Abstract
Exocytosis of Weibel-Palade bodies (WPB) represents a distinct response of endothelial cells to stressors, and local release of WPB contents leads to systemic escalation of this response. We synthesized a glycine-(Nα-Et)lysine-proline-arginine (ITF 1697) peptide that has a potential to inhibit exocytosis of WPB and protect microcirculation. Here, we confirmed an inhibitory effect of ITF 1697 using intravital videoimaging and point-tracking of individual organelles. In an in vivo study, mice were implanted with Alzet osmotic pumps (10 μg ITF 1697·kg−1·min−1 at volume of 1 μl/h) and subjected to renal ischemia (IRI). IRI resulted in marked renal injury and elevation of serum creatinine in mice treated with a vehicle. In contrast, renal injury and elevation of creatinine were significantly ameliorated in mice subjected to IRI and receiving ITF 1697. ITF 1697 prevented a systemic response to IRI: a significant surge in the levels of eotaxin and IL-8 (KC; both components of WPB), IL-1α, IL-1β, and RANTES was all prevented or blunted by the administration of ITF 1697, whereas the levels of an anti-inflammatory, IL-10, and macrophage inflammatory protein-1α were upregulated in ITF 1697-treated animals. En face staining of aortic endothelial cells showed that WPB were depleted after 40–180 min post-IRI, and this was significantly blunted in aortic preparations obtained from mice treated with ITF 1697. WPB exocytosis contributed to IRI-associated mobilization of endothelial progenitor cells and hematopoietic stem cells, and ITF 1697 blunted their mobilization. Unexpectedly, 1 mo after IRI, mice treated with ITF 1697 showed a significantly more pronounced degree of scarring than nontreated animals. In conclusion, 1) application of ITF 1697 inhibits exocytosis of WPB and IRI; 2) the systemic inflammatory response of IRI is in part due to the exocytosis of WPB and its blockade blunts it; and 3) ITF 1697 improves short-term renal function after IRI, but not the long-term fibrotic complications.
Keywords: cytokines, chemokines, mobilization of stem cells, fibrosis
weibel-palade bodies (WPB) are rod-shaped organelles (0.2 × 2–3 μm) characteristic of endothelial cells and containing an array of proteins, peptides, and cytokines, which can be released emergently on demand. Among these biologically active compounds are von Willebrand factor, P-selectin, IL-8, endothelin-1, angiopoietin-2 (Ang-2), and many others, which contribute to the induction of a proinflammatory milieu on the one hand but also induce mobilization of stem cells on the other. A list of compounds known to induce exocytosis of WPB is long and includes thrombin, histamine, peptido-leukotrienes, complement components, superoxide anion, VEGF, sphingosine-1-phosphate, ceramide, purine nucleotides, serotonin, vasopressin, and epinephrine (15, 21, 9). Notably, ischemia-reperfusion injury to various organs has been shown to release WPB (14, 20).
The possibility of exacerbated tissue injury and inflammation as a result of exocytosis of WPB has been explored in several studies. Matsushita et al. (16) developed fusion polypeptides composed of a 22-amino acid N-ethyl-maleimide-sensitive factor (a regulator of exocytosis) and a carrier peptide derived from the human immunodeficiency virus transactivating regulatory protein domain and demonstrated that inhibition of WPB exocytosis decreased leukocyte trafficking and peritonitis (7). de Leeuw et al. (6) demonstrated that a small GTP-binding protein, Ral, is involved in exocytosis of WPB and that expression of a dominant-negative Ral variant prevented it. More recently, Bertuglia et al. (4) explored a lysine-proline motif encountered in several biologically active small peptides and synthesized a glycine-(Nα-Et)lysine-proline-arginine (ITF 1697) peptide, with the biological half-life of 20–120 min, and demonstrated that it inhibits ischemia-reperfusion-induced exocytosis of WPB and protected pulmonary microcirculation by preventing increase in permeability, leukocyte and platelet adhesion, P-selectin, and von Willebrand factor secretion. This peptide has in its core the Lys-Pro-motif found in tuftsin (a γ-globulin-derived peptide stimulator of phagocytosis), a C-terminal region of α-melanocyte-stimulating hormone endowed with anti-inflammatory properties, and an IL-1β-derived peptide antagonist of hyperalgesic effect on the parent molecule (4). In the present study, we selected ITF 1697 peptide to investigate its effects on the course of renal ischemia-reperfusion. Specifically, we confirmed the inhibitory action of ITF 1697 on exocytosis of WPB, demonstrated that the peptide salvages renal function and morphology in the postischemic period, and showed that inhibition of WPB exocytosis is accompanied by the exacerbated profibrotic changes in the kidney 1 mo after ischemia.
MATERIALS AND METHODS
Cell culture.
Human umbilical vein endothelial cells (HUVEC; ATCC, Manassas, VA), were maintained in endothelial basal medium-2 (EBM-2, Clonetics) supplemented with 2% FBS and growth factors (Lonza, Walkersville, MD), under conditions of 37°C and 5% CO2, and used between passages 2 and 5. Cells were grown to 80% confluency on pronectin-coated glass-bottom dishes (MatTek, Ashland, MA) and incubated with 4 μM FM1–43 (Calbiochem, Gibbstown, NJ) for 1 h at 37°C. For inhibition studies, cells were preincubated with 10 μg/ml ITF 1697 for 30 min at 37°C. For imaging, culture media were replaced by fresh Krebs-Ringer HEPES (KRH) buffer (119 mM NaCl, 4.75 mM KCl, 2.5 mM CaCl2, 1.2 mM MgCl2, 1.2 mM KH2PO4, 10 mM HEPES, pH 74, and 2 mM d-glucose) supplemented with 10 μg/ml ITF 1697 or 1 μM ionomycin for the ITF and ionomycin group, respectively.
Intravital videomicroscopy and image analysis.
A Nikon Y-FL epifluorescence intravital microscope (Nikon, Melville, NY) equipped with an intensified CoolSNAP HQ tube camera (Photometrics, Tucson, AZ) and a ×60 Nikon Plan Apo objective was used for microscopy and image acquisition. Intravital videomicroscopy was performed over a period of 28 min, with a time-lapse interval set to 10 s. Particle/point-tracking analysis was performed using Fiji/ImageJ software (Version 1.45m, National Institutes of Health, Bethesda, MD) and the plugin “Manual Tracking” (Fabrice Cordelières, Institut Curie, Orsay, France). Each experiment was repeated three times. On average, 15 cells/experiment were tracked.
Animal studies.
Male FVB/NJ mice were obtained from the Jackson Laboratory and used at the age of 8–9 wk. All animal protocols were conducted in accord with the National Institutes of Health (NIH) guidelines and were approved by the Institutional Animal Care and Use Committee. Alzet osmotic pumps (model 1003D, Cupertino, CA) with constant delivery rates of 1 μl/h for the duration of 3 days were filled with ITF 1697 (10 μg·kg−1·min−1) and implanted into the peritoneal cavity 24 h before renal artery clamping. This dosing regimen is based on the previously reported optimal dose of ITF 1697 used for a continuous intravenous infusion (4). For induction of renal ischemia-reperfusion injury, mice were anesthetized with an intraperitoneal injection of ketamine hydrochloride (16 mg/100 g) and xylazine hydrochloride (0.77 mg/100 g) and placed on a heated surgical pad. Rectal temperature was maintained at 37°C. After a median section, the kidneys were exposed and clamping of the renal pedicles performed with microserrefines (Fine Science Tools, Foster City, CA) for 30 min. Mice (n = 6/group) were euthanized at 40 min, 180 min, and 48 h after renal ischemia, and the aorta, bone marrow, blood, kidneys, and spleen were collected for further analysis. For sham operation, a median section without vascular clamping was performed.
Histochemical protocols and renal injury score.
Kidneys were collected 2 days and 4 wk after renal ischemia for morphological analysis. Midcoronal kidney sections were fixed in 4% paraformaldehyde (PFA) and embedded in paraffin. Paraffin sections (4 μm thick) were stained with hematoxylin and eosin, periodic acid-Schiff, and Masson's trichrome and examined by a nephropathologist blinded to the origin of individual preparations. Semiquantitative grading of injury, designed to evaluate the degree of tubulointerstitial injury (tubular casts, debris, necrosis, and interstitial fibrosis) was used. The degree of injury and fibrosis score ranging from 0 to 3 were determined as follows: 0, normal kidney; 1, mild changes; 2, moderate changes; and 3, severe changes. The scores were determined in each section selected at random, and >20 fields were examined under ×100 magnification. Data are presented as an average of individual pathological scores, each obtained in four different mice.
En face aorta staining of von Willebrand factor.
Postischemia mice with and without ITF 1697 or sham operation were euthanized at 40 min, 180 min, and 48 h later and perfused with PBS followed by 4% PFA. Thoracic aortas were gently removed, sectioned longitudinally, and postfixed. Tissue wedges were dissected carefully and stored in PBS overnight at 4°C. For immunolabeling, aortas were incubated in the blocking buffer for 30 min, anti-von Willebrand factor (at 1:50) in blocking buffer for 1 h, and washed three times for 5 min each in blocking buffer, followed by the secondary antibodies rabbit anti-rabbit Alexa Fluor 594 at 1:200 for 1 h. Images were obtained using a compound Nikon microscope (TE-2000U) equipped with a Spot Insight digital camera (Diagnostic Instruments) and analyzed using NIS Elements AR software (Nikon).
Measurement of cyto- and chemokines and serum creatinine.
Profiling of cyto- and chemokines was accomplished using multiplex analysis of the plasma obtained from experimental animals (Luminex, Austin, TX). The following parameters were determined: IL-1α, IL-1β, IL-6, IL-8 (KC), IL-10, IL-12 (p70); IFN-γ, IFN-γ-inducible protein (IP-10); granulocyte-colony stimulating factor, granulocyte-macrophage-colony stimulating factor; TNF-α, macrophage inflammatory protein (MIP-1α); and eotaxin and regulated on activation normal T cell expressed and secreted (RANTES). The plates were analyzed using a Luminex IS100 analyzer. The data were saved and evaluated as median fluorescence intensity (MFI) using appropriate curve-fitting software (100IS software version 2.3, Luminex). A five-parameter logistical method with weighting was used as part of the software routine. Serum creatinine concentration was measured using a commercially available kit (Abcam, Cambridge, MA).
Isolation of endothelial progenitors and hematopoietic stem cells and their FACS analysis.
For stem cell isolation, single-cell suspensions were prepared from the bone marrow, blood, spleen, and kidney. A whole spleen and kidney from each animal of each experimental group were placed in 2 ml of ice-cold RPMI 1640 (Invitrogen, Carlsbad, CA) and minced using a sterile scalpel. Digestion of the tissue was performed in collagenase II (Invitrogen) solution (1 mg/ml of RPMI 1640) for 30 min at 37°C in a 5% CO2 incubator. Cell suspensions were passed through a 35-μm nylon sieve. Repeated digestions were performed until microscopic evaluation showed a suspension of single cells. Finally, cells were washed in PBS-BSA [1% (wt/vol)], counted, and kept on ice in the dark.
FACS analysis was performed to quantify the dynamics of endothelial progenitor cells (EPC) and hematopoietic stem cells (HSC). For this analysis, 1 × 106 cells from the single-cell suspensions were incubated with specified primary antibodies for 1 h at 4°C in the dark. The following antibodies were used for incubation: FITC-conjugated anti-mouse CD34, PE-conjugated anti-mouse Flk-1, PE-conjugated anti-mouse CD150, and FITC-conjugated anti-mouse CD117 (c-Kit) (BD Pharmingen, San Diego, CA). After each incubation step, cells were washed with PBS-BSA [1% (wt/vol)] and finally fixed in 1% PFA. Data were acquired using a FACScan cytometer equipped with a 488-nm argon laser and a 635-nm red diode laser and analyzed using CellQuest software (Becton Dickinson, Franklin Lakes, NJ). The setup of FACScan was performed using unstained cells. For quantification of EPC and HSC, the number of CD34/Flk-1 and CD150/c-Kit double-positive cells within the monocytic cell population was counted.
Statistical analysis.
Statistical analysis was performed with GraphPad Prism 4.0 (GraphPad Software, San Diego, CA). Statistical significance was determined by an unpaired, two-tailed Student's t-test or ANOVA, as appropriate. Post hoc analysis for multiple group comparisons was performed using the Bonferroni method. A P value of <0.05 was considered statistically significant. All data are expressed as means ± SE.
RESULTS
In vitro effects of ITF 1697.
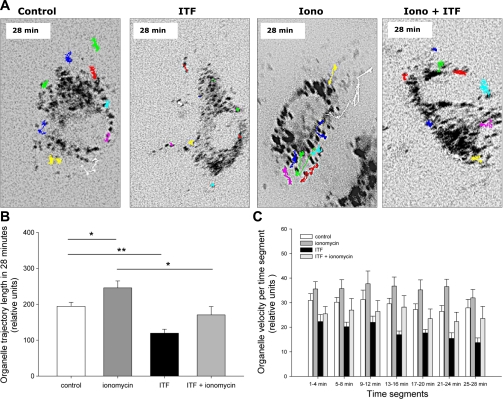
Cultured HUVEC loaded with FM1–43 were stimulated with a calcium ionophore, ionomycin (5 uM), a universal trigger of exocytosis, in the presence or absence of ITF 1697, and the fluorescence was monitored using time-lapse videomicroscopy. Figure 1A illustrates the results of point-tracking of individual peripheral organelles after 28 min of observation. Tracking experiments were performed 4 times, and on average 15 cells/experiment were studied. In control and ITF 1697-treated cells, WPB exhibited mostly a Brownian motion (Supplemental Movies 1 and 2; all supplemental material for this article is available on the journal website). Ionomycin resulted in exocytosis of WPB, which was significantly halted by pretreatment with ITF 1697 (Supplemental Movies 2 and 3, respectively). Figure 1B represents the total length of organellar trajectory during the study period of 28 min. Ionomycin increased the distance of organellar movement, whereas ITF 1697 consistently reduced it. Organelles of cells cultured in the presence of ionomycin demonstrate constantly higher velocities (in each of the studied 4-min time segments) compared with the control cells, as summarized in Fig. 1C. The addition of ITF alone or in combination with ionomycin exhibited an inhibitory effect on organellar velocity. Similar results were obtained for organellar velocity (pixels/s): control: 0.12 ± 0.02; ITF 1697: 0.08 ± 0.02; ionomycin: 0.15 ± 0.03; ionomycin+ITF 1697: 0.13 ± 0.04. Collectively, these data confirmed ITF 1697-mediated inhibition of exocytosis of WPB.
Fig. 1.
Effect of ITF 1697 and ionomycin treatment on organellar movement in human umbilical endothelial cells (HUVEC). A: representative images demonstrating organelle trajectories in HUVEC during a 28-min study period in the presence of ionomycin, ITF 1697, or both. HUVEC cultured in regular culture medium served as a control. B: total organellar trajectories lengths during a 28-min study period in the presence of ionomycin, ITF 1697, or both. Organelles of cells cultured in the presence of ionomycin demonstrate significantly longer trajectories compared with control cells. The addition of ITF 1697 or the combination of ITF and ionomycin exhibits an opposite effect on organellar trajectory length. C: organellar velocities in each of the studied 4-min segments. Organelles of cells cultured in the presence of ionomycin demonstrate constantly higher velocities (in each of the studied 4-min time segments) compared with the control cells. The addition of ITF alone or in combination with ionomycin exhibited inhibitory effects on organellar velocities. Data are means ± SE. *P < 0.05, **P < 0.01; n = 4.
ITF 1697 ameliorates ischemia-induced renal injury.
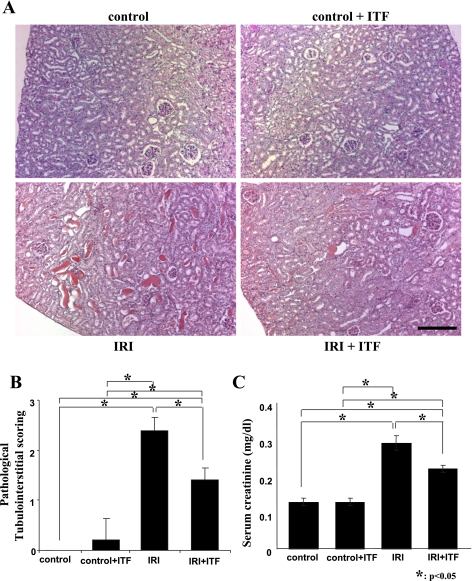
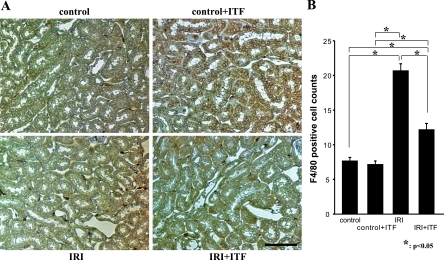
Ischemia-reperfusion resulted in a marked renal injury and elevation of serum creatinine in mice treated with a vehicle (Fig. 2). In contrast, renal injury and elevation of serum creatinine level were significantly ameliorated in mice subjected to renal ischemia and receiving ITF 1697. This functional improvement was associated with the reduced infiltration of the kidney with F4/80-positive cells (Fig. 3).
Fig. 2.
Morphological and functional parameters of renal ischemic injury. A and B: representative images of periodic acid-Schiff (PAS)-stained kidney sections and pathological scores. A: normal control (top left), ITF-treated control (top right), ischemia-reperfusion injury (IRI; bottom left), and ITF-treated IRI (bottom right) groups. See text for detailed description. B: semiquantitative analysis of pathological score. All kidneys were harvested on day 2 post-IRI. C: serum creatinine concentration; n = 6. Scale bar = 200 μm. *P < 0.05.
Fig. 3.
Infiltration of postischemic kidneys with F4/80-positive cells is reduced in ITF 1697-treated mice. A: representative images. B: quantitative summary of F4/80-positive cells/field.
In vivo effects of ITF 1697 on exocytosis of WPB.
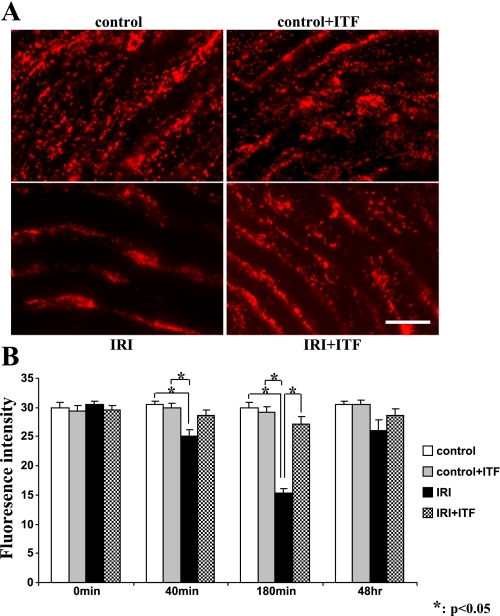
To detect the effect of ITF 1697 on WPB exocytosis in vivo, animals were subjected to renal ischemia, euthanized at different times postischemia, and en face aortic preparations, a surrogate for the systemic vascular bed, were stained for von Willebrand factor, a universal marker of these organelles. As shown in Fig. 4, control aortic endothelial cells showed a rich endowment with WPB, which was depleted after 40 and 180 min postischemia, but recovered quantitatively at 48 h. The ischemia-induced depletion of WPB, most probably reflecting their exocytosis, was significantly blunted in aortic preparations obtained from mice with ITF 1697 delivery via osmotic minipumps. Collectively, these studies demonstrated that application of ITF 1697 inhibits exocytosis of WPB in response to renal ischemia.
Fig. 4.
Representative images of immunodetectable von Willebrand factor in en face aorta and analysis of fluorescence intensity. A: endothelial layer of en face aorta 3 h after IRI from normal control (top left), ITF 1697-treated control (top right), IRI (bottom left), and ITF 1697-treated IRI (bottom right) groups stained with anti- von Willebrand factor antibodies. Scale bar = 30 μm. B: graphs summarizing fluorescence intensity in each group; n = 6. *P < 0.05.
ITF 1697 ameliorates ischemia-induced systemic inflammatory response.
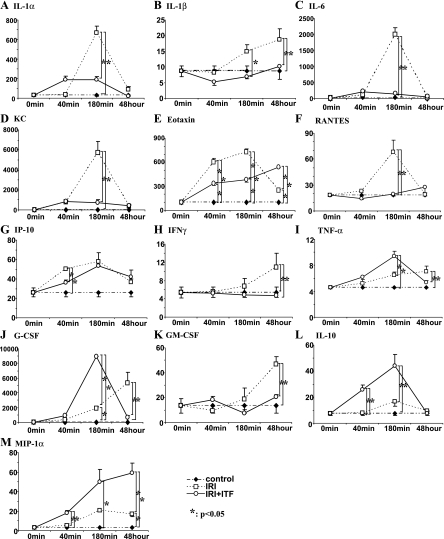
These findings raised the next question: could ITF 1697, by virtue of inhibiting exocytosis of WPB, prevent the systemic inflammatory response to renal ischemia? While renal ischemia-reperfusion was associated with a systemic cyto- and chemokine response, as summarized in Fig. 5, in vivo administration of ITF 1697 reversed it. Data demonstrated that renal ischemia resulted in a significant surge in the levels of eotaxin and IL-8 (KC; both components of WPB), IL-1α, and IL-1β, and RANTES, which were all prevented or blunted by the administration of ITF 1697 (Fig. 5). Furthermore, the levels of anti-inflammatory IL-10 and MIP-1α were upregulated in ITF 1697-treated animals with renal ischemia. Collectively, data demonstrate that the systemic inflammatory response of renal ischemia is in part due to the exocytosis of WPB and its blockade by and large prevents it.
Fig. 5.
Analysis of cyto- and chemokine concentrations in the circulation of mice subjected to renal IRI in combination with ITF 1697 pretreatment. A–M: multiplex analysis of plasma samples obtained from control sham-operated, IRI, and mice with IRI treated with ITF 1697 (only parameters that reached statistical significance are presented). See text for definitions. Samples were collected at 0, 40, and 180 min and 48 h after IRI; n = 4/group. *P < 0.05.
FACS analysis of stem cell mobiization.
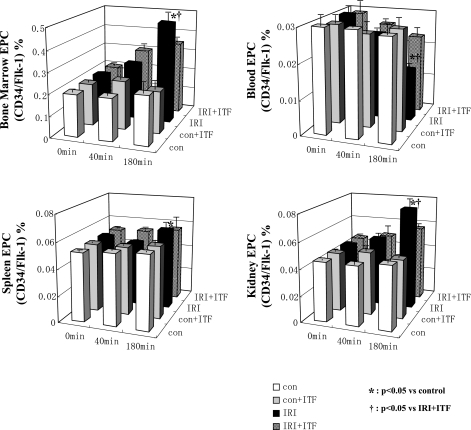
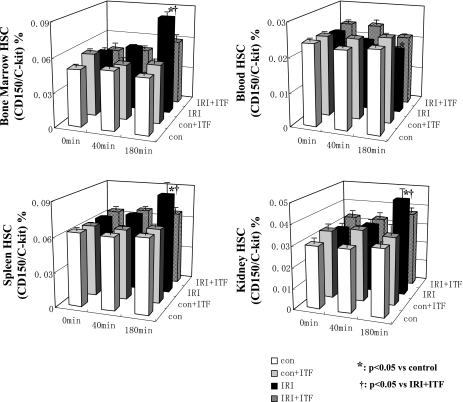
Concerning the role of several components of WPB in ischemia-induced mobilization of stem cells, we next inquired whether inhibition of exocytosis of WPB would affect this process. Toward this end, HSC and EPC were isolated from the bone marrow, spleen, kidney, and blood, labeled with specific markers, and quantified using FACS analysis. The abundance of putative EPC (CD34+/Flk-1+ cells) in the bone marrow and kidney was increased after renal ischemia, but the amplitude was significantly higher in animals that did not receive ITF 1697 (Fig. 6). A similar rise in HSC postischemia was detected in the kidney and spleen, but was curtailed in mice treated with ITF 1697 (Fig. 7). This data indicate that exocytosis of WPB significantly contributes to renal ischemia-associated mobilization of EPC and HSC and its blockade blunts stem/progenitor cell mobilization.
Fig. 6.
Dynamics of epithelial progenitor cells (EPCs) after renal IRI. The ratio of double-positive cells in dot plots (anti-CD34-FITC and anti-Flk-1-PE fluorescence) of FACS analysis is shown at 0, 40, and 180 min postischemia. The proportion of EPCs in control, ITF 1697-treated control, IRI, ITF 1697-treated IRI group in bone marrow (top left), blood (top right), spleen (bottom left), and kidney (bottom right) is shown. White bars, control; gray bars, ITF 1697-treated control; black bars, IRI; patterned bars, ITF 1697-treated IRI. *P < 0.05 vs. control. †P < 0.05 vs. ITF 1697- treated IRI (n = 6/group).
Fig. 7.
Dynamics of hematopoietic stem cells (HSCs) after renal IRI. The ratio of double-positive cells in dot plot (anti-c-kit-FITC and anti-CD150-PE fluorescence) of FACS analysis is shown at 0, 40, and 180 min postischemia. The proportion of HSCs in control, ITF 1697-treated control, IRI, ITF 1697-treated IRI group in The proportion of EPCs in control, ITF 1697-treated control, IRI, ITF 1697-treated IRI group in bone marrow (top left), blood (top right), spleen (bottom left), and kidney (bottom right) is shown. White bars indicate control; gray bars, ITF 1697-treated control; black bars, IRI; patterned bars, ITF 1697-treated IRI. *P < 0.05 vs. control. †P < 0.05 vs. ITF 1697-treated IRI (n = 6/group).
Long-term postischemic consequences of inhibiting exocytosis of WPB.
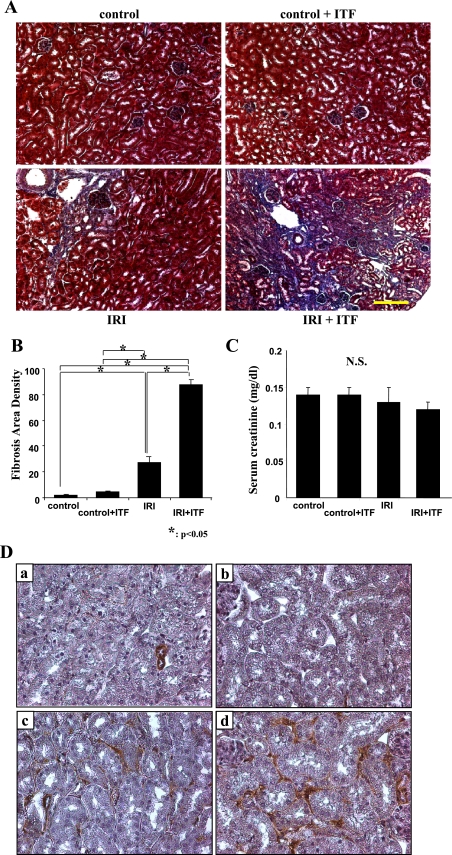
In a series of studies, Basile and colleagues (2, 3) have convincingly demonstrated that the recovery from ischemic kidney injury is incomplete and carries with it a high probability of scarring. Could inhibition of the systemic inflammatory response prevent or diminish developing tubulointerstitial scarring? This question was addressed by examining kidneys 1 mo after ischemia. As shown in Fig. 8, serum creatinine was not different from control in postischemic animals either treated or untreated with ITF 1697. Despite this, there were differences in the degree of scarring: postischemic mice treated with ITF 1697 showed a significantly more pronounced degree of scarring and accumulation of α-smooth muscle actin-stained myofibroblasts than nontreated animals by 1 mo after induction of ischemia.
Fig. 8.
Long-term effects of inhibiting exocytosis of Weibel-Palade bodies. Representative Masson's trichrome staining of kidney sections and corresponding levels of serum creatinine are shown. A: normal control (top left), ITF 1697-treated control (top right); IRI (bottom left); ITF 1697-treated IRI (bottom right). B: graphs summarizing fibrosis area and density in each group are shown. Image analysis was performed using National Institutes of Health Image J software. The procedure included both area and density estimation that enabled identification of fibrosis and quantification of pixels. Y-axis summarizes the number of pixels. Kidneys were harvested 1 mo post-IRI. C: serum creatinine concentration; n = 6 each, *P < 0.05. Scale bar = 200 μm. D: representative images of α-smooth muscle actin-stained kidney sections demonstrating an increase in myofibroblastic detection in the ITF 1697-treated IRI group. Kidneys were harvested 1 mo postischemia. Normal control (a); ITF-treated control (b); IRI (c); ITF-treated IRI (d). Magnification ×400.
DISCUSSION
Data presented herein confirmed the efficacy of ITF 1697 in inhibiting exocytosis of WPB both in vitro and in vivo. Using this inhibitor, we demonstrated that acute ischemic kidney injury could be curtailed. The systemic inflammatory response to renal ischemia was also suppressed by inhibiting exocytosis of WPB, as was the mobilization of EPC and HSC. However, these benefits were short-lived and the ensuing postischemic tubulointerstitial scarring, 1 mo after the ischemic episode, was found to be more pronounced in mice receiving ITF 1697.
Ischemia-induced stem cell mobilization has been well documented (5, 14). It is mediated at least in part via generation of hypoxia-inducible factor-1-regulated release of vascular endothelial growth factor, erythropoietin, and stromal cell-derived factor-1 (SDF-1), as well as by placental growth factor, and granulocyte- and granulocyte-macrophage colony-stimulating factors (5, 8, 10, 11, 12). Rookmaaker and colleagues (7) observed a more than fourfold increase in the number of bone marrow-derived glomerular endothelial cells by day 7 after anti-Thy-1.1 injection of bone marrow-transplanted rats. The participation of donor-derived cells in glomerular endothelial cell turnover has also been shown in bone marrow-transplanted rats with unilateral nephrectomy and anti-Thy-1.1 injection (13). In addition, we have previously demonstrated that stem cells derived from skeletal muscle of Tie-2/GFP mice, ex vivo expanded and differentiated to EPC, and injected into mice with acute renal ischemia were associated with significant functional and structural preservation (1). An alarmin, acute, and short-lived postischemic surge in uric acid induced stem cell mobilization and improved renal function (18, 19). At least two components of WBP can be a priori considered as potential mediators of stem cell recruitment: Ang-2 and IL-8. Ang-2 is stored in WPB of endothelial cells and is released upon cell activation (7). A relative increase in the Ang-2/Ang-1 ratio has been proposed as a proangiogenic stimulus resulting from mobilization of stem cells in brain tumors (24). IL-8 is another constituent of WPB, which is released upon their exocytosis (21). Secretion of IL-8 after myocardial infarction was found to be associated with mobilization of EPC (23). Hence, it is possible that the reduced level of EPC and HSC mobilization and recruitment to the ischemic kidneys in ITF 1697-treated animals is a manifestation of curtailed release of IL-8 and Ang-2. However, the fact that the injury to the kidney was ameliorated by ITF 1697 may explain the reduction in the homing signaling. The fact that inhibition of exocytosis after renal ischemia resulted in exacerbation of the fibrotic process indirectly argues in favor of the former possibility. Nevertheless, data cannot rule out a possibility that ITF 1697 per se inhibits stem/progenitor cell mobilization, although the Lys-Pro-motif has not been identified as such.
An intriguing corollary that emerges from this study is related to the potential importance of the postischemic systemic inflammatory response in orchestrating the resolution of tissue injury and restoration of tissue architecture. This would explain the dissociation between the short-term benefits of inhibiting exocytosis of WPB and the long-term disadvantages of this strategy in preventing tubulointerstitial scarring. Two possible solutions of this conundrum could be envisaged. In the first, inhibition of exocytosis of WPB should be kept to a minimum, necessary to only mildly inhibit this process rather than to attempt a profound inhibition. The second solution may be found in substituting some components of WPB critical for tissue regeneration (possibly, such as Ang-2 and/or IL-8 participating in stem cell mobilization) from exogenous sources. Future work will focus on these two approaches.
GRANTS
These studies were supported in part by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK54602, DK052783, and DK45462 and the Westchester Artificial Kidney Foundation (M. S. Goligorsky).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: K.Y. and M.S.G. provided conception and design of research; K.Y., R.V., P.H., B.B.R., H.B., J.M., P.M., and M.S.G. performed experiments; K.Y., R.V., P.H., B.B.R., H.B., J.M., S.M., S.B., P.M., and M.S.G. analyzed data; K.Y., R.V., P.H., B.B.R., H.B., J.M., S.M., S.B., P.M., and M.S.G. interpreted results of experiments; K.Y., R.V., P.H., B.B.R., H.B., S.M., S.B., P.M., and M.S.G. prepared figures; K.Y., R.V., S.M., and M.S.G. drafted manuscript; K.Y., R.V., and M.S.G. edited and revised manuscript; K.Y. approved final version of manuscript.
REFERENCES
- 1. Arriero M, Brodsky SV, Gealekman O, Lucas PA, Goligorsky MS. Adult skeletal muscle stem cells differentiate into endothelial lineage and ameliorate renal dysfunction after acute ischemia. Am J Physiol Renal Physiol 287: F621–F627, 2004 [DOI] [PubMed] [Google Scholar]
- 2. Basile DP, Donohoe D, Roethe K, Osborn JL. Renal ischemic injury results in permanent damage to peritubular capillaries and influences long-term function. Am J Physiol Renal Physiol 281: F887–F899, 2001 [DOI] [PubMed] [Google Scholar]
- 3. Basile DP. Rarefaction of peritubular capillaries following ischemic acute renal failure: a potential factor predisposing to progressive nephropathy. Curr Opin Nephrol Hypertens 13: 1–7, 2004 [DOI] [PubMed] [Google Scholar]
- 4. Bertuglia S, Ichimura H, Fossati G, Parthasarathi K, Leoni F, Modena D, Cremonesi P, Bhattacharya J, Mascagni P. ITF 1697, a stable Lys-Pro-containing peptide, inhibits Weibel-Palade body exocytosis induced by ischemia/reperfusion and pressure elevation. Mol Med 13: 615–624, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ceradini D, Kulkarni A, Callaghan M, Tepper O, Bastidas N, Kleinman M, Capla J, Galiano R, Levine J, Gurtner G. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nature Med 10: 858–864, 2004 [DOI] [PubMed] [Google Scholar]
- 6. de Leeuw H, Fernandez-Borja M, Reits E, de Wit T, Wijers-Koster P, Hordijk P, Neefjes J, van Mourik J, Voorberg J. Small GTP-binding protein Ral modulates regulated exocytosis of von Willebrand factor by endothelial cells. Arterioscler Thromb Vasc Biol 21: 899–904, 2001 [DOI] [PubMed] [Google Scholar]
- 7. Fiedler U, Scharpfenecker M, Koidl S, Hegen A, Grunow V, Schmidt JM, Kriz W, Thurston G, Augustin HG. The Tie-2 ligand angiopoietin-2 is stored in and rapidly released upon stimulation from endothelial cell Weibel-Palade bodies. Blood 103: 4150–56, 2004 [DOI] [PubMed] [Google Scholar]
- 8. Gill M, Dias S, Hattori K, Rivera M, Hicklin D, Witte L, Girardi L, Yurt R, Himel H, Rafii S. Vascular trauma induces rapid but transient mobilization of VEGFR2+AC133+ endothelial precursor cells. Circ Res 88: 167–174, 2001 [DOI] [PubMed] [Google Scholar]
- 9. Goligorsky MS, Patschan D, Kuo MC. Weibel-Palade bodies—sentinels of acute stress. Nat Rev Nephrol 5: 423–426, 2009 [DOI] [PubMed] [Google Scholar]
- 10. Hattori K, Heissig B, Wu Y, Dias S, Tejada R, Ferris B, Hicklin D, Zhu Z, Bohlen P, Witte L, Hendrikx J, Hackett N, Crystal R, Moore M, Werb Z, Lyden D, Rafii S. Placental growth factor reconstitutes hematopoiesis by recruiting VEGFR1+ stem cells from bone marrow microenvironment. Nature Med 8: 841–849, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hattori K, Dias S, Heissig B, Hackett N, Lyden D, Tateno M, Hicklin D, Zhu Z, Witte L, Crystal R, Moore M, Rafii S. Vascular endothelial growth factor and angiopoietin-1 stimulate postnatal hematopiesis by recruitment of vasculogenic and hematopoietic stem cells. J Exp Med 193: 1005–1014, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Heeschen C, Aicher A, Lehmann R, Fichtlscherer S, Vasa M, Urbich C, Mildner-Rihm C, Martin H, Zeiher A, Dimmeler S. Erythropoietin is a potent physiologic stimulus for endothelial progenitor cell mobilization. Blood 102: 1340–1346, 2003 [DOI] [PubMed] [Google Scholar]
- 13. Ikarashi K, Li B, Suwa M, Kawamura K, Morioka T, Yao J, Khan F, Uchiyama M, Oite T. Bone marrow cells contribute to regeneration of damaged glomerular endothelial cells. Kidney Int 67: 1925–1933, 2005 [DOI] [PubMed] [Google Scholar]
- 14. Kuo MC, Patschan D, Patschan S, Cohen-Gould L, Park HC, Ni J, Addabbo F, Goligorsky MS. Ischemia-induced exocytosis of Weibel-Palade bodies mobilizes stem cells. J Am Soc Nephrol.19: 2321–2330, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Loewenstein C, Morrell C, Yamakuchi M. Regulation of Weibel-Palade body exocytosis. Trends Cardiovasc Med 15: 302–308, 2005 [DOI] [PubMed] [Google Scholar]
- 16. Matsushita K, Morrell C, Loewenstein C. A novel class of fusion polypeptides inhibits exocytosis. Mol Pharmacol 67: 1137–1144, 2005 [DOI] [PubMed] [Google Scholar]
- 17. Morrell C, Matsushita K, Loewenstein C. A novel inhibitor of N-ethylmaleimide-sensitive factor decreases leukocyte trafficking and peritonitis. J Pharmacol Exp Ther 314: 155–161, 2005 [DOI] [PubMed] [Google Scholar]
- 18. Patschan D, Krupincza K, Patschan S, Zhang Z, Hamby C, Goligorsky MS. Dynamics of mobilization and homing of endothelial progenitor cells after acute renal ischemia: modulation by ischemic preconditioning. Am J Physiol Renal Physiol 291: F176–F185, 2006 [DOI] [PubMed] [Google Scholar]
- 19. Patschan D, Patschan S, Gobe GG, Chintala S, Goligorsky MS. Uric acid heralds ischemic tissue injury to mobilize endothelial progenitor cells. J Am Soc Nephrol 18: 1516–1524, 2007 [DOI] [PubMed] [Google Scholar]
- 20. Pinsky DJ, Naka Y, Liao H, Oz MC, Wagner DD, Mayadas TN, Johnson RC, Hynes RO, Heath M, Lawson CA, Stem DM. Hypoxia-induced exocytosis of endothelial cell Weibel-Palade bodies. A mechanism for rapid neutrophil recruitment after cardiac preservation. J Clin Invest 97:493–500, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rondaij M, Bierings R, Kragt A, van Mourik J, Voorberg J. Dynamics and plasticity of Weibel-Palade bodies in endothelial cells. Atheroscl Thrombosis Vasc Biol 26: 1002–1007, 2006 [DOI] [PubMed] [Google Scholar]
- 22. Rookmaaker MB, Verhaar MC, van Zonneveld AJ, Rabelink TJ. Progenitor cells in the kidney: biology and therapeutic perspectives. Kidney Int 66: 518–22, 2004 [DOI] [PubMed] [Google Scholar]
- 23. Schomig K, Busch G, Steppich B, Sepp D, Kaufmann J, Stein A, Schomig A, Ott I. Interleukin-8 is associated with circulating CD133+ progenitor cells in acute myocardial infarction. Eur Heart J 27: 1032–1037, 2006 [DOI] [PubMed] [Google Scholar]
- 24. Udani V, Santarelli J, Yung Y, Cheshier S, Andrews A, Kasad Z, Tse V. Differential expression of angiopoietin-1 and -2 may enhance recruitment of bone marrow-derived endothelial precursor cells into brain tumors. Neurol Res 27: 801–806, 2005 [DOI] [PubMed] [Google Scholar]