Abstract
Signal transduction pathways play diverse, context-dependent roles in vertebrate development. In studies of human embryonic stem cells (hESCs), conflicting reports claim Wnt/β-catenin signaling promotes either self-renewal or differentiation. We use a sensitive reporter to establish that Wnt/β-catenin signaling is not active during hESC self-renewal. Inhibiting this pathway over multiple passages has no detrimental effect on hESC maintenance, whereas activating signaling results in loss of self-renewal and induction of mesoderm lineage genes. Following exposure to pathway agonists, hESCs exhibit a delay in activation of β-catenin signaling, which led us to postulate that Wnt/β-catenin signaling is actively repressed during self-renewal. In support of this hypothesis, we demonstrate that OCT4 represses β-catenin signaling during self-renewal and that targeted knockdown of OCT4 activates β-catenin signaling in hESCs. Using a fluorescent reporter of β-catenin signaling in live hESCs, we observe that the reporter is activated in a very heterogeneous manner in response to stimulation with Wnt ligand. Sorting cells on the basis of their fluorescence reveals that hESCs with elevated β-catenin signaling express higher levels of differentiation markers. Together these data support a dominant role for Wnt/β-catenin signaling in the differentiation rather than self-renewal of hESCs.
Embryonic stem cells (ESCs) derived from the inner cell mass of preimplantation-stage mammalian embryos are pluripotent cells capable of proliferating in their undifferentiated state in vitro while maintaining the ability to give rise to all three primary germ layers. Once established in culture, ESC lines can be propagated indefinitely. Whereas human and mouse ESCs share many key characteristics, these species differ in the signal transduction pathways that influence self-renewal. This may be partly due to the fact that human ESCs (hESCs) more closely resemble epiblast stem cells from the mouse, which correspond to a slightly later developmental stage than inner cell mass cells (1, 2).
The Wnt gene family encodes evolutionarily conserved, secreted glycoproteins that act as ligands to stimulate several signal transduction pathways, thereby regulating processes in both embryonic development and in adults (3–5). Signaling through the best understood pathway, the Wnt/β-catenin pathway, is mediated through posttranslational regulation of the stability of β-catenin. Activation of Wnt signaling leads to the accumulation of β-catenin in nuclei, where it binds to high mobility group (HMG) box transcription factors of the T-cell factor (TCF) and lymphoid enhancing factor (LEF) families and promotes context-dependent changes in transcription (5).
Wnt/β-catenin signaling has been implicated in the maintenance of both mouse and human ESCs in vitro (6–13). Wnt signaling has also been reported to promote the acquisition of a pluripotent state during reprogramming of somatic cells to induced pluripotent stem cells (14, 15). Many studies have shown that activating Wnt/β-catenin signaling promotes self-renewal of mouse ESCs (mESCs) (6, 7, 10–13), whereas reciprocal loss-of-function (LOF) studies indicate that β-catenin is required for multilineage differentiation but is dispensable for self-renewal (13, 16, 17).
The role of Wnt/β-catenin signaling in hESCs is less clear due to contradictory results among published studies. Sato et al. (7) found that activating the Wnt/β-catenin pathway with either Wnt3A or a GSK3 inhibitor, BIO, maintained the self-renewal of hESCs under feeder-free conditions. Conversely, others have reported that Wnt3A or GSK3 inhibitors lead to differentiation of hESCs toward primitive streak and definitive endoderm lineages (18, 19). Ullmann et al. (20) found that BIO promoted undifferentiated cellular morphology and maintained expression of pluripotency markers in short-term assays, but was not sufficient to expand undifferentiated hESCs over multiple passages. In other studies, Wnt3A and Wnt1 transiently stimulated proliferation and/or increased clonal survival of hESCs, but failed to maintain other functional measures of pluripotency over several passages (21–23). Whether Wnt/β-catenin signaling maintains hESCs in an undifferentiated and self-renewing state, or whether it promotes differentiation, remains controversial.
Results
Activation of Wnt/β-Catenin Signaling Promotes Loss of Self-Renewal of hESCs.
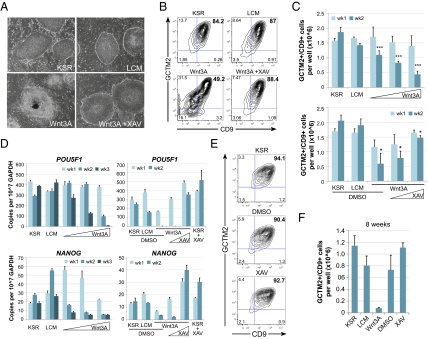
To resolve the roles of Wnt/β-catenin signaling in hESCs, we first investigated the consequences of activating the pathway during multiple passages of the cells. hESCs cultured with Wnt3A conditioned medium (CM) adopt a morphology characteristic of differentiation (Fig. 1A) and down-regulate expression of stem cell surface markers, GCTM2 and CD9 (Fig. 1B). If Wnt/β-catenin signaling were promoting the maintenance of hESCs, then one would expect the application of exogenous Wnt3A ligand should promote expansion of phenotypically undifferentiated cells via self-renewal. Therefore, we measured the total number of GCTM2+/CD9+ cells present when hESCs were cultured with Wnt3A CM at 1- and 2-wk time points. We observed that increasing concentrations of Wnt3A CM led to significantly fewer undifferentiated hESCs after 2 wk compared with control CM (Fig. 1C). Remarkably, this change occurred despite cells being cultured on mouse embryonic fibroblasts (MEFs) and in medium otherwise supportive of maintenance. Likewise, treatment of hESCs with Wnt3A CM also led to down-regulation of POU5F1 (OCT4) and NANOG mRNA levels relative to control L cell CM (LCM) (Fig. 1D). Importantly, the loss of maintenance markers occurred in a dose-dependent manner. We also activated Wnt/β-catenin signaling with recombinant Wnt3A or with the GSK3-β inhibitor, BIO, and found that these treatments led to similar results as Wnt3A CM (Fig. S1A).
Fig. 1.
Wnt3A fails to maintain hESCs. (A) H1 cells cultured for 1 wk on MEFs in KSR media, 50% (vol/vol) control L cell CM (LCM) in KSR, 50% (vol/vol) Wnt3A CM, or 50% Wnt3A CM + 2.5 μM XAV. DMSO vehicle control was included in all media without XAV. (B) Representative flow cytometric plots showing GCTM2 and CD9 coexpression in hESCs cultured in the same conditions as A for 1 wk. (C) Graph showing the total number of GCTM2+/CD9+ cells each week per well of a 12-well plate. Graphs of mean + SEM from three independent experiments. Differences at week two (wk2) were significant by ANOVA with a posttest for linear trend (*P < 0.05, ***P < 0.001). (D) Representatitve qRT-PCR of stem cell pluripotency-associated transcripts. Graphs of mean + SEM of experimental replicates. (E) Representative flow cytometric plots of GCTM2 and CD9 coexpression for hESCs cultured continuously with 2.5 μM XAV for 6 wk. For C and D: concentrations of Wnt3a CM tested were 12.5, 25, and 50%, with LCM added to yield 50% total CM; XAV concentrations tested were 0.2 μM and 2.5 μM in 50% Wnt3A CM. (F) Graph showing the total number of GCTM2+/CD9+ cells per well of a 12-well plate after 8 wk of continual exposure to 50% L or Wnt3A CM, DMSO (vehicle control), or 2.5 μM XAV. Graphs of mean + SEM from two independent experiments.
We hypothesized that if Wnt3A CM promotes loss of hESC maintenance through Wnt/β-catenin signaling, then we should be able to block this effect using downstream inhibitors of the pathway. XAV939 (XAV) is a Tankyrase inhibitor that effectively antagonizes Wnt/β-catenin signaling downstream of receptor activation by stabilizing levels of Axin, a negative regulator of β-catenin (Fig. S2) (24). We found that the ability of Wnt3A CM to promote loss of maintenance of hESCs was blocked by coincubating cells with XAV in a dose-dependent manner (Fig. 1 A–C). This observation suggests that the effects of Wnt3A CM are due specifically to Wnt/β-catenin signaling and not to other components of the CM. Down-regulation of POU5F1 and NANOG transcripts was also inhibited by XAV (Fig. 1D). Collectively, these experiments demonstrate that activating Wnt/β-catenin signaling leads to loss of hESC maintenance under conditions that are permissive for self-renewal.
Endogenous Wnt/β-Catenin Signaling Is Not Required for Self-Renewal of hESCs.
We next investigated the effects of long-term inhibition of Wnt/β-catenin signaling in hESCs. We cultured hESCs with 2.5 μM XAV for 8 wk and found no significant difference from hESCs cultured in standard hESC medium (KSR) on MEFs (Fig. 1 E and F). The weekly split ratio for XAV-cultured hESCs was stable and the hESCs remain phenotypically undifferentiated (>90% GCTM2+/CD9+, indistinguishable from controls; Fig. 1E). Transcripts for POU5F1 and NANOG are equal or higher in XAV- versus vehicle-treated hESCs (Fig. 1D). These observations show that endogenous Wnt/β-catenin signaling is dispensable for hESC self-renewal.
Wnt/β-Catenin Signaling Drives Mesoderm Lineage Differentiation of hESCs.
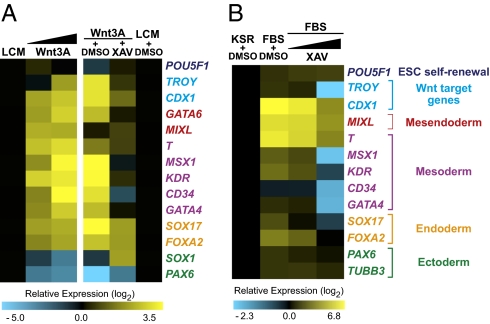
Our observation that cell morphologies change in the presence of exogenous Wnt3A (Fig. 1A) suggested that Wnt/β-catenin signaling promotes differentiation of hESCs. To investigate this directly we asked whether Wnt/β-catenin signaling promotes changes in expression of a panel of lineage-specific markers assessed by qRT-PCR. We found that Wnt3A leads to elevation of transcripts associated with early mesoderm, endoderm, and primitive streak cell fates (Fig. 2A). Comparable results are observed following treatment of hESCs with either recombinant Wnt3A protein or with Wnt3A CM (Fig. S3). As a control we showed that XAV blocks induction of mesoderm and primitive streak transcripts in hESCs treated with Wnt3A CM and reduces levels of endoderm transcripts (Fig. 2A). Interestingly, serum alone, without adding exogenous Wnt, also induces transcripts associated with multilineage differentiation. We found that inhibition of β-catenin signaling via XAV is also sufficient to block serum-induced mesoderm transcripts in a dose-dependent manner (Fig. 2B). Taken together, these results suggest that Wnt/β-catenin signaling drives transcriptional changes indicative of mesoderm lineage differentiation of hESCs. This finding is in agreement with previous publications suggesting a role for Wnt in differentiation rather than self-renewal of hESCs (18, 19, 22).
Fig. 2.
Wnt3A induces lineage-specific transcripts of differentiation. (A) Heat maps of qRT-PCR results from H1 cells cultured for 6 d on MEFs in KSR media plus the conditions listed: LCM (50%), Wnt3A CM (Left,12.5 and 50%; Right, 50%), and XAV (2.5 μM). Wnt3A leads to increased transcripts associated with mesoderm and endoderm lineages in a dose-dependent manner (Left). Transcriptional changes resulting from Wnt3A treatment are blocked by coadministration of XAV (Right). (B) Medium containing 20% FBS also induces transcripts associated with multilineage differentiation. Inhibition of β-catenin signaling in serum leads to a dose-dependent reduction in mesoderm and endoderm transcripts. XAV concentrations, 0.2 μM and 2.5 μM. Representative data are shown from one experiment.
Endogenous Wnt/β-Catenin Signaling Is Inactive in Undifferentiated hESCs and Active in Differentiating hESCs.
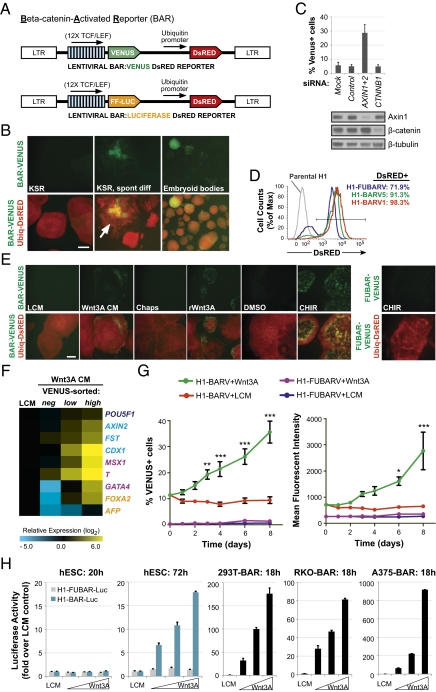
To monitor β-catenin/TCF signaling in live cells, we generated multiple hESC lines stably transduced with a fluorescence-based β-catenin activated reporter (BAR-VENUS; Fig. 3A). Remarkably, hESCs stably transduced with the BAR reporter have low/undetectable levels of β-catenin signaling in mostly undifferentiated colonies, whereas endogenous BAR activity is observed primarily in cases of morphological differentiation (Fig. 3B). The levels of VENUS in undifferentiated hESCs were similar to those produced by a control reporter that contained mutant TCF-binding sites, denoted as found unresponsive BAR (FUBAR) (25). Moreover, we found that CTNNB1 (β-catenin) siRNA transfection did not significantly reduce basal levels of VENUS expression in undifferentiated hESCs (Fig. 3C). This result suggests that endogenous Wnt/β-catenin signaling is less active in undifferentiated hESCs during self-renewal.
Fig. 3.
β-catenin reporter hESCs. (A) Schematic of the BAR lentiviral vector used to generate stable H1-BAR-Venus (BARV) and H1-BAR-Luciferase (BAR-Luc) cell lines. The construct contains 12× TCF binding sites upstream of a minimal promoter driving VENUS fluorescent protein or Firefly Luciferase, followed by a Ubiquitin promoter driving DsRED fluorescent protein. (B) Undifferentiated H1-BARV cells exhibit low/absent endogenous reporter activity when cultured on MEFs in KSR medium. Endogenous VENUS expression is observed under various contexts of differentiation (spont diff, spontaneous differentiated colony, marked with an arrow). (Scale bar, 400 μm.) (C) H1-BARV cells show no significant reduction in VENUS expression versus control at 72 h posttransfection with CTNNB1 siRNA. AXIN1+2 siRNAs serve as a positive control. Graph of mean + SEM of three independent experiments. (D) After mechanical selection of DsRED-positive cells, H1-BARV lines are ≥90% DsRED+. (E) hESCs respond to Wnt pathway stimuli heterogeneously, both within and between colonies. H1-FUBARV cells containing mutated TCF-binding sites are unresponsive to CHIR. (L or Wnt3A CM, 50%; 1% Chaps is the vehicle control for rWnt3a; rWnt3A, 500 ng/mL recombinant Wnt3a; DMSO is the vehicle control for CHIR; CHIR, 6 μM). (Scale bar, 400 μm.) (F) 72 h Wnt3A-treated hESCs were sorted on the basis of VENUS expression into negative-, low-, and high-expressing subpopulations, then analyzed by qRT-PCR. Results from a representative experiment are shown. Data show that reporter heterogeneity in response to Wnt reflects transcriptional differences indicative of true variations in levels of β-catenin signaling. (G) Reporter activity, as measured by percent of VENUS-positive cells (Left) and mean fluorescent intensity of these cells (Right), over time of Wnt3A treatment (50% CM). Graph of mean ± SEM from three independent experiments. Reporter response following Wnt3A treatment was significantly different from the control treatment by two-way ANOVA with Bonferroni posttest (*P < 0.05, **P < 0.01, ***P < 0.001). H1-FUBARV cells containing mutated TCF-binding sites are unresponsive to Wnt3A. (H) Luciferase reporter assay results from stable BAR-Luciferase transduced hESCs and nonpluripotent cell lines stimulated with control LCM or increasing concentrations of Wnt3A CM for the period (hours) noted for each graph.
Heterogeneous Activation of Wnt/β-Catenin Signaling in Response to Pathway Agonists Reflects Intrinsic Differences in hESCs.
To confirm that BAR could be activated, we stimulated hESCs with exogenous Wnt3A or the GSK3 inhibitor, CHIR99021 (CHIR). We also confirmed that β-catenin protein levels were stabilized, as expected, with pathway agonists (Fig. S4). We observed a heterogeneous pattern of VENUS expression both within and between colonies of H1-BARV hESCs exposed to pathway agonists, even though expression of the Ubiquitin-DsRED portion of the integrated construct was strongly maintained in all colonies (Fig. 3 D and E). We then tested whether heterogeneity observed with Wnt-induced VENUS expression was due to differences in the intrinsic ability of the cells to respond to Wnt signaling or due to transient silencing of the BAR-VENUS portion of the integrated reporter. Specifically, H1-BAR-VENUS cells were stimulated with Wnt3A CM for 72 h to induce VENUS expression, then FACS-sorted cells into VENUS-negative, -low, and -high subpopulations for transcriptional analysis. In this context, the VENUS-negative cells had been exposed to the same Wnt3A signal as the VENUS-positive cells. If VENUS-negative cells have silenced the VENUS reporter but actively signal through β-catenin, then we would expect their transcriptional response to be equivalent to VENUS-positive cells. Instead, we found that VENUS-negative cells have a much lower Wnt transcriptional signature versus VENUS-positive cells (Fig. 3F), suggesting that these cells in fact have low levels of β-catenin signaling. Compared with control LCM-treated hESCs, Wnt-treated VENUS-negative hESCs express similar or lower levels of transcripts for Wnt/β-catenin target genes, as well as mesoderm and endoderm lineages (Fig. 3F). Furthermore, VENUS-high cells express higher levels of Wnt3A-induced transcripts compared with VENUS-low cells, indicating that the fluorescent intensity of our BAR-VENUS reporter correlates with quantitative transcriptional measures of Wnt/β-catenin signaling. These results confirm that the heterogeneous reporter response observed in BAR-VENUS cells reflects genuine transcriptional differences in levels of Wnt/β-catenin signaling in cultures of hESCs.
Interestingly, we observe that Wnt-induced reporter expression is reversible if VENUS-positive hESCs are replated back into self-renewal conditions without Wnt (Fig. S5). hESCs treated with Wnt3A CM for 1 wk, then replated in KSR media without Wnt for another week also express an undifferentiated phenotype, comparable to controls (Fig. S5).
hESCs Exhibit a Delay in Activating Wnt/β-Catenin Signaling Compared with Nonpluripotent Cells.
When we examined the time frame of VENUS expression in hESCs following stimulation with Wnt3A, we found that it takes at least 3 d to detect robust and statistically significant increases in the percentage of VENUS-positive cells above control LCM-treated cells (Fig. 3G). We also observed consistent results regarding the kinetics of Wnt3A-induced reporter activation using H1-BAR-Luciferase (H1-BAR-Luc) cells. In comparison with nonpluripotent cells, hESCs require longer to activate the BAR-Luciferase reporter following stimulation with Wnt3A (72 h for hESCs versus 18 h for nonpluripotent HEK293T, RKO, and A375 cells; Fig. 3H). These observations are consistent with the hypothesis that undifferentiated hESCs are refractory to Wnt/β-catenin signaling due to active repression of the pathway and that the delay in reporter response reflects the gradual alleviation of this repression.
Oct4 Functionally Represses Wnt/β-Catenin Signaling in Undifferentiated hESCs.
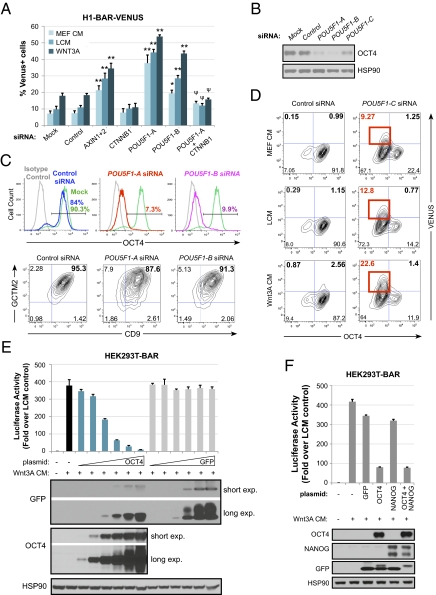
A key stem cell pluripotency factor, OCT4, has been previously reported to regulate levels of β-catenin (26). Thus, we examined whether OCT4 functionally regulates Wnt/β-catenin signaling in hESCs by carrying out LOF experiments with siRNAs. Interestingly, transient knockdown of POU5F1 (OCT4) results in activation of the BAR reporter (Fig. 4A) and stabilization of β-catenin protein levels (Fig. S4) in hESCs independent of exogenous Wnt. Two of our POU5F1 siRNAs (POU5F1-A and -B) achieved complete OCT4 knockdown in ≥90% of hESCs (Fig. 4 B and C), whereas one POU5F1 siRNA sequence (POU5F1-C) was less efficient at knocking down OCT4 protein (Fig. 4 B and D). Levels of reporter activation resulting from OCT4 knockdown with POU5F1-A and -B were statistically significant compared with control siRNA and similar to, or higher than, that achieved by knockdown of AXIN1 and AXIN2, known negative regulators of β-catenin. BAR-VENUS expression could be blocked by cotransfection of CTNNB1 siRNA with POU5F1 siRNA, indicating that β-catenin is required for reporter activation in cells depleted of OCT4.
Fig. 4.
Oct4 functionally represses β-catenin/TCF signaling in hESCs. (A) H1-BAR-VENUS cells transfected with POU5F1 siRNA activate the reporter. BAR reporter response for both POU5F1 siRNAs was significantly different from control siRNA by two-way ANOVA with Bonferroni posttest (*P < 0.01, **P < 0.001). CTNNB1 in combination with POU5F1-A siRNA significantly blocks reporter activation induced by POU5F1-A siRNA alone (ψP < 0.001). Graph of mean + SEM from three independent experiments analyzed at 72 h post-siRNA transfection with 24 h stimulation with 50% L or Wnt3A CM in MEF CM. (B) Western blots validating that POU5F1 siRNAs lead to targeted knockdown of OCT4 protein in hESCs at 72 h posttransfection. (C) Flow plots showing OCT4 (Upper), CGTM2, and CD9 labeling (Lower) of siRNA-transfected H1-BAR-VENUS cells. (D) Flow plots showing expression of VENUS and OCT4 in H1-BAR-VENUS cells. Red boxes indicate OCT4−/VENUS+ subpopulation. (E) Graph of HEK293T cells transiently transfected with pBAR-Luciferase reporter and increasing concentrations of GFP or OCT4 expression plasmids, then stimulated overnight with Wnt3A CM. Bars denote SEM from experimental replicates. Western blots confirm that the protein of interest was expressed for each sample. The Oct4 plasmid contained GFP and Oct4 connected by a 2A-linker peptide, previously described (38). (F) Graph of HEK293T cells transiently transfected with pBAR-Luciferase reporter and expression plasmids noted, then stimulated overnight with Wnt3A CM. Bars denote SEM from experimental replicates. Western blots confirm that proteins of interest were expressed for each sample.
Although reduced OCT4 expression will eventually lead to differentiation of hESCs (27), at 72 h post-siRNA transfection OCT4-negative hESCs are still positive for other stem cell markers, GCTM2 and CD9 (Fig. 4C). This combination of cell surface markers was chosen because coexpression of GCTM2 and CD9 is reported to correlate with OCT4 protein coexpression, increased levels of pluripotency transcripts, and decreased levels of lineage-specific transcripts in hESCs (28). Therefore, activation of the BAR reporter by POU5F1 knockdown at this time point is unlikely to be due to overt differentiation. Interestingly, when we treat hESCs with the inefficient POU5F1 siRNA (POU5F1-C), which results in a mixed population of OCT4-positive and OCT4-negative cells, we observe that all of the VENUS expression lies within the OCT4-negative subpopulation (Fig. 4D). These experiments further support the hypothesis that OCT4 functionally represses Wnt/β-catenin signaling in hESCs.
We next investigated whether OCT4 gain-of-function can repress Wnt/β-catenin signaling in nonpluripotent cells. Indeed, transient expression of OCT4 in HEK293T cells inhibits BAR reporter activity induced by Wnt3A CM (Fig. 4E), consistent with other reports (26, 29). OCT4 and NANOG are known to regulate each others’ promoters (30–32). Not surprisingly, we found that knockdown of POU5F1 in hESCs results in concurrent decrease of NANOG protein and vice versa (Fig. S6). Thus, we also examined whether NANOG contributes to Wnt/β-catenin repression in HEK293T cells. Our results indicate that expression of NANOG had no significant effect on Wnt-induced BAR reporter activity, alone or in combination with OCT4, implying that OCT4-mediated repression of Wnt/β-catenin signaling does not require NANOG (Fig. 4F).
Discussion
One explanation for variations in the effects of Wnt/β-catenin activation in hESCs observed in different studies may involve the dose and duration of Wnt stimulus. The fact that Wnt3A required more than 1 wk to significantly diminish self-renewal of hESCs in the present study may explain why other studies that used short-term assays did not observe the same effect. We also tested multiple doses of Wnt3A and XAV to confirm effects were dose dependent versus studies that used a single dose of Wnt3A. Interestingly, similar to Bone et al. (18), we found that the concentration of BIO published to maintain hESCs (2 μM) (7) was not sufficient to robustly activate the β-catenin reporter in the absence of exogenous Wnt (Fig. S1B). hESC maintenance was diminished to a level similar to that observed with Wnt3A when cells were cultured with a higher, reporter-activating concentration of BIO (4 μM) for 3 wk (Fig. S1A). Similarly, we found that concentrations of recombinant Wnt3A that activated the BAR reporter lead to loss of hESC maintenance, whereas lower concentrations that did not robustly activate the reporter had no effect on maintenance (Fig. S1 A and C).
Although other studies have concluded that Wnt/β-catenin signaling is active in hESCs due to nuclear β-catenin immunostaining, subcellular localization of β-catenin in undifferentiated versus differentiated hESCs is inconsistent between studies (7, 20). Moreover, steady-state levels of nuclear β-catenin do not always correlate with TCF-mediated transcriptional activity (33), and the ability of β-catenin to facilitate transcriptional activation with TCF may also be modulated by other interacting proteins, such as ICAT for example (34). Therefore, our study used detection of β-catenin/TCF transcriptional activity to monitor whether Wnt/β-catenin signaling was active in live hESCs. Whereas negligible endogenous β-catenin signaling was observed in undifferentiated hESCs, endogenous signaling was subsequently elevated in hESCs undergoing differentiation. Our demonstration that hESCs can be sorted on the basis of a fluorescent β-catenin/TCF transcriptional reporter provides a platform by which cells can be sorted on the basis of live signaling activity, thereby allowing for more precise interrogation of Wnt/ β-catenin signaling in a variety of downstream applications.
Regarding the heterogeneity observed in β-catenin reporter hESCs in response to exogenous Wnt3a, we cannot exclude the possibility that some hESCs may be more primed for differentiation and this may account for differences in their sensitivity to Wnt3a. Indeed, such heterogeneity has been previously reported in hESC cultures (35). However, Wnt-induced reporter expression is reversible if the exogenous Wnt3a is withdrawn, and after withdrawal the hESCs retain an undifferentiated phenotype and self-renew, suggesting that VENUS-positive cells are not irreversibly committed to differentiate under these conditions.
Although Wnt/β-catenin signaling has been implicated in promoting self-renewal of hESCs (7), several studies have found β-catenin pathway activation transiently enhances hESC proliferation and/or self-renewal, but ultimately fails to maintain hESCs after multiple passages (9, 20–23). Our study calls for a revised interpretation of the role of Wnt/β-catenin signaling in hESCs and supports a primary role for Wnt/β-catenin signaling in the differentiation, rather than self-renewal, of hESCs in vitro. The long-term expansion achieved with sustained inhibition of the pathway suggests that endogenous Wnt/β-catenin signaling is in fact not required for the self-renewal of undifferentiated hESCs. Furthermore, activating β-catenin signaling leads to induction of mesoderm lineage transcripts and an eventual loss of self-renewal after several passages. Given that activation of the Wnt pathway leads to loss of hESC self-renewal, we speculate that intrinsic inhibition of β-catenin/TCF signaling may actually be critical for the maintenance of undifferentiated hESCs. In support of this theory, we also find that Oct4, a key pluripotency factor highly expressed in hESCs, functionally represses endogenous Wnt/β-catenin signaling in self-renewing hESCs. Taken together, our results support a model whereby Wnt/β-catenin signaling is repressed by Oct4 in the context of self-renewing hESCs and is derepressed when hESCs differentiate.
Materials and Methods
hESC Culture.
H1 hESCs were cultured on irradiated MEF feeders (2.5 × 104 MEF/cm2) in 20% (vol/vol) knockout serum replacement medium + 8 ng/mL basic (b)FGF (36). Colonies were passaged ∼1:12 weekly as small clusters using dispase (1.75 units/mL; Invitrogen). This split ratio corresponds to 30,000–50,000 hESCs per centimeter squared. MEF carryover was minimized during passage by gravity settling hESC clusters in a conical tube, and then aspirating the medium above the settled pellet before seeding hESCs. For feeder-free experiments, hESCs were seeded onto matrigel (Invitrogen; coated at 35 μg/cm2) and cultured in KSR medium + 4 ng/mL bFGF conditioned overnight on irradiated MEFs (6 × 104 MEF/cm2) (hereby referred to as MEF CM). MEF CM was sterile filtered and freshly supplemented with 8 ng/mL bFGF before use. All hESC lines used for this study were confirmed to be karyotypically normal and mycoplasma-free. For manual selection of hESC, see Fig. S7.
Additional experimental procedures are provided in SI Materials and Methodsand Table S1.
Supplementary Material
Acknowledgments
We thank Mick Bhatia for the pMIN-Ub-DsRED-WPRE construct (37). This work was supported by the National Institutes of Health (NIH) Grants 1P01–GM081619 and NIH U01 vHL100395 and T.L.B. is funded in part through NIH/National Institute of Arthritis and Musculoskeletal and Skin Diseases Training Grant T32AR056969. R.T.M. is an Investigator of the Howard Hughes Medical Institute.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1118777109/-/DCSupplemental.
References
- 1.Brons IG, et al. Derivation of pluripotent epiblast stem cells from mammalian embryos. Nature. 2007;448:191–195. doi: 10.1038/nature05950. [DOI] [PubMed] [Google Scholar]
- 2.Tesar PJ, et al. New cell lines from mouse epiblast share defining features with human embryonic stem cells. Nature. 2007;448:196–199. doi: 10.1038/nature05972. [DOI] [PubMed] [Google Scholar]
- 3.Kohn AD, Moon RT. Wnt and calcium signaling: Beta-catenin-independent pathways. Cell Calcium. 2005;38:439–446. doi: 10.1016/j.ceca.2005.06.022. [DOI] [PubMed] [Google Scholar]
- 4.Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 5.Moon RT. Wnt/beta-catenin pathway. Sci STKE. 2005;2005:cm1. doi: 10.1126/stke.2712005cm1. [DOI] [PubMed] [Google Scholar]
- 6.Hao J, Li TG, Qi X, Zhao DF, Zhao GQ. WNT/beta-catenin pathway up-regulates Stat3 and converges on LIF to prevent differentiation of mouse embryonic stem cells. Dev Biol. 2006;290:81–91. doi: 10.1016/j.ydbio.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 7.Sato N, Meijer L, Skaltsounis L, Greengard P, Brivanlou AH. Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor. Nat Med. 2004;10:55–63. doi: 10.1038/nm979. [DOI] [PubMed] [Google Scholar]
- 8.Miyabayashi T, et al. Wnt/beta-catenin/CBP signaling maintains long-term murine embryonic stem cell pluripotency. Proc Natl Acad Sci USA. 2007;104:5668–5673. doi: 10.1073/pnas.0701331104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singla DK, Schneider DJ, LeWinter MM, Sobel BE. wnt3a but not wnt11 supports self-renewal of embryonic stem cells. Biochem Biophys Res Commun. 2006;345:789–795. doi: 10.1016/j.bbrc.2006.04.125. [DOI] [PubMed] [Google Scholar]
- 10.Berge DT, et al. Embryonic stem cells require Wnt proteins to prevent differentiation to epiblast stem cells. Nat Cell Biol. 2011;13:1070–1075. doi: 10.1038/ncb2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ogawa K, Nishinakamura R, Iwamatsu Y, Shimosato D, Niwa H. Synergistic action of Wnt and LIF in maintaining pluripotency of mouse ES cells. Biochem Biophys Res Commun. 2006;343:159–166. doi: 10.1016/j.bbrc.2006.02.127. [DOI] [PubMed] [Google Scholar]
- 12.Takao Y, Yokota T, Koide H. Beta-catenin up-regulates Nanog expression through interaction with Oct-3/4 in embryonic stem cells. Biochem Biophys Res Commun. 2007;353:699–705. doi: 10.1016/j.bbrc.2006.12.072. [DOI] [PubMed] [Google Scholar]
- 13.Wagner RT, Xu X, Yi F, Merrill BJ, Cooney AJ. Canonical Wnt/β-catenin regulation of liver receptor homolog-1 mediates pluripotency gene expression. Stem Cells. 2010;28:1794–1804. doi: 10.1002/stem.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marson A, et al. Wnt signaling promotes reprogramming of somatic cells to pluripotency. Cell Stem Cell. 2008;3:132–135. doi: 10.1016/j.stem.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lluis F, Pedone E, Pepe S, Cosma MP. Periodic activation of Wnt/beta-catenin signaling enhances somatic cell reprogramming mediated by cell fusion. Cell Stem Cell. 2008;3:493–507. doi: 10.1016/j.stem.2008.08.017. [DOI] [PubMed] [Google Scholar]
- 16.Lyashenko N, et al. Differential requirement for the dual functions of β-catenin in embryonic stem cell self-renewal and germ layer formation. Nat Cell Biol. 2011;13:753–761. doi: 10.1038/ncb2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Soncin F, et al. Abrogation of E-cadherin-mediated cell-cell contact in mouse embryonic stem cells results in reversible LIF-independent self-renewal. Stem Cells. 2009;27:2069–2080. doi: 10.1002/stem.134. [DOI] [PubMed] [Google Scholar]
- 18.Bone HK, Nelson AS, Goldring CE, Tosh D, Welham MJ. A novel chemically directed route for the generation of definitive endoderm from human embryonic stem cells based on inhibition of GSK-3. J Cell Sci. 2011;124:1992–2000. doi: 10.1242/jcs.081679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakanishi M, et al. Directed induction of anterior and posterior primitive streak by Wnt from embryonic stem cells cultured in a chemically defined serum-free medium. FASEB J. 2009;23:114–122. doi: 10.1096/fj.08-111203. [DOI] [PubMed] [Google Scholar]
- 20.Ullmann U, et al. GSK-3-specific inhibitor-supplemented hESC medium prevents the epithelial-mesenchymal transition process and the up-regulation of matrix metalloproteinases in hESCs cultured in feeder-free conditions. Mol Hum Reprod. 2008;14:169–179. doi: 10.1093/molehr/gan001. [DOI] [PubMed] [Google Scholar]
- 21.Hasegawa K, Fujioka T, Nakamura Y, Nakatsuji N, Suemori H. A method for the selection of human embryonic stem cell sublines with high replating efficiency after single-cell dissociation. Stem Cells. 2006;24:2649–2660. doi: 10.1634/stemcells.2005-0657. [DOI] [PubMed] [Google Scholar]
- 22.Dravid G, et al. Defining the role of Wnt/beta-catenin signaling in the survival, proliferation, and self-renewal of human embryonic stem cells. Stem Cells. 2005;23:1489–1501. doi: 10.1634/stemcells.2005-0034. [DOI] [PubMed] [Google Scholar]
- 23.Cai L, et al. Promoting human embryonic stem cell renewal or differentiation by modulating Wnt signal and culture conditions. Cell Res. 2007;17:62–72. doi: 10.1038/sj.cr.7310138. [DOI] [PubMed] [Google Scholar]
- 24.Huang SM, et al. Tankyrase inhibition stabilizes axin and antagonizes Wnt signalling. Nature. 2009;461:614–620. doi: 10.1038/nature08356. [DOI] [PubMed] [Google Scholar]
- 25.Biechele TL, Moon RT. Assaying beta-catenin/TCF transcription with beta-catenin/TCF transcription-based reporter constructs. Methods Mol Biol. 2008;468:99–110. doi: 10.1007/978-1-59745-249-6_8. [DOI] [PubMed] [Google Scholar]
- 26.Abu-Remaileh M, et al. Oct-3/4 regulates stem cell identity and cell fate decisions by modulating Wnt/β-catenin signalling. EMBO J. 2010;29:3236–3248. doi: 10.1038/emboj.2010.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zafarana G, Avery SR, Avery K, Moore HD, Andrews PW. Specific knockdown of OCT4 in human embryonic stem cells by inducible short hairpin RNA interference. Stem Cells. 2009;27:776–782. doi: 10.1002/stem.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laslett AL, et al. Transcriptional analysis of early lineage commitment in human embryonic stem cells. BMC Dev Biol. 2007;7:12. doi: 10.1186/1471-213X-7-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anton R, Kestler HA, Kühl M. Beta-catenin signaling contributes to stemness and regulates early differentiation in murine embryonic stem cells. FEBS Lett. 2007;581:5247–5254. doi: 10.1016/j.febslet.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 30.Loh YH, et al. The Oct4 and Nanog transcription network regulates pluripotency in mouse embryonic stem cells. Nat Genet. 2006;38:431–440. doi: 10.1038/ng1760. [DOI] [PubMed] [Google Scholar]
- 31.Pan G, Thomson JA. Nanog and transcriptional networks in embryonic stem cell pluripotency. Cell Res. 2007;17:42–49. doi: 10.1038/sj.cr.7310125. [DOI] [PubMed] [Google Scholar]
- 32.Rodda DJ, et al. Transcriptional regulation of nanog by OCT4 and SOX2. J Biol Chem. 2005;280:24731–24737. doi: 10.1074/jbc.M502573200. [DOI] [PubMed] [Google Scholar]
- 33.Rosin-Arbesfeld R, Cliffe A, Brabletz T, Bienz M. Nuclear export of the APC tumour suppressor controls beta-catenin function in transcription. EMBO J. 2003;22:1101–1113. doi: 10.1093/emboj/cdg105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Daniels DL, Weis WI. ICAT inhibits beta-catenin binding to Tcf/Lef-family transcription factors and the general coactivator p300 using independent structural modules. Mol Cell. 2002;10:573–584. doi: 10.1016/s1097-2765(02)00631-7. [DOI] [PubMed] [Google Scholar]
- 35.Hough SR, Laslett AL, Grimmond SB, Kolle G, Pera MF. A continuum of cell states spans pluripotency and lineage commitment in human embryonic stem cells. PLoS ONE. 2009;4:e7708. doi: 10.1371/journal.pone.0007708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Amit M, et al. Clonally derived human embryonic stem cell lines maintain pluripotency and proliferative potential for prolonged periods of culture. Dev Biol. 2000;227:271–278. doi: 10.1006/dbio.2000.9912. [DOI] [PubMed] [Google Scholar]
- 37.Stewart MH, et al. Clonal isolation of hESCs reveals heterogeneity within the pluripotent stem cell compartment. Nat Methods. 2006;3:807–815. doi: 10.1038/nmeth939. [DOI] [PubMed] [Google Scholar]
- 38.Papapetrou EP, et al. Stoichiometric and temporal requirements of Oct4, Sox2, Klf4, and c-Myc expression for efficient human iPSC induction and differentiation. Proc Natl Acad Sci USA. 2009;106:12759–12764. doi: 10.1073/pnas.0904825106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.