Abstract
P2X receptors for ATP have a wide range of physiological roles and comprise a structurally distinct family of ligand-gated trimeric ion channels. The crystal structure of a P2X4 receptor, in combination with mutagenesis studies, has provided a model of the intersubunit ATP-binding sites and identified an extracellular lateral portal, adjacent to the membrane, that funnels ions to the channel pore. However, little is known about the extent of ATP-induced conformational changes in the extracellular domain of the receptor. To address this issue, we have used MTSEA-biotinylation (N-Biotinoylaminoethyl methanethiosulfonate) to show ATP-sensitive accessibility of cysteine mutants at the human P2X1 receptor. Mapping these data to a P2X1 receptor homology model identifies significant conformational rearrangement. Electron microscopy of purified P2X1 receptors showed marked changes in structure on ATP binding, and introducing disulphide bonds between adjacent subunits to restrict intersubunit movements inhibited channel function. These results are consistent with agonist-induced rotation of the propeller-head domain of the receptor, sliding of adjacent subunits leading to restricted access to the upper vestibule, movement in the ion conducting lateral portals, and gating of the channel pore.
Keywords: purinergic receptor, cysteine-scanning
ATP functions not only as an energy carrier within cells but also acts extracellularly at P2 receptors (G protein-coupled P2Y receptors and ligand-gated P2X receptor cation channels) (1) to mediate signaling between cells. A role for purinergic signaling was first suggested in the 1970s (2) and it is now known that one or more P2X receptor subtypes are expressed on virtually every cell type in the body (3, 4). Given the almost ubiquitous expression of the receptors, it is not surprising that they have been shown to have widespread physiological roles, ranging from taste sensation to bone formation (3, 5). In addition, P2X receptors contribute to pathophysiological states, including thromboembolism (6) and pain (7), highlighting the considerable therapeutic potential of P2X receptor-selective drugs (4).
Genes encoding seven mammalian P2X receptors have been identified (P2X1 to -7). Functional receptors form from the homo- or heterotrimeric assembly of subunits with intracellular amino and carboxyl termini, two transmembrane segments, and a large extracellular ligand-binding loop (8). This topology and stoichiometry is different from that of the cys loop and glutamate gated ion channels (9). The crystalisation of a zebrafish P2X4 receptor in an ATP-free, closed, resting state (10) provided a major advance in understanding the molecular basis of receptor function. The individual subunits have been likened to the shape of a dolphin (Fig. S1), with the transmembrane domains corresponding to the fluke and the extracellular region to the body, with a cysteine-rich head domain, flippers, and a dorsal fin. The three subunits are entwined in a right-handed twist surrounding the upper, central, and extracellular vestibules (Figs. 1A and 2B). The 3D structure supports many previous mutagenesis-based predictions (for reviews, see refs. 11–13) and was a catalyst for recent studies that have further extended the understanding of the molecular basis of the ligand-binding site (14, 15) and ionic permeation through the extracellular vestibule (lateral portal) of the extracellular domain to the transmembrane pore (15–17). ATP binding induces a conformational change that leads to gating of the ion-channel pore. However, the zebrafish P2X4 receptor structure provides only a snapshot of the receptor in an agonist-free closed state. A major unanswered question is the nature and extent of agonist-induced conformational changes on receptor activation in this distinct family of ligand-gated ion channels.
Fig. 1.
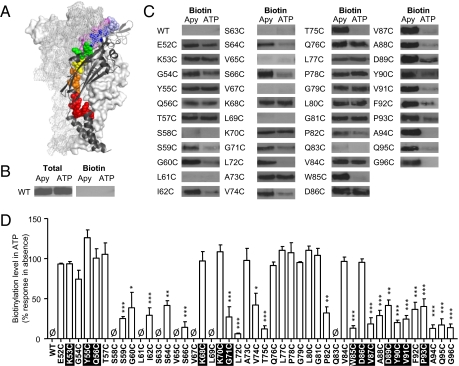
MTSEA-biotinylation reveals the extent of ATP-induced changes in accessibility of the cysteine mutants E52C to G96C. (A) Schematic representation of cysteine-scanned region. Mutated residues are shown as spheres with rainbow colors (red, E52-S59; orange, G60-S64; yellow, V65-V67; green, K68-K70; blue, G71-T75; indigo, Q76-L80; and violet, G81-G96) for the P2X1 subunit in the front of the picture. The overall structure of this subunit is shown in black cartoon representation and the two P2X1 subunits in the background are shown in surface and grid representation, respectively. (B) To determine the surface accessibility of cysteine residues, oocytes were incubated with MTSEA-biotin, biotinylated proteins isolated, separated by SDS/PAGE, and blotted with anti-P2X1 receptor antibody. Blots show MTSEA biotinylation was not detected from WT P2X1 receptors either when pretreated with apyrase (to break down endogenous nucleotides) or ATP (to activate the receptor). Total levels of the WT P2X1 receptor are shown for the oocytes before isolation of biotinylated proteins. (C) Representative blots of MTSEA-biotinylation of WT and P2X1 receptor mutants. Blots are representative of those from three to eight separate batches of oocytes. Controls for the expression of total P2X1 receptor protein for mutants where biotinylation was below the limit of detection are shown in Fig. S2. (D) Densitometic analysis using ImageJ calculated any change in MTSEA biotin accessibility at each point mutation following receptor activation with ATP. Inaccessibility of particular mutants to MTSEA biotin is indicated by Ø. Conserved residues highlighted in black. Statistical analysis was performed by one-way ANOVA followed by Bonferroni's post test (*P < 0.05, **P < 0.01, ***P < 0.001).
Fig. 2.
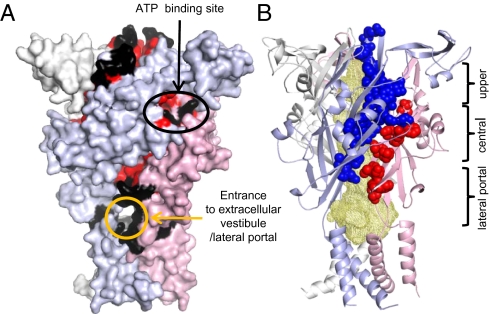
Mapping ATP sensitivity of cysteine mutant accessibility indicates conformational change and restricted access to the upper and central vestibules. (A) Homology model of the hP2X1 receptor; surface representation shows the three subunits in white, light blue, and light pink. Cysteine mutants that were accessible (as assessed by MTSEA-biotinylation) in the absence or presence of ATP are shown in black and are on the cell surface at the apex of the receptor and surrounding the entrance to the extracellular vestibule/lateral portal (orange circle). Cysteine mutants that were reactive with MTSEA-biotin, and this was reduced following binding ATP, are shown in red. These residues broadly correspond to those around the ATP binding site (black elipse). (B) Many of the cysteine mutants that were accessible are not on the cell surface but present within the receptor (shown in red and blue spheres for adjacent subunits) lining the upper and central vestibules (wheat-colored mesh shows a rendering of the open space within the receptor). MTSEA-biotinylation at these mutants was reduced following ATP binding and indicates reduced accessibility on channel activation.
In this study we have used cysteine mutagenesis, N-Biotinoylaminoethyl methanethiosulfonate (MTSEA-biotinylation), and electron microscopy to map the extent of ATP-induced structural rearrangement in the human P2X1 receptor. Introduction of disulphide bonds between the P2X1 receptor subunits showed that subunit rearrangement is essential for receptor activation. These studies provide an insight into agonist-induced changes in the extracellular ligand binding domain of a P2X receptor and suggest that receptor activation involves movement of the subunits, with rotation of the cysteine-rich head, restricted access to the upper vestibule, and gating of the ion-conducting pore.
Results
ATP-Sensitive Accessibility of Cysteine Mutant P2X1 Receptors.
The predicted ATP binding site forms a groove/open jaw between adjacent subunits with ATP coordinated by N290, F291, R292, and K309 of the left-hand subunit and K68 and K70 of the right-hand subunit (Fig. S1). It has been suggested that agonist binding induces movement between the subunits, closing the agonist-binding pocket (10). Such rearrangement is also likely to result in changes in access to the vestibules within the extracellular domain bordered/enclosed by the subunits. To test for a conformational change, we have used MTSEA-biotinylation to determine the accessibility of a series of individual cysteine mutants (E52C to G96C) in the extracellular domain of the human P2X1 receptor (and whether this is ATP-sensitive). These mutants, which are all functional and expressed at the cell surface (15), stretch from the first transmembrane segment to the receptor apex, and incorporate residues lining the upper, central, and extracellular/lateral vestibules and part of the ligand binding site (Fig. 1A).
MTSEA-biotin binds to exposed cysteine residues and provides a biochemical measure of their accessibility. In control experiments on WT human P2X1 receptors expressed in Xenopus oocytes, MTSEA-biotinylation was not detected either in the absence (15 U/mL apyrase to break down any endogenous ATP) or presence of ATP (1 mM) (Fig. 1 B–D). This finding is consistent with the 10 conserved native cysteine residues in the extracellular loop forming five disulphide bonds (10). MTSEA-biotinylation was also below detectable levels for alternate mutants in the β-strand L61-K70, as well as for mutants S58C and Q83C (Fig. 1 C and D, and Fig. S2). For the mutants E52C-T57C, K68C, K70C, A73C, Q76C-G81C, V84C, and D86C, MTSEA-biotinylation was detected at similar levels in both the absence and presence of ATP. For the remainder of the mutants, S59C, G60C, I62C, S64C, S66C, G71C, L72C, V74C, T75C, P82C, W85C, and V87C-G96C MTSEA-biotinylation was detected in the absence and reduced in the presence of ATP (Fig. 1 C and D). These decreases demonstrate agonist-induced changes in accessibility.
Mapping ATP-Sensitive Changes in Accessibility of Cysteine Mutants Reveals Extensive Conformational Rearrangement.
To gain a mechanistic insight into the patterns of biotinylation, the results for E52C-G96C, as well as data from previous studies [E181C-V200C (18) and S286C-I329C (19)], have been mapped onto a P2X1 receptor homology model (15) (Fig. 2). Inaccessible residues are predominantly buried within the receptor (Fig. S3). Cysteine mutants that were biotinylated can be divided into three groups: (i) those on the receptor surface around the mouth of the lateral portal, and at the apex of the receptor that are accessible in the presence/absence of ATP; (ii) residues around the agonist binding site, where MTSEA-biotinylation was generally reduced by ATP consistent with an allosteric reduction in accessibility; and (iii) residues that line, or are at the interface between, subunits that form the upper and central vestibules, where ATP reduced MTSEA-biotinylation. These results demonstrate that ATP binding leads to reduced access to the upper and central vestibules and suggests that ATP binding evokes significant conformational movement in the extracellular domain of the receptor.
The P2X1 receptor shows marked desensitization during the continued presence of agonist (time to 50% desensitization: ∼1 s) and the changes in biotinylation in response to ATP application may reflect a distinct desensitized conformation of the receptor. We have shown recently that desensitization is abolished upon replacement of amino acids 16–49 in P2X1 with those from the human P2X2 receptor (chimera P2X1-2NβTM1, corresponding to the second half of the intracellular amino terminus and the first transmembrane domain) (20). We therefore generated cysteine mutants on the nondesensitizing P2X1-2NβTM1 receptor background corresponding to the base/mouth of the lateral portal (S56C), apex of the receptor (L80C), and residues in the upper and central vestibules (Q95C and S64C). In these studies, ATP was applied for 1 min before a 5-min coincubation with ATP and MTSEA-biotin; currents evoked by 6-min application of ATP at either the P2X1-NβTM1 receptor or cysteine mutants on this background showed <10% desensitization (Fig. S4). Biotinylation data from the P2X1-2NβTM1 receptor and mutants in the presence of ATP are therefore likely to correspond to an agonist-bound open form of the receptor.
Comparison of data for the cysteine mutants on the P2X1 and P2X1-NβTM1 backgrounds allowed us to test whether the reduction in access to the upper and central vestibules at P2X1 receptor mutants corresponded to channel opening or a subsequent desensitized state. The pattern of MTSEA-biotinylation for the P2X1-2NβTM1 background (not detected), P2X1-2NβTM1 mutants S56C, L80C (detected but unaffected by ATP), and Q95C (biotinylation reduced by ATP) was the same as for the WT and corresponding cysteine mutants at the P2X1 receptor (Fig. S4). In contrast, ATP application had no significant effect on the level of MTSEA-biotin access to the central vestibule (residue S64C) at the nondesensitizing P2X1-2NβTM1 channel, but was significantly reduced in the desensitized P2X1 receptor background (Fig. S4). These results suggest that (i) ATP binding and channel opening leads to reduced access to the upper vestibule, and (ii) access to the central vestibule is reduced for the desensitized receptor.
Electron Microscopy of P2X1 Receptors Demonstrates ATP-Induced Rotation of the Cysteine-Rich Head and Visualization of Lateral Portals.
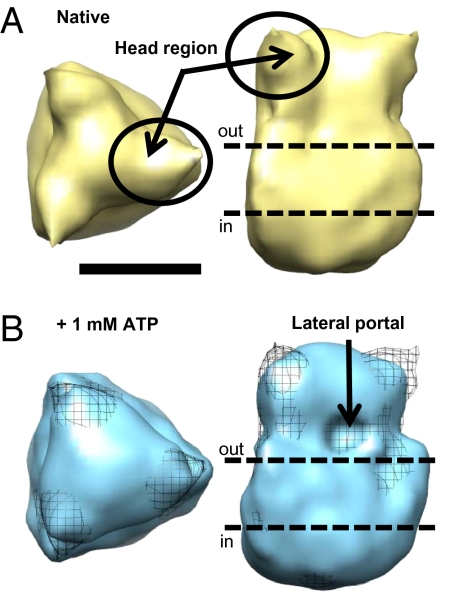
To gain a structural insight into ATP-sensitive conformational changes, we used transmission electron microscopy (TEM) to image negatively stained single purified P2X1 receptors either in the absence or presence of ATP (Fig. 3 and Fig. S5). Briefly, HEK293 cells stably expressing human P2X1 receptors with a C-terminal FLAGHis6 tag were lysed, the receptor was solubilized in 1% n-Octyl glucoside, isolated with an anti-FLAG M2 affinity agarose gel/column, and purity was confirmed by mass spectrometry. Samples were adsorbed on to carbon-coated TEM grids in either the presence or absence of 1 mM ATP (1 min), and single protein particles were imaged as detailed in SI Methods. The overall dimensions of the native structure (∼7 × 10 nm; 290 nm3) were consistent with those from human P2X4 (21) and zebrafish P2X4.1 (10) receptors, and the extracellular domain “propeller” [also observed in the low-resolution human P2X4 (21) structure] that most likely corresponds to the cysteine-rich head region of the protein was clearly visible (Fig. 3A). Because of the use of negative stain and presence of a detergent micelle, which stabilizes the transmembrane domains in solution, it was not possible to gain any structural information concerning the transmembrane domains (21).
Fig. 3.
Electron microscopy of P2X1 receptors shows ATP induced twisting of the subunits. TEM of negatively stained single purified P2X1 receptor protein particles [top view (Left) and side view (Right)] the absence (A) and presence (B) of ATP. The differences between the two structures are shown in B, with the structure in the absence of ATP shown as a mesh representation. (Scale bar, 5 nm.)
In the presence of 1 mM ATP (Fig. 3B), the propeller-shaped domain at the top of the structure was reduced in size, implying an inward movement of the head region upon ATP binding. In addition, we observed the appearance of an indent in the surface of the structure that could potentially represent the lateral portal pathway above the transmembrane domains. The differences between the two structures are most clearly observed in the combined fit displayed in Fig. 3B. We did not observe the opening of a central pore in the extracellular domain, consistent with the lateral pores being the sites of ion entry (15–17). These images provide structural insight into agonist-induced conformational changes at a P2X receptor.
Introduced Disulphide Bonds Between P2X1 Receptor Subunits Inhibit Channel Activation.
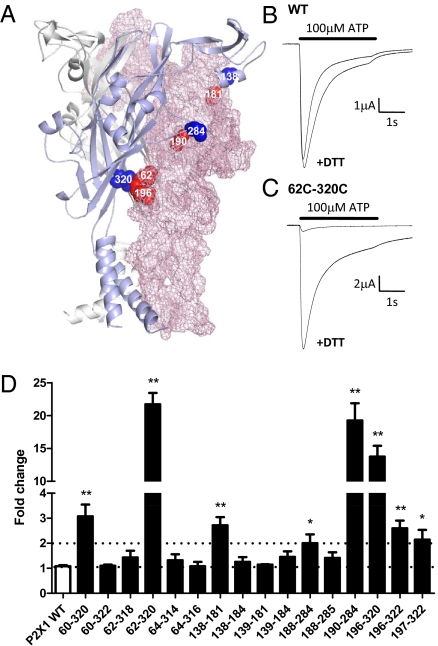
The MTSEA-biotinylation and TEM studies suggested marked ATP-induced conformational changes consistent with movement at the subunit interfaces and rotation of the cysteine-rich head region. We have therefore used the P2X1 receptor homology model (15) to predict pairs of cysteine mutants that could form disulphide bonds between subunits (Fig. 4A). The first set of mutants was designed to cross-link two subunits via the core β-sheets of the body region. The β-sheets comprise strands L61-K70, T186-F195, K199-R203 from one subunit and strands G251-D262, I271-G278 (this strand leads to the “right flipper”), and K309-A323 from the adjacent subunit. Disulphide formation would “lock” these sheets relative to each other. A second set of pairs of cysteine mutants attempted to tag the cysteine-rich head domain to the adjacent subunit. Treatment with the reducing agent DTT (10 mM) had no effect on ATP-evoked responses at WT P2X1 receptors (Fig. 4B). DTT also had no effect at several double-cysteine mutants (e.g., 60C-322C) and suggests that the introduced cysteine residues are not in the correct orientation to form a disulphide bond. In contrast, DTT potentiated ATP-evoked currents at the double-cysteine mutants at the base (60C/320C, 62C/320C, 196C/320, 196C/322C, and 197C/322C) of the β-sheets of the body region, between the top of the central β-sheet region and the “right flipper” (190C-284C), and between the cysteine-rich head region and the adjacent subunit (138C-181C). DTT had no effect on the time course of responses, and no potentiating effect on the constituent single cysteine mutants (Fig. S6). These results are consistent with the introduced cysteine residues forming disulphide bonds between the adjacent subunits that constrained channel function, and suggest that conformational changes throughout the extracellular domain of the receptor are required for normal channel function.
Fig. 4.
Introduced disulphide bonds restrict intersubunit movement and inhibit P2X receptor currents. (A) Homology model of the hP2X1 receptor, cartoon (gray, light blue) and mesh (light pink) representation of three subunits. Double-cysteine mutants 320C-62C, 320C-196C, 190C-284C, and 138-181 that were sensitive to the reducing agent DTT are shown as spheres (blue and red from two separate subunits). (B) DTT (10 mM) had no effect on currents at WT P2X1 receptors evoked by a maximal concentration of ATP. (C) Currents at the 62C-320C mutant were reduced compared with WT and potentiated by DTT treatment. (D) Summary of results. WT and mutant P2X1 receptors were expressed in Xenopus oocytes and ATP-evoked currents recorded with two-electrode voltage clamp. Data are expressed as fold-change following DTT treatment. *P < 0.01, **P < 0.001.
Discussion
The determination of the crystal structure of the zebrafish P2X4 receptor represented a major advance in our understanding of the molecular properties of P2X receptors (10), but because the structure was solved in the absence of ligand, little is known about the conformational changes induced by ATP binding. In this study we demonstrate that ATP binding results in extensive movement between the three receptor subunits.
The crystal structure of zebrafish P2X4 represents an agonist-free closed conformation, and shows a series of three vestibules (formed by the entwined subunits) that pass through the center of the extracellular domain of the receptor. In the structure, the dimensions of the constrictions to the upper vestibule are too narrow for hydrated ions to pass (10). Accordingly, in our P2X1 receptor homology model based on the P2X4 structure, both the upper and central vestibules are predicted to be too narrow for MTSEA-biotin to gain access. However, in our biochemical studies we show extensive MTSEA-biotinylation of cysteine mutants lining the upper and central vestibules, as well as the extracellular vestibule/lateral portal. This finding suggests that in the absence of agonist, the P2X1 receptor can also, at least temporarily, adopt a more open conformation, giving access to the upper and central vestibules of the extracellular domain.
MTSEA-biotinylation of cysteine mutants in the upper vestibule of both the rapidly desensitizing P2X1 and nondesensitizing P2X1-2NβTM1 receptor was reduced by ATP. This result shows that agonist binding and channel opening results in a conformational change, restricting access to the upper vestibule, and supports recent studies showing that the upper vestibule does not contribute to ionic permeation (15–17). The head region and right flipper (the loop incorporating the N-linked glycosylation site Asn184) of one subunit border the outer reaches of the left-hand side of the ATP-binding pocket, and have been proposed to move relative to the adjacent subunit to close the agonist binding site (10). The DTT sensitivity of double-cysteine mutants between the head, as well as the right flipper, and the adjacent subunit (138C/181C and 284C/190C, respectively) supports agonist-induced movement of adjacent subunits and demonstrates conformational changes are functionally important. It seems unlikely that the conformational change would result in the subunits moving away from each other, as this would open the ATP-binding pocket and would not be expected to result in a decrease in MTSEA-biotinylation of residues lining the upper vestibule. The simplest explanation is that ATP binding leads to a counter-clockwise rotation of the subunits, closing the ligand-binding pocket and gating the channel. This finding is consistent with ATP-induced changes in both MTSEA-biotinylation and TEM images of the purified P2X1 receptor.
The predicted ATP-binding pocket is ∼40 Å from the transmembrane pore-forming domains and raises the question as to what extent the movements “initiated” in the head and right flipper around the binding site are transmitted to the rest of the receptor. Key residues for ATP binding are lysines K68, K70, and K309 of adjacent subunits. These residues are part of the phosphate-binding moiety at the entry of the ATP-binding pocket and directly connected to the transmembrane segments via β-strands. Each of these strands is in turn part of a β-sheet L61-K70, T186-F195, K199-R203 and G251-D262, I271-G278, and K309-A323, respectively (Fig. 5A). Within a single subunit these two β-sheets are closely packed together. MTSEA-biotinylation data for residues at the interface between the two sheets within a subunit (L61, S63, V65, V67, L187, I189, N191, I193) demonstrate that they remain inaccessible under both ATP-bound and ATP-free conditions, giving no indication of a conformational change occurring at this interface. However, cross-linking the two β-sheets of adjacent subunits by introducing double-cysteine mutations via strands I271-G278 and T186-F195 at the top to the right flipper (190C/284C, see above) or via strands K309-A323 and I62-K70 at the base (60C/320C, 62C/320C, 196C/320, 196C/322C, and 197C/322C) inhibited channel function (Fig. 4). The inhibition was reversed by the reducing agent DTT. The observation that intersubunit “locking” inhibited channel function suggests that conformational change involving concerted movement of the two cross-linked β-sheets relative to each other is required for opening the channel (Fig. 5B, sites of disulphide locking indicated by key-hole symbol). Combining this finding with the fact that key residues of the ATP-binding site, K68 and K309, are positioned at the upper end of each of the β-sheets but the transmembrane helices forming the channel pore are connected to the lower end, gives an intriguing model for signal transmission. ATP binding triggers movement of the head region and right flipper, and in particular conformational changes of residues K68, K70, and K309. In turn, this process leads to movement of the two β-sheets of adjacent subunits relative to each other. Both β-sheets are connected to transmembrane helices so it seems plausible that this will affect the conformation of the transmembrane region and result in channel opening. This finding is also consistent with our previous studies, suggesting that G60 movement was important for channel gating of the P2X1 receptor (15) and studies on the P2X2 receptor that proposed a salt bridge between adjacent subunits at the top of the lateral portal as being important for channel function (22) and the DTT sensitivity of double-cysteine mutants in this region (G63C/R274C and E59C/Q321C) (16, 22).
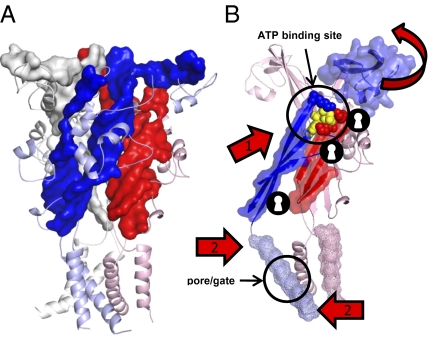
Fig. 5.
Model of agonist induced conformational changes and channel gating. (A) Homology model of the hP2X1 receptor shown as cartoon (gray, light blue, light pink). The core β-strands of the extracellular domain are illustrated in backbone surface representation (gray, blue, red). (B) One complete subunit in light pink and the TM2 (light blue, mesh), the linker region from TM2 to Lys309 with neighboring beta-strands (blue), and the cysteine rich head region (blue surface) of a second subunit are shown. Residues Lys68, Lys70, and Lys309 that coordinate the phosphate tail of ATP in the agonist binding pocket are shown as red and blue spheres, respectively, at one subunit interface, a docked ATP molecule is shown in yellow. The β-sheets are shown as red and blue backbone surfaces and the positions where introduced disulphide bonds inhibited channel function as shown as keyhole symbols indicating locking of a receptor conformation. Movement of the β-strand 309-320 (arrow 1) relative to the two β-strands 62-70 and 186-195 (red) of the adjacent subunit leads to movement in the TM2 (arrow 2) leading to opening of the channel gate.
The electron microscopy and cysteine mutant data highlight important changes at the interface between the subunits in the P2X1 receptor following ATP binding. Based on these experiments, we propose a model (Fig. 5B) whereby agonist binding leads to movement of the head region and right flipper to close the ATP-binding pocket and restricts access to the upper vestibule. The counter-clockwise movement of the subunit “pulling” on the β-strand 309–320 results in movement in the lateral ion-conducting portals, and in the connected TM2 (that lines the channel pore) that results in channel opening.
Methods
Methods are fully described in SI Methods. Briefly, cRNAs encoding either human P2X1 receptor WT or cysteine point mutants were expressed in Xenopus oocytes. The accessibility of introduced cysteine residues was assessed with an MTSEA-biotinylation assay. DTT sensitivity of ATP-evoked currents from WT and double-cysteine mutants was determined using a two-electrode voltage-clamp recording. C-terminally FlagHis6-tagged P2X1 receptors stably expressed in HEK293 cells were purified using an anti-FLAG gel column and prepared for electron microscopy. Data are plotted onto a P2X1 receptor homology using PyMol.
Supplementary Material
Acknowledgments
We thank Prof. Robert C. Ford for advice with single-particle analysis; Drs. Andrew Powell and Kelvin Agboh for initial work on purification; Drs. G. Willars and M. Viskaduraki for advice on statistical analysis; Manijeh Maleki-Dizaji for technical assistance; and Drs. Claudia Blindauer and Peter Moody and Profs. Martyn Mahaut Smith and Andrew Tobin for comments on the paper. This work was supported by the Wellcome Trust and the British Heart Foundation. M.T.Y. was supported by a Wellcome Trust Advanced Training Fellowship and an Evans-Huber Fellowship from Cardiff University.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1201872109/-/DCSupplemental.
References
- 1.Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- 2.Burnstock G. Historical review: ATP as a neurotransmitter. Trends Pharmacol Sci. 2006;27:166–176. doi: 10.1016/j.tips.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 3.Surprenant A, North RA. Signaling at purinergic P2X receptors. Annu Rev Physiol. 2009;71:333–359. doi: 10.1146/annurev.physiol.70.113006.100630. [DOI] [PubMed] [Google Scholar]
- 4.Burnstock G. Pathophysiology and therapeutic potential of purinergic signaling. Pharmacol Rev. 2006;58:58–86. doi: 10.1124/pr.58.1.5. [DOI] [PubMed] [Google Scholar]
- 5.Roberts JA, et al. Molecular properties of P2X receptors. Pflugers Arch. 2006;452:486–500. doi: 10.1007/s00424-006-0073-6. [DOI] [PubMed] [Google Scholar]
- 6.Hechler B, et al. A role of the fast ATP-gated P2X1 cation channel in thrombosis of small arteries in vivo. J Exp Med. 2003;198:661–667. doi: 10.1084/jem.20030144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsuda M, et al. P2X4 receptors induced in spinal microglia gate tactile allodynia after nerve injury. Nature. 2003;424:778–783. doi: 10.1038/nature01786. [DOI] [PubMed] [Google Scholar]
- 8.Coddou C, Yan Z, Obsil T, Huidobro-Toro JP, Stojilkovic SS. Activation and regulation of purinergic P2X receptor channels. Pharmacol Rev. 2011;63:641–683. doi: 10.1124/pr.110.003129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Surprenant A, Buell G, North RA. P2X receptors bring new structure to ligand-gated ion channels. Trends Neurosci. 1995;18:224–229. doi: 10.1016/0166-2236(95)93907-f. [DOI] [PubMed] [Google Scholar]
- 10.Kawate T, Michel JC, Birdsong WT, Gouaux E. Crystal structure of the ATP-gated P2X(4) ion channel in the closed state. Nature. 2009;460:592–598. doi: 10.1038/nature08198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Evans RJ. Structural interpretation of P2X receptor mutagenesis studies on drug action. Br J Pharmacol. 2010;161:961–971. doi: 10.1111/j.1476-5381.2010.00728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Browne LE, Jiang LH, North RA. New structure enlivens interest in P2X receptors. Trends Pharmacol Sci. 2010;31:229–237. doi: 10.1016/j.tips.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Young MT. P2X receptors: Dawn of the post-structure era. Trends Biochem Sci. 2010;35:83–90. doi: 10.1016/j.tibs.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang R, et al. Agonist trapped in ATP-binding sites of the P2X2 receptor. Proc Natl Acad Sci USA. 2011;108:9066–9071. doi: 10.1073/pnas.1102170108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Allsopp RC, El Ajouz S, Schmid R, Evans RJ. Cysteine scanning mutagenesis (residues Glu52-Gly96) of the human P2X1 receptor for ATP: Mapping agonist binding and channel gating. J Biol Chem. 2011;286:29207–29217. doi: 10.1074/jbc.M111.260364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kawate T, Robertson JL, Li M, Silberberg SD, Swartz KJ. Ion access pathway to the transmembrane pore in P2X receptor channels. J Gen Physiol. 2011;137:579–590. doi: 10.1085/jgp.201010593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Samways DS, Khakh BS, Dutertre S, Egan TM. Preferential use of unobstructed lateral portals as the access route to the pore of human ATP-gated ion channels (P2X receptors) Proc Natl Acad Sci USA. 2011;108:13800–13805. doi: 10.1073/pnas.1017550108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roberts JA, Valente M, Allsopp RC, Watt D, Evans RJ. Contribution of the region Glu181 to Val200 of the extracellular loop of the human P2X1 receptor to agonist binding and gating revealed using cysteine scanning mutagenesis. J Neurochem. 2009;109:1042–1052. doi: 10.1111/j.1471-4159.2009.06035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roberts JA, Evans RJ. Cysteine substitution mutants give structural insight and identify ATP binding and activation sites at P2X receptors. J Neurosci. 2007;27:4072–4082. doi: 10.1523/JNEUROSCI.2310-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Allsopp RC, Evans RJ. The intracellular amino terminus plays a dominant role in desensitization of ATP-gated P2X receptor ion channels. J Biol Chem. 2011;286:44691–44701. doi: 10.1074/jbc.M111.303917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Young MT, et al. Molecular shape, architecture, and size of P2X4 receptors determined using fluorescence resonance energy transfer and electron microscopy. J Biol Chem. 2008;283:26241–26251. doi: 10.1074/jbc.M804458200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang R, et al. A putative extracellular salt bridge at the subunit interface contributes to the ion channel function of the ATP-gated P2X2 receptor. J Biol Chem. 2010;285:15805–15815. doi: 10.1074/jbc.M110.101980. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.