Abstract
LacY mutant Cys154 → Gly exhibits a periplasmic-closed crystal structure identical to the WT, but is periplasmic-open in the membrane. The mutant hardly catalyzes transport, but binds galactosides from either side of the membrane with the same affinity and is resistant to site-directed proteolysis relative to the pseudo-WT. Site-directed alkylation was also applied to 11 single-Cys mutants in Cys154 → Gly LacY in right-side-out membrane vesicles or after solubilization and purification in dodecyl-β-D-maltopyranoside (DDM). Unlike the pseudo-WT, Cys replacements on the periplasmic side of the Cys154 → Gly mutant label rapidly in the membrane without sugar, but labeling decreases markedly after the mutant proteins are purified. Thus, Cys154 → Gly LacY likely favors a higher-energy intermediate periplasmic-open conformation in situ, but collapses to a lower-energy periplasmic-closed conformation in DDM after purification. Notably, branched-chain or neopentyl glycol maltoside detergents stabilize Cys154 → Gly LacY in the membrane-embedded form.
Keywords: lactose permease, membrane proteins, site-directed alkylation, symport, membrane transport
The lactose permease of Escherichia coli (LacY), a paradigm for the Major Facilitator Superfamily of membrane transport proteins, catalyzes stoichiometric translocation of a galactoside and an H+ across the cytoplasmic membrane (galactoside/H+ symport) (reviewed in refs. 1, 2). LacY has been solubilized, purified, and reconstituted into proteoliopsomes in a fully functional state (3). Initial X-ray structures of the conformationally restricted C154G mutant (4, 5) and a subsequent crystal structure of WT LacY (6), as well as a single-Cys construct with a covalently bound inactivator (7), exhibit the same inward-facing conformation. In both structures, twelve mostly irregular transmembrane α-helices are organized into two pseudosymmetrical six-helix bundles surrounding a cavernous hydrophilic cleft open to the cytoplasm only. A single sugar-binding site is located at the apex of the cavity in approximately the middle of the molecule across from residues that form an H+-binding site; both sites are inaccessible from the sealed periplasmic side (Fig. 1). However, LacY is highly dynamic, and sugar binding induces a global conformational change in which the cytoplasmic cavity closes with opening of a comparable hydrophilic cavity on the periplasmic side (2, 4, 8). A wealth of biophysical and spectroscopic data, which includes site-directed alkylation (SDA) (9–12), single-molecule fluorescence resonance energy transfer (13), double electron-electron resonance (14), site-directed cross-linking (15) and Trp fluorescence studies (8, 16, 17) provide converging evidence that the sugar- and H+-binding site(s) in LacY are alternatively accessible from either side of the membrane [the alternating access model (reviewed in refs. 18, 19.)]
Fig. 1.
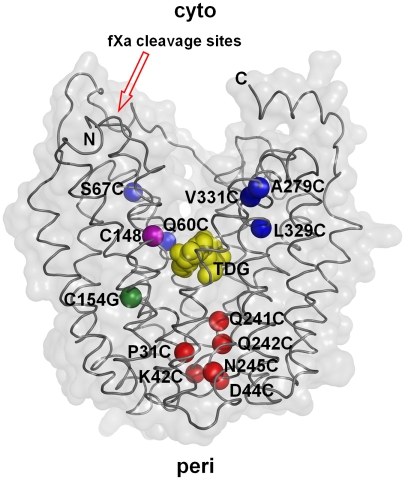
Single-Cys replacements in C154G LacY. Positions of single-Cys replacements are shown superimposed on the backbone of C154G LacY (PDB ID: 1PV7). LacY is viewed perpendicular to the membrane with the N-terminal helix bundle on the left and C-terminal helix bundle on the right. Blue spheres represent single-Cys replacements on the cytoplasmic side: Q60C, S67C (helix II), A279C (helix VIII), L329C, and V331C (helix X); red spheres represent single-Cys replacements on the periplasmic side: P31C (helix I), K42C, D44C (helix II), Q241C, Q242C, and N245C (helix VII). The position of Gly154 is shown in green, and the position of Cys148 is shown in purple. Yellow spheres at the apex of cavity represent TDG. The tandem factor Xa protease sites are indicated with a red arrow.
Recently, the reactivity/accessibility of single-Cys replacements in Cysless LacY (henceforth Cysless LacY is referred to as ‘WT’) was examined in RSO membrane vesicles and after solubilization and purification in the detergent dodecyl-β-d-maltopyranoside (DDM) by SDA with the hydrophobic, membrane-permeant thiol reagent teramethylrhodmine-5-maleimide (TMRM) (12). The results indicate that ‘WT’ LacY in the native bacterial membrane or solubilized and purified in DDM retains much the same periplasmic-closed conformation in the absence of sugar as in the crystal structure. Moreover, binding of galactopyranoside induces a similar global conformational change to a periplasmic-open conformation in right-side-out (RSO) membrane vesicles or with solubilized, purified protein. Thus, upon sugar binding to ‘WT’ LacY, Cys replacements on the periplasmic side become 6- to 10-fold more reactive/accessible, while Cys replacements on the cytoplasmic side become 6- to 10-fold less so.
The conformationally restricted mutant C154G, which was crystallized initially (4, 5), exhibits unique properties compared to wild-type LacY. Although transport is almost negligible, the mutant binds galactopyranosides as well as or even better than the wild type (20–23). The crystal structures yield a clue as to why the C154G mutant is conformationally restricted. Helices I and V cross in the approximate middle of the membrane where Cys154 (helix V) and Gly24 (helix I) are close (5, 6). Abutting Gly residues in adjacent helices may lead to significantly tighter helix packing (24–27), which may partially explain the lack of conformational flexibility of the mutant (28). Most remarkably, several lines of evidence (11, 13, 14) demonstrate that, unlike wild-type LacY, the mutant favors a periplasmic-open conformation in contradistinction to the X-ray structures currently available.
We demonstrate here that C154G LacY likely represents an intermediate conformation of LacY in the native bacterial membrane by showing that although transport activity is minimal, galactopyranoside can access the sugar-binding site from either side of the membrane with essentially the same affinity. Moreover, in contradistinction to ‘WT’ LacY, single-Cys replacements on the periplasmic side of the mutant label rapidly in the membrane in the absence of sugar, and galactoside binding has little or no effect on labeling rates. Surprisingly, however, the reactivity/accessibility of periplasmic single-Cys replacements is much lower after solubilization and purification in DDM, and the effect of sugar on labeling is minimal or reversed at some positions. The findings are consistent with the conclusion that the membrane-embedded conformation of C154G LacY collapses from a higher-energy intermediate conformation with a periplasmic-open conformation to a lower-energy periplasmic-closed conformation. Because crystals are formed from LacY purified in DDM, the periplasmic-closed conformer may be selected preferentially. Importantly, solubilization and purification with branched-chain or neopentyl glycol maltoside detergents stabilize C154G LacY in the membrane-embedded conformation, as judged by SDA, which holds promise with regard to obtaining a crystal structure of the mutant in an intermediate conformation(s).
Results
Sugar Binding.
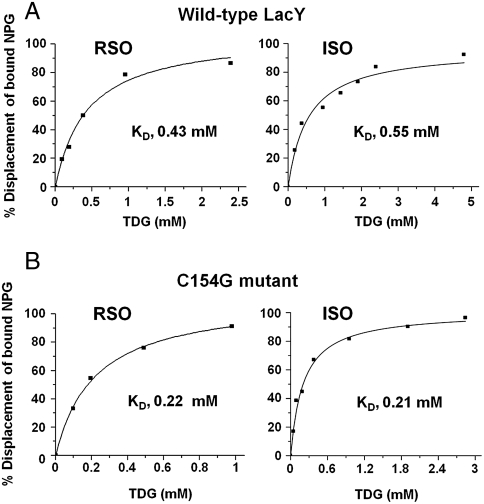
Binding of the lactose homologue β-d-galactopyranosyl-1-thio-β-d-galactopyransoside (TDG) by wild-type or C154G LacY in RSO or inside-out (ISO) membrane vesicles was measured by flow dialysis (Fig. 2). As shown previously (29), RSO or ISO membrane vesicles containing wild-type LacY bind TDG under conditions where transport is minimal with KD values of 0.43 or 0.55 mM, respectively, an insignificant difference (Fig. 2A). Vesicles of either orientation with C154G LacY bind TDG with slightly better affinity (in addition see refs. 21–23, 28), exhibiting KD values of 0.22 or 0.21 mM in RSO or ISO vesicles, respectively (Fig. 2B). In this regard, it is notable that the rate of binding by C154G LacY in reconstituted proteoliposomes or in detergent is at least as fast or faster than wild-type (8).
Fig. 2.
TDG binding by wild type LacY and C154G LacY. Binding of TDG to RSO or ISO membrane vesicles containing wild-type LacY (A) or C154G LacY (B) was assayed by flow dialysis as described in Materials and Methods.
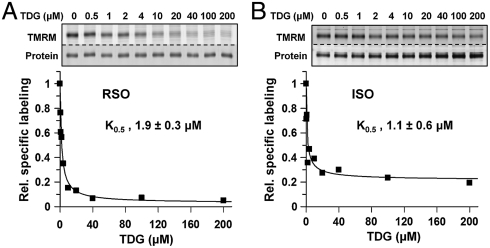
Ligand protection against alkylation of Cys148, a position in close proximity to bound galactoside, was also utilized to test binding (30, 31). RSO or ISO vesicles containing C154G LacY with a single native Cys at position 148 bind TDG with K0.5 values of 1.9 μM (Fig. 3A) or 1.1 μM (Fig. 3B), respectively, which is within experimental error. Therefore, despite the almost negligible ability of the C154G mutant to catalyze translocation across the membrane (22), the mutant binds sugar as well as wild-type LacY from either side of the membrane.
Fig. 3.
Protection of C154G/Cys148 LacY against alkylation by sugar binding. RSO (A) or ISO (B) membrane vesicles were incubated with a given concentration of TDG and then labeled with 40 μM TMRM for 5 min at 0 °C. K0,5 values were obtained by quantifying the labeling intensity as described in Materials and Methods. The concentration of TDG in each sample is indicated at the top of the gels.
Proteolysis.
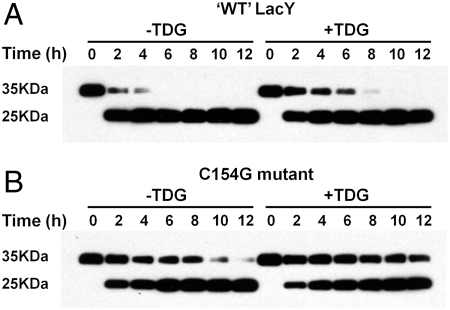
Previous studies show that LacY remains functional after insertion of tandem factor Xa protease sites between Ser136 and Asn137 on the cytoplasmic loop between helix IV and V (15, 32) (Fig. 1). As shown in Fig. 4, factor Xa protease cleaves ‘WT’ LacY at this site much faster than what it does with the Cysless C154G mutant. ‘WT’ LacY is almost completely digested by 4 h (Fig. 4A), while complete digestion of the C154G mutant takes ∼12 h (Fig. 4B). Furthermore, addition of 10 mM TDG markedly decreases the rate of proteolysis for both ‘WT’ LacY and the C154G mutant. The data clearly indicate that the conformation of the C154G mutant is different from ‘WT’ LacY and suggest that sugar binding induces similar conformations in both proteins.
Fig. 4.
Proteolysis of ‘WT’ LacY and C154G mutant. ‘WT’ LacY (A) or C154G LacY (B) with tandem factor Xa sites in loop IV/V were digested with factor Xa protease in the absence or presence of 10 mM TDG for given times at 0 °C and Western-blots were performed with anti-C-terminal antibody as described in Materials and Methods.
SDA on the Periplasmic Side of C154G LacY.
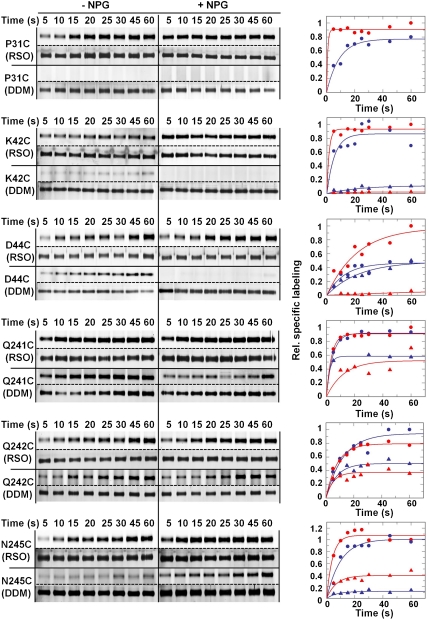
To approximate the open probability of the periplasmic cavity, the reactivity/accessibility of six single-Cys replacement mutants lining the potential periplasmic cavity in the C154G mutant (Fig. 1) was tested by using the fluorescent alkylating agent TMRM (10) (Fig. 5). With the exception of C154G/D44C LacY, which labels slowly and exhibits about a twofold increase in the presence of p-nitrophenyl-α,d-galactopyranoside (NPG), labeling of the other single-Cys replacements occurs so rapidly that it is not possible to obtain initial rates. For example, labeling of C154G/N241C in RSO membrane vesicles is almost complete at 5 s, the earliest time that can be assayed, and plateaus by approximately 10 s in the absence of NPG (Fig. 5). In the presence of NPG, essentially the same kinetics are observed. Each of the other five mutants labels relatively rapidly with TMRM in the absence ligand. NPG binding increases the rate of labeling with mutants C154G/P31C, C154G/K42C, C154G/D44C, and C154G/N245C while the labeling rate with mutants C154G/Q241C or C154G/Q242C is essentially the same whether or not NPG is present. Maximum intensity of the bands at 60 s with each mutant is approximately the same in the absence or presence of NPG with the exception of C154G/D44C where maximum labeling increases by about twofold in the presence of NPG. Relative to similar studies with ‘WT’ LacY (12), the data presented here demonstrate clearly that the periplasmic side of C154G LacY is in an open conformation in the native bacterial membrane.
Fig. 5.
SDA of periplasmic C154G/single-Cys mutants. Single-Cys mutants C154G/Q241C, C154G/Q242C, C154G/N245C, C154G/P31C, C154G/K42C, or C154G/D44C in RSO membrane vesicles (solid circles) or after solubilization and purification in DDM (solid triangles) were incubated with 40 μM TMRM for given times at 0 °C in the absence (blue) or presence of 1 mM NPG (red). RSO membrane vesicles containing a given mutant and labeled with TMRM were solubilized and purified in DDM by monomeric avidin chromatography and then subjected to SDS-PAGE. Alternatively, protein was solubilized and purified in DDM prior to labeling with TMRM. The gels were assayed for TMRM fluorescence (bands above the dotted lines) and silver stained for protein (bands below the dotted lines). The data were treated semiquantitatively as described in Materials and Methods by setting the highest 60 s point obtained with RSO vesicles to 1.
Unexpectedly, labeling of each single-Cys replacement mutant is markedly decreased after solubilization and purification (Fig. 5; triangles). At 60 s, labeling is diminished by 50%–90% depending on the position of the Cys replacement, and with mutant C154G/P31C, there is no labeling whatsoever. Moreover, NPG binding increases labeling of mutant C154G/N245C only, but inhibits labeling with the other five single-Cys mutants. Clearly, a significant structural change likely occurs with C154G LacY after the protein is solubilized and purified in DDM.
SDA on the Cytoplasmic Side of C154G LacY.
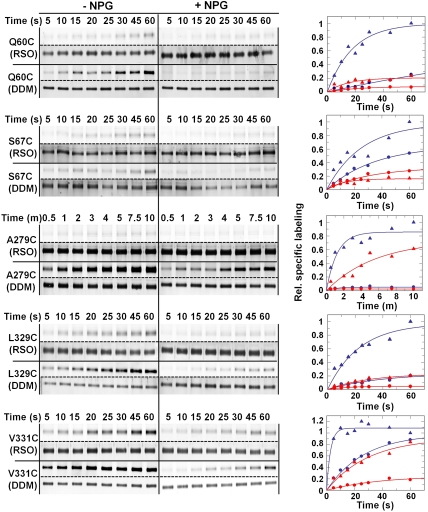
Single-Cys mutants at positions 60, 67, 279, 329, and 331 on the cytoplasmic side (Fig. 1) label with TMRM. As observed with both ‘WT’ LacY (9) and the C154G mutant (11), galactopyranoside binding inhibits labeling of the cytoplasmic single-Cys mutants (Fig. 6). As an example, C154G/L329C LacY reacts relatively faster with TMRM in RSO membrane vesicles in the absence of NPG. As observed in the ‘WT’ background (12), incubation with a galactopyranoside reduces the labeling rate and decreases maximum labeling by about 75%. Mutants C154G/Q60C, C154G/S67C, C154G/A279C, and C154G/V331C in RSO membrane vesicles also exhibit decreased rates of labeling in the presence of NPG. These effects are considerably more dramatic when the mutant proteins are solubilized and purified in DDM (triangles) in all probably due to the absence of the membrane, which represents a permeability barrier to TMRM. The findings are consistent with the conclusion that galactopyranoside binding induces closure of the cytoplasmic cavity despite paralysis of the C154G mutant, which has an open conformation on the periplasmic side.
Fig. 6.
SDA of cytoplasmic single-Cys mutants. Single-Cys mutants C154G/Q60C, C154G/S67C, C154G/A279C, C154G/L329C, or C154G/V331C were labeled in RSO membrane vesicles (solid circles) or in DDM as purified protein (solid triangles) for indicated times at 0 °C in the absence (blue) or presence of 1 mM NPG (red) as described in Fig. 5. Relative labeling was calculated and plotted as described in Materials and Methods.
Branched-Chain or Neopentyl Glycol Maltoside Detergents.
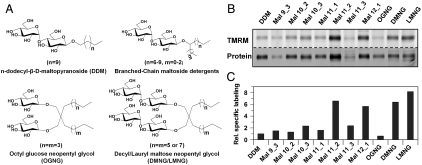
A new group of branched-chain or neopentyl glycol maltoside detergents appears to be useful for membrane protein solubilization and crystallization (33, 34) (Fig. 7A). Therefore, single-Cys N245C/C154G LacY was solubilized and purified in given branched-chain or neopentyl glycol maltoside detergents and labeled with TMRM in the absence of sugar (Fig. 7B). Specific labeling in DDM is about 10% of that observed in RSO vesicles, as shown above (Fig. 5). However, when solubilized and purified with the branched-chain detergents, specific labeling is increased to various levels. Compared to DDM, Mal 11_2 or Mal 12_1 give significant increases in specific labeling by about seven and sixfold, respectively. Furthermore, specific labeling of single-Cys N245C/C154G after solubilization and purification in decyl maltose neopentyl glycol (DMNG) or lauryl maltose neopentyl glycol (LMNG) is about six or eightfold higher than in DDM, respectively (Fig. 7 B and C), which is comparable to that observed in RSO vesicles. In contrast, solubilization and purification in octyl glucose neopentyl glycol (OGNG) leads to poor specific labeling.
Fig. 7.
SDA of periplasmic C154G/N245C in branched-chain maltoside detergents and glucose or maltose neopentyl glycols. (A) Structures of DDM, branched-chain maltoside detergents and maltose or glucose neopentyl glycols used. (B) SDA of C154G/N245C LacY in branched-chain maltoside detergents and maltose or glucose neopentyl glycols. RSO membrane vesicles containing C154G/N245C (0.1 mg total protein) were incubated with given detergents at a final concentration 20-fold above the critical micelle concentration. LacY was purified with monomeric avidin followed by labeling with 40 μM TMRM for 1 min at 0 °C in the absence of NPG. The concentrations (%; wt/vol) of detergents used in washing and chromatography were: DDM, 0.02; Mal 9_3, 0.06; Mal 10_2, 0.06; Mal 10_3, 0.06; Mal 11_1, 0.06; Mal 11_2, 0.02; Mal 11_3, 0.02; Mal 12_1, 0.02; OGNG, 0.2; DMNG, 0.02; LMNG, 0.02. The variability observed with respect to the proteins bands with each detergent is due primarily to differences in the retention of LacY on the monovalent avidin affinity columns under the conditions used for elution. (C) Relative specific labeling of C154G/N245 LacY in selected detergents. Specific labeling in each detergent was calculated by dividing the TMRM signal by the intensity of the protein band, and the specific labeling in DDM was set as 1.
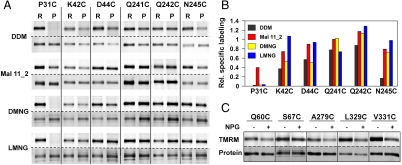
Cytoplasmic single-Cys mutants P31C, K42C, D44C, Q241C, and Q242C were also purified and labeled in DDM, Mal 11_2, DMNG, and LMNG (Fig. 8 A and B). In Mal 11_2 (red), each mutant exhibits higher specific labeling relative to DDM (black). With DMNG (yellow), specific labeling increases at positions 42, 241, 242, and 245, and with LMNG (blue) increases in specific labeling at positions 42, 44, 242, and 245. Moreover, labeling of each single-Cys mutant on the cytoplasmic side in Mal 11_2 exhibits a clear decrease in specific labeling upon NPG binding (Fig. 8C), as observed in RSO vesicles or with the purified proteins in DDM (Fig. 6). Thus, C154G LacY clearly binds sugar in Mal 11_2.
Fig. 8.
SDA of periplasmic single-Cys mutants in DDM, Mal 11_2, DMNG and LMNG. (A) Single-Cys replacements P31C, K42C, D44C, Q241C, Q242C, and N245C were labeled with TMRM in RSO membrane vesicles (R) or as purified protein (P) in the given detergent. RSO membrane vesicles were solubilized with DDM, Mal 11_2, DMNG, and LMNG at a final concentration 20-times the critical micelle concentration and the single-Cys LacY mutants were purified by monomeric avidin affinity chromatography. RSO membrane vesicles or purified proteins were labeled with 40 μM of TMRM for 1 min at 0 °C in the absence of NPG. (B) Relative specific labeling of periplasmic single-Cys mutants after purification in DDM (black), Mal 11_2 (red), DMNG (yellow) or LMNG (blue). For each detergent, relative specific labeling in RSO membrane vesicles was set at 1. (C) Effect of NPG on labeling of cytoplasmic single-Cys mutants in Mal 11_2. RSO membrane vesicles containing single-Cys replacement Q60C, S67C, A279C, L329C, or V331C were solubilized with 0.12% Mal 11_2 (wt/vol), and the proteins were purified with monomeric avidin affinity chromatography. Purified LacY in 0.02% Mal 11_2 was labeled with 40 μM of TMRM for 1 min at 0 °C in the absence or presence of 1 mM NPG as indicated.
Discussion
Mutant C154G exhibits radically different properties from wild-type LacY (11, 13, 14), a conclusion confirmed and extended here. However, the X-ray crystal structures are indistinguishable from wild-type. As shown by direct binding measurements (Fig. 2) or by sugar protection against alkylation of Cys148 (Fig. 3), the sugar-binding site is accessible from either side of the membrane, as shown previously for ‘WT’ LacY (29). However, the C154G mutant exhibits extremely low transport activity and is not leaky to H+, thereby suggesting that the C154G mutant likely assumes an intermediate conformation upon sugar binding. The conclusion is supported by site-directed proteolysis experiments with ‘WT’ and C154G LacY (Fig. 4). Clearly, C154G LacY is proteolyzed approximately 3-times slower than ‘WT’ LacY, and both proteins are stabilized by ligand binding.
Use of SDA has provided decisive information regarding structure/function relationships in LacY: (i) In the absence of sugar, the predominant population of ‘WT’ LacY is in an inward-facing or cytoplasmic-open conformation in both RSO membrane vesicles (9, 12) and after purification in DDM (12). (ii) In both RSO membrane vesicles and after purification in DDM, sugar binding to ‘WT’ LacY causes closing of the cytoplasmic cavity with opening of a complementary hydrophilic pathway on the periplasmic side, thereby allowing alternating access of the sugar-binding site to either side of the membrane, a conclusion supported by a number of converging lines of evidence (reviewed in refs. 18, 19). (iii) Initial findings (11) indicate that the conformationally restricted mutant C154G is paralyzed in a periplasmic-open conformation in the membrane and relatively unaffected by sugar binding. The observations presented here strongly reinforce the preliminary observations; however, the conclusion clearly conflicts with X-ray crystal structures of the mutant where the periplasmic side is closed (4, 5). Thus, unlike ‘WT’ LacY (12), Cys replacements on the periplasmic side of the sugar-binding site in C154G LacY are highly reactive/accessible in the absence of sugar (Fig. 5). But on the cytoplasmic side, labeling of the single-Cys mutants decreases markedly upon sugar binding (Fig. 6), as observed with ‘WT’ LacY. Taken together with the binding data, it seems likely that sugar binding causes the mutant protein to assume an intermediate conformation that is periplasmic-open and cytoplasmic-closed from which it escapes only rarely.
Remarkably, when the C154G/single-Cys mutants on the periplasmic side are solubilized from the membrane and purified in DDM, labeling is markedly decreased relative to that in the native membrane with or without sugar (Fig. 5). Thus, the structure of C154G LacY is altered when the mutant protein is solubilized and purified in DDM in such a manner that the periplasmic pathway collapses, and the molecule assumes a conformation that is likely at a lower free energy than the membrane-embedded structure.
The observations reported here for C154G LacY may also be relevant to wild-type LacY. With wild-type, isothermal calorimetry indicates that galactopyranoside binding induces an increase in entropy predominantly, in contrast to increased enthalpy with the C154G mutant (23). Thus, sugar binding with wild-type LacY induces multiple conformations, while the C154G protein is limited in the number of conformations that can be occupied upon sugar binding. As discussed above, this conclusion is consistent with a number of independent experimental approaches demonstrating that sugar binding induces a global change in the conformation of wild-type LacY, while the C154G mutant remains in a periplasmic-open conformation.
Taken as a whole, the findings indicate that, unlike wild-type LacY, mutant C154G is adversely affected by solubilization and purification in DDM. In the membrane, C154G LacY is in a periplasmic-open conformation, and upon sugar binding, the protein assumes another probably intermediate conformation that is open on the periplasmic side and closed on the cytoplasmic side. However, the periplasmic-closed conformation observed in the crystal structure predominates after solubilization and purification in DDM. Therefore, it seems reasonable to deduce that the periplasmic-closed conformation is selected during crystallization. Because it is likely that wild-type LacY also partially populates a similar conformation(s) (14), solubilization and crystallization in DDM may also preferentially select against an intermediate conformation(s) with wild-type LacY.
In order to obtain a crystal structure that represents the membrane-embedded structure of C154G LacY, detergents other than DDM may be useful. Thus, maltose neopentyl glycol (33) or branched-chain maltoside (35) detergents can improve solubility and stability of membrane proteins and may lead to different crystal structures relative to DDM (34, 35). As shown here, solubilization and purification of mutant C154G with such detergents, Mal 11_2, Mal 12_1, DMNG, and LMNG in particular, lead to labeling behavior very similar to that observed in situ. Apparent stabilization by these detergents may be due to the size of the micelles, their compactness, enhanced hydrophobicity, or a combination of all three. In any case, we anticipate that use of these new types of detergents will be useful with respect to obtaining an X-ray crystal structure(s) of the C154G mutant in a membrane-embedded intermediate conformation.
Materials and Methods
Materials.
[14C]NEM was purchased from DuPont/New England Nuclear. p-Nitrophenyl-α,d-[6-3H]galactopyranoside ([3H]NPG) was generously provided by G. Leblanc (Laboratoire J. Maetz/Commissariat a l’Energie Atomique). TMRM (T-6027) was obtained from Molecular Probes, Invitrogen Corp.. ImmunoPure immobilized monomeric avidin was obtained from Pierce, Thermo Scientific Inc.. Branched-chain detergents were synthesized as described (34). OGNG, DMNG, and LMNG were obtained from Affymetrix Inc.. All other materials were reagent grade and obtained from commercial sources.
Plasmid Construction.
Plasmids encoding single-Cys replacements in the C154G/Cysless LacY background with a C-terminal biotin acceptor domain followed by a 6-His tag were constructed as described (10, 11).
Growth of Bacteria.
E. coli T184 (lacy- z-) (36) transformed with plasmid pT7-5 encoding wild-type LacY, C154G LacY or a given single-Cys replacements were grown, induced, and harvested for the preparation of RSO or ISO membrane vesicles as previously described (10, 12).
Preparation of RSO and ISO Membrane Vesicles.
RSO membrane vesicles were prepared from 1.0 L cultures of E. coli T184 expressing a specific mutant by lysozyme-EDTA treatment and osmotic lysis (37, 38). ISO membrane vesicles were prepared by passage of cells through a French press at 5–8,000 psi as described (39). RSO or ISO membrane vesicles were resuspended to a protein concentration of 10–20 mg/mL in 100 mM potassium phosphate (KPi; pH 7.5) containing 10 mM MgSO4, frozen in liquid nitrogen and stored at -80 °C until use.
Flow Dialysis.
Binding of TDG to membrane vesicles containing LacY (20 mg protein/ml; total volume, 0.15 mL) was measured by flow dialysis as described (40). Briefly, [6-3H]NPG (840 mCi/mM) was added to the upper chamber at a final concentration of 10 μM, and a given concentration of TDG was added to displace bound [3H]NPG. NPG displaced from C154G LacY was expressed as percentage of total bound NPG, and plotted as a function of given TDG concentrations. KD values were determined by fitting the data to a hyperbolic function using the ORIGIN computer program (Microcal Software) as described previously (29).
Protection of Cys148 Against Alkylation.
Protection of C154G LacY single-Cys148 against TMRM labeling in RSO and ISO membrane vesicles was carried out at 0 °C as described (41). RSO or ISO membrane vesicles (50 μL final volume containing 0.1 mg protein) preincubated with TDG at given concentrations were labeled with 40 μM TMRM for 5 min at 0 °C. Reactions were then terminated by addition of 20 mM dithiothreitol (DTT), and LacY was solubilized and purified as described (42). Fluorescent signals were imaged after SDS-PAGE and analyzed by ImageQuant 5.0 (Molecular Dynamics, GE Healthcare Bio-Sciences Corp). K0.5 values were calculated by GraFit 6 (Erithacus Software Limited).
Proteolysis.
RSO vesicles [40 μg total protein] containing ‘WT’ LacY or C-less C154G LacY with two tandem factor Xa protease sites between Ser136 and Asn137 (loop IV/V) were mixed with 20 mM Tris-HCl (pH 7.5)/100 mM NaCl/2.0 mM CaCl2/1% DDM. Factor Xa protease (2 μg) and 10 mM TDG (where indicated) were added to a final volume of 80 μL. The mixture was incubated at 0 °C, and 2 μL samples [1.0 μg total protein] were taken at each time point for Western-blotting with anti-C-terminal antibody.
TMRM Labeling of LacY in RSO and DDM.
Labeling experiments were carried out following the procedure described previously (12, 42). RSO membrane vesicles [0.1 mg total protein in 50 μL 100 mM KPi (pH 7.5)/10 mM MgSO4] containing a given single-Cys replacement were incubated with 40 μM TMRM in the absence or presence of 1 mM NPG at 0 °C. After adding DTT (20 mM, final concentration) at given times to stop the reaction, vesicles were solubilized with 2.0% DDM and incubated with 50 μL monomeric avidin beads for 10 min at room temperature. The mixture was loaded on to a mini column followed by washing with 3 mL of washing buffer [50 mM NaPi (pH 7.5), 0.1 M NaCl, 0.02% DDM] and biotinylated LacY was eluted with 50 μL of elution buffer [50 mM NaPi (pH 7.5)/0.1 M NaCl/5 mM biotin/0.02% DDM]. For labeling purified proteins in DDM, given single-Cys mutants were purified from the same amount of RSO membrane vesicles as described and then labeled with 40 μM TMRM in the absence or presence of 1 mM NPG at 0 °C for given times. Aliquots (10 μL out of 50 μL total of purified protein) were subjected to SDS-PAGE. The gels were immediately scanned by using Amersham Typhoon 9410 Workstation (λex = 532 nm and λem = 580 nm for TMRM), and the protein bands were developed subsequently by silver staining.
Density and area of fluorescent signals from TMRM labeled or silver-stained LacY bands were quantified by using ImageQuant 5.0 (Molecular Dynamics, GE Healthcare Bio-Sciences Corp). A background from the appropriate gel was subtracted. Maximum specific labeling at 60 s was calculated by dividing the fluorescent signal by the protein signal and normalizing to 1. Each set of data was fit by using GraFit 6 (Erithacus Software Limited) with the first order kinetics equation (Eq. 1):
| [1] |
Detergent Screening.
RSO membrane vesicles [0.1 mg total protein in 50 μL 100 mM KPi (pH 7.5)/10 mM MgSO4] containing a single-Cys replacement on the periplasmic side were incubated with the selected detergent at a final concentration of 20-times the critical micelle concentration, and biotinylated LacY was purified by immobilized monomeric avidin Sepharose chromatography. Purified single-Cys LacY was labeled with 40 μM TMRM for 1 min at 0 °C in the absence of a galactoside and the reaction was terminated by adding 20 mM DTT. After addition of sample buffer, 10 μL samples were subjected to SDS-PAGE. Fluorescent and protein band intensities were quantified by using ImageQuant 5.0, and relative labeling was calculated as described.
Acknowledgments.
We thank Irina Smirnova and Vladimir Kasho for many helpful discussions and for editorial help with the manuscript. We also thank Sir John Walker for suggesting the use of the neopentyl glycol detergents. This work was supported by National Institutes of Health (NIH) Grant DK51131, DK069463 to H.R.K., and NIH Roadmap Grant P50 GM074929 to H.R.K. and P50 GM073197 to Q.Z.
Footnotes
The authors declare no conflict of interest.
See Author Summary on page 4349 (volume 109, number 12).
References
- 1.Kaback HR, Sahin-Toth M, Weinglass AB. The kamikaze approach to membrane transport. Nat Rev Mol Cell Biol. 2001;2:610–620. doi: 10.1038/35085077. [DOI] [PubMed] [Google Scholar]
- 2.Guan L, Kaback HR. Lessons from lactose permease. Annu Rev Biophys Biomol Struct. 2006;35:67–91. doi: 10.1146/annurev.biophys.35.040405.102005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Viitanen P, Newman MJ, Foster DL, Wilson TH, Kaback HR. Purification, reconstitution, and characterization of the lac permease of Escherichia coli. Methods Enzymol. 1986;125:429–452. doi: 10.1016/s0076-6879(86)25034-x. [DOI] [PubMed] [Google Scholar]
- 4.Abramson J, et al. Structure and mechanism of the lactose permease of Escherichia coli. Science. 2003;301:610–615. doi: 10.1126/science.1088196. [DOI] [PubMed] [Google Scholar]
- 5.Mirza O, Guan L, Verner G, Iwata S, Kaback HR. Structural evidence for induced fit and a mechanism for sugar/H(+) symport in LacY. Embo J. 2006;25:1177–1183. doi: 10.1038/sj.emboj.7601028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guan L, Mirza O, Verner G, Iwata S, Kaback HR. Structural determination of wild-type lactose permease. Proc Nat'l Acad Sci USA. 2007;104:15294–15298. doi: 10.1073/pnas.0707688104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chaptal V, et al. Crystal structure of lactose permease in complex with an affinity inactivator yields unique insight into sugar recognition. Proc Nat'l Acad Sci USA. 2011;108:9361–9366. doi: 10.1073/pnas.1105687108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smirnova I, Kasho V, Sugihara J, Kaback HR. Opening the periplasmic cavity in lactose permease is the limiting step for sugar binding. Proc Nat'l Acad Sci USA. 2011;108:15147–15151. doi: 10.1073/pnas.1112157108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaback HR, et al. Site-directed alkylation and the alternating access model for LacY. Proc Nat'l Acad Sci USA. 2007;104:491–494. doi: 10.1073/pnas.0609968104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nie Y, Ermolova N, Kaback HR. Site-directed alkylation of LacY: effect of the proton electrochemical gradient. J Mol Biol. 2007;374:356–364. doi: 10.1016/j.jmb.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nie Y, Sabetfard FE, Kaback HR. The Cys154 → Gly mutation in LacY causes constitutive opening of the hydrophilic periplasmic pathway. J Mol Biol. 2008;379:695–703. doi: 10.1016/j.jmb.2008.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nie Y, Kaback HR. Sugar binding induces the same global conformational change in purified LacY as in the native bacterial membrane. Proc Nat'l Acad Sci USA. 2010;107:9903–9908. doi: 10.1073/pnas.1004515107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Majumdar DS, et al. Single-molecule FRET reveals sugar-induced conformational dynamics in LacY. Proc Nat'l Acad Sci USA. 2007;104:12640–12645. doi: 10.1073/pnas.0700969104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smirnova I, et al. Sugar binding induces an outward facing conformation of LacY. Proc Nat'l Acad Sci USA. 2007;104:16504–16509. doi: 10.1073/pnas.0708258104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou Y, Guan L, Freites JA, Kaback HR. Opening and closing of the periplasmic gate in lactose permease. Proc Nat'l Acad Sci USA. 2008;105:3774–3778. doi: 10.1073/pnas.0800825105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smirnova IN, Kasho VN, Kaback HR. Direct sugar binding to LacY measured by resonance energy transfer. Biochemistry. 2006;45:15279–15287. doi: 10.1021/bi061632m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smirnova I, Kasho V, Sugihara J, Kaback HR. Probing of the rates of alternating access in LacY with Trp fluorescence. Proc Nat'l Acad Sci USA. 2009;106:21561–21566. doi: 10.1073/pnas.0911434106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaback HR, Smirnova I, Kasho V, Nie Y, Zhou Y. The alternating access transport mechanism in LacY. J Membr Biol. 2011;239:85–93. doi: 10.1007/s00232-010-9327-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smirnova I, Kasho V, Kaback HR. Lactose permease and the alternating access mechanism. Biochemistry. 2011;50:9684–9693. doi: 10.1021/bi2014294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Menick DR, Sarkar HK, Poonian MS, Kaback HR. Cys154 is important for lac permease activity in Escherichia coli. Biochem Biophys Res Commun. 1985;132:162–170. doi: 10.1016/0006-291x(85)91002-2. [DOI] [PubMed] [Google Scholar]
- 21.van Iwaarden PR, Driessen AJ, Lolkema JS, Kaback HR, Konings WN. Exchange, efflux, and substrate binding by cysteine mutants of the lactose permease of Escherichia coli. Biochemistry. 1993;32:5419–5424. doi: 10.1021/bi00071a017. [DOI] [PubMed] [Google Scholar]
- 22.Smirnova IN, Kaback HR. A mutation in the lactose permease of Escherichia coli that decreases conformational flexibility and increases protein stability. Biochemistry. 2003;42:3025–3031. doi: 10.1021/bi027329c. [DOI] [PubMed] [Google Scholar]
- 23.Nie Y, Smirnova I, Kasho V, Kaback HR. Energetics of ligand-induced conformational flexibility in the lactose permease of Escherichia coli. J Biol Chem. 2006;281:35779–35784. doi: 10.1074/jbc.M607232200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cosson P, Bonifacino JS. Role of transmembrane domain interactions in the assembly of class II MHC molecules. Science. 1992;258:659–662. doi: 10.1126/science.1329208. [DOI] [PubMed] [Google Scholar]
- 25.Lemmon MA, Flanagan JM, Treutlein HR, Zhang J, Engelman DM. Sequence specificity in the dimerization of transmembrane alpha-helices. Biochemistry. 1992;31:12719–12725. doi: 10.1021/bi00166a002. [DOI] [PubMed] [Google Scholar]
- 26.Smith SO, Bormann BJ. Determination of helix-helix interactions in membranes by rotational resonance NMR. Proc Nat'l Acad Sci USA. 1995;92:488–491. doi: 10.1073/pnas.92.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Javadpour MM, Eilers M, Groesbeek M, Smith SO. Helix packing in polytopic membrane proteins: role of glycine in transmembrane helix association. Biophys J . 1999;77:1609–1618. doi: 10.1016/S0006-3495(99)77009-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ermolova NV, Smirnova IN, Kasho VN, Kaback HR. Interhelical packing modulates conformational flexibility in the lactose permease of Escherichia coli. Biochemistry. 2005;44:7669–7677. doi: 10.1021/bi0502801. [DOI] [PubMed] [Google Scholar]
- 29.Guan L, Kaback HR. Binding affinity of lactose permease is not altered by the H+ electrochemical gradient. Proc Nat'l Acad Sci USA. 2004;101:12148–12152. doi: 10.1073/pnas.0404936101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frillingos S, Kaback HR. Probing the conformation of the lactose permease of Escherichia coli by in situ site-directed sulfhydryl modification. Biochemistry. 1996;35:3950–3956. doi: 10.1021/bi952601m. [DOI] [PubMed] [Google Scholar]
- 31.Sahin-Toth M, Lawrence MC, Nishio T, Kaback HR. The C-4 hydroxyl group of galactopyranosides is the major determinant for ligand recognition by the lactose permease of Escherichia coli. Biochemistry. 2001;40:13015–13019. doi: 10.1021/bi011233l. [DOI] [PubMed] [Google Scholar]
- 32.Wolin CD, Kaback HR. Thiol cross-linking of transmembrane domains IV and V in the lactose permease of Escherichia coli. Biochemistry. 2000;39:6130–6135. doi: 10.1021/bi0001269. [DOI] [PubMed] [Google Scholar]
- 33.Chae PS, et al. Maltose-neopentyl glycol (MNG) amphiphiles for solubilization, stabilization and crystallization of membrane proteins. Nat Methods. 2010;7:1003–1008. doi: 10.1038/nmeth.1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hong WX, et al. Design, synthesis, and properties of branch-chained maltoside detergents for stabilization and crystallization of integral membrane proteins: human connexin 26. Langmuir. 2010;26:8690–8696. doi: 10.1021/la904893d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang Q, Tao H, Hong WX. New amphiphiles for membrane protein structural biology. Methods. 2011;55:318–323. doi: 10.1016/j.ymeth.2011.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Teather RM, et al. Lactose carrier protein of Escherichia coli. Structure and expression of plasmids carrying the Y-gene of the lacoperon. Eur J Biochem. 1980;108:223–231. doi: 10.1111/j.1432-1033.1980.tb04715.x. [DOI] [PubMed] [Google Scholar]
- 37.Kaback HR. Bacterial Membranes. In: Kaplan NP, Jakoby WB, Colowick NP, editors. Methods in Enzymol. Vol XXII. New York: Elsevier; 1971. pp. 99–120. [Google Scholar]
- 38.Short SA, Kaback HR, Kohn LD. Localization of D-lactate dehydrogenase in native and reconstituted Escherichia coli membrane vesicles. J Biol Chem. 1975;250:4291–4296. [PubMed] [Google Scholar]
- 39.Nagamori S, Vazquez-Ibar JL, Weinglass AB, Kaback HR. In vitro synthesis of lactose permease to probe the mechanism of membrane insertion and folding. J Biol Chem. 2003;278:14820–14826. doi: 10.1074/jbc.M300332200. [DOI] [PubMed] [Google Scholar]
- 40.Rudnick G, Schuldiner S, Kaback HR. Equilibrium between two forms of the lac carrier protein in energized and nonenergized membrane vesicles from Escherichia coli. Biochemistry. 1976;15:5126–5131. doi: 10.1021/bi00668a028. [DOI] [PubMed] [Google Scholar]
- 41.Sahin-Tóth M, Gunawan P, Lawrence MC, Toyokuni T, Kaback HR. Binding of hydrophobic D-galactopyranosides to the lactose permease of Escherichia coli. Biochemistry. 2002;41:13039–13045. doi: 10.1021/bi0203076. [DOI] [PubMed] [Google Scholar]
- 42.Jiang X, Nie Y, Kaback HR. Site-directed alkylation studies with LacY provide evidence for the alternating access model of transport. Biochemistry. 2011;50:1634–1640. doi: 10.1021/bi101988s. [DOI] [PMC free article] [PubMed] [Google Scholar]