Abstract
Despite continuous progress toward tissue engineering of functional articular cartilage, significant challenges still remain. Advances in morphogens, stem cells, and scaffolds have resulted in enhancement of the bulk mechanical properties of engineered constructs, but little attention has been paid to the surface mechanical properties. In the near future, engineered tissues will be able to withstand and support the physiological compressive and tensile forces in weight-bearing synovial joints such as the knee. However, there is an increasing realization that these tissue-engineered cartilage constructs will fail without the optimal frictional and wear properties present in native articular cartilage. These characteristics are critical to smooth, pain-free joint articulation and a long-lasting, durable cartilage surface. To achieve optimal tribological properties, engineered cartilage therapies will need to incorporate approaches and methods for functional lubrication. Steady progress in cartilage lubrication in native tissues has pushed the pendulum and warranted a shift in the articular cartilage tissue-engineering paradigm. Engineered tissues should be designed and developed to possess both tribological and mechanical properties mirroring natural cartilage. In this article, an overview of the biology and engineering of articular cartilage structure and cartilage lubrication will be presented. Salient progress in lubrication treatments such as tribosupplementation, pharmacological, and cell-based therapies will be covered. Finally, frictional assays such as the pin-on-disk tribometer will be addressed. Knowledge related to the elements of cartilage lubrication has progressed and, thus, an opportune moment is provided to leverage these advances at a critical step in the development of mechanically and tribologically robust, biomimetic tissue-engineered cartilage. This article is intended to serve as the first stepping stone toward future studies in functional tissue engineering of articular cartilage that begins to explore and incorporate methods of lubrication.
Introduction
Pain-free ambulation and joint movement are important quality-of-life issues for all healthy adults. However, for more than 26 million adults in the United States alone,1 degenerative joint disease or osteoarthritis (OA) impairs these daily activities and reduces their quality of life.2 Regenerative medicine, through tissue engineering, aims at producing functional, engineered synovial tissues to replace and restore these damaged joints. Despite its seemingly simple structure, articular cartilage regeneration has been elusive and presents an important clinical challenge. As the connective tissue located on the ends of long bones, articular cartilage is able to support and distribute large mechanical loads while providing a nearly frictionless surface for joint movement. Recently, exciting progress has been made in engineering tissues with mechanical properties, such as compressive and tensile strength, approaching native tissue levels.3–5 The next grand challenge in cartilage engineering is to address perhaps the most important functional attribute of cartilage: lubrication.
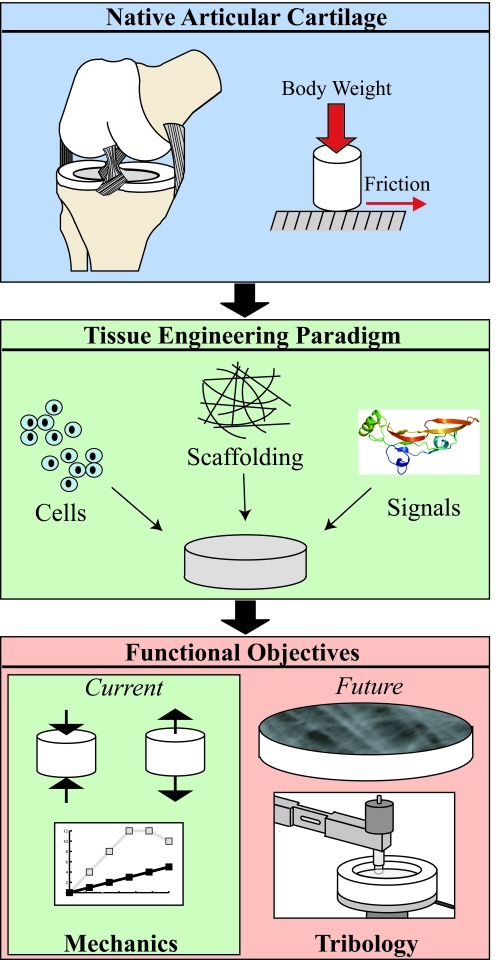
The lubrication mechanisms of articular cartilage impart the tissue with its low friction properties. Maintenance of these tribological properties is crucial to reducing wear and ensuring healthy and functional cartilage for the lifetime of the individual. For example, ineffective joint lubrication has been demonstrated to play an important role in the development of precocious joint degeneration and OA.6,7 Therefore, engineered cartilage should possess both mechanical and frictional properties to function and endure in vivo. Historically, the tribological aspects of cartilage tissue engineering have been a secondary concern compared with achieving the necessary mechanical properties. This approach was a practical prioritization, as the bulk of the engineered tissue needed to withstand large mechanical loads before surface characteristics became a concern. However, with this goal closer to being realized, the state of tissue engineering has advanced sufficiently to make a case for a shift in this paradigm. In this age of mechanically functional cartilage, tribological characterization and development need to become a key component of any articular cartilage tissue-engineering strategy (Fig. 1). The aims of this article are to provide a synopsis of the biology and engineering of lubrication in articular cartilage, and present approaches to lubrication engineering. In addition to tissue-engineering-based approaches, other methods of cartilage lubrication augmentation such as tribosupplementation and pharmacological therapies will also be discussed. In presenting this information, we wish to demonstrate that the foundations have been laid for expansion into this exciting frontier of engineering cartilage lubrication. The studies described in this article should serve as a vital stepping stone in directing tissue engineers of articular cartilage along the path toward mechanically and tribologically robust, engineered cartilage.
FIG. 1.
The tissue engineering of articular cartilage traditionally follows a paradigm of utilizing primary chondrocytes or stem cells, growth factors and morphogens, and scaffolding material to develop constructs that aim to replace natural cartilage. Current functional approaches rely on experimental methods driven by biomechanical properties. Advancement toward artificial, biomimetic cartilage will require future developments in the tribological properties of engineered cartilage.
Structure-function relationships in articular cartilage
Articular cartilage distributes forces and lubricates the contact surfaces between articulating long bones, as in the knee.8,9 Cartilage is an avascular and aneural tissue composed of water (60%–85%), rich in extracellular matrix (ECM), and a relatively small volume of cells, the chondrocytes.9,10 The major ECM constituents consist of type II collagen and the proteoglycan aggrecan.11 Other ECM components include cartilage oligomeric protein (COMP), cartilage link protein, hyaluronan, biglycan, and decorin. The maintenance and homeostasis of the ECM is regulated by chondrocytes by enzymatic degradation and secretion of matrix. Cells comprise only 2%–10% of cartilage by volume, and this cell density decreases with age.12,13 These cells possess low proliferative and metabolic abilities.13 Nutrient and oxygen transport is mediated by diffusion. Transport within the tissue is augmented by convection driven by cyclic compression.9 Inherent deficiencies in cellularization and vascularization prevent articular cartilage in adults from engaging in any substantial self-repair.
Articular cartilage is an anisotropic and heterogeneous tissue at multiple levels. The ECM structure, chondrocyte phenotype, and cell shape varies between each zone.14,15 Macroscopically, cartilage is divided into three zones: superficial, middle, and deep.8 The surface layer, or superficial zone, possesses type II collagen fibrils aligned parallel with the articular surface.16 Collagen fibrils are, thus, oriented in the superficial zone to resist shearing forces at the articular surface generated by joint articulation. Cell density is the greatest in the superficial zone,12 where chondrocytes assume a flattened shape.17 The central 40% to 60% of cartilage that comprises the middle zone contains randomly aligned type II collagen fibrils and rounded cells. The remaining 30% makes up the deep zone, which is mineralized in the depths of this zone. The so called “tide mark” is the interface between the unmineralized and mineralized ECM. In the deep zone of articular cartilage, collagen fibrils are aligned perpendicularly to the articular surface. Chondrocytes are aligned similarly in columns of ellipsoidal cells. Proteoglycan content increases from the superficial zone to the deep zone, whereas hydration decreases through each zone.17
Since cartilage is mechanically loaded, the forces are supported by a combination of ECM and water. Water is retained in the ECM by aggrecan, a post-translationally modified proteoglycan with negatively charged glycosylaminoglycans (GAG), such as chondroitin sulfate and keratan sulfate, which attract cations into the ECM. The resulting osmotic pressures hydrate articular cartilage and provide resistance to compression. This support is complemented by type II collagen fibrils, which provide the tensile strength.18 Stress relaxation in human cartilage due to fluid exudation and ECM interactions occurs over 10,800 s.19 Articular cartilage is a mechanically robust material, as it must endure localized loading of nearly 18 MPa in the hip.20 Over two decades ago, Mow et al.10,21 developed a biphasic theory to mathematically characterize the material properties of this complex, viscoelastic tissue. Using this biphasic model, the aggregate compressive modulus of cartilage has been found to vary between 0.53 and 1.82 MPa. Tensile modulus measurements have varied between 1 and 20 MPa, depending on the cartilage zone measured.13
Articular Cartilage Lubrication
The functional lifetime of articular cartilage is dependent on minimizing friction and wear. Friction (F) is the opposing force generated from the relative movement of two contacting surfaces. The magnitude of friction is linearly proportional to the applied normal load (W) by the coefficient of friction (μ), and is the product of multiplying the two variables: F=μ·W.22 Hyaline cartilage possesses the lowest measured friction coefficient of any material, ranging from 0.005 to 0.02.10 Wear occurs when asperities (microscopic surface roughness) from opposing surfaces come into contact and deform, resulting in removal of material.23 Frictional forces can be moderated by fluid films or lubricants that separate the interfacing materials, preventing solid–solid contact and reducing material deformation. Lubrication of articular surfaces can be divided into two categories: fluid film and boundary lubrication.
Fluid film-lubricated interfaces are separated by a viscous fluid with a thickness greater than the surface roughness to prevent contact.24 The fluid forces that support the applied loads from the interacting surfaces are generated through hydrostatic and hydrodynamic mechanisms. Under hydrostatic lubrication, the opposing surfaces are separated by an externally pressurized fluid film.24,25 For hydrodynamic lubrication, fluid film forces are generated by the entraining or sliding speed of the articulating surfaces. The thickness of a hydrodynamic film is a function of multiple factors such as fluid viscosity, geometry and roughness of the articulating surfaces, applied normal load, and sliding speed.23 In the elastohydrodynamic lubrication regime, the fluid film thickness approaches the order of asperity heights.26 The applied loads and fluid pressures result in elastic deformation of the articulating surfaces.10,24
Under conditions not conducive for fluid film lubrication such as low sliding speeds, high loads, and low fluid viscosity, articulating surfaces are separated by a molecular film or boundary lubrication.10,27,28 Asperities are separated by this one- to two-molecule thick layer of boundary lubricant.29 According to one model, surfaces are protected by a sacrificial layer of strongly adsorbed boundary lubricant that is sheared away during sliding.30 Boundary lubricants should, therefore, be quickly and continuously replenished to prevent wear from the next round of solid–solid contact.24 During the walking cycle, the articulating surfaces of the knee bear a wide variety of contact stresses and sliding speeds. For example, the sliding speed becomes zero during each directional change in the leg swing.31 Thus, biological joints operate under a mixed lubrication regime where articulating surfaces are subjected to both fluid film and boundary lubrication.10,24,27,28,31 Within mixed lubrication, weeping and boosted lubrication may occur. Weeping lubrication is generated by the release of interstitial fluid from compressed cartilage.32,33 Stresses transmitted through asperity-asperity contacts compress the cartilage matrix, pressurizing the ECM and inducing interstitial fluid exudation. Boosted lubrication occurs under joint loading conditions that force fluid back into the ECM, effectively increasing (or “boosting”) the concentration of lubricant confined at the articular surface.34 For detailed information on lubrication mechanisms and wear of articular joints, the reader is directed to a review on biotribology by Neu et al.24
Boundary Mode Lubricants
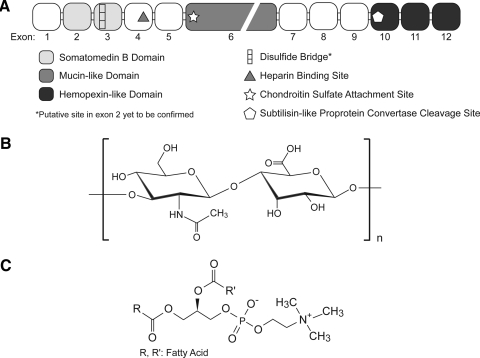
Fluid film lubrication relies on fluid forces and rheological phenomena generated from joint motion. However, after finding the lubricity of synovial fluid was not entirely dependent on viscosity, investigators began searching for the lubricating component of synovial fluid. This research led to the important discoveries on boundary lubrication, which is unique in that it relies on molecular interactions and biochemical properties to provide the last layer of defense in preventing solid–solid contact and degradation of the articular surface.28 Researchers have focused on three molecules as putative boundary lubricants: hyaluronic acid (HA), surface active phospholipids (SAPLs), and superficial zone protein (SZP)/lubricin/PRG4 (Fig. 2). There is a debate in the literature regarding the importance and relevance of these putative lubricants and boundary mode lubrication in general.24,35–37 Despite these disagreements, compelling results from many meticulous studies merit the consideration of these physiologically important biomolecules.
FIG. 2.
The molecular structures of three putative boundary lubricants, (A) superficial zone protein (SZP), (B) hyaluronic acid, and (C) surface active phospholipids (e.g., phosphatidylcholine) are represented. SZP is a 345 kDa proteoglycan and a product of the gene prg4. Hyaluronic acid is a disaccharide polymer consisting of repeating units of glucuronic acid and N-acetylglucosamine. Phosphatidylcholine can be composed of a variety of fatty acid structures.
Hyaluronic acid
HA, or hyaluronan (Fig. 2B), is a nonsulfated GAG composed of the repeating sugars glucuronic acid and N-acetylglucosamine.38 Lacking a protein core, HA is synthesized at the plasma membrane into the extracellular space. HA is a major constituent of the ECM and is also involved with cell signaling by binding with the cell surface receptor CD44.38 The compressive and viscoelastic properties of cartilage tissue are due in part to the aggregating complexes resulting from HA association with aggrecan and link protein.38 HA plays a major role in fluid film lubrication by providing viscosity to synovial fluid through its high molecular weight (0.5–3.8×106 Da) and concentration (0.1–5 mg/mL).10,39 However, the role of HA in boundary lubrication is under debate.
Radin et al.40,41 found that digestion or separation of HA from bovine synovial fluid reduced the viscosity, but did not affect the boundary lubrication of the treated fluid. These results have been confirmed most recently by friction measurements of hyaluronidase-treated cartilage with an atomic force microscope.28 In addition, HA has not been found to bind or adsorb to the cartilage surface, a requirement for boundary lubrication.42,43 However, in contrast, HA decreased friction in a cartilage–cartilage interface. In combination with PRG4, the reduction was additive.44 Experiments using the surface force apparatus suggest that HA serves a chondroprotective role by preventing wear of the articular surface, rather than reducing the coefficient of friction.45,46 The utility of HA varies by the frictional test system employed. Additional work is needed to pinpoint the exact role of HA in the boundary lubrication regime.
Surface active phospholipids
Phospholipids such as phosphatidylcholine (Fig. 2C), phosphatidylethanolamine, and sphingomyelin have been identified as constituents of synovial fluid and bound to the articular surface.47,48 Inspired by their role in reducing surface tension (surface active or surfactant) in the lungs, SAPLs have been proposed as a boundary lubricant for articular cartilage.47 A strongly adsorbed layer of SAPLs could provide hydrophobicity to the articular surface and shield asperities from solid–solid contact. The literature contains conflicting reports on the boundary lubricity of SAPLs in synovial joints. Enzymatic digestion of SAPLs with phospholipase was shown to eliminate the lubricating ability of synovial fluid.49 However, these results were disputed in a later report, suggesting phospholipase may contain traces of protease activity due to trypsin.35 Phospholipase supplemented with trypsin inhibitors was found to have a minimal effect on the lubricity of synovial fluid. Other studies that examined the effects of SAPL degradation on the cartilage surface found no effect on the frictional coefficient.28 While lacking universal agreement,37 the current consensus is that SAPLs do not contribute to articular cartilage boundary lubrication.44
Lubricin/SZP/PRG4
A hyaluronate-free protein fraction of bovine synovial fluid was purified by Radin et al. in 1970.40 This protein possessed similar boundary lubricating properties to that of whole synovial fluid. The name lubricin was christened after its purification a decade later.50 Lubricin (227 kDa) is one of the several proteins encoded by the gene prg4. Other homologous proteins include SZP, PRG4, megakaryocyte-stimulating factor precursor, and hemangiopoietin.51–54 Differences between these homologous proteins appear to result from post-translational O-linked glycosylation.53 Lubricin is typically expressed by synovial fibroblasts, whereas SZP is produced by articular chondrocytes in the superficial zone. Much work has been performed in characterizing the biochemical properties, and biological and mechanical mechanisms of these lubricating proteins. SZP is a heavily glycosylated protein with an apparent molecular weight of 345 kDa (Fig. 2A). Middle- and deep-zone chondrocytes express little to no SZP.55,56 SZP has also been found to be expressed in the infrapatellar fat pad, meniscus, tendon, and ligaments.57–60 It is hypothesized that SZP forms a nanofilm that reduces and smoothens asperities on articular cartilage, reducing “stick-slip motion” and subsequent friction.7,61
SZP: Critical Boundary Lubricant
There is a general consensus in the cartilage lubrication field that SZP is a critical lubricant for articular cartilage. This hypothesis is based on an abundant number of studies involving a human genetic disorder,6,62 rodent gene knockout models,63,64 animal models of arthritis,65–68 and functional tribological assays.61,69 In addition to serving as a boundary lubricant, SZP has been found to prevent hyper-proliferation of synovial cells and fouling of the articular surface.64 These functions are particularly important. Patients with camptodactyly-arthropathy-coxa vara-pericarditis syndrome (CACP), a genetic autosomal recessive disease, endure excessive synovial growth, fouling of the articular surface, and precocious joint failure.6,64,70 A study by Sah and coworkers71 also suggested that SZP expression inhibits integration of apposed, articular cartilage surfaces, and, by extension, cartilage repair.
SZP expression is modulated by growth factors, morphogens, and other cytokines. Transforming growth factor-β (TGF-β) and bone morphogenetic protein (BMP) upregulate SZP production in both superficial zone chondrocytes and synoviocytes.72–74 These effects are additive in both populations.72 Upregulation of SZP production in articular chondrocytes by insulin-like growth factor-1, a stimulator of aggrecan expression, appears to be context dependent.74 Interleukin-1 (IL-1) and tumor necrosis factor-α (TNF-α), catabolic cytokines, decrease SZP expression in synoviocytes and articular chondrocytes.73–76 This modulation of SZP also mirrors tribological effects observed in cartilage. TGF-β1 treatment increases SZP deposition and decreases the coefficient of friction in bovine cartilage explants.69 Although IL-1 also decreases the coefficient of friction by increasing articular surface roughness, in the long term, this damage accelerates wearing of the articular surface.
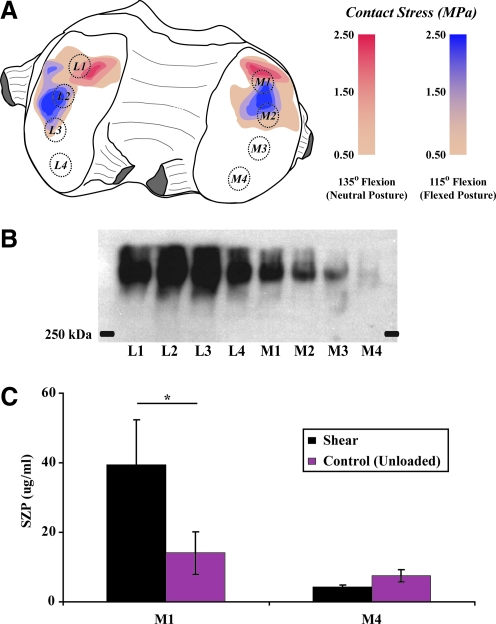
SZP is heterogeneously distributed across the articular surface.61,77 The topographical variation can be partially attributed to in vivo mechanical loading. In the bovine stifle joint, expression was found to be primarily localized to load bearing, anterior regions (M1) of the femoral condyles (Fig. 3A, 3B).61 Nonloaded, more posterior locations (M4) produced significantly less SZP. Increased amounts of SZP from load-bearing regions were found to correlate with decreased coefficients of friction. This localization within load-bearing regions suggests that SZP production is mechanosensitive. In addition, when cartilage explants were isolated from M1 and M4 and subjected to reciprocal sliding, SZP protein was upregulated in M1 explants but not M4 tissue (Fig. 3C). This sliding shear-mediated upregulation of SZP was inhibited by SB431542, an inhibitor of TGF-β receptor type I kinase, suggesting a role for TGF-β signaling in SZP mechanotransduction.61 Taken together, the functional phenotype of articular chondrocytes differs across the geometry of condyle in addition to depth.
FIG. 3.
Expression of SZP in (A) regions of high in vivo loading (M1) in the femoral condyles of bovine stifle joints is (B) significantly greater than in regions experiencing lower contact stresses (M4). L, lateral condyle; M, medial condyle; 1–4, locations on condyle numbered from anterior to posterior. SZP expression is (C) increased by sliding shear loading in cartilage explants obtained from M1. SZP synthesis in M4 explants is unaffected by shear. (Mean±SEM, *p=0.021).
The mechanical regulation of SZP has been investigated using other loading methods. Nugent et al.78 found SZP expression to be modulated by unconfined, compressive, mechanical forces. Patellofemoral, articular cartilage explants were compressed for 1 day using static conditions of 6 and 100 kPa, or oscillatory compressive loads (0.01 Hz) of 3–10 kPa and 3–300 kPa. All loading conditions induced a significant decrease in SZP protein immediately following treatment.78 Compared with unloaded controls, only the dynamic loading of 3–300 kPa induced a significant 46% increase in SZP secretion at day 1 post-treatment. In addition, static treatment of 100 kPa induced a significant decrease of SZP production by day 3 post-treatment. All other treatments were insignificant at all other time points.78 In another study, no changes were observed in prg4 gene and protein expression when bovine articular chondrocytes seeded in porous polyurethane scaffolds were dynamically compressed at 10%–20% strain at 0.1 Hz.79 It is difficult to draw firm conclusions from a limited sample set. Although SZP synthesis is responsive to compressive loading, the lack of a robust response suggests that compression may not act as a major mechanical regulator of expression.
In monolayer cultures, 0.5% and 3.0% biaxial strain of primary human chondrocytes induced a nearly twofold increase in transcription of prg4.80 However, identical treatment of chondrocytes passaged thrice resulted in 5-fold and 2.5-fold decreases, respectively. This study corroborates other publications demonstrating a change in chondrocyte gene expression due to dedifferentiation of chondrocytes during monolayer culture.81 An earlier study looked at the effects of cyclic hydrostatic pressure (5 MPa) and cyclic tension at 9% strain (0.5 Hz, 3 h/day for 3 days) on hypertrophic and matrix proteins in primary bovine chondrocytes seeded in 2% alginate gel. Cyclic tension significantly upregulated SZP mRNA, whereas hydrostatic pressure had no effect.82
Due to its anatomical location at the articular surface, superficial zone chondrocytes experience shearing forces. Bovine cartilage explants dynamically loaded (0.1 Hz) under 3% shear strain, and 20% compressive strain produced over three-times greater PRG4 protein than either unloaded or compressively loaded controls.83 These experimental observations utilizing tensile, shear, sliding shear, and compressive mechanical stimuli suggest that SZP expression is sensitive to multiple types of mechanical loading conditions (Table 1).
Table 1.
Effects of Mechanical Stimulation on Superficial Zone Protein Expression in Articular Chondrocytes
| Load type | Study | Cells | Culture type | Loading regimen | Effects on SZP expression |
|---|---|---|---|---|---|
| Compression | Nugent et al.78 | 1- to 3-w/o bovine chondrocytes | Patellofemoral groove explants | 3–300 kPa, 0.01 Hz, for 24 h | Secretion into media increased 46%, 1 day after loading |
| Tension | Wong et al.82 | Calf (bovine) humeral head chondrocytes | Primary MZ/DZ cells in 2% alginate gel | 9% strain (0.004 MPa), 0.5 Hz, 3 h/day for 3 days | Gene expression increased 1.5-fold, 1 day after loading |
| Kamiya et al.116 | 6- to 8-m/o porcine chondrocytes | Confluent monolayer of mandibular condyle cells | 7% or 21% strain, 0.5 Hz, for 12, 24, or 48 h | 7% strain increased gene and protein expression; 21% strain decreased protein secretion | |
| Simple shear | Nugent et al.83 | 1- to 3-w/o bovine chondrocytes | Patellofemoral groove explants | 3% strain, 0.1 Hz (with 20% static compression) for 1 day | Secretion into media increased three- to fourfold during loading, 1 day and 2 days after loading |
| Sliding shear | Neu et al.61 | 3-m/o bovine chondrocytes | Femoral condyle explants | 0.5 mm/s, 0.1 MPa, for 5 min | Secretion into media increased two- to threefold, 2 days after loading |
| Hydrostatic pressure | Wong et al.82 | Calf (bovine) humeral head chondrocytes | Primary MZ/DZ cells in 2% alginate gel | 5 MPa, 0.5 Hz, 3 h/day for 3 days | No significant change in gene expression, 1 day after loading |
| Applied surface motion | Grad et al.100 | 3- to 4-m/o bovine chondrocytes | Metacarpal joint cells in porous polyurethane scaffold | Hip ball rotated 60° 0.6 Hz, applied 10%–20% comp. strain, for 1 h, 2x/day, for 3 days | Gene expression increased sevenfold, protein secretion detected and increased during loading cycle |
| Continuous passive motion | Nugent-Derfus et al.77 | 1- to 3-w/o bovine chondrocytes | Patellofemoral groove and femoral condyle explants | 10°–46° flexion, 110°/min (0.025 Hz), for 1 day | Increased % of PRG4 positive cells in regions subjected to continuous sliding, no significant differences in secretion |
y/o, year-old; m/o, month-old; w/o, week-old; MZ/DZ, middle zone/deep zone; 2x, twice; SZP, superficial zone protein.
SZP plays a key role in the development and homeostasis of the joint as illustrated in prg4 knockout mice and CACP patients.6,62–64 Mice lacking prg4 were born with ordinary joints. However, during maturation, Rhee et al.64 observed a loss of superficial zone chondrocytes, fouling of the articular surface, and synovial hyperplasia; characteristics observed in CACP. Mechanical tests of articular cartilage from prg4 knockout mice demonstrated an increase in the coefficient of friction and a decrease in superficial zone cartilage stiffness compared with wild-type mice.63 These results suggest that SZP dysfunction may play a role in OA. Indeed, numerous animal models of OA demonstrate a downregulation in lubricin synthesis and increase in friction coefficient after knee injuries such as anterior cruciate ligament (ACL) transection and meniscectomy.65–68,84,85 These animal models were confirmed by human studies where lubricin expression was found to be decreased in individuals afflicted with ACL injuries.86 In vitro models of post-traumatic OA, such as hyper-physiological impact loading of articular cartilage, similarly display decreased levels of prg4 expression.87 Inflammatory or rheumatoid arthritis animal models also follow this response.68,88
SZP protein levels were upregulated in cartilage explants obtained from human knees with advanced OA. This study complements other reports by suggesting an overactive recovery in SZP expression later in the progression of OA.89 However, this rebound could be classified as “too little, too late” given the ruinous state of the cartilage. Taken together, there is persuasive evidence linking OA disease development with dysfunctional lubricin regulation. The downregulation of SZP leads to increases in joint friction and wear.7,69,90 Replenishment of boundary lubricants by supplementation may inhibit wear and prevent significant cartilage degradation from occurring.
Approaches to Enhancing Cartilage Lubrication
To successfully recapitulate cartilage lubrication, fluid film and boundary modes need to be established. Due to complex loads and sliding speeds, articular cartilage should function over mixed regimes of lubrication. However, modulation of hydrostatic and hydrodynamic lubrication is a difficult task due to the multifactorial nature of these regimes. Small alterations to the joint such as fibrillations on the articular surface can impede the formation of fluid films.10 Boundary lubrication requires only a molecular monolayer preventing asperity-asperity contact, and as such is conceptually simpler. Regeneration of functional and enduring articular cartilage will require the restoration of boundary lubrication. Potential OA treatments exploiting this approach include intra-articular injections of lubricants or tribosupplementation, pharmacological agents, and cartilage tissue engineering. Each of these therapeutic strategies has advantages and limitations that will be discussed.
Tribosupplementation
The underlying hypothesis of boundary lubricant-centered treatments is that maintaining or increasing boundary lubricant levels (tribosupplementation) may slow or prevent the onset of cartilage degradation. Accordingly, the effects of lubricin were examined in rat models of OA. In two separate studies, purified lubricin, full-length recombinant human PRG4, and modified recombinant lubricin (LUB:1) were injected into knees 7 days after surgical induction of OA.84,85 Compared with controls, increased localization of lubricin at the articular surface and decreased cartilage degradation were observed. Long-term studies are needed to determine whether intra-articular supplementation of SZP halts or simply slows the progression of OA.
In clinical practice, intra-articular injections of HA have been approved by the FDA for the symptomatic relief of OA.91 This treatment is considered “viscosupplementation” due to the effect of increasing the viscosity of synovial fluid and supporting fluid film lubrication.92 However, HA may also function as a tribosupplement according to results from a boundary lubrication study in a cartilage-cartilage system.44 HA and SZP could form a potential combination therapy for OA, improving both the rheological properties of synovial fluid and boundary mode lubricity.84,93
In addition to LUB:1, other tribosupplements have been proposed and experimentally tested in vitro. Chawla et al.94 functionalized PRG4 with an aldehyde group to increase binding to the cartilage surface. Other GAG such as chondroitin sulfate have also been studied as boundary lubricants.95 Based on SAPLs, an Israeli group engineered phospholipid-based liposomes to behave as boundary lubricants.96 Factors to be considered in designing a tribosupplementation therapy include cartilage surface binding affinity, reservoir kinetics, and delivery route. Intra-articular injections would provide the easiest, direct method of administration and minimize systemic interactions. The tribosupplement reservoir in the synovial fluid would depend on the enzymatic degradation rate as well as synovial capsule retention, which would affect the dosage frequency. Lubricant reservoir (synovial fluid) concentrations could have a large effect on binding kinetics and replenishment of the boundary lubricant. Lastly, the lubricant itself will need to strongly bind or adsorb to the cartilage surface so that interfacial contact is continuously prevented with a lubricating, molecular monolayer.
Boundary lubrication is the final, defensive guard against wear. SZP or other boundary lubricants have not been found to repair the ECM or induce cell signaling for cell-mediated regeneration. Additional lubricant (SZP) was observed to have no beneficial effects in severely damaged cartilage.89 Thus, tribosupplementation would likely be most effective as a preventative treatment of OA.
Pharmacological therapy
Another approach toward lubricant replenishment is stimulating endogenous, cellular production. Much work has been published on the cellular pathways controlling expression of boundary lubricants. For example, growth factors such as TGF-β and BMP have been demonstrated to upregulate the expression of SZP, whereas catabolic cartilage cytokines such as IL-1β and TNF-α depress expression.72–75 More importantly, modulation of SZP protein expression levels has a direct functional significance, as it led to corresponding changes in the frictional properties of cartilage explants.69,90 This experimental evidence strongly suggests that a pharmacological or bio-therapeutic agent that stimulates the production of SZP or some other boundary lubricant could provide chondroprotection and serve as a bioactive method of tribosupplementation. Supporting this theory, Elsaid et al.97 found that administration of etanercept, a TNF-α inhibitor, increased the synovial fluid concentration and cartilage surface localization of lubricin in ACL-transected rats. In summary, several pathways of SZP regulation have been identified in the literature that may serve as suitable targets for pharmacological intervention.
There are several barriers that should be surmounted to enable the success of a pharmacological therapy. First, signaling pathways such as TGF-β are pleiotropic and utilized by many other different cell phenotypes and cellular processes. Any potential drug candidates would need to specifically regulate SZP synthesis without disrupting other cellular functions that could lead to deleterious side effects. In addition, therapies would need to be successfully delivered to the articular cartilage. Drug delivery could potentially be achieved through oral medications, intra-articular injections, or a hydrogel carrier for controlled release. The method of delivery selected would depend on several factors such as compound stability and cross-reactivity with other cellular processes. Similar to any other drug therapy, it could take many years and millions of dollars of research to develop a working pharmaceutical compound to regulate SZP expression. However, with a disease such as OA that affects a significant percentage of the population, the possible payoff is large.
Incorporating boundary lubrication into engineered cartilage
Boundary lubrication enhancement would in all likelihood be unable to ameliorate the symptoms of OA due to extensive degradation of the articular surface. Since cartilage is recalcitrant to self-repair, the only recourse would be regeneration of the articular surface. Tissue-engineered cartilage has the potential to fill this need by providing replacement tissues that enable pain-free joint articulation. Features that prevent wear such as boundary lubrication will be key to the success of these constructs. Since work progresses on enhancing mechanical characteristics such as aggregate and elastic moduli, newer generations of engineered tissues are beginning to address frictional properties.98–102
Since native cartilage operates under mixed modes of lubrication, a boundary lubricant will need to be incorporated into any cartilage engineering approach. Independent of a scaffold or cellular self-assembly approach, the construct should facilitate binding and concentration of the boundary lubricant at the interfacial surface.99 Lubricant localization at the articular surface is crucial in two other respects. One, bulk retention of the lubricant in the matrix may diminish surface availability.98 Two, the boundary lubricant may interfere with in vivo implantation and tissue integration as PRG4 has been observed to prevent cell- and cartilage-cartilage adhesion.71
Another design consideration is lubricant source. The most common method will likely be a biomimetic approach that incorporates a resident population of cells into the construct. In accordance with the constraints just defined, boundary lubricant secreting cells would be located along the articular surface of the construct. Challenges of limited cell availability are especially acute for SZP-based approaches, as only superficial zone cells possess the requisite chondrocyte phenotype.55,56 Stem cells have the potential to provide a plentiful cell source, as mesenchymal progenitors have been induced to express PRG4 at the protein level.58,99,103 With the appropriate treatment and differentiation protocol, mesenchymal stem cells or embryonic stem cells could also serve as sources of SZP-secreting, articular chondrocytes. Middle- and deep-zone chondrocytes may have the ability to secrete SZP at superficial zone levels.104 However, induction is not achievable with current methods, as little is known about the mechanism behind the zone-specific expression of SZP.
Methods of lubrication assessment
The functional tissue engineering of articular cartilage will require careful analysis and modulation of characteristics such as friction and wear to replicate the tribological properties of native tissue. Many tools exist for measuring the friction properties of tissue engineered constructs, each offering unique capabilities and limitations. Perhaps the most widely used frictional assay is the pin-on-disk tribometer, useful for determining macroscopic friction.61,105 Numerous configurations have been implemented106,107 with the same, basic operating principle: the sample surface is placed in contact with an opposing surface such as glass. Normal loads are applied to the sample from a fixed weight or actuator. As one surface reciprocates or rotates about the other, the opposing frictional force or torque is measured.61,99
Macroscale coefficients of friction can also be assessed using the surface force apparatus.46 The SFA 2000 with friction device attachment operates similarly to a pin-on-disk tribometer: a cartilage sample is linearly reciprocated against an opposing surface such as glass. Displacement is controlled by either a motorized micrometer or bimorph (piezoelectric) slide. The resistive, friction force is measured by a Wheatstone bridge strain gauge system. A “3D/XYZ” configuration permits measuring independent forces in all orthogonal directions.108
Friction forces can also be measured at the nanoscale using atomic force microscopy (AFM).109,110 An advantage of this approach is that boundary lubrication is measured without the confounding effects of fluid film lubrication or interstitial fluid release.31 As the AFM cantilever is scanned across the surface, surface interactions are probed by measuring the deflection of the cantilever.111 Frictional forces and surface topography/roughness can be obtained using this method. Electron microscopy can also be employed to determine nanoscale features.31 Through a combination of these tests, wear can be gauged through measured changes in friction and roughness. In addition, wear products such as proteins released into the lubricating solution (i.e., phosphate-buffered saline) can be quantified through biochemical tests such as the bicinchoninic acid assay.112,113
As a greater number of investigators turn their attention toward lubrication engineering, it will be important for the field of cartilage tissue engineering to come to an agreement regarding issues such as assay operating parameters and a set of success criteria. For example, it is difficult to compare friction measurements when publications have used different sets of normal loads and sliding speeds, as these parameters are important for determining the mode and mechanism of lubrication. The evaluation of different treatments could be simplified greatly by a set of measurement standards. Finally, to determine the efficacy of lubrication, methods of scoring for both friction and wear are needed. For instance, a mutually accepted set of success criteria could apply equal weights to low friction and low wear. It is imperative that both sets of measurements be performed, as wear particles can reduce friction and, thus, bias the tribological data.69
Perspectives in the Emerging Field of Engineering Lubrication
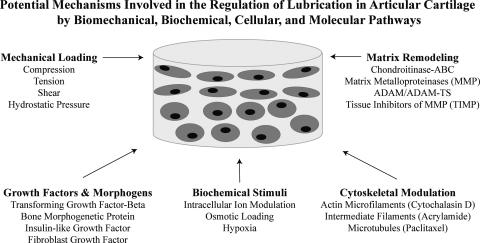
It is not clear whether tribological characteristics can be adjusted after mechanical maturation, or whether tribological properties need to be developed alongside mechanical characteristics. Since the iron is still hot with regard to bulk properties, it is a good time to strike and address these and other important questions (Fig. 4). For example, it remains to be determined exactly how mechanical and biochemical properties, such as compressive modulus and collagen II content, will influence variables such as surface roughness and lubricant binding/entrapment. Will a homogeneous construct replicate these features, or is a particular ECM distribution and zonal heterogeneity essential for cartilage lubrication? In addition, as synthetic scaffold technology matures and attains the mechanical characteristics of native cartilage tissue, how will the tribological properties of the scaffold evolve as well? Either the scaffolding will be natively suited for low friction,102 or the material may need to be modified with lubricious molecules such as SZP. With the recent advances in engineering increasingly stiffer constructs, it is now time to shift the existing cartilage tissue-engineering paradigm and begin addressing these questions in anticipation of meeting the next major challenge, cartilage lubrication.
FIG. 4.
Mechanical and tribological properties of engineered cartilage constructs may be developed sequentially or in parallel from a variety of potential stimuli and regulatory mechanisms involved in articular cartilage lubrication.
Beyond lubrication, additional significant obstacles remain. Outstanding questions include the integrity and stability of engineered tissues in the demanding and complex biological and mechanical environment of living articular joints. Work is already being performed in this area, as synovial bioreactors capable of simulating these conditions are currently in development.114,115 In addition, identification of suitable in vivo animal models is needed, as it is unknown whether normal or OA-induced animal joints are capable of replicating the conditions of injured or diseased human joints. However, before these issues become relevant, it is paramount that biomimetic, engineered cartilage be developed in vitro that possesses both native tissue-level mechanical and frictional properties.
Acknowledgments
The authors wish to thank Dr. Corey Neu for his help with illustrations in Figure 3. Funds from the Lawrence J. Ellison Endowed Chair supported the initial experimental work. The current research is supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, NIH (NIAMS 1R01 AR061496).
Disclosure Statement
No competing financial interests exist.
References
- 1.Lawrence R.C. Felson D.T. Helmick C.G. Arnold L.M. Choi H. Deyo R.A., et al. Estimates of the prevalence of arthritis, other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58:26. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hunter D.J. Pharmacologic therapy for osteoarthritis—the era of disease modification. Nat Rev Rheumatol. 2011;7:13. doi: 10.1038/nrrheum.2010.178. [DOI] [PubMed] [Google Scholar]
- 3.Natoli R.M. Revell C.M. Athanasiou K.A. Chondroitinase ABC treatment results in greater tensile properties of self-assembled tissue-engineered articular cartilage. Tissue Eng Part A. 2009;15:3119. doi: 10.1089/ten.tea.2008.0478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elder B.D. Athanasiou K.A. Synergistic and additive effects of hydrostatic pressure and growth factors on tissue formation. PLoS One. 2008;3:e2341. doi: 10.1371/journal.pone.0002341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hu J.C. Athanasiou K.A. A self-assembling process in articular cartilage tissue engineering. Tissue Eng. 2006;12:969. doi: 10.1089/ten.2006.12.969. [DOI] [PubMed] [Google Scholar]
- 6.Marcelino J. Carpten J.D. Suwairi W.M. Gutierrez O.M. Schwartz S. Robbins C., et al. CACP, encoding a secreted proteoglycan, is mutated in camptodactyly-arthropathy-coxa vara-pericarditis syndrome. Nat Genet. 1999;23:319. doi: 10.1038/15496. [DOI] [PubMed] [Google Scholar]
- 7.Jay G.D. Torres J.R. Rhee D.K. Helminen H.J. Hytinnen M.M. Cha C.J., et al. Association between friction and wear in diarthrodial joints lacking lubricin. Arthritis Rheum. 2007;56:3662. doi: 10.1002/art.22974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eleswarapu S.V. Leipzig N.D. Athanasiou K.A. Gene expression of single articular chondrocytes. Cell Tissue Res. 2007;327:43. doi: 10.1007/s00441-006-0258-5. [DOI] [PubMed] [Google Scholar]
- 9.Knobloch T.J. Madhavan S. Nam J. Agarwal S., Jr. Agarwal S. Regulation of chondrocytic gene expression by biomechanical signals. Crit Rev Eukaryot Gene Expr. 2008;18:139. doi: 10.1615/critreveukargeneexpr.v18.i2.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mow V.C. Ratcliffe A. Poole A.R. Cartilage and diarthrodial joints as paradigms for hierarchical materials and structures. Biomaterials. 1992;13:67. doi: 10.1016/0142-9612(92)90001-5. [DOI] [PubMed] [Google Scholar]
- 11.Fitzgerald J.B. Jin M. Grodzinsky A.J. Shear and compression differentially regulate clusters of functionally related temporal transcription patterns in cartilage tissue. J Biol Chem. 2006;281:24095. doi: 10.1074/jbc.M510858200. [DOI] [PubMed] [Google Scholar]
- 12.Quinn T.M. Hunziker E.B. Hauselmann H.J. Variation of cell and matrix morphologies in articular cartilage among locations in the adult human knee. Osteoarthritis Cartilage. 2005;13:672. doi: 10.1016/j.joca.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 13.Almarza A.J. Athanasiou K.A. Design characteristics for the tissue engineering of cartilaginous tissues. Ann Biomed Eng. 2004;32:2. doi: 10.1023/b:abme.0000007786.37957.65. [DOI] [PubMed] [Google Scholar]
- 14.Dunn W. DuRaine G. Reddi A.H. Profiling microRNA expression in bovine articular cartilage and implications for mechanotransduction. Arthritis Rheum. 2009;60:2333. doi: 10.1002/art.24678. [DOI] [PubMed] [Google Scholar]
- 15.Darling E.M. Hu J.C. Athanasiou K.A. Zonal and topographical differences in articular cartilage gene expression. J Orthop Res. 2004;22:1182. doi: 10.1016/j.orthres.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 16.Becerra J. Andrades J.A. Guerado E. Zamora-Navas P. Lopez-Puertas J.M. Reddi A.H. Articular cartilage: structure and regeneration. Tissue Eng Part B Rev. 2010;16:617. doi: 10.1089/ten.TEB.2010.0191. [DOI] [PubMed] [Google Scholar]
- 17.Darling E.M. Athanasiou K.A. Rapid phenotypic changes in passaged articular chondrocyte subpopulations. J Orthop Res. 2005;23:425. doi: 10.1016/j.orthres.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 18.Lin Z. Willers C. Xu J. Zheng M.H. The chondrocyte: biology and clinical application. Tissue Eng. 2006;12:1971. doi: 10.1089/ten.2006.12.1971. [DOI] [PubMed] [Google Scholar]
- 19.Athanasiou K.A. Rosenwasser M.P. Buckwalter J.A. Malinin T.I. Mow V.C. Interspecies comparisons of in situ intrinsic mechanical properties of distal femoral cartilage. J Orthop Res. 1991;9:330. doi: 10.1002/jor.1100090304. [DOI] [PubMed] [Google Scholar]
- 20.Hodge W.A. Fijan R.S. Carlson K.L. Burgess R.G. Harris W.H. Mann R.W. Contact pressures in the human hip joint measured in vivo. Proc Natl Acad Sci U S A. 1986;83:2879. doi: 10.1073/pnas.83.9.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mow V.C. Kuei S.C. Lai W.M. Armstrong C.G. Biphasic creep and stress relaxation of articular cartilage in compression: Theory and experiments. J Biomech Eng. 1980;102:73. doi: 10.1115/1.3138202. [DOI] [PubMed] [Google Scholar]
- 22.Dowson D. History of Tribology. second. London: Professional Engineering Publishing; 1998. [Google Scholar]
- 23.Scherge M. Gorb S.N. Biological Micro- and Nanotribology: Nature's Solutions. Germany: Springer-Verlag Berlin Heidelberg; 2001. [Google Scholar]
- 24.Neu C.P. Komvopoulos K. Reddi A.H. The interface of functional biotribology and regenerative medicine in synovial joints. Tissue Eng Part B Rev. 2008;14:235. doi: 10.1089/ten.teb.2008.0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Elwell R. Hydrostatic lubrication. In: Booser E.R., editor. CRC Handbook of Lubrication. New York: CRC Press; 1983. pp. 105–120. [Google Scholar]
- 26.Cheng H. Elastohydrodynamic lubrication. In: Booser E.R., editor. CRC Handbook of Lubrication. New York: CRC Press; 1983. pp. 139–162. [Google Scholar]
- 27.Gleghorn J.P. Bonassar L.J. Lubrication mode analysis of articular cartilage using Stribeck surfaces. J Biomech. 2008;41:1910. doi: 10.1016/j.jbiomech.2008.03.043. [DOI] [PubMed] [Google Scholar]
- 28.Chan S.M. Neu C.P. Duraine G. Komvopoulos K. Reddi A.H. Atomic force microscope investigation of the boundary-lubricant layer in articular cartilage. Osteoarthritis Cartilage. 2010;18:956. doi: 10.1016/j.joca.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 29.Heshmat H. Tribology of Interface Layers. New York: CRC Press; 2010. [Google Scholar]
- 30.Hsu S.M. Boundary lubrication: current understanding. Tribology Letters. 1997;3:1. [Google Scholar]
- 31.Chan S.M. Neu C.P. Komvopoulos K. Reddi A.H. Dependence of nanoscale friction and adhesion properties of articular cartilage on contact load. J Biomech. 2011;44:1340. doi: 10.1016/j.jbiomech.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 32.Lewis P.R. McCutchen C.W. Mechanism of animal joints: experimental evidence for weeping lubrication in mammalian joints. Nature. 1959;184:1285. doi: 10.1038/1841285a0. [DOI] [PubMed] [Google Scholar]
- 33.McCutchen C.W. Mechanism of animal joints: sponge-hydrostatic and weeping bearings. Nature. 1959;184:1284. doi: 10.1038/1841284a0. [DOI] [PubMed] [Google Scholar]
- 34.Walker P.S. Dowson D. Longfield M.D. Wright V. “Boosted lubrication” in synovial joints by fluid entrapment and enrichment. Ann Rheum Dis. 1968;27:512. doi: 10.1136/ard.27.6.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jay G.D. Cha C.J. The effect of phospholipase digestion upon the boundary lubricating ability of synovial fluid. J Rheumatol. 1999;26:2454. [PubMed] [Google Scholar]
- 36.Krishnan R. Caligaris M. Mauck R.L. Hung C.T. Costa K.D. Ateshian G.A. Removal of the superficial zone of bovine articular cartilage does not increase its frictional coefficient. Osteoarthritis Cartilage. 2004;12:947. doi: 10.1016/j.joca.2004.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hills B.A. Identity of the joint lubricant. J Rheumatol. 2002;29:200. [PubMed] [Google Scholar]
- 38.Knudson C.B. Knudson W. Hyaluronan and CD44: modulators of chondrocyte metabolism. Clin Orthop Relat Res. 2004;427(Suppl):S152. [PubMed] [Google Scholar]
- 39.Swann D.A. Radin E.L. Nazimiec M. Weisser P.A. Curran N. Lewinnek G. Role of hyaluronic acid in joint lubrication. Ann Rheum Dis. 1974;33:318. doi: 10.1136/ard.33.4.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Radin E.L. Swann D.A. Weisser P.A. Separation of a hyaluronate-free lubricating fraction from synovial fluid. Nature. 1970;228:377. doi: 10.1038/228377a0. [DOI] [PubMed] [Google Scholar]
- 41.Linn F.C. Radin E.L. Lubrication of animal joints. 3. The effect of certain chemical alterations of the cartilage and lubricant. Arthritis Rheum. 1968;11:674. doi: 10.1002/art.1780110510. [DOI] [PubMed] [Google Scholar]
- 42.Chang D.P. Abu-Lail N.I. Guilak F. Jay G.D. Zauscher S. Conformational mechanics, adsorption, and normal force interactions of lubricin and hyaluronic acid on model surfaces. Langmuir. 2008;24:1183. doi: 10.1021/la702366t. [DOI] [PubMed] [Google Scholar]
- 43.Tadmor R. Chen N. Israelachvili J.N. Thin film rheology and lubricity of hyaluronic acid solutions at a normal physiological concentration. J Biomed Mater Res. 2002;61:514. doi: 10.1002/jbm.10215. [DOI] [PubMed] [Google Scholar]
- 44.Schmidt T.A. Gastelum N.S. Nguyen Q.T. Schumacher B.L. Sah R.L. Boundary lubrication of articular cartilage: role of synovial fluid constituents. Arthritis Rheum. 2007;56:882. doi: 10.1002/art.22446. [DOI] [PubMed] [Google Scholar]
- 45.Benz M. Chen N. Israelachvili J. Lubrication and wear properties of grafted polyelectrolytes, hyaluronan and hylan, measured in the surface forces apparatus. J Biomed Mater Res A. 2004;71:6. doi: 10.1002/jbm.a.30123. [DOI] [PubMed] [Google Scholar]
- 46.Greene G.W. Banquy X. Lee D.W. Lowrey D.D. Yu J. Israelachvili J.N. Adaptive mechanically controlled lubrication mechanism found in articular joints. Proc Natl Acad Sci U S A. 2011;108:5255. doi: 10.1073/pnas.1101002108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hills B.A. Butler B.D. Surfactants identified in synovial fluid and their ability to act as boundary lubricants. Ann Rheum Dis. 1984;43:641. doi: 10.1136/ard.43.4.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sarma A.V. Powell G.L. LaBerge M. Phospholipid composition of articular cartilage boundary lubricant. J Orthop Res. 2001;19:671. doi: 10.1016/S0736-0266(00)00064-4. [DOI] [PubMed] [Google Scholar]
- 49.Schwarz I.M. Hills B.A. Surface-active phospholipid as the lubricating component of lubricin. Br J Rheumatol. 1998;37:21. doi: 10.1093/rheumatology/37.1.21. [DOI] [PubMed] [Google Scholar]
- 50.Swann D.A. Slayter H.S. Silver F.H. The molecular structure of lubricating glycoprotein-I, the boundary lubricant for articular cartilage. J Biol Chem. 1981;256:5921. [PubMed] [Google Scholar]
- 51.Flannery C.R. Hughes C.E. Schumacher B.L. Tudor D. Aydelotte M.B. Kuettner K.E., et al. Articular cartilage superficial zone protein (SZP) is homologous to megakaryocyte stimulating factor precursor and Is a multifunctional proteoglycan with potential growth-promoting, cytoprotective, and lubricating properties in cartilage metabolism. Biochem Biophys Res Commun. 1999;254:535. doi: 10.1006/bbrc.1998.0104. [DOI] [PubMed] [Google Scholar]
- 52.Ikegawa S. Sano M. Koshizuka Y. Nakamura Y. Isolation, characterization and mapping of the mouse and human PRG4 (proteoglycan 4) genes. Cytogenet Cell Genet. 2000;90:291. doi: 10.1159/000056791. [DOI] [PubMed] [Google Scholar]
- 53.Jay G.D. Tantravahi U. Britt D.E. Barrach H.J. Cha C.J. Homology of lubricin and superficial zone protein (SZP): products of megakaryocyte stimulating factor (MSF) gene expression by human synovial fibroblasts and articular chondrocytes localized to chromosome 1q25. J Orthop Res. 2001;19:677. doi: 10.1016/S0736-0266(00)00040-1. [DOI] [PubMed] [Google Scholar]
- 54.Liu Y.J. Lu S.H. Xu B. Yang R.C. Ren Q. Liu B., et al. Hemangiopoietin, a novel human growth factor for the primitive cells of both hematopoietic and endothelial cell lineages. Blood. 2004;103:4449. doi: 10.1182/blood-2003-06-1825. [DOI] [PubMed] [Google Scholar]
- 55.Schumacher B.L. Block J.A. Schmid T.M. Aydelotte M.B. Kuettner K.E. A novel proteoglycan synthesized and secreted by chondrocytes of the superficial zone of articular cartilage. Arch Biochem Biophys. 1994;311:144. doi: 10.1006/abbi.1994.1219. [DOI] [PubMed] [Google Scholar]
- 56.Schmidt T.A. Schumacher B.L. Klein T.J. Voegtline M.S. Sah R.L. Synthesis of proteoglycan 4 by chondrocyte subpopulations in cartilage explants, monolayer cultures, and resurfaced cartilage cultures. Arthritis Rheum. 2004;50:2849. doi: 10.1002/art.20480. [DOI] [PubMed] [Google Scholar]
- 57.Sun Y. Berger E.J. Zhao C. Jay G.D. An K.N. Amadio P.C. Expression and mapping of lubricin in canine flexor tendon. J Orthop Res. 2006;24:1861. doi: 10.1002/jor.20239. [DOI] [PubMed] [Google Scholar]
- 58.Lee S.Y. Nakagawa T. Reddi A.H. Induction of chondrogenesis and expression of superficial zone protein (SZP)/lubricin by mesenchymal progenitors in the infrapatellar fat pad of the knee joint treated with TGF-beta1 and BMP-7. Biochem Biophys Res Commun. 2008;376:148. doi: 10.1016/j.bbrc.2008.08.138. [DOI] [PubMed] [Google Scholar]
- 59.Schumacher B.L. Schmidt T.A. Voegtline M.S. Chen A.C. Sah R.L. Proteoglycan 4 (PRG4) synthesis and immunolocalization in bovine meniscus. J Orthop Res. 2005;23:562. doi: 10.1016/j.orthres.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 60.Rees S.G. Davies J.R. Tudor D. Flannery C.R. Hughes C.E. Dent C.M., et al. Immunolocalisation and expression of proteoglycan 4 (cartilage superficial zone proteoglycan) in tendon. Matrix Biol. 2002;21:593. doi: 10.1016/s0945-053x(02)00056-2. [DOI] [PubMed] [Google Scholar]
- 61.Neu C.P. Khalafi A. Komvopoulos K. Schmid T.M. Reddi A.H. Mechanotransduction of bovine articular cartilage superficial zone protein by transforming growth factor beta signaling. Arthritis Rheum. 2007;56:3706. doi: 10.1002/art.23024. [DOI] [PubMed] [Google Scholar]
- 62.Rhee D.K. Marcelino J. Al-Mayouf S. Schelling D.K. Bartels C.F. Cui Y., et al. Consequences of disease-causing mutations on lubricin protein synthesis, secretion, and post-translational processing. J Biol Chem. 2005;280:31325. doi: 10.1074/jbc.M505401200. [DOI] [PubMed] [Google Scholar]
- 63.Coles J.M. Zhang L. Blum J.J. Warman M.L. Jay G.D. Guilak F., et al. Loss of cartilage structure, stiffness, and frictional properties in mice lacking PRG4. Arthritis Rheum. 2010;62:1666. doi: 10.1002/art.27436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rhee D.K. Marcelino J. Baker M. Gong Y. Smits P. Lefebvre V., et al. The secreted glycoprotein lubricin protects cartilage surfaces and inhibits synovial cell overgrowth. J Clin Invest. 2005;115:622. doi: 10.1172/JCI200522263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Teeple E. Elsaid K.A. Fleming B.C. Jay G.D. Aslani K. Crisco J.J., et al. Coefficients of friction, lubricin, and cartilage damage in the anterior cruciate ligament-deficient guinea pig knee. J Orthop Res. 2008;26:231. doi: 10.1002/jor.20492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wei L. Fleming B.C. Sun X. Teeple E. Wu W. Jay G.D., et al. Comparison of differential biomarkers of osteoarthritis with and without posttraumatic injury in the Hartley guinea pig model. J Orthop Res. 2010;28:900. doi: 10.1002/jor.21093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Young A.A. McLennan S. Smith M.M. Smith S.M. Cake M.A. Read R.A., et al. Proteoglycan 4 downregulation in a sheep meniscectomy model of early osteoarthritis. Arthritis Res Ther. 2006;8:R41. doi: 10.1186/ar1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Elsaid K.A. Jay G.D. Warman M.L. Rhee D.K. Chichester C.O. Association of articular cartilage degradation and loss of boundary-lubricating ability of synovial fluid following injury and inflammatory arthritis. Arthritis Rheum. 2005;52:1746. doi: 10.1002/art.21038. [DOI] [PubMed] [Google Scholar]
- 69.DuRaine G. Neu C.P. Chan S.M. Komvopoulos K. June R.K. Reddi A.H. Regulation of the friction coefficient of articular cartilage by TGF-beta1 and IL-1beta. J Orthop Res. 2009;27:249. doi: 10.1002/jor.20713. [DOI] [PubMed] [Google Scholar]
- 70.Bahabri S.A. Suwairi W.M. Laxer R.M. Polinkovsky A. Dalaan A.A. Warman M.L. The camptodactyly-arthropathy-coxavara-pericarditis syndrome: clinical features and genetic mapping to human chromosome 1. Arthritis Rheum. 1998;41:730. doi: 10.1002/1529-0131(199804)41:4<730::AID-ART22>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 71.Englert C. McGowan K.B. Klein T.J. Giurea A. Schumacher B.L. Sah R.L. Inhibition of integrative cartilage repair by proteoglycan 4 in synovial fluid. Arthritis Rheum. 2005;52:1091. doi: 10.1002/art.20986. [DOI] [PubMed] [Google Scholar]
- 72.Niikura T. Reddi A.H. Differential regulation of lubricin/superficial zone protein by transforming growth factor beta/bone morphogenetic protein superfamily members in articular chondrocytes and synoviocytes. Arthritis Rheum. 2007;56:2312. doi: 10.1002/art.22659. [DOI] [PubMed] [Google Scholar]
- 73.Jones A.R. Flannery C.R. Bioregulation of lubricin expression by growth factors and cytokines. Eur Cell Mater. 2007;13:40. doi: 10.22203/ecm.v013a04. [DOI] [PubMed] [Google Scholar]
- 74.Schmidt T.A. Gastelum N.S. Han E.H. Nugent-Derfus G.E. Schumacher B.L. Sah R.L. Differential regulation of proteoglycan 4 metabolism in cartilage by IL-1alpha, IGF-I, and TGF-beta1. Osteoarthritis Cartilage. 2008;16:90. doi: 10.1016/j.joca.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 75.Khalafi A. Schmid T.M. Neu C. Reddi A.H. Increased accumulation of superficial zone protein (SZP) in articular cartilage in response to bone morphogenetic protein-7 and growth factors. J Orthop Res. 2007;25:293. doi: 10.1002/jor.20329. [DOI] [PubMed] [Google Scholar]
- 76.Lee S.Y. Niikura T. Reddi A.H. Superficial zone protein (lubricin) in the different tissue compartments of the knee joint: modulation by transforming growth factor beta 1 and interleukin-1 beta. Tissue Eng Part A. 2008;14:1799. doi: 10.1089/ten.tea.2007.0367. [DOI] [PubMed] [Google Scholar]
- 77.Nugent-Derfus G.E. Takara T. O'Neill J K. Cahill S.B. Gortz S. Pong T., et al. Continuous passive motion applied to whole joints stimulates chondrocyte biosynthesis of PRG4. Osteoarthritis Cartilage. 2007;15:566. doi: 10.1016/j.joca.2006.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nugent G.E. Schmidt T.A. Schumacher B.L. Voegtline M.S. Bae W.C. Jadin K.D., et al. Static and dynamic compression regulate cartilage metabolism of PRoteoGlycan 4 (PRG4) Biorheology. 2006;43:191. [PubMed] [Google Scholar]
- 79.Grad S. Gogolewski S. Alini M. Wimmer M.A. Effects of simple and complex motion patterns on gene expression of chondrocytes seeded in 3D scaffolds. Tissue Eng. 2006;12:3171. doi: 10.1089/ten.2006.12.3171. [DOI] [PubMed] [Google Scholar]
- 80.Das R.H. Jahr H. Verhaar J.A. van der Linden J.C. van Osch G.J. Weinans H. In vitro expansion affects the response of chondrocytes to mechanical stimulation. Osteoarthritis Cartilage. 2008;16:385. doi: 10.1016/j.joca.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 81.Hayman D.M. Blumberg T.J. Scott C.C. Athanasiou K.A. The effects of isolation on chondrocyte gene expression. Tissue Eng. 2006;12:2573. doi: 10.1089/ten.2006.12.2573. [DOI] [PubMed] [Google Scholar]
- 82.Wong M. Siegrist M. Goodwin K. Cyclic tensile strain and cyclic hydrostatic pressure differentially regulate expression of hypertrophic markers in primary chondrocytes. Bone. 2003;33:685. doi: 10.1016/s8756-3282(03)00242-4. [DOI] [PubMed] [Google Scholar]
- 83.Nugent G.E. Aneloski N.M. Schmidt T.A. Schumacher B.L. Voegtline M.S. Sah R.L. Dynamic shear stimulation of bovine cartilage biosynthesis of proteoglycan 4. Arthritis Rheum. 2006;54:1888. doi: 10.1002/art.21831. [DOI] [PubMed] [Google Scholar]
- 84.Flannery C.R. Zollner R. Corcoran C. Jones A.R. Root A. Rivera-Bermudez M.A., et al. Prevention of cartilage degeneration in a rat model of osteoarthritis by intraarticular treatment with recombinant lubricin. Arthritis Rheum. 2009;60:840. doi: 10.1002/art.24304. [DOI] [PubMed] [Google Scholar]
- 85.Jay G.D. Fleming B.C. Watkins B.A. McHugh K.A. Anderson S.C. Zhang L.X., et al. Prevention of cartilage degeneration and restoration of chondroprotection by lubricin tribosupplementation in the rat following anterior cruciate ligament transection. Arthritis Rheum. 2010;62:2382. doi: 10.1002/art.27550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Elsaid K.A. Fleming B.C. Oksendahl H.L. Machan J.T. Fadale P.D. Hulstyn M.J., et al. Decreased lubricin concentrations and markers of joint inflammation in the synovial fluid of patients with anterior cruciate ligament injury. Arthritis Rheum. 2008;58:1707. doi: 10.1002/art.23495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jones A.R. Chen S. Chai D.H. Stevens A.L. Gleghorn J.P. Bonassar L.J., et al. Modulation of lubricin biosynthesis and tissue surface properties following cartilage mechanical injury. Arthritis Rheum. 2009;60:133. doi: 10.1002/art.24143. [DOI] [PubMed] [Google Scholar]
- 88.Elsaid K.A. Jay G.D. Chichester C.O. Reduced expression and proteolytic susceptibility of lubricin/superficial zone protein may explain early elevation in the coefficient of friction in the joints of rats with antigen-induced arthritis. Arthritis Rheum. 2007;56:108. doi: 10.1002/art.22321. [DOI] [PubMed] [Google Scholar]
- 89.Neu C.P. Reddi A.H. Komvopoulos K. Schmid T.M. Di Cesare P.E. Increased friction coefficient and superficial zone protein expression in patients with advanced osteoarthritis. Arthritis Rheum. 2010;62:2680. doi: 10.1002/art.27577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gleghorn J.P. Jones A.R. Flannery C.R. Bonassar L.J. Alteration of articular cartilage frictional properties by transforming growth factor beta, interleukin-1beta, and oncostatin M. Arthritis Rheum. 2009;60:440. doi: 10.1002/art.24259. [DOI] [PubMed] [Google Scholar]
- 91.Goldberg V.M. Buckwalter J.A. Hyaluronans in the treatment of osteoarthritis of the knee: evidence for disease-modifying activity. Osteoarthritis Cartilage. 2005;13:216. doi: 10.1016/j.joca.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 92.Balazs E.A. Denlinger J.L. Viscosupplementation: a new concept in the treatment of osteoarthritis. J Rheumatol Suppl. 1993;39:3. [PubMed] [Google Scholar]
- 93.Jay G.D. Torres J.R. Warman M.L. Laderer M.C. Breuer K.S. The role of lubricin in the mechanical behavior of synovial fluid. Proc Natl Acad Sci U S A. 2007;104:6194. doi: 10.1073/pnas.0608558104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chawla K. Ham H.O. Nguyen T. Messersmith P.B. Molecular resurfacing of cartilage with proteoglycan 4. Acta Biomater. 2010;6:3388. doi: 10.1016/j.actbio.2010.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Katta J. Jin Z. Ingham E. Fisher J. Chondroitin sulphate: an effective joint lubricant? Osteoarthritis Cartilage. 2009;17:1001. doi: 10.1016/j.joca.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 96.Sivan S. Schroeder A. Verberne G. Merkher Y. Diminsky D. Priev A., et al. Liposomes act as effective biolubricants for friction reduction in human synovial joints. Langmuir. 2010;26:1107. doi: 10.1021/la9024712. [DOI] [PubMed] [Google Scholar]
- 97.Elsaid K.A. Machan J.T. Waller K. Fleming B.C. Jay G.D. The impact of anterior cruciate ligament injury on lubricin metabolism and the effect of inhibiting tumor necrosis factor alpha on chondroprotection in an animal model. Arthritis Rheum. 2009;60:2997. doi: 10.1002/art.24800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Klein T.J. Schumacher B.L. Blewis M.E. Schmidt T.A. Voegtline M.S. Thonar E.J., et al. Tailoring secretion of proteoglycan 4 (PRG4) in tissue-engineered cartilage. Tissue Eng. 2006;12:1429. doi: 10.1089/ten.2006.12.1429. [DOI] [PubMed] [Google Scholar]
- 99.Gleghorn J.P. Jones A.R. Flannery C.R. Bonassar L.J. Boundary mode frictional properties of engineered cartilaginous tissues. Eur Cell Mater. 2007;14:20. doi: 10.22203/ecm.v014a02. [DOI] [PubMed] [Google Scholar]
- 100.Grad S. Lee C.R. Gorna K. Gogolewski S. Wimmer M.A. Alini M. Surface motion upregulates superficial zone protein and hyaluronan production in chondrocyte-seeded three-dimensional scaffolds. Tissue Eng. 2005;11:249. doi: 10.1089/ten.2005.11.249. [DOI] [PubMed] [Google Scholar]
- 101.Morita Y. Tomita N. Aoki H. Sonobe M. Wakitani S. Tamada Y., et al. Frictional properties of regenerated cartilage in vitro. J Biomech. 2006;39:103. doi: 10.1016/j.jbiomech.2004.10.031. [DOI] [PubMed] [Google Scholar]
- 102.Moutos F.T. Guilak F. Functional properties of cell-seeded three-dimensionally woven poly(epsilon-caprolactone) scaffolds for cartilage tissue engineering. Tissue Eng Part A. 2010;16:1291. doi: 10.1089/ten.tea.2009.0480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lee S.Y. Nakagawa T. Reddi A.H. Mesenchymal progenitor cells derived from synovium and infrapatellar fat pad as a source for superficial zone cartilage tissue engineering: analysis of superficial zone protein/lubricin expression. Tissue Eng Part A. 2010;16:317. doi: 10.1089/ten.TEA.2009.0104. [DOI] [PubMed] [Google Scholar]
- 104.Li Z. Yao S. Alini M. Grad S. Different response of articular chondrocyte subpopulations to surface motion. Osteoarthritis Cartilage. 2007;15:1034. doi: 10.1016/j.joca.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 105.Swann D.A. Hendren R.B. Radin E.L. Sotman S.L. Duda E.A. The lubricating activity of synovial fluid glycoproteins. Arthritis Rheum. 1981;24:22. doi: 10.1002/art.1780240104. [DOI] [PubMed] [Google Scholar]
- 106.Jay G.D. Elsaid K.A. Zack J. Robinson K. Trespalacios F. Cha C.J., et al. Lubricating ability of aspirated synovial fluid from emergency department patients with knee joint synovitis. J Rheumatol. 2004;31:557. [PubMed] [Google Scholar]
- 107.Schmidt T.A. Sah R.L. Effect of synovial fluid on boundary lubrication of articular cartilage. Osteoarthritis Cartilage. 2007;15:35. doi: 10.1016/j.joca.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 108.Israelachvili J. Min Y. Akbulut M. Alig A. Carver G. Greene W., et al. Recent advances in the surface forces apparatus (SFA) technique. Rep Prog Phys. 2010;73 [Google Scholar]
- 109.Chang D.P. Abu-Lail N.I. Coles J.M. Guilak F. Jay G.D. Zauscher S. Friction force microscopy of lubricin and hyaluronic acid between hydrophobic and hydrophilic surfaces. Soft Matter. 2009;5:3438. doi: 10.1039/b907155e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Park S. Costa K.D. Ateshian G.A. Microscale frictional response of bovine articular cartilage from atomic force microscopy. J Biomech. 2004;37:1679. doi: 10.1016/j.jbiomech.2004.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hansma P.K. Elings V.B. Marti O. Bracker C.E. Scanning tunneling microscopy and atomic force microscopy: application to biology and technology. Science. 1988;242:209. doi: 10.1126/science.3051380. [DOI] [PubMed] [Google Scholar]
- 112.Graindorge S. Ferrandez W. Ingham E. Jin Z. Twigg P. Fisher J. The role of the surface amorphous layer of articular cartilage in joint lubrication. Proc Inst Mech Eng H. 2006;220:597. doi: 10.1243/09544119JEIM122. [DOI] [PubMed] [Google Scholar]
- 113.Katta J. Jin Z. Ingham E. Fisher J. Effect of nominal stress on the long term friction, deformation and wear of native and glycosaminoglycan deficient articular cartilage. Osteoarthritis Cartilage. 2009;17:662. doi: 10.1016/j.joca.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 114.Blewis M.E. Lao B.J. Schumacher B.L. Bugbee W.D. Sah R.L. Firestein G.S. Interactive cytokine regulation of synoviocyte lubricant secretion. Tissue Eng Part A. 2010;16:1329. doi: 10.1089/ten.tea.2009.0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Blewis M.E. Lao B.J. Jadin K.D. McCarty W.J. Bugbee W.D. Firestein G.S., et al. Semi-permeable membrane retention of synovial fluid lubricants hyaluronan and proteoglycan 4 for a biomimetic bioreactor. Biotechnol Bioeng. 2010;106:149. doi: 10.1002/bit.22645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kamiya T. Tanimoto K. Tanne Y. Lin Y.Y. Kunimatsu R. Yoshioka M., et al. Effects of mechanical stimuli on the synthesis of superficial zone protein in chondrocytes. J Biomed Mater Res A. 2009;92:801. doi: 10.1002/jbm.a.32295. [DOI] [PubMed] [Google Scholar]