Abstract
Background
Absorbance of near-infrared (600–800 nm) light by the tissue components water, melanin, and hemoglobin is minimal. This property allows the use of near-infrared-emitting fluorophores for noninvasive, in vivo, real-time imaging of tissue, without the interference of autofluorescence experienced with imaging in other wavelength ranges. Near-infrared (NIR) fluorescence imaging has been used to noninvasively image lymphatic architecture and pumping function in animals, as well as in humans. The effects of different doses of a NIR dye, indocyanine green (ICG), on lymphatic function have been questioned. This study aims to address these concerns in the context of a mouse inguinal-to-axillary lymphatic imaging model.
Methods and Results
We measured lymph propulsive velocity and frequency using an imaging system composed of a laser diode for excitation of the dye, an image intensifier, and an intensified charge-coupled device (ICCD) camera to capture real-time images. At 0.32, 0.645, and 1.3 mM ICG, no significant differences in lymphatic propulsive velocity or frequency were observed. Additionally, the use of other NIR imaging agents did not result in significant differences.
Conclusions
The use of different concentrations of ICG and the use of other near-infrared fluorophores for optical imaging of lymphatics does not significantly affect lymphatic propulsive velocity or frequency.
Introduction
The lymphatic system plays a critical role in fluid homeostasis, returning approximately four liters of fluid to the human blood circulatory system each day.1 Unfortunately, lymph fluid is clear and is difficult to image using conventional techniques. Recently, near-infrared (NIR) fluorescence imaging has emerged as a new technology for imaging the movement of lymph in humans.2–8 The technique depends upon low-energy NIR light that diffusely propagates into tissues. Fluorescent dyes that are selectively taken up by the lymphatics absorb the light, and upon relaxation, emit red-shifted photons. These photons are collected as they emanate from the body, and are captured by an ICCD camera, yielding images and movies of fluorescent dye-laden lymph traveling through lymphatic vessels and lymph nodes. These images are exquisite, and allow real-time imaging with much better spatial resolution than conventional lymphoscintigraphy, which typically produces grainy and low-resolution images.
Indocyanine green (ICG) has been used safely in humans for over 50 years on the basis of its dark green color for assessing hepatic clearance and diabetic retinopathy. Studies have shown that ICG associates with serum proteins, making ICG an excellent vascular contrast agent that can also be used on the basis of its fluorescent properties.9–11 When administered intradermally, ICG rapidly enters normally functioning lymphatic vessels. ICG is a very weak fluorescent dye compared to other NIR fluorescent dyes now available, and ICG has no reactive functional groups that would enable linking of molecularly targeting moieties, such as molecular therapeutic agents. Several other near-infrared fluorescent agents, such as HSA-IRDye800 CW (human serum albumin-IRDye800 CW)12 and albumin-binding domain-IRDye 800 (cABD-IRDye 800),11 have been developed, and offer much higher fluorescence intensity and chemical coupling potential.
The goal of this study was to evaluate the impact of ICG concentration in a mouse model that simulates our human studies.13,14 Because our FDA-approved investigational human studies are restricted to microdose administrations (25 ug ICG in 100 uL) to form a wheal or intradermal depot that drains into the initial lymphatics, we examined the effects of different concentrations of ICG on lymphatic pulsatile function in the mouse model. In addition, we explored the effects of different fluorescent dye entities on lymphatic pulsatile frequency.
Materials and Methods
Mice
10–12-week-old female C57BL/6 mice were obtained from Charles River (Wilmington, MA) and housed in an Association for Assessment and Accreditation of Laboratory Animal Care-approved facility, according to institutional guidelines. All animal protocols were reviewed and approved by the Institutional Animal Care and Use Committee at the University of Texas Health Science Center-Houston.
Fluorophores
Indocyanine green (ICG) was obtained from Akorn, Inc. (Lake Forest, IL) and diluted with sterile saline (Hospira, Lake Forest, IL) to 0.32, 0.50, 0.625, and 1.3 mM for use. IRDye800 CW was purchased from Li-Cor (Lincoln, NE) and diluted with sterile saline to 100 μM for use. Cyclic albumin-binding domain peptide (cABD)-IRDye800 was produced as follows, and was diluted with sterile saline to 100 μM for use. The cABD peptide, RLIEDICLPRWGCLWEDDK, with an added disulfide bond bridging the cysteines, was purchased from Bachem (Torrance, CA), stored as received at −20°C, and reconstituted immediately before use in Sorenson's Phosphate Buffer (pH 8–8.5). cABD peptide conjugation to IRDye800CW was performed by combining equimolar amounts of IRDye800CW in DMSO (dimethylsulfoxide) and cABD peptide in phosphate buffer. Conjugation occurred overnight at 4°C on a rotator platform under dark conditions. The conjugated cABD peptide was successfully purified using a semi-prep HPLC (high performance liquid chromatography) system equipped with a Zorbax column (C-18, 250×10 mm) using a mobile phase of 0.1% trifluoroacetic acid (TFA) in H2O/0.1% TFA in CH3CN (acetonitrile) gradient and flow rate of 1 mL/min. A retention time of 32.3 min was measured using EzChrome Elite software. The conjugate was then dried using a vacuum evaporator (Thermo Scientific Inc.), and stored at −20°C, protected from light.
Concentrations of IRDye800 CW and cABD-IRDye800 used were lower than the ICG concentrations, because previous measurements have shown that fluorescence yield is many times higher for IRDye and the cABD conjugate.
Lymphatic imaging agent injections
Mice were anesthetized with isoflurane, shaved, and covered with depilatory cream (Nair Sensitive) for 3 min. The cream was then rinsed off with warm water. Several days later, mice were anesthetized with isoflurane, and 10 μL per injection site of ICG, IRDye800 CW, or cABD-IRDye were injected intradermally with a 31-gauge needle/syringe (BD #328438, Fisher Scientific) at the base of the tail on both right and left sides of the mice (20 μL total per mouse). Images of lymphatics were then captured.
We used 12 animals per group for 0.32, 0.645, and 1.3 mM ICG. We did not pool left and right sides of each animal in these groups, in order to determine if there were sided or systemic effects on lymphatic function due to ICG concentration. Left- and right-side results in the measurements using different fluorescent dyes were pooled.
Imaging system
Images were acquired using a home-built system as described by Kwon and Sevick-Muraca.13,14 Images were analyzed with V++ software (Total Turnkey Solutions, Sydney, Australia). The integration time for fluorescence images was 200 milliseconds. 300 images were collected per side per mouse for lymph velocity and propulsive frequency measurements.
Data analysis/statistics
Images were loaded into ImageJ software (NIH), and fluorescence intensities were quantified and imported into Microsoft Excel for propulsive frequencies and velocities as previously described.5,15 Statistical significance for comparisons of propulsive frequencies was determined using ANOVA. Statistical significance for comparisons of propulsive velocities was determined using a linear mixed effects model.
Results
Typical near-infrared image of lymphatic vessels and propulsive data collection
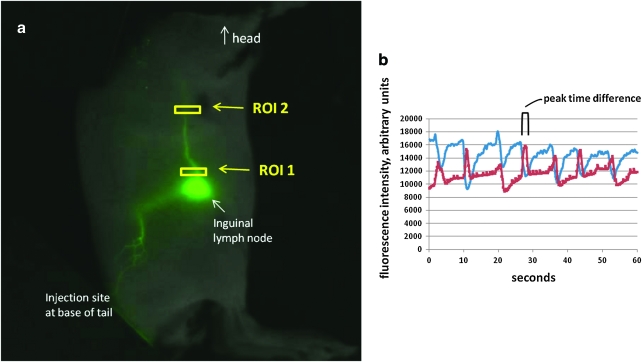
Figure 1a shows a typical fluorescent image of an inguinal-to-axillary lymphatic collector, overlaid with a white light image. The rear flank and inguinal-to-axillary lymphatic vessels appear green. Regions of interest (ROIs) are denoted with small boxes. Figure 1b is a graph of typical fluorescent intensity results as a function of time. Fluorescent intensities at two regions of interest (ROI) are measured, and lymphatic propulsions are represented by fluorescent intensity peaks. Propulsive velocities are calculated from the distance between peaks of the two ROIs (time) and the distance between the two ROIs.
FIG. 1.
Fluorescent imaging outputs. (a) Typical fluorescent image of inguinal-to-axillary collecting lymph vessel (flank lymphatics also appear) with regions of interest (ROIs) selected (yellow boxes). (b) Fluorescence intensity in arbitrary units plotted against seconds. ROI 1 values are shown in blue, and ROI 2 values are shown in red. Each peak represents one pulse. The difference between ROI 1 and ROI 2 time peaks is used to calculate lymph propulsive velocity. A color version of this figure is available in the online article at www.liebertpub.com/lrb.
ICG concentration does not affect lymphatic propulsive frequency or velocity
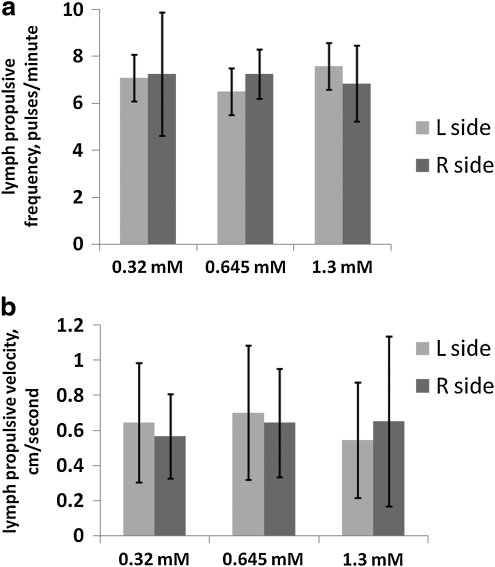
Figure 2 displays results of imaging mice using 20 uL per mouse of 0.32, 0.645, and 1.3 mM ICG as lymph imaging agent. Use of all three concentrations of ICG resulted in lymph propulsive velocities of 0.55–0.70 centimeters/second, with no significant differences, and lymph propulsive frequencies of 6.5–7.6 pulses/min, with no significant differences (n=12 mice per ICG concentration). Mean pulses per minute were 7.1, 6.5, and 7.6 for 0.32, 0.645, and 1.3 mM ICG, respectively, on the left sides of the mice (standard deviations were 2.2, 1.8, and 2.4, respectively), and 7.3, 7.3, and 6.9 for 0.32, 0.645, and 1.3 mM ICG, respectively, on the right sides of the mice (standard deviations were 2.6, 1.1, and 1.6, respectively). Mean pulsatile velocities were 0.65, 0.70, and 0.55 centimeters/second for 0.32, 0.645, and 1.3 mM ICG, respectively, on the left sides (standard deviations were 0.34, 0.38, and 0.33, respectively), and 0.57, 0.64, and 0.65 centimeters/second for 0.32, 0.645, and 1.3 mM ICG, respectively, on the right sides of the mice (standard deviations were 0.24, 0.31, and 0.48, respectively). Due to the large variances, the results are statistically analyzed, not statistically powered.
FIG. 2.
Lymphatic function is not affected by indocyanine green (ICG) concentration. (a) Lymphatic propulsive frequencies (pulses/minute) and (b) lymph propulsive velocities (centimeters/second) observed in inguinal-to-axillary postnodal efferent transport lymphatic vessels using 0.32, 0.645, and 1.3 mM ICG as fluorescent dye. Light gray bars denote left side values, while dark gray bars denote right side values. N=12 mice per group for 0.32, 0.645, and 1.3 mM ICG. No statistically significant differences between/among groups were found by linear mixed effects model (velocities) or ANOVA (frequencies).
Near-infrared fluorescent dye does not affect lymphatic propulsive frequency
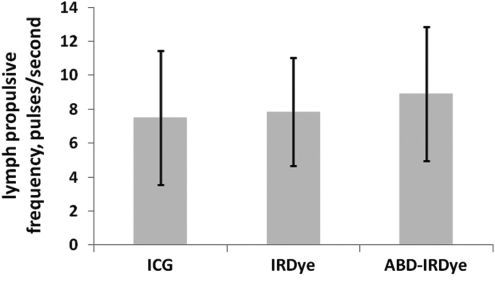
Figure 3 shows that the use of three different near-infrared agents (20 uL per mouse of 500 μM ICG, 100 μM IRDye800 CW, or 100 μM cABD-IRDye800) resulted in no significant differences in lymph propulsive frequencies, with values between 7.5 and 8.9 propulsions/minute (n=6–7). IRDye800 CW and cABD-IRDye800 displayed similar frequencies to that of 500 μM ICG, as well as to those of the concentrations of ICG used in Figure 2.
FIG. 3.
Lymphatic propulsive frequency is similar with different fluorescent dyes. Lymph propulsive frequencies (pulses/minute) in inguinal-to-axillary collecting lymphatic vessels using 500 μM ICG, 100 μM IRDye800 CW, and 100 μM cABD-IRDye as fluorescent dyes. N=6–7 mice per group. No statistically significant differences among groups were found by ANOVA.
Conclusions
Administration of any exogenous agent can possibly affect lymphatic function. We hypothesized that the ICG concentration and use of other NIR fluorophores would not affect our lymphatic imaging results. Using an intact inguinal-to-axillary mouse model, we found no significant differences in lymphatic propulsive frequency or lymphatic propulsive velocity when ICG concentration was changed, or if we used cABD-IRDye800 or IRDye800, in this model.
The lymphatic motility we observe with NIR fluorescence in this model is not consistent with the phasic contractile activity documented in most species, since the second signal peak (at the second ROI) depicts a rapid increase in the integrated fluorescence signal. This motility may represent a bolus movement of fluid ejected from the node or lymphatic near the node, perhaps coupled with a myogenic constriction similar to those experienced by the intestine associated with the bulk movement via peristalsis, or may indicate the propagation of a bolus of lymph propelled downstream in a highly compliant vessel without any active contraction involvement. The concentrations of ICG used in this study, however, did not produce a dose-dependent change in frequency or velocity of lymph movement in this model. Only the use of other in vivo imaging modalities that do not use fluorescent dyes and excitation can verify that our results are valid. Unfortunately, no such imaging methods presently exist. Nonstriated myocytes of tunica media around the basal lamina on which LECs (lymphatic endothelial cells) rest in lymphatic collectors, ducts, and trunks contract spontaneously approximately 6–12 times per minute.16 In this study, we found pulsing of approximately 6–7 pulses per minute in the inguinal-to-axillary postnodal efferent transport lymphatic vessels of mice. We have also imaged human lymphatics using ICG as the fluorescent agent, and have measured propulsive velocity rates ranging from 0.4 to 1.3 cm/second and propulsive frequencies between 0.7 and 3.3 pulses/min in healthy human controls.9 The imaging is conducted over relatively long periods of time (2 hours). During that time, we see continuous pulsing of lymph. While intradermal injection is responsible for creating a wheal, and increased local interstitial pressure may be responsible for influx of dye into the lymphatics, the technique nonetheless has enabled us to detect changes in pumping function far away from the site of dye injection in humans, mice, and swine.
Our quantitative analysis of lymphatic pumping is limited to lymphatic propulsive function (or apparent velocity), not volume flow, since our images are two-dimensional, and the flow is a result of periodic propulsions. To date, with the exception of lymphoscintigraphy, from which one can compute an effective transit velocity from “time-to-groin” measurements,” there is no other published method to assess lymphatic volume flow noninvasively.
It is likely that the published results by another group17 showing changes in lymphatic function with varying ICG concentrations could be attributed to the use of very different experimental conditions than ours. The other group perfused ICG through ex vivo isolated mesenteric rat vessels at a fixed pressure and an imposed flow, while our studies noninvasively measured murine in vivo peripheral, inguinal-to-axillary postnodal efferent transport. Mesenteric and peripheral lymphatic vessel contractile activities are very likely different,18 so the results of the other study are difficult to compare to ours.
Our studies used irradiated ICG, by necessity, for NIR imaging. A previous study showed laser-induced photo-oxidation and cellular uptake of ICG using much more intense irradiation than our studies (80 mW/cm2 of 805 nm light versus our 1.5 mW/cm2).19 It is most likely that laser irradiation alone induced free radical damage in the previous study, since ICG's fluorescence lifetime is 0.58 nanoseconds, and achieving the triplet state of an electron for free radical generation requires milliseconds. Photo-oxidation of tissue by ICG alone, then, is improbable. A study using laser illumination of skin or muscle as an adjuvant for vaccination found little alteration in skin histology, and no overt cell death or leukocyte infiltration using 0.3 W of 532 nm laser for 2 min,20 so it is unlikely that the levels of laser irradiation used in our studies, which were 200 times lower, caused overt lymphatic tissue alterations.
Three recent articles, “A novel method of measuring human lymphatic pumping using indocyanine green fluorescence lymphography,”21 “Quantitative imaging of lymphatic function with liposomal indocyanine green,”22 and “Characteristic indocyanine green lymphography findings in lower extremity lymphedema: The generation of a novel lymphedema severity staging system using dermal backflow patterns”23 provide evidence that ICG is being used as a tool to assess lymphatic function by groups other than our own. Our studies and these by other groups illustrate the importance of determining fluorophore effects on lymphatic function. In our studies using a mouse model that simulates our human studies, ICG concentration, as well as the use of other NIR fluorophores, did not affect lymphatic function. Using this NIR technique developed in our laboratories, we have shown its use to direct therapy17 as well as to detect improvement in lymphatic function immediately after therapy.6,8 Noninvasive methods to investigate the lymphatic system can provide new avenues to discovery, diagnosis, and emerging therapies of lymphatic disorders and diseases.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Kirkman E. Sawdon M. Capillary dynamics and interstitial fluid lymphatic system. Anaesth Intensive Care Med. 2004;5:38–42. [Google Scholar]
- 2.Sevick-Muraca EM. Rasmusssen JC. Molecular imaging with optics: Primer and case for near-infrared fluorescence techniques in personalized medicine. J Biomed Opt. 2008;13:041303. doi: 10.1117/1.2953185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Unno N. Suzuki M. Yamamoto N. Inuzuka K. Sagara D. Nishiyama M. Tanaka H. Konno H. Indocyanine green fluorescence angiography for intraoperative assessment of blood flow: A feasibility study. Eur J Vasc Endovasc Surg. 2008;35:205–207. doi: 10.1016/j.ejvs.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 4.Rasmussen JC. Tan I-C. Marshall MV. Fife CE. Sevick-Muraca EM. Lymphatic imaging in humans with near-infrared fluorescence. Curr Opin Biotechnol. 2009;20:74–82. doi: 10.1016/j.copbio.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rasmussen JC. Tan I-C. Marshall MV. Adams KE. Kwon S. Fife CE. Maus EA. Smith LA. Covington KR. Sevick-Muraca EM. Human lymphatic architecture and dynamic transport imaged using near-infrared fluorescence. Transl Oncol. 2010;3:362–372. doi: 10.1593/tlo.10190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tan I-C. Maus EA. Rasmussen JC. Marshall MV. Adams KE. Fife CE. Smith LA. Chan W. Sevick-Muraca EM. Assessment of lymphatic contractile function following manual lymphatic drainage using near-infrared fluorescence imaging. Arch Phys Med Rehabil. 2011;92:756–764.e1. doi: 10.1016/j.apmr.2010.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maus EA. Tan I-C. Rasmussen JC. Marshall MV. Fife CE. Smith LA. Guilliod R. Sevick-Muraca EM. Near-infrared fluorescence imaging of lymphatics in head and neck lymphedema. Head Neck. 2012;34:448–453. doi: 10.1002/hed.21538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adams KE. Rasmussen JC. Darne C. Tan I-C. Aldrich MB. Marshall MV. Fife CE. Maus EA. Smith LA. Guilliod R. Hoy S. Sevick-Muraca EM. Direct evidence of lymphatic function improvement after advanced pneumatic compression device treatment of lymphedema. Biomed Opt Express. 2010;1:114–125. doi: 10.1364/BOE.1.000114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marshall MV. Rasmussen JC. Tan I-C. Aldrich MB. Adams KE. Wang X. Fife CE. Maus EA. Smith LA. Sevick-Muraca EM. Near-infrared fluorescence imaging in humans with indocyanine green: A review and update. Open Surg Oncol J. 2010;2:12–25. doi: 10.2174/1876504101002010012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cherrick GR. Stein SW. Leevy CM. Davidson CS. Indocyanine green: Observations on its physical properties, plasma decay and hepatic extraction. J Clin Investig. 1960;39:592–600. doi: 10.1172/JCI104072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davies-Venn CA. Angermiller B. Wilganowski N. Ghosh P. Harvey BR. Wu G. Kwon S. Aldrich MB. Sevick-Muraca EM. Albumin-binding domain conjugate for near-infrared fluorescence lymphatic imaging. Mol Imaging Biol. 2011 Jun 18; doi: 10.1007/s11307-011-0499-x. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ohnishi S. Lomnes SJ. Laurence RG. Gogbashian A. Mariani G. Frangioni JV. Organic alternatives to quantum dots for intraoperative near-infrared fluorescent sentinel lymph node mapping. Mol Imag. 2005;4:172–181. doi: 10.1162/15353500200505127. [DOI] [PubMed] [Google Scholar]
- 13.Kwon S. Sevick-Muraca EM. Noninvasive quantitative imaging of lymph function in mice. Lymph Res Biol. 2007;5:219–231. doi: 10.1089/lrb.2007.1013. [DOI] [PubMed] [Google Scholar]
- 14.Kwon S. Sevick-Muraca EM. Functional lymphatic imaging in tumor-bearing mice. J Immunol Meth. 2010;360:167–172. doi: 10.1016/j.jim.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sharma R. Wendt JA. Rasmussen JC. Adams KE. Marshall MV. Sevick-Muraca EM. New horizons for imaging lymphatic function. Ann NY Acad Sci. 2008;1131:13–36. doi: 10.1196/annals.1413.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilting J. Becker J. Buttler K. Weich HA. Lymphatics and inflammation. Curr Med Chem. 2009;16:4581–4592. doi: 10.2174/092986709789760751. [DOI] [PubMed] [Google Scholar]
- 17.Gashev AA. Nagai T. Bridenbaugh EA. Indocyanine green and lymphatic imaging: Current problems. Lymph Res Biol. 2010;8:127–130. doi: 10.1089/lrb.2010.0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gashev AA. Davis MJ. Delp MD. Zawieja DC. Regional variations of contractile activity in isolated rat lymphatics. Microcirculation. 2004;11:477–492. doi: 10.1080/10739680490476033. [DOI] [PubMed] [Google Scholar]
- 19.Abels C. Fickweiler S. Weiderer P. Baumler W. Hofstadter F. Landthaler M. Szeimies RM. Indocyanine green (ICG) and laser irradiation induce photooxidation. Arch Dermatol Res. 2000;292:404–411. doi: 10.1007/s004030000147. [DOI] [PubMed] [Google Scholar]
- 20.Chen X. Kim P. Farinelli B. Doukas A. Yun SH. Gelfand JA. Anderson RR. Wu M.X. A novel laser vaccine adjuvant increases the motility of antigen presenting cells. PLoS One. 2010;5:e13776. doi: 10.1371/journal.pone.0013776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Unno N. Nishiyama M. Suzuki M. Tanaka H. Yamamoto N. Sagara D. Mano Y. Konno H. A novel method of measuring human lymphatic pumping using indocyanine green fluorescence lymphography. J Vasc Surg. 2010;52:946–952. doi: 10.1016/j.jvs.2010.04.067. [DOI] [PubMed] [Google Scholar]
- 22.Proulx ST. Luciani P. Derzsi S. Rinderknecht M. Mumprecht V. Leroux JC. Detmar M. Quantitative imaging of lymphatic function with liposomal indocyanine green. Cancer Res. 2010;70:7053–7062. doi: 10.1158/0008-5472.CAN-10-0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamamoto T. Narushima M. Doi K. Oshima A. Ogata F. Mihara M. Koshima I. Mundinger GS. Characteristic indocyanine green lymphography findings in lower extremity lymphedema: The generation of a novel lymphedema severity staging system using dermal backflow patterns. Plast Reconstr Surg. 2011;127:1979–1986. doi: 10.1097/PRS.0b013e31820cf5df. [DOI] [PubMed] [Google Scholar]