Abstract
Nautiloidea is the oldest group within the cephalopoda, and modern Nautilus differs much in its outer morphology from all other recent species; its external shell and pinhole camera eye are the most prominent distinguishing characters. A further unique feature of Nautilus within the cephalopods is the lack of suckers or hooks on the tentacles. Instead, the animals use adhesive structures present on the digital tentacles. Earlier studies focused on the general tentacle morphology and put little attention on the adhesive gland system. Our results show that the epithelial parts on the oral adhesive ridge contain three secretory cell types (columnar, goblet, and cell type 1) that differ in shape and granule size. In the non-adhesive aboral epithelium, two glandular cell types (cell types 2 and 3) are present; these were not mentioned in any earlier study and differ from the cells in the adhesive area. The secretory material of all glandular cell types consists mainly of neutral mucopolysaccharide units, whereas one cell type in the non-adhesive epithelium also reacts positive for acidic mucopolysaccharides. The present data indicate that the glue in Nautilus consists mainly of neutral mucopolysaccharides. The glue seems to be a viscous carbohydrate gel, as known from another cephalopod species. De-attachment is apparently effectuated mechanically, i.e., by muscle contraction of the adhesive ridges and tentacle retraction.
Keywords: Adhesive system, Cephalopods, Digital tentacles, Glue, Nautiloidea
1. Introduction
Nautiloidea is the oldest group within the cephalopoda, originating about 500 million years ago and persisting until the present time (House, 1988). The genus Nautilus is well known as a “living fossil” (Teichert and Matsumoto, 1987) and was already described by Aristotle in his Historia Animalium, page 622b (Thompson, 1910). Nautilus differs in many aspects from other modern cephalopod groups; the most prominent character is its brown and white externalized shell, whose main function is to adjust buoyancy.
In contrast to other cephalopod groups, the tentacles in Nautilus lack suckers or hooks. Instead, the animals produce glue in a specialized glandular structure on the oral side of the digital tentacles (Fukuda, 1988). They use these sticky tentacles to pick up food or attach to vertical/horizontal substrata or other individuals for mating (Kakinuma et al., 1995).
Although the gross anatomy and morphology of the tentacles has been previously described in detail (Owen, 1843; Willey, 1898; Bidder, 1962; Fukuda, 1988; Muntz and Wentworth, 1995; Ruth et al., 2002), our knowledge about the Nautilus adhesive system and its secretory composition is still limited.
The digital tentacles are slender, oval in cross-section, up to 10 cm long, 0.4 cm thick at their base, and slightly tapered, with a blunt, rounded tip. An axial nerve cord is surrounded by a large network of radial muscle fibers (Fig. 1). The longitudinal muscles are divided into bundles and form the core of the tentacle; several layers of oblique muscle fibers and connective tissue enclose the muscle layers (Kier, 1987). Tentacle retraction into a sheath is effected by the longitudinal muscles, while elongation is performed by the radial musculature (Kier, 1987). One large, thick-walled artery and one small, thinner-walled vein run close to the oral surface.
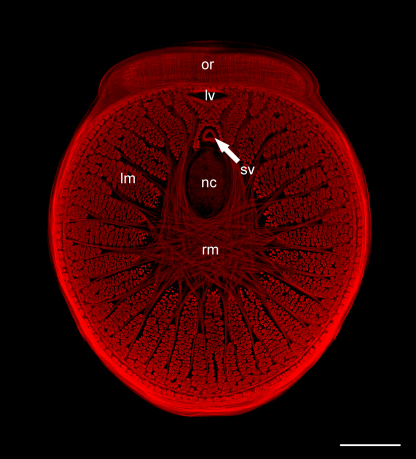
Fig. 1.
Cross-section of a digital tentacle of Nautilus pompilius, light microscopy image. An axial nerve cord (nc) is visible in the center of the tentacles. One large, thick-walled vein (lv) and one small, thinner-walled vein (sv) run close to the oral ridges (or). A large network of radial muscle fibers (rm) surrounds the central nerve bundle. The longitudinal muscles (lm) are divided into bundles and form the main body of the tentacle. Scale bar = 500 μm.
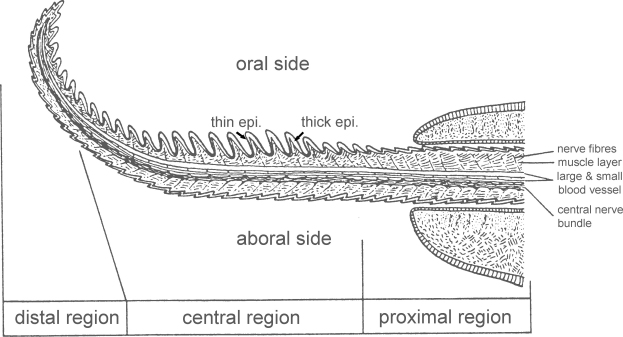
Tongue-like ridges surround the tentacle trunk and are more prominent on the oral side than the aboral side (Stenzel, 1964) (Fig. 2). The epithelium of the oral ridges is heterogeneous, thick on the proximal surface but thin on the distal side, whereas the epithelium on the aboral tentacle lacks height variations.
Fig. 2.
Schematic longitudinal section of the digital tentacle of Nautilus pompilius showing the different compartments. The ridges on the oral side of the tentacle are thicker and longer than those on the aboral side.
Source: Adapted from Fukuda (1988) and reproduced with permission.
According to the literature, only the oral side of the digital tentacles is specialized for producing the glue for adhesion (Fukuda, 1988). Two glandular cell types are described in these epithelia, columnar cells (in the thick epithelium) and goblet cells (in the thin epithelium). The secretory material of both cell types consists of neutral mucopolysaccharides, whereas the goblet cells also stain weakly positive for protein (Millon reaction) (Fukuda, 1988; Muntz and Wentworth, 1995).
In an earlier review article (von Byern et al., 2010a) we provided some basic information on the epithelial glands and secretory composition of the Nautilus adhesive system. Apart from the two cell types already described by Fukuda (1988) we found an additional cell type (named cell type 1), which had never been mentioned before, in the thick epithelium of the oral side. That review also provided the first brief description of the two gland cell types (named cell types 2 and 3) in the aboral epithelium. The review was done to provide a concise overview of the glandular diversity in the digital tentacle of Nautilus pompilius and presented only a selection of our research data.
The present research article closes the gaps and provides a detailed ultrastructural, histochemical and immunocytochemical description of the Nautilus digital tentacle. This improves our knowledge about the gland cells and the nature of glandular material of the adhesive and non-adhesive epithelium. Here, we also add a schematic drawing of the adhesive epithelium of the oral ridge to more clearly illustrate the cellular composition and arrangement (Fig. 3).
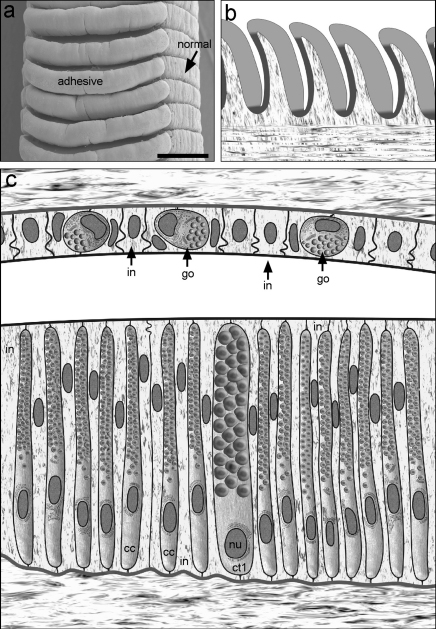
Fig. 3.
Schematic drawing of the adhesive area in Nautilus pompilius. (A) SEM image of the digital tentacle. Scale bar = 500 μm. (B) Details showing the thick epithelium (light grey) and thin epithelium (dark grey) of the oral ridge as well as the non-glandular interstitial cells (in). (C) The thick epithelium (bottom) consists of two cell types, columnar cells (cc) and cell type 1 (ct1), which vary in size and secretory content. In the thin epithelium (top) only one cell type, the goblet cell (go), is present. nu, nucleus.
2. Materials and methods
Adult specimens of N. pompilius were collected in waters off the coast of Tagnan, Panglao Island, in the Bohol Province of the Philippines (Wani, 2004) with the permission of the Bureau of Fisheries and Aquatic Resources, Department of Agriculture, Republic of the Philippines (Permit No. 09-2001).
2.1. Preparation and fixation
The animals were anesthetized with 3% (v/v) ethanol–seawater solution, and parts of the digital tentacles were cut off and fixed using the following methods: for histological/histochemical analyses, in an acetic–alcohol–formalin (AAF) mixture (Böck, 1989) for 3 h at 25 °C or in a Carnoy solution (Kiernan, 1999) for 3 h at 25 °C; for ultrastructural analyses, in 2.5% glutaraldehyde in sodium cacodylate buffer (0.1 M; pH 7.4, plus 10% sucrose) for 6 h at 25 °C. For cryostat investigations, samples were prefixed in 4% formalin–seawater (v/v) or embedded directly in a tissue freezing medium (Tissue-Tek O.C.T.; Sakura Finetek, Torrance, CA, USA) and frozen in liquid nitrogen.
2.2. Histology, histochemistry, and immunocytochemistry
AAF- and Carnoy-fixed material was dehydrated in a graded ethanol series, cleared three times for 20 min each in methylbenzoate as well as in benzene and infiltrated overnight with paraffin. Sections (5–7 μm thick) were cut, mounted on glass slides with Ruyter solution (Ruyter, 1931) and dried at room temperature before use. Frozen samples were cut with a Leica CM3050 S (Leica Microsystems, Wetzlar, Germany) into 15 μm sections, mounted on slides and used for the subsequent analysis.
The trichrome staining method AZAN (Heidenhain, 1905) was used to provide an overview of the glandular system and structural details. The periodic acid-Schiff (PAS) method (McManus and Mowry, 1960) was used to detect the presence of neutral mucopolysaccharides. Control and blocking of PAS was effected by prior treatment in dimedone for 3 h (Bulmer, 1959), borohydride reduction and phenylhydrazine for 3 h, acetylation for 2 and 9 h, and acetylation–deacetylation for 24 h (Kiernan, 1999). Acid mucopolysaccharides were stained by alcian blue 8GX (McManus and Mowry, 1960) at pH 1.0 and 2.5 for 2 h at 20 °C. Basic proteins were detected by staining with Biebrich scarlet (0.04%) for 1 h at 20 °C in phosphate buffer at pH 6.0 (Spicer and Lillie, 1961), and in Laskey's glycine buffer at pH 8.0, 9.5 and 10.5 (McManus and Mowry, 1960). Sudan black B (Böck, 1989) was used to identify lipids.
Carbohydrates were characterized enzymatically using the following lectins (50 mg/ml; incubated for 30 min at room temperature): FITC-labeled concanavalin agglutinin (ConA) (EY Laboratories, San Mateo, CA, USA), specific for α-d-mannose/α-d-glucose; Texas Red labeled peanut agglutinin (PNA), specific for lactose/β-galactose; TRITC-labeled soybean agglutinin (SBA) (EY Laboratories), specific for N-acetyl-d-galactosamine; FITC-labeled wheat germ agglutinin (WGA) (EY Laboratories), specific for N-acetyl-d-glucosamine; TRITC-labeled Galanthus nivalis lectin (GNA) (EY Laboratories), specific for mannose; and FITC-labeled Ulex europaeus agglutinin (UEA), specific for α-l-fucose. All lectins were diluted with the respective buffers specified by the manufacturers. Inhibition was carried out by incubating diluted fluorescent-labeled lectin with 0.2 M inhibitory carbohydrate for 60 min at room temperature before application to the sections. Autofluorescence was controlled for by incubating sections in buffer solution without fluorescent-labeled lectin.
Immunocytochemical analysis of muscles and nerve fibers was carried out on 100 μm thick vibratome sections prepared with a microtome (Leica VT 1200S; Leica Microsystems, Wetzlar, Germany), and incubated with 2.5% Alexa Fluor TRITC-conjugated phalloidin (R415; Invitrogen, Carlsbad, CA, USA) and 1:100 diluted acetylated α-tubulin (T-6793; Sigma–Aldrich, St. Louis, MO, USA) with FITC-labeled secondary antibody (M308012; Invitrogen) (see protocol in Wollesen et al., 2009, 2010).
2.3. Ultrastructure
Glutaraldehyde-fixed samples were washed three times for 30 min in buffer solution at room temperature and stored for further processing. For post-fixation, the samples were immersed for 1.5 h in 1% osmium tetroxide in 0.1 M sodium cacodylate buffer (same pH as above) and dehydrated in a graded series of ethanol.
For transmission electron microscopy (TEM) examination, the samples were embedded in epon; ultrathin sections (50–70 nm) were mounted on copper slot grids coated with formvar in dioxane, stained with uranyl acetate and lead citrate (Reynolds, 1963), and examined in a Zeiss EM 9S-2 and EM 902 (Carl Zeiss AG, Oberkochen, Germany).
For scanning electron microscopy (SEM) examination, the samples were washed several times in 100% acetone, immersed in hexamethyldisilazane (HMDS), dried in air overnight, mounted on stubs, coated with gold in a Polaron 5800 sputter coater (Quorum Technologies, East Grinstead, UK), and viewed in the SEM Philips XL 20 (Philips, Amsterdam, The Netherlands).
3. Results
The tongue-shaped ridges on the oral side of the digital tentacles are low and shallow in the distal region, but are well developed (about 500–700 μm in height and approx. 200 μm in width) in the central region (Fig. 3). The single epithelium layer of the oral ridges is heterogeneous, being thick on the proximal side (approx. 40 μm wide at its base, up to 150 μm wide at the ridge top) but thin on the distal surface (about 8 μm wide at the base, increasing to 30 μm at the top) (Fig. 4A).
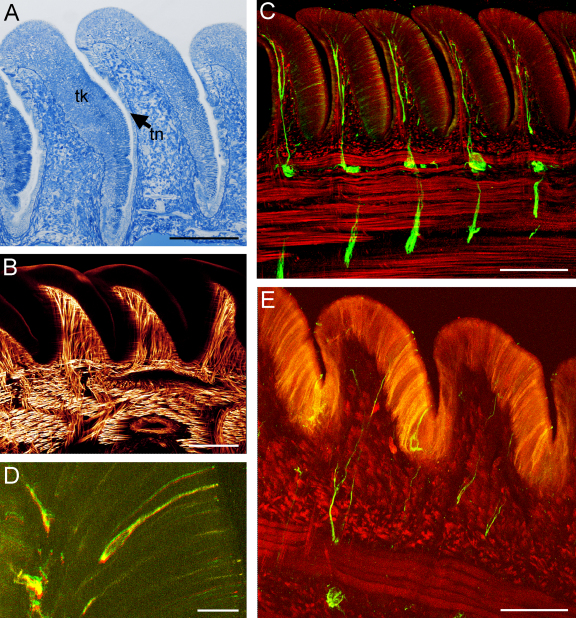
Fig. 4.
Longitudinal section of the oral ridges, light microscopy images. (A) The oral ridges of the digital tentacle are serrate and slightly concave anteriorly. The epithelium of the ridges is heterogeneous, thick (tk) on the proximal side but thin (tn) on the distal surface. Scale bar = 200 μm. (B) In the central part of the oral ridges and aboral ridges (not shown) there are large bundles of muscular fibers which fuse with the circular and longitudinal tentacle musculature. Scale bar = 150 μm. (C) Below each ridge is a sublamellar nerve plexus, interconnected with the others by a longitudinal as well as a central nerve cord. Scale bar = 150 μm. (D and E) From this plexus, nerves proceed into the ridges and end in ciliated cells on the tentacle surface. Scale bar in (D) = 15 μm, in (E) = 50 μm. Muscles were stained with phalloidin and are visualized in red; nerves were stained with acetylated α-tubulin and show up in green (D and E) or yellow (C).
In contrast, the ridges on the aboral side are low, flat, and more hump-shaped (about 200 μm tall and wide) and likewise consist of a single epithelium layer (50 μm thick) which is homogeneous throughout the ridge.
Although an earlier description denied the presence of muscles within the aboral ridge (Fukuda, 1988), the present re-examination revealed a large network of circular muscle fibers and nerves in the centre part of the oral (Fig. 4B) and aboral ridges. Below each ridge a sublamellar nerve plexus is present; these plexuses are connected to each other as well as to the central nerve cord (Fig. 4D).
Additionally, ciliated cells with nerve fibers are widely distributed on the surface of the digital tentacle and were detected by α-tubulin labeling (Fig. 4C–E).
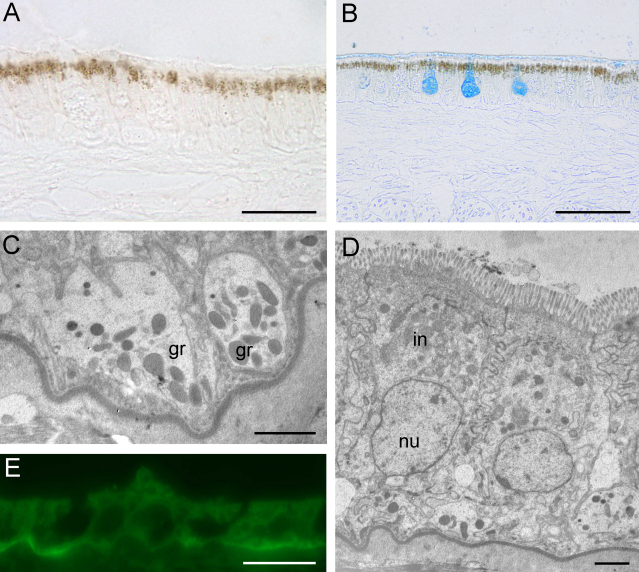
The aboral epithelium contains a pigment layer (about 10 μm thick) near the cell surface, which consists of brown-colored granules (Fig. 5A and B); this pigment layer does not react to any histochemical test that is applied. Additionally, a thin pigment layer (about 5 μm thick) filled with small granules (Fig. 5C) is located near the basal membrane of the oral and aboral ridge.
Fig. 5.
Light microscopy (A, B, and E) and TEM images (C and D) of the aboral epithelium. (A) A thin layer (∼10 μm) of brown pigments is visible on the surface of the aboral epithelium cells. Scale bar = 20 μm. (B) Only the glandular cells (here cell type 2), but not the pigment layer itself, react to any histochemical test (e.g., alcian blue at pH 2.5). Scale bar = 50 μm. (C) A second pigment layer (∼5 μm) is visible near the basal membrane of the oral and aboral ridges and contains electron-dense granules (gr). Scale bar = 2 μm. (D) Interstitial cells (in) with densely arranged microvilli and a centrally orientated nucleus (nu) are visible between the glandular cells of the oral and aboral epithelia. Scale bar = 2 μm. (E) The basal lamina of the aboral epithelium revealed strong reactivity to UEA (staining for fucose). Scale bar = 20 μm.
Interstitial cells are positioned between the glandular cells of the oral and aboral ridges; they are characterized by densely arranged microvilli (1–1.5 μm long and Ø 80–160 nm) (Fig. 5D). The oblong nucleus (10 μm × 5 μm) of this cell type is located in the middle of the epithelium and contains 2–4 nucleoli. The mitochondria are predominantly located near the cell periphery. Although the interstitial cells contain vesicles, they form the PAS-positive glycocalyx (also reactive for UEA and WGA) rather than secrete substances for adhesion/release.
Both the surface layer and the basal membrane of the oral side as well as the aboral side are strongly reactive to neutral sugars, identified by PAS and UEA lectin (Fig. 5E).
3.1. Oral side
The oral side contains three glandular cell types – two in the thick (proximal) epithelium and one in the thin (distal) epithelium.
3.1.1. Thick epithelium (proximal side of the ridges)
The columnar cells are elongate, slender (about 2.5 μm wide), and abundant throughout the epithelium (Fig. 6A and B). The basally located nucleus (globular to oval, Ø 5–8 μm) contains several nucleoli. Numerous Golgi bodies are present around the nucleus. The cells contain small, globular to polygonal granules (about 1 μm in diameter) with an electron-dense central core (Fig. 6D and E) that is already visible during granule synthesis. At the basal cell pole, the granules are round and lined like a string of beads, but they become polygonal and arranged pair-wise near the apical pole (Fig. 6B). Secretion is effected by pinching off droplets filled with several granules (Fig. 6C).
Fig. 6.
Light microscopy (A, C, F, and G) and TEM images (B, D, and E) of the thick epithelium of the oral ridges, showing two glandular cell types (A–F) columnar cells (cc), and (F and G) cell type 1 (ct1). (A and B) The columnar cells are slender and contain small, round to polygonal granules with an electron-dense core. Scale bar in (A) = 10 μm, in (B) = 2 μm. (C) For secretion, small vesicles filled with several granules are pinched off. Scale bar = 10 μm. (D) When the granules are synthesized in the basal area of the cells, they already contain the electron-dense core. Scale bar = 2 μm. (E) Columnar cell granules in high magnification. Scale bar = 500 nm. (F) Cell type 1 (ct1) and the columnar cells (cc) show a positive reaction to PAS staining (neutral sugars), as confirmed by Fig. 7. Scale bar = 10 μm. (G) Semithin section of cell type 1 (ct1) and the columnar cells (cc). Scale bar = 10 μm.
The histochemical analysis confirms that the secretory material is moderately periodate-reactive for neutral sugar units (PAS staining) (Fig. 6F, and in particular shown in Fig. 7) and unreactive to any blocking or control tests. The secretory material of the granular cells was strongly labeled by UEA and only moderately by WGA, demonstrating the presence of fucose and glucosamine. Biebrich scarlet and alcian blue at all pH levels showed no positive reactions for basic or acidic proteins. Sudan black B, which would indicate the presence of lipid components, also produced no staining. The small size of this cell type, however, makes it more difficult to achieve a clear and positive histochemical reaction as given for the other cell types. The chemical nature and function of the electron-dense core has not yet been characterized.
Fig. 7.
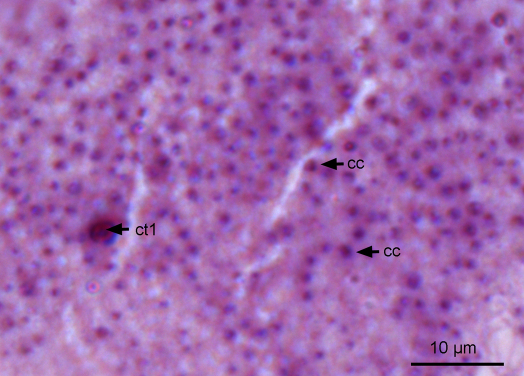
Horizontal section of the thick epithelium, light microscopy image. Cell type 1 (ct1) and the columnar cells (cc) react strongly to PAS (neutral sugars). Scale bar = 10 μm.
Another cell type (cell type 11) has been identified in the thick epithelium. This cell type is rare and can be detected only near the base of the ridge. Like the columnar cells, it traverses the complete height of the epithelium. Similar to the columnar cells, the nucleus of cell type 1 is also located basally, is globular to oval in shape (Ø 6–9 μm), and is surrounded by endoplasmatic reticulum (ER) and Golgi bodies.
Type 1 cells (about 7 μm wide) are wider than columnar cells and loosely packed with granules (Ø 1.5–1.8 μm) that are almost polygonal in shape (Fig. 6F and G). As in the columnar cells, the granules of cell type 1 have an electron-dense central core. Like those in the columnar cells, the granules of cell type 1 reacted strongly to PAS staining (Figs. 6F and 7) and U. europaeus lectin (UEA) and showed no positive reaction to other stains (Biebrich scarlet, alcian blue and Sudan black B).
3.1.2. Thin epithelium (distal side of the ridges)
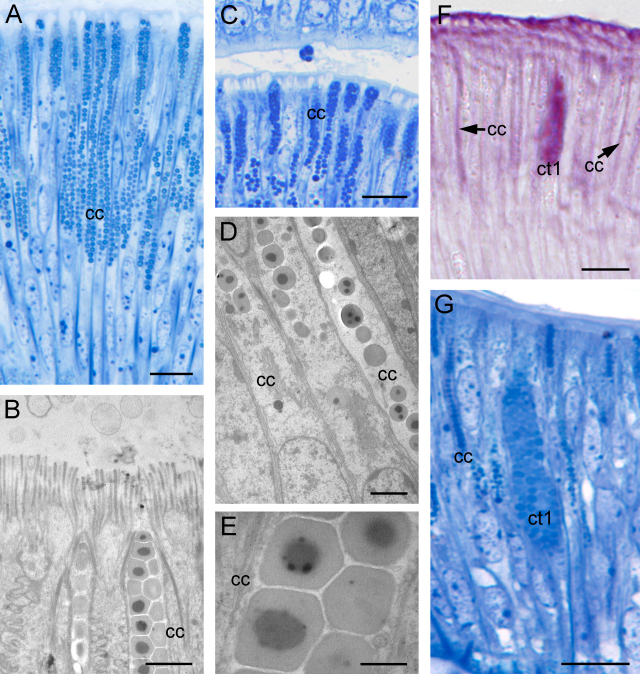
The goblet cells are rare and scattered within the epithelium from the base of the ridge to its top. As in the cell types in the thick epithelium, their nuclei are located basally and are surrounded by several layers of rough ER (rER) and Golgi bodies. The cells are round to oval near the base of the ridge (Fig. 8A) and elongated at the distal tip of the ridge (Fig. 8B) due to the height of the epithelium. They are filled with loosely arranged round granules (Ø 1.2–1.7 μm) (Fig. 8C), which, in contrast to the secretory material of both cell types of the thick epithelium, lack an electron-dense central core. As in the two cell types of the thick epithelium, the goblet cells also secrete the most apical part of their cell content (Fig. 8E–G).
Fig. 8.
Thin epithelium of the oral ridges, light microscopy (A, B, D, E, and G) and TEM (C and F) images. The goblet cells (go) in the thin epithelium of the oral ridges show different cell shapes depending on the epithelium height. (A) Near the ridge base the cells are round to oval. Scale bar = 10 μm. (B) Close to the ridge top the cells are slender. Scale bar = 10 μm. (C) Granule synthesis occurs in the basal part, supported by several layers of rER and a large Golgi network. Scale bar = 2 μm. (D) The cells are strongly periodate-reactive (PAS staining). Scale bar = 10 μm. (E–G) Secretion process in the goblet cells: as in the columnar cells, the apical part also is pinched off and several granules are released into the gap between the ridges. Scale bars = 10 μm, except in (F) = 2 μm.
The histochemical analysis indicated that the granules consist solely of neutral mucopolysaccharides (Fig. 8D). Incubation with U. europaeus lectin (UEA) revealed strong reactivity to fucose but no reactivity to any other lectins that were applied. Although earlier descriptions reported a weak positive reaction to protein staining (Millon reaction) for this secretory material, this could not be verified with the staining methods that were applied in this study.
3.2. Aboral side
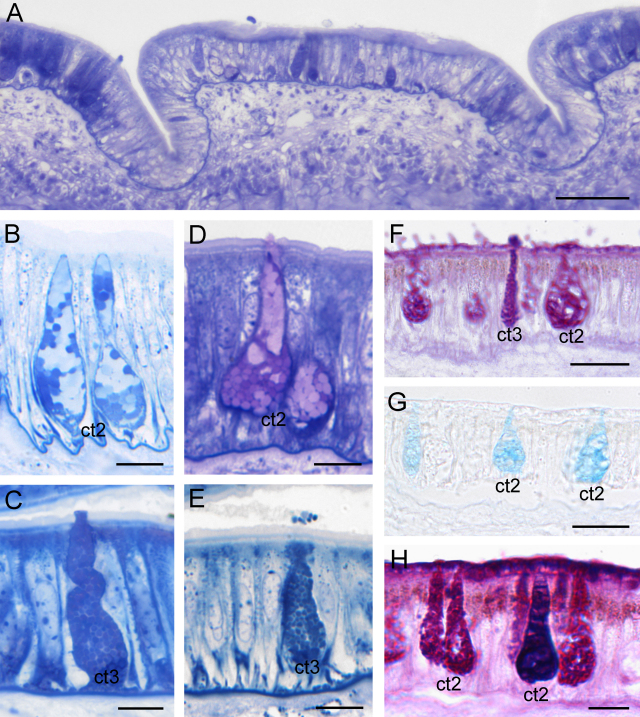
Two thus far unknown distinct cell types (type 2 and type 3) are present in the aboral epithelium (Fig. 9A).
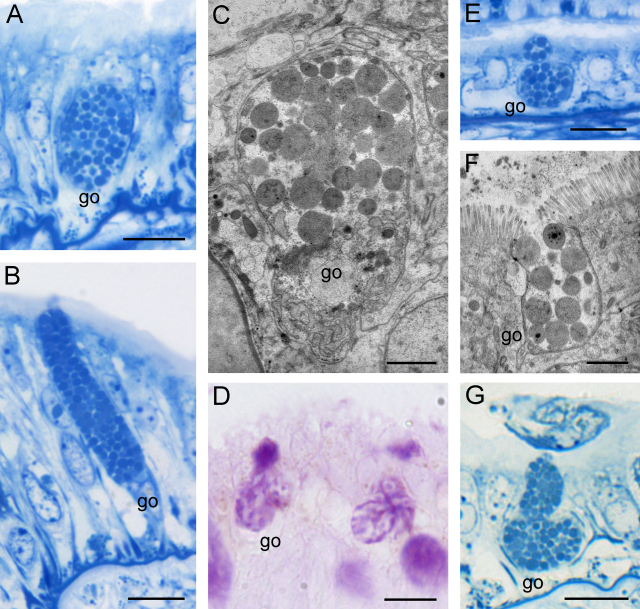
Fig. 9.
Epithelium of the aboral side, light microscopy images. (A) In contrast to the oral ridges, the aboral ridges are flat and hump-shaped. Scale bar = 50 μm. (B and C) The epithelium consists of two cell types: (B) cell type 2 (ct2), which contains large polygonal granules of different density, and (C) cell type 3 (ct3) filled with small, round to polygonal granules. Scale bars = 10 μm. (D and E) Secretion processes of cell type 2 (D) and cell type 3 (E). Scale bars = 10 μm. (F) Both cell types react strongly to PAS (neutral sugars). Scale bar = 20 μm. (G) Cell type 2 also shows a strong affinity to alcian blue at pH 2.5 (acid proteins). Scale bar = 20 μm. (H) Combined staining of cell type 2 with PAS and alcian blue (pH 2.5). Scale bar = 10 μm.
Cell type 2 is pear-shaped and tightly packed with large polygonal granules (Ø 2 μm) of different density (Fig. 9B and D). The nucleus (globular to oval, Ø 5–8 μm) is located basally and surrounded by several layers of rER. When tightly packed with secretory material, the nucleus becomes compressed and has a sickle-shaped form.
The secretory content of this cell type consists of acidic mucosubstances (pH 1.0; 2.5) detected by alcian blue and PAS staining (Fig. 9F–H). Like the columnar cells and cell type 1, this type also showed a strong reactivity with UEA, indicating the presence of fucose, whereas no reaction was detected by any other lectin. Sole staining with alcian blue (Fig. 9G and H) showed that not all of the secretory material is reactive to this staining; some areas clearly lacked acid proteins and remained unstained. We were unable to correlate the different granular density with the histochemical staining results. A more precise chemical differentiation at the biochemical level is necessary in order to explain these observations.
Cell type 3 corresponds in shape to cell type 1 from the thick epithelium of the oral tentacle side. However, its granules are slightly smaller (Ø 1.3–1.5 μm) (Fig. 9C and E) and more loosely packed. These granules also lack the prominent electron-dense core visible in cell type 1. The nucleus of cell type 3 (globular to oval, Ø 5–8 μm) is also located basally and surrounded by Golgi bodies and rER.
As in cell type 1, the secretory product of cell type 3 is strongly periodate-reactive (Fig. 9F) and moderately reactive for N-acetyl-d-glucosamine as shown by wheat germ lectin. Staining without periodate oxidation or conversion of the hydroxyl groups by acetylation produced no reactions. The secretory content did not react to staining for proteins (alcian blue and Biebrich scarlet staining) or lipids (Sudan black B staining).
4. Discussion
Although numerous studies are available on the tentacular system in Nautilus spp., its bonding mechanism remains unknown and speculative. Early observations postulated an exclusively mechanical mechanism similar to the laminated sucker of the fish Remora (Owen, 1843). Willey (1902) described the adhesive power in Nautilus as a “powerful grip” – so strong that the tentacles still remain fixed to the object even when they are torn off the animal. Many subsequent morphological studies favored the hypothesis of a chemical bonding effect, induced either by the columnar cells alone (Fukuda, 1988) or by a combination of the columnar and goblet cells (Muntz and Wentworth, 1995).
4.1. Chemical attachment
The present results support the hypothesis of Fukuda (1988) and Muntz and Wentworth (1995) that attachment in Nautilus is chemical. Adhesion generated by reduced pressure as in Octopus or Remora can definitely be excluded for Nautilus. Its ridges differ clearly from the laminated plates in Remora by their close arrangement, flexibility and lack of embedded spinules (Fulcher and Motta, 2006). Moreover, the ridges in Nautilus cannot form a sub-ambient pressure chamber for suction as indicated for the suckers of Octopus and the bonding behavior of Sepia tuberculata (von Byern et al., 2010b).
Instead, the adhesive system in Nautilus exhibits different epithelial layers and gland cells in the oral ridges, which produce an adhesive substance. Whether the glue is produced by the columnar cells only, by two cell types, or even by all three types of glandular cells (columnar cells, cell type 1, goblet cells) in the oral ridges remains to be determined.
The large amounts secreted in goblet cells and cell type 1 would indicate these cell types as major contributors; on the other hand, these cells are basally positioned and/or release their secretory content in a direction opposite to the attachment plane. Moreover, ridge deflection during attachment would close the gap between the thick and thin epithelium and thereby prevent a mixing of their secretions with those of the columnar cells. Therefore, participation of these two glandular cell types in the adhesion process seems improbable.
In our opinion, several attributes (location within the oral ridge, direct contact with the bonding surface, numerous cells and abundant secretion) indicate the columnar cells as being the solitary player in the attachment process – although the secretory amount each cell synthesizes appears to be small.
Based on our ultrastructural and histochemical data, we assume that the goblet cells of the thin epithelium and cell type 3 within the epithelium of the aboral ridges represent the same cell type. Variations in cell shape and granule size could be structural adaptations to the different thicknesses of the epithelia and cellular densities. The histochemical data show that both cell types contain neutral sugar secretions. Cell type 1 also corresponds structurally and chemically to these two cell types, except that its granules possess electron-dense inclusions.
The remaining cell types (columnar cells in the oral epithelium and cell type 2 of the aboral epithelium) strongly differ from these common cell types (goblet cells and cell type 3) by their form (columnar cells) or secretory composition (cell type 2).
Since the other glue-producing cephalopods, the coleoids Idiosepius, Euprymna, and S. tuberculata, also possess a gland cell type which occurs in the adhesive as well as the normal epithelium (Cyran and von Byern, 2010; Klinger et al., 2010; von Byern et al., 2010b), such a condition is also conceivable for this common cell type in the digital tentacles of Nautilus. In the three coleoids, this cell type is supposed to produce normal mucus (Cyran et al., 2010). Based on our hypothesis of a common cell type in Nautilus, such a function is also conceivable for the goblet cells and cell type 1. Whether cell type 1 is really identical with this common cell type, however, still needs to be clarified.
4.2. Muscular release
Nautilus is a bottom-dwelling scavenger and opportunistic predator inhabiting fore-reef slopes in tropical waters (Saunders and Landman, 1987; Ward, 1988). The animals are slow swimmers and avoid fast movements or strong water currents (Arnold and Carlson, 1986; Hayasaka et al., 1995). Although the tentacles exert a strong bonding force, the attachment and release mechanisms are no doubt slow processes, as in the other glue-producing cephalopods (von Byern and Klepal, 2006) or gastropods (Smith et al., 1993; Smith, 2002).
Based on the histochemical findings, chemical attachment and release by a so-called duo-gland system, which comprises two types of secretory cells, i.e., cells responsible for adhesion and cells releasing solvents (e.g., acidic or basic proteins) (Hermans, 1983), seems improbable for Nautilus. All cell types in the oral ridges contain mainly neutral sugar units and lack proteins as found in cell type 2.
The pronounced musculature within the oral and aboral ridges as well as the main tentacle musculature rather suggests a muscular-triggered release. This would represent a faster and more coordinated mechanism, in which each ridge is controlled or moved separately and able to increase or reduce the adhesion force. This form of release mechanism is not restricted to Nautilus but is also proposed for the glue-producing cephalopods Euprymna and S. tuberculata (Micossi et al., 2008).
4.3. Related adhesive systems in cephalopods
At least four cephalopod taxa (Idiosepius spp., Euprymna scolopes, S. tuberculata, Nautilus spp.) are known to adhere by means of chemical substances (Cyran and von Byern, 2010; Klinger et al., 2010; von Byern et al., 2010b). Although their systems vary with regard to the gland location (ventral mantle side in S. tuberculata, dorsal mantle side in Idiosepius and Euprymna, digital tentacle in Nautilus), the glands show some similarities.
Generally, two specific glandular cells are exclusively present in the adhesive epithelia, which are mainly distinguishable by the size of their granula. One long slender cell type always contains small granules (0.05–1 μm) of neutral sugars (not verified for Nautilus and Sepia). The second cell type synthesizes large, membrane-bound secretory granules (0.5–2 μm in diameter) which likewise consist of neutral sugars (Cyran and von Byern, 2010; Klinger et al., 2010; von Byern et al., 2010b). In two cases (Idiosepius and Sepia), both cell types are associated with aggregations of different cell numbers (1:8 in Idiosepius, 1:2 in S. tuberculata).
Histochemical data indicate that the glue in Idiosepius, Euprymna, and S. tuberculata (Cyran and von Byern, 2010; Klinger et al., 2010; von Byern et al., 2010b) consists of a viscous carbohydrate-based gel as known in gastropods (Smith, 2002, 2006). Regardless of whether the glue in Nautilus is derived from the columnar cells alone or from all three cell types of the oral ridges, the histochemical results show that all cell types contain mainly neutral sugars and therefore the animals probably adhere via such a carbohydrate-based gel.
Nevertheless, the adhesives of the other glue-producing cephalopods also contain a small proportion of protein components (Singley, 1982; von Byern and Grunwald, 2008; von Byern et al., 2008) which we have not yet detected in the columnar cells of Nautilus. One reason for this may be the small size of the cells and granules that makes a positive identification difficult.
For S. tuberculata (von Byern et al., 2010b) as well as Nautilus, muscular de-adhesion seems to be the primary mechanism, whereas in Idiosepius the absence of an explicit mantle musculature beneath the adhesive area argues against such a release mechanism (von Byern et al., 2008; Cyran et al., 2011).
From another perspective, it remains questionable whether the kind of glue found in the living fossil Nautilus is comparable to that of the recent coleoids Idiosepius, Euprymna and Sepia. Both the phylogenetic relationship (Nautilus versus the coleoids) as well as the location of the adhesive system (tentacle epithelium in Nautilus, mantle epithelium in Idiosepius, Euprymna, and Sepia) indicate strong differences between the different systems. Moreover, in the coleoids two gland cell types participate in glue formation, whereas in Nautilus only the columnar cells seem to be responsible for bonding. From an evolutionary standpoint, this raises the question as to whether the adhesives of the recent cephalopod species are derived from the Nautilus glue and became modified later, or whether the different bonding systems evolved independently. Our morphological results and the chemical glue data reported here provide no indication of a potential relationship between the gland systems in glue-producing cephalopods. Molecular biological and biochemical investigations are necessary in order to characterize the different systems in more detail and to define the “original” glue of this mollusk group.
Acknowledgments
This work was funded by the Austrian Science Fund (FWF, Project No. P 211 35 – B 17). The authors would like to thank Sylvia Nürnberger (Dept. of Traumatology, Medical University of Vienna, Austria) and Dr. Michael Stachowitsch (Dept. of Marine Biology, University of Vienna, Austria) for their editorial assistance and Dr. Tomoki Kase (National Museum of Nature and Science, Tokyo, Japan) for his cooperation with the fieldwork in the Philippines. In particular, we would like to thank the two anonymous reviewers for their critical but useful comments, proofreading of the manuscript and their support for publishing this study in Zoology.
Footnotes
As pointed out by von Byern and Klepal (2006), von Byern et al. (2008), and Cyran et al. (2011), there is some confusion regarding the terminology for glandular adhesive cell types in cephalopods. For this reason we avoided naming the characterized granular cells, but rather numbered them consecutively as has also been done in gastropods (Grenon and Walker, 1978). While this makes reading the text more difficult, it does avoid misleading name designations.
References
- Arnold J.M., Carlson B.A. Living Nautilus embryos: preliminary observations. Science. 1986;232:73–76. doi: 10.1126/science.232.4746.73. [DOI] [PubMed] [Google Scholar]
- Bidder A.M. Use of the tentacles, swimming and buoyancy control in the pearly Nautilus. Nature. 1962;196:451–454. [Google Scholar]
- Böck P. Urban und Schwarzenberg; München: 1989. Romeis Mikroskopische Technik. [Google Scholar]
- Bulmer D. Dimedone as an aldehyde blocking reagent to facilitate the histochemical demonstration of glycogen. Stain Technol. 1959;34:95–98. doi: 10.3109/10520295909114656. [DOI] [PubMed] [Google Scholar]
- Cyran N., von Byern J. Idiosepius. In: von Byern J., Grunwald I., editors. Biological Adhesive Systems: From Nature to Technical and Medical Application. Springer Verlag; Wien: 2010. pp. 61–66. [Google Scholar]
- Cyran N., Klinger L., Scott R., Griffiths C., Schwaha T., Zheden V., Ploszczanski L., von Byern J. Characterization of the adhesive systems in cephalopods. In: von Byern J., Grunwald I., editors. Biological Adhesive Systems: From Nature to Technical and Medical Application. Springer Verlag; Wien: 2010. pp. 53–86. [Google Scholar]
- Cyran N., Klepal W., von Byern J. Ultrastructural characterization of the adhesive organ of Idiosepius biserialis (Voss, 1962) and Idiosepius pygmaeus (Steenstrup, 1881) (Mollusca, Cephalopoda) J. Mar. Biol. Assoc. U. K. 2011 [Google Scholar]
- Fukuda Y. Histology of the long digital tentacles. In: Saunders W.B., Landman N.H., editors. Nautilus. The Biology and Paleobiology of a Living Fossil. Plenum Press; New York: 1988. pp. 249–256. [Google Scholar]
- Fulcher B.A., Motta P.J. Suction disk performance of echeneid fishes. Can. J. Zool. 2006;84:42–50. [Google Scholar]
- Grenon J.F., Walker G. The histology and histochemistry of the pedal glandular system of two limpets. Patella vulgata and Acmaea tessulata (Gastropoda: Prosobranchia) J. Mar. Biol. Assoc. U. K. 1978;58:803–816. [Google Scholar]
- Hayasaka S., Oki K., Suzuki H., Shinomiya A. Environmental background of the habitat of Nautilus belauensis off the Southeast coast of the Malakal Island, Palau. In: Kakinuma Y., editor. Studies of Nautilus belauensis in Palau. Kagoshima University; Kagoshima: 1995. pp. 5–10. [Google Scholar]
- Heidenhain M. Über die Anwendung des Azokarmins und der Chromotrope. Z. wiss. Mikrosk. u. mikroskop. Technik. 1905;XXII:337–343. [Google Scholar]
- Hermans C.O. The duo-gland adhesive system. Oceanogr. Mar. Biol. Annu. Rev. 1983;21:283–339. [Google Scholar]
- House M.R. Major features of cephalopod evolution. In: Wiedmann J., Kullmann J., editors. Cephalopods Present and Past. O. H. Schindewolf Symposium; Tübingen. E. Schweizerbart'sche Verlagsbuchhandlung, Stuttgart; 1988. pp. 1–16. [Google Scholar]
- Kakinuma K., Hisanaga K., Tsukahara J., Tabata M. The predatory activity of captured Nautilus belauensis. In: Kakinuma Y., editor. Studies of Nautilus belauensis in Palau. Kagoshima University; Kagoshima: 1995. pp. 83–90. [Google Scholar]
- Kier W.M. The functional morphology of the tentacle musculature of Nautilus pompilius. In: Saunders W.B., Landman N.H., editors. Nautilus. The Biology and Paleobiology of a Living Fossil. Plenum Press; New York: 1987. pp. 257–269. [DOI] [PubMed] [Google Scholar]
- Kiernan J.A. Butterworth Heinemann; Oxford: 1999. Histological and Histochemical Methods: Theory and Practice. [Google Scholar]
- Klinger L., von Byern J., Cyran N. Euprymna. In: von Byern J., Grunwald I., editors. Biological Adhesive Systems: From Nature to Technical and Medical Application. Springer Verlag; Wien: 2010. pp. 54–61. [Google Scholar]
- McManus J.F.A., Mowry R.W. Paul Hoeber Inc.; New York: 1960. Staining Methods: Histological and Histochemical. [Google Scholar]
- Micossi A., Klepal W., von Byern J. Ultrastructural characterization of the adhesive area of Sepia tuberculata (Lamarck, 1798). In: Faber A., Weiss R., Fuchs D., editors. Proceedings of the 3rd International Symposium Coleoid Cephalopods Through Time; Musée National d’Histoire Naturelle Luxembourg, Luxembourg; 2008. pp. 33–35. [Google Scholar]
- Muntz W.R.A., Wentworth S.L. Structure of the adhesive surface of the digital tentacles of Nautilus pompilius. J. Mar. Biol. Assoc. U. K. 1995;75:747–750. [Google Scholar]
- Owen R. On the structure and homology of the cephalic tentacles in the pearly Nautilus. Ann. Nat. Hist. 1843;XII:305–311. [Google Scholar]
- Reynolds E.S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J. Cell Biol. 1963;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruth P., Schmidtberg H., Westermann B., Schipp R. The sensory epithelium of the tentacles and the rhinophore of Nautilus pompilius L. (Cephalopoda, Nautiloidea) J. Morphol. 2002;251:239–255. doi: 10.1002/jmor.1086. [DOI] [PubMed] [Google Scholar]
- Ruyter J.H.C. Eine einfache Methode für das Aufkleben von Zelloidin-Paraffinschnitten. Z. wissenschaftl. Mikrosk. u. mikroskop. Technik. 1931;48:226–227. [Google Scholar]
- Saunders W.B., Landman N.H. Plenum Press; New York: 1987. Nautilus. The Biology and Paleobiology of a Living Fossil. [Google Scholar]
- Singley C.T. Histochemistry and fine structure of the ectodermal epithelium of the sepiolid squid Euprymna scolopes. Malacologia. 1982;23:177–192. [Google Scholar]
- Smith A.M. The structure and function of adhesive gels from invertebrates. Integr. Comp. Biol. 2002;42:1164–1171. doi: 10.1093/icb/42.6.1164. [DOI] [PubMed] [Google Scholar]
- Smith A.M. The biochemistry and mechanics of gastropod adhesive gels. In: Smith A.M., Callow J.A., editors. Biological Adhesives. Springer Verlag; Heidelberg: 2006. pp. 167–182. [Google Scholar]
- Smith A.M., Kier W.M., Johnsen S. The effect of depth on the attachment force of limpets. Biol. Bull. 1993;184:338–341. doi: 10.2307/1542452. [DOI] [PubMed] [Google Scholar]
- Spicer S.S., Lillie R.D. Histochemical identification of basic proteins with Biebrich Scarlet at alkaline pH. Stain Technol. 1961;6:365–370. [Google Scholar]
- Stenzel H.B. Living Nautilus. In: Moore R.C., editor. Treatise on Invertebrate Paleontology. Geological Society of America and University of Kansas Press; Lawrence, KA: 1964. pp. 59–93. [Google Scholar]
- Teichert C., Matsumoto T. The ancestry of the genus Nautilus. In: Saunders W.B., Landman N.H., editors. Nautilus. The Biology and Paleobiology of a Living Fossil. Plenum Press; New York: 1987. pp. 25–32. [Google Scholar]
- Thompson D.W. Historia Animalium. In: Ross D., editor. The Works of Aristotle. Clarendon Press; Oxford: 1910. pp. 486a–633a. [Google Scholar]
- von Byern J., Grunwald I. Glue components in Idiosepius (Mollusca, Cephalopoda) In: Gelpey J.C., Hamers R.J., Muralt P., Orme C.A., editors. From Biological Materials to Biomimetic Material Synthesis. Material Research Society, Spring Meeting 2008; San Francisco, USA: 2008. p. 645. [Google Scholar]
- von Byern J., Klepal W. Adhesive mechanisms in cephalopods: a review. Biofouling. 2006;22:329–338. doi: 10.1080/08927010600967840. [DOI] [PubMed] [Google Scholar]
- von Byern J., Rudoll L., Cyran N., Klepal W. Histochemical characterization of the adhesive organ of three Idiosepius spp. species. Biotech. Histochem. 2008;83:29–46. doi: 10.1080/10520290801999316. [DOI] [PubMed] [Google Scholar]
- von Byern J., Schwaha T., Ploszczanski L., Cyran N. Nautilus. In: von Byern J., Grunwald I., editors. Biological Adhesive Systems: From Nature to Technical and Medical Application. Springer Verlag; Wien: 2010. pp. 66–73. [Google Scholar]
- von Byern J., Scott R., Griffiths C., Zheden V., Cyran N. Sepia. In: von Byern J., Grunwald I., editors. Biological Adhesive Systems: From Nature to Technical and Medical Application. Springer Verlag; Wien: 2010. pp. 73–86. [Google Scholar]
- Wani R. Experimental fragmentation patterns of modern Nautilus shells and the implications for fossil cephalopod taphonomy. Lethaia. 2004;37:113–123. [Google Scholar]
- Ward P.D. Simon & Schuster; New York: 1988. In Search of Nautilus. [Google Scholar]
- Willey A. The adhesive tentacles of Nautilus, with some notes on its pericardium and spermatophores. Q. J. Microsc. Sci. 1898;40:207–209. [Google Scholar]
- Willey A. Contribution to the natural history of the pearly Nautilus. In: Willey A., editor. part VI. University Press; Cambridge: 1902. pp. 691–830. (Zoological Results Based on Material from New Britain, New Guinea, Loyalty Islands and Elsewhere Collected During the Years 1895, 1896 and 1897). [Google Scholar]
- Wollesen T., Loesel R., Wanninger A. Pygmy squid and giant brains: mapping the complex cephalopod CNS by phalloidin staining of vibratome sections and whole-mount preparations. J. Neurosci. Methods. 2009;179:63–67. doi: 10.1016/j.jneumeth.2009.01.021. [DOI] [PubMed] [Google Scholar]
- Wollesen T., Cummins S.F., Degnan B.M., Wanninger A. FMRFamide gene and peptide expression during central nervous system development of the cephalopod mollusk, Idiosepius notoides. Evol. Dev. 2010;12:113–130. doi: 10.1111/j.1525-142X.2010.00398.x. [DOI] [PubMed] [Google Scholar]