Abstract
The coevolution of female mate preferences and exaggerated male traits is a fundamental prediction of many sexual selection models, but has largely defied testing due to the challenges of quantifying the sensory and cognitive bases of female preferences. We overcome this difficulty by focusing on postcopulatory sexual selection, where readily quantifiable female reproductive tract structures are capable of biasing paternity in favor of preferred sperm morphologies and thus represent a proximate mechanism of female mate choice when ejaculates from multiple males overlap within the tract. Here, we use phylogenetically controlled generalized least squares and logistic regression to test whether the evolution of female reproductive tract design might have driven the evolution of complex, multivariate sperm form in a family of aquatic beetles. The results indicate that female reproductive tracts have undergone extensive diversification in diving beetles, with remodeling of size and shape of several organs and structures being significantly associated with changes in sperm size, head shape, gains/losses of conjugation and conjugate size. Further, results of Bayesian analyses suggest that the loss of sperm conjugation is driven by elongation of the female reproductive tract. Behavioral and ultrastructural examination of sperm conjugates stored in the female tract indicates that conjugates anchor in optimal positions for fertilization. The results underscore the importance of postcopulatory sexual selection as an agent of diversification.
Keywords: ornaments, sperm competition, heteromorphism, genitalia, spermatheca
Darwin attributed the evolution of many elaborate male traits to selection exerted by female mate discrimination (1). Female choosiness remains a foundation of sexual selection theory, with most models predicting a pattern of coevolution between female preference and exaggerated male traits (2). The role of cognition, however, renders preferences notoriously difficult to quantify, with constraints on the timing of reproduction, risks associated with mate evaluation, and environmental influences on female perception of mate quality further complicating matters (3). Consequently, few studies have attempted to test macroevolutionary patterns of codiversification of female preference and male traits, and those that do have very limited taxon sampling (4, 5).
As with male traits important for mate choice, some sperm attributes exhibit high levels of morphological variation within species (6, 7) and dramatic divergence among species (7). This variation has been widely attributed to postcopulatory sexual selection (8–11), occurring whenever females mate with multiple males within a breeding cycle (12). Experimental and comparative evidence indicates that female reproductive tract architecture can influence competitive male fertilization success and generate selection on sperm form (2, 13–16), thus representing the proximate basis of female sperm choice (17). Reproductive tract dimensions are easily quantifiable and, because they are relatively invariant over the reproductive lifespan of a female, represent a consistent female preference unaffected by external environmental conditions.
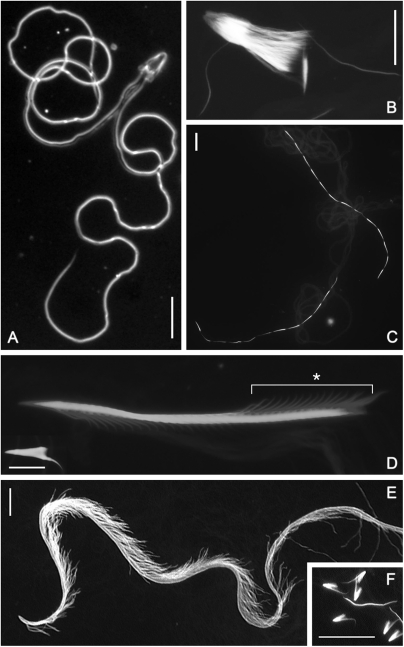
Comparative analyses of diverse taxa [e.g., beetles (18, 19), birds (20), flies (21–24), mammals (25), moths (26), and snails (27)] have revealed a widespread pattern of correlated morphological evolution between sperm and the female tract (but see refs. 28, 29). These studies have primarily explored a single axis of variation: sperm length and the length of the female sperm-storage organ(s) or its duct, whereas sperm and female reproductive tracts can differ among species in a multitude of ways (7, 15). For example, our comparative investigations of sperm form in diving beetles (Dytiscidae) have revealed an astonishing diversity including (i) total length (128–4,493 μm), (ii) head shape, (iii) flagellum length, (iv) length and head shape dimorphism (e.g., Fig. 1 B and F), and (v) conjugation (30). Conjugation is an unusual, yet taxonomically widespread, phenomenon in which two or more sperm physically unite for motility or transport through the female reproductive tract (31). Among diving beetle species with conjugation, the size and organization of conjugates vary greatly and include at least three distinct forms: (i) pairs (Fig. 1A), (ii) aggregates (Fig. 1B), and (iii) orderly stacks of sperm called rouleaux (Fig. 1 C–E) (30). Finally, although the evolutionary origins of sperm dimorphism and conjugation are independent across the diving beetle lineage (Fig. 2), the two character states sometimes co-occur (Fig. 1 B and E) (30).
Fig. 1.
Types of sperm conjugation. Diving beetles exhibit three forms of conjugation: (i) pairing with heads tightly bound along corresponding sides; (ii) aggregations of varying size (typically <25) with heads in register; and (iii) sperm stacked into structures called rouleaux, where the tip of one head slips into a hollow pocket at the base of the head of another sperm cell and results in conjugates with greater total length than the sperm they contain (up to three times longer; Dataset S1). (A) Sperm pairing of Graphoderus liberus. (B) Aggregate, sperm dimorphic (sperm with broad, flattened heads on the interior, surrounded by filamentous headed sperm) conjugate of Ilybius oblitus. (C) Sperm rouleaux of Uvarus lacustris. (D) Composite image of a single sperm head (Lower Left corner) and a rouleau of Neoporus undulatus. Sperm heads stack tightly with basal spurs exposed (indicated by *). (E) Rouleau-type sperm dimorphic conjugate of Hygrotus sayi. (F) Dimorphic sperm heads of H. sayi. Sperm with broad heads and basal spurs stack to form the scaffolding to which sperm with filamentous heads attach (E). (A) Darkfield microscopy; heads and flagella visible. (B–F) Epifluorescence microscopy with only DNA-stained heads visible. (Scale bars, 20 μm.)
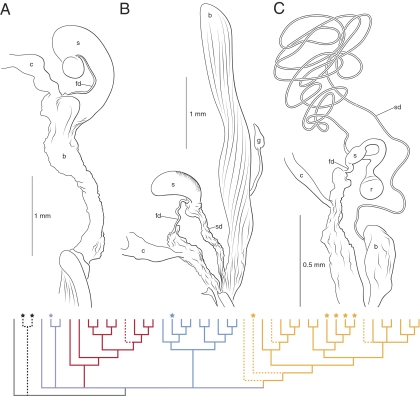
Fig. 2.
Phylogeny and representatives of three basic designs of female reproductive tracts. Female diving beetles have “conduit”-type reproductive tracts: sperm enter and exit storage through different ducts. (A) Large spermatheca without a distinct spermathecal duct; G. liberus. (B) Clearly defined spermathecal duct, spermatheca, and fertilization duct; Rhantus binotatus. (C) Typically narrowed and lengthened spermathecal ducts and, in some species, the addition of a receptacle; Nebrioporus rotundatus. b, bursa; c, common oviduct; fd, fertilization duct; g, gland; r, receptacle; s, spermatheca; sd, spermathecal duct. Colored branches indicate in-group taxa (see Fig. S1 for branch support). Clade 1 (red) is characterized by species with paired sperm and large sperm-storage organs (type A). Clade 2 (blue) contains species with paired sperm or larger aggregate–type conjugates and type A or B female tracts. Clade 3 (yellow) is characterized by sperm that form rouleaux and type C tracts. Dashed lines indicate species where sperm do not conjugate and stars show species with sperm dimorphism. Out-group taxa are shown in black or gray. Gray is used where sperm data are missing.
Although sperm competition has not been confirmed in any species of diving beetle, several lines of evidence suggest that sexual selection has been important during the evolutionary history of this lineage and might have contributed to diversification of sperm form. First, males of some species invest heavily in sperm production (up to 13% of total body mass in Dytiscus sharpi) (32). Second, males of numerous species display behavioral adaptations to reduce sperm competition (i.e., mate guarding and mating plugs) (32–35). Third, comparative studies have identified coevolutionary arms races between female mating resistance and male persistence traits (36, 37), consistent with a history of polyandry. Female diving beetles have “conduit”-type reproductive tracts where sperm enter and exit through separate ducts (Fig. 2). If females do mate multiply, such reproductive tract architecture might favor sperm that can maintain position or displace rival sperm near the site of fertilization (38).
Here we investigate whether the evolution of female reproductive tract design might have driven the evolution of such complex sperm forms (i.e., female preference–male ornament correlated evolution) by quantifying female tract morphology for 42 species of diving beetles. We then used phylogenetically controlled generalized least squares (39) and logistic regression (40) to explore potential coevolutionary relationships with sperm form (30). Additionally, we used Bayesian methods (41) to infer the probable sequence of sperm and female character transitions to test the prediction that changes in female preference precede those of male traits and subsequently trigger diversification of male reproductive characters.
Results
Female Reproductive Tract and Individual Sperm Traits.
With the exception of the fertilization duct, we found that any of the main features of the female tract (i.e., the spermathecal duct, spermatheca, or receptacle; Fig. 2) may be absent or highly elaborated, with dimensions of every component varying substantially among species (e.g., spermathecal ducts; Fig. 2 and Dataset S1). Correlations between sperm and female reproductive tract traits suggest that either they are evolving in response to a common selective force or that one trait exerts selection on the other. Across the entire diving beetle lineage, the length of individual sperm was only associated with the presence of a female receptacle (an organ of unknown function that sometimes contains sperm and thus might act as a secondary sperm-storage organ, Table 1 and Fig. 2C). Of the species possessing receptacles (n = 11), sperm length was positively correlated with the smallest dimension of the organ and negatively correlated with the largest dimension (Table 1). Additionally, in species where males produce two distinct types of sperm (e.g., Fig. 1 B, E, and F), both sperm morphs are transferred to females, but on the basis of findings in other insect species (23, 26, 42), only the long morph is expected to participate in fertilization. We found that neither the presence of dimorphism, nor the length of the short sperm morph was correlated with any aspect of female morphology (P > 0.05).
Table 1.
Results from generalized least squares stepwise multiple regression
| Trait | Taxa | R2 | F | df | p | Predictors | Coefficient | t | p |
| Sperm length | Dytiscidae | 0.09 | 3.76 | 1,40 | 0.06 | Presence of a receptacle | + | 1.94 | 0.06 |
| Sperm length | Species with receptacles | 0.52 | 4.32 | 2,8 | 0.05 | Receptacle min width | + | 2.94 | 0.02 |
| Receptacle max width | − | 2.57 | 0.03 | ||||||
| Sperm length | Clade 1 | 0.65 | 14.97 | 1,8 | <0.01 | Body size | + | 3.87 | <0.01 |
| Sperm length | Clade 2 | 0.92 | 17.43 | 3,5 | <0.01 | Fertilization duct length | + | 6.43 | 0.001 |
| Spermathecal length | + | 3.84 | 0.01 | ||||||
| Spermathecal area | − | 3.79 | 0.01 | ||||||
| Sperm length | Clade 3 | 0.54 | 6.33 | 3,16 | <0.01 | Spermathecal length | − | 3.98 | 0.001 |
| Spermathecal area | + | 1.19 | 0.25 | ||||||
| Interaction | − | 3.36 | <0.01 | ||||||
| Head length | Clade 2 | 0.75 | 21.13 | 1,7 | <0.01 | Fertilization duct length | + | 4.62 | <0.01 |
| Conjugate length | Species with receptacles | 0.84 | 15.3 | 2,6 | <0.01 | Receptacle min width | + | 5.52 | 0.002 |
| Receptacle max width | − | 4.95 | 0.003 | ||||||
| Conjugate length | Clade 3 | 0.75 | 15.12 | 3,15 | <0.001 | Spermathecal length | − | 6.02 | <0.001 |
| Spermathecal area | + | 3.06 | <0.01 | ||||||
| Interaction | − | 4.93 | <0.001 | ||||||
| Conjugate head length | Clade 3 | 0.63 | 12.87 | 2,15 | <0.001 | Spermathecal duct max width | + | 5.07 | <0.001 |
| Body size | − | 2.72 | 0.02 |
Body size and dimensions of fourteen measures of female reproductive tract morphology were considered: presence/absence of a spermathecal duct; length, minimum and maximum width of the spermathecal duct; presence/absence of a receptacle; length, area, minimum and maximum width of the receptacle when present; spermathecal length, area, minimum and maximum width; and fertilization duct length. Sperm length equals that of the long sperm morph in instances of sperm dimorphism. All variables were log transformed.
We also performed separate analyses on each of the three major subclades in our phylogeny because (i) lineage-wide analyses of correlated trait evolution can obscure important relationships when these differ in direction and/or magnitude among sublineages (43), (ii) there were qualitative among-clade differences in female tract and sperm design (Fig. 2), and (iii) there is uncertainty of evolutionary relationships in the basal branches of the diving beetle lineage (Fig. S1). Within clades, variation in female reproductive tract form further explained a significant amount of the interspecific variation in sperm length and head length (Table 1). In two of the three major clades in our phylogeny, dimensions of the spermatheca and/or fertilization duct explained 92% (clade 2) and 54% (clade 3) of the variation in sperm length. In clade 1, sperm length was associated only with body size (clade 1 shows comparatively little variation in sperm and reproductive tract dimensions relative to clades 2 and 3; e.g., sperm length ranges from 177–283 μm in clade 1 to 298–1,965 and 241–3,581 μm in clades 2 and 3, respectively; see Dataset S1). Sperm head length, width, and basal spur length were not associated with dimensions of the female tract in clades 1 and 3, but head length showed strong positive correlation with fertilization duct length in clade 2.
Conjugation and the Sequence of Transitions in Sperm and Female Form.
Similar to individual sperm morphology, correlations between conjugate form and female reproductive tracts suggest that these traits functionally interact and that one may exert selection on the other. Because the formation of rouleaux results in conjugates longer than the individual sperm they contain, we examined the relationship between conjugate length (the distance from the tip of the conjugate to the end of the tails; only differs from sperm length in clade 3) and female reproductive tract dimensions. This approach increased the variation in sperm unit length explained by spermathecal morphology to 75% in clade 3 (Table 1). We also found a strong relationship between the total length of heads in a conjugate (distance from the first to the last sperm head in a rouleau) and both the maximum width of the spermathecal duct and body size in clade 3.
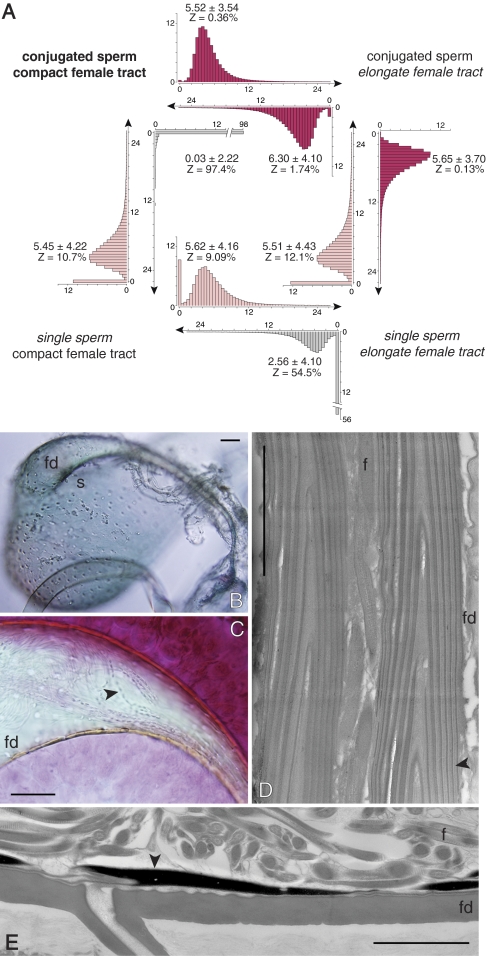
Results of logistic regression revealed that sperm conjugation was significantly explained by the presence of compact female reproductive tracts (i.e., relatively short fertilization ducts (standardized mean coefficient −1.55, bootstrapped 95% CI: −3.70 to −0.20, P = 0.03) and round spermathecae (i.e., negatively associated with spermathecal length, −2.20, 95% CI: −5.86 to −0.15, P = 0.04, but positively associated with spermathecal area, 3.29, 95% CI: 0.29–8.20, P = 0.04). Bayesian inference (41) of character evolution supported the regression-based results, showing strong support for correlated evolution of sperm conjugation and female reproductive tract architecture (i.e., models of correlated evolution have a greater likelihood than models of independent evolution, Bayes factor (BF) > 7). Ancestral trait reconstruction indicates the presence of sperm conjugation and compact female reproductive tracts as the basal condition in diving beetles (BF > 2). On the basis of evolutionary transition rates, the female reproductive tracts appear to change in advance of sperm form (reproductive tract 5.52 ± 3.54 > sperm 0.03 ± 2.22 changes per unit branch length ± SD) such that reproductive tract evolution elicits corresponding modification in sperm morphology (Fig. 3A).
Fig. 3.
Conjugate–female interactions. (A) Diagram showing evolutionary transitions in sperm and reproductive tract form. Histograms show the posterior distribution of evolutionary transition rates per unit of branch length (y axis: percentage of models). Transition rates that are rarely assigned to zero (Z < 5% of models of trait evolution) are considered probable events (shown in dark red; marginal events, Z ∼10%, are shown in light red). The bold Upper Left text indicates the ancestral condition for sperm and reproductive tract form in diving beetles; italicized text indicates character transitions. Female reproductive tract evolution away from the ancestral state is more probable than changes in sperm form (Z: reproductive tract 0.36% < sperm form 97.4%). Change in reproductive tract design results in a correlated loss of sperm conjugation (Far Right histogram, Z < 5%). Transition rates and Z values are based on 100,000 observations from 10,000,000 iterations from each of three independent runs of the Markov chain. (B) Sperm storage organ of Hydrovatus pustulatus stained with chlorazol black. (C) Orcein-stained rouleau-type conjugates within the fertilization duct of H. pustulatus. (D) Sagital section showing two conjugates of N. undulatus occupying the fertilization duct and oriented toward the site of fertilization. Vertical lines are the margins of the stacked heads (see Fig. 1D for explanation of rouleaux formation). Flagella can be seen between the two rouleaux and extend into the spermatheca. (E) Sperm of A. mediatus are paired within the spermatheca but are mostly single within the fertilization duct and tightly associated with the duct walls. Similar to N. undulatus, the sperm heads are oriented toward the exit of the fertilization duct. (Differential interference micrographs and scale bars in B and C, 50 μm.) (Transmission electron micrographs and scale bars in D and E, 2 μm.) f, flagellum; fd, fertilization duct; s, spermatheca; arrow, sperm head(s).
To determine the functional basis for correlated evolution, we examined sperm–female interactions in females. Intact, motile conjugates with their tips positioned in fertilization ducts were found in the spermatheca in 34 of 35 field-collected females among four species (Hygrotus sayi, 15/15; Nebrioporus rotundatus, 3/3; Neoporus dimidiatus, 5/5; and Neoporus undulatus, 16/17; Fig. 2 B–C and Movie S1). Furthermore, the sperm of Acilius mediatus remained paired in the spermatheca but were primarily single within the fertilization duct and tightly associated with the duct walls (Fig. 3E), whereas sperm remained associated in rouleaux within the fertilization duct of N. undulatus (Fig. 3D). In all species examined, individual sperm detached from conjugates only when positioned for fertilization (but see ref. 44 for an example of paired sperm dissociating within the spermatheca).
Discussion
Our results suggest female reproductive tract form drives the evolution of multivariate sperm morphology in diving beetles through physical interaction resulting in a macroevolutionary pattern of correlated evolution between dimensions of the female tract and sperm traits. Variation in sperm morphology and conjugation was significantly explained by female reproductive tract architecture, and elongation of specific components of the female reproductive tract preceded loss of sperm conjugation. Sperm heads were observed to interact with the fertilization duct pre- and postconjugate dissociation (rouleaux of N. undulatus and formerly paired sperm of A. mediatus, respectively). Additionally, the paucity of significant correlations between sperm morphology or conjugation and the presence/dimensions of the spermathecal duct suggests that selection for enhanced speed of arrival in storage has not been the primary factor influencing sperm evolution in diving beetles.
Female reproductive tract architecture can be an important determinant of the outcome of sperm competition. For example, male crickets from populations experimentally evolved to have longer sperm have no competitive fertilization advantage over males with shorter sperm within the short, round spermathecae of females (45). By contrast, investigations with Drosophila have shown that (i) physical displacement by competitor sperm is a critical determinant of competitive fertilization success in the long, narrow female sperm-storage organ (46); (ii) longer sperm are better at displacing and resisting being displaced by shorter sperm from the proximal end of the organ closest to the site of egg fertilization (14); (iii) sperm and female tract morphology interact such that the fitness advantage to males of producing relatively long sperm increases with increasing length of narrow sperm-storage organs (13); and as a consequence, (iv) the evolution of longer sperm-storage organs drives the evolution of longer sperm (13).
The association of sperm conjugation with short fertilization ducts and round spermathecae in diving beetles would be explained if the physical structure of conjugated heads enhances anchoring within the fertilization duct. Here, rouleaux would provide a further selective advantage: those with anterior ends anchored in the fertilization duct could maintain a “queue” for fertilization despite a voluminous spermatheca. As predicted by this hypothesis, we found that individual sperm detached from conjugates only when positioned for fertilization (Fig. 3 C–E, but see ref. 44). On the basis of these observations, we propose that conjugation in diving beetles is an adaptation for positional advantage in the displacement-based system of sperm competition observed in many insects (47, 48). Such interpretation, however, will remain highly speculative until detailed investigations of postcopulatory sexual selection, including the relationships between variation in sperm form, female tract form, and competitive fertilization success, are conducted in diving beetles and other taxa with diverse sperm and female reproductive tract morphology.
The inferred sequence of evolutionary transitions indicate that, whereas female reproductive tract form drives the evolution of sperm morphology, changes in sperm form do not necessarily elicit changes in female reproductive tracts. Such an evolutionary pattern might result if female reproductive tracts evolve for reasons other than sperm selection. For example, ecological factors such as patchy habitat distribution or mate availability might result in selection on females to maintain large sperm stores, potentially outweighing any fitness benefits to discrimination among stored sperm. Alternatively, female reproductive tracts might be more evolutionary labile than sperm, switching phenotypes before sperm can respond. Rapid evolution could result if female reproductive tracts are composed of multiple component traits, thereby facilitating exploration of morphospace, particularly if tracts evolve in a relatively flat fitness landscape, where many morphological variants have equivalent fitness. Consideration of fluctuating selective environments over a lineage's history might provide insight to the origin and subsequent modification of female preference in the absence of direct fitness benefits or sensory bias, one of the most perplexing questions in the study of sexual selection (2).
Across the metazoa, sperm have diverse and often complex morphology (7). Our results show that understanding the evolutionary origin and maintenance of this variation requires consideration of the largely neglected selective environment of the female reproductive tract (15). They further provide a general explanation for the relatively dramatic and multivariate diversification of sperm morphology in internally versus externally fertilizing species (7). Additionally, our results suggest that conjugation in diving beetles helps sperm maintain optimal positions for fertilization within the reproductive tract. Selection to increase the likelihood of sperm being present in an appropriate location for fertilization might be a generalizable principle of sperm evolution, equally applicable to internally and externally fertilizing species. When considered alongside recent studies using experimental evolution to manipulate a putative postcopulatory female preference trait to examine preference heritability, quantify preference cost, and experimentally discern the microevolutionary impact of preference shift on male trait evolution (13, 49, 50), the present analyses illustrate the utility of shifting attention to postcopulatory sexual selection for advancing our understanding of female preference evolution and ornament-preference coevolution.
Materials and Methods
Morphological Characters.
Sperm were harvested from the seminal vesicles of field-collected or alcohol-preserved specimens, DAPI or Hoechst's stained, and imaged using darkfield and epifluorescence microscopy. Female reproductive tracts were dissected from preserved specimens, processed as described by Miller (51), and imaged using differential interference microscopy. Sperm length and female reproductive tract dimensions were measured from digital images using Image J (52). To permit inference of probable evolutionary pathways of sperm and female reproductive tract coevolution, female multivariate morphology was categorized as a binary trait by examining the predicted values produced by our logistic regression equation and assigning species falling above or below the mean a value of one and zero, respectively. Total body length was used as a measure of body size. See Dataset S1 for species mean values and sample sizes.
Transmission Electron Microscopy.
Reproductive tracts were dissected from wild-caught females, fixed in 2.5% glutaraldehyde and 1% tannic acid, postfixed with 1% osmium tetroxide, embedded in plastic, and sectioned with a Leica EM UC6 microtome. Sections were observed with a JEOL JSM-2000EX transmission electron microscope at 100 kV.
Phylogenetic Inference.
Evolutionary relationships were inferred from partial DNA sequences of two mitochondrial (CoI and 16s) and three nuclear (H3, Wnt1, and 18s) genes (see Dataset S2 for accession numbers). Ribosomal genes were aligned using PRANK+F (53) and hypervariable regions removed using Gblocks (54); the remaining genes were aligned by eye (available from TreeBASE). Models of sequence evolution were determined using DT-ModSel (55). Evolutionary relationships among species were inferred using MRBAYES (56). We used uninformative priors for all of the models’ parameters (i.e., MRBAYES defaults). Four independent runs of Markov chain Monte Carlo (MCMC) of 100,000,000 generations, consisting of six chains each, were used to sample phylogenetic tree space. After a burn-in period (assessed using AWTY; ref. 57), trees are visited in proportion to their probability of being true, given the model, priors, and data and can be used to determine the posterior probability of a branching event and branch lengths. The MCMC conditions included chain heating (temperature = 0.01) with two attempted swaps between chains at each generation.
Statistical Analyses.
A majority consensus tree (Fig. S1), derived from 20,800 post burn-in trees (57), was used to create a variance–covariance matrix to account for correlation resulting from evolutionary relationships among species. We performed separate analyses on each of the three major subclades in our phylogeny because (i) lineage-wide analyses of correlated trait evolution can obscure important relationships when these differ in direction and/or magnitude among sublineages (43), (ii) there were qualitative among-clade differences in female tract and sperm design (Fig. 2), and (iii) uncertainty of evolutionary relationships in the basal branches of the diving beetle lineage (Fig. S1).
Forward and backward stepwise factor selection was used for both phylogenetic generalized least squares (39) and logistic regression (40), with only significant explanatory variables retained in the final models. The results were robust to the assumed model of evolution (e.g., Brownian motion, stabilizing or accelerating/decelerating evolution) and produced qualitatively or quantitatively similar results regardless of the method used to generate the variance–covariance matrix from the consensus tree. To explore rates of evolutionary transitions among correlated traits and infer probable evolutionary pathways (among all three clades) we used reversible-jump Markov chain Monte Carlo (41) analyses and 1,000 post burn-in trees (available on TreeBASE). We used a β-distributed prior with its parameters seeded from uniform hyperpriors (distributions: 0–30 and 0–5) and a rate deviation of 6, which resulted in mean acceptance of 24% of the rate parameter proposals. The chain was run for 10,050,000 iterations with the first 50,000 discarded as burn-in. Each run was repeated three times to check stability of the harmonic means.
Supplementary Material
Acknowledgments
We thank M. Pagel, A. Meade, A. Ives, and W. T. Starmer for statistical advice; R. P. Smith for technical assistance with transmission electron microscopy; and the Institute for Bioinformatics and Evolutionary Studies (IBEST) (National Institutes of Health/National Center for Research Resources P20RR16448 and P20RR-16454) for providing computing resources. E. M. Droge-Young provided stimulating conversation regarding reproductive tract evolution. Additionally, we thank E. M. Droge-Young, S. Lüpold, M. K. Manier, J. A. C. Uy, two anonymous reviewers, and the editor for their comments on an earlier version of this manuscript. This work was supported by the National Science Foundation (DDIG-0910049, DEB-0743101, DEB-0814732, and DEB-6990357), Natural Sciences and Engineering Research Council (Canada) (PGS-D 331458), and the Systematics Research Fund.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. R.S. is a guest editor invited by the Editorial Board.
Data deposition: Accession numbers are provided in Dataset S2. Alignments and trees are available from http://purl.org/phylo/treebase/phylows/study/TB2:S12334.
See Commentary on page 4341.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1111474109/-/DCSupplemental.
References
- 1.Darwin C. The Descent of Man, and Selection in Relation to Sex. London: Murray; 1871. [Google Scholar]
- 2.Andersson M. Sexual Selection. Princeton: Princeton Univ Press; 1994. p. 443. [Google Scholar]
- 3.Wagner WEJ. Measuring female mating preferences. Anim Behav. 1998;55:1029–1042. doi: 10.1006/anbe.1997.0635. [DOI] [PubMed] [Google Scholar]
- 4.Basolo AL. Phylogenetic evidence for the role of a pre-existing bias in sexual selection. Proc R Soc Lond. Ser B Biol Sci. 1995;259:307–311. doi: 10.1098/rspb.1995.0045. [DOI] [PubMed] [Google Scholar]
- 5.Ryan MJ, Rand AS. Female responses to ancestral advertisement calls in tungara frogs. Science. 1995;269:390–392. doi: 10.1126/science.269.5222.390. [DOI] [PubMed] [Google Scholar]
- 6.Ward PI. Intraspecific variation in sperm size characters. Heredity (Edinb) 1998;80:655–659. doi: 10.1046/j.1365-2540.1998.00401.x. [DOI] [PubMed] [Google Scholar]
- 7.Pitnick S, Hosken DJ, Birkhead TR. In: Sperm morphological diversity. Sperm Biology, an Evolutionary Perspective. Birkhead TR, Hosken DJ, Pitnick S, editors. San Diego: Academic; 2009. pp. 69–149. [Google Scholar]
- 8.Birkhead TR, Pizzari T. Postcopulatory sexual selection. Nat Rev Genet. 2002;3:262–273. doi: 10.1038/nrg774. [DOI] [PubMed] [Google Scholar]
- 9.Pizzari T, Parker GA. In: Sperm competition and sperm phenotype. Sperm Biology, an Evolutionary Perspective. Birkhead TR, Hosken DJ, Pitnick S, editors. London: Elsevier; 2009. pp. 205–244. [Google Scholar]
- 10.Fitzpatrick JL, et al. Female promiscuity promotes the evolution of faster sperm in cichlid fishes. Proc Natl Acad Sci USA. 2009;106:1128–1132. doi: 10.1073/pnas.0809990106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Immler S, et al. Resolving variation in the reproductive tradeoff between sperm size and number. Proc Natl Acad Sci USA. 2011;108:5325–5330. doi: 10.1073/pnas.1009059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Birkhead TR, Møller AP, editors. 1998. Sperm competition and sexual selection (Academic Press, San Diego), p 826. [Google Scholar]
- 13.Miller GT, Pitnick S. Sperm-female coevolution in Drosophila. Science. 2002;298:1230–1233. doi: 10.1126/science.1076968. [DOI] [PubMed] [Google Scholar]
- 14.Pattarini JM, Starmer WT, Bjork A, Pitnick S. Mechanisms underlying the sperm quality advantage in Drosophila melanogaster. Evolution. 2006;60:2064–2080. [PubMed] [Google Scholar]
- 15.Pitnick S, Wolfner MF, Suarez SS. In: Ejaculate- and sperm-female interactions. Sperm Biology, an Evolutionary Perspective. Birkhead TR, Hosken DJ, Pitnick S, editors. London: Elsevier; 2009. pp. 247–304. [Google Scholar]
- 16.Schärer L, Littlewood DTJ, Waeschenbach A, Yoshida W, Vizoso DB. Mating behavior and the evolution of sperm design. Proc Natl Acad Sci USA. 2011;108:1490–1495. doi: 10.1073/pnas.1013892108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Birkhead TR. Cryptic female choice: Criteria for establishing female sperm choice. Evolution. 1998;52:1212–1218. doi: 10.1111/j.1558-5646.1998.tb01848.x. [DOI] [PubMed] [Google Scholar]
- 18.Dybas LK, Dybas HS. Coadaptation and taxonomic differentiation of sperm and spermathecae in featherwing beetles. Evolution. 1981;35:168–174. doi: 10.1111/j.1558-5646.1981.tb04869.x. [DOI] [PubMed] [Google Scholar]
- 19.Rugman-Jones PF, Eady PE. Co-evolution of male and female reproductive traits across the Bruchidae (Coleoptera) Funct Ecol. 2008;22:880–886. [Google Scholar]
- 20.Briskie JV, Montgomerie R, Birkhead TR. The evolution of sperm size in birds. Evolution. 1997;51:937–945. doi: 10.1111/j.1558-5646.1997.tb03674.x. [DOI] [PubMed] [Google Scholar]
- 21.Minder AM, Hosken DJ, Ward PI. Co-evolution of male and female reproductive characters across the Scathophagidae (Diptera) J Evol Biol. 2005;18:60–69. doi: 10.1111/j.1420-9101.2004.00799.x. [DOI] [PubMed] [Google Scholar]
- 22.Pitnick S, Markow TA, Spicer GS. Evolution of multiple kinds of female sperm-storage organs in Drosophila. Evolution. 1999;53:1804–1822. doi: 10.1111/j.1558-5646.1999.tb04564.x. [DOI] [PubMed] [Google Scholar]
- 23.Presgraves DC, Baker RH, Wilkinson GS. Coevolution of sperm and female reproductive tract morphology in stalk-eyed flies. Proc R Soc Lond. Ser B Biol Sci. 1999;266:1041–1047. [Google Scholar]
- 24.Holman L, Freckleton RP, Snook RR. What use is an infertile sperm? A comparative study of sperm-heteromorphic Drosophila. Evolution. 2008;62:374–385. doi: 10.1111/j.1558-5646.2007.00280.x. [DOI] [PubMed] [Google Scholar]
- 25.Anderson MJ, Dixson AS, Dixson AF. Mammalian sperm and oviducts are sexually selected: Evidence for co-evolution. J Zool (Lond) 2006;270:682–686. [Google Scholar]
- 26.Morrow EH, Gage MJG. The evolution of sperm length in moths. Proc R Soc Lond Ser B Biol Sci. 2000;267:307–313. doi: 10.1098/rspb.2000.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beese K, Armbruster GFJ, Beier K, Baur B. Evolution of female sperm-storage organs in the carrefour of stylommatophoran gastropods. J Zoological Syst Evol Res. 2009;47:49–60. [Google Scholar]
- 28.Gage MJG. Mammaliam sperm morphometry. Proc R Soc Lond Ser B Biol Sci. 1998;265:97–103. doi: 10.1098/rspb.1998.0269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hosken DJ. Testes mass in megachiropteran bats varies in accordance with sperm competition theory. Behav Ecol Sociobiol. 1998;44:169–177. [Google Scholar]
- 30.Higginson DM, Miller KB, Segraves KA, Pitnick S. Convergence, recurrence and diversification of complex sperm traits in diving beetles (Dytiscidae) Evolution. 2012 doi: 10.1111/j.1558-5646.2011.01532.x. 10.1111/j.1558-5646.2011.01532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Higginson DM, Pitnick S. Evolution of intra-ejaculate sperm interactions: Do sperm cooperate? Biol Rev Camb Philos Soc. 2011;86:249–270. doi: 10.1111/j.1469-185X.2010.00147.x. [DOI] [PubMed] [Google Scholar]
- 32.Inoda T, et al. Temperature-dependent regulation of reproduction in the diving beetle Dytiscus sharpi (Coleoptera: Dytiscidae) Zoolog Sci. 2007;24:1115–1121. doi: 10.2108/zsj.24.1115. [DOI] [PubMed] [Google Scholar]
- 33.Aiken RB. The mating behaviour of a boreal water beetle, Dytiscus alaskanus (Coleopotera Dytiscidae) Ethol Ecol Evol. 1992;4:245–254. [Google Scholar]
- 34.Aiken RB, Wilkinson W. Bionomics of Dytiscus alaskanus J. Balfour-Browne (Coleoptera: Dytiscidae) in a central Alberta lake. Can J Zool. 1985;63:1316–1323. [Google Scholar]
- 35.Cleavall LM. 2009. Description of Thermonectus nigrofasciatus and Rhantus binotatus (Coleoptera: Dytiscidae) mating behavior. MS thesis (University of New Mexico, Alburquerque) [Google Scholar]
- 36.Bergsten J, Miller KB. Phylogeny of diving beetles reveals a coevolutionary arms race between the sexes. PLoS ONE. 2007;2:e522. doi: 10.1371/journal.pone.0000522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miller KB. The phylogeny of diving beetles (Coleoptera: Dytiscidae) and the evolution of sexual conflict. Biol J Linn Soc Lond. 2003;79:359–388. [Google Scholar]
- 38.Austad SN. In: Evolution of sperm priority patterns in spiders. Sperm Competition and the Evolution of Animal Mating Systems. Smith RL, editor. London: Academic; 1984. pp. 233–249. [Google Scholar]
- 39.Lavin SR, Karasov WH, Ives AR, Middleton KM, Garland T., Jr Morphometrics of the avian small intestine compared with that of nonflying mammals: A phylogenetic approach. Physiol Biochem Zool. 2008;81:526–550. doi: 10.1086/590395. [DOI] [PubMed] [Google Scholar]
- 40.Ives AR, Garland TJ., Jr Phylogenetic logistic regression for binary dependent variables. Syst Biol. 2010;59:9–26. doi: 10.1093/sysbio/syp074. [DOI] [PubMed] [Google Scholar]
- 41.Pagel M, Meade A. Bayesian analysis of correlated evolution of discrete characters by reversible-jump Markov chain monte carlo. Am Nat. 2006;167:808–825. doi: 10.1086/503444. [DOI] [PubMed] [Google Scholar]
- 42.Snook RR, Markow TA, Karr TL. Functional nonequivalence of sperm in Drosophila pseudoobscura. Proc Natl Acad Sci USA. 1994;91:11222–11226. doi: 10.1073/pnas.91.23.11222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Immler S, Birkhead TR. Sperm competition and sperm midpiece size: No consistent pattern in passerine birds. Proc R Soc Lond Ser B Biol Sci. 2007;274:561–568. doi: 10.1098/rspb.2006.3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dallai R, Afzelius BA. Sperm ultrastructure in the water beetles (Insecta, Coleoptera) Boll Zool. 1987;54:301–306. [Google Scholar]
- 45.Morrow EH, Gage MJG. Sperm competition experiments between lines of crickets producing different sperm lengths. Proc R Soc Lond Ser B Biol Sci. 2001;268:2281–2286. doi: 10.1098/rspb.2001.1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Manier MK, et al. Resolving mechanisms of competitive fertilization success in Drosophila melanogaster. Science. 2010;328:354–357. doi: 10.1126/science.1187096. [DOI] [PubMed] [Google Scholar]
- 47.Parker GA, Pizzari T. Sperm competition and ejaculate economics. Biol Rev Camb Philos Soc. 2010;85:897–934. doi: 10.1111/j.1469-185X.2010.00140.x. [DOI] [PubMed] [Google Scholar]
- 48.Parker GA, Immler S, Pitnick S, Birkhead TR. Sperm competition games: Sperm size (mass) and number under raffle and displacement, and the evolution of P2. J Theor Biol. 2010;264:1003–1023. doi: 10.1016/j.jtbi.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 49.Miller GT, Pitnick S. Functional significance of seminal receptacle length in Drosophila melanogaster. J Evol Biol. 2003;16:114–126. doi: 10.1046/j.1420-9101.2003.00476.x. [DOI] [PubMed] [Google Scholar]
- 50.Miller GT, Starmer WT, Pitnick S. Quantitative genetics of seminal receptacle length in Drosophila melanogaster. Heredity (Edinb) 2001;87:25–32. doi: 10.1046/j.1365-2540.2001.00903.x. [DOI] [PubMed] [Google Scholar]
- 51.Miller KB. On the phylogeny of the Dytiscidae (Insecta: Coleoptera) with emphasis on the morphology of the female reproductive system. Insect Syst Evol. 2001;31:45–92. [Google Scholar]
- 52.Rasband WS. ImageJ (National Institutes of Health, Bethesda, MD), version 1.43u. 1997–2008 [Google Scholar]
- 53.Löytynoja A, Goldman N. Phylogeny-aware gap placement prevents errors in sequence alignment and evolutionary analysis. Science. 2008;320:1632–1635. doi: 10.1126/science.1158395. [DOI] [PubMed] [Google Scholar]
- 54.Talavera G, Castresana J. Improvement of phylogenies after removing divergent and ambiguously aligned blocks from protein sequence alignments. Syst Biol. 2007;56:564–577. doi: 10.1080/10635150701472164. [DOI] [PubMed] [Google Scholar]
- 55.Minin V, Abdo Z, Joyce P, Sullivan J. Performance-based selection of likelihood models for phylogeny estimation. Syst Biol. 2003;52:674–683. doi: 10.1080/10635150390235494. [DOI] [PubMed] [Google Scholar]
- 56.Ronquist F, Huelsenbeck JP. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19:1572–1574. doi: 10.1093/bioinformatics/btg180. [DOI] [PubMed] [Google Scholar]
- 57.Nylander JAA, Wilgenbusch JC, Warren DL, Swofford DL. AWTY (are we there yet?): A system for graphical exploration of MCMC convergence in Bayesian phylogenetics. Bioinformatics. 2008;24:581–583. doi: 10.1093/bioinformatics/btm388. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.