Abstract
We aimed to investigate the potential relationship between alarmins [acting via Toll-like receptor-4 (TLR4)], uric acid (UA), and high-mobility group box-1 protein (HMGB1) during acute kidney injury. UA, which is significantly increased in the circulation following renal ischemia-reperfusion injury (IRI), was used both in vitro and in vivo as an early response-signaling molecule to determine its ability to induce the secretion of HMGB1 from endothelial cells. Treatment of human umbilical vein endothelial cells (HUVEC) with UA resulted in increased HMGB1 mRNA expression, acetylation of nuclear HMGB1, and its subsequent nuclear-cytoplasmic translocation and release into the circulation, as determined by Western blotting and immunofluorescence. Treatment of HUVEC with UA and a calcium mobilization inhibitor (TMB-8) or a MEK/Erk pathway inhibitor (U0126) prevented translocation of HMGB1 from the nucleus, resulting in reduced cytoplasmic and circulating levels of HMGB1. Once released, HMGB1 in autocrine fashion promoted further HMGB1 release while also stimulating NF-κB activity and increased angiopoietin-2 expression and protein release. Transfection of HUVEC with TLR4 small interfering (si) RNA reduced HMGB1 levels during UA and HMGB1 treatment. In summary, UA after IRI mediates the acetylation and release of HMGB1 from endothelial cells by mechanisms that involve calcium mobilization, the MEK/Erk pathway, and activation of TLR4. Once released, HMGB1 promotes its own further cellular release while acting as an autocrine and paracrine to activate both proinflammatory and proreparative mediators.
Keywords: secretion, acetylation, ethyl pyruvate, systemic inflammatory response
one of the default mechanistic responses in mammals to ischemia-reperfusion injury (IRI) is represented by the activation of xanthine oxidoreductase and a surge of purine metabolites in the circulation (15, 42). We have previously demonstrated that renal ischemia induces a rapid, transient elevation in the level of uric acid (UA) (50). Acting as a prototypical “alarm-signaling” alarmin, UA acts through Toll-like receptor-4 (TLR4) to signal exocytosis of Weibel-Palade bodies in vitro and in vivo. This in turn leads to the release of their constituents, such as von Willebrand factor, IL-8, and angiopoietin-2 (ANG 2), while also stimulating stem cell mobilization (34).
Another well-established alarmin that also acts via TLR4 is the high-mobility group box-1 protein (HMGB1). It is released by necrotic cells, but also by non-lethally injured cells (2–4, 56). Recent reports have indicated that HMGB1 is released by the kidney following IRI (7, 10, 35, 38, 70). HMGB1 and UA both participate in the systemic inflammatory response, but the possible existence of cooperative interactions between the two remains obscure. Exploring this possibility represented the main goal of this study.
Studies in monocytes and macrophages have indicated that when activated by various stressors, HMGB1 is actively translocated from the nucleus to the cytoplasm, utilizing various signaling cascades (2, 21, 50). For instance, the oxidative stress of macrophages leads to HMGB1 release by mechanisms involving MEK-Erk (64). HMGB1 release by oxidative stress, however, is markedly reduced by inhibition of calcium-dependent pathways (65). In fact, reports are emerging that suggest intracellular calcium concentration plays a variety of roles in nuclear-cytoplasmic shuttling of HMGB1 (51, 58, 59). Regardless of which signaling pathway is activated, the active nuclear-to-cytoplasmic translocation of HMGB1 appears to require the acetylation of HMGB1 at its two lysine-rich nuclear localization signals (4, 24). Here, we examined whether mechanisms of HMGB1 secretion utilized by immune cells are functional in endothelial cells and whether these mechanisms are involved in the interaction between UA and HMGB1.
While HMGB1 has been shown to promote autophagy, cell survival, angiogenesis, wound healing, stem cell homing, and DNA repair in some injury models (5, 22, 37, 46, 60, 61, 63), it also has a strong cytokine-like effect that induces a proinflammatory, immunostimulatory, and chemotactic response (1, 44, 48, 56). In essence, HMGB1 proteins function as universal sentinels for nucleic acid-mediated innate immune responses (72). Once released, HMGB1 has been shown to interact with at least five different receptors on target immune cells: RAGE, TLR4, TLR2, TREM-1, and CD24 (6, 16, 30, 33, 47, 49, 74). Feedback mechanisms involving released HMGB1 may synergistically activate these receptors, causing further secretion of intracellular HMGB1. Along these lines, one of the aims of this study was to examine the ability of exogenous HMGB1 to stimulate further release of HMGB1 from endothelial cells while also activating proinflammatory and angiogenic responses, thus ensuring the propagation of systemic inflammatory response signaling. We demonstrated that 1) UA acting via TLR4 induces acetylation, translocation, and release of HMGB1; 2) HMGB1 translocation is calcium and MEK/Erk dependent and can be blocked by ethyl pyruvate in cultured endothelial cells; and 3) released HMGB1 acts in a positive feedback loop to enhance its own synthesis and release, thus amplifying, expanding, and chronologically extending inflammatory response.
MATERIALS AND METHODS
Endothelial cell culture UA treatment.
Human umbilical vein endothelial cells (HUVEC) were obtained from ATCC (Manassas, VA) and cultured in EBM-2 medium (Lonza, Allendale, NJ) supplemented with (per 500 ml of EGM-2) 10 ml FBS, 0.2 ml hydrocortisone, 2 ml hFGF, 0.5 ml VEGF, 0.5 ml IGF-1, 0.5 ml ascorbic acid, 0.5 ml hEGF, 0.5 ml GA-1000, and 0.5 ml heparin. HUVEC were maintained at 37°C with 5% CO2 and were used in experiments when cultures reached 80% confluence. UA was prepared as previously reported (34). Briefly, UA sodium salt at varying concentrations (10–100 μg/ml) was dissolved in EBM-2 medium, then filtered through a sterile 0.22-um filter. HUVEC were treated with varying concentrations of UA (10, 50, and 100 μg/ml) for up to 60 min. We have previously demonstrated that endothelial cells are activated when treated with 10–100 μg/ml of UA, with optimal activation occurring with 50 μg/ml (34).
Calcium and MEK/Erk experiments.
HUVEC were incubated for 60 min with either 10 μM TMB-8 (a nonspecific inhibitor of intracellular calcium mobilization) (Sigma, St. Louis, MO) (9, 40, 41) or 10 μM U0126 (inhibitor of MEK/Erk) (Sigma) (13, 14, 17, 26, 31, 69) diluted in PBS supplemented with 4.1 mM KCl, 2.5 mM NaHCO3, 1.0 mM MgCl2, 5.0 mM glucose, and 1.4 mM CaCl2.
Recombinant HMGB1 and ethyl pyruvate experiments.
HUVEC were incubated in EBM-2 medium for 60 min with either 50 μg/ml UA (with or without 25 mM ethyl pyruvate, Sigma) (10, 11, 28, 39, 66, 67) or various concentrations (10, 50 and 100 ng/ml) (19) of recombinant HMGB1 (rHMGB1; with or without 25 mM ethyl pyruvate, R&D Systems, Minneapolis, MN). The pharmacological ethyl pyruvate dose of 25 mM was selected based on reports in the literature that identify this concentration as within the range that inhibits HMGB1 release from cells (11, 18, 28, 55, 57, 67, 73). Due to the acidic nature of ethyl pyruvate, 15 mM HEPES was added and the resulting solution was set to a pH of 7.3 using NaOH.
RNA extraction and real-time PCR.
Total RNA was extracted from HUVEC by TRIzol reagent (Invitrogen, Carlsbad, CA) and quantified by a Quant-iT RNA assay kit (Invitrogen). In brief, for each sample, 2 μg of total RNA were used to synthesize cDNA by a SuperScript III reverse transcriptase kit according to the supplier's protocol (Invitrogen). cDNA was amplified using iQ SYBR Green Supermix (Bio-Rad Laboratories, Hercules, CA). cDNA and primers were added to the PCR mixture to a final volume of 20 μl. PCR was performed using the MiniOpticon real-time PCR system (Bio-Rad Laboratories). Primers were designed using Beacon Designer 4 software (Premier Biosoft, Palo Alto, CA). The forward primer for HMGB1 was 5′-AgATATggCAAAAgCggACAAg-3′, and the reverse primer was 5′-TCAgAgCAgAAgAggAAgAAgg-3′. For ANG 2, the forward primer was 5′-TCggAAgAgCATggACAgCATAg-3′, and the reverse primer was 5′-TggAggAAgAgCggCAgTTg-3′. β-Actin mRNA expression was used as an internal control.
Isolation of cytoplasmic and nuclear proteins.
To obtain whole kidney and whole cell lysates, samples were homogenized in RIPA buffer with a protease inhibitor. To obtain cytoplasmic and nuclear fractions from either kidney tissue or HUVEC, Abcam's subcellular and nuclear fractionation protocols with modifications (71) were utilized. In brief, samples were incubated on ice for 10 min in cytoplasmic lysis buffer [10 mM Tris·HCl (pH 8.0), 60 mM KCl, 1 mM EDTA, 1 mM DTT, protease inhibitor, and 0.5% NP-40] and gently triturated using a 26-gauge needle (BD, Franklin, NJ). After centrifugation at 2,300 rpm for 5 min at 4°C, the supernatant fraction was removed and the pellet was resuspended in nuclear lysis buffer (20 mM Tris·HCl, pH 8.0, 420 mM NaCl, 0.2 mM EDTA, 25% glycerol, 1.5 mM MgCl2, Ultra pure water, protease inhibitor, 1 mM DTT, and 1% Triton X-100), incubated on ice for 30 min, repeatedly triturated, centrifuged at 14,000 rpm for 5 min at 4°C, and the supernatant containing the nuclear fraction was retained. Three Kunitz units of DNase (Qiagen, Valencia, CA) was added to nuclear fractions.
Analysis of acetylated HMGB1 by immunoprecipitation.
In experiments here, nuclear and cytoplasmic fractions and medium/blood samples were examined for total HMGB1 (both acetylated and nonacetylated) by Western blotting using standard HMGB1 antibodies. To distinguish what fraction of total HMGB1 was acetylated, HMGB1 was immunoprecipitated out of samples and Western blotting using an antibody specific for acetylated lysine residues was used. For immunoprecipitation, Santa Cruz Biotechnology's immunoprecipitation protocol was used. In brief, lysates were precleared by adding 1:2 protein A/G PLUS agarose bead slurry (Santa Cruz Biotechnology, Santa Cruz, CA), incubated with agitation for 10 min, centrifuged, and the resulting supernatant was retained. The protein concentration of the supernatant was determined by a Bradford assay (Bio-Rad Laboratories). For immunoprecipitation of HMGB1, rabbit polyclonal HMGB1 antibody (Abcam, Cambridge, MA) was added to the supernatant at a concentration of 10 μg of antibody/500 μg of total cellular protein and incubated overnight at 4°C. The resulting antibody-HMGB1 protein immunocomplex was captured from the solution by adding 10 μl of protein A/G PLUS agarose bead slurry and incubated overnight at 4°C. Samples were centrifuged at 14,000 rpm, and the pellet was washed with PBS, resuspended in lysis buffer, and boiled for 5 min to remove immunocomplexes from beads followed by centrifugation to pellet beads. The supernatant was mixed with Laemmli buffer (Bio-Rad Laboratories) and subjected to Western blot analysis.
Western blot analysis.
HMGB1 and ANG 2 were examined in tissue and cell samples by Western blotting (24) and in conditioned medium by ELISA (R&D Systems). Medium samples were concentrated using cellulose 10,000 MWCO concentrating centrifugal filter units (Millipore, Billerica, MA) before Western blotting. In brief, samples were dissolved in Laemmli buffer, boiled at 80°C for 10 min, and separated on 4–20% polyacrylamide Mini-Protean TGX gels (Bio-Rad Laboratories). Proteins were electrotransferred to a polyvinylidene difluoride membrane (Millipore, Medford, MA). After blocking with 5% wt/vol nonfat milk, membranes were incubated at 4°C for 48 h with a primary antibody, followed by incubation with a secondary antibody. A polyclonal rabbit antibody to HMGB1 (Abcam), polyclonal goat ANG 2 antibody from Santa Cruz Biotechnology, polyclonal rabbit antibody to TLR4 (Abcam), monoclonal mouse antibody to β-tubulin, monoclonal antibody to β-actin (Sigma), and polyclonal rabbit antibody to histone (Calbiochem, Gibbstown, NJ) were used as primary antibodies, according to the manufacturers′ recommendation (including dilutions). The polyclonal rabbit antibody to acetyl lysine (Abcam) was used as on immunoprecipitated samples. Anti-rabbit, anti-goat, and anti-mouse antibodies (GE Healthcare Lifesciences, Piscataway, NJ) conjugated to horseradish peroxidase were used as secondary antibodies, according to the manufacturer's recommendation. Detection was performed using enhanced chemiluminescence (Thermo Scientific, Rockford, IL) and X-ray exposure. Relative protein levels were calculated as densitometric ratios to histone, β-tubulin, or β-actin. To ensure the purity of cytoplasmic fractions, histone antibodies were used in Western blotting of cytoplasmic fraction samples. To ensure the purity of nuclear fractions, cytoplasmic tubulin antibodies were used in Western blotting of nuclear fraction samples. Furthermore, for Western blotting of plasma samples, equal amounts of total protein (as determined by a Bradford assay) of each sample were loaded onto Western blot gels and utilized as a loading control.
To differentiate between endogenous and exogenous HMGB1 in medium samples, 50 ng/ml of exogenous HMGB1 was added to medium (without cells) and quantified by Western blotting to establish a control baseline of the signal produced in samples by exogenous HMGB1. The resulting Western blot quantification of exogenous HMGB1 in the medium (without cells) was then subtracted from values obtained from experiments that quantified the amount of endogenous HMGB1 released from HUVEC stimulated by exogenous HMGB1. The resulting difference between the HMGB1 signal in the medium with and without cells was defined as the amount of endogenous HMGB1 released into the medium by HUVEC.
Immunocytochemical staining for HMGB1.
HUVEC were grown in EGM-2 medium until 80% confluent. Cells were incubated at 37°C with 50 μg/ml (34) UA for up to 1 h. After treatment, cells were fixed with 4% paraformaldehyde (Electron Microscopy Sciences, Hatfield, PA), permeabilized by 0.25% Triton X-100 in PBS-BSA (1%, Sigma), and incubated with rabbit polyclonal antibody to HMGB1 (Abcam), according to the manufacturer's recommendation, followed by labeling with Alexa Fluor 594-conjugated donkey anti-rabbit secondary antibody (Invitrogen). Cells were imaged on an Axiovert fluorescence microscope at ×100 with an Axiocam MRm camera using Axiovision 4.6.3SP1 Software (Zeiss). Fluorescence intensity was measured using NIS Elements AR 3.00, SP5 (Nikon).
In vivo UA treatment.
In brief, male FVB/NJ mice (Jackson Laboratory, Bar Harbor, ME), ages 10–12 wk, were anesthetized via intraperitoneal injection of 60 mg/kg ketamine and 6.6 mg/kg xylazine. A flank incision was made to expose the left kidney. The renal artery and the aorta were cleaned of connective tissue. A 33-gauge needle (Hamilton, Reno, NV) was inserted through the aorta into the renal artery and 50 μg/25 g body wt of UA (34) was infused. After removal of the needle, the vasculature was sealed with BioGlue surgical adhesive (CryoLife, Kennesaw, GA) and the flank incision was closed by suturing. Mice were euthanized 1 and 3 h following UA treatment with kidneys and whole blood collected for further analysis. The animal study protocol was in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee.
Immunohistochemical staining for HMGB1.
Upon euthanasia, kidneys were fixed by perfusion with 4% paraformaldehyde. After excision, kidneys were dehydrated with 30% sucrose, frozen in O.C.T. (Tissue-Tek, Torrance, CA), and cryosectioned (10-μm-thick sections). Staining of kidney sections followed the same protocol as previously described here for immunocytochemistry. Briefly, sections were permeabilized with 0.25% Triton X-100 in PBS-BSA (1%, Sigma), blocked with PBS-BSA (1%), and stained using rabbit polyclonal antibody to HMGB1 followed by Alexa Fluor 594-conjugated donkey anti-rabbit (Invitrogen) and Alexa Fluor 488-conjugated goat anti-rat (Invitrogen) secondary antibodies, according to the manufacturer's recommendation. Nuclei were stained by adding 10 μg/ml of 4,6-diamidino-2-phenylindole (DAPI) for 1 min followed by washing and addition of antifade reagent in PBS (Invitrogen).
NF-κB reporter activity.
HUVEC were transfected with NF-κB firefly luciferase, Renilla luciferase plasmids, MyD88 small interfering (si) RNA, and/or control scrambled siRNA (Promega, Madison, WI) using the Amaxa Biosystems Nucleofector II Transfection Unit (Lonza). After transfections, cell suspensions were cultured in EBM-2. Following 24 h of incubation at 37°C/5% CO2, cells were incubated for an additional 24 h with either UA (50 μg/ml) or rHMGB1 (50 ng/ml) with and without ethyl pyruvate (25 mM). After treatment, NF-κB luciferase activity was determined using the Promega Dual Luciferase Reporter Assay (Promega). Luciferase chemiluminescence activity was measured using the Mithras LB 940 Chemiluminescence Microplate Reader (Berthold Technologies, Oak Ridge, TN).
TLR4 siRNA.
TLR4 siRNA, control scrambled siRNA, and transfection reagents were purchased from Santa Cruz Biotechnology, and the manufacturer's protocol was used for transfections. HUVEC were cultured in EBM-2 medium and grown to 80% confluence before transfections. After transfection of either TLR4 siRNA or control scrambled siRNA, cells were incubated for 24 h at 37°C/5% CO2 in EBM-2 medium. After incubation, fresh EBM-2 was added and cells were treated with either UA (50 μg/ml) or rHMGB1 (50 ng/ml), followed by Western blot analysis.
Statistical analysis.
Data are presented as means ± SE. For multiple comparisons between groups, one-way ANOVA with Tukey's posttest or two-way ANOVA with a Bonferroni posttest was performed using NCSS 2007 statistical package (NCSS, Kaysville, UT) or GraphPad Prism 5.0 (GraphPad Software, San Diego CA). Differences were considered significant at P < 0.05.
RESULTS
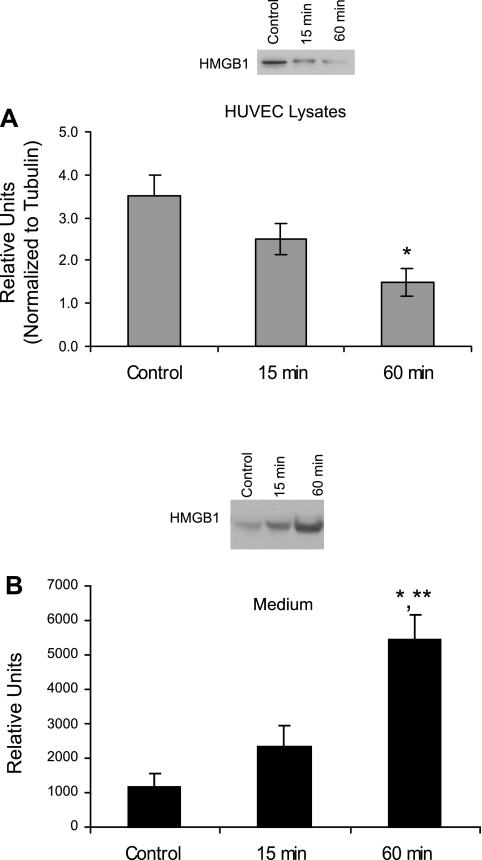
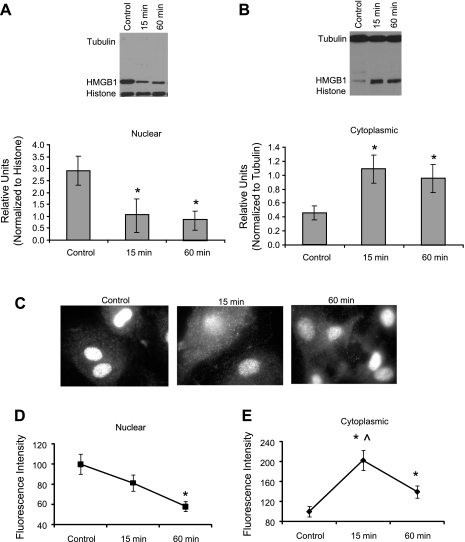
Treatment with UA (50 μg/ml for 1 h) was associated with a significant decrease in HMGB1 in HUVEC (Fig. 1A), accompanied by its increased appearance in the culture medium (Fig. 1B). This finding indicated endothelial cells contain HMGB1 and upon stimulation with UA, HMGB1 is released from these cells into the culture medium. To determine whether nuclear-cytoplasmic translocation occurred during UA stimulation, HUVEC were subjected to cell fractionation and isolation of nuclear and cytoplasmic fractions followed by Western blot analysis. We observed a decrease in nuclear accompanied by a significant increase in cytoplasmic HMGB1 after just 15 min of UA treatment (Fig. 2, A and B). Consistent with this, immunocytochemical analysis showed that cells treated with 50 μg/ml UA displayed fluorescently tagged HMGB1 translocation from the nucleus to the cytoplasm within 15 min (Fig. 2, C–E). In addition to its effects on HMGB1 protein release, UA also upregulated HMGB1 mRNA expression within 30 min in HUVEC (Fig. 3).
Fig. 1.
High-mobility group box-1 protein (HMGB1) expression in human umbilical vein endothelial cells (HUVEC) treated with uric acid (UA). Western blot analysis with representative gels and quantified graphs of HMGB1 in HUVEC lysates and culture medium after treatment of HUVEC with 50 μg/ml UA for varying times. A: HMGB1 protein expression after 15 and 60 min of UA treatment. B: HMGB1 protein release in the cell culture medium. Within 1 h of UA treatment, HMGB1 decreased in HUVEC lysates while concomitantly increasing in the medium, suggesting HUVEC were stimulated by UA to release HMGB1. *P < 0.05 vs. control. **P < 0.05 vs. 15 min; n = 5.
Fig. 2.
HMGB1 translocation in HUVEC treated with UA. Quantified graphs and representative gels depict HMGB1 protein abundance in nuclear and cytoplasmic fractions after UA (50 μg/ml) treatment. HMGB1 decreased in nuclear fractions (A) while increasing in cytoplasmic fractions (B) after 15 and 60 min of UA treatment. Immunocytochemistry images (C) of HUVEC treated with UA for 15 and 60 min, fixed, and stained with fluorescent HMGB1 antibodies show an increase in cytoplasmic fluorescence 15 and 60 min after UA treatment, indicative of HMGB1 movement from nucleus to cytoplasm. Graphs depicting quantified fluorescence intensity of HMGB1 in nuclei (D) and cytoplasm (E) indicate that upon UA treatment HMGB1 translocates from the nucleus to the cytoplasm. *P < 0.05 vs. control. ∧P < 0.05 vs. 60; n = 5. Magnification ×100.
Fig. 3.
HMGB1 mRNA expression in HUVEC treated with UA. HUVEC treated with 50 and 100 μg/ml of UA demonstrated increased HMGB1 mRNA expression within 1 h of treatment. Treatment with 50 μg/ml of UA resulted in a more robust increase in HMGB1 mRNA compared with 100 μg/ml of UA, with a peak in expression at 30 min. HMGB1 mRNA expression was normalized to β-actin expression. *P < 0.05 vs. 0 min. #P < 0.05 vs. all time points.
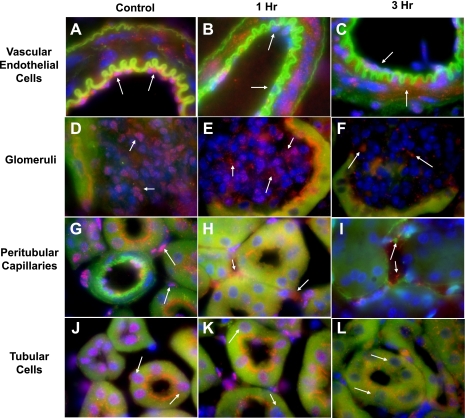
Having demonstrated that in vitro UA stimulates HMGB1 translocation and release into the culture medium of endothelial cells, we next examined its release in vivo. After bilateral renal ischemia in mice, circulating UA increases 2.4-fold (50). We have previously shown a single injection of 50 μg/25 g body wt of UA is sufficient to more than double its plasma concentration, which is comparable to the detected amount in the postischemic period (50). In in vivo experiments here, we perfused 50 μg/25 g body wt of UA into the renal circulation of mice to mimic the transient surge of UA that occurs during IRI. After injection, mice were subsequently killed and plasma and kidneys were examined for HMGB1. Western blot analysis of nuclear and cytoplasmic kidney fractions demonstrate HMGB1 in the nucleus declines while its levels in the cytoplasm and plasma increase within 1–3 h after UA injection (Fig. 4, A and B). These data strongly suggest that UA infusion, mimicking its surge in the postischemic circulation, results in translocation of HMGB1 from the nucleus to the cytoplasm and its secretion into the circulation. These findings support our immunohistochemical analysis of kidneys after intrarenal injection of UA. In control kidneys, HMGB1 was localized to the nuclei of endothelial cells of the renal vasculature (Fig. 5A), the glomerular capillary beds (Fig. 5D), and the peritubular capillaries (Fig. 5G), and to the nuclei of tubular epithelial cells (Fig. 5J). One hour after UA injection, HMGB1 staining in the nuclei of vascular (including glomerular and peritubular) endothelial (Fig. 5, B, E, and H) and tubular cells (Fig. 5K) was diminished. Three hours after UA injection, HMGB1 staining was significantly reduced in the nuclei of endothelial cells of the renal vasculature (Fig. 5C) including the glomerular (Fig. 5F) and peritubular capillaries (Fig. 5I) and in the nuclei of tubular cells (Fig. 5L).
Fig. 4.
HMGB1 after intrarenal UA injection. HMGB1 was measured by Western blot analysis in nuclear and cytoplasmic kidney fractions (A) and whole blood (B) 1 and 3 h after intrarenal UA injection (50 μg/25 g body wt) in mice. HMGB1 decreased in the nucleus and increased in the cytoplasm (A) and was enriched in the systemic circulation (B) within 1–3 h after UA treatment. Nuclear HMGB1 was normalized to histone, cytoplasmic HMGB1 was normalized to tubulin, and whole blood HMGB1 was normalized to total protein content. Absence of tubulin in nuclear samples and absence of histone in cytoplasmic samples confirmed purity. *P < 0.05 vs. control; n = 5.
Fig. 5.
Renal HMGB1 immunohistochemistry staining 1 and 3 h after UA intrarenal injection. Immunohistochemistry of kidney sections indicated HMGB1 in the nucleus of vascular endothelium (A), glomerular endothelial cells (D), peritubular capillary endothelial cells (G), and tubular epithelial cells (J), as indicated by colocalization of 4,6-diamidino-2-phenylindole (DAPI) nuclear stain (blue) and HMGB1 staining with antibody (red). One hour after UA injection, colocalization of DAPI and HMGB1 staining in the nuclei of vascular (B), glomerular (E), and peritubular endothelial (H) and tubular cells (K) had declined, indicating HMGB1 translocation from the nucleus to the cytoplasm. Three hours after UA injection, the decrease in colocalization of nuclear and HMGB1 staining was even more pronounced than at 1 h in cells of the renal vasculature (C), glomerular capillary bed (F), peritubular capillaries (I), and tubular epithelial cells (I). Magnification ×100.
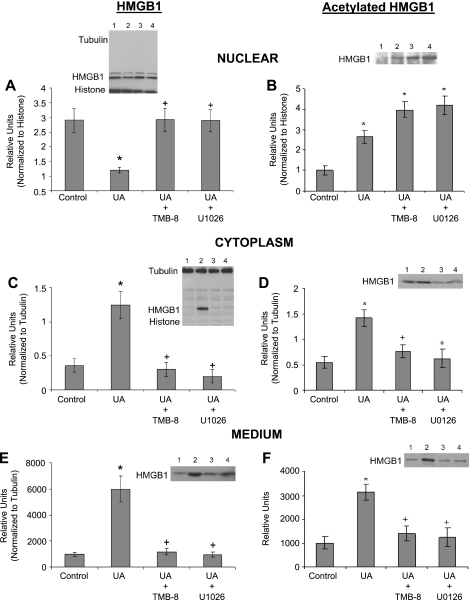
We next explored the mechanisms by which UA may cause nuclear-to-cytoplasmic HMGB1 translocation and eventual passage out of the cell in vitro. Western blot analysis indicated HMGB1 remains in the nucleus of HUVEC cells upon treatment with UA when intracellular calcium mobilization was inhibited by the nonspecific intracellular calcium mobilization inhibitor TMB-8 (Fig. 6A) or when the MEK/Erk pathway was blocked with U0126. As a consequence of HMGB1 nuclear retention, HMGB1 levels in the cytoplasm and medium remained unchanged compared with control (Fig. 6, C and E). Western blot analysis of immunoprecipitates of nuclear and cytoplasmic fractions and conditioned medium indicate UA treatment increased levels of acetylated HMGB1 in the nucleus (Fig. 6B), leading to its translocation and subsequent increase in the cytoplasm (Fig. 6D) and in the culture medium (Fig. 6F). However, the increase in both acetylated and nonacetylated HMGB1 in the cytoplasm and medium suggests UA provokes HMGB1 movement out of the nucleus by both passive and active mechanisms. When intracellular calcium mobilization or the MEK/Erk pathway was inhibited with 10 μM TMB-8 or 10 μM U0126, respectively, during UA treatment, acetylated HMGB1 remained elevated in the nucleus (Fig. 6B), but did not translocate to the cytoplasm (Fig. 6D) or the culture medium (Fig. 6F). Data taken collectively from Fig. 6 suggest UA initiates a cascade of events that leads to nuclear HMGB1 acetylation, nuclear-cytoplasmic translocation, and cellular release, a process that can be disrupted by blocking intracellular calcium mobilization or the MEK/Erk pathway.
Fig. 6.
HMGB1 translocation in HUVEC treated with inhibitors of calcium mobilization and the MEK/Erk pathway. Western blot analysis with representative gels and quantified graphs of HMGB1 and acetylated HMGB1 after 1 h of treatment with UA (50 μg/ml) in the presence or absence of 10 μM TMB-8 (a nonspecific intracellular calcium release blocker) or 10 μM U0126 (MEK/Erk pathway inhibitor) in nuclear fractions (A and B), cytoplasmic fractions (C and D), and culture medium (E and F) is shown. HMGB1, initially retained in the nucleus (A), decreased in the nucleus upon UA treatment while concomitantly increasing in the cytoplasm (C) and medium (E), indicating UA-induced HMGB1 nuclear-cytoplasm translocation. However, movement of HMGB1 out the nucleus was blocked by TMB-8 and U0126, suggesting calcium mobilization and the MEK/Erk pathway are involved in UA-stimulated HMGB1 release from endothelial cells. Treatment with UA increased the fraction of total HMGB1 that was acetylated in the nucleus (B), cytoplasm (D), and medium (F). HMGB1 is normally acetylated in the nucleus, leading to its active translocation to the cytoplasm and out of the cell. When HUVEC were treated TMB-8 or U0126 in addition to UA, acetylated HMGB1 remained elevated in the nucleus (B), but not in the cytoplasm (D) or the culture medium (F), suggesting nuclear-cytoplasmic translocation had been disrupted. Representative gel lanes: lane 1, control; lane 2, UA; lane 3, UA+TMB-8; and lane 4, UA+U0126. *P < 0.05 vs. control. +P < 0.05 vs. UA; n = 5.
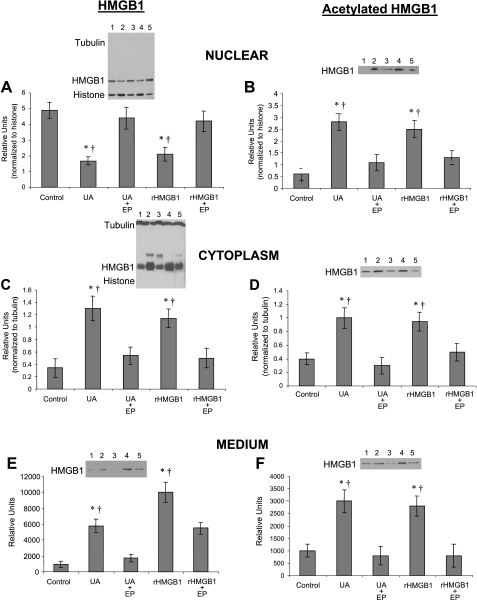
Ethyl pyruvate is a simple aliphatic ester of the metabolic intermediate pyruvate (18) that possesses effective anti-inflammatory properties in a variety of in vitro and in vivo model systems (18). Administration of ethyl pyruvate ameliorates organ damage and improves survival in animal models of mesenteric ischemia and reperfusion, hemorrhagic shock, endotoxemia, and sepsis (41, 55, 57). Ethyl pyruvate has also been found to be a pharmacological inhibitor of HMGB1 secretion (10, 11, 18, 28, 39, 55, 57, 66, 67, 73); however, the mechanistic actions that mediate its inhibitory effects have not been identified as yet. Reports indicating ethyl pyruvate inhibits HMGB1 release from a variety of cells and experimental settings prompted us to study its effect in UA-stimulated HUVEC. We treated HUVEC with 25 mM ethyl pyruvate after stimulation by 50 μg/ml UA or 50 ng/ml rHMGB1. Western blot analysis of HUVEC indicated UA-induced translocation of HMGB1 from the nucleus (Fig. 7A) to the cytoplasm (Fig. 7C) and subsequent cellular release (Fig. 7E) were sensitive to ethyl pyruvate blockade. Treatment of HUVEC with rHMGB1 (50 ng/ml) also led to nuclear-cytoplasmic translocation and release of endogenous HMGB1, an effect also blocked by ethyl pyruvate (Fig. 7, A, C, and E). Further analysis revealed that treatment with UA or rHMGB1 increased levels of acetylated HMGB1 in the nucleus (Fig. 7B), causing its translocation and subsequent increase in the cytoplasm (Fig. 7D) and culture medium (Fig. 7F). However, the increase in both acetylated and nonacetylated HMGB1 in the cytoplasm and medium suggests rHMGB1 provokes HMGB1 movement out of the nucleus by both passive and active mechanisms. However, ethyl pyruvate pretreatment abolished the UA- and rHMGB1-stimulated increase in acetylated HMGB1 in the nucleus (Fig. 7B), cytoplasm (Fig. 7D), and culture medium (Fig. 7F). Taken together, these data suggest that similar to UA, rHMGB1 can stimulate acetylation of nuclear HMGB1, its nuclear-cytoplasmic translocation, and release from endothelial cells, an effect inhibited by ethyl pyruvate.
Fig. 7.
UA- and HMGB1-mediated HMGB1 translocation is blocked by ethyl pyruvate. Similar to UA, HUVEC treatment with 50 ng/ml of rHMGB1 reduced nuclear (A) while increasing cytoplasmic (C) and medium (E) HMGB1, an effect that was blocked by 25 mM ethyl pyruvate (EP). Treatment with rHMGB1 (indicative of a positive feedback mechanism) increased acetylation of nuclear HMGB1 (B), and following translocation, acetylated HMGB1 subsequently increased in the cytoplasm (D) and medium (F), an effect also inhibited by EP. Representative gel lanes: lane 1, control; lane 2, UA; lane 3, UA+EP; lane 4, rHMGB1; and lane 5, rHMGB1+EP. *P < 0.05 vs. control. †P < 0.05 vs. EP; n = 8.
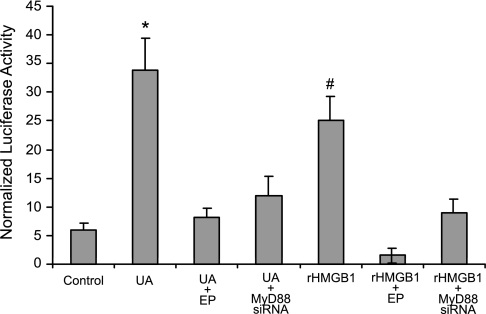
HUVEC, when treated with UA or exogenous rHMGB1, demonstrated significantly increased proinflammatory NF-κB activity, as measured by NF-κB luciferase reporter assay in transfected cells (Fig. 8). This effect was also blocked by ethyl pyruvate (Fig. 8). When HUVEC were transfected with MyD88 siRNA, UA- or rHMGB1-induced increases in NF-κB activity were drastically attenuated (Fig. 8). Transfection with control scrambled siRNA did not affect NF-κB activity. Data suggest that released HMGB1 may function in an autocrine manner by activating a MyD88-dependent pathway causing upregulation of NF-κB activity in endothelial cells.
Fig. 8.
NF-κB activity in HUVEC is elevated by UA and rHMGB1, an effect inhibited by EP. NF-κB luciferase reporter assay was measured after HUVEC treatment with either UA (50 μg/ml) or rHMGB1 (50 ng/ml; with or without 25 mM EP) for 24 h. NF-κB activity increased upon UA and rHMGB1 treatment, an effect significantly attenuated by EP treatment or MyD88 small interfering siRNA. Transfection of control scrambled siRNA did not affect UA- or rHMGB1-mediated increase in NF-κB activity (data not shown). *P < 0.05 vs. control, EP, MyD88 siRNA. #P < 0.05 vs. control, EP; n = 6.
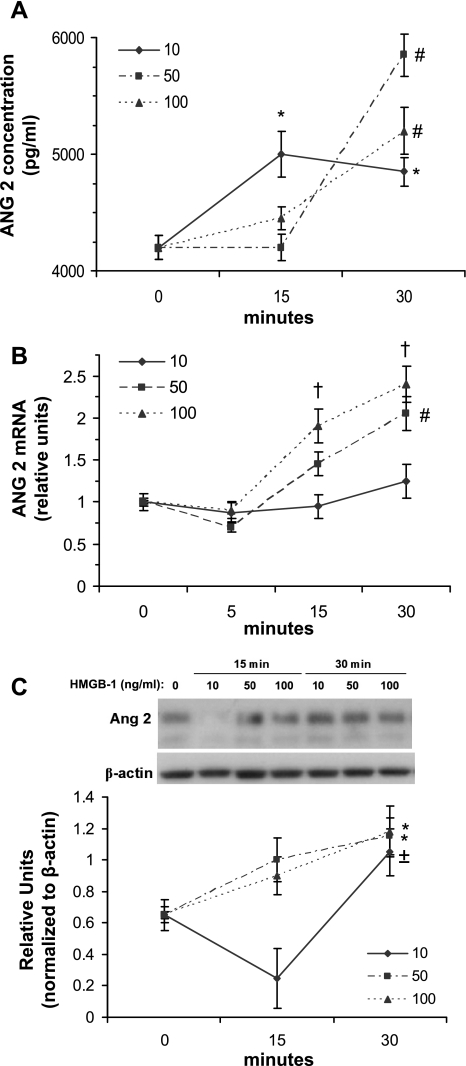
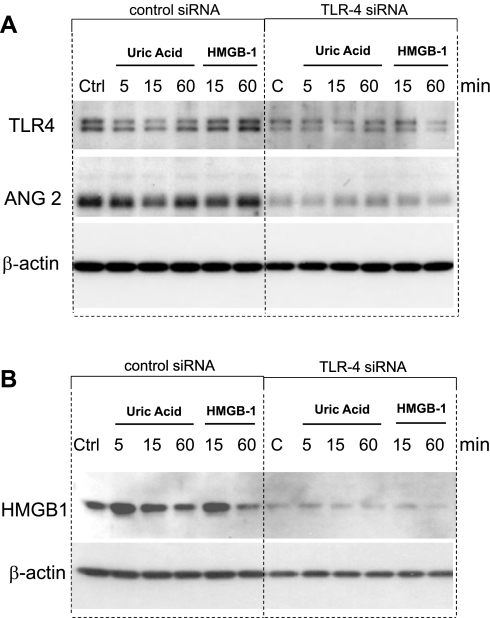
When HUVEC were treated with rHMGB1, ANG 2 (which is stored exclusively in Weibel-Palade bodies of endothelial cells) was released from cells into the culture medium within 30 min (Fig. 9A). The increase in ANG 2 release was accompanied by upregulation of ANG 2 mRNA expression (Fig. 9B) and protein expression (Fig. 9C). UA and rHMGB1 upregulation of ANG 2 was greatly attenuated when siRNA for TLR4 was introduced into HUVEC (Fig. 10A). Transfection of TLR4 siRNA resulted in attenuation of TLR4 protein in HUVEC compared with HUVEC transfected with control scrambled siRNA instead (Fig. 10A). Furthermore, UA and exogenous rHMGB1 upregulation of HMGB1 in HUVEC was also greatly attenuated by TLR4 siRNA transfection (Fig. 10B). These data suggest the involvement of TLR4 in mediating the UA and/or HMGB1 signaling cascade in stimulated endothelial cells.
Fig. 9.
Upregulation of angiopoietin-2 (ANG 2) by HMGB1 in HUVEC. HUVEC were treated with 3 different concentrations of rHMGB1: 10, 50, and 100 ng/ml. Treatment of HUVEC with 10 ng/ml of HMGB1 resulted in a significant increase in ANG 2 release into the medium within 15 min (A). However, a more robust increase was observed at 30 min with 50 and 100 ng/ml (A). ANG 2 mRNA expression in HUVEC was increased within 30 min of treatment with 50 and 100 ng/ml of rHMGB1 protein, but not with 10 ng/ml (B). ANG 2 mRNA was normalized to β-actin. ANG 2 protein in HUVEC increased within 30 min of treatment with all 3 concentrations of rHMGB1 (C), but treatment with 10 ng/ml of rHMGB1 caused an initial decline in HUVEC ANG 2 at 15 min, suggesting initial stimulated ANG 2 release from HUVEC at this concentration. *P < 0.05 vs. 0 min. #P < 0.05 vs. all time points. †P < 0.05 vs. 0 and 5 min. ±P < 0.05 vs. 15 min; n = 4.
Fig. 10.
Western blotting of ANG 2 and HMGB1 in HUVEC transfected with Toll-like receptor-4 (TLR4) siRNA. HUVEC were transfected with either TLR4 siRNA or control scrambled siRNA. Transfection of TLR4 siRNA resulted in successful attenuation of TLR4 in HUVEC (A). Downregulation of TLR4 by siRNA resulted in reduced levels of ANG 2 (A) and HMGB1 (B) in HUVEC when cells were treated with either UA (50 μg/ml) or rHMGB1 (50 ng/ml) for up to 1 h, suggesting TLR4 is involved in UA- and HMGB1-mediated upregulation of ANG 2 and HMGB1.
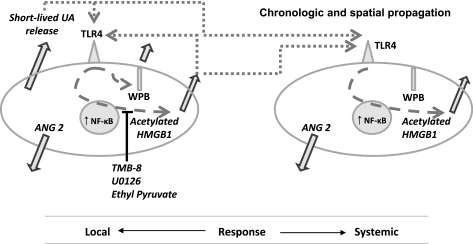
Figure 11 is a schematic representation of the chronologically and spaciously coordinated systemic inflammatory response that accompanies the local ischemic processes.
Fig. 11.
Schematic summary of the proposed interactions between UA and HMGB1 and their local and systemic effects. Release of UA by stressed cells activates TLR4 on endothelial cells. Once TLR4 is activated, nuclear HMGB1 becomes acetylated, translocates to the cytoplasm, and is released by mechanisms that are sensitive to blockade by TMB-8, U0126, and EP. Once released, HMGB1 can act as an autocrine signal or as a paracrine signal on distant cells to enhance release of HMGB1, increase exocytosis of Weibel-Palade bodies (WPB) and ANG 2, and increase NF-κB activity.
DISCUSSION
The data presented herein demonstrated that UA, usually released from an ischemic organ within a few minutes of reperfusion (50), activated endothelial cells to release HMGB1 within 1 h. HMGB1 translocated from the nucleus to the cytoplasm of HUVEC in a calcium- and MEK/Erk-dependent process, resulting in its secretion both in vitro and in vivo. Translocation of HMGB1 involved its acetylation, while blockade of calcium- and MEK/Erk-dependent pathways prevented acetylation and translocation. Furthermore, the prevention of acetylation could be also achieved with ethyl pyruvate. rHMGB1 also increased the acetylation and translocation of the endogenous pool. However, the nuclear-cytoplasmic movement of both acetylated and nonacetylated HMGB1 suggests both active and passive mechanisms are involved in HMGB1 shuttling during UA and rHMGB1 stimulation. These findings provide a groundwork for a hypothetical scenario whereby the ischemia-reperfusion-induced release of UA acts both locally and systemically on vascular endothelia 1) to mediate activation of TLR4 exocytosis of Weibel-Palade bodies and their constituents, as we have previously demonstrated (34); and as shown here 2) to ignite translocation of HMGB1 and its secretion into the circulation; and 3) released HMGB1 further activates endothelial cells in an autocrine positive feedback loop to enhance its own synthesis, translocation, and secretion. These mechanistic steps account for the chronologically and spaciously coordinated systemic inflammatory response that accompanies the local ischemic processes, as schematically depicted in Fig. 11.
While release of HMGB1 has been reported after IRI (7, 10, 35, 38, 70), the extent and the locality of release remain to be fully examined. The most relevant report was a recent study by Li et al. (35) using horseradish peroxidase immunohistochemical staining of kidneys after IRI. In the study by Li et al., the renal tubules, peritubular capillaries, and glomeruli all stained positive for HMGB1 release (35). After we observed a significant release of HMGB1 into the circulation after renal IRI, we attempted to investigate HMGB1 release in the kidney.
While it is well established that monocytes and macrophages are prime candidates for HMGB1 release, Degryse et al. (12) showed that endothelial cells are also capable of HMGB1 release. Findings by Mullins et al. (45) supported the idea that HMGB1 may be released from activated endothelial cells when they reported HMGB1 is translocated from the nucleus to the cytoplasm and released from HUVEC upon stimulation by LPS or TNF-α. We attempted to expand on these findings by examining HMGB1 release by renal endothelial cells in vivo. Our immunostaining results of IRI kidneys demonstrated abundant HMGB1 to be localized in vascular endothelial cells (Fig. 5), confirming the reports of other investigators (12, 20, 45, 52).
UA is emerging as a crucial very early mediator of damage response after injury, including renal ischemic insult. UA is currently recognized as a prototypical alarm signal, which undergoes a surge after IRI (50). The UA release after IRI is accompanied by a relatively fast damage response, suggesting UA acts on cells in the kidney to quickly mediate exocytosis of molecules from internalized storage. Kuo et al. (34) demonstrated that within minutes after IRI, UA causes exocytosis of Weibal-Palade bodies and release of their constituents (including von Willebrand factor, ANG 2, and IL-8) into the circulation. Based on this rationale, we reasoned the surge in UA after IRI may also lead to the exocytosis of other internalized molecules, including the release of HMGB1 from endothelial cells. We demonstrate here in in vivo experiments that intrarenal injection of UA leads to renal endothelial cell nuclear-cytoplasmic HMGB1 translocation and subsequent release into the circulation within 1–3 h (Figs. 3 and 4), a finding further supported by our in vitro studies utilizing HUVEC that also demonstrated HMGB1 release within the first hour after UA treatment (Figs. 1 and 2).
Many groups have focused their efforts on investigating the properties of HMGB1 nuclear-cytoplasmic translocation and release out of the cell, but these investigative efforts have primarily focused on specific immune cells. It is generally believed in monocytes and macrophages that HMGB1 relinquishes DNA binding and releases from the nucleus by two distinct mechanisms (4, 24). HMGB1 can be passively released or actively released from activated cells following acetylation of HMGB1 by nuclear proteins such as histone acetyltransferases (4, 24). HMGB1 contains two lysine-rich nuclear localization signals (NLSs) (4, 62), and acetylation of both NLSs is involved in its translocation (4, 24). Here, we found UA stimulation of HUVEC triggers HMGB1 acetylation in the nucleus (Figs. 6 and 7). The acetylation of HMGB1 at active DNA binding sites decreases its affinity for DNA, allowing for its nuclear release. The nuclear exporter protein chromosome region maintenance 1 (CRM1) shuttles acetylated HMGB1 from nucleus to cytoplasm (25). When internal mobilization of calcium or MEK/Erk was inhibited in HUVEC in experiments here, nuclear HMGB1 acetylation was still detected but its nuclear export and release was prevented (Figs. 6 and 7), suggesting calcium mobilization and MEK/Erk play a role in translocation of HMGB1.
Recent reports implicate calcium as an influential player in the translocation and release of HMGB1. In the absence of calcium, HMGB1 DNA binding properties may be enhanced, an effect dependent on the C-terminal domain of HMGB1 (51, 58, 59). In other reports, calcium appears to also play a role in the shuttling of HMGB1. Zhang et al. (75) demonstrated in macrophages that calcium/calmodulin-dependent protein kinase (CaMK) IV mediates nuclear-cytoplasmic shuttling. Recently, Oh et al. (45a) showed in monocytes that HMGB1 secretion can be induced by calcium ionophores and inhibited by calcium chelators. The involvement of calcium in HMGB1 secretion from monocytes was further demonstrated by Gardella et al. (21), who showed that after HMGB1 translocalization from the nucleus to the cytoplasm the secretory granules and/or lysosomes that contain HMGB1 are exocytosed in a calcium-regulated secretory pathway. All of these calcium-dependent kinases and organelles may take part in the shuttling of HMGB1 within various cell types, thus highlighting the importance of calcium signaling in HMGB1 release and secretion.
We examined some of the potential mechanisms by which UA may induce translocation and secretion of HMGB1 from endothelial cells. Our findings indicate intracellular mobilization of calcium from internal storage pools sensitive to inositol 1,4,5-trisphosphate is possibly a critical component of UA-mediated HMGB1 translocation and release. However, in experiments here, we used the nonspecific intracellular calcium release inhibitor TMB-8, which may also block other modes of calcium mobilization; therefore, influence from extracellular calcium influx cannot be ruled out as a factor involved in HMGB1 translocation (9, 40, 41). Furthermore, other laboratories have shown the MEK/Erk pathway is activated by UA in a variety of cell types (8, 23, 27). Our investigations here revealed that inhibition of the MEK/Erk pathway by U0126 also prevents UA-induced HMGB1 nuclear-cytoplasmic shuttling and release, thus indicating that the mechanism of UA-induced secretion of HMGB1 involves both calcium mobilization and the MEK/Erk pathway (Fig. 6).
The cellular release of HMGB1 has also been implicated in a variety of proinflammatory responses after tissue injury (1, 44, 48, 56). Here, we observed HMGB1 stimulates proinflammatory NF-κB activity in cultured endothelial cells (Fig. 8). However, despite the reparative/regenerative effects HMGB1 indirectly promotes, HMGB1 has been directly implicated in regeneration and repair in various injury models including cardiac injury and epithelial cell damage (1, 32, 36, 44, 48, 53, 54, 56, 68, 74). The ability of HMGB1 to act not only as a signal of tissue damage but also to promote proliferation, migration, and differentiation of several cell types, particularly those involved in angiogenesis (43), prompted us to examine the ability of HMGB1 to induce ANG 2 upregulation and release from endothelial cells. HMGB1 stimulation of HUVEC resulted in upregulated ANG 2 mRNA expression and protein translation and release (Fig. 9), an effect inhibited by TLR4 siRNA (Fig. 10). Interestingly, TLR4 siRNA also reduced HMGB1 protein in both UA- and rHMGB1-stimulated HUVEC (Fig. 10). Our findings suggest secreted endogenous HMGB1 activates TLR4 on endothelial cells in a positive feedback loop, thus amplifying its own synthesis and secretion.
In summary (Fig. 11), we show UA activates endothelial cells causing nuclear HMGB1 acetylation initiating its translocation and release. The UA-induced translocation and release of HMGB1 is dependent on activation of calcium mobilization and the MAP/Erk pathway. Once released, HMGB1 can act as an autocrine and a paracrine to stimulate further HMGB1 acetylation and release, while also activating NF-κB activity and upregulating ANG 2 expression and release. Thus, once UA induces the release of HMGB1 from endothelial cells, operating in a feedback loop mechanism, HMGB1 may further stimulate its own release while also activating various proinflammatory and proreparative mediators, both locally and systemically. These findings have the potential to explain the development of systemic inflammatory response after a localized injury and outline potential strategies for therapeutic interventions.
GRANTS
These studies were supported in part by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK54602, DK052783, and DK45462, the Westchester Artificial Kidney Foundation (M. S. Goligorsky), a Kaohsiung Medical University grant (KMU-QA098001), and a National Science Council of Taiwan grant (NSC-98-2314-B-037-010-MY3).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: M.M.R., M.-C.K., M.S.G., and B.B.R. provided conception and design of research; M.M.R., M.-C.K., T.G., S.M.R., M.W., and B.B.R. performed experiments; M.M.R., M.-C.K., and B.B.R. analyzed data; M.M.R., M.S.G., and B.B.R. interpreted results of experiments; M.M.R., M.-C.K., T.G., M.S.G., and B.B.R. prepared figures; M.M.R., M.S.G., and B.B.R. drafted manuscript; M.M.R., M.-C.K., M.S.G., and B.B.R. edited and revised manuscript; M.-C.K., M.S.G., and B.B.R. approved final version of manuscript.
REFERENCES
- 1. Andersson U, Wang H, Palmblad K, Aveberger AC, Bloom O, Erlandsson-Harris H, Janson A, Kokkola R, Zhang M, Yang H, Tracey KJ. High mobility group 1 protein (HMG-1) stimulates proinflammatory cytokine synthesis in human monocytes. J Exp Med 192: 565–570, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bianchi ME. DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol 81: 1–5, 2006 [DOI] [PubMed] [Google Scholar]
- 3. Bianchi ME, Celona B. Ancient news: HMGBs are universal sentinels. J Mol Cell Biol 2: 116–117, 2010 [DOI] [PubMed] [Google Scholar]
- 4. Bonaldi T, Talamo F, Scaffidi P, Ferrera D, Porte A, Bachi A, Rubartelli A, Agresti A, Bianchi ME. Monocytic cells hyperacetylate chromatin protein HMGB1 to redirect it towards secretion. EMBO J 22: 5551–5560, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chavakis E, Hain A, Vinci M, Carmona G, Bianchi M, Vajkoczy P, Zeiher A, Chavakis T, Dimmeler S. High-mobility group box 1 activates integrin-dependent homing of endothelial progenitor cells. Circ Res 100: 204–212, 2007 [DOI] [PubMed] [Google Scholar]
- 6. Chen GY, Tang J, Zheng P, Liu Y. CD24 and Siglec-10 selectively repress tissue damage-induced immune responses. Science 323: 1722–1725, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen J, John R, Richardson JA, Shelton JM, Zhou XJ, Wang Y, Wu QQ, Hartono JR, Winterberg PD, Lu CY. Toll-like receptor 4 regulates early endothelial activation during ischemic acute kidney injury. Kidney Int 79: 288–299, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cheng TH, Lin JW, Chao HH, Chen YL, Chen CH, Chan P, Liu JC. Uric acid activates extracellular signal-regulated kinases and thereafter endothelin-1 expression in rat cardiac fibroblasts. Int J Cardiol 139: 42–49, 2010 [DOI] [PubMed] [Google Scholar]
- 9. Chiou CY, Malagodi MH. Studies on the mechanism of action of a new Ca2+ antagonist, 8-(N,N-diethylamino)octyl 3,4,5-trimethoxybenzoate hydrochloride in smooth and skeletal muscles. Br J Pharmacol 53: 279–285, 1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chung KY, Park JJ, Kim YS. The role of high-mobility group box-1 in renal ischemia and reperfusion injury and the effect of ethyl pyruvate. Transplant Proc 40: 2136–2138, 2008 [DOI] [PubMed] [Google Scholar]
- 11. Dave SH, Tilstra JS, Matsuoka K, Li F, DeMarco RA, Beer-Stolz D, Sepulveda AR, Fink MP, Lotze MT, Plevy SE. Ethyl pyruvate decreases HMGB1 release and ameliorates murine colitis. J Leukoc Biol 86: 633–643, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Degryse B, Bonaldi T, Scaffidi P, Muller S, Resnati M, Sanvito F, Arrigoni G, Bianchi ME. The high mobility group (HMG) boxes of the nuclear protein HMG1 induce chemotaxis and cytoskeleton reorganization in rat smooth muscle cells. J Cell Biol 152: 1197–1206 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. DeSilva DR, Jones EA, Favata MF, Jaffee BD, Magolda RL, Trzaskos JM, Scherle PA. Inhibition of mitogen-activated protein kinase blocks T cell proliferation but does not induce or prevent anergy. J Immunol 160: 4175–4181, 1998 [PubMed] [Google Scholar]
- 14. Duncia JV, Santella JB, 3rd, Higley CA, Pitts WJ, Wityak J, Frietze WE, Rankin FW, Sun JH, Earl RA, Tabaka AC, Teleha CA, Blom KF, Favata MF, Manos EJ, Daulerio AJ, Stradley DA, Horiuchi K, Copeland RA, Scherle PA, Trzaskos JM, Magolda RL, Trainor GL, Wexler RR, Hobbs FW, Olson RE. MEK inhibitors: the chemistry and biological activity of U0126, its analogs, and cyclization products. Bioorg Med Chem Lett 8: 2839–2844, 1998 [DOI] [PubMed] [Google Scholar]
- 15. Ejaz AA, Mu W, Kang DH, Roncal C, Sautin YY, Henderson G, Tabah-Fisch I, Keller B, Beaver TM, Nakagawa T, Johnson RJ. Could uric acid have a role in acute kidney failure? Clin J Am Soc Nephrol 2: 16–21, 2007 [DOI] [PubMed] [Google Scholar]
- 16. El Mezayen R, El Gazzar M, Seeds MC, McCall CE, Dreskin SC, Nicolls MR. Endogenous signals released from necrotic cells augment inflammatory responses to bacterial endotoxin. Immunol Lett 111: 36–44, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Favata MF, Horiuchi KY, Manos EJ, Daulerio AJ, Stradley DA, Feeser WS, Van Dyk DE, Pitts WJ, Earl RA, Hobbs F, Copeland RA, Magolda RL, Scherle PA, Trzaskos JM. Identification of a novel inhibitor of mitogen-activated protein kinase. J Biol Chem 273: 18623–18632, 1998 [DOI] [PubMed] [Google Scholar]
- 18. Fink MP. Ethyl pyruvate: a novel anti-inflammatory agent. J Intern Med 261: 349–362, 2007 [DOI] [PubMed] [Google Scholar]
- 19. Fiuza C, Bustin M, Talwar S, Tropea M, Gerstenberger E, Shelhamer JH, Suffredini AF. Inflammation-promoting activity of HMGB1 on human microvascular endothelial cells. Blood 101: 2652–2660, 2003 [DOI] [PubMed] [Google Scholar]
- 20. Gao M, Hu Z, Zheng Y, Zeng Y, Shen X, Zhong D, He F. PPARγ agonist troglitazone inhibits HMGB1 expression in endothelial cells via suppressing transcriptional activity of NF-κB and AP-1. Shock 36: 228–234, 2011 [DOI] [PubMed] [Google Scholar]
- 21. Gardella S, Andrei C, Ferrera D, Lotti LV, Torrisi MR, Bianchi ME, Rubartelli A. The nuclear protein HMGB1 is secreted by monocytes via a non-classical, vesicle-mediated secretory pathway. EMBO Rep 3: 995–1001, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Goula AV, Berquist BR, Wilson DM, 3rd, Wheeler VC, Trottier Y, Merienne K. Stoichiometry of base excision repair proteins correlates with increased somatic CAG instability in striatum over cerebellum in Huntington's disease transgenic mice. PLoS Genet 5: e1000749, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Griffith JW, Sun T, McIntosh MT, Bucala R. Pure hemozoin is inflammatory in vivo and activates the NALP3 inflammasome via release of uric acid. J Immunol 183: 5208–5220, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Harris HE, Andersson U. The nuclear protein HMGB1 as a proinflammatory mediator. Eur J Immunol 34: 1503–1512, 2004 [DOI] [PubMed] [Google Scholar]
- 25. Hayakawa K, Arai K, Lo EH. Role of ERK map kinase and CRM1 in IL-1beta-stimulated release of HMGB1 from cortical astrocytes. Glia 58: 1007–1015, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hotokezaka H, Sakai E, Kanaoka K, Saito K, Matsuo K, Kitaura H, Yoshida N, Nakayama K. U0126 and PD98059, specific inhibitors of MEK, accelerate differentiation of RAW2647 cells into osteoclast-like cells. J Biol Chem 277: 47366–47372, 2002 [DOI] [PubMed] [Google Scholar]
- 27. Jaramillo M, Naccache PH, Olivier M. Monosodium urate crystals synergize with IFN-gamma to generate macrophage nitric oxide: involvement of extracellular signal-regulated kinase 1/2 and NF-kappa B. J Immunol 172: 5734–5742, 2004 [DOI] [PubMed] [Google Scholar]
- 28. Johansson AS, Johansson-Haque K, Okret S, Palmblad J. Ethyl pyruvate modulates acute inflammatory reactions in human endothelial cells in relation to the NF-kappaB pathway. Br J Pharmacol 154: 1318–1326, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kang R, Tang D, Schapiro NE, Livesey KM, Loughran P, Bierhaus A, Lotze MT, Zeh HJ. The receptor for advanced glycation end products (RAGE) sustains autophagy and limits apoptosis, promoting pancreatic tumor cell survival. Cell Death Differ 17: 666–676, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kim SK, Abdelmegeed MA, Novak RF. The mitogen-activated protein kinase kinase (MEK) inhibitor PD98059 elevates primary cultured rat hepatocyte glutathione levels independent of inhibiting MEK. DMD 34: 683–689, 2006 [DOI] [PubMed] [Google Scholar]
- 32. Kohno T, Anzai T, Naito K, Miyasho T, Okamoto M, Yokota H, Yamada S, Maekawa Y, Takahshi T, Yoshikawa T, Ishizaka A, Ogawa S. Role of high-mobility group box 1 protein in post-infarction healing process and left ventricular remodeling. Cardiovasc Res 81: 565–573, 2009 [DOI] [PubMed] [Google Scholar]
- 33. Kokkola R. RAGE is the major receptor for the proinflammatory activity of HMGB1 in rodent macrophages. Scand J Immunol 61: 1–9, 2005 [DOI] [PubMed] [Google Scholar]
- 34. Kuo MC, Patschan D, Patschan S, Cohen-Gould L, Park HC, Ni J, Addabbo F, Goligorsky MS. Ischemia-induced exocytosis of Weibel-Palade bodies mobilizes stem cells. J Am Soc Nephrol 19: 2321–2330, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Li J, Gong Q, Zhong S, Wang L, Guo H, Xiang Y, Ichim TE, Wang CY, Chen S, Gong F, Chen G. Neutralization of the extracellular HMGB1 released by ischemic damaged renal cells protects against renal ischemia-reperfusion injury. Nephrol Dial Transplant 26: 469–478, 2011 [DOI] [PubMed] [Google Scholar]
- 36. Limana F, Germani A, Zacheo A, Kajstura J, Di Carlo A, Borsellino G, Leoni O, Palumbo R, Battistini L, Rastaldo R, Muller S, Pompilio G, Anversa P, Bianchi M, Capogrossi M. Exogenous high-mobility group box 1 protein induces myocardial regeneration after infarction via enhanced cardiac c-kit cell proliferation and differentiation. Circ Res 97: e73–e83, 2005 [DOI] [PubMed] [Google Scholar]
- 37. Liu Y, Prasad R, Wilson SH. HMGB1: roles in base excision repair and related function. Biochem Biophys Acia 1799: 119–130, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lu CY, Hartono J, Senitko M, Chen J. The inflammatory response to ischemic acute kidney injury: a result of the ‘right stuff’ in the ‘wrong place’? Curr Opin Nephrol Hypertens 16: 83–89, 2007 [DOI] [PubMed] [Google Scholar]
- 39. Luan ZG, Zhang H, Yang PT, Ma XC, Zhang C, Guo RX. HMGB1 activates nuclear factor-κB signaling by RAGE and increases the production of TNF-α in human umbilical vein endothelial cells. Immunobiology 215: 956–962, 2010 [DOI] [PubMed] [Google Scholar]
- 40. Malagodi MH, Chiou CY. Pharmacological evaluation of a new Ca++ antagonist, 8-(N,N-diethylamino)octyl 3,4,5-trimethoxybenzoate hydrochloride (TMB-8): studies in skeletal muscles. Pharmacology 12: 20–31, 1974 [DOI] [PubMed] [Google Scholar]
- 41. Malagodi MH, Chiou CY. Pharmacological evaluation of a new Ca2+ antagonist, 8-(N,N-diethylamino)-octyl-3,4,5-trimethoxybenzoate hydrochloride (TMB-8): studies in smooth muscles. Eur J Pharmacol 27: 25–33, 1974 [DOI] [PubMed] [Google Scholar]
- 42. Miller WL, Thomas RA, Berne RM, Rubio R. Adenosine production in the ischemic kidney. Circ Res 43: 390–397, 1978 [DOI] [PubMed] [Google Scholar]
- 43. Mitola S, Belleri M, Urbinati C, Coltrini D, Sparatore B, Pedrazzi M, Melloni E, Presta M. Cutting edge: extracellular high mobility group box-1 protein is a proangiogenic cytokine. J Immunol 176: 12–15, 2006 [DOI] [PubMed] [Google Scholar]
- 44. Muller S, Ronfani L, Bianchi ME. Regulated expression and subcellular localization of HMGB1, a chromatin protein with a cytokine function. J Intern Med 255: 332–343, 2004 [DOI] [PubMed] [Google Scholar]
- 45. Mullins G, Sunden-Cullberg J, Johansson A, Rouhiainen A, Erlandsson-Harriss H, Yang H, Tracey Rauvala H K, Palmbald J, Andersson J, Treutiger C. Activation of human umbilical vein endothelial cells leads to relocation and release of high-mobility group box chromosomal protein 1. Scand J Immunol 60: 566–573, 2004 [DOI] [PubMed] [Google Scholar]
- 45a. Oh YJ, Youn JH, Ji Y, Lee SE, Lim KJ, Choi JE, Shin JS. HMGB1 is phosphorylated by classical protein kinase c and is secreted by a calcium-dependent mechanism. J Immunol 182: 5800–5809, 2009 [DOI] [PubMed] [Google Scholar]
- 46. Palumbo R, Sampaolesi M, De Marchis F, Tonlorenzi R, Colombetti S, Mondino A, Cossu G, Bianchi M. Extracellular HMGB1, a signal of tissue damage, induces mesoangioblast migration and proliferation. J Cell Biol 164: 447–449, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Park JS. High mobility group box 1 protein interacts with multiple toll-like receptors. Am J Physiol Cell Physiol 290: C917–C924, 2006 [DOI] [PubMed] [Google Scholar]
- 48. Park JS, Arcaroli J, Yum HK. Activation of gene expression in human neutrophils by high mobility group box 1 protein. Am J Physiol Cell Physiol 284: C870–C879, 2003 [DOI] [PubMed] [Google Scholar]
- 49. Park JS, Svetkauskaite D, He Q, Kim JY, Strassheim D, Ishizaka A, Abraham E. Involvement of TLR2 and TLR4 in cellular activation by high mobility group box 1 protein (HMGB1). J Biol Chem 279: 7370–7377, 2004 [DOI] [PubMed] [Google Scholar]
- 50. Patschan D, Patschan S, Gobe GG, Chintala S, Goligorsky MS. Uric acid heralds ischemic tissue injury to mobilize endothelial progenitor cells. J Am Soc Nephrol 18: 1516–1524, 2007 [DOI] [PubMed] [Google Scholar]
- 51. Polianichko AM, Chikhirzhina EV, Andrushchenko VV, Kostyleva EI, Wieser H, Vorob'ev VI. The effect of Ca2+ ions on DNA compaction in the complex with non-histone chromosomal protein HMGB1. Mol Biol (Mosk) 38: 701–712, 2004 [PubMed] [Google Scholar]
- 52. Porto A, Palumbo R, Pieroni M, Aprigliano G, Chiesa R, Sanvito F, Maseri A, Bianchi ME. Smooth muscle cells in human atherosclerotic plaques secrete and proliferate in response to high mobility group box 1 protein. FASEB J 20: 2565–2566, 2006 [DOI] [PubMed] [Google Scholar]
- 53. Ranzato E, Patrone M, Pedrazzi M, Burlando B. HMGB1 promotes scratch wound closure of HaCaT keratinocytes via ERK1/2 activation. Mol Cell Biochem 332: 199–205, 2009 [DOI] [PubMed] [Google Scholar]
- 54. Ranzato E, Patrone M, Pedrazzi M, Burlando B. HMGB1 promotes wound healing of 3T3 mouse fibroblasts via RAGE-dependent ERK1/2 activation. Cell Biochem Biophys 57: 9–17, 2010 [DOI] [PubMed] [Google Scholar]
- 55. Sappington PL, Fink ME, Yang R, Delude RL, Fink MP. Ethyl pyruvate provides durable protection against inflammation-induced intestinal epithelial barrier dysfunction. Shock 20: 521–528, 2003 [DOI] [PubMed] [Google Scholar]
- 56. Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature 418: 191–195, 2002 [DOI] [PubMed] [Google Scholar]
- 57. Sims CA, Wattanasirichaigoon S, Menconi MJ, Ajami AM, Fink MP. Ringer's ethyl pyruvate solution ameliorates ischemia/reperfusion-induced intestinal mucosal injury in rats. Crit Care Med 29: 1513–1518, 2001 [DOI] [PubMed] [Google Scholar]
- 58. Stros M, Bernues J, Querol E. Calcium modulates the binding of high-mobility-group protein 1 to DNA. Biochem Int 21: 891–899, 1990 [PubMed] [Google Scholar]
- 59. Stros M, Reich J, Kolibalova A. Calcium binding to HMG1 protein induces DNA looping by the HMG-box domains. FEBS Lett 344: 201–206, 1994 [DOI] [PubMed] [Google Scholar]
- 60. Tang D, Kang R, Cheh CW, Livesey KM, Liang X, Schapiro NE, Benschop R, Sparvero LJ, Amoscato AA, Tracey KJ, Zeh HJ, Lotze MT. HMGB1 release and redox regulates autophagy and apoptosis in cancer cells. Oncogene 29: 5299–5310, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Tang D, Kang R, Livesey KM, Cheh C, Adam Farkas A, Loughran P, Hoppe G, Bianchi ME, Tracey KJ, Zeh HJ, Lotze MT. Endogenous HMGB1 regulates autophagy. J Cell Biol 190: 881–892, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Tang D, Kang R, Zeh H, Lotze M. HMGB1, oxidative stress and disease. Antioxid Redox Signal 14: 1315–1335, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Tang D, Lotze MT, Zeh HJ, 3rd, Kang R. The redox protein HMGB1 regulates cell death and survival in cancer treatment. Autophagy 7: 904–906, 2010 [DOI] [PubMed] [Google Scholar]
- 64. Tang D, Shi Y, Kang R, Li T, Xiao W, Wang H, Xiao X. Hydrogen peroxide stimulates macrophages and monocytes to actively release HMGB1. J Leukoc Biol 81: 741–747, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Tsung A, Klune JR, Zang X, Jeyabalan G, Cao Z, Peng X, Stelz DB, Geller DA, Rosengart MR, Billiar TR. HMGB1 release induced by liver ischemia involves Toll-like receptor 4 dependent reactive oxygen species production and calcium-mediated signaling. J Exp Med 204: 2913–2923, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Uchiyama T, Delude RL, Fink MP. Dose-dependent effects of ethyl pyruvate in mice subjected to mesenteric ischemia and reperfusion. Intensive Care Med 29: 2050–2058, 2003 [DOI] [PubMed] [Google Scholar]
- 67. Ulloa L, Ochani M, Yang H, Tanovic M, Halperin D, Yang R, Czura C, Fink M, Tracey K. Ethyl pyruvate prevents lethality in mice with established lethal sepsis and systemic inflammation. Proc Natl Acad Sci USA 99: 12351–12356, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Wake H, Mori S, Liu K, Takahashi HK, Nishibori M. High mobility group box 1 complexed with heparin induced angiogenesis in a matrigel plug assay. Acta Med Okayama 63: 249–262, 2009 [DOI] [PubMed] [Google Scholar]
- 69. Wei S, Wang M, Teitelbaum S, Ross F. Interleukin-4 reversibly inhibits osteoclastogenesis via inhibition of NF-kappa B and mitogen-activated protein kinase signaling. J Bio Chem 277: 6622–6630, 2002 [DOI] [PubMed] [Google Scholar]
- 70. Wu H, Ma J, Wang P, Corpuz TM, Panchapakesan U, Wyburn KR, Chadban SJ. HMGB1 contributes to kidney ischemia reperfusion injury. J Am Soc Nephrol 21: 1878–1890, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Xavier S, Piek E, Fujii M, Javelaud D, Mauviel A, Flanders KC, Samuni AM, Felici A, Reiss M, Yarkoni S, Sowers A, Mitchell JB, Roberts AB, Russo A. Amelioration of radiation-induced fibrosis. J Biol Chem 279: 15167–15176, 2004 [DOI] [PubMed] [Google Scholar]
- 72. Yanai H, Ban T, Wang Z, Choi MK, Kawamura T, Negishi H, Nakasato M, Lu Y, Hangai S, Koshiba R, Savitsky D, Ronfani L, Akira S, Bianchi ME, Honda K, Tamura T, Kodama T, Taniguchi T. HMGB proteins function as universal sentinels for nucleic-acid-mediated innate immune responses. Nature 462: 99–103, 2009 [DOI] [PubMed] [Google Scholar]
- 73. Yu DH, Noh DH, Song RH, Park J. Ethyl pyruvate downregulates tumor necrosis factor alpha and interleukin (IL)-6 and upregulates IL-10 in lipopolysaccharide-stimulated canine peripheral blood mononuclear cells. J Vet Med Sci 72: 1379–1381, 2010 [DOI] [PubMed] [Google Scholar]
- 74. Yu M, Wang H, Ding A, Golenbock DT, Latz E, Czura CJ, Fenton MJ, Tracey KJ, Yang H. HMGB1 signals through toll-like receptor (TLR) 4 and TLR2. Shock 26: 174–179, 2006 [DOI] [PubMed] [Google Scholar]
- 75. Zhang X, Wheeler D, Tang Y, Guo L, Shapiro RA, Ribar TJ, Means AR, Billiar TR, Angus DC, Rosengart MR. Calcium/calmodulin-dependent protein kinase (CaMK) IV mediates nucleocytoplasmic shuttling and release of HMGB1 during lipopolysaccharide stimulation of macrophages. J Immunol 181: 5015–5023, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]