Abstract
We examined the effect of angiotensin II (ANG II) on epithelial Na+ channel (ENaC) in the rat cortical collecting duct (CCD) with single-channel and the perforated whole cell patch-clamp recording. Application of 50 nM ANG II increased ENaC activity, defined by NPo (a product of channel numbers and open probability), and the amiloride-sensitive whole cell Na currents by twofold. The stimulatory effect of ANG II on ENaC was absent in the presence of losartan, suggesting that the effect of ANG II on ENaC was mediated by ANG II type 1 receptor. Moreover, depletion of intracellular Ca2+ with 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (BAPTA)-AM failed to abolish the stimulatory effect of ANG II on ENaC but inhibiting protein kinase C (PKC) abolished the effect of ANG II, suggesting that the effect of ANG II was the result of stimulating Ca2+-independent PKC. This notion was also suggested by the experiments in which stimulation of PKC with phorbol ester derivative mimicked the effect of ANG II and increased amiloride-sensitive Na currents in the principal cell, an effect that was not abolished by treatment of the CCD with BAPTA-AM. Also, inhibition of NADPH oxidase (NOX) with diphenyleneiodonium chloride abolished the stimulatory effect of ANG II on ENaC and application of superoxide donors, pyrogallol or xanthine and xanthine oxidase, significantly increased ENaC activity. Moreover, addition of ANG II or H2O2 diminished the arachidonic acid (AA)-induced inhibition of ENaC in the CCD. We conclude that ANG II stimulates ENaC in the CCD through a Ca2+-independent PKC pathway that activates NOX thereby increasing superoxide generation. The stimulatory effect of ANG II on ENaC may be partially the result of blocking AA-induced inhibition of ENaC.
Keywords: angiotensin II type 1 receptor, protein kinase C, superoxide anions, Na transport
the cortical collecting duct (CCD) is responsible for the hormone-regulated Na absorption and plays a significant role in determining the amount of the final renal Na excretion. Na enters the cells across the apical membrane via epithelial Na+ channel (ENaC) and is extruded from the cell across the basolateral membrane through Na-K-ATPase. Two types of Na channels, 4–6 pS and 28 pS, have been identified in the collecting duct (24, 30, 31, 34). However, it is generally accepted that the 4–6 pS ENaC is mainly responsible for the Na entry across the apical membrane in the CCD (1, 13). The rate-limiting step of transepithelial Na transport in the CCD is the apical Na conductance (20). This is determined by the number of ENaC in the apical membrane and the channel open probability. An increase in either ENaC number or channel open probability should augment the apical Na conductance and lead to the stimulation of Na absorption. For instance, Liddle's syndrome, an inherited form of hypertension, is the result of increase in the apical ENaC in the CCD (36, 37).
A large body of evidence indicates that ANG II plays an important role in the regulation of NaCl transport in the distal nephron segments (6, 11, 32, 33). Luminal application of ANG II has been shown to stimulate the apical entry of Na in the CCD, an effect that was blocked by benzamil and losartan, suggesting that ANG II stimulates ENaC activity via angiotensin II type 1 receptor (AT1R) in the CCD (33). Although the role of ENaC in ANG II-induced increase in Na absorption in the CCD has been established, the mechanism by which ANG II stimulates ENaC is not completely understood. Systemic infusion of ANG II has been reported to increase protein abundance of ENaC α-subunit (6). However, it is unlikely that the stimulation of Na transport induced by acute application of ANG II in the isolated CCD is the result of increasing ENaC transcription or translation. ANG II has been shown to stimulate NADPH oxidase (NOX) and increase superoxide generation in a variety of tissues and the role of superoxide anions in stimulating ENaC has been demonstrated by several investigators (16, 35, 45). Yu et al. (45) reported that increasing superoxide production induced by application of aldosterone diminished the nitric oxide (NO)-mediated inhibition of ENaC thereby increasing ENaC activity. Similar observation has been reported in lung epithelial cells in which superoxide anions antagonize the inhibitory effect of NO on ENaC (16). We previously demonstrated that Cyp-epoxygenase-dependent metabolism of arachidonic acid (AA) inhibited ENaC activity in the CCD (41). The aim of the present study is to examine whether ANG II and superoxide anions counter the inhibitory effect of ENaC by Cyp-epoxygenase-dependent metabolism of AA.
METHODS
Preparation of CCDs.
Male Sprague-Dawley rats (5 to 6 wk, <90 g) were used in the experiments and were fed with control diet (1% K and 0.4% Na) for 7 days. In some experiments as indicated in the figure legends, animals were fed with a high-K (2.5% KCl) diet for 7 days to increase the probability of finding ENaC. Animals were killed by cervical dislocation and kidneys were immediately removed. The kidney was then cut into several 1-mm-thin slices from which the CCDs were isolated. The isolated CCDs were placed on a 5 × 5-mm cover glass coated with poly-lysine and the cover glass was transferred to a chamber (1,000 μl) mounted on an inverted Nikon microscope. The CCDs were superfused with HEPES-buffered NaCl solution. To gain access to the apical membrane, the CCDs were cut open with a sharpened micropipette. The protocol was reviewed and approved by an independent Animal Use Committee from New York Medical College.
Patch-clamp technique.
For single-channel recording, an Axon200B patch-clamp amplifier was used to record channel current. The Na current was recorded and digitized by an Axon interface (Digidata 1322). Data were analyzed using the pClamp software system 9.0 (Axon). Channel activity defined as NPo was calculated from data samples of 60-s duration. This 60-s trace was selected as a representative channel activity from an at least 5-min-long steady state. NPo = ∑(t1 + 2t2 +. . . . . . iti), where ti was the fractional open time spent at each of the observed current levels. The bath solution for single-channel recording contains (in mM) 140 NaCl, 5 KCl, 1.8 CaCl2, 1.8 MgCl2, and 10 HEPES (pH 7.4). The pipette solution was composed of (in mM) 140 NaCl, 5 KCl, 1.8 Mg2Cl, and 5 HEPES (pH 7.4). The principal cells (PCs) were recognized by their typical hexagonal shape and large flat surface. For whole cell clamp measurements, tubules were superfused with solutions containing (in mM) 135 Na methanesulfonate, 5 KCl, 2 CaCl2, 1 MgCl2, 2 glucose, 5 mM BaCl2, and 10 HEPES adjusted to pH 7.4 with NaOH. The patch-clamp pipettes were filled with solutions containing (in mM) 7 KCl, 123 aspartic acid, 20 CsOH, 20 TEAOH, 5 EGTA, 10 HEPES, 3 MgATP, and 0.3 NaGDPβS with the pH adjusted to 7.4 with KOH. For conducting the perforated whole cell recording, the pipettes were back filled with amphotericin A (20 mg/ml) containing pipette solution. The total concentration of K+ was 120 mM. Amiloride-sensitive currents were measured as the difference in current with and without 10 μM amiloride in the bath solution.
Chemicals and statistics.
All the chemicals used in the present experiments were purchased from Sigma (St. Louis, MO). The data are presented as means ± SE. We used Student's t-tests or one-way ANOVA to determine the statistical significance. If the P value was <0.05, the difference will be considered to be significant.
RESULTS
ANG II stimulates ENaC activity.
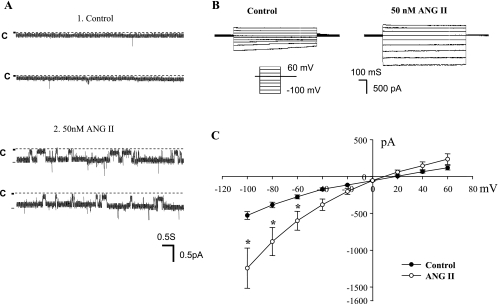
We first used the single-channel patch-clamp recording to examine the effect of ANG II on ENaC in the CCD of rats on a normal rat chow (0.4% Na). Figure 1A is a channel recording showing the effect of 50 nM ANG II on ENaC in a cell-attached patch. We used 50 nM ANG II because a previous study demonstrated that 50 nM ANG II caused a maximal stimulation of AT1R (46). We confirmed that ENaC activity in the CCD of rats on a normal-Na diet was low (the mean NPo in 10 patches was 0.01 ± 0.01) (29, 38). However, application of ANG II (50 nM) increased ENaC activity to 0.4 ± 0.08 (N = 12). Moreover, the effect of ANG II could be observed in the CCD treated with aldosterone antagonist (10 μM spironolactone; data not shown). Thus, the stimulatory effect of ANG II on ENaC was most likely achieved by an aldosterone-independent mechanism.
Fig. 1.
A: channel recording shows the effect of 50 nM ANG II on epithelial Na+ channel (ENaC) activity in the cortical collecting duct (CCD). The experiments were performed in a cell-attached patch and ANG II was directly added to the bath. The top 2 traces show the channel activity before ANG II and the bottom 2 traces demonstrate ENaC activity after addition of ANG II. The channel closed level is indicated by “C” and the holding potential was 60 mV (hyperpolarization). B: perforated whole cell recording shows the effect of ANG II (50 nM) on the amiloride-sensitive whole cell Na currents in a principal cell (PC) of the CCD. The bath solution contains (in mM) 135 Na methanesulfonate, 5 KCl, 2 CaCl2, 1 MgCl2, 2 glucose, 5 BaCl2, and 10 HEPES adjusted to pH 7.4 with NaOH. The pipette solution was composed of (in mM) 7 KCl, 123 aspartic acid, 20 CsOH, 20 TEAOH, 5 EGTA, 10 HEPES, 3 MgATP, and 0.3 NaGDPβS with the pH adjusted to 7.4 with KOH. The PC was clamped from −100 to 60 mV with 20-mV increment. The Na currents were determined by adding amiloride (10 μM) at the end of experiments. C: current-voltage (I-V) plot summarizes 6 experiments similar to those demonstrated in B. *Significant difference between ANG II's group and the corresponding control value.
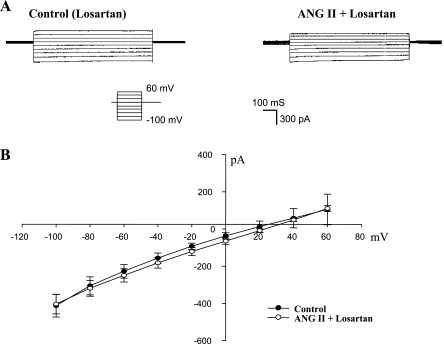
After showing that application of ANG II stimulates ENaC channel activity at a single-channel level, we also used the perforated whole cell patches to measure the amiloride-sensitive Na currents in principal cell (PC) treated with ANG II. The advantage of using the whole cell recording is to allow us quantitatively to analyze the effect of ANG II on ENaC at the whole cell level. Figure 1B is a typical whole cell recording showing the effect of ANG II on the amiloride-sensitive Na currents in the PC of rats on a normal-Na diet. After a high-resistance seal was formed, cell membrane was perforated with amphotericin B. We measured Na currents by clamping the cell membrane from −100 to 60 mV in the presence of or in the absence of 50 nM ANG II. At the end of the experiments, we added amiloride to dissect the amiloride-sensitive Na currents. Figure 1B shows the amiloride-sensitive whole cell Na currents before (left) and after ANG II (right). It is apparent that application of ANG II increased the amiloride-sensitive Na currents in PC. Figure 1C is the current-voltage (I-V) plot summarizing experiments in which the effect of ANG II (50 nM) on the amiloride-sensitive Na currents was examined with the whole cell recording. ANG II increased amiloride-sensitive whole cell Na currents from 528 ± 52 to 1,244 ± 272 pA at −100 mV (N = 6) within 5–10 min. The stimulatory effect of ANG II on ENaC was mediated by AT1R because inhibition of AT1R with losartan abolished the effect of ANG II on ENaC. Figure 2A is a whole cell recording demonstrating the effect of ANG II (50 nM) on the Na currents in the CCD treated with losartan (10 μM). It is apparent that ANG II failed to activate ENaC in the presence of losartan since the amiloride-sensitive Na currents were similar between the control group (410 ± 50 pA, N = 5) and ANG II's group (420 ± 50 pA, N = 5; Fig. 2B). Therefore, single-channel and the whole cell patch-clamp experiments confirmed the previous reports that ANG II stimulates ENaC by an aldosterone-independent and AT1R-dependent mechanism (6, 33).
Fig. 2.
A: perforated whole cell recording shows the effect of ANG II (50 nM) on the amiloride-sensitive Na currents of PC in the presence of losartan (10 μM). The PC was clamped from −100 to 60 mV with 20-mV increment. The Na currents were determined by adding amiloride at the end of experiments. B: I-V plot summarizes 5 experiments similar to those demonstrated in A.
Effect of ANG II on ENaC is mediated by a Ca2+-independent protein kinase C.
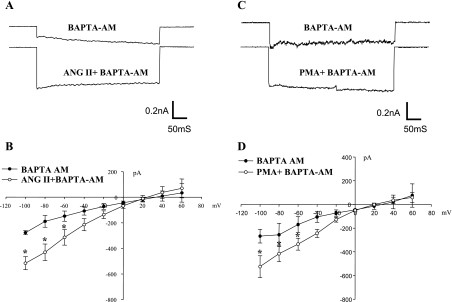
After demonstrating that ANG II stimulated ENaC through activating AT1R, we explored the cellular signaling pathway by which ANG II activated ENaC. The stimulation of AT1R has been shown to activate protein kinase C (PKC) and to increase the intracellular Ca2+ (39). However, since an increase in intracellular Ca2+ has been shown to inhibit ENaC activity through activating the Ca2+-dependent PKC pathway (12), it is unlikely that ANG II-induced stimulation of ENaC is the result of an increase in intracellular Ca2+. This hypothesis was tested by reexamining the effect of ANG II on Na currents in the CCD treated with 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (BAPTA)-AM (50 μM), a membrane-permeable form of BAPTA that is an intracellular Ca2+ chelator. Figure 3A is a recording showing the amiloride-sensitive Na currents measured at −100 mV with perforated whole cell recording before and after ANG II in the CCD treated with BAPTA-AM. Although the whole cell Na current in PC treated with BAPTA-AM was slightly smaller (276 ± 15 pA) than those in untreated cells (Fig. 1), application of ANG II still significantly increased Na currents to 515 ± 50 pA at −100 mV (n = 4; Fig. 3B). This suggests that the stimulatory effect of ANG II on ENaC was mediated by a Ca2+-independent mechanism. To test whether a Ca2+-independent PKC might mediate the effect of ANG II on ENaC, we examined the effect of phorbol-12-myristate 13-acetate (PMA; 5 μM) on ENaC in the CCD treated with BAPTA-AM. Figure 3C is a whole cell recording showing the effect of PMA on Na currents measured at −100 mV. It is apparent that the stimulation of PKC with PMA activated Na channels in the CCD treated with BAPTA. The effect of PMA on ENaC was mediated by a Ca2+-independent PKC because PMA failed to activate ENaC in the CCD treated with 100 nM calphostin C (data not shown). Data summarized in Fig. 3D show that 5 μM PMA significantly increased the amiloride-sensitive Na currents from 268 ± 58 to 525 ± 92 pA at −100 mV (N = 4).
Fig. 3.
A: whole cell recording shows the effect of ANG II (50 nM) on amiloride-sensitive Na currents at −100 mV in the presence of 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (BAPTA)-AM (50 μM). B: I-V plot summarizes results of the experiments in which the effect of ANG II on the whole cell Na currents was examined in the presence of BAPTA-AM. C: whole cell recording shows the effect of phorbol-12-myristate 13-acetate (PMA; 5 μM) on amiloride-sensitive Na currents at −100 mV in the presence of BAPTA-AM. D: I-V plot summarizes the results of experiments in which the effect of PMA on whole cell Na currents was examined in the presence of BAPTA-AM. The CCD was incubated with BAPTA-AM-containing solution for 10 min before adding ANG II or PMA. The whole cell Na currents in PC were measured with the perforated whole cell recording from −100 to 60 mV with 20-mV increment. The Na currents were determined by adding amiloride at the end of experiments. *Significant difference between control and experimental groups.
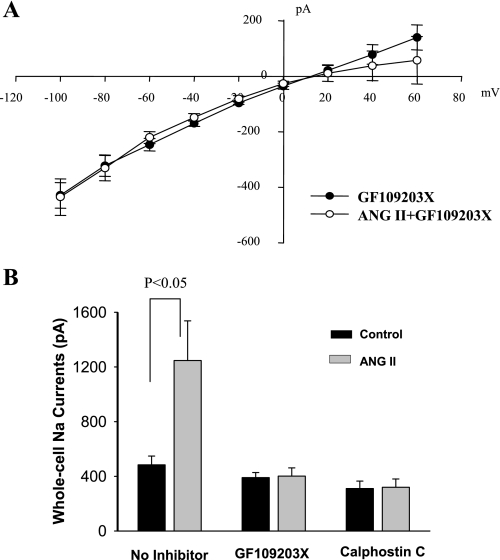
The notion that a Ca2+-independent PKC may mediate the stimulatory effect of ANG II on ENaC was further tested by examining the effect of ANG II on ENaC in the CCD treated with PKC inhibitors. Figure 4A is an I-V curve summarizing five experiments in which amiloride-sensitive Na currents were measured from −100 to 60 mV before and after ANG II (50 nM) in the CCD treated with GF109203X (5 μM). It is apparent that inhibition of PKC abolished the stimulatory effect of ANG II on ENaC. Application of ANG II failed to increase the amiloride-sensitive Na currents in the presence of GF109203X (control 405 ± 50 pA; ANG II 415 ± 60 pA). We also repeated the experiments with other PKC inhibitors since GF109203s could also inhibit kinases other than PKC. Results are summarized in Fig. 4B showing that application of calphostin C (100 nM) also abolished the stimulatory effect of ANG II on ENaC (Fig. 4B). Taken together, results indicate that ANG II stimulates ENaC through a Ca2+-independent PKC pathway.
Fig. 4.
A: I-V plot summarizes experiments in which the effect of ANG II on the whole cell Na currents was examined in the presence of GF109203 (5 μM). The whole cell Na currents in the PC were measured with the perforated whole cell recording from −100 to 60 mV with 20-mV increment. The Na currents were determined by adding amiloride at the end of experiments. B: bar graph summarizes results of ANG II's effect on Na currents in the absence of or in the presence of PKC inhibitors (5 μM GF109203X, 100 nM calphostin C).
Superoxide mediates the effect of ANG II to ENaC in the CCD.
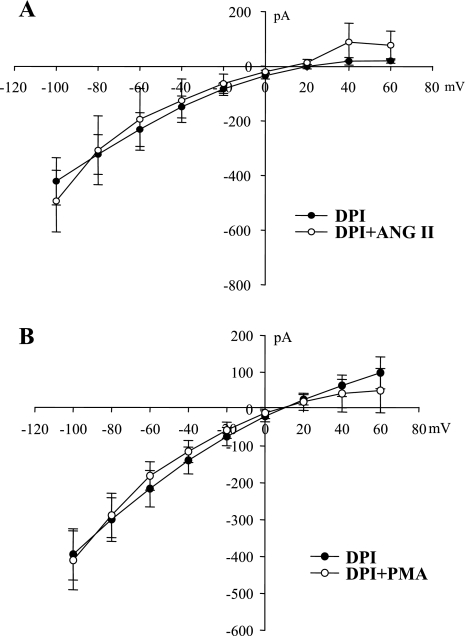
ANG II has been shown to stimulate the generation of superoxide and related products by activating NADPH oxidase (NOX) in a variety of tissues including the kidney (35, 43, 48). Moreover, superoxide anions have been shown to stimulate Na channels in A6 cells (45). Thus, we next examined the role of NOX in mediating the effect of ANG II on ENaC by exploring the effect of ANG II on ENaC in the CCD treated with diphenyleneiodonium chloride (DPI; 10 μM), an agent that inhibits NOX. From inspection of the I-V plot shown in Fig. 5A, it is clearly demonstrated that inhibition of NOX completely abolished the effect of ANG II on ENaC. In the presence of DPI, ANG II did not significantly increase the amiloride-sensitive Na currents (493 ± 113 pA, a value not significantly different from the control currents, 421 ± 87 pA; N = 3), suggesting that ANG II stimulates ENaC by activating NOX. We also tested the possibility that stimulation of PKC-induced increase in Na currents was the result of activation of NOX by examining the effect of PMA, which increased Na currents in the presence of BAPTA-AM (Fig. 3C) with the whole cell recording. Data summarized in Fig. 5B demonstrate that inhibition of NOX also abolished the effect of PMA (5 μM) on ENaC in the CCD (N = 4). This suggests the possibility that ANG II increases ENaC activity by stimulating PKC-NOX pathway.
Fig. 5.
A: I-V curve summarizes experiments in which the effect of ANG II on the whole cell Na currents was examined in the presence of diphenyleneiodonium chloride (DPI; 10 μM). B: I-V curve summarizes experiments in which the effect of PMA (5 μM) on the whole cell Na currents was examined in the presence of DPI.
Superoxide anions activate ENaC.
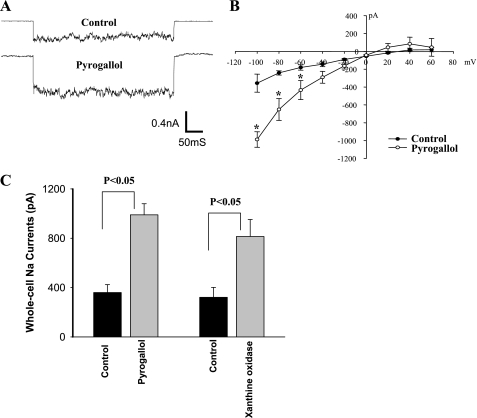
To further demonstrate the role of superoxide anions in stimulating ENaC activity, we examined the effect of pyrogallol, a superoxide donor, on the amiloride-sensitive Na currents with the perforated whole cell recording. Figure 6 is a whole cell recording of Na current measured at −100 mV, demonstrating that pyrogallol increased Na currents compared with the control. The I-V plot (Fig. 6B) summarizes four experiments in which the effect of pyrogallol on the amiloride-sensitive Na currents was examined. Application of pyrogallol (100 μM) increased Na currents from 360 ± 64 to 990 ± 90 pA at −100 mV. Similar results were observed in experiments in which we measured the Na currents in the PC of the CCD treated with xanthine (0.3 mM) and xanthine oxidase (7 μU/ml). Figure 6C is a bar graph summarizing five experiments demonstrating that addition of xanthine/xanthine oxidase increased Na currents from 322 ± 80 to 815 ± 138 pA at −100 mV. The results support the role of superoxide anions in mediating the stimulatory effect of ANG II on ENaC.
Fig. 6.
A: whole cell recording shows the effect of pyrogallol (100 μM) on amiloride-sensitive Na currents at −100 mV. B: I-V plot summarizes the effect of pyrogallol (100 μM) on the whole cell Na currents in the PC. C: bar graph summarizes the effect of pyrogallol and xanthine (0.3 mM)/xanthine oxidase (7 μU/ml) on the amiloride-sensitive whole cell Na currents at −100 mV measured with perforated patches.
ANG II and superoxide-related products diminished the AA-induced inhibition of ENaC.
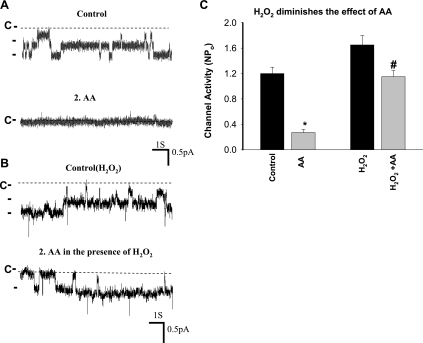
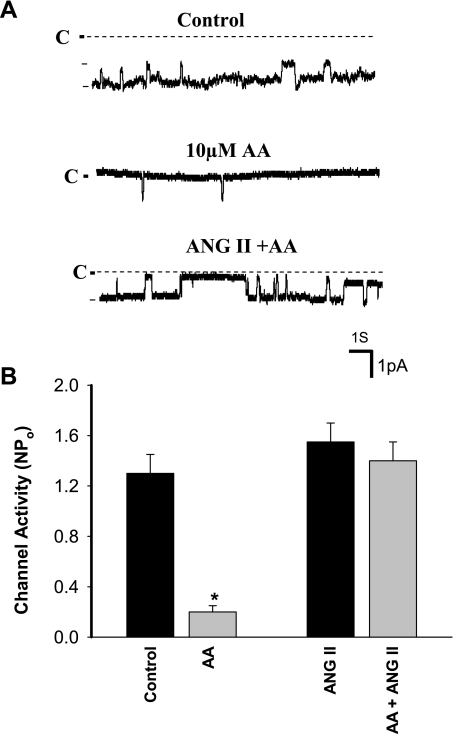
After demonstrating that ANG II stimulated ENaC through a superoxide-dependent mechanism, we explored the mechanism by which superoxide-related products activated ENaC. Superoxide-related products such as H2O2 have been shown to inhibit CYP epoxygenase activity (22). Since CYP epoxygenase-dependent metabolites of AA inhibit ENaC in the CCD (42), we suspect that superoxide-related products might stimulate ENaC activity through blocking AA-induced inhibition of ENaC in the CCD. To test this possibility, we examined whether H2O2 could interfere with the inhibitory effect of AA on ENaC. Figure 7A is a channel recording in a cell-attached patch of the CCD showing that application of 10 μM AA inhibited ENaC activity in the CCD. However, treatment of the CCD with H2O2 (100 μM) attenuated the effect of AA on ENaC (Fig. 7B). In the presence of H2O2, AA decreased channel activity from 1.65 ± 0.13 to 1.15 ± 0.1 or by a modest 30 ± 5%, whereas the AA reduced ENaC NPo from 1.2 ± 0.1 to 0.25 ± 0.05 or by 80 ± 10% (N = 5) in the absence of H2O2 (Fig. 7C; the patches with similar NPo were selected for the experiments). We next examined whether ANG II-induced stimulatory effect was also, at least in part, the result of attenuating the inhibitory effect of AA on ENaC by examining the effect of AA on ENaC in the absence of or in the presence of ANG II. Figure 8A is a channel recording in a cell-attached patch showing that application of 10 μM AA almost completely inhibited ENaC. However, adding 50 nM ANG II reversed the AA-induced inhibition in the presence of AA. In four similar experiments, adding AA failed to inhibit ENaC in the presence of 50 nM ANG II (control NPo 1.3 ± 0.15; AA 0.19 ± 0.05; ANG II 1.5 ± 0.13; AA + ANG II 1.4 ± 0.1; Fig. 8B). Therefore, ANG II may activate ENaC by attenuating the inhibition of Na channel by CYP-epoxygenase-dependent AA metabolism.
Fig. 7.
H2O2 diminishes the arachidonic acid (AA)-induced inhibition of ENaC in the rat CCD. A: channel recording in a cell-attached patch shows that AA (10 μM) inhibited ENaC but the effect of AA was attenuated in the presence of 100 μM H2O2 (B). C: bar graph summarizes the effect of AA on the ENaC activity in the presence or absence of H2O2. *Significant difference from the rest of groups and #significant difference compared with its control. The experiments were performed in the CCD of rats on a high-K (2.5% K) diet for 7 days.
Fig. 8.
ANG II diminishes the AA-induced inhibition of ENaC. A: channel recording demonstrates that AA-induced inhibition of ENaC (middle trace) is reversed by adding 50 nM ANG II (bottom panel). The experiments were performed in a cell-attached patch of the rat CCD. B: bar graph summarizes the effect of AA on the ENaC activity in the presence or absence of 50 nM ANG II. *Significant difference compared with the control. The experiments were performed in the CCD of rats on a high-K (2.5% K) diet for 7 days.
DISCUSSION
The main finding of the present study is that ANG II stimulates ENaC through the activation of AT1R. Because the stimulatory effect of ANG II on ENaC was observed in single PC of the isolated CCD, the effect of ANG II on ENaC is most likely aldosterone independent. Relevant to the present observation is the report that luminal application of ANG II stimulated the apical entry of Na in the CCD, an effect that was blocked by benzamil and losartan (33). A large body of evidence indicates that ANG II may stimulate NaCl transport in the distal nephron segments by an aldosterone-independent mechanism (6, 11, 32, 33). Chronic ANG II infusion decreased urinary Na excretion and increased Na absorption in the distal nephron in mice. This effect was not abolished by spironolactone, suggesting that ANG II-induced stimulation of Na transport was not the result of high aldosterone (47). Moreover, systemic infusion of ANG II has been reported to increase protein abundance of ENaC α-subunit (6). Because the effect was not blocked by spironolactone, ANG II should have a direct effect on the expression of ENaC α-subunit. Also, ANG II has been shown to stimulate Na absorption and to increase the transcription of ENaC in the colon (15). However, in the present study, we observed the stimulatory effect of ANG II on ENaC within 5–10 min. Therefore, it is unlikely that ANG II-induced increase in Na currents is due to facilitating protein translation in the CCD.
Instead, the present study indicates that the stimulatory effect of ANG II on ENaC was mediated by a Ca2+-independent PKC because inhibition of PKC abolished the effect of ANG II on ENaC. The role of PKC in regulating ENaC is well-established (2, 8, 12); however, these studies focused on the role of PKC in inhibiting ENaC rather than in stimulating ENaC. For instance, classic (Ca2+-dependent) PKC was responsible for mediating the inhibitory effect of Ca2+ on ENaC in the CCD (12, 25). Also, PKC has been shown to stimulate endocytosis of ENaC (2) and to promote β- and γ-subunit degradation through activation of the ERK1/2 (8). Two lines of evidence suggest that the stimulatory effect of ANG II on ENaC was mediated by Ca2+-independent PKC isoforms: 1) ANG II was still able to activate ENaC in the CCD treated with BAPTA-AM and 2) PMA mimicked the effect of ANG II and increased ENaC activity in the CCD treated with the intracellular Ca2+ chelator. Therefore, it is conceivable that Ca2+-independent PKC is responsible for activation of ENaC. Moreover, the stimulatory effect of PKC on ENaC was the result of activation of NOX, although we could not completely exclude the possibility that PKC phosphorylates ENaC thereby stimulating ENaC. This notion is supported by the observation that inhibition of NOX abolished the effect of PMA on Na channels in the CCD. Also, the finding that inhibition of NOX abolished the effect of ANG II on ENaC strongly suggests that superoxide anions or their related products are involved in mediating the stimulatory effect of ANG II on ENaC.
A large body of evidence demonstrated that ANG II stimulates the generation of superoxide anions and related products in a variety of tissues including the nervous system, heart, vascular system, and kidney (26, 35, 43, 48). Also, ANG II-induced increase in superoxide anions plays an important role in ANG II-mediated hypertension and organ damages (21, 35, 43). ANG II-induced stimulation of superoxide generation is through the activation of AT1R (10, 35, 43). We previously demonstrated that inhibition of AT1R significantly decreased superoxide generation in renal tubules (17). Five isoforms of NOX have been cloned and identified in different tissues (5). However, NOXII and NOX4 are mainly expressed in the kidney (35). Immunocytochemistry demonstrated that p22Phox, p67Phox, and p47Phox, three important components of NOXII, are expressed in the collecting duct (9). The role of PKC in mediating the stimulatory effect of ANG II on NOXII activity is well-established (28). One mechanism by which PKC activates NOX is to phosphorylate p47Phox (7), thereby facilitating the translocation of phosphorylated p47Phox from cytosolic complexes to the plasma membrane (5) to interact with gp91Phox and p22Phox complex (4, 14). We previously demonstrated that the generation of superoxide and related products was significantly attenuated in gp91Phox (−/−) mice (3), suggesting that NOXII plays a role in generating superoxide in the renal tubule including the CCD.
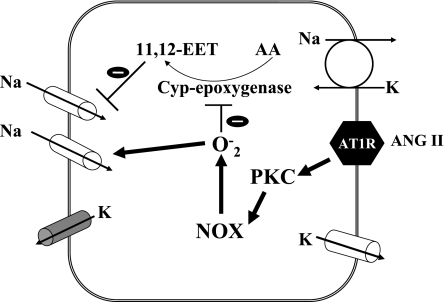
Accumulated evidences indicated that superoxide anion-related products play a role in stimulating renal Na absorption (44). It has been reported that H2O2 stimulated luminal Na/H exchange and Na-K-2Cl cotransporter in the thick ascending limb (18, 19). The effect of superoxide anions on ENaC depends on tissue types: superoxide-related products inhibited ENaC in lung epithelial cells (23, 40) while they stimulated ENaC activity in A6 cells (45). However, the mechanism by which superoxide anions stimulate ENaC in renal tissue is not clear. H2O2 increases ENaC activity through stimulating PI3K in A6 cells (27). In the present study, we showed that the presence of superoxide or its related products diminished the AA-induced inhibition of ENaC. Our previous study demonstrated that AA inhibited ENaC in the CCD by a CYP-epoxygenase-dependent pathway and that 11,12-EET mediated the effect of AA on ENaC (41). To our interest, it has been reported that superoxide-related products inhibited the CYP-epoxygenase activity (22). Thus, it is conceivable that superoxide-induced stimulation of ENaC might be the result of inhibiting CYP-epoxygenase and diminishing AA-mediated inhibition of Na channels. Figure 9 is cell scheme illustrating a possible mechanism by which ANG II activates ENaC in the CCD. ANG II activates Ca2+-independent PKC through AT1R and, consequently, stimulates NOX. An increase in superoxide and related products could suppress the CYP-epoxygenase-dependent AA metabolism thereby activating ENaC.
Fig. 9.
Scheme illustrating the mechanism by which ANG II stimulates ENaC in PCs of the CCD. Arrows mean stimulation. 11,12-EET, epoxyeicosatrienoic acid; NOX, NADPH oxidase.
We conclude that ANG II stimulates ENaC in the CCD by activating a Ca2+-independent PKC that stimulates NOX and increasing the generation of superoxide anions. Also, ANG II-induced activation of ENaC is partially induced by reversing the inhibition of ENaC induced by CYP-epoxygenase-dependent AA metabolism.
GRANTS
The work is supported by the National Institutes of Health Grant HL34100.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: P.S. and W.-H.W. conception and design of research; P.S. and P.Y. performed experiments; P.S., P.Y., and W.-H.W. analyzed data; P.S., P.Y., and W.-H.W. interpreted results of experiments; P.S. and W.-H.W. prepared figures; P.S. and W.-H.W. drafted manuscript; P.S., P.Y., and W.-H.W. approved final version of manuscript; W.-H.W. edited and revised manuscript.
REFERENCES
- 1. Ahn YJ, Brooker DR, Kosari F, Harte BJ, Li J, Mackler SA, Kleyman TR. Cloning and functional expression of the mouse epithelial sodium channel. Am J Physiol Renal Physiol 277: F121–F129, 1999 [DOI] [PubMed] [Google Scholar]
- 2. Awayda MS. Specific and nonspecific effects of protein kinase C on the epithelial Na+ channel. J Gen Physiol 115: 559–570, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Babilonia E, Lin D, Zhang Y, Wei Y, Yue P, Wang WH. Role of gp91phox-containing NADPH oxidase in mediating the effect of K restriction on ROMK channels and renal K excretion. J Am Soc Nephrol 18: 2037–2045, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Babior BM. NADPH oxidase: an update. Blood 93: 1464–1476, 1999 [PubMed] [Google Scholar]
- 5. Bedard K, Krause KH. The Nox family of ROS-generating NADPH oxidase: physiology and pathophysiology. Physiol Rev 87: 245–313, 2007 [DOI] [PubMed] [Google Scholar]
- 6. Beutler KT, Masilamani S, Turban S, Nielsen J, Brooks HL, Ageloff S, Fenton RA, Packer RK, Knepper MA. Long-term regulation of ENaC expression in kidney by angiotensin II. Hypertension 41: 1143–1150, 2003 [DOI] [PubMed] [Google Scholar]
- 7. Bey EA, Xu B, Bhattacharjee A, Oldfield CM, Zhao X, Li Q, Subbulakshumi V, Feldman GM, Wientjes FB, Cathcart MK. Protein kinase C delta is required for p47phox phosphorylation and translocation in activated human monocytes. J Immunol 173: 5730–5738, 2004 [DOI] [PubMed] [Google Scholar]
- 8. Booth RE, Stockand JD. Targeted degradation of ENaC in response to PKC activation of the ERK1/2 cascade. Am J Physiol Renal Physiol 284: F938–F947, 2003 [DOI] [PubMed] [Google Scholar]
- 9. Chabrashvili T, Tojo A, Onozato ML, Kitiyakara C, Quinn MT, Fujita A, Welch WJ, Wilcox CS. Expression and cellular location of classic NADPH oxidase subunits in the spontaneously hypertensive rat kidney. Hypertension 39: 269–274, 2002 [DOI] [PubMed] [Google Scholar]
- 10. Chabrashvili T, Kitiyakara C, Blau J, Karber A, Aslam S, Welch WJ, Wilcox CS. Effects of ANG II type 1 and 2 receptors on oxidative stress, renal NADPH oxidase, and SOD expression. Am J Physiol Regul Integr Comp Physiol 285: R117–R124, 2003 [DOI] [PubMed] [Google Scholar]
- 11. Dickson ME, Sigmund CD. Genetic basis of hypertension: revisiting angiotensinogen. Hypertension 48: 14–20, 2006 [DOI] [PubMed] [Google Scholar]
- 12. Frindt G, Palmer LG, Windhager EE. Feedback regulation of Na channels in rat CCT IV. Mediation by activation of protein kinase C. Am J Physiol Renal Fluid Electrolyte Physiol 270: F371–F376, 1996 [DOI] [PubMed] [Google Scholar]
- 13. Garty H, Palmer LG. Epithelial sodium channel: function, structure, and regulation. Physiol Rev 77: 359–396, 1997 [DOI] [PubMed] [Google Scholar]
- 14. Geiszt M, Leto TL. The Nox family of NAD(P)H oxidases: host defense and beyond. J Biol Chem 279: 51715–51718, 2004 [DOI] [PubMed] [Google Scholar]
- 15. Hatch M, Freel RW. Increased colonic sodium absorption in rats with chronic renal failure is partially mediated by AT1 receptor agonism. Am J Physiol Gastrointest Liver Physiol 295: G348–G356, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Helms MN, Jain L, Self JL, Eaton DC. Redox regulation of epithelial sodium channels examined in alveolar type 1 and 2 cells patch-clamped in lung slice tissue. J Biol Chem 283: 22875–22883, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jin Y, Wang Y, Wang ZJ, Lin DH, Wang WH. Inhibition of angiotensin type 1 receptor impairs renal ability of K conservation in response to K restriction. Am J Physiol Renal Physiol 296: F1179–F1184, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Juncos R, Garvin JL. Superoxide enhances Na-K-2Cl cotransporter activity in the thick ascending limb. Am J Physiol Renal Physiol 288: F982–F987, 2005 [DOI] [PubMed] [Google Scholar]
- 19. Juncos R, Hong NJ, Garvin JL. Differential effects of superoxide on luminal and basolateral Na+/H+ exchange in the thick ascending limb. Am J Physiol Regul Integr Comp Physiol 290: R79–R83, 2006 [DOI] [PubMed] [Google Scholar]
- 20. Koeppen BM, Stanton BA. Sodium chloride transport. In: The Kidney: Physiology and Pathophysiology, edited by Seldin DW, Giebisch G. New York: Raven, 1992, p. 2003–2039 [Google Scholar]
- 21. Kopkan L, Castillo A, Navar LG, Majid DSA. Enhanced superoxide generation modulates renal function in ANG II-induced hypertensive rats. Am J Physiol Renal Physiol 290: F80–F86, 2006 [DOI] [PubMed] [Google Scholar]
- 22. Larsen BT, Gutterman DD, Sato A, Toyama K, Campbell WB, Zeldin DC, Manthati VL, Falck JR, Miura H. Hydrogen peroxide inhibits cytochrome P450 epoxygenases: interaction between two endothelium-derived hyperpolarizing factors. Circ Res 102: 59–67, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lazrak A, Iles KE, Liu G, Noah DL, Noah JW, Matalon S. Influenza virus M2 protein inhibits epithelial sodium channels by increasing reactive oxygen species. FASEB J 23: 3829–3842, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Light DB, McCann FV, Keller TM, Stanton BA. Amiloride-sensitive cation channel in apical membrane of inner medullary collecting duct. Am J Physiol Renal Fluid Electrolyte Physiol 255: F278–F286, 1988 [DOI] [PubMed] [Google Scholar]
- 25. Ling BN, Kokko KE, Eaton DC. Inhibition of apical Na channels in rabbit cortical collecting tubules by basolateral prostaglandin E2 is modulated by protein kinase C. J Clin Invest 90: 1328–1334, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lopez B, Salom MG, Arregui B, Valero F, Fenoy FJ. Role of superoxide in modulating the renal effects of angiotensin II. Hypertension 42: 1150–1156, 2003 [DOI] [PubMed] [Google Scholar]
- 27. Markadieu N, Crutzen R, Boom A, Erneux C, Beauwens R. Inhibition of insulin-stimulated hydrogen peroxide production prevents stimulation of sodium transport in A6 cell monolayers. Am J Physiol Renal Physiol 296: F1428–F1438, 2009 [DOI] [PubMed] [Google Scholar]
- 28. Ohtsu H, Suzuki H, Nakashima H, Dhobale S, Frank GD, Motley ED, Eguchi S. Angiotensin II signal transduction through small GTP-binding proteins: mechanism and significance in vascular smooth muscle cells. Hypertension 48: 534–540, 2006 [DOI] [PubMed] [Google Scholar]
- 29. Palmer LG, Antonian L, Frindt G. Regulation of apical K and Na channels and Na/K pumps in rat cortical collecting tubule by dietary K. J Gen Physiol 104: 693–710, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Palmer LG, Frindt G. Amiloride-sensitive Na channels from the apical membrane of the rat cortical collecting duct. Proc Natl Acad Sci USA 83: 2767–2770, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Palmer LG, Frindt G. Effect of cell Ca and pH on Na channels from rat cortical collecting tubule. Am J Physiol Renal Fluid Electrolyte Physiol 253: F333–F339, 1987 [DOI] [PubMed] [Google Scholar]
- 32. Pech V, Kim YH, Weinstein AM, Everett LA, Pham TD, Wall SM. Angiotensin II increases chloride absorption in the cortical collecting duct in mice through a pendrin-dependent mechanism. Am J Physiol Renal Physiol 292: F914–F920, 2007 [DOI] [PubMed] [Google Scholar]
- 33. Peti-Peterdi J, Warnock DG, Bell PD. Angiotensin II directly stimulates ENaC activity in the cortical collecting duct via AT1 receptors. J Am Soc Nephrol 13: 1131–1135, 2002 [DOI] [PubMed] [Google Scholar]
- 34. Pochynyuk O, Tong Q, Medina J, Vandewalle A, Staruschenko A, Bugaj V, Stockand JD. Molecular determinants of PI(4,5)P2 and PI(3,4,5)P3 regulation of the epithelial Na+ channel. J Gen Physiol 130: 399–413, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sachse A, Wolf G. Angiotensin II induced reactive oxygen species and the kidney. J Am Soc Nephrol 18: 2439–2446, 2007 [DOI] [PubMed] [Google Scholar]
- 36. Shimkets RA. Liddle's syndrome: heritable human hypertension caused by mutations in the beta subunit of the epithelial sodium channel. Cell 79: 407–414, 1994 [DOI] [PubMed] [Google Scholar]
- 37. Snyder PM, Price MP, McDonald FJ, Adams CM, Volk KA, Zeiher BG, Stokes JB, Welsh MJ. Mechanism by which Liddle's Syndrome mutations increase activity of a human epithelial Na+ channel. Cell 83: 969–978, 1995 [DOI] [PubMed] [Google Scholar]
- 38. Sun P, Lin DH, Yue P, Jiang H, Gotlinger KH, Schwartzman ML, Falck JR, Goli M, Wang WH. High potassium intake enhances the inhibitory effect of 11,12-EET on ENaC. J Am Soc Nephrol 21: 1667–1677, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Timmermans PBMWM, Wong PC, Chiu AT, Herblin WF, Benfield P, Carini DJ, Lee RJ, Wexler RR, Saye JAM, Smith RD. Angiotensin II receptors and angiotensin II receptor antagonists. Pharm Rev 45: 205–251, 1993 [PubMed] [Google Scholar]
- 40. Wang HC, Zentner MD, Deng HT, Kim KJ, Wu R, Yang PC, Ann DK. Oxidative stress disrupts glucocorticoid hormone-dependent transcription of the amiloride-sensitive epithelial sodium channel α-subunit in lung epithelial cells through ERK-dependent and thioredoxin-sensitive pathways. J Biol Chem 275: 8600–8609, 2000 [DOI] [PubMed] [Google Scholar]
- 41. Wei Y, Lin DH, Kemp R, Yaddanapudi GSS, Nasjletti A, Falck JR, Wang WH. Arachidonic acid inhibits epithelial Na channel via cytochrome P450 (CYP) epoxygenase-dependent metabolic pathways. J Gen Physiol 124: 719–727, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wei Y, Sun P, Wang ZJ, Yang BF, Wang WH. Adenosine inhibits epithelial Na channels (ENaC) by cytochrome P-450 (CYP)-epoxygenase-dependent metabolites of arachidonic acid. Am J Physiol Renal Physiol 290: F1163–F1168, 2006 [DOI] [PubMed] [Google Scholar]
- 43. Welch WJ. Angiotensin II-dependent superoxide: effects on hypertension and vascular dysfunction. Hypertension 52: 51–56, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wilcox CS. Oxidative stress and nitric oxide deficiency in the kidney: a critical link to hypertension? Am J Physiol Regul Integr Comp Physiol 289: R913–R935, 2005 [DOI] [PubMed] [Google Scholar]
- 45. Yu L, Bao HF, Self JL, Eaton DC, Helms MN. Aldosterone-induced increases in superoxide production counters nitric oxide inhibition of epithelial Na channel activity in A6 distal nephron cells. Am J Physiol Renal Physiol 293: F1666–F1677, 2007 [DOI] [PubMed] [Google Scholar]
- 46. Yue P, Sun P, Lin DH, Pan C, Xing W, Wang W. Angiotensin II diminishes the effect of SGK1 on the WNK4-mediated inhibition of ROMK1 channels. Kidney Int 79: 423–431, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zhao D, Seth DM, Navar LG. Enhanced distal nephron sodium reabsorption in chronic angiotensin II-infused mice. Hypertension 54: 120–126, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zimmerman MC, Lazartigues E, Sharma RV, Davisson RL. Hypertension caused by angiotensin II infusion involves increased superoxide production in the central nervous system. Circ Res 95: 210–216, 2004 [DOI] [PubMed] [Google Scholar]