Abstract
Cytosolic proteins can be selectively delivered to lysosomes for degradation through a type of autophagy known as chaperone-mediated autophagy (CMA). CMA contributes to intracellular quality control and to the cellular response to stress. Compromised CMA has been described in aging and in different age-related disorders. CMA substrates cross the lysosomal membrane through a translocation complex; consequently, changes in the properties of the lysosomal membrane should have a marked impact on CMA activity. In this work, we have analyzed the impact that dietary intake of lipids has on CMA activity. We have found that chronic exposure to a high-fat diet or acute exposure to a cholesterol-enriched diet both have an inhibitory effect on CMA. Lysosomes from livers of lipid-challenged mice had a marked decrease in the levels of the CMA receptor, the lysosome-associated membrane protein type 2A, because of loss of its stability at the lysosomal membrane. This accelerated degradation of lysosome-associated membrane protein type 2A, also described as the mechanism that determines the decline in CMA activity with age, results from its increased mobilization to specific lipid regions at the lysosomal membrane. Comparative lipidomic analyses revealed qualitative and quantitative changes in the lipid composition of the lysosomal membrane of the lipid-challenged animals that resemble those observed with age. Our findings identify a previously unknown negative impact of high dietary lipid intake on CMA and underscore the importance of diet composition on CMA malfunction in aging.
Keywords: cathepsins, lipid load, lyso-bis phosphatidic acid, membrane microdomains, membrane proteins
Autophagy is the process that mediates degradation of intracellular components in lysosomes (1). Different autophagic pathways have been described to coexist in most mammalian cells, but they differ in the mechanisms involved in the delivery of cargo to the lysosomal compartment (2, 3). This study focuses on chaperone-mediated autophagy (CMA), a type of lysosomal degradation for a selective pool of cytosolic proteins all bearing a targeting motif (4, 5). Once this motif is recognized by the cytosolic chaperone heat shock cognate protein 70 (hsc70), it is delivered to the surface of the lysosomal membrane (6, 7), where it binds to the lysosome-associated membrane protein type 2A (LAMP-2A) (8). Binding of the substrate to the cytosolic tail of LAMP-2A promotes the assembly of this protein into a multimeric protein complex that mediates the translocation of the substrate protein across the lysosomal membrane (9). Translocation is attained on substrate unfolding, and it requires the assistance of a variant of hsc70 (lys-hsc70) that resides in the lysosomal lumen (10).
Basal levels of CMA activity are detectable in almost all cells (11) and contribute to the maintenance of cellular homeostasis, as well as to specialized functions depending on the cell type and substrate degraded. For example, CMA has been shown to participate in antigen presentation (12), regulation of cellular growth (13), modulation of neuronal survival (11), and control of specific transcriptional programs in response to nutritional challenges (14). CMA is maximally activated as part of the cellular response to different stressors, such as prolonged starvation (15), oxidative stress (16) and exposure to agents that lead to protein damage (17). In fact, compromised CMA in cultured cells renders cells more susceptible to stressors, such as oxidants, prooxidants, and UV light (18). CMA activity declines with age, and it is compromised in different age-related disorders, such as neurodegenerative diseases, metabolic disorders, and nephropathies, as well as in some lysosomal storage disorders (19).
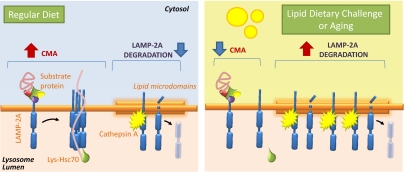
Levels of LAMP-2A at the lysosomal membrane have been shown to be limiting for CMA activity because they directly determine rates of substrate binding and translocation through this pathway (20). Multiple nonexclusive mechanisms regulate LAMP-2A levels at the lysosomal membrane, including de novo synthesis of the protein, mobilization of a luminal pool of LAMP-2A to the lysosomal membrane, and its regulated degradation in this compartment (20–22). Mobilization of LAMP-2A to specific membrane microdomains of particular lipid composition (enriched in cholesterol and glycosphingolipids) initiates its sequential cleavage by a yet to be identified metalloprotease and cathepsin A, a luminal protease that dynamically associates with these microdomains (23). CMA regulation depends on the dynamic interaction of LAMP-2A with the lysosomal membrane microdomains. Under conditions of low CMA activity, part of the membrane pool of LAMP-2A is mobilized to these regions for degradation, whereas when maximal activation of CMA occurs, LAMP-2A is excluded from these regions because its assembly into the multimeric translocation complex only occurs outside the microdomains (22). Alterations in the recruitment of LAMP-2A to these regions of selective cleavage have been identified as the main reason for the pronounced decrease in LAMP-2A levels in the lysosomal membrane of old organisms and the subsequent decline in the activity of this pathway (24). However, the exact mechanism that determines the enhanced instability of LAMP-2A at the lysosomal membrane with age remains unknown.
We have recently identified an inhibitory effect of different lipid challenges on another autophagic pathway, namely, macroautophagy, and we have narrowed the defect to changes in the lipid composition, particularly to the cholesterol content of the membranes of the vesicular compartments involved in that process (25). Given that lateral mobility of proteins at the lysosomal membrane is essential for CMA, we hypothesized that modifications of the lysosomal membrane as a result of changes in the cellular availability of lipids could have a marked impact on CMA activity. In this work, we have analyzed the effect of different lipid challenges on CMA, both in cultured cells and in vivo by subjecting mice to defined lipid content diets. Our results have revealed reduced CMA activity in cells exposed to different lipid challenges because of accelerated degradation of the CMA receptor under these conditions in lysosomes. Qualitative and quantitative changes in the lipid composition of the membranes of lysosomes from animals exposed to dietary lipid challenges resemble those observed in old animals and favor higher mobilization of LAMP-2A toward the lipid membrane microdomains, where its degradation occurs. This negative effect of dietary lipids on the stability of LAMP-2A at the lysosomal membrane could be one of the main reasons for reduced CMA activity in aging.
Results
Different Lipid Challenges Exert an Inhibitory Effect on CMA.
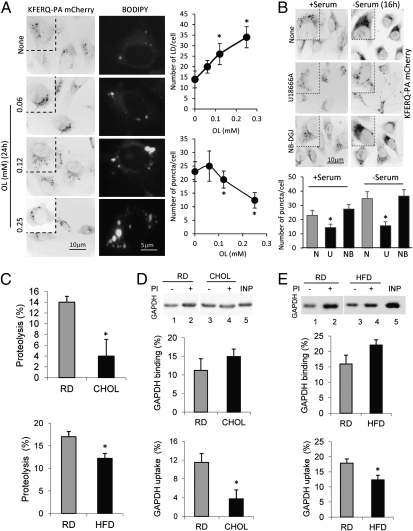
We first analyzed the effect on CMA of increasing concentrations of oleate in mouse fibroblasts in culture using a recently developed photoactivable CMA reporter (KFERQ-PA-mCherry1) (11). Photoactivation converts the reporter protein present at that time in the cell to fluoresce in red, but any reporter synthesized de novo after photoactivation will not fluoresce. This strategy allows tracking changes with time in the fluorescence pattern and degradation of the reporter protein independent of possible changes in synthesis. As CMA is activated, the reporter is delivered to lysosomes, resulting in a gradual change from a cytosolic diffuse pattern to a lysosomal punctate pattern. Comparison of the number of fluorescent puncta per cell provides a good estimate of the amount of substrate bound to the lysosomal membrane at any given time, which we have found to correlate well with CMA activity in cultured cells (11). As shown in Fig. 1A, exposure to increasing concentrations of oleate sufficient to induce intracellular accumulation of lipids in the form of lipid droplets [visualized by staining with BODIPY 493/503; Molecular Probes (Invitrogen)] had an inhibitory effect on the levels of CMA reporter bound to lysosomes in the treated cells. We confirmed that treatment with oleic acid also resulted in reduced lysosomal uptake of the reporter by blocking lysosomal proteolysis with leupeptin and using an antibody against the reporter (this is necessary because once internalized, the CMA reporter is no longer fluorescent as a result of its unfolding) (Fig. S1A). A similar inhibitory effect on CMA was observed when cells were exposed to palmitic acid, a saturated fatty acid (Fig. S1B), although in this case, only lower concentrations could be tested because of the higher toxicity of this lipid. Treatment with the inhibitor of desmosterol Δ24-reductase, U18666A, which has been shown to alter cholesterol trafficking by preventing its exit from late endosomes and lysosomes (Fig. S2), had a similar inhibitory effect on basal and even inducible CMA (activated by prolonged serum removal) (Fig. 1B). In contrast, we did not observe any significant effect on CMA activity on treatment with the inhibitor of glycosphingolipid synthesis, N-butyldeoxygalactonojirimycin (Fig. 1B), which also leads to accumulation of intracellular lipids, mainly in the form of lipid droplets (Fig. S2B), without a significant effect on lysosomal/endosomal lipid content (Fig. S2C). These results support that intracellular buildup of lipids may not be enough to interfere with CMA activity but that direct changes in the cholesterol content at the lysosomal compartment may instead be responsible for the observed compromise in CMA.
Fig. 1.
Effect of lipid load on CMA activity. (A) Mouse fibroblasts stably expressing KFERQ-PA-mCherry1 were exposed to the indicated concentrations of oleate (OL), and the number of fluorescent puncta per cell was calculated after photoactivation. Parallel cells were stained with BODIPY to quantify the number of lipid droplets (LD) per cell after each treatment. Values are the mean ± SEM of three different experiments with >50 cells quantified per experiment. (B) Mouse fibroblasts stably expressing KFERQ-PA-mCherry1 maintained in the presence or absence of serum were treated with the indicated compounds, and activation of CMA was quantified as in A. N, none; NB-DGJ or NB, N-butyldeoxygalactonojirimycin; U, U1866A. (C) Freshly isolated lysosomes from livers of mice maintained on the RD, CHOL, or HFD were incubated with a pool of radiolabeled cytosolic proteins, and proteolysis was expressed as the percentage of the acid-insoluble radioactivity transformed into acid-soluble radioactivity at the end of the incubation. Values are the mean ± SEM of three independent experiments. Freshly isolated lysosomes from livers of mice maintained on the RD, CHOL (D), or HFD (E) were isolated, treated or not treated with a protease inhibitor (PI) as indicated, and incubated with GAPDH for 20 min at 37 °C in isotonic medium. Lysosomes collected at the end of the incubation by centrifugation were subjected to SDS/PAGE and immunoblotting for GAPDH. Uptake was calculated as the amount of GAPDH associated with lysosomes treated with protease inhibitors (association) after discounting the amount associated with untreated lysosomes (binding) for each experiment. Values are the mean ± SEM of four to eight independent experiments. *P < 0.05 compared with untreated cells or the RD. INP, input.
To investigate the effect of lipid challenges on CMA further and to address the physiological relevance of this effect, we moved to an in vivo model and analyzed the consequences of changes in dietary lipid intake in mice on the rates of hepatic CMA activity. To this purpose, we analyzed the effect of acute (3-wk) exposure to a diet enriched in cholesterol (2% CHOL) and of chronic (16-wk) exposure to a lipid challenge in animals fed a high-fat diet (HFD; 60% calories from fat and ∼5% calories from cholesterol), and compared these animals with control groups maintained on a regular diet (RD). We first analyzed the ability of intact lysosomes isolated from livers of these two groups of animals to degrade a radiolabeled pool of cytosolic proteins enriched in CMA substrates. We validated that purity of the fractions was comparable in the three groups of animals (determined as hexosaminidase enrichments of 28.1 ± 1.5, 21.4 ± 4.5, and 23.7 ± 1.9 in the RD, CHOL, and HFD, respectively) and that diets did not reduce the stability of the lysosomal membrane (determined as the percentage of hexosaminidase released into the media of 8.1 ± 1.2, 7.8 ± 1.4, and 4.1 ± 1.1 in the RD, CHOL, and HFD, respectively). Incubation of intact lysosomes with radiolabeled proteins recapitulates the three main steps of CMA: binding, uptake, and degradation once in the lysosomal lumen (26). Degradation of the cytosolic proteins was significantly lower in lysosomes isolated from animals exposed to either the CHOL or HFD (Fig. 1C). These observed changes in CMA seem to be primary at the level of binding/uptake rather than degradation, because we did not find differences in proteolysis rates when the same experiments were performed with lysosomes in which the membranes have been disrupted to allow direct access of the lysosomal proteases to the substrates (Fig. S3). Interestingly, and confirming that the effect of the lipid diets was primarily on CMA, this treatment did not have an effect on the degradation of proteins by a subpopulation of lysosomes unable to perform CMA because they lack the luminal chaperone required for substrate translocation (Fig. S3B).
In agreement with reduced CMA activity in the lysosomes from the treated groups, levels of endogenous cytosolic proteins previously identified as CMA substrates, such as GAPDH (27), were higher in the cytosolic fraction (Fig. S4A) and markedly lower inside lysosomes (Fig. S4B) from CHOL- and HFD-treated animals compared with those maintained on the RD. Despite the higher cytosolic content of GAPDH, the specific activity of this enzyme was lower in the CHOL and HFD groups (Fig. S4C), supporting a possible gradual loss of function associated with the lower turnover of the enzyme under these conditions. To address the effect of the diets on substrate binding and uptake via CMA directly, we used a second well-established in vitro assay with isolated lysosomes incubated with specific CMA substrate proteins that allows us to analyze these two steps separately independent of proteolysis (26). When substrates are incubated with isolated intact lysosomes, the substrate translocated into the lumen is rapidly degraded and only that bound to the lysosomal membrane is detected. However, if lysosomes have been previously treated with protease inhibitors, the protein translocated into the lumen remains intact and the total amount of substrate recovered with lysosomes corresponds to that bound to the membrane and that present in the lumen. Substrate uptake can be calculated as the difference in levels of substrate in lysosomes treated or not treated with protease inhibitors. Comparison of binding and uptake of GAPDH in lysosomes isolated from the different groups of mice revealed no differences in the rate of lysosomal binding of the protein (Fig. 1 D and E). In contrast, uptake was significantly reduced in lysosomes isolated from the HFD and CHOL groups (Fig. 1 D and E). A similar decrease in lysosomal uptake was also observed for RNase A, another well-characterized CMA substrate (Fig. S4D). These results support that the reduced rates of CMA observed in the treated animals are mainly attributable to a reduced ability of these lysosomes to translocate cytosolic substrates into their lumen.
Effect of Different Lipid Challenges on CMA Components in Lysosomes.
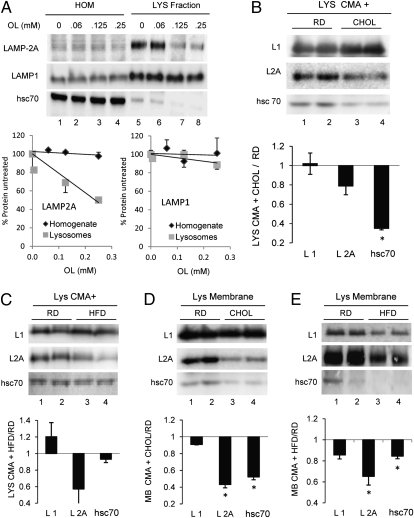
The essential components involved in substrate translocation via CMA are LAMP-2A, which organizes into the multimeric translocation complex (9), and lys-hsc70, which assists the substrate from the luminal side of the membrane (10) and also mediates the active dissociation of LAMP-2A from the multimeric complex (9). Analysis of the levels of these two proteins in lysosomes isolated from the oleate-treated cells in which CMA activity was gradually compromised revealed a dose-dependent decrease in the levels of these two proteins in lysosomes (Fig. 2A). Levels of other lysosome membrane components, such as LAMP-1, remained unchanged.
Fig. 2.
CMA components change in response to lipid challenges. Homogenates (HOM) and lysosomes (LYS) isolated from mouse fibroblasts were treated with the indicated concentrations of oleate (OL) (A), and lysosomes isolated from livers of mice maintained on the RD, CHOL, or HFD (B–E) were isolated and collected by centrifugation (A–C) or, where indicated, subjected to hypotonic shock to separate lysosomal membranes (D and E). (Upper) Immunoblots for LAMP-1 (L1), LAMP-2A (L2A), and hsc70. (Lower) Changes in protein content calculated by densitometric analysis of immunoblots as the ones shown here and expressed relative to the RD. Values are the mean ± SEM of six experiments. *P < 0.05 compared with the RD group.
Similar reductions in lysosomal levels of LAMP-2A and hsc70 were observed in the subgroup of CMA-active lysosomes isolated from animals maintained on the CHOL or HFD (Fig. 2 B and C). Isolation of lysosomal membranes using hypotonic shock and high-speed centrifugation confirmed that changes in the CMA-related proteins were more pronounced at the lysosomal membrane (Fig. 2 D and E). The reduction in levels of LAMP-1, the most abundant protein at the lysosomal membrane, was less pronounced (25% compared with the 50% observed for LAMP-2A) (Fig. 2 D and E).
Dietary Lipids Reduce the Stability of LAMP-2A at the Lysosomal Membrane.
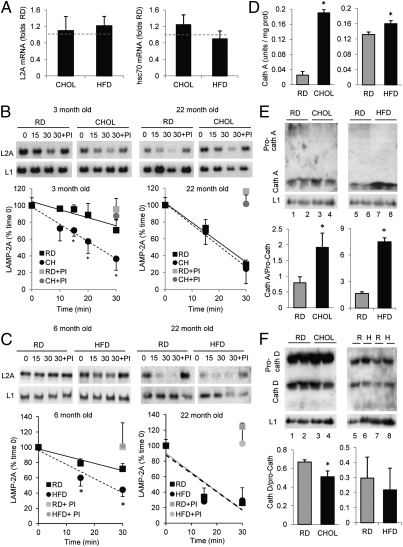
Because LAMP-2A is the limiting component for CMA at the lysosomal membrane, and changes in the levels of CMA chaperones in these membranes can be secondary to the reduced levels of this receptor, we investigated the mechanism behind the reduced levels of LAMP-2A observed in the treated animal groups. We did not find significant differences in the levels of LAMP-2A or hsc70 mRNA between controls and any of the treated groups of mice, supporting the lack of differences in de novo synthesis of these proteins (Fig. 3A). In contrast, analysis of the stability of LAMP-2A at the lysosomal membrane at different times of incubation in an isotonic buffer revealed a faster reduction in the level of this protein in the membrane of lysosomes isolated from animals maintained on either the CHOL (Fig. 3B) or HFD (Fig. 3C). The differences with control mice were completely abolished when lysosomes were incubated in the presence of protease inhibitors, supporting that the marked reduction in protein levels was mainly attributable to accelerated degradation in these compartments (Fig. 3 B and C).
Fig. 3.
Mechanisms of lipid-mediated decrease in LAMP-2A levels. (A) Changes in levels of LAMP-2A (Left) and hsc70 (Right) mRNA in livers of animals subjected to the CHOL or HFD, determined by quantitative real-time PCR assay. Values were corrected for actin in each sample and are expressed relative to the RD. Stability of LAMP-2A in lysosomes isolated from 3- and 22-mo-old animals maintained on the RD and CHOL (CH) (B) and from 6- and 22-mo-old animals maintained on the HFD (C). Lysosomes were incubated at 37 °C in isotonic medium in the presence or lack of a protease inhibitor (PI) and pelleted at the indicated times, and were then subjected to SDS/PAGE and immunoblotting for LAMP-2A (L2A) or LAMP-1 (L1). (Upper) Representative immunoblots. (Lower) Densitometric quantification of the immunoblots. Values are expressed as the percentage of protein present at time 0 and are the mean ± SEM of four different experiments. Where indicated, protease inhibitors were added during the incubation and lysosomes were collected at 30 min (gray symbols). The two-way ANOVA showed interaction (P < 0.05) between the diet and the incubation time in the young animals but not in the old ones in both the CHOL and HFD. The incubation time was the major source of variation in the old groups. *P < 0.05 compared with the RD group by a Bonferroni posttest. (D) Cathepsin A (Cath A) activity in lysosomal membranes isolated from RD-, CHOL-, and HFD-maintained mice. Values are expressed as units per milligram of protein and are the mean ± SEM of three different experiments. Representative immunoblots and densitometric quantification for cathepsin A (E) and cathepsin D (Cath D) (F) in the same lysosomes as in D are shown. Values are expressed as the ratio of the active lower band vs. the precursor upper band and are the mean ± SEM of four different experiments. *P < 0.05 compared with the RD group. R, RD; H, HFD.
Interestingly, reduced stability of LAMP-2A because of its accelerated degradation in lysosomes has been described as the main cause for the functional decline of CMA in aging (24). To determine the possible contribution of dietary lipids to LAMP-2A instability with age, we compared the kinetics of degradation of LAMP-2A in lysosomes isolated from 22-mo-old mice subjected or not subjected to the CHOL for 3 wk (Fig. 3B) or to the HFD for 4 mo (initiated at 18 mo of age) (Fig. 3C). Changes in LAMP-1 were only noticeable in the older group when these animals were subjected to the diet and were rather discrete compared with changes in LAMP-2A. As expected, CMA substrate uptake was reduced (Fig. S5) and degradation of LAMP-2A was markedly accelerated (Fig. 3 B and C) in the 22-mo-old group compared with 4-mo-old mice [about 65% decrease in LAMP-2A stability in the old group, comparable to the 60% decrease previously reported in old rat livers (24)]. However, subjecting these animals to the CHOL or HFD did not have an additive effect on the rates of substrate uptake (Fig. S5) or of LAMP-2A degradation (Fig. 3 B and C), supporting that both aging and lipid load likely share common mechanisms for their effect on LAMP-2A stability at the lysosomal membrane.
To investigate the mechanisms behind the lipid-mediated accelerated degradation of LAMP-2A at the lysosomal membrane further, we analyzed the different components previously described to participate in the regulated degradation of this protein. Discrete cleavage of LAMP-2A by cathepsin A at the lysosomal membrane is the trigger that initiates the degradation of this protein in the lysosomal compartment (23). Measurement of the specific activity of cathepsin A in the isolated lysosomal membranes (Fig. 3D) and immunoblot analysis for cathepsin A revealed a marked increase in the levels of the active form of this hydrolase in CHOL and HFD lysosomes (Fig. 3E). In fact, the low levels of the inactive precursor form of cathepsin A present in the lysosomes from control animals were almost undetectable in the lysosomes from the lipid-challenged groups (Fig. 3E), supporting accelerated processing of this enzyme. However, this rapid maturation does not seem to be a generalized feature of all hydrolases in these lysosomes but, instead, something specific for cathepsin A, because analysis of other lysosomal cathepsins (cathepsin D shown in Fig. 3F) did not reveal a significant increase in the lysosomal levels of the mature and precursor forms of this enzyme among the different groups of animals.
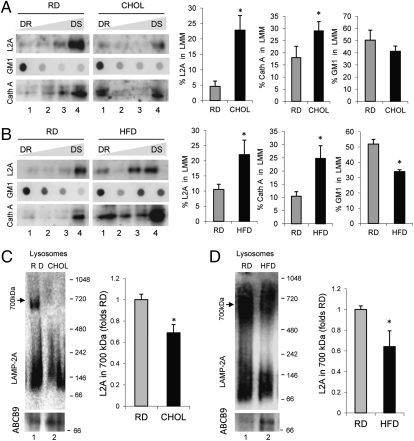
Cathepsin A-mediated cleavage of LAMP-2A in lysosomes occurs in discrete lipid microdomain regions at the lysosomal membrane (22). Molecules of LAMP-2A destined for degradation are retrieved to these regions, where cathepsin A preferentially binds to the lysosomal membrane. Using previously established detergent extraction and flotation in sucrose density gradient procedures (22), we isolated the lipid microdomains where LAMP-2A degradation occurs from lysosomal membranes of RD-, CHOL-, or HFD-maintained mice and analyzed LAMP-2A distribution in these fractions. The resistance of the microdomains containing LAMP-2A to detergent extraction allows for their recovery in the regions of lower density of the sucrose gradient. As shown in Fig. 4, we found a consistent increase in the percentage of LAMP-2A present in these detergent-resistant regions at any given time in animals on both the CHOL (Fig. 4A) and HFD (Fig. 4B). The increased association of LAMP-2A with these regions was even evident under normal feeding conditions, when LAMP-2A is usually degraded faster (Fig. S6), suggesting that continuous enhanced degradation in these regions may be the main reason for the low levels of lysosomal LAMP-2A during the lipid challenges. In support of higher degradation, levels of cathepsin A were also considerably higher in the lipid microdomains isolated from CHOL- and HFD-maintained animals (Fig. 4 A and B). Analysis of the content of ganglioside GM1, previously shown to locate preferentially in these regions, demonstrated not only higher absolute GM1 levels in the large lipid microdomains from CHOL- and HFD-treated animals but a broader distribution of this ganglioside into other smaller detergent-resistant regions (lower flotation ability) (Fig. 4 A and B).
Fig. 4.
Effect of dietary lipids on LAMP-2A dynamics at the lysosomal membrane. (A and B) Lysosomes from livers of 24-h starved mice maintained on the RD, CHOL, or HFD were extracted with 1% Triton X-114 and then subjected to flotation in discontinuous sucrose density gradients. Four aliquots collected from the detergent-resistant (DR) to the detergent-soluble (DS) region of the gradient were subjected to immunoblotting for LAMP-2A (L2A) and cathepsin A (Cath A) or to dot blot analysis for GM1 using cholera toxin and an antibody against this toxin. (Left) Representative immunoblots and immunodot blots. (Right) Densitometric quantification of blots as the ones shown here. Values are expressed as the percentage of the total lysosomal LAMP-2A present in the lysosomal membrane microdomain (LMM). Note that each fraction is collected, precipitated in acid, and loaded in its totality, which allows for calculation of the total amount of LAMP-2A in the membrane by adding the amount of LAMP-2A detected in each of the fractions. Values are the mean ± SEM of three to four experiments. (C and D) Isolated lysosomes from the same group of animals were solubilized with 0.5% octyl glucoside and subjected to blue-native electrophoresis and immunoblotting for LAMP-2A. (Left) Representative immunoblot. (Right) Levels of LAMP-2A in the 700-kDa complex expressed relative to levels in the RD. Values are the mean ± SEM of three different experiments. *P < 0.05 compared with the RD group.
Association of LAMP-2A with lipid microdomains has been shown to determine both the degradation rate of this receptor at the lysosomal membrane and its ability to organize into the multimeric complex required for substrate translocation, which only occurs outside these regions (9, 22). Analysis of the multimeric status of LAMP-2A at the lysosomal membrane using blue-native electrophoresis revealed a marked reduction in the amount of LAMP-2A organized into the 700-kDa multimeric complex required for substrate translocation in lysosomes from the CHOL-treated mice (Fig. 4C) and HFD-treated mice (Fig. 4D). These findings are directly in agreement with the observed increased partition of LAMP-2A into lipid microdomains in those animals.
Overall, our results support that dietary lipids exert a marked effect on the dynamics of LAMP-2A at the lysosomal membrane and, consequently, on the capability to degrade cytosol substrates via CMA.
Effect of Dietary Lipids on the Lipid Composition of Lysosomal Membranes.
To analyze the impact that dietary lipids had on the lipid composition of the lysosomal membrane directly and to compare these changes with those associated with aging, we isolated lysosomes from animals on the CHOL and HFD and from 22-mo-old mice maintained on the RD. To be able to analyze changes in the lipid composition at the lysosomal membrane, independent of the lysosomal content, we subjected lysosomes to hypotonic shock and separated the membrane fraction by high-speed centrifugation. In contrast to lysosomes involved in macroautophagy, the subgroup of lysosomes active for CMA does not participate in degradation of organelles under normal conditions (28); consequently, contamination by membranes or organelles sequestered for degradation is unlikely. In fact, the lumen of these lysosomes contains mainly amorphous material, luminal membranes are not often observed (Fig. S7A), and immunoblot analysis reveals that only minimal traces of structural membrane proteins of different organelles are detected in this subgroup of lysosomes (Fig. S7B).
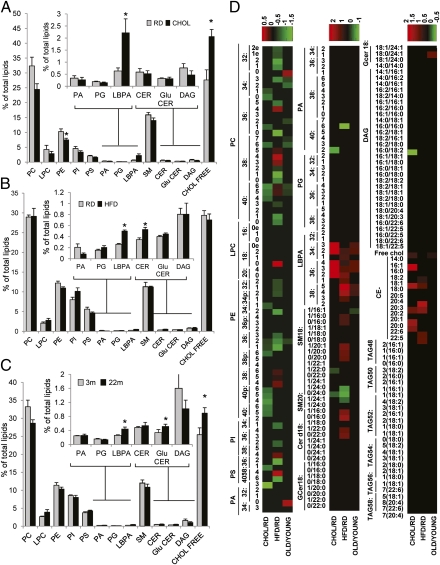
We performed comparative lipidomic analysis of lysosomal membranes from these four groups (RD, CHOL, HFD, and 22-mo-old mice) to gain a better understanding of the qualitative and quantitative changes in lipid composition imposed by dietary lipids and by aging. Analysis of the lysosomal membranes from the CHOL-treated animals revealed a statistically significant increase (∼20%) in the percentage of free cholesterol in these membranes along with a statistically significant ∼25% decrease in the percentage of phosphatidylethanolamine (PE) as trends for a reduction in major lipid groups at the lysosomal membrane, such as phosphatidylcholine (PC), phosphatidylinositol (PI), phosphatidylserine (PS), and sphingomyelin (SM) (Fig. 5A). Interestingly, we found a marked increase (∼75%) in the percentage of lysobisphosphatidic acid (LBPA) (Fig. 5A, Inset), a phospholipid known for its ability to enhance transfer of cholesterol from and to membranes and to mediate the formation of intraluminal vesicles in the endolysosomal system (29). We then analyzed possible changes in lipid species composition (Fig. 5D shows the heat map of the comparative lipid profiles) and found that CHOL-treated animals have a significant decrease in saturated SM and ceramide (CER) chains and a significant increase in the forms with unsaturated chains, both in SM and in CER (Fig. 5D and Fig. S8 A–D). In contrast, we observed a significant increase in the percentage of long-chain saturated PC, along with a decrease in the PC chains, with a higher number of unsaturations (Fig. 5D and Fig. S9 A–D).
Fig. 5.
Changes in the lipid composition of the lysosomal membrane in response to dietary challenges and aging. Lysosomal membranes isolated from livers of mice maintained on the RD and CHOL (A), on the RD and HFD (B), or from 3- and 22-mo-old mice (C) were subjected to lipidomic analysis. Graphs show the mean values of the molar percentage of every lipid class with respect to the total amount of lipids (after excluding triglycerides and cholesterol esters). Values are the mean ± SEM of five different experiments. (D) Heat map showing the comparative lipid profile of lysosomal membranes isolated from livers of mice maintained on the CHOL vs. RD, on the HFD vs. RD, or in 22-mo-old vs. 3-mo-old mice (OLD/YOUNG). The three columns represent the normalized values of the individual lipid species. The color bar represents the log2 value of the ratio of each lipid species. Only changes that are statistically significant in the relative lipid amount are highlighted in the heat map. Relative increases and decreases are shown in red and green, respectively (n = 5). *P < 0.05 compared with the RD group. CE, cholesterol esther; CHOL FREE, free cholesterol; DAG, diacylglyceride; LPC, lysophosphatidyl choline; PA, phosphatydic acid; PG, phosphatidylglycerol; TAG, triglyceride.
Comparative lipidomics in the lysosomal membranes from HFD-treated mice revealed that some of the changes in their lipid composition were shared with the CHOL group, whereas others were specific for this treatment. For example, we did not find changes in the percentage of free cholesterol in HFD lysosomal membranes but noticed that CER levels were significantly increased instead (Fig. 5B). These changes may have a similar effect on membrane organization, because CER has been shown to promote the order of lipid membranes and to segregate laterally into rigid, gel-like domains to a higher extent than cholesterol (30, 31). Interestingly, as in the CHOL group, the membranes from HFD-treated mice contained significantly higher levels of LBPA (Fig. 5B). This increase in LBPA cannot be attributed to a mere higher content of contaminating late endosomal compartments in the lysosomal fraction isolated from CHOL- or HFD-treated mice, because analysis of mannose-6-phosphate receptor, a marker of the recycling endocytic compartment, was comparable in lysosomes from mice maintained on the RD and high-lipid content diets (Fig. S7C). Also shared with the CHOL group was the decrease in PE (Fig. 5B), and some of the changes in SM and CER, such as those pertinent to long-saturated lipid species (heat map in Fig. 5D and Fig. S8 E–H). However, in clear contrast to the CHOL group, we observed a significant decrease of long-chain saturated and monounsaturated PC, PE, and PI, along with an increase in the PC, PE and PI chains, with a high number of unsaturations (heat map in Fig. 5D and Fig. S9 E–H). Changes in monounsaturated SM and CER species and in some of the forms with both chains unsaturated were also the reverse of those observed in CHOL-treated mice (Fig. 5D and Fig. S8 E–H). These results support that both diets favor formation of organized lipid microdomains at the lysosomal membrane, but of different lipid composition. This may explain why although both diets exert an inhibitory effect on CMA, the effect of the CHOL was consistently more pronounced (compare substrate uptake, LAMP-2A levels, and LAMP-2A stability in Fig. 1 D and E, Fig. 2 D and E, and Fig. 3 B and C).
Finally, we performed a similar lipidomic analysis in lysosomal membranes from 22-mo-old mice maintained on the RD. As in the case of the lipid diets, the lipid composition of the membranes from the older age group had some distinctive features unique for this group, but it also reproduced many of the changes observed in the lipid-challenged animals, overall being closer to the changes in the CHOL group. Thus, we observed a small (∼5%) but significant increase in free cholesterol levels, as in the CHOL group, and increases in CER (∼15%), glucosyl ceramide (∼35%), and LysoPC (∼45%) comparable to those observed in the HFD group (Fig. 5C). Strikingly, the increase in LBPA observed with both diets was also noticeable in the aged group (Fig. 5C). Overall, aging promoted changes in the composition of the acyl chains that resemble those of animals on the CHOL: an increase in SM, CER, and glucoceramide unsaturated in both chains (Fig. S8 I–L), an increase in the percentage of saturated PC, and a decrease in the percentage of polyunsaturated PC (Fig. S9 I–L). The three treatments resulted in comparable changes in long saturated SM species (Fig. S8 A, E, and I), and the aging group shared with the HFD-treated animals an increase in the levels of short saturated species of SM (Fig. S8 I–L). This increase in short saturated species of PC and SM with age, along with the increase in free cholesterol, may promote the formation of cholesterol-enriched, detergent-resistant microdomains.
Overall, our findings are consistent with lipid challenges through the diet having a direct impact on the organization of the lysosomal membrane through qualitative and quantitative changes in its lipid composition. The lipidomic analysis also reveals high similarity between the diet-induced changes at the lysosomal membrane and those occurring with age. Among the numerous consequences that these changes can have on lysosomal function, in this work, we have characterized the negative effect that the high enrichment in cholesterol and perturbation of the lysosomal membrane lipid composition has on CMA, a pathway heavily dependent on membrane lateral mobility.
Discussion
In this work, we have identified a negative effect of dietary lipid challenges on CMA activity that is mediated, for the most part, by changes in the lipid composition of the lysosomal membrane as a result of the lipid exposure. The decreased stability of lysosomal membrane proteins observed under these conditions, particularly the higher susceptibility of LAMP-2A to these changes, unveils a unique mechanism for CMA compromise under these conditions, which could also be behind the functional loss in this pathway under different pathological conditions and in aging.
Our analysis also emphasizes the modulatory role of lipids on autophagy as part of the recently described interplay between this catabolic process and lipid metabolism. Thus, we have recently reported that macroautophagy can contribute to the mobilization of intracellular lipid deposits; in fact, this process, known as macrolipophagy, is up-regulated in response to moderate lipid challenges (32). However, chronic lipid challenges or acute exposure to abnormally high lipid concentrations exerts an inhibitory effect on macroautophagy (28). The main defect identified as responsible for macroautophagy failure under these conditions is the reduced fusion ability between the vesicular compartments involved in that process: autophagosomes and lysosomes. Interestingly, the compromise in vesicular fusion can be attributed to changes in the lipid composition of the membrane of these vesicles and, in fact, can be reproduced by artificially mimicking these lipid changes in isolated autophagosomes (i.e., using chemical extractors of cholesterol) (28). Here, we show that high concentrations of cholesterol at the lysosomal membrane also exert a marked inhibitory effect on CMA, mainly by enhancing LAMP-2A mobilization to the specific microdomains where this protein is normally degraded. This dual compromise of macroautophagy and CMA in response to an abnormally high content of dietary lipids may underlie the basis of part of the cellular toxicity observed under these conditions. These autophagic pathways, along with the ubiquitin/proteasome system, are mainly responsible for the maintenance of cellular quality control. Thus, compromise of the two autophagic pathways may render cells particularly susceptible to stressors, such as oxidative stress, because of the inability to handle the damage associated with these stressors. In this respect, and in agreement with previous reports (33, 34), we found a moderate increase in the levels and some of the proteolytic activities of the proteasome in the liver of animals exposed to the diets with a high lipid content (Fig. S10 A and B). Up-regulation of the proteasome may be a cellular attempt to compensate for the loss of activity of the autophagic systems. However, the fact that levels of polyubiquitinated proteins increase during the high-lipid diets (Fig. S10C) already points toward an overall deficient cellular quality control.
In addition to the expected problems in cellular quality control, it is possible that the inhibitory effect of lipid challenges on CMA described in this work may have important implications in cellular metabolism. Thus, in studies with cancer cells, we have recently identified a role for CMA in the regulation of glycolysis and the need for functional CMA to maintain proper β-oxidation in these cells (35). Although the mechanisms behind this effect of CMA in β-oxidation remain unknown, it is interesting that CMA was consistently up-regulated in response to exposure to low concentrations of oleic acid (Fig. 1A). This up-regulation of CMA could be linked to the increase in β-oxidation necessary to accommodate the higher affluence of lipids under these conditions. In this respect, the inhibitory effect of dietary lipids on CMA may further contribute to intracellular lipid accumulation by reducing the mitochondrial catabolism.
Future studies are required to elucidate the reason for the higher susceptibility of LAMP-2A to lysosomal membrane lipid challenges, compared with other lysosomal membrane proteins. We have previously shown differences between the membrane dynamics of LAMP-2A and the other variants of this protein and of LAMP-1 (9, 21, 22). Although these other proteins can also be associated with lipid microdomains, they do not seem to coincide in the same ones in which LAMP-2A undergoes degradation (22). Likewise, LAMP-2B and LAMP-1 can also be detected in oligomeric complexes at the lysosomal membrane, but they are usually of smaller size than the 700-kDa complex enriched in LAMP-2A that is required for substrate translocation (9). It is possible that the diet-induced changes in the lipid composition of the lysosomal membrane affect specific microdomain regions where LAMPs locate differently.
We have found a marked change in the amount of cholesterol at the lysosomal membrane of animals maintained on the CHOL. When used in model membranes, similar concentrations of cholesterol have been shown to form mainly a liquid-ordered phase, equivalent to that in the LAMP-2A–enriched lysosomal microdomains (36). However, as revealed by the lipidomic analysis, changes in other lipids at the lysosomal membrane also contribute to decreased CMA. In this respect, we have found that not only quantitative but qualitative changes in different lipid species occur at the lysosomal membrane during the dietary cholesterol lipid challenge. Some of the noted changes seem to favor more compact cholesterol packing inside microdomains, as is the case with the relative increase in saturated PC, which occupies less space than saturated SM (which is actually decreased in CHOL lysosomes). In contrast, other changes may help to maintain the fluidity of the membrane outside the microdomain regions despite the high increase in cholesterol. For example, the observed switch toward unsaturated forms of SM and CER would allow for such high concentrations of cholesterol while maintaining fluidity, because unsaturated lipids with a double bond in the middle of the acyl chain remain fluid even in the presence of cholesterol (37).
The comparative analyses of lipid changes in both diets support that different lipid combinations may have a similar impact on the final properties of the lysosomal membrane. Thus, in the HFD group, we did not observe an increase in cholesterol levels; instead, the elevated levels of short saturated CER and SM species detected in this group may promote the formation of even tighter domains (38, 39). It is well established that CER and phospholipids with polyunsaturated fatty acid chains, which are also elevated in the lysosomal membrane of HFD mice, do not mix well with cholesterol and form domains with different lipid packing than those of cholesterol, which may promote the concentration of specific proteins in these domains (40). Therefore, although HFD- and CHOL-induced changes in the lipid composition of the lysosomal membrane were not identical, both diets favor the formation of detergent-resistant microdomains in which LAMP-2A is degraded. The qualitative differences in the composition of the microdomains promoted by each diet may explain why the inhibitory effect of the HFD on CMA is less pronounced than the one observed after the CHOL.
The age-related changes in the lipid composition of the lysosomal membrane revealed features also observed in the other two groups (i.e., increase in LBPA or changes in the long saturated SM chains); however, overall, the changes in the aged group more closely resemble those induced by the CHOL, which are also the ones with a higher negative impact on CMA activity. As for both diets, age-dependent changes in lysosomal membrane lipids also favor the formation of microdomains. In this case, along with high CHOL, the increases in glycosylceramide and other glycosphingolipids and the enrichment in short and saturated forms of SM and PC also promote the formation of detergent-resistant microdomains. In previous studies, we have reported that in addition to the alteration in the regulated degradation of LAMP-2A that occurs in the microdomains, part of the membrane-resistant LAMP-2A is abnormally internalized into the lumen, where it is rapidly degraded (24). It is plausible that some of the changes in the lipid composition of the lysosomal membrane with age observed in this study also contribute to that abnormal internalization. In this respect, the marked increase in LBPA observed in the three interventions is of great interest because this unconventional phospholipid can induce formation of small vesicles and invaginations in other membranes (29).
The different properties of the lipid microdomains could have a direct impact on the ability of different LAMPs to associate with these regions. Although all LAMPs have transmembrane regions of comparable length and highly homologous luminal regions that predict similar structural features (41), differences in posttranslational modifications among them, which are likely changes in the glycosylation pattern, could modulate their interaction with the lipids in different microdomains. Another possibility is that yet unidentified membrane proteins actively mobilize LAMPs in and out of these microdomain regions and that changes in the packing density of lipids or length of their lateral chains determine the affinity of these LAMP-targeting proteins for the lipid microdomain regions. In this respect, membrane-associated hsc70 has been previously shown to be necessary for the active insertion of LAMP-2A at the lysosomal membrane, because blocking antibodies against this chaperone are enough to inhibit this process (20). Finally, it is also possible that the increase in the content of known lysosomal lipidic cargos, such as cholesterol esters, contributes to the abnormal dynamics of LAMP-2A in the lysosomes of animals exposed to the CHOL. In fact, a fraction of lysosomal LAMP-2A that resides in the lumen associated with lipids can be retrieved back to the lysosomal membrane under conditions requiring maximal CMA activation (20). It is plausible that changes in the lysosomal luminal lipids affect the efficiency of retrieval of this subfraction of lysosomal LAMP-2A toward the lysosomal membrane.
In this study, we have also identified a marked decrease in levels of hsc70 in the membranes of lysosomes from both CHOL- and HFD-maintained mice. It is possible that this decrease is just a consequence of the reduced levels of LAMP-2A at the lysosomal membrane in these animals, because hsc70 interacts with this receptor for substrate translocation. However, in light of the recently described direct association of hsc70 with lipids described at the membrane of late endosomes (42), we cannot discard the possibility that the reduced levels of hsc70 at the lysosomal membrane after the lipid challenges reflect reduced direct binding to membrane lipids. In the case of late endosomes, hsc70 binds directly to PS in the outer leaflet of the membrane. Although, we have not found significant differences in the PS content at the lysosomal membrane after the lipid challenges, we cannot discard the possibility that changes in other lipids could affect the organization of PS at the lysosomal membrane and, in this way, interfere with chaperone binding. In addition, the observed increase in LBPA could affect the association of hsc70 with the lysosomal membranes by competition with hsp70, which has been demonstrated to bind LBPA directly in membranes (29).
The pathophysiological implications of our findings are multiple, because these could help in elucidating the basis for CMA malfunction in different conditions. From the physiological point of view, this modulatory effect of intracellular lipids on CMA may directly or indirectly contribute to the previously described bidirectional cross-talk between macroautophagy and CMA (18, 43). Most cells respond to blockage in macroautophagy by up-regulating CMA (43), which has been proven beneficial because it helps in preserving cellular resistance to particular stressors (44). Based on the recently described contribution of macroautophagy to mobilization of intracellular lipid stores (32), it is anticipated that a blockage in macroautophagy will reduce the availability of intracellular cholesterol and reduce its levels in organelle membranes, including lysosomes. This reduction in lysosomal membrane cholesterol may contribute to the up-regulation of CMA observed when macroautophagy is compromised. Aging is associated with a decline in CMA activity, which seems, for the most part, to be attributable to reduced stability of LAMP-2A at the lysosomal membrane (24). Our early studies support that the rapid degradation of LAMP-2A in lysosomes from old animals is mainly attributable to changes in the lysosomal membrane with age rather than to direct changes in LAMP-2A, because the increased instability can be reproduced when the recombinant LAMP-2A is incorporated in resealed membranes from old mice lysosomes but not if the membranes originate from young mice (24). Our current study supports that changes in the lipid composition of the membrane with age are responsible for this destabilizing effect on LAMP-2A. In fact, the accelerated degradation of LAMP-2A in lysosomes isolated from animals maintained on the CHOL or HFD is comparable to the one observed in lysosomes from old mice (24). The fact that maintaining old animals on the HFD for 4 mo or on the CHOL diet for 3 wk did not have an additive effect on LAMP-2A instability further supports that changes in lipid composition of the lysosomal membrane with age are behind the observed decline in CMA activity in aging. Reduced CMA activity has also been described in different neurodegenerative diseases, such as Parkinson disease and some tauopathies (45–47), and in metabolic disorders, such as diabetes (13). Because aging has been shown to be an aggravating factor in all these diseases, it has been proposed that the primary defect in CMA could be further aggravated by the age-dependent decrease in the activity of this pathway, and thus contributes to accelerate the pathological changes. In this respect, modulation of dietary lipid intake may provide a way to slow down the decline in CMA with age and, consequently, to delay disease onset. Interestingly, alterations in intracellular lipids and in lipid metabolism in general have also been described in several of these conditions, leaving open the possibility of a perpetuating negative feedback between intracellular lipids and CMA in these pathological conditions and further reinforcing the possible beneficial effect of interventions aimed at modulating dietary lipid intake.
Methods
A detailed description of all methods is provided in SI Methods.
Animals, Cells, and Reagents.
Male C57BL/6 mice (6–8 wk old) from the Jackson Laboratory were maintained on the RD (2018, Global 18% Protein Rodent Diet; Teklad), the HFD (D12492, 60% kcal in fat; Research Diets) for 16 wk once they reached 8 wk of age, or the 2% CHOL (2018 + 2% cholesterol TD.01383; Teckland) for 3 wk. For the aging studies, 3- and 22-mo-old mice from the National Institute on Aging age-controlled colony were used. For lysosomal isolation, the livers of two animals were pooled per condition in each experiment. Mouse fibroblasts [National Institutes of Health (NIH) 3T3] were from the American Type Culture Collection. Sources of chemicals are as described previously (8, 18, 20, 25) and as detailed in SI Methods.
Lysosome Isolation and CMA Measurements.
Lysosomes were isolated from mouse liver and cultured cells by centrifugation in a discontinuous metrizamide density gradient as described (48). Uptake assays were performed by incubation of isolated lysosomes with radiolabeled cytosolic proteins and analysis of protein breakdown (49) or with single purified proteins and analysis of protein association by immunoblot as described (26). The stability of LAMP-2A was measured in intact lysosomes on incubation in isotonic medium for increasing periods of time (20). Detergent-resistant microdomains were isolated from lysosomal membranes by detergent extraction and flotation in sucrose gradients as described (22). Blue-native electrophoresis of solubilized lysosomal membranes was used to visualize the CMA translocation complex utilizing 3–12% (wt/vol) NativePAGE Novex bis-Tris precast gels (Invitrogen).
Measure of CMA Activity in Intact Cells.
Direct fluorescence microscopy was used to determine CMA activity in fibroblasts expressing the CMA reporter (KFERQ-photoactivable mCherry1) after photoactivation (11). Images were acquired with an Axiovert 200 fluorescence microscope (Carl Zeiss Ltd.), subjected to deconvolution with the manufacturer's software, and prepared using Photoshop 6.0 software (Adobe Systems, Inc.).
Lipid Extraction and Analysis.
A modified Bligh/Dyer extraction procedure was used for lipid extraction from organelle fractions before analysis by liquid chromatography-mass spectrometry utilizing multiple reaction monitoring (50, 51). Separated lipid classes were quantified via multiple reaction monitoring (MRN) mode on a triple-quadrupole instrument (API 3200; Applied Biosystems) using previously reported MRM transition pairs and instrument settings (50).
General Methods.
BODIPY 493/503 was used to visualize lipid droplets in cultured cells (32). RT-PCR was used for mRNA quantification in total RNA prepared with the SuperScript II RNase H Reverse Transcriptase (Invitrogen) and oligo-(dT)12–18 primers. Details of amplification primers are provided in SI Methods.
Statistical Analysis.
Two-way ANOVA, followed by the Bonferroni post hoc and Student t tests for unpaired data, was used for statistical analysis.
Supplementary Material
Acknowledgments
We thank Ms. Samantha J. Orenstein for critically reviewing the manuscript and Robin Chan for her help with the analysis of lipids. This work was supported by National Institutes of Health Grants AG021904 and AG031782 (to A.M.C.), Grant NS056049 (to G.D.P.), and Grant AG08702 (to G.D.P. and C.D.). J.A.R.-N. is supported by a Spanish Ministerio de Educacion y Ciencia Fellowship, and S.K. is supported by National Institutes of Health/National Institute on Aging Training Grant T32AG023475.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. D.J.K. is a guest editor invited by the Editorial Board.
See Author Summary on page 4351 (volume 109, number 12).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1113036109/-/DCSupplemental.
References
- 1.Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature. 2008;451:1069–1075. doi: 10.1038/nature06639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wong E, Cuervo AM. Autophagy gone awry in neurodegenerative diseases. Nat Neurosci. 2010;13:805–811. doi: 10.1038/nn.2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaushik S, Singh R, Cuervo AM. Autophagic pathways and metabolic stress. Diabetes Obes Metab. 2010;12(Suppl 2):4–14. doi: 10.1111/j.1463-1326.2010.01263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaushik S, et al. Chaperone-mediated autophagy at a glance. J Cell Sci. 2011;124:495–499. doi: 10.1242/jcs.073874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cuervo AM. Chaperone-mediated autophagy: Selectivity pays off. Trends Endocrinol Metab. 2010;21(3):142–150. doi: 10.1016/j.tem.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chiang HL, Terlecky SR, Plant CP, Dice JF. A role for a 70-kilodalton heat shock protein in lysosomal degradation of intracellular proteins. Science. 1989;246:382–385. doi: 10.1126/science.2799391. [DOI] [PubMed] [Google Scholar]
- 7.Dice JF. Peptide sequences that target cytosolic proteins for lysosomal proteolysis. Trends Biochem Sci. 1990;15:305–309. doi: 10.1016/0968-0004(90)90019-8. [DOI] [PubMed] [Google Scholar]
- 8.Cuervo AM, Dice JF. A receptor for the selective uptake and degradation of proteins by lysosomes. Science. 1996;273:501–503. doi: 10.1126/science.273.5274.501. [DOI] [PubMed] [Google Scholar]
- 9.Bandyopadhyay U, Kaushik S, Varticovski L, Cuervo AM. The chaperone-mediated autophagy receptor organizes in dynamic protein complexes at the lysosomal membrane. Mol Cell Biol. 2008;28:5747–5763. doi: 10.1128/MCB.02070-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Agarraberes FA, Terlecky SR, Dice JF. An intralysosomal hsp70 is required for a selective pathway of lysosomal protein degradation. J Cell Biol. 1997;137:825–834. doi: 10.1083/jcb.137.4.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koga H, Martinez-Vicente M, Macian F, Verkhusha VV, Cuervo AM. A photoconvertible fluorescent reporter to track chaperone-mediated autophagy. Nat Commun. 2011;2:386. doi: 10.1038/ncomms1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou D, et al. Lamp-2a facilitates MHC class II presentation of cytoplasmic antigens. Immunity. 2005;22:571–581. doi: 10.1016/j.immuni.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 13.Sooparb S, Price SR, Shaoguang J, Franch HA. Suppression of chaperone-mediated autophagy in the renal cortex during acute diabetes mellitus. Kidney Int. 2004;65:2135–2144. doi: 10.1111/j.1523-1755.2004.00639.x. [DOI] [PubMed] [Google Scholar]
- 14.Cuervo AM, Hu W, Lim B, Dice JF. IkappaB is a substrate for a selective pathway of lysosomal proteolysis. Mol Biol Cell. 1998;9:1995–2010. doi: 10.1091/mbc.9.8.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wing SS, Chiang HL, Goldberg AL, Dice JF. Proteins containing peptide sequences related to Lys-Phe-Glu-Arg-Gln are selectively depleted in liver and heart, but not skeletal muscle, of fasted rats. Biochem J. 1991;275(Pt 1):165–169. doi: 10.1042/bj2750165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kiffin R, Christian C, Knecht E, Cuervo AM. Activation of chaperone-mediated autophagy during oxidative stress. Mol Biol Cell. 2004;15:4829–4840. doi: 10.1091/mbc.E04-06-0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cuervo AM, Hildebrand H, Bomhard EM, Dice JF. Direct lysosomal uptake of alpha 2-microglobulin contributes to chemically induced nephropathy. Kidney Int. 1999;55:529–545. doi: 10.1046/j.1523-1755.1999.00268.x. [DOI] [PubMed] [Google Scholar]
- 18.Massey AC, Kaushik S, Sovak G, Kiffin R, Cuervo AM. Consequences of the selective blockage of chaperone-mediated autophagy. Proc Natl Acad Sci USA. 2006;103:5905–5910. doi: 10.1073/pnas.0507436103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kon M, Cuervo AM. Chaperone-mediated autophagy in health and disease. FEBS Lett. 2010;584:1399–1404. doi: 10.1016/j.febslet.2009.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cuervo AM, Dice JF. Regulation of lamp2a levels in the lysosomal membrane. Traffic. 2000;1:570–583. doi: 10.1034/j.1600-0854.2000.010707.x. [DOI] [PubMed] [Google Scholar]
- 21.Cuervo AM, Dice JF. Unique properties of lamp2a compared to other lamp2 isoforms. J Cell Sci. 2000;113:4441–4450. doi: 10.1242/jcs.113.24.4441. [DOI] [PubMed] [Google Scholar]
- 22.Kaushik S, Massey AC, Cuervo AM. Lysosome membrane lipid microdomains: Novel regulators of chaperone-mediated autophagy. EMBO J. 2006;25:3921–3933. doi: 10.1038/sj.emboj.7601283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cuervo AM, Mann L, Bonten EJ, d'Azzo A, Dice JF. Cathepsin A regulates chaperone-mediated autophagy through cleavage of the lysosomal receptor. EMBO J. 2003;22:47–59. doi: 10.1093/emboj/cdg002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kiffin R, et al. Altered dynamics of the lysosomal receptor for chaperone-mediated autophagy with age. J Cell Sci. 2007;120:782–791. doi: 10.1242/jcs.001073. [DOI] [PubMed] [Google Scholar]
- 25.Koga H, Kaushik S, Cuervo AM. Inhibitory effect of intracellular lipid load on macroautophagy. Autophagy. 2010;6:825–827. doi: 10.1096/fj.09-144519. [DOI] [PubMed] [Google Scholar]
- 26.Kaushik S, Cuervo AM. Methods to monitor chaperone-mediated autophagy. Methods Enzymol. 2009;452:297–324. doi: 10.1016/S0076-6879(08)03619-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cuervo AM, Terlecky SR, Dice JF, Knecht E. Selective binding and uptake of ribonuclease A and glyceraldehyde-3-phosphate dehydrogenase by isolated rat liver lysosomes. J Biol Chem. 1994;269:26374–26380. [PubMed] [Google Scholar]
- 28.Koga H, Kaushik S, Cuervo AM. Altered lipid content inhibits autophagic vesicular fusion. FASEB J. 2010;24:3052–3065. doi: 10.1096/fj.09-144519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hullin-Matsuda F, Luquain-Costaz C, Bouvier J, Delton-Vandenbroucke I. Bis(monoacylglycero)phosphate, a peculiar phospholipid to control the fate of cholesterol: Implications in pathology. Prostaglandins Leukot Essent Fatty Acids. 2009;81:313–324. doi: 10.1016/j.plefa.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 30.Goñi FM, Alonso A. Effects of ceramide and other simple sphingolipids on membrane lateral structure. Biochim Biophys Acta. 2009;1788:169–177. doi: 10.1016/j.bbamem.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 31.Silva LC, de Almeida RF, Castro BM, Fedorov A, Prieto M. Ceramide-domain formation and collapse in lipid rafts: Membrane reorganization by an apoptotic lipid. Biophys J. 2007;92:502–516. doi: 10.1529/biophysj.106.091876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Singh R, et al. Autophagy regulates lipid metabolism. Nature. 2009;458:1131–1135. doi: 10.1038/nature07976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arizti P, Arribas J, Castaño JG. Modulation of the multicatalytic proteinase complex by lipids, interconversion and proteolytic processing. Enzyme Protein. 1993;47:285–295. doi: 10.1159/000468686. [DOI] [PubMed] [Google Scholar]
- 34.Vigouroux S, Farout L, Clavel S, Briand Y, Briand M. Increased muscle proteasome activities in rats fed a polyunsaturated fatty acid supplemented diet. Int J Biochem Cell Biol. 2003;35:749–755. doi: 10.1016/s1357-2725(02)00389-8. [DOI] [PubMed] [Google Scholar]
- 35.Kon M, et al. Chaperone-mediated autophagy is required for tumor growth. Sci. Trans Med. 2011;3 doi: 10.1126/scitranslmed.3003182. 109ra117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goñi FM, et al. Phase diagrams of lipid mixtures relevant to the study of membrane rafts. Biochim Biophys Acta. 2008;1781:665–684. doi: 10.1016/j.bbalip.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martinez-Seara H, et al. Interplay of unsaturated phospholipids and cholesterol in membranes: Effect of the double-bond position. Biophys J. 2008;95:3295–3305. doi: 10.1529/biophysj.108.138123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jaikishan S, Slotte JP. Effect of hydrophobic mismatch and interdigitation on sterol/sphingomyelin interaction in ternary bilayer membranes. Biochim Biophys Acta. 2011;1808:1940–1945. doi: 10.1016/j.bbamem.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 39.Pinto SN, Silva LC, Futerman AH, Prieto M. Effect of ceramide structure on membrane biophysical properties: The role of acyl chain length and unsaturation. Biochim Biophys Acta. 2011;1808:2753–2760. doi: 10.1016/j.bbamem.2011.07.023. [DOI] [PubMed] [Google Scholar]
- 40.Wassall SR, et al. Order from disorder, corralling cholesterol with chaotic lipids. The role of polyunsaturated lipids in membrane raft formation. Chem Phys Lipids. 2004;132(1):79–88. doi: 10.1016/j.chemphyslip.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 41.Eskelinen EL, et al. Unifying nomenclature for the isoforms of the lysosomal membrane protein LAMP-2. Traffic. 2005;6:1058–1061. doi: 10.1111/j.1600-0854.2005.00337.x. [DOI] [PubMed] [Google Scholar]
- 42.Sahu R, et al. Microautophagy of cytosolic proteins by late endosomes. Dev Cell. 2011;20(1):131–139. doi: 10.1016/j.devcel.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kaushik S, Massey AC, Mizushima N, Cuervo AM. Constitutive activation of chaperone-mediated autophagy in cells with impaired macroautophagy. Mol Biol Cell. 2008;19:2179–2192. doi: 10.1091/mbc.E07-11-1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang Y, Schattenberg JM, Rigoli RM, Storz P, Czaja MJ. Hepatocyte resistance to oxidative stress is dependent on protein kinase C-mediated down-regulation of c-Jun/AP-1. J Biol Chem. 2004;279:31089–31097. doi: 10.1074/jbc.M404170200. [DOI] [PubMed] [Google Scholar]
- 45.Cuervo AM, Stefanis L, Fredenburg R, Lansbury PT, Sulzer D. Impaired degradation of mutant alpha-synuclein by chaperone-mediated autophagy. Science. 2004;305:1292–1295. doi: 10.1126/science.1101738. [DOI] [PubMed] [Google Scholar]
- 46.Mak SK, McCormack AL, Manning-Bog AB, Cuervo AM, Di Monte DA. Lysosomal degradation of alpha-synuclein in vivo. J Biol Chem. 2010;285:13621–13629. doi: 10.1074/jbc.M109.074617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang Y, et al. Tau fragmentation, aggregation and clearance: The dual role of lysosomal processing. Hum Mol Genet. 2009;18:4153–4170. doi: 10.1093/hmg/ddp367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cuervo AM, Dice JF, Knecht E. A population of rat liver lysosomes responsible for the selective uptake and degradation of cytosolic proteins. J Biol Chem. 1997;272:5606–5615. doi: 10.1074/jbc.272.9.5606. [DOI] [PubMed] [Google Scholar]
- 49.Ohsumi Y, Ishikawa T, Kato K. A rapid and simplified method for the preparation of lysosomal membranes from rat liver. J Biochem. 1983;93:547–556. [PubMed] [Google Scholar]
- 50.Chan R, et al. Retroviruses human immunodeficiency virus and murine leukemia virus are enriched in phosphoinositides. J Virol. 2008;82:11228–11238. doi: 10.1128/JVI.00981-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dall'Armi C, et al. The phospholipase D1 pathway modulates macroautophagy. Nat Commun. 2010;1:142. doi: 10.1038/ncomms1144. [DOI] [PMC free article] [PubMed] [Google Scholar]