BE. Effects of angiotensin II on renal dopamine metabolism: synthesis, release, catabolism and turnover. Nephron Physiol 115: 1–7, 2010 [DOI] [PubMed] [Google Scholar]
- 11. Choi MR, Medici C, Gironacci MM, Correa AH, Fernandez BE. Angiotensin II regulation of renal dopamine uptake and Na+,K+-ATPase activity. Nephron Physiol 111: 53–58, 2009 [DOI] [PubMed] [Google Scholar]
- 12. De Luca Sarobe V, Nowicki S, Carranza A, Levin G, Barontini M, Arrizurieta E, Ibarra FR. Low sodium intake induces an increase in renal monoamine oxidase activity in the rat. Involvement of an angiotensin II dependent mechanism. Acta Physiol Scand 185: 161–167, 2005 [DOI] [PubMed] [Google Scholar]
- 13. Eadington DW, Swainson CP, Frier BM, Johnston N, Samson RR, Lee MR. Urinary dopamine response to angiotensin II is not abnormal in type 1 (insulin-dependent) diabetes mellitus. Nephrol Dial Transplant 8: 36–40, 1993 [DOI] [PubMed] [Google Scholar]
- 14. Eklof AC, Holtback U, Sundelof M, Chen S, Aperia A. Inhibition of COMT induces dopamine-dependent natriuresis and inhibition of proximal tubular Na+, K+-ATPase. Kidney Int 52: 742–747, 1997 [DOI] [PubMed] [Google Scholar]
- 15. Gesek FA, Schoolwerth AC. Hormone responses of proximal Na+-H+ exchanger in spontaneously hypertensive rats. Am J Physiol Renal Fluid Electrolyte Physiol 261: F526–F536, 1991 [DOI] [PubMed] [Google Scholar]
- 16. Gildea JJ, Wang X, Jose PA, Felder RA. Differential D1 and D5 receptor regulation and degradation of the angiotensin type 1 receptor. Hypertension 51: 360–366, 2008 [DOI] [PubMed] [Google Scholar]
- 17. Gogos JA, Morgan M, Luine V, Santha M, Ogawa S, Pfaff D, Karayiorgou M. Catechol-O-methyltransferase-deficient mice exhibit sexually dimorphic changes in catecholamine levels and behavior. Proc Natl Acad Sci USA 95: 9991–9996, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Harris RC, McKanna JA, Akai Y, Jacobson HR, Dubois RN, Breyer MD. Cyclooxygenase-2 is associated with the macula densa of rat kidney and increases with salt restriction. J Clin Invest 94: 2504–2510, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Helkamaa T, Finckenberg P, Louhelainen M, Merasto S, Rauhala P, Lapatto R, Cheng ZJ, Reenila I, Mannisto P, Muller DN, Luft FC, Mervaala EM. Entacapone protects from angiotensin II-induced inflammation and renal injury. J Hypertens 21: 2353–2363, 2003 [DOI] [PubMed] [Google Scholar]
- 20. Lautrette A, Li S, Alili R, Sunnarborg SW, Burtin M, Lee DC, Friedlander G, Terzi F. Angiotensin II and EGF receptor cross-talk in chronic kidney diseases: a new therapeutic approach. Nat Med 11: 867–874, 2005 [DOI] [PubMed] [Google Scholar]
- 21. Li H, Armando I, Yu P, Escano C, Mueller SC, Asico L, Pascua A, Lu Q, Wang X, Villar VA, Jones JE, Wang Z, Periasamy A, Lau YS, Soares-da-Silva P, Creswell K, Guillemette G, Sibley DR, Eisner G, Gildea JJ, Felder RA, Jose PA. Dopamine 5 receptor mediates Ang II type 1 receptor degradation via a ubiquitin-proteasome pathway in mice and human cells. J Clin Invest 118: 2180–2189, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Miller PL, Rennke HG, Meyer TW. Glomerular hypertrophy accelerates hypertensive glomerular injury in rats. Am J Physiol Renal Fluid Electrolyte Physiol 261: F459–F465, 1991 [DOI] [PubMed] [Google Scholar]
- 23. Narkar V, Kunduzova O, Hussain T, Cambon C, Parini A, Lokhandwala M. Dopamine D2-like receptor agonist bromocriptine protects against ischemia/reperfusion injury in rat kidney. Kidney Int 66: 633–640, 2004 [DOI] [PubMed] [Google Scholar]
- 24. Salomone LJ, Howell NL, McGrath HE, Kemp BA, Keller SR, Gildea JJ, Felder RA, Carey RM. Intrarenal dopamine D1-like receptor stimulation induces natriuresis via an angiotensin type-2 receptor mechanism. Hypertension 49: 155–161, 2007 [DOI] [PubMed] [Google Scholar]
- 25. Schnuelle P, Gottmann U, Hoeger S, Boesebeck D, Lauchart W, Weiss C, Fischereder M, Jauch KW, Heemann U, Zeier M, Hugo C, Pisarski P, Kramer BK, Lopau K, Rahmel A, Benck U, Birck R, Yard BA. Effects of donor pretreatment with dopamine on graft function after kidney transplantation: a randomized controlled trial. JAMA 302: 1067–1075, 2009 [DOI] [PubMed] [Google Scholar]
- 26. Singh AB, Sugimoto K, Harris RC. Juxtacrine activation of EGFR by membrane-anchored HB-EGF protects epithelial cells from anoikis while maintaining an epithelial phenotype. J Biol Chem 282: 32890–32901, 2007 [DOI] [PubMed] [Google Scholar]
- 27. Wang X, Li F, Jose PA, Ecelbarger CM. Reduction of renal dopamine receptor expression in obese Zucker rats: role of sex and angiotensin II. Am J Physiol Renal Physiol 299: F1164–F1170, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wang X, Villar VA, Armando I, Eisner GM, Felder RA, Jose PA. Dopamine, kidney, and hypertension: studies in dopamine receptor knockout mice. Pediatr Nephrol 23: 2131–2146, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yang Z, Asico LD, Yu P, Wang Z, Jones JE, Escano CS, Wang X, Quinn MT, Sibley DR, Romero GG, Felder RA, Jose PA. D5 dopamine receptor regulation of reactive oxygen species production, NADPH oxidase, and blood pressure. Am J Physiol Regul Integr Comp Physiol 290: R96–R104, 2006 [DOI] [PubMed] [Google Scholar]
- 30. Yao B, Harris RC, Zhang MZ. Interactions between 11β-hydroxysteroid dehydrogenase and COX-2 in kidney. Am J Physiol Regul Integr Comp Physiol 288: R1767–R1773, 2005 [DOI] [PubMed] [Google Scholar]
- 31. Yasunari K, Kohno M, Kano H, Minami M, Yoshikawa J. Dopamine as a novel antioxidative agent for rat vascular smooth muscle cells through dopamine D(1)-like receptors. Circulation 101: 2302–2308, 2000 [DOI] [PubMed] [Google Scholar]
- 32. Yatsu T, Aoki M, Inagaki O. Preventive effect of zelandopam, a dopamine D1 receptor agonist, on cisplatin-induced acute renal failure in rats. Eur J Pharmacol 461: 191–195, 2003 [DOI] [PubMed] [Google Scholar]
- 33. Zelenina M, Zelenin S, Bondar AA, Brismar H, Aperia A. Water permeability of aquaporin-4 is decreased by protein kinase C and dopamine. Am J Physiol Renal Physiol 283: F309–F318, 2002 [DOI] [PubMed] [Google Scholar]
- 34. Zeng C, Sanada H, Watanabe H, Eisner GM, Felder RA, Jose PA. Functional genomics of the dopaminergic system in hypertension. Physiol Genomics 19: 233–246, 2004 [DOI] [PubMed] [Google Scholar]
- 35. Zeng C, Villar VA, Yu P, Zhou L, Jose PA. Reactive oxygen species and dopamine receptor function in essential hypertension. Clin Exp Hypertens 31: 156–178, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhang MZ, Wang JL, Cheng HF, Harris RC, McKanna JA. Cyclooxygenase-2 in rat nephron development. Am J Physiol Renal Physiol 273: F994–F1002, 1997 [DOI] [PubMed] [Google Scholar]
- 37. Zhang MZ, Yao B, Cheng HF, Wang SW, Inagami T, Harris RC. Renal cortical cyclooxygenase 2 expression is differentially regulated by angiotensin II AT(1) and AT(2) receptors. Proc Natl Acad Sci USA 103: 16045–16050, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhang MZ, Yao B, Fang X, Wang S, Smith JP, Harris RC. Intrarenal dopaminergic system regulates renin expression. Hypertension 53: 564–570, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhang MZ, Yao B, McKanna JA, Harris RC. Cross talk between the intrarenal dopaminergic and cyclooxygenase-2 systems. Am J Physiol Renal Physiol 288: F840–F845, 2005 [DOI] [PubMed] [Google Scholar]
- 40. Zhang MZ, Yao B, Wang S, Fan X, Wu G, Yang H, Yin H, Yang S, Harris RC. Intrarenal dopamine deficiency leads to hypertension and decreased longevity in mice. J Clin Invest 121: 2845–2854, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]