Abstract
Efficient behavior involves the coordinated activity of large-scale brain networks, but the way in which these networks interact is uncertain. One theory is that the salience network (SN)—which includes the anterior cingulate cortex, presupplementary motor area, and anterior insulae—regulates dynamic changes in other networks. If this is the case, then damage to the structural connectivity of the SN should disrupt the regulation of associated networks. To investigate this hypothesis, we studied a group of 57 patients with cognitive impairments following traumatic brain injury (TBI) and 25 control subjects using the stop-signal task. The pattern of brain activity associated with stop-signal task performance was studied by using functional MRI, and the structural integrity of network connections was quantified by using diffusion tensor imaging. Efficient inhibitory control was associated with rapid deactivation within parts of the default mode network (DMN), including the precuneus and posterior cingulate cortex. TBI patients showed a failure of DMN deactivation, which was associated with an impairment of inhibitory control. TBI frequently results in traumatic axonal injury, which can disconnect brain networks by damaging white matter tracts. The abnormality of DMN function was specifically predicted by the amount of white matter damage in the SN tract connecting the right anterior insulae to the presupplementary motor area and dorsal anterior cingulate cortex. The results provide evidence that structural integrity of the SN is necessary for the efficient regulation of activity in the DMN, and that a failure of this regulation leads to inefficient cognitive control.
Efficient behavior involves the coordinated activity of large-scale brain networks (1, 2). These networks can be identified by studying patterns of brain activity measured by functional MRI (fMRI) (3). A key question is whether—and in what way—the interactions between these networks control behavior. Regions on the medial wall of the frontal lobe, including the anterior cingulate cortex (ACC) and presupplementary motor area (preSMA), often show highly correlated brain activity with the anterior insula (AI) (4–6), and together they have been termed the salience network (SN) (4). In many situations, activity within this network appears to signal the need for behavioral change (7–9), and one proposal is that the SN may operate to dynamically control changes of activity in other networks (6).
The role of the SN in mediating the function of other networks is likely to be most evident when a rapid change in behavior is required. We have previously studied this type of behavior in the context of motor control using the stop-signal task (SST) (10). The SST probes the ability to inhibit an action that has already been initiated. Stop trials require a switch from relatively automatic to highly controlled behavior. Parts of the right inferior frontal cortex, including the right AI (rAI), are important for the attentional processes involved in responding to an unexpected event (the “stop signal”) (10). In addition, coupled deactivation of the default mode network (DMN) appears important for efficient behavioral performance. A lack of deactivation within the DMN is associated with slower and less successful inhibitory control (11, 12), and, more generally, the failure to deactivate the DMN is associated with attentional lapses (13). The precise contribution of these brain regions to cognitive control remains controversial (14, 15), but coordinated activity between “task-positive” networks, including the SN and other “executive” regions, and the DMN appears to be a feature of rapid and efficient engagement of motor control.
Insight into how the SN interacts with other networks can be gained from observing how white-matter damage to the network's connections affects the functioning of other networks such as the DMN. Traumatic brain injury (TBI) often results in impairments of executive function and attention (16). A key reason appears to be the impact of traumatic axonal injury (TAI) on large-scale brain networks (17, 18). TAI is commonly observed after TBI, although until recently it has been difficult to study in vivo. Diffusion tensor imaging (DTI) provides a way of sensitively quantifying the degree of white-matter disruption after TBI (19, 20). This white-matter damage has the effect of partially disconnecting brain networks, which has an impact on their function and also affects the behavior they support (21). Although TAI may be widespread, damage to specific tracts can be an important predictor of a particular cognitive function (19, 21).
The study of TBI patients thus provides an opportunity to investigate whether damage to specific white matter tracts is associated with brain network dysfunction and consequent behavioral deficits. Here, we use fMRI to study brain function during inhibitory control and DTI to quantify white matter integrity within tracts connecting nodes in networks that are modulated by the task. We test the hypothesis that damage to the connections between the nodes of the SN specifically predicts abnormalities in the pattern of brain activation and behavioral performance during the SST.
Results
Behavioral Results.
Fifty-seven TBI patients and 25 healthy volunteers performed two runs of the SST (Fig. S1). As a group, the TBI patients showed an expected pattern of neuropsychological impairment, with evidence of slow information processing speed, impaired inhibition, and reduced cognitive flexibility (SI Results and Table S1). Both patients and controls performed the SST as expected (∼50% accuracy on stop trials). Accuracy on go trials was high for both groups (>95%), although patients were slightly less accurate than controls (t = 2.81, df = 80, P = 0.006) (Table S2). Performance on the task can be used to derive the stop signal reaction time (SSRT), which provides an estimate of the efficiency of an individual‘s inhibitory processing. SSRT could be estimated in a subset of 46 patients and 22 control subjects (SI Methods). SSRT was significantly higher in patients than age-matched controls (t = 2.01, df = 66, P = 0.014) (Table S2). There was no other behavioral difference between the groups on SST performance. The pattern of patients’ performance on the task as a whole suggests that SSRT abnormalities were related in part to attentional impairments (SI Results).
Functional Imaging.
TBI patients and controls show similar activation of brain regions involved in attentional capture and response inhibition.
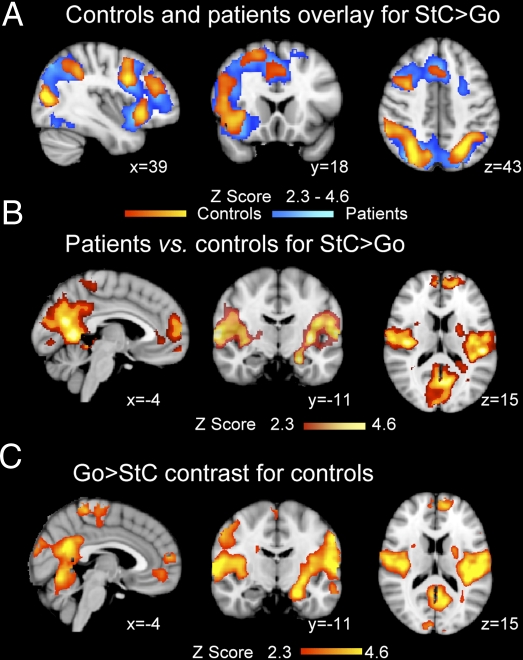
Brain regions involved in attending to the stop signal and inhibiting the go response are demonstrated by comparing the pattern of activation seen during correct stop and go trials (StC > Go). For both controls and patients, event-related analysis revealed an expected pattern of brain activation (10) (Fig. 1A), with activity in the right inferior frontal gyrus (rIFG), the rAI, the middle and superior frontal gyri, and within the dorsal ACC (dACC). More posteriorly, activation was observed within the right supramarginal gyrus, the temporo-parietal junction (TPJ), the intraparietal sulcus (IPS), and the lateral occipital cortices. A direct group comparison showed no significant group difference in these task-positive regions.
Fig. 1.
Abnormal DMN deactivation after TBI. (A) Overlay of brain activation associated with correct stop (StC) vs. go trials for patients (blue) and controls (yellow-red). (B) Brain regions where patients show greater brain activation for StC > Go than controls. (C) Brain regions showing greater activation for go than stop trials in the control group. Results are superimposed on the MNI 152 T1 1-mm brain template. Cluster corrected Z = 2.3, P < 0.05.
TBI patients show less deactivation of the DMN during stopping.
In contrast to the normal activation seen in task-positive regions, patients showed less deactivation in parts of the DMN, including the precuneus and posterior cingulate cortex (precu/PCC), the ventromedial prefrontal cortex (vmPFC), and the left hippocampus, compared with controls (Fig. 1B and Table S3). Less deactivation was also observed in the left amygdala as well as bilateral secondary somatosensory and posterior insulae regions. These differences were due to controls showing extensive deactivation during stopping in parts of the DMN (Fig. 1C), which was not observed in patients. There was no group difference in the contrast go vs. rest.
Failure of DMN deactivation is associated with impaired inhibitory control.
Failure to deactivate the DMN may lead to the ”intrusion” of DMN activity during normal task performance, resulting in inefficient behavior (22). Therefore, patients were split into two subgroups based on the presence or absence of relative deactivation within the precu/PCC, with deactivation defined as a negative percentage signal change on correct stop relative to go trials. We focused on the precu/PCC area because of its central role within the DMN (23, 24) and the relationship observed between its activation and fluctuations of attention (13, 21, 25). Precu/PCC activators (n = 20) had slower response inhibition (higher SSRT) compared with both patients who deactivated (n = 26) (t = −2.155, df = 44, P = 0.037) and controls (t = −3.069, df = 40, P = 0.004) (Fig. S2A). The two patients’ groups showed no difference in any other behavioral measures or in injury severity, lesion load, or age. Deactivators and controls showed no differences in SSRT (P > 0.35). No relationship between DMN deactivation and SSRT was observed in controls (SI Results). Precu/PCC activators also showed a failure of deactivation in other parts of the DMN, whereas the deactivating patients and controls showed similar activation across the brain (Fig. S2). In patients, the precu/PCC activity associated with stopping showed a correlation with SSRT of only borderline significance (one-tailed Spearman, r = 0.2, P = 0.091). The marginal significance of the correlation may be because relative activation within the DMN is qualitatively different from relative deactivation.
Structural Imaging.
We next investigated the relationship between white matter tract integrity, network function, and behavior. We focused our analysis on white matter tracts connecting regions either activated or deactivated during response inhibition (Fig. 2 A and C). Fractional anisotropy (FA) provided a validated marker of white matter integrity after TBI (26), and we tested whether damage to network connections predicted: (i) abnormal activation within the DMN and (ii) behavioral impairment.
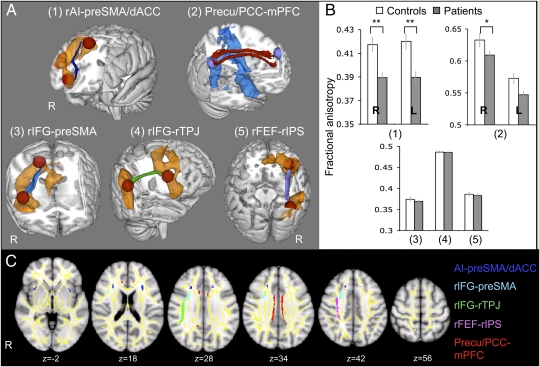
Fig. 2.
Comparison of white matter structure between patients and controls. (A and C) White matter tracts of interest are represented in 3D on the MNI-152 T1 1-mm brain 3D template (A) and in 2D on axial MNI-152 T1 1-mm brain views, overlaid on tract-based spatial statistics (TBSS) white matter skeleton (yellow) (C). (A) The resulting probabilistic tractography tracts are shown for the connections between the rAI and the preSMA/dACC (dark blue) (1), the precu/PCC and vmPFC bilaterally (red) (2), the rIFG and the preSMA (light blue) (3), the rIFG and the right TPJ (rTPJ) (green; 4) and the rFEF and rIPS (lilac/pink; 5). Orange and blue areas represent the mean BOLD signal change in patients and controls for the contrasts StC > Go and Go > StC, respectively. (B) The bar charts show FA ± SEM within each tract compared between TBI patients (gray) and 30 age-matched controls (white). R, right; L, left. *P < 0.05; **P < 0.005.
Connections within the SN and DMN are damaged after TBI.
White matter tracts connecting nodes within the SN and DMN showed significantly lower FA in patients, especially for the connections between the preSMA/dACC and the rAI (t = 3.62, df = 85, P = 0.001) and left AI (lAI; t = 3.59, df = 85, P = 0.001) (Fig. 2B). The right cingulum bundle connecting the vmPFC to the precu/PCC (t = 2.43, df = 85, P = 0.017) and more marginally the left cingulum (t = 1.90, df = 85, P = 0.076) were also more impaired in patients (Fig. 2B). The presence of focal brain damage such as contusions did not explain these results, because the tracts were abnormal when 21 patients with focal cortical injury were removed [rAI–preSMA/dACC: t = 3.29, df = 64, P = 0.012; lAI–preSMA/dACC: t = 3.59, df = 64, P = 0.006; right precu (rprecu)–mPFC: t = 2.82, df = 64, P = 0.036 (Bonferroni corrected statistics)].
Structural connectivity within the SN predicts DMN function.
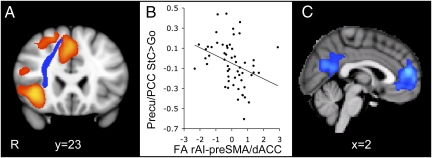
We next used a binary logistic regression to investigate whether white matter tracts’ structure predicted DMN function. Measures of FA for all of the tracts assessed were simultaneously included in an analysis aimed at classifying patients into those who did or did not show DMN deactivation during stopping. A significant model was generated whereby only the structure of the rAI–preSMA/dACC connection remained (see Fig. 3A and Figs. S3 and S4 for tract location in individual subjects). The model resulted in classification of the two groups of patients with 78.3% accuracy (χ2 = 16.9, df = 1, P < 0.0005). This result was not an effect of focal brain injury, because the model remained significant after removal of the subjects with focal damage. It was also not due to the overall amount of white matter damage, because there was no relationship between average white matter FA and DMN activity, and the model remained significant after whole-brain white matter damage was controlled for (SI Results). The rAI–preSMA/dACC tract FA was linearly correlated with the amount of precu/PCC activation during stopping (r = −0.472, P < 0.0005) (Fig. 3B), and a confirmatory whole-brain analysis strikingly demonstrated that structural integrity of this tract negatively correlates with activity only within the core nodes of the DMN (Fig. 3C).
Fig. 3.
The integrity of the rAI–preSMA/dACC white matter tract predicts DMN deactivation. (A) Coronal view of the white matter connection between rAI and preSMA/dACC (blue) overlaid on the activation map for the contrast StC > Go in patients (orange), superimposed on the MNI 152 T1 1-mm brain template. (B) FA of the rAI–preSMA/dACC tracts in patients plotted against the percent signal change within the precu/PCC ROI on correct stop trials relative to go trials. FA measures are corrected for age and whole-brain mean FA and normalized. (C) Sagittal view of the brain regions showing negatively correlated activation with FA measures within the rAI–preSMA/dACC tract for StC relative to go trials, superimposed on the MNI 152 T1 2-mm brain template. Cluster corrected Z = 2.3, P < 0.05.
We did not observe a relationship between rAI–preSMA/dACC structural integrity and activity in task-positive regions. To investigate whether the rAI interacts with regions of the executive network (EN) via other pathways, two additional tracts connecting the rAI to regions of the EN [right frontal eye field (rFEF) and right IPS (rIPS)] were generated. FA within those tracts showed no significant damage in patients compared with controls and no significant relation with activation within the task-positive regions (SI Results).
Structural connectivity is correlated with behavioral impairments.
Motivated by these results, we next specifically investigated whether the structure of the rAI–preSMA/dACC tract was related to SSRT. FA within this tract (corrected for age and whole-brain white matter damage) was negatively correlated with SSRT, albeit relatively weakly (one-tailed Spearman correlation: r = −0.263, P = 0.043, uncorrected), and this result remained significant after excluding patients with focal lesions (r = −0.386, P = 0.019, n = 29, uncorrected). There was no significant correlation between FA and SSRT in the other tracts studied. In addition, integrity within this tract also correlated with patients’ performance on a separate neuropsychological task commonly used to assess cognitive flexibility and inhibitory control (SI Results).
Discussion
Efficiently changing behavior requires rapid and coordinated activity across brain networks. The SN is thought to be important for signaling the need to change behavior (27). We studied its function in a large group of TBI patients using the SST. In this task, subjects must attend to an infrequent stop signal, process its meaning, and inhibit an ongoing motor response. The SST involves a change from a relatively automatic go response to highly controlled response inhibition, which requires coordinated changes in the activity of a number of distributed brain networks. TBI frequently produces TAI, which disconnects brain networks. Therefore, studying TBI patients provides an opportunity to investigate the impact of structural disconnection on brain network function.
The SN comprises paralimbic structures—most prominently the AI and medial prefrontal areas such as the dACC and preSMA—which are anatomically and functionally interconnected (4, 28). We show that the structural integrity of one of the tracts connecting the rAI to the dorsomedial wall of the frontal lobe predicts DMN function, as well as behavior. This result is unlikely to be a nonspecific effect of injury severity, because the structural integrity of other tracts studied did not predict DMN function. The key tract connects the rAI to a region around the boundary of the dorsal part of the ACC and the preSMA. It is similar to a tract described by van den Heuvel and colleagues (28), connecting medial and lateral nodes of the SN (the Core Network in their terminology). It is important to note that our probabilistic tractography method is constrained to demonstrate the structural connection between peaks of activation associated with SST performance. There are a number of other pathways connecting the AI to the medial frontal lobe, including parts of the uncinate fasiculus and external capsule (29–32). Further studies will be required to provide a comprehensive description of the organization of these tracts and the effects of their damage on behavior.
The link between SN abnormality and DMN function is in keeping with work showing that SN dysfunction in patients with fronto-temporal dementia is associated with abnormalities in the DMN (33). Regulating activity within the DMN appears particularly important for efficient cognitive function (22, 24, 34). The DMN shows rapid and highly reactive deactivation during attentionally demanding tasks (35, 36), and greater anticorrelation between activity in the DMN and in brain regions involved in executive function is associated with more efficient behavior (34). The precu/PCC region is the central node of the DMN (23) and forms part of what has been termed the structural core of the brain (37). Activity within the precu/PCC appears very sensitive to changes elsewhere in the brain, and a failure to deactivate this region during task performance is associated with cognitive impairment (38, 39). We have reported that abnormalities of precu/PCC activity following TBI are correlated with behavioral impairments (21, 25). Here we extend these results by showing that rapid changes in activity within the DMN can also be impaired after TBI and may result in cognitive impairment.
The results are broadly in line with the proposed functions for the SN, which include initiation of cognitive control (27), maintenance and implementation of task sets (9, 40), and coordination of behavioral response (41). In healthy subjects, increased SN activity is observed during the suppression of unwanted thoughts (42) and more generally in the self-regulation of internal states (43, 44). In contrast, attentional lapses and “stimulus-independent” thoughts are associated with increased activity within the DMN (13, 45). These observations have been integrated by Sridharan and colleagues (6), who provide evidence that the rAI acts as a “causal outflow hub” coordinating activity between the DMN and brain regions involved in executive control. In their study, activity in the rAI preceded that in the DMN and EN, and Granger causality analysis provided some evidence that it directly influenced the EN and DMN. However, causality can be difficult to infer from fMRI data alone (46), and by demonstrating the link between structural damage to the rAI– preSMA/dACC white matter connections and DMN deactivation, we provide converging evidence that structural integrity of the SN is required for the efficient deactivation of the DMN during cognitively demanding behavior.
It is interesting that we do not observe differences in brain activity in regions activated by the SST. This finding suggests that the effects of TAI may particularly affect functioning of the DMN—or at least that these effects are most easily observed in this network. It is particularly noteworthy that activation within the SN was normal, despite the presence of structural damage to its connections and the presence of a clear relationship between SN tract integrity and activity within the DMN. It is possible that our analysis techniques are insensitive to subtle changes in SN activity—perhaps as a consequence of the limitations of fMRI in resolving rapid activity fluctuations. Alternatively, the effects of subtle dysfunction within the SN may be amplified within the DMN, possibly because of its highly interconnected organization.
Neuropsychological studies have demonstrated that damage to parts of the right inferior frontal cortex produce impairments in the monitoring of task performance and the inhibition of inappropriate behavior (47–49). The amount of damage in this area has specifically been shown to correlate with the SSRT (48). Work of this type has tended to focus on the relationship between cortical damage and behavior. However, the lesions studied almost always result in a degree of damage to large white matter tracts, and the contribution of this damage to cognitive impairment has been difficult to differentiate from that of damage to the overlying cortex (50). Previous work has begun to show that frontal white matter damage influences SN function (51), and, by using DTI, we have been able to show that connections between the rAI and the preSMA/dACC also relate to SSRT. This relationship is present in a subgroup of patients without clear frontal cortical damage, demonstrating the importance of studying the contribution of structural connections within networks to cognitive function.
Our results do not necessarily provide evidence for a direct link between the SN and inhibitory processing. The SSRT is not a pure measure of motor response inhibition, because stopping in response to an unexpected cue requires attentional capture and processing of the stop signal (10) and can be influenced by other factors including motivation (52, 53). In our patients, SSRT correlated with go accuracy and IIV, both of which reflect attentional processing rather than response inhibition. Other work has also shown that paying attention to task-relevant stimuli can play an important role in determining SSRT (54). Hence, the changes in SSRT observed after TBI might reflect impairments of attention rather than pure motor inhibition.
In summary, we provide evidence that structural integrity of the SN is necessary for the efficient regulation of activity in the DMN. Patients who fail to deactivate their DMN during focused task performance show deficits of inhibitory control, which provides a way in which damage to the SN might directly influence behavior. We propose that this mechanism is not unique to TBI and that an inability to down-regulate the DMN due to SN dysfunction may underlie behavioral deficits observed in other clinical populations.
Subjects and Methods
Patients: Demographic and Clinical Details.
Fifty-seven patients with persistent neurological problems after head injury were included in the analyses we report (11 females, mean age 36.7 ± 11.5, range 18–62 y). They were all in the postacute/chronic phase postinjury (mean 20 mo, range 2–96 mo). A battery of standardized neuropsychological tests designed to be sensitive to cognitive impairments commonly observed after TBI was used to compare TBI patients with an age-matched control group (19, 25) (SI Methods, SI Results, and Table S1). Clinical details about the patients, including injury cause, severity, presence of contusions or microbleeds, and a lesion overlay map, are in Fig. S5. The study was approved by the Hammersmith and Queen Charlotte's and Chelsea Research ethics committee, and all participants gave written informed consent.
Control Groups.
Two age-matched control groups were used. Twenty-five controls were recruited for the fMRI study (17 males, mean age 34.2 ± 9.6) and 30 controls for the DTI study (14 males, mean age 37.2 ± 8.9). Subjects had no history of neurological or psychiatric disorders. An additional group of 10 young healthy volunteers was used to perform the initial tractography in an unbiased way (6 males, mean age 23 ± 2.5).
SST.
The SST is a two-choice reaction time task in which participants are required to respond to visually presented go stimuli with a button press and to attempt to withhold their response when a stop signal is presented (Fig. S1). Here, we used a similar version of the task to that described (10). The SSRT, a measure of inhibition, was estimated by using standard procedures (SI Methods).
Scanning Protocol and MRI Acquisition.
Patients had a structural and a functional scanning session. The structural session included DTI, T1, and T2* scans. During the functional session, participants performed two runs of the SST, preceded by one run of a simple choice reaction time task, identical to the SST, except that only go trials were presented. MRI data were obtained by using a Philips Intera 3.0 Tesla MRI scanner. MRI parameters for T1, fMRI, and DTI were similar to those presented (10, 21) (SI Methods).
fMRI Analysis of the SST.
fMRI analysis was performed by using FSL, as described (10, 21) (SI Methods). Briefly, the following contrast images were generated: StC vs. Go, Go vs. StC, and Go vs. Rest. The two SST runs were first analyzed separately and then combined by using fixed effects analysis. Group effects were analyzed by using FLAME [Oxford Centre for Functional MRI of the Brain (FMRIB) Local Analysis of Mixed Effects]. The final statistical images were thresholded by using Gaussian random field-based cluster inference with a height threshold of Z > 2.3 and a cluster significance threshold of P < 0.05. Individual gray matter density maps were included in the FMRI Expert Analysis Tool (FEAT) GLM as a confound regressor (SI Methods) (55). To further investigate the change in activation within the DMN in patients, a region of interest (ROI) analysis was performed by using Featquery. To provide an unbiased way of sampling DMN activity in the patients, a 10-mm radius sphere ROI was defined based on peaks of “deactivation” in controls for the contrast StC > Go within the precu/PCC (MNI coordinates: x = −4, y = −60, z = 20).
DTI Analysis.
Our analysis of white matter structure was focused on tracts connecting brain regions activated during response inhibition. Therefore, we defined the connections between loci of activation observed during SST performance, in an approach similar to the one used by Aron and colleagues (56). Tracts between all possible combinations of the activated brain regions were not investigated. Instead, the analysis was constrained to connections between theoretically relevant regions. We studied the connections between key cortical nodes of the SN, the DMN, and the dorsal and ventral attentional networks (57), as well as between key regions for inhibitory control. The following tracts were studied: (i) preSMA/dACC to rAI and lAI (comprising the anterior part of the inferior fronto-occipital fasciculus and the superior region of the corona radiata); (ii) vmPFC to precu/PCC (cingulum bundle); (iii) rIFG to preSMA; (iv) rIFG to right TPJ (part of the superior longitudinal fasciculus); and (v) rFEF to rIPS (part of the superior longitudinal fasciculus). Two additional tracts were also generated, connecting the rAI to the rFEF and the rIPS (SI Results). The subthalamus nucleus was not included in the analysis because it was not a locus of significant peak of activation. Tracts were generated between 10-mm radius spherical ROI defined based on the peak activation or deactivation during correct stop trials vs. go trials (Table S4). To avoid the potential problems associated with probabilistic tractography in patients with abnormal white matter, we followed the approach described by Hua et al. (58). Individual tractography was performed in a separate group of 10 young normal controls (6 males, mean age = 23 ± 2.5), before the generation of a mean tract, which was back projected in individual space to “sample” the white matter in the patient group and the age-matched control group. Linear regression was then used to derive the age-corrected FA values used in the analyses reported. Further details are provided in SI Methods. Tracts of interest produced by using this approach can be viewed in three dimensions and overlaid on standard brain axial views and tract-based spatial statistics (TBSS) skeleton in Fig. 2 A and C. Figs. S3 and S4 show the overlay of the TBSS skeleton and rAI–pSMA/dACC tract onto each subject's FA map.
Supplementary Material
Acknowledgments
This work was supported by grants from the Medical Research Council (to D.J.S.) and the Imperial College Healthcare Charity (to D.J.S.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1113455109/-/DCSupplemental.
References
- 1.Bressler SL, Menon V. Large-scale brain networks in cognition: Emerging methods and principles. Trends Cogn Sci. 2010;14:277–290. doi: 10.1016/j.tics.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 2.Mesulam M. Defining neurocognitive networks in the BOLD new world of computed connectivity. Neuron. 2009;62:1–3. doi: 10.1016/j.neuron.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 3.Damoiseaux JS, et al. Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci USA. 2006;103:13848–13853. doi: 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seeley WW, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beckmann CF, DeLuca M, Devlin JT, Smith SM. Investigations into resting-state connectivity using independent component analysis. Philos Trans R Soc Lond B Biol Sci. 2005;360:1001–1013. doi: 10.1098/rstb.2005.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sridharan D, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci USA. 2008;105:12569–12574. doi: 10.1073/pnas.0800005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carter CS, et al. Parsing executive processes: strategic vs. evaluative functions of the anterior cingulate cortex. Proc Natl Acad Sci USA. 2000;97:1944–1948. doi: 10.1073/pnas.97.4.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kerns JG, et al. Anterior cingulate conflict monitoring and adjustments in control. Science. 2004;303:1023–1026. doi: 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- 9.Dosenbach NU, et al. A core system for the implementation of task sets. Neuron. 2006;50:799–812. doi: 10.1016/j.neuron.2006.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sharp DJ, et al. Distinct frontal systems for response inhibition, attentional capture, and error processing. Proc Natl Acad Sci USA. 2010;107:6106–6111. doi: 10.1073/pnas.1000175107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li CS, Yan P, Bergquist KL, Sinha R. Greater activation of the “default” brain regions predicts stop signal errors. Neuroimage. 2007;38:640–648. doi: 10.1016/j.neuroimage.2007.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Congdon E, et al. Engagement of large-scale networks is related to individual differences in inhibitory control. Neuroimage. 2010;53:653–663. doi: 10.1016/j.neuroimage.2010.06.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weissman DH, Roberts KC, Visscher KM, Woldorff MG. The neural bases of momentary lapses in attention. Nat Neurosci. 2006;9:971–978. doi: 10.1038/nn1727. [DOI] [PubMed] [Google Scholar]
- 14.Duann JR, Ide JS, Luo X, Li CS. Functional connectivity delineates distinct roles of the inferior frontal cortex and presupplementary motor area in stop signal inhibition. J Neurosci. 2009;29:10171–10179. doi: 10.1523/JNEUROSCI.1300-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends Cogn Sci. 2004;8:170–177. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 16.McDonald BC, Flashman LA, Saykin AJ. Executive dysfunction following traumatic brain injury: Neural substrates and treatment strategies. NeuroRehabilitation. 2002;17:333–344. [PubMed] [Google Scholar]
- 17.Niogi SN, et al. Extent of microstructural white matter injury in postconcussive syndrome correlates with impaired cognitive reaction time: A 3T diffusion tensor imaging study of mild traumatic brain injury. AJNR Am J Neuroradiol. 2008;29:967–973. doi: 10.3174/ajnr.A0970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kraus MF, et al. White matter integrity and cognition in chronic traumatic brain injury: A diffusion tensor imaging study. Brain. 2007;130:2508–2519. doi: 10.1093/brain/awm216. [DOI] [PubMed] [Google Scholar]
- 19.Kinnunen KM, et al. White matter damage and cognitive impairment after traumatic brain injury. Brain. 2011;134:449–463. doi: 10.1093/brain/awq347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Niogi SN, Mukherjee P. Diffusion tensor imaging of mild traumatic brain injury. J Head Trauma Rehabil. 2010;25:241–255. doi: 10.1097/HTR.0b013e3181e52c2a. [DOI] [PubMed] [Google Scholar]
- 21.Bonnelle V, et al. Default mode network connectivity predicts sustained attention deficits after traumatic brain injury. J Neurosci. 2011;31:13442–13451. doi: 10.1523/JNEUROSCI.1163-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sonuga-Barke EJ, Castellanos FX. Spontaneous attentional fluctuations in impaired states and pathological conditions: A neurobiological hypothesis. Neurosci Biobehav Rev. 2007;31:977–986. doi: 10.1016/j.neubiorev.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 23.Fransson P, Marrelec G. The precuneus/posterior cingulate cortex plays a pivotal role in the default mode network: Evidence from a partial correlation network analysis. Neuroimage. 2008;42:1178–1184. doi: 10.1016/j.neuroimage.2008.05.059. [DOI] [PubMed] [Google Scholar]
- 24.Leech R, Kamourieh S, Beckmann CF, Sharp DJ. Fractionating the default mode network: Distinct contributions of the ventral and dorsal posterior cingulate cortex to cognitive control. J Neurosci. 2011;31:3217–3224. doi: 10.1523/JNEUROSCI.5626-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sharp DJ, et al. Default mode network functional and structural connectivity after traumatic brain injury. Brain. 2011;134:2233–2247. doi: 10.1093/brain/awr175. [DOI] [PubMed] [Google Scholar]
- 26.Mac Donald CL, Dikranian K, Bayly P, Holtzman D, Brody D. Diffusion tensor imaging reliably detects experimental traumatic axonal injury and indicates approximate time of injury. J Neurosci. 2007;27:11869–11876. doi: 10.1523/JNEUROSCI.3647-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Menon V, Uddin LQ. Saliency, switching, attention and control: A network model of insula function. Brain Struct Funct. 2010;214:655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van den Heuvel MP, Mandl RC, Kahn RS, Hulshoff Pol HE. Functionally linked resting-state networks reflect the underlying structural connectivity architecture of the human brain. Hum Brain Mapp. 2009;30:3127–3141. doi: 10.1002/hbm.20737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Uddin LQ, Supekar KS, Ryali S, Menon V. Dynamic reconfiguration of structural and functional connectivity across core neurocognitive brain networks with development. J Neurosci. 2011;31:18578–18589. doi: 10.1523/JNEUROSCI.4465-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schmahmann JD, et al. Association fibre pathways of the brain: Parallel observations from diffusion spectrum imaging and autoradiography. Brain. 2007;130:630–653. doi: 10.1093/brain/awl359. [DOI] [PubMed] [Google Scholar]
- 31.Petrides M, Pandya DN. Efferent association pathways from the rostral prefrontal cortex in the macaque monkey. J Neurosci. 2007;27:11573–11586. doi: 10.1523/JNEUROSCI.2419-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schmahmann JD, Pandya DN. Fiber Pathways of the Brain. New York: Oxford University Press; 2006. [Google Scholar]
- 33.Zhou J, et al. Divergent network connectivity changes in behavioural variant frontotemporal dementia and Alzheimer's disease. Brain. 2010;133:1352–1367. doi: 10.1093/brain/awq075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kelly AM, Uddin LQ, Biswal BB, Castellanos FX, Milham MP. Competition between functional brain networks mediates behavioral variability. Neuroimage. 2008;39:527–537. doi: 10.1016/j.neuroimage.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 35.Singh KD, Fawcett IP. Transient and linearly graded deactivation of the human default-mode network by a visual detection task. Neuroimage. 2008;41:100–112. doi: 10.1016/j.neuroimage.2008.01.051. [DOI] [PubMed] [Google Scholar]
- 36.Pyka M, et al. Impact of working memory load on FMRI resting state pattern in subsequent resting phases. PLoS ONE. 2009;4:e7198. doi: 10.1371/journal.pone.0007198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hagmann P, et al. Mapping the structural core of human cerebral cortex. PLoS Biol. 2008;6:e159. doi: 10.1371/journal.pbio.0060159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fassbender C, et al. A lack of default network suppression is linked to increased distractibility in ADHD. Brain Res. 2009;1273:114–128. doi: 10.1016/j.brainres.2009.02.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Frings L, et al. Reduced precuneus deactivation during object naming in patients with mild cognitive impairment, Alzheimer's disease, and frontotemporal lobar degeneration. Dement Geriatr Cogn Disord. 2010;30:334–343. doi: 10.1159/000320991. [DOI] [PubMed] [Google Scholar]
- 40.Nelson SM, et al. Role of the anterior insula in task-level control and focal attention. Brain Struct Funct. 2010;214:669–680. doi: 10.1007/s00429-010-0260-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Medford N, Critchley HD. Conjoint activity of anterior insular and anterior cingulate cortex: Awareness and response. Brain Struct Funct. 2010;214:535–549. doi: 10.1007/s00429-010-0265-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wyland CL, Kelley WM, Macrae CN, Gordon HL, Heatherton TF. Neural correlates of thought suppression. Neuropsychologia. 2003;41:1863–1867. doi: 10.1016/j.neuropsychologia.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 43.Lerner A, et al. Involvement of insula and cingulate cortices in control and suppression of natural urges. Cereb Cortex. 2009;19:218–223. doi: 10.1093/cercor/bhn074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Posner MI, Rothbart MK. Toward a physical basis of attention and self regulation. Phys Life Rev. 2009;6:103–120. doi: 10.1016/j.plrev.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mason MF, et al. Wandering minds: The default network and stimulus-independent thought. Science. 2007;315:393–395. doi: 10.1126/science.1131295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smith SM, et al. Network modelling methods for FMRI. Neuroimage. 2011;54:875–891. doi: 10.1016/j.neuroimage.2010.08.063. [DOI] [PubMed] [Google Scholar]
- 47.Stuss DT, Alexander MP. Is there a dysexecutive syndrome? Philos Trans R Soc Lond B Biol Sci. 2007;362:901–915. doi: 10.1098/rstb.2007.2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aron AR, Fletcher PC, Bullmore ET, Sahakian BJ, Robbins TW. Stop-signal inhibition disrupted by damage to right inferior frontal gyrus in humans. Nat Neurosci. 2003;6:115–116. doi: 10.1038/nn1003. [DOI] [PubMed] [Google Scholar]
- 49.Floden D, Stuss DT. Inhibitory control is slowed in patients with right superior medial frontal damage. J Cogn Neurosci. 2006;18:1843–1849. doi: 10.1162/jocn.2006.18.11.1843. [DOI] [PubMed] [Google Scholar]
- 50.Corbetta M, Shulman GL. Spatial neglect and attention networks. Annu Rev Neurosci. 2011;34:569–599. doi: 10.1146/annurev-neuro-061010-113731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hogan AM, Vargha-Khadem F, Saunders DE, Kirkham FJ, Baldeweg T. Impact of frontal white matter lesions on performance monitoring: ERP evidence for cortical disconnection. Brain. 2006;129:2177–2188. doi: 10.1093/brain/awl160. [DOI] [PubMed] [Google Scholar]
- 52.Boehler CN, Appelbaum LG, Krebs RM, Hopf JM, Woldorff MG. Pinning down response inhibition in the brain—conjunction analyses of the stop-signal task. Neuroimage. 2010;52:1621–1632. doi: 10.1016/j.neuroimage.2010.04.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Leotti LA, Wager TD. Motivational influences on response inhibition measures. J Exp Psychol Hum Percept Perform. 2010;36:430–447. doi: 10.1037/a0016802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bekker EM, Böcker KB, Van Hunsel F, van den Berg MC, Kenemans JL. Acute effects of nicotine on attention and response inhibition. Pharmacol Biochem Behav. 2005;82:539–548. doi: 10.1016/j.pbb.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 55.Oakes TR, et al. Integrating VBM into the General Linear Model with voxelwise anatomical covariates. Neuroimage. 2007;34:500–508. doi: 10.1016/j.neuroimage.2006.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Aron AR, Behrens TE, Smith S, Frank MJ, Poldrack RA. Triangulating a cognitive control network using diffusion-weighted magnetic resonance imaging (MRI) and functional MRI. J Neurosci. 2007;27:3743–3752. doi: 10.1523/JNEUROSCI.0519-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- 58.Hua K, et al. Tract probability maps in stereotaxic spaces: Analyses of white matter anatomy and tract-specific quantification. Neuroimage. 2008;39:336–347. doi: 10.1016/j.neuroimage.2007.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.