Abstract
The role of endocytosis in the control of EGF receptor (EGFR) activation and cell signaling was explored by using mouse fibroblasts in which dynamin was conditionally depleted. Dynamin is a GTPase shown to play an important role in the control clathrin mediated endocytosis of EGFR and other cell surface receptors. In this report, we demonstrate that EGF binding activity and the display of high and low affinity EGFRs on the cell surface are not affected by dynamin depletion. By contrast, dynamin depletion leads to a strong inhibition of EGFR endocytosis, robust enhancement of EGFR autophosphorylation and ubiquitination, and slower kinetics of EGFR degradation. Surprisingly, MAPK stimulation induced by either low or high EGF concentrations is not affected by dynamin depletion. While a similar initial Akt response is detected in control or dynamin depleted fibroblasts, a somewhat more sustained Akt stimulation is detected in the dynamin depleted cells. These experiments demonstrate that dynamin-mediated endocytosis leads to attenuation of EGFR activation and degradation and that stimulation of the MAPK response and Akt activation are primarily mediated by activated EGFR located in the plasma membrane.
Keywords: membrane receptors, tyrosine kinases
Epidermal growth factor receptor (EGFR) and other receptor tyrosine kinases (RTK) undergo rapid internalization and degradation following ligand induced activation (1–3). At low physiological concentrations, EGF induced EGFR internalization is primarily mediated by clathrin mediated endocytosis; a process blocked by siRNA silencing of clathrin heavy chain expression or by overexpression of a dominant interfering dynamin mutant (K44) (4–6). By contrast, when high EGF concentrations are applied, it is proposed that EGFR endocytosis is primarily mediated by clathrin-independent mechanisms (3, 7, 8).
It was initially thought that the function of EGFR endocytosis and degradation was to terminate the signal initiated by EGF binding to EGFRs located at the plasma membrane (1, 2). Subsequent studies demonstrated that EGFR and other RTKs, are also capable of recruiting signaling molecules and transmitting signals from endosomes (7, 9, 10). Moreover, it was proposed that following EGFR activation clathrin mediated endocytosis plays an important regulatory role in control of sustained activation of the MAPK/ERK signaling pathway and in Akt stimulation (4, 11). The current prevailing view is that signals induced by activated EGFR and other RTKs can be transmitted from the plasma membrane as well as from endosomes and that the spatial localizations of RTKs play an important role in the control of signal specificity, duration, and robustness (4, 9–12).
Dynamin is large GTPase, that mediates the endocytic fission of coated pits (13, 14). In this report we describe the analysis of the role played by dynamin in EGFR activation, endocytosis, and cell signaling. Using dynamin conditional knockout mouse fibroblasts we demonstrate that the ligand binding characteristics and the typical display of high and low affinity EGFR binding sites on the cell surface are maintained and not affected by dynamin depletion. However, dynamin depletion leads to a strong inhibition of EGFR endocytosis that is accompanied by enhanced tyrosine autophosphorylation, enhanced and prolonged EGFR ubiquitination, and reduced EGFR degradation. We also demonstrate that depletion of dynamin expression results in selective enhancement in tyrosine phosphorylation of the p66 isoform of the adaptor protein Shc. Moreover, while MAPK stimulation induced by low or high concentrations of EGF are not affected by dynamin depletion, a somewhat more sustained Akt activation is observed in these cells. These experiments demonstrate that dynamin-mediated endocytosis results in attenuation of EGFR activation, autophosphorylation, and ubiquitination as well as in enhanced EGFR degradation. Furthermore, stimulation of the MAPK/ERK pathway and Akt activation can be effectively activated by EGFR located in the plasma membrane.
Results
The role of dynamin in the control of EGFR activation, endocytosis, and downstream signaling was explored by using fibroblasts derived from Dnm1flox/flox; Dnm2flox/flox; Cre-Esr1 + /-mice (15, 16) following tamoxifen-induced gene recombination in vitro. The Dnm1 and Dnm2 genes encode for dynamin 1 and 2, respectively, both of which are expressed in murine fibroblasts while the third dynamin isoform, dynamin 3, is largely undetectable in these cells. We therefore refer to these cells as dynamin depleted cells, or dynamin knockout cells (DKO).
Ligand Binding Activity of EGFR Is Not Influenced by Dynamin Depletion.
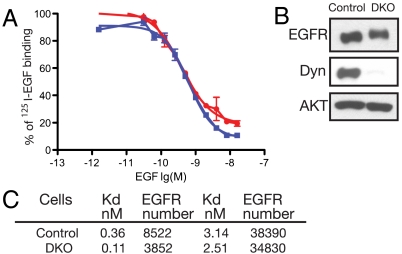
In order to evaluate the effect of dynamin depletion on the ligand binding characteristics of cell surface EGFRs, murine fibroblasts were either pretreated with 4-hydroxy-tamoxifen or buffer alone and then subjected to quantitative binding experiments with 125I-EGF. The effect of dynamin depletion on the ligand binding characteristics of EGFR were determined by comparing displacement curves or Scatchard analyzes (17–19) of quantitative 125I-EGF binding experiments to live cells (Fig. 1). To confirm that dynamin 1 and 2 were indeed depleted by tamoxifen treatment, samples of tamoxifen treated or untreated cells were lysed and subjected to immunoblotting with anti-dynamin antibodies (Fig. 1B). Control or dynamin depleted fibroblasts were incubated with 5 ng/mL of 125I-EGF for 1 h at room temperature in the presence of increasing concentrations of unlabeled EGF. The treated cells were lysed and the radioactive contents of the samples were determined using a scintillation counter. The experiment presented in Fig. 1A depicts displacement curves of 125I-EGF binding to control fibroblasts or to dynamin depleted fibroblasts. This experiment reveals similar 125I-EGF displacement curves for control and dynamin depleted fibroblasts (Fig. 1B) with concentrations required for 50% inhibition, IC50 of 0.62 and 0.40 nM, respectively. This experiment demonstrates that the display of EGFR on the plasma membrane and the overall binding affinity of EGFR towards EGF was not affected by dynamin loss. A similar conclusion was reached from Scatchard analysis of quantitative binding experiments with concentrations ranging from 0.1 to 100 ng/mL 125I-EGF to control or dynamin depleted fibroblasts. Similar numbers of EGFRs were present on the cell surfaces of control and dynamin depleted cells and both cells displayed a typical pattern of low and high affinity EGFRs on their cell surfaces (Fig. 1C). It is noteworthy that under the conditions in which the 125I-EGF binding experiments were performed a substantial fraction of bound 125I-EGF molecules become internalized into dynamin depleted cells and even more into WT cells indicating that the profile of EGF binding characteristic is not sensitive to EGFR internalization. These experiments support the conclusion that the display of EGFR on the cell surface and the ligand binding characteristics of EGFRs are not affected by dynamin loss. However, these conclusions contradict an earlier study demonstrating that overexpression of a dominant interfering dynamin mutant (K44A) prevent high affinity EGF binding and reduces EGF stimulation of EGFR autophosphorylation (20).
Fig. 1.
Ligand binding characteristics of EGFR are not influenced by dynamin depletion. (A). Binding experiments were performed using a single concentration of 125I-labeled EGF in the presence of increasing concentration on unlabeled native EGF. The graphs depict competition experiments of 125I-labeled EGF binding to control (blue squares) or DKO (red circles) fibroblasts in the presence of increasing concentration of native EGF. Curve fitting to binding data shown by lines and bars indicate standard deviation values. (B). Immunoblotting analysis of total cell lysates of control or DKO fibroblasts with anti-dynamin antibodies. Immunobloting with anti-AKT antibodies was used as loading control. (C). Dissociation constants (Kd) and numbers of low and high affinity EGFR binding sites on control and DKO fibroblasts were determined using Scatchard analysis of quantitative 125I-EGF binding experiments to these cells. Similar results were obtained in three separate 125I-EGF binding experiments.
Endocytosis of EGFR Is Strongly Impaired in Dynamin Depleted Cells.
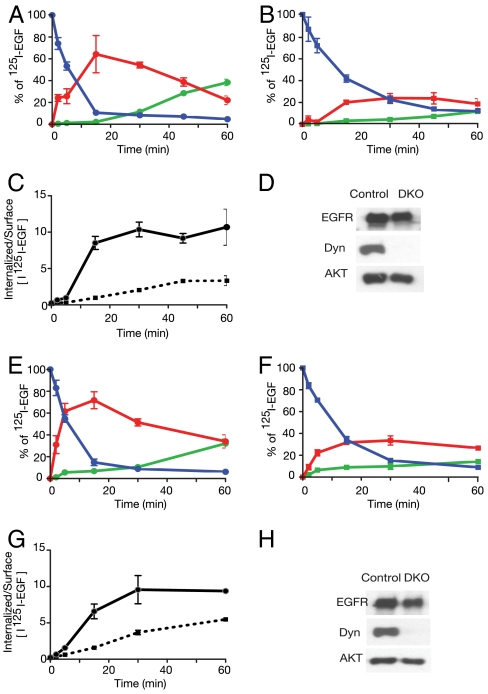
It was previously reported that clathrin-mediated endocytosis is the primary route for internalization of EGFR at low EGF concentrations (1.5 ng/mL), while at higher ligand concentrations EGFR internalization may occur via both clathrin-dependent and clathrin-independent mechanisms (4, 8). To investigate whether the absence of dynamin affects EGFR internalization, we next analyzed the kinetics of internalization of 125I-EGF using a previously described, well established quantitative procedure (21, 22). To this end, control or dynamin depleted fibroblasts were incubated with either low (1.5 ng/mL) or high (100 ng/mL) 125I-EGF concentrations for 90 min at 4 °C. At the end of the incubations, the cells were washed and further incubated at 37 °C for different times. To quantitatively determine the amount of EGFRs located at the cell membrane, the cells were subjected to a mild acid wash at different time points to selectively release only cell surface bound 125I-EGF molecules into the medium (21). The washed cells were then solubilized and the radioactive contents of the cell surface bound and the internalized 125I-EGF molecules were separately determined using a scintillation counter (21).
The experiments presented in Fig. 2 A–H show that endocytosis of EGFR is strongly impaired in dynamin depleted fibroblasts. The results show that after 15 min most cell surface EGFR in control fibroblasts became internalized in response to stimulation with either low or high EGF concentrations (Fig. 2 A–C and Fig. 2 E–G). In contrast, a robust inhibition of EGFR internalization was detected in dynamin depleted fibroblasts stimulated with 1.5 ng/mL (low dose) of EGF (Fig. 2B). Internalization of EGFR was also compromised in dynamin depleted cells stimulated with 100 ng/mL (high dose) of EGF (Fig. 2E). Under these conditions approximately 70% inhibition of EGFR internalization was observed in dynamin depleted cells (Fig. 2G). These experiments also show that EGFR internalization is not completely blocked in dynamin depleted cells. The partial internalization that takes place in dynamin depleted cells is probably not caused by the residual expression of dynamins in these cells, because residual dynamin expression is extremely low (Fig. 2H). For the same reason, it is also unlikely that the incomplete block of EGFR endocytosis may reflect the presence of few cells where recombination did not occur. A plausible explanation is that EGF induced internalization of EGFR can follow both dynamin-dependent and dynamin-independent routes of internalization, especially when the cells are stimulated with high EGF concentrations.
Fig. 2.
Endocytosis of EGFR is strongly impaired in dynamin depleted cells. Quantitative ligand internalization experiments using 1.5 ng/mL (A–D) or 20 ng/mL (E–H) of 125I-EGF to control (A, E) and DKO (B, F) fibroblasts are shown. Surface bound 125I-EGF (blue), internalized 125I-EGF (red) and degraded 125I-EGF (green) are shown. The TCA precipitatable radioactivity representing intact released or recycled 125I-EGF molecules are not included in the figures. The ratio of the amount of 125I EGF internalized vs. surface bound 125I-EGF molecules are shown in (C) and (G) for control (solid line) and DKO (dashed line) fibroblasts. Each data point is the average value of duplicate results. Data are presented as mean ± SD, as indicated by the bars. Also shown immunobloting analyzes with anti-dynamin antibodies or anti-EGFR antibodies to reveal dynamin or EGFR expression respectively, in control or DKO fibroblasts. Similar results were obtained in three different experiments.
Ligand Induced Degradation of EGFR Is Compromised in Dynamin Depleted Cells.
Following EGF induced receptor activation and endocytosis, a fraction of the internalized EGFRs are degraded (3, 23, 24). The process of ligand induced EGFR elimination from the cell surface and ensuing degradation that terminates signaling is known as “receptor down regulation” (25, 26). To explore the effect of dynamin depletion on EGFR degradation, control or dynamin depleted fibroblasts were first incubated with cycloheximide for an hour to block protein synthesis and block the formation of new EGFRs. The cells were then treated with 1, 10, or 100 ng/mL of EGF, solubilized at different time points and subjected to immunoblotting analyses with anti-EGFR antibodies to reveal the amount of EGFR. Because minimal EGFR degradation was detected in cells stimulated with 1 ng/mL of EGF after 4 h, the experiment presented in Fig. 3 shows the effect of stimulation with 10 or 100 ng/mL of EGF. As previously described (27), stimulation of control fibroblasts with 100 ng/mL of EGF led to robust degradation of EGFR with a half-time of approximately 45 min (Fig. 3B). Slower kinetics of EGFR degradation were detected in control fibroblasts treated with 10 ng/mL of EGF with a half-time of 2–3 h (Fig. 3A). Previously described degradation products of EGFR were clearly detected after 30 min of ligand stimulations in both experiments. The experiments presented in Fig. 3 A and B demonstrate that the stability of EGFR is increased in dynamin depleted fibroblasts that were stimulated with either 10 or 100 ng/mL EGF. In dynamin depleted fibroblasts that were stimulated with 100 ng/mL the half-time of EGFR degradation was extended to approximately 2 h without the appearance of EGFR degradation products seen in control stimulated cells. It is noteworthy that the typical EGFR degradation products detected in control fibroblasts stimulated with 100 ng/mL EGF (21, 27) were not detected in dynamin depleted fibroblasts stimulated with the same EGF concentration suggesting that EGF induced degradation of EGFR may proceed via a different mechanism in dynamin deficient cells.
Fig. 3.
Ligand induced degradation of EGFR is compromised in dynamin depleted cells. Serum starved control or DKO fibroblasts were pretreated with 10 μM of cycloheximide for 1 h followed by stimulation with 10 ng/mL (A) or 100 ng/mL (B) of EGF for indicated times. Equal amounts of cell lysates were subjected to immunobloting with anti-EGFR antibodies and as controls with anti-dynamin or anti-AKT antibodies. Arrow points to an EGFR degradation product that is detected in EGF stimulated WT cells and not in dynamin depleted cells. Similar results were obtained in three different experiments.
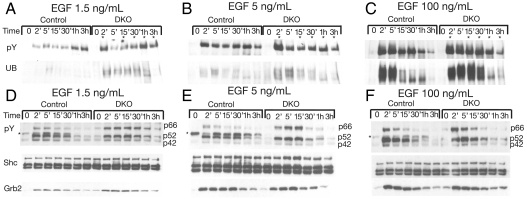
Enhanced Ligand Induced Autophosphorylation and Ubiquitination of EGFR in Dynamin Deficient Cells.
We next examined the effect of dynamin depletion on EGF stimulated EGFR tyrosine phosphorylation and ubiqutination (Fig. 4 A–C). Control or dynamin depleted fibroblasts were stimulated with 1.5, 5, or 100 ng/mL of EGF. Cell lysates of EGF stimulated or unstimulated cells were subjected to immunoprecipitation with anti-EGFR antibodies followed by immunoblotting with either anti-pTyr or anti-ubiquitin antibodies. The experiments presented in Fig. 4 A–C show an overall strong increase in tyrosine phosphorylation and ubiquitination of EGFR in dynamin depleted cells stimulated with 1.5 ng/mL of EGF. Interestingly bimodal activation of EGFR autophosphorylation was reproducibly observed in cells stimulated with 1.5 or 5 ng/mL of EGF. The experiment presented in Fig. 4A shows that in WT cells enhanced EGFR autophosphorylation occurs after 5 min of stimulation with 1.5 ng/mL of EGF followed by reduced autophosphorylation at the 15 and 30 min time points that is followed after 1 and 3 h by a second peak. In dynamin depleted fibroblasts stimulated with 1.5 ng/mL EGF, a stronger and earlier onset of the first peak was seen after 2 min followed by reduced autophosphorylation at the 5, 15, and 30 min time points that is followed after 1 and 3 h by a strong second peak of autophosphorylation. These experiments also show that the levels of EGFR ubiquitination, which is weakly detected in control fibroblasts, is strongly stimulated in dynamin depleted fibroblasts stimulated with 1.5 ng/mL of EGF (Fig. 4A).
Fig. 4.
Enhanced EGF induced autophosphorylation and ubiquitination of EGFR and altered pattern of phosphorylation of Shc isoforms in dynamin depleted cells. Serum starved control or DKO fibroblasts were stimulated with 1.5 ng/mL (A, D), 5 ng/mL (B, E) or 100 ng/mL (C, F) of EGF for different times. Equal amounts of cell lysates were subjected to immunoprecipitation with either anti-EGFR or anti-Shc antibodies. (A–C) The anti-EGFR immunoprecipitates were immunobloted with anti-phosphotyrosine (pY) and reprobed with anti-ubiquitin (UB) antibodies. Enhanced tyrosine phosphorylation of EGFR is marked with asterisks. (D–E) Anti-Shc immunoprecipitates were subjected to immunobloting with anti-pY antibodies (asterisk marks the presence of an nonspecific protein band observed in control cells) and reprobed using anti-Shc or anti-Grb2 antibodies. The efficiency of tamoxifen induced dynamin depletion as well as the levels of EGFR expression in control and DKO fibroblasts were determined by immunobloting with anti-dynamin or anti-EGFR antibodies, respectively (not shown for clarity). Similar results were obtained in three different experiments.
Upon stimulation with 5 ng/mL EGF stronger enhancement of autophosphorylation of EGFR was detected in control and dynamin depleted fibroblasts (Fig. 4B) with a robust early onset after 2 min stimulation of the dynamin depleted cells. Similarly, enhanced ubiquitination of EGFR was also detected in cells stimulated with 5 ng/mL of EGF (Fig. 4B).
While autophosphorylation and ubiquitination of EGFR were much more robust in both control and dynamin depleted fibroblasts during the first thirty minutes of stimulation with 100 ng/mL EGF (Fig. 4C) the initial decline and the second elevated phase of autophosphorylation that was seen in 1.5 or 5 ng/mL EGF stimulated cells at the 1 and 3 h time points were not detected at this higher EGF concentration.
We also examined the effect of pretreatment with cycloheximide on ligand induced EGFR autophosphorylation and ubiquitination. These experiments showed minor effects of cycloheximide treatment on these posttranslational modifications (Fig. S1), indicating that preexisting pools of EGFR play a major role in the control of EGFR autophosphorylation and ubiquitination during the first 3 h of ligand stimulation.
Altered Pattern of Tyrosine Phosphorylation of Shc Isoforms in Dynamin Depleted Fibroblasts.
Shc is an adapter protein containing an SH2 and PTB domain that functions upon tyrosine phosphorylation by EGFR and other RTKs as an important link between EGFR and the RAS/MAP kinase signaling pathway by recruitment of Grb2/Sos complexes (28). Grb2 is an adaptor protein composed of one SH2 domain flanked by two SH3 domains which plays an important role via direct or indirect interactions with EGFR and other RTKs to link between RTK stimulation and the Ras/MAP kinase signaling pathway (2, 28). Most cells express three known Shc isoforms designated p42, p52, and p66 (29, 30). The experiments presented in Fig. 4 D–F show that both the p42 and p52 isoforms of Shc are similarly tyrosine phosphorylated in control or dynamin depleted fibroblasts in response to low (1.5 ng/mL) or high (100 ng/mL) EGF concentrations (Fig. 4 D–F, lower boxes). Interestingly, a more robust and sustained tyrosine phosphorylation of the p66 isoform of Shc was detected in dynamin depleted fibroblasts stimulated by either low or high EGF concentrations (Fig. 4 D–F, lower boxes). These experiments showed similar Grb2 recruitment in Shc immunoprecipitates in lysates from stimulated control or dynamin depleted fibroblasts suggesting that Grb2 becomes associated primarily with the tyrosine phosphorylated p42 and/or p52 Shc isoforms and that the p66 isoform may be involved in mediating a Grb2 independent process (31).
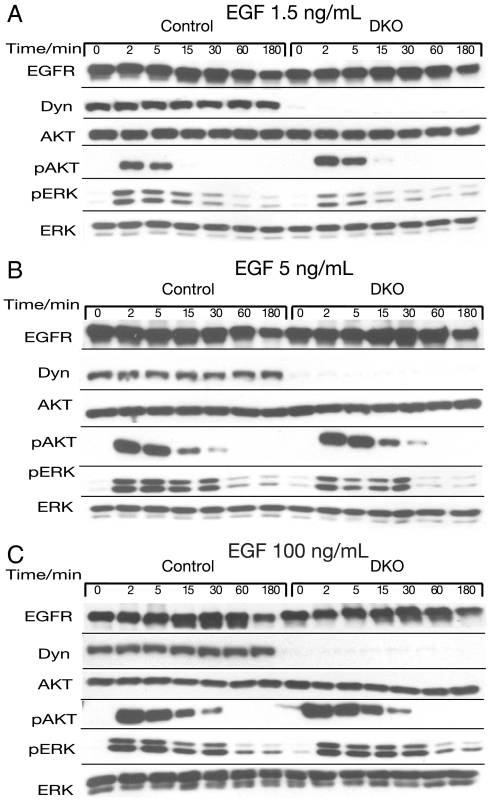
Similar Stimulation of MAP Kinase and Akt Responses in Dynamin Depleted Cells.
We next examined the effect of dynamin depletion on EGF stimulation of the MAP kinase (ERK) and Akt signaling pathways in response to either low (1.5 ng/mL) or high (100 ng/mL) EGF concentrations (Fig. 5). Control or dynamin depleted fibroblasts were stimulated with EGF. At different time points, the cells were solubilized and subjected to immunoblotting with antibodies that recognized either MAPK (anti-ERK) or activated MAPK (anti-pERK). The samples were also subjected to immunoblotting with antibodies that selectively recognize total Akt and the activated form of Akt (anti-pAkt). The experiments presented in Fig. 5 show a very similar profile of MAPK stimulation in control and dynamin depleted fibroblasts over a 3 h period in response to 1.5, 5, or 100 ng/mL of EGF stimulation. A similar Akt response was also observed in control or dynamin depleted fibroblasts stimulated with either low or high EGF concentrations. However, a somewhat more sustained Akt activation was reproducibly detected in dynamin depleted cells stimulated with any of these EGF concentrations (Fig. 5).
Fig. 5.
Similar stimulation of MAP kinase and AKT responses in dynamin depleted cells. Serum starved control or DKO fibroblasts were stimulated with 1.5 ng/mL (A), 5 ng/mL (B), or 100 ng/mL (C) of EGF. Cells were collected at the indicated time points and equal amounts of lysates were subjected to immunobloting with anti-AKT or anti-ERK antibodies. Membranes were reprobed with anti-pAKT or anti-pERK antibodies to monitor their enzymatic activities. EGFR and dynamin levels were monitored by inmunoblotting with anti-EGFR or anti-dynamin antibodies respectively. Similar results were obtained in three different experiments.
Discussion
It is well established that clathrin-mediated endocytosis of EGFR or other RTKs plays an important role in the control of receptor down regulation; a process mediated by intracellular degradation of both EGF and EGFR which results in signal termination (1, 2, 21, 25, 26). Subsequent studies reporting experiments in which clathrin mediated endocytosis of EGFR was blocked by either ectopic overexpression of a dominant interfering dynamin mutant (5, 32) or by silencing the expression of clathrin heavy chain using specific siRNAs (4, 6) concluded that EGFR molecules internalized by means of clathrin mediated endocytosis are capable of recruitment and activation of critical intracellular signaling pathways from endosomal compartments (5, 12, 33).
In this report we use dynamin depleted murine fibroblasts to explore the role played by endocytosis in the control of EGFR display on the cell surface, in regulation of EGFR activation, EGFR degradation, and in cell signaling via EGFR. Our experiments demonstrate that the expression and display of high and low affinity EGFRs on the cell surface are not affected by dynamin loss. These experiments contradict earlier studies demonstrating that overexpression of a dominant interfering dynamin mutant prevent high affinity EGF binding and reduces EGFR autophosphorylation (20). Ligand induced endocytosis of EGFR is strongly impaired in dynamin depleted fibroblasts stimulated with either low EGF concentrations [1-1.5 ng/mL, conditions under which internalization of EGFR is primarily driven by clathrin mediated endocytosis (4, 8)], or high EGF concentrations (100 ng/mL, a condition under which EGFR endocytosis is thought to be mediated by both clathrin-dependent and clathrin-independent mechanisms). The experiments performed with dynamin depleted fibroblasts stimulated with low EGF concentration provide an opportunity to address the role of clathrin-mediated endocytosis in the control of EGFR activation and signaling via EGFR. These experiments clearly demonstrate that during the early phase of EGF stimulation under conditions in which the majority of EGFR are still located at the cell surface, autophosphorylation and ubiquitination of EGFR are strongly enhanced indicating that autophosphorylation and ubiquitination of EGFR are taking place primarily by activated EGFR located at the cell membrane. Autophosphorylation of EGFR leads to recruitment of Cbl which is responsible for the ubiquitination and subsequent degradation of internalized EGFR molecules (34–36). The enhanced tyrosine autophosphorylation and ubiquitination of EGFR observed under conditions in which EGFR endocytosis is strongly compromised suggests that tyrosine phosphatases and deubiquitinating enzymes (DUB) may start to act shortly after activated EGFR become internalized and that Cbl activity is reduced prior to the onset of EGFR degradation by lysosomal enzymes. It is noteworthy that similar results were obtained using HeLa cells in which endocytosis of EGFR was impaired by siRNA silencing of clathrin heavy chain. Namely, cells in which endocytosis is impaired by depletion of clathrin heavy chain expression also exhibit enhanced EGFR autophosphorylation and ubiquitination (Fig. S2). Moreover, similar MAPK stimulation was observed in control cells or in cells in which EGFR endocytosis was impaired by clathrin heavy chain depletion (Fig. S3). Similar EGF stimulation of Map Kinase of ERK Kinase (MEK) and ERK activation was previously described in HeLa cells in which clatharin expression was silenced by specific siRNA (37). These experiments provide further support to the conclusion that MAP kinase stimulation is primarily mediated by activated EGFR located at the plasma membrane.
We have also shown that unaltered tyrosine phosphorylation of the p42 and p52 isoforms of the adapter protein Shc and complex formation between Shc and Grb2 are detected both in control and in dynamin depleted fibroblasts. By contrast, selective tyrosine phosphorylation of the p66 isoform of Shc is detected in dynamin depleted cells in which the majority of activated EGFR are located on the cell membrane.
Surprisingly and in contrast to earlier publications demonstrating that MAPK signaling is compromised when endocytosis was prevented (4, 5), MAP kinase stimulation by EGF was comparable in control and in dynamin depleted cells. Thus, the experiments presented in this report demonstrate that MAP kinase stimulation is driven primarily by activated EGFR located at the plasma membrane rather then by activated EGFR located inside the cell. Although MAP kinase activation is primarily mediated by activated EGFR located on the cell surface, the fact that a similar MAP kinase response is observed in cells in which the majority of EGFR are internalized suggests that the fraction of activated EGFR that are located on the cell surface are capable stimulating the entire MAP kinase response.
Interestingly, EGF stimulation of Akt differs from the MAP kinase response, as we observed a somewhat more sustained Akt stimulation in dynamin depleted fibroblasts stimulated with either low or high EGF concentrations. This experiment suggests that Akt stimulation may be driven both by activated EGFR located at the cell membrane and by activated EGFR located in the membranes of intracellular organelles such as endosomes. These results also suggest, however, that termination of Akt signaling is enhanced by endocytosis of the EGFR.
All in all, while the experiments presented in this report do not rule out the possibility that activated EGFRs are capable of stimulating signaling pathways from within the cells; i.e., from endosomes, it is clear that the MAP kinase response is primarily mediated by activated EGFR located at the cell membrane. Moreover, one of the functions of endocytosis is to suppress EGFR autophosphorylation and ubiquitination which are primarily taking place at the cell membrane likely by reduced action of Cbl and possibly by the action of tyrosine phosphatases and DUB that operate during endocytosis.
Material and Methods
Cells and Culture.
Tamoxifen-inducible dynamin conditional knockout mouse fibroblasts (dnm1flox/flox; dnm2flox/flox; Cre-Esr1+/0) were previously described (38). Mouse fibroblasts were grown in Dulbecco modified Eagle medium (DMEM) containing 10% serum and 1% streptomycin and penicillin mixture. Dynamin depleted fibroblasts were generated by treating the cells twice with 2 μM 4-hydroxy-tamoxifen (Sigma) on sequential days. Six days after initiating tamoxifen treatment, cells were either plated into six-well plates coated with collagen (BD Biosciences) or serum starved overnight prior to EGF stimulation.
125I-Labeled EGF Binding and Internalization Experiments.
Murine EGF (Biomedical Technologies) was labeled with 125I using the Iodogen method (Pierce) according to the manufacturer’s instructions. Tamoxifen treated (DKO) and nontreated (control) cells were plated into six-well plates and allowed to grow overnight. 125I-EGF binding and internalization experiments were performed as previously described (19, 39, 40). For binding experiments, cells were incubated with the indicated concentration of 125I-EGF at room temperature for 1 h in the presence of increasing concentration of nonlabeled EGF; conditions permitting internalization of bound 125I-EGF molecules. Cells were lysed in 0.5 M of NaOH overnight and their radioactive content quantified in 10 mL Optifluor (Perkin Elmer) with a scintillation counter (LS6500, Beckman Coulter). The half time of displacement curves of 125I-EGF binding to control and DKO fibroblasts were calculated by curve fitting using PRISM3 software (GraphPad).
Scatchard analysis of 125I-EGF binding experiments was carried out in triplicate using concentrations of 125I-EGF ranging from 0.1 to 100 ng/mL. A 100-fold excess of nonlabeled EGF was added for each assayed concentration to measure nonspecific binding. The bound radioactivity was quantified as described above. The average values per well for control and DKO cells were determined from two independent counts and used to calculate values of Bmax (number of receptors per cell) using nonlinear curve fitting to saturation binding according to Scatchard analysis as previously described (15, 17).
Quantitative analyzes of EGF internalization were performed by incubating cells with 1.5 or 20 ng/mL of 125I-EGF for 90 min at 4 °C. The labeled cells were then washed twice with cold PBS to remove ligand excess followed by addition of prewarmed medium and incubation at 37 °C for the indicated times. After an acid wash to remove cell surface bound ligand molecules, the cell surface bound and intracellular 125I-EGF molecules were collected for each time point and quantified using a scintillation counter. The incubation medium was precipitated with 10% TCA (tri-chloroacetic acid) in order to quantify the amount of degraded 125I-EGF molecules in the medium. All the results are presented as a percentage of total cell associated 125I-EGF radioactivity after 90 min incubation (time 0) at 4 °C (mean ± SD). To reveal the efficiency of dynamin depletions, cell lysates were collected from control and DKO cells plated in extra wells from each binding and internalization experiment followed by immunoblotting with anti-dynamin or anti-EGFR antibodies.
Immunoblotting and Immunoprecipitation Analysis.
After serum starvation, control or DKO fibroblasts were stimulated with 1.5, 5, 10, or 100 ng/mL of EGF at 37 °C for the indicated time points and collected in lysis buffer (50 mM Hepes, 150 mM NaCL, 1 mM EDTA, 1 mM EGTA, 10% glycerol, 1% TritonX-100, 25 mM NaF, 10 μM ZnCl2, 1 mM NaVO4) that includes a protease inhibitor cocktail (Roche). To carry out the degradation experiments, control and DKO fibroblasts were preincubated prior to ligand stimulation with 10 μM of cycloheximide for 1 h. Identical amounts of total cell lysates were subjected to inmunoprecipitation with anti-EGFR antibodies. The samples were also subjected to immunoblotting analysis with anti-AKT, anti-pAKT (Cell Signaling Technology), anti-ERK, anti-pERK, anti-ubiquitin (Santa Cruz Biotechnology), anti-dynamin (clone 41, BD Biosciences), and anti-4G10, anti-phosphotyrosine antibodies. The anti-EGFR (ab328) antibodies used in the study were generated in our laboratory. Primary antibodies were detected using anti-mouse HRP and Protein A-HRP (Santa Cruz Biotechnology), and visualized by a chemiluminescence kit (Denville Scientific Inc.). Equal loading of proteins analyzed by immunoblotting or immunoprecipitation analyzes were confirmed by reprobing the stripped membranes (0.2 M NaOH, 5 min) with anti-AKT antibodies.
Supplementary Material
Acknowledgments.
The authors thank members of the Schlessinger group for helpful discussion.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1200164109/-/DCSupplemental.
References
- 1.Carpenter G, Cohen S. Epidermal growth factor. Annu Rev Biochem. 1979;48:193–216. doi: 10.1146/annurev.bi.48.070179.001205. [DOI] [PubMed] [Google Scholar]
- 2.Lemmon MA, Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2010;141:1117–1134. doi: 10.1016/j.cell.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sorkin A, Goh LK. Endocytosis and intracellular trafficking of ErbBs. Exp Cell Res. 2009;315:683–696. doi: 10.1016/j.yexcr.2008.07.029. [DOI] [PubMed] [Google Scholar]
- 4.Sigismund S, et al. Clathrin-mediated internalization is essential for sustained EGFR signaling but dispensable for degradation. Dev Cell. 2008;15:209–219. doi: 10.1016/j.devcel.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 5.Vieira AV, Lamaze C, Schmid SL. Control of EGF receptor signaling by clathrin-mediated endocytosis. Science. 1996;274:2086–2089. doi: 10.1126/science.274.5295.2086. [DOI] [PubMed] [Google Scholar]
- 6.Huang F, Khvorova A, Marshall W, Sorkin A. Analysis of clathrin-mediated endocytosis of epidermal growth factor receptor by RNA interference. J Biol Chem. 2004;279:16657–16661. doi: 10.1074/jbc.C400046200. [DOI] [PubMed] [Google Scholar]
- 7.von Zastrow M, Sorkin A. Signaling on the endocytic pathway. Curr Opin Cell Biol. 2007;19:436–445. doi: 10.1016/j.ceb.2007.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sigismund S, et al. Clathrin-independent endocytosis of ubiquitinated cargos. Proc Natl Acad Sci USA. 2005;102:2760–2765. doi: 10.1073/pnas.0409817102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murphy JE, Padilla BE, Hasdemir B, Cottrell GS, Bunnett NW. Endosomes: a legitimate platform for the signaling train. Proc Natl Acad Sci USA. 2009;106:17615–17622. doi: 10.1073/pnas.0906541106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miaczynska M, Pelkmans L, Zerial M. Not just a sink: endosomes in control of signal transduction. Curr Opin Cell Biol. 2004;16:400–406. doi: 10.1016/j.ceb.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 11.Taub N, Teis D, Ebner HL, Hess MW, Huber LA. Late endosomal traffic of the epidermal growth factor receptor ensures spatial and temporal fidelity of mitogen-activated protein kinase signaling. Mol Biol Cell. 2007;18:4698–4710. doi: 10.1091/mbc.E07-02-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hupalowska A, Miaczynska M. The new faces of endocytosis in signaling. Traffic. 2011;3:9–18. doi: 10.1111/j.1600-0854.2011.01249.x. [DOI] [PubMed] [Google Scholar]
- 13.Ferguson S, De Camilli P. Dynamin, a membrane remodeling GTPase. Nat Rev Mol Cell Biol . 2012;13:75–88. doi: 10.1038/nrm3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmid SL, Frolov VA. Dynamin: functional design of a membrane fission catalyst. Annu Rev Cell Dev Biol. 2011;27:79–105. doi: 10.1146/annurev-cellbio-100109-104016. [DOI] [PubMed] [Google Scholar]
- 15.Ferguson SM, et al. Coordinated actions of actin and BAR proteins upstream of dynamin at endocytic clathrin-coated pits. Dev Cell. 2009;17:811–822. doi: 10.1016/j.devcel.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shen H, et al. Constitutive activated Cdc42-associated kinase (Ack) phosphorylation at arrested endocytic clathrin-coated pits of cells that lack dynamin. Mol Biol Cell. 2011;22:493–502. doi: 10.1091/mbc.E10-07-0637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schlessinger J. Allosteric regulation of the epidermal growth factor receptor kinase. J Cell Biol. 1986;103:2067–2072. doi: 10.1083/jcb.103.6.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mattoon D, Klein P, Lemmon MA, Lax I, Schlessinger J. The tethered configuration of the EGF receptor extracellular domain exerts only a limited control of receptor function. Proc Natl Acad Sci USA. 2004;101:923–928. doi: 10.1073/pnas.0307286101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ozcan F, Klein P, Lemmon MA, Lax I, Schlessinger J. On the nature of low- and high-affinity EGF receptors on living cells. Proc Natl Acad Sci USA. 2006;103:5735–5740. doi: 10.1073/pnas.0601469103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ringerike T, et al. High-affinity binding of epidermal growth factor (EGF) to EGF receptor is disrupted by overexpression of mutant dynamin (K44A) J Biol Chem. 1998;273:16639–16642. doi: 10.1074/jbc.273.27.16639. [DOI] [PubMed] [Google Scholar]
- 21.Honegger AM, et al. Point mutation at the ATP binding site of EGF receptor abolishes protein-tyrosine kinase activity and alters cellular routing. Cell. 1987;51:199–209. doi: 10.1016/0092-8674(87)90147-4. [DOI] [PubMed] [Google Scholar]
- 22.Sorkin A, Duex JE. Quantitative analysis of endocytosis and turnover of epidermal growth factor (EGF) and EGF receptor. Current Protocols in Cell Biology. 2010;46:15.14.1–15.14.20. doi: 10.1002/0471143030.cb1514s46. Chapter 15:Unit 15.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kirisits A, Pils D, Krainer M. Epidermal growth factor receptor degradation: an alternative view of oncogenic pathways. Int J Biochem Cell Biol. 2007;39:2173–2182. doi: 10.1016/j.biocel.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 24.Madshus IH, Stang E. Internalization and intracellular sorting of the EGF receptor: a model for understanding the mechanisms of receptor trafficking. J Cell Sci. 2009;122:3433–3439. doi: 10.1242/jcs.050260. [DOI] [PubMed] [Google Scholar]
- 25.Beguinot L, Lyall RM, Willingham MC, Pastan I. Down-regulation of the epidermal growth factor receptor in KB cells is due to receptor internalization and subsequent degradation in lysosomes. Proc Natl Acad Sci USA. 1984;81:2384–2388. doi: 10.1073/pnas.81.8.2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stoscheck CM, Carpenter G. Down regulation of epidermal growth factor receptors: direct demonstration of receptor degradation in human fibroblasts. J Cell Biol. 1984;98:1048–1053. doi: 10.1083/jcb.98.3.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Honegger AM, Schmidt A, Ullrich A, Schlessinger J. Separate endocytic pathways of kinase-defective and -active EGF receptor mutants expressed in same cells. J Cell Biol. 1990;110:1541–1548. doi: 10.1083/jcb.110.5.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ravichandran KS. Signaling via Shc family adapter proteins. Oncogene. 2001;20:6322–6330. doi: 10.1038/sj.onc.1204776. [DOI] [PubMed] [Google Scholar]
- 29.Pelicci G, et al. A family of Shc related proteins with conserved PTB, CH1 and SH2 regions. Oncogene. 1996;13:633–641. [PubMed] [Google Scholar]
- 30.Migliaccio E, et al. Opposite effects of the p52shc/p46shc and p66shc splicing isoforms on the EGF receptor-MAP kinase-fos signalling pathway. EMBO J. 1997;16:706–716. doi: 10.1093/emboj/16.4.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xi G, Shen X, Clemmons DR. p66shc inhibits insulin-like growth factor-I signaling via direct binding to Src through its polyproline and Src homology 2 domains, resulting in impairment of Src kinase activation. J Biol Chem. 2010;285:6937–6951. doi: 10.1074/jbc.M109.069872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johannessen LE, Ringerike T, Molnes J, Madshus IH. Epidermal growth factor receptor efficiently activates mitogen-activated protein kinase in HeLa cells and Hep2 cells conditionally defective in clathrin-dependent endocytosis. Exp Cell Res. 2000;260:136–145. doi: 10.1006/excr.2000.5004. [DOI] [PubMed] [Google Scholar]
- 33.Roepstorff K, Grøvdal L, Grandal M, Lerdrup M, van Deurs B. Endocytic downregulation of ErbB receptors: mechanisms and relevance in cancer. Histochem Cell Biol. 2008;129:563–578. doi: 10.1007/s00418-008-0401-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bertelsen V, Breen K, Sandvig K, Stang E, Madshus IH. The Cbl-interacting protein TULA inhibits dynamin-dependent endocytosis. Exp Cell Res. 2007;313:1696–1709. doi: 10.1016/j.yexcr.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 35.Argenzio E, et al. Proteomic snapshot of the EGF-induced ubiquitin network. Mol Syst Biol. 2011;7:462. doi: 10.1038/msb.2010.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Visser Smit GD, et al. Cbl controls EGFR fate by regulating early endosome fusion. Science Signaling. 2009;2:ra86. doi: 10.1126/scisignal.2000217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Galperin E, Sorkin A. Endosomal targeting of MEK2 requires RAF, MEK kinase activity and clatharin-dependent endocytosis. Traffic. 2008;9:1776–1790. doi: 10.1111/j.1600-0854.2008.00788.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ferguson SM, et al. A selective activity-dependent requirement for dynamin 1 in synaptic vesicle endocytosis. Science. 2007;316:570–574. doi: 10.1126/science.1140621. [DOI] [PubMed] [Google Scholar]
- 39.Yang Y, Yuzawa S, Schlessinger J. Contacts between membrane proximal regions of the PDGF receptor ectodomain are required for receptor activation but not for receptor dimerization. Proc Natl Acad Sci USA. 2008;105:7681–7686. doi: 10.1073/pnas.0802896105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mattoon DR, Lamothe B, Lax I, Schlessinger J. The docking protein Gab1 is the primary mediator of EGF-stimulated activation of the PI-3K/Akt cell survival pathway. BMC Biol. 2004;2:24. doi: 10.1186/1741-7007-2-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.