Abstract
DNA double-strand breaks (DSBs) are essential intermediates in Ig gene rearrangements: V(D)J and class switch recombination (CSR). In contrast to V(D)J recombination, which is almost exclusively dependent on nonhomologous end joining (NHEJ), CSR can occur in NHEJ-deficient cells via a poorly understand backup pathway (or pathways) often termed alternative end joining (A-EJ). Recently, several components of the single-strand DNA break (SSB) repair machinery, including XRCC1, have been implicated in A-EJ. To determine its role in A-EJ and CSR, Xrcc1 was deleted by targeted mutation in the CSR proficient mouse B-cell line, CH12F3. Here we demonstrate that XRCC1 deficiency slightly increases the efficiency of CSR. More importantly, Lig4 and XRCC1 double-deficient cells switch as efficiently as Lig4-deficient cells, clearly indicating that XRCC1 is dispensable for A-EJ in CH12F3 cells during CSR.
A DNA double-strand break (DSB) is one of the most severe forms of DNA damage and can result in chromosome loss or translocations. A variety of endogenous and exogenous sources can induce DSBs, including ionizing radiation, reactive oxygen species, and some chemicals. On the other hand, physiological processes during lymphocyte development such as V(D)J and Ig class switch recombination (CSR) rely on DSBs to rearrange genetic information in somatic cells.
V(D)J recombination is a site-specific DNA recombination initiated by the RAG proteins, which are evolved from an ancient DNA transposase. The RAG complex recognizes specific DNA sequences called recombination signal sequences (RSS) and cuts the DNA on one side of the RSS. The ensuing repair of the four DNA ends that are produced from a pair of cleavage events results in joining of subexonic coding fragments to form an exon encoding the antigen-binding domain of a B- or T-cell receptor. In contrast, CSR in antigen-stimulated mature B cells is a regionally specific recombination between two repetitive regions [called switch (S) regions] that precede each of the constant regions (1). Looping out intervening sequences between two S regions allows the expression of a new constant region that was further downstream and results in a switch of Ig class (or isotype) from IgM to IgG, IgE, or IgA (2). CSR is initiated by activation-induced cytidine deaminase (AID) that converts DNA cytosines into uracils at S regions. Through mechanisms that are not yet fully understood, repair of AID-generated uracils in the S region ultimately results in DSBs (2), which serve as critical intermediates in an overall cut-and-paste chromosomal deletion (2).
In vertebrate cells, DSB repair mechanisms generally fall into two major categories: homologous recombination (HR) and nonhomologous end joining (NHEJ) (3). HR relies on the presence of another copy of DNA sequences that are highly similar to the one harboring the DSB. Copying genetic information from the intact copy allows high-fidelity repair of the DSB. In complex genomes rich in repetitive DNA sequences, HR is restricted to S and G2 phase of the cell cycle when sister chromatids are available as a source of homology. In contrast, NHEJ is the major DSB repair pathway that operates throughout the cell cycle. The core NHEJ components include the Ku70/86 heterodimer that binds to the DNA end, the DNA-dependent protein kinase (DNA-PKcs) that regulates end joining by phosphorylating other proteins (including itself), and the ligase complex containing XLF, XRCC4, and DNA ligase 4. Also involved is a growing list of auxiliary factors, including end processing nucleases (e.g., Artemis) and polymerases (μ and λ), polynucleotide kinases, 53BP1, and many DNA damage response proteins (ATM, H2AX, Chk1, etc.).
Although both V(D)J and class switch recombination rely on the generation and repair of DSBs, the dependence on NHEJ is distinctively different between these two reactions. Whereas RAG-generated DSBs are almost exclusively joined by NHEJ, S region breaks can be joined in NHEJ-deficient cells at a reduced but still considerable rate (4–6). DSB repair in the absence of an intact NHEJ system has been collectively termed alternative end joining (A-EJ) (3, 4). A-EJ could be a component-substitution form of NHEJ or a distinct pathway (or pathways) (7–9). So far, components of A-EJ have not been conclusively defined. A-EJ has attracted much research attention recently because of its implication in chromosomal translocations that could lead to oncogenic transformations (10). Many translocation junctions have microhomology (DNA sequences that can be assigned to either of the two ends), which is characteristic of A-EJ. A-EJ is sometimes called microhomology-mediated end joining (9). However, the presence of microhomology at the junction is not a criterion to distinguish A-EJ from NHEJ, as NHEJ also prefers short homology between the two ends (9, 11, 12).
The final stage of DSB repair depends on DNA ligases. Vertebrates have three ATP-dependent ligases (I, III, and IV) (13). Lig4 appears to be dedicated to NHEJ as no other function has been described for Lig4 outside the NHEJ realm. XRCC4 complexes with and stabilizes Lig4. Deficiency of either Lig4 or XRCC4 essentially abolishes NHEJ. Conceivably, joining of S region breaks in Lig4-deficient cells must depend on Lig1 and/or Lig3. Normally, Lig1 complexes with proliferating cell nuclear antigen and is recruited to the replication fork to join Okazaki fragments during DNA replication (13). Lig3 complexes with XRCC1 and was generally considered the ligase involved in single-strand break (SSB) repair pathways. It has been shown that cellular Lig3 activity is dependent on XRCC1 (14), which is a scaffold protein that interacts with many other DNA repair factors (e.g., Parp1, Pol β, APE1, PNKP, aprataxin, etc.) (13). Although the traditional view of Lig3 in nuclear DNA repair has been recently challenged (15, 16), the importance of XRCC1 in SSB repair has been well established.
Because A-EJ relies on microhomology and because both Lig1 and Lig3 are SSB ligases, we considered the possibility that A-EJ results from a pair of XRCC1-dependent SSB ligations at DNA ends containing long nucleotide overlaps. In addition, XRCC1 has recently been implicated in A-EJ (17, 18), along with other SSB repair factors (19). To assess the role of XRCC1 in CSR and A-EJ, we deleted Xrcc1 in wild-type and Lig4-deficient CH12F3 cells by targeted mutation. Here we demonstrate that XRCC1 deficiency alone slightly enhances the efficiency of CSR. More importantly, deletion of both Lig4 and Xrcc1 genes does not abolish CSR. In fact, cells deficient in both XRCC1 and Lig4 switch as efficiently, or slightly better than, Lig4-deficient cells. These data demonstrate unequivocally that XRCC1 is dispensable for A-EJ during CSR.
Results and Discussion
Gene Targeting of XRCC1.
XRCC1 is a DNA repair factor that has recently been implicated as a component of A-EJ (17, 18). Because XRCC1 is essential for mouse embryonic development (20), there is a lack of direct genetic evidence for its involvement in CSR and A-EJ. XRCC1-deficient Chinese hamster ovary cell lines are viable (21), suggesting that XRCC1 is dispensable for somatic cell growth. We therefore attempted gene targeting of Xrcc1 in a mouse B-cell line (CH12F3) that is capable of robust cytokine-dependent CSR. DNA sequences flanking exons 4–6 of Xrcc1 were amplified from CH12F3 genomic DNA and used as homology blocks for gene targeting (Fig. 1A). In successfully targeted clones, exons 4–6 are replaced with a floxed puromycin-resistant gene expression cassette that can be excised by Cre-LoxP reaction (Fig. 1 A and B). Removal of exons 4–6 results in a frameshift of all downstream exons and yields a null allele as evidenced by the complete lack of XRCC1 as shown by Western blot analysis (Fig. 1C). In contrast to XRCC1 haplodeficient mouse primary B cells (18), deletion of one copy of Xrcc1 in CH12F3 cells does not alter XRCC1 protein levels. Although there is a moderate reduction of Lig3 protein in XRCC1Δ/Δ cells (consistent with a role of XRCC1 in stabilizing Lig3) (14), Lig3 protein levels in XRCC1+/Δ cells (Fig. 1C) are indistinguishable from XRCC1+/+ cells, consistent with the lack of effect haplodeficiency has on XRCC1 expression levels in these cells.
Fig. 1.
Gene targeting of Xrcc1. (A) Genomic organization of the wild-type and targeted Xrcc1 loci. Gray boxes indicate exons. Open block arrow (Puro) indicates expression cassette of puromycin-resistant gene. Shaded block arrow (DTA) indicates diphtheria toxin A chain. BamH I restriction sites are indicated by “B.” Probes used in Southern blot analysis are depicted at the Top. Plus indicates wild-type allele. P and Δ indicate targeted allele with or without the puro cassette, respectively. (B) Southern blot analysis of BamH I-digested genomic DNA from wild-type and targeted cells. Genotypes, sizes of bands, and probes are indicated. (C) Western blot analysis of protein expression in wild type (WT), XRCC1 haplodeficient (+/Δ), and XRCC1-deficient (Δ/Δ) cells. The same blot was stripped and reprobed with different monoclonal antibodies as indicated.
Slightly Increased CSR in XRCC1-Deficient Cells.
To determine whether XRCC1 is involved in CSR, cell growth and CSR efficiency were compared between XRCC1 proficient (+/Δ) and deficient (Δ/Δ) cells. XRCC1Δ/Δ cells grow more slowly than wild-type or XRCC1+/Δ cells, as assessed by live cell counting, regardless of the presence or absence of cytokine stimulation (Fig. 2A). We considered that the apparent slow growth of XRCC1Δ/Δ cells might be attributable to increased cell death because of the inability of XRCC1Δ/Δ cells to repair oxidation-associated DNA damage. However, shifting cells from 20% oxygen to 3% only partially improve the growth of XRCC1Δ/Δ cells.
Fig. 2.
Proliferation and CSR of XRCC1-proficient and -deficient cells. (A) Propagation of live cells (trypan exclusion) over a span of 3 d in unstimulated (−CIT) or stimulated (+CIT) cultures for wild-type (WT), XRCC1-proficient (XRCC1+/Δ), and -deficient (XRCC1Δ/Δ) cells, respectively. Error bars indicate SD of three independent experiments. (B) Representative FACS analysis of CSR by surface staining of IgA after 72 h of growth in the absence or presence of cytokines (CIT). Numbers in boxed areas indicate percentages.
Although the mechanism underlying the slow growth of XRCC1Δ/Δ cells has not been determined, it is unlikely that XRCC1Δ/Δ cells have an intrinsic proliferation defect because XRCC1Δ/Δ cells undergo robust CSR (Fig. 2B), which is known to be cell-proliferation dependent. In fact, XRCC1Δ/Δ cells switch more efficiently than XRCC1+/Δ cells (Figs. 2B and 3D). The increase in CSR efficiency in the absence of XRCC1 is small (∼19%), but consistent (P = 0.014). The mechanism by which XRCC1 deficiency promotes CSR efficiency is unknown. One possible explanation is that XRCCΔ/Δ cells accumulate more SSBs in S regions that enhance the chance of DSB formation. In this regard, our data are also consistent with previous reports regarding an inhibitory effect on CSR by several SSB repair factors (22, 23).
Fig. 3.
CSR in cells deficient in both XRCC1 and Lig4. (A) Western blot analysis of protein expression. (Lane 1) Wild type; (lane 2) XRCC1Δ/Δ; (lane 3) Lig4Δ/Δ; (lane 4) Lig4Δ/Δ XRCC1Δ/Δ. The same blot was stripped and reprobed with different antibodies as indicated. (B) Propagation of live cells (trypan exclusion) over a span of 3 d in unstimulated (−CIT) or stimulated (+CIT) cultures. Error bars indicate SD of three independent experiments. (C) Representative FACS analysis of CSR by surface staining of IgM (PE labeled) and IgA (FITC labeled) after 72 h growth in the absence or presence of cytokines (CIT). Numbers in boxed areas indicate percentages. (D) Relative CSR efficiency of various genotypes. Error bars indicated SD of at least three independent experiments (wild-type CSR efficiency set to 100%). P value was calculated by two-tailed paired t test. Comparison between XRCC1+/Δ and XRCC1Δ/Δ is based on five independent experiments.
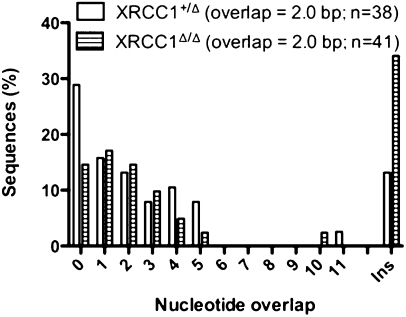
To determine whether XRCC1 deficiency affects switch junction microhomology, postswitched clones of XRCC1+/Δ and XRCC1Δ/Δ cells were isolated and each junction was amplified individually (Fig. S1). The average nucleotide overlap is 2 bp regardless of the genotype, and there is no obvious difference with regard to switch junction microhomology (Fig. 4). This observation is consistent with a recent report from Boboila et al. (24), but is at odds with another study that showed a slight decrease of nucleotide overlaps from 2.5 bp in wild-type to 1.7 bp in XRCC1 haplodeficient cells (18).
Fig. 4.
Length of microhomology in switch junctions. Percentage of switch junctions with the indicated nucleotide overlap (excluding nucleotide additions).
In this study, XRCC1Δ/Δ junctions have more insertions; this modest effect must be interpreted cautiously. In these analyses, it is difficult to differentiate a 1-bp insertion from a point mutation. There are six 1-bp insertions in the XRCC1Δ/Δ group compared with one in the XRCC1+/Δ group. In addition, junction 10 in the XRCC1Δ/Δ group also has this ambiguity (insertion vs. mutation), and junction 19 could have an insertion or a small deletion in the Sα nearby the junction. These ambiguities are intrinsic in analyzing switch junction; this is even more problematic when analyzing junctions between highly homologous S regions, as is the case in our study. Although XRCC1 deficiency might minimally alter the frequency of mutations (18) or small deletions/insertions because of its role in single-strand break repair, these data suggest that this is not a major affect.
CSR in XRCC1 and Lig4 Double-Deficient Cells.
XRCC1 was recently implicated as a component of A-EJ (17, 18). If that were the case, deletion of Xrcc1 in NHEJ-deficient cells (e.g., Lig4Δ/Δ) should inhibit CSR. To test this hypothesis, we targeted Xrcc1 in Lig4-deficient cells (Lig4Δ/Δ) (5). Lack of both Lig4 and XRCC1 protein in XRCC1Δ/Δ Lig4Δ/Δ cells was confirmed by Western blot analysis (Fig. 3A). XRCC1Δ/Δ Lig4Δ/Δ cultures grow considerably slower than XRCC1Δ/Δ cultures based on live cell counting (Fig. 3B). Consistent with previous studies (4, 5), CSR in Lig4Δ/Δ cells is reduced to 25–40% compared with wild-type cells after 3 d of cytokine stimulation. As can be seen, deletion of Xrcc1 in Lig4Δ/Δ cells does not further reduce CSR efficiency (Fig. 3 C and D); we concluded that XRCC1 is dispensable for A-EJ. In fact, CSR efficiency in XRCC1Δ/Δ Lig4Δ/Δ cells is slightly higher than in cells deficient in only Lig4 (Fig. 3 C and D), consistent with our earlier observation that XRCC1 deficiency modestly promotes CSR. Staining for both IgM and IgA isotypes revealed a population of double-negative (IgM−IgA−) cells (Fig. 3C). These cells are likely the ones that are undergoing CSR because they are more prominent in Lig4-deficienct cells (Fig. 3C), consistent with our previous observations that CSR in Lig4-deficient cells is kinetically slower than in wild-type cells. This double-negative population is even more exaggerated in XRCC1Δ/Δ Lig4Δ/Δ cells, which raises the possibility that some of these cells harbor large deletions due to the excessive DNA damage in the absence of XRCC1 and insufficient end joining in the absence of Lig4. This possibility might explain our difficulty in obtaining switch junctions from this genotype.
To rule out the possibility that the observed effects were due to a clonal variation, genetic complementation experiments were carried out to transduce Lig4 or Xrcc1 cDNA into XRCC1Δ/Δ Lig4Δ/Δ cells by recombinant retroviruses. Expression of Lig4 or XRCC1 from transduced cDNA was confirmed by Western blot analysis (Fig. 5A). As expected, complementation by Lig4 cDNA restored robust CSR (Fig. 5B). In contrast, transduction with Xrcc1 cDNA resulted in slightly diminished CSR efficiency compared with an empty virus control (Fig. 5B), consistent with our earlier observations.
Fig. 5.
Genetic complementation of Lig4Δ/Δ XRCC1Δ/Δ cells. (A) Western blot analysis of protein expression in wild-type (WT) cells and Lig4Δ/Δ XRCC1Δ/Δ cells infected with retroviruses carrying Lig4, XRCC1, or no cDNA, as indicated. The same blot was stripped and reprobed with different antibodies as indicated. (B) Representative FACS analysis of CSR by surface staining of IgM (PE labeled) and IgA (FITC labeled) after 72 h growth in the absence or presence of cytokines (CIT). Numbers in boxed areas indicate percentages.
A recent study implied that XRCC1 might be involved in A-EJ during CSR. The implication was based on a small decrease of switch junction microhomology between wild-type and XRCC1 haplodeficient primary B cells (18); however, CSR efficiency was not affected by XRCC1 haplodeficiency. Our study reaches a different conclusion. Here, we have introduced targeted deletions of Xrcc1 on both alleles in CH12F3 cells; we find (unequivocally) that XRCC1 is not required for CSR efficiency and does not affect the fine structure of joined switch regions, strongly arguing that XRCC1 is also dispensable for A-EJ. Moreover, these conclusions are in excellent agreement with those in a recent study from Alt and colleagues who also disrupted both alleles of Xrcc1 in switching B cells (24).
The moderate increase in CSR efficiency in the absence of XRCC1 suggests that accumulation of unrepaired SSBs in S regions promotes CSR, perhaps by increasing the chance of DSBs. Our data do not rule out a possible role of Lig3 in A-EJ and CSR. However, given the recent findings regarding the nonessential role of Lig3 in nuclear DNA repair (15, 16) and the dispensability of its cofactor XRCC1 in A-EJ shown in this study, it seems more likely that Lig1 is the ligase that operates in A-EJ in the absence of intact NHEJ. Alternatively, both Lig1 and Lig3 may participate in A-EJ, and elimination of either one alone may not reveal a defect.
Materials and Methods
Reagents.
Lig4 antibody was kindly provided by David Schatz (Yale University, New Haven, CT). Lig3 antibody was purchased from BD Biosciences (611876). XRCC1 antibody was purchased from Abcam (ab1838). Antibody against mouse β-actin was purchased from Santa Cruz (sc-47778).
Cell Culture and CSR Assay.
CH12F3 cells were cultured in RPMI1640 medium supplemented with 10% (vol/vol) FBS and 50 μM of β-mercaptoethanol. For CSR assay, healthy CH12F3 cells were seeded at 5 × 104 cells/mL in the presence of 1 μg/mL anti-CD40 antibody (eBioscience; 16–0402-86), 5 ng/mL of IL-4 (R&D Systems; 404-ML), and 0.5 ng/mL TGF-β1 (R&D Systems; 240-B) and grown for 72 h. Cells were stained with a FITC-conjugated antimouse IgA antibody (BD Biosciences; 559354) and analyzed on a flow cytometer (LSR II; BD Biosciences). CSR efficiency was determined as the percentage of IgA+ cells.
XRCC1 Gene Targeting.
Two homology blocks (2.1 and 5.8 kb) were PCR amplified from CH12F3 genomic DNA and used as homology blocks for gene targeting. Gene targeting procedures have been reported previously (5, 25, 26).
Genetic Complementation.
Lig4 or XRCC1 coding region sequences were cloned into the retroviral transfer vector pMSCV-puro (Clontech Laboratories). Recombinant retroviruses were packaged in the Phoenix cell line. Viral supernatants were harvested 48 h after transfection and used to infect XRCC1Δ/Δ Lig4Δ/Δ cells. Infected cells were selected by puromycin and subjected to CSR assays.
Switch Junction Analysis.
Individual IgA+ clones were isolated by limiting dilutions of cytokine-stimulated cultures in 96-well plates. Switch junctions were amplified with primers KY761 (5′-AACTCTCCAGCCACAGTAATGACC-3′) and KY743 (5′-GAGCTCGTGGGAGTGTCAGTG-3′). PCR products were sequenced at the genomic core facility at Michigan State University.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grant R01 AI081817 (to K.Y.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1120743109/-/DCSupplemental.
References
- 1.Lieber MR. Site-specific recombination in the immune system. FASEB J. 1991;5:2934–2944. doi: 10.1096/fasebj.5.14.1752360. [DOI] [PubMed] [Google Scholar]
- 2.Yu K, Lieber MR. Nucleic acid structures and enzymes in the immunoglobulin class switch recombination mechanism. DNA Repair (Amst) 2003;2:1163–1174. doi: 10.1016/j.dnarep.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 3.Lieber MR. The mechanism of double-strand DNA break repair by the nonhomologous DNA end-joining pathway. Annu Rev Biochem. 2010;79:181–211. doi: 10.1146/annurev.biochem.052308.093131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yan CT, et al. IgH class switching and translocations use a robust non-classical end-joining pathway. Nature. 2007;449:478–482. doi: 10.1038/nature06020. [DOI] [PubMed] [Google Scholar]
- 5.Han L, Yu K. Altered kinetics of NHEJ and CSR in DNA ligase IV-deficient B cells. J Exp Med. 2008;205:2745–2753. doi: 10.1084/jem.20081623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Soulas-Sprauel P, et al. Role for DNA repair factor XRCC4 in immunoglobulin class switch recombination. J Exp Med. 2007;204:1717–1727. doi: 10.1084/jem.20070255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boboila C, et al. Alternative end-joining catalyzes robust IgH locus deletions and translocations in the combined absence of ligase 4 and Ku70. Proc Natl Acad Sci USA. 2010;107:3034–3039. doi: 10.1073/pnas.0915067107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boboila C, et al. Alternative end-joining catalyzes class switch recombination in the absence of both Ku70 and DNA ligase 4. J Exp Med. 2010;207:417–427. doi: 10.1084/jem.20092449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lieber MR. The mechanism of human nonhomologous DNA end joining. J Biol Chem. 2008;283:1–5. doi: 10.1074/jbc.R700039200. [DOI] [PubMed] [Google Scholar]
- 10.Nussenzweig A, Nussenzweig MC. A backup DNA repair pathway moves to the forefront. Cell. 2007;131:223–225. doi: 10.1016/j.cell.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 11.Grawunder U, Zimmer D, Fugmann S, Schwarz K, Lieber MR. DNA ligase IV is essential for V(D)J recombination and DNA double-strand break repair in human precursor lymphocytes. Mol Cell. 1998;2:477–484. doi: 10.1016/s1097-2765(00)80147-1. [DOI] [PubMed] [Google Scholar]
- 12.Gerstein RM, Lieber MR. Extent to which homology can constrain coding exon junctional diversity in V(D)J recombination. Nature. 1993;363:625–627. doi: 10.1038/363625a0. [DOI] [PubMed] [Google Scholar]
- 13.Ellenberger T, Tomkinson AE. Eukaryotic DNA ligases: Structural and functional insights. Annu Rev Biochem. 2008;77:313–338. doi: 10.1146/annurev.biochem.77.061306.123941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caldecott KW, Tucker JD, Stanker LH, Thompson LH. Characterization of the XRCC1-DNA ligase III complex in vitro and its absence from mutant hamster cells. Nucleic Acids Res. 1995;23:4836–4843. doi: 10.1093/nar/23.23.4836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simsek D, et al. Crucial role for DNA ligase III in mitochondria but not in Xrcc1-dependent repair. Nature. 2011;471:245–248. doi: 10.1038/nature09794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gao Y, et al. DNA ligase III is critical for mtDNA integrity but not Xrcc1-mediated nuclear DNA repair. Nature. 2011;471:240–244. doi: 10.1038/nature09773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Della-Maria J, et al. Human Mre11/human Rad50/Nbs1 and DNA ligase IIIalpha/XRCC1 protein complexes act together in an alternative nonhomologous end joining pathway. J Biol Chem. 2011;286:33845–33853. doi: 10.1074/jbc.M111.274159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saribasak H, et al. XRCC1 suppresses somatic hypermutation and promotes alternative nonhomologous end joining in Igh genes. J Exp Med. 2011;208:2209–2216. doi: 10.1084/jem.20111135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robert I, Dantzer F, Reina-San-Martin B. Parp1 facilitates alternative NHEJ, whereas Parp2 suppresses IgH/c-myc translocations during immunoglobulin class switch recombination. J Exp Med. 2009;206:1047–1056. doi: 10.1084/jem.20082468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tebbs RS, et al. Requirement for the Xrcc1 DNA base excision repair gene during early mouse development. Dev Biol. 1999;208:513–529. doi: 10.1006/dbio.1999.9232. [DOI] [PubMed] [Google Scholar]
- 21.Thompson LH, West MG. XRCC1 keeps DNA from getting stranded. Mutat Res. 2000;459:1–18. doi: 10.1016/s0921-8777(99)00058-0. [DOI] [PubMed] [Google Scholar]
- 22.Wu X, Stavnezer J. DNA polymerase beta is able to repair breaks in switch regions and plays an inhibitory role during immunoglobulin class switch recombination. J Exp Med. 2007;204:1677–1689. doi: 10.1084/jem.20070756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shockett P, Stavnezer J. Inhibitors of poly(ADP-ribose) polymerase increase antibody class switching. J Immunol. 1993;151:6962–6976. [PubMed] [Google Scholar]
- 24.Boboila C, et al. Robust chromosomal DNA repair via alternative end-joining in the absence of X-ray repair cross-complementing protein 1 (XRCC1) Proc Natl Acad Sci USA. 2012;109:2473–2478. doi: 10.1073/pnas.1121470109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Han L, Masani S, Yu K. Cutting edge: CTNNBL1 is dispensable for Ig class switch recombination. J Immunol. 2010;185:1379–1381. doi: 10.4049/jimmunol.1001643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Han L, Masani S, Yu K. Overlapping activation-induced cytidine deaminase hotspot motifs in Ig class-switch recombination. Proc Natl Acad Sci USA. 2011;108:11584–11589. doi: 10.1073/pnas.1018726108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.