Abstract
Regenerative therapies possess high theoretical potential for medical advance yet their success as commercial therapeutics is still open to debate. Appropriate data on target opportunities that provide perspective and enable strategic decision making is necessary for both efficient and effective translation. Up until now, this data have been out of reach to research scientists and many start-up companies—the very groups currently looked to for the critical advance of these therapies. The target-based estimate of opportunity presented in this report demonstrates its importance in evaluating medical need and technology feasibility. In addition, analysis of U.S. research spending, productivity, and innovation reveals that U.S. basic research in this field would benefit from greater interdisciplinarity. Overcoming the barriers that currently prevent translation into high value therapies that are quickly clinically adopted requires simultaneous integration of engineering, science, business, and clinical practice. Achieving this integration is nontrivial.
Introduction
In 2009, demonstrating the value of medical innovation was identified as the primary risk factor in life science development going forward.1 There is an increasing challenge in biotechnology and pharmaceutical research to not only push into new technologies but also translate those efforts into true medical breakthroughs.
The pressing need for innovative therapies in pharmaceutical pipelines,2 the move to more personalized medicine as an area for future pharmaceutical company growth,3 and the growing interest in orphan indications4 suggest that the time is right for regenerative therapies. The tissue engineering or broader regenerative medicine, and regenerative medicine, and stem cell research (RMSCR) fields have been long viewed by governments5,6 and researchers as areas where the potential for medical breakthroughs is high.
Interestingly, commercial translation remains significantly less than many other areas of biotechnology and medical devices. Over the last 25 years, large medical device and pharmaceutical companies have struggled to generate a return on investment in regenerative therapies. While many companies wait on the sidelines for a clearer indication that regenerative therapies are medically and commercially feasible, it would behoove those engaged in the field to determine the underlying reasons for the differences between expectations and deliverables. Manufacturing and regulatory pathways for regenerative therapies are less clear than for traditional pharmaceuticals and the fit between large company core competencies and the needs of regenerative therapy development is weak. As a new technology area, RMSCR still represents uncharted territory for most pharmaceutical and medical device companies. This implies that not only must the scientific basis be sound, but also the supporting technology, medical use, and commercial strategy must be developed enough to seem plausible in a field where some issues will be addressed for the first time. Any disruptive technology faces the same challenges that RMSCR faces, and addressing them head on is, we believe, the most effective way to drive progress forward. Fortunately interest in RMSCR has existed for quite some time, and a wealth of science and technology to build upon has been generated.
The U.S. clinical use of laboratory-grown cells as transplants dates back to the 1980s.7 The first living engineered tissue gained U.S. Food and Drug Administration (FDA) approval in 1998.8 Also, beginning in 1998, the possibilities for regenerative therapies progressively expanded with the ability to grow human embryonic stem cells,9 the realization that adult stem cells retained multipotency,10 and now, the technical developments of being able to induce pluripotency in mature adult cells by a variety of methods.11–13
From a translational perspective, the most valuable data is actionable—capable of driving a science forward, not simply expanding it outward. Over the last decade, we have primarily seen the expansion of possibilities in RMSCR, particularly in the stem cell area. However, the reality of translation is that not every possibility will be equally feasible or reasonable. Eventually, a line of effort must focus on meeting a specific medical objective that also fulfills commercial requirements. Not all objectives will be of equal medical importance or value. We hypothesize that a clearer understanding of the merits of those objectives early on will increase the efficiency and effectiveness of translation from the start.
As we discuss herein, highly pertinent data on target opportunities, where indications can be studied in perspective, can be a valuable tool in translational RMSCR research. This type of information is generally unavailable to most research scientists and small start-up companies. A recent survey of those involved in tissue engineering in academic and industry listed “orienting research to market needs” as the second most difficult self-ranked hurdle for academics, and “maintaining focus in an evolving market” as the most difficult for development-stage companies.14 A 2008 report by the U.K.'s Bioscience Innovation & Growth Team (BIGT)6 noted that “industry cannot deliver continued healthcare improvements by itself” and that a cultural shift in university biomedical research where translation of research and business engagement becomes a core mission is important both to the future of bioscience and economic development. In our view, it is not a matter of limiting scientific freedom or abandoning basic research for mundane practicality or purely commercial considerations, but rather acknowledging the most suitable endpoints and challenging even those in basic research to be cognizant of the medical targets in a way that helps them to look ahead to the pivotal issues that will either enable or prevent translation. As we learn more it will be vital to also point out where basic research can play a key role, and to couple that to further developments.
This report is not meant to be encyclopedic nor an exhaustive list of all possible applications of tissue engineering or stem cells. Rather, this first report in our series of studies is meant to supply important objective data in perspective regarding the medical need for regenerative therapies in the strictest sense, that is, for the most part homologous therapies that are formed or work through a regenerative mode of action to repair, restore, enhance, or replace lost tissue function. This report is intended to act as a stimulant for discussion of these issues and the sharing of critical data.
The underlying premise of the first objective of this study is that effective translation of science and medicine requires the identification of appropriate targets in a way that enables strategic decisions. Therefore our first goal was to study disease and procedure incidence of regenerative therapy targets in a way that (1) provided a true estimate of the medical need for a regenerative therapy within a diagnosis or procedure group, (2) could be used to determine the target population within that group, (3) could be used to better match a treatment approach to the medical need, and (4) could illuminate the opportunities for leveraging scientific knowledge, expertise, and technology (likely to be a key factor in achieving a sufficient return on investment for many RMSCR applications).
The impetus for research and innovation in RMSCR currently falls on government funding and academic science in the United States. Therefore, the second goal of our study was to assess the current status of U.S. RMSCR research and innovation using newly available government data. This data can objectively measure the present level of scientific productivity and innovation in relevant government-funded RMSCR research. Of interest was determining how U.S. basic research matched the principal medical needs identified in the estimate of opportunity analysis (EOA). By making data from a detailed EOA specific to regenerative therapies broadly accessible and establishing a baseline of current regenerative medicine output and innovation, we would now be in a position to test our hypothesis that a clearer perspective of the relative target opportunities can improve the connectivity between scientific, medical, and commercial objectives. Establishing this connectivity will be vital to the future of regenerative medicine since the ultimate value to society lies in what benefits the resulting therapy will mean for the patient. The ability to get the therapy to the patient who needs it requires commercial feasibility, or at the very least financial feasibility.
Going forward our plan is to develop metrics that can objectively measure changes in this connectivity and its impact on productivity and commercial translation over time. To further test the benefits of this knowledge and foster the evolution of how regenerative medicine is tackled, we will continue to develop more in-depth opportunity analyses in key medical areas identified by the EOA. In the second phase of our work we are studying the gap between regenerative technology and specific medical needs as well as the state of the science across the multiple disciplines likely to be needed to close these gaps. Our hypothesis is that this growing body of knowledge and information will make it ever easier to recognize not only the best medical and commercial opportunities for regenerative therapies but also the best and most efficient way forward.
Methods
Estimate of target opportunities
Limits to the incidence data
The estimate of target opportunities, while comprehensive, does not attempt to quantify the entire market for RMSCR and everything cells and/or biomaterials could be used for. Rather, we focused on the opportunities where cells and/or biomaterials might be used to enhance, augment, restore, regrow, and re-establish normal tissue, which we refer to as “regenerative therapies” in recognition of their principal biological action. For this reason, the use of cells simply as drugs or in nonhomologous applications, such as their use for the purpose of immune suppression, was considered beyond the scope of the current study even though we recognize it as an active area of research with several potential medical uses.
In addition, because we have relied on data from the National Hospital Discharge Survey (NHDS), incidence analysis is limited to disorders requiring hospitalization. Therefore disorders treated on an outpatient basis will not be captured in the NHDS data, such as many wound healing procedures not requiring skin grafting and most ophthalmic procedures.
To collect relevant NHDS data for regenerative therapies required, forming a detailed list of regenerative targets based on International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) codes down to billable code. We recognize that ICD-9-CM codes are by nature not patient specific, but they represent an excellent starting point for differentiating between clinically adoptable opportunities. Most therapies that could in some way enable or achieve tissue repair and regeneration were included without distinction of type of technology that is, or could be, used to achieve the therapeutic goal. However, the scope of the opportunity analysis was limited to targets that could be helped through a regenerative mechanism. Due to the size of the study, analysis of the intersection of regenerative medicine and cancer was not included. In addition, discussion of the medical application of stem cell therapies often includes the treatment of inflammatory and autoimmune disorders, but their different biologic, mechanistic, and therapeutic considerations also necessitated placing these disorders outside the scope of the current study. Nevertheless, our more stringent parameters included activities currently categorized as regenerative medicine, tissue engineering, and stem cell therapy—encompassing opportunities for factors, biological matrices, cells, and combination products.
Discharges are reported in the NHDS by first-listed diagnosis, which is also the principal diagnosis and primary reason for hospitalization. Up to seven diagnoses and up to four procedures are listed for each discharge. Because many of the treatment targets are as yet unmet, incidence based on principal diagnoses alone may list as treatment of sequelae rather than the treatment target, which may only appear as a secondary diagnosis. To allow for this possibility, data for the incidence of culled ICD-9-CM codes captured both first- and second-listed (secondary) diagnoses. Data for all listed diagnoses were collected for comparison.
Medical information about patients includes diagnoses and procedures coded using the ICD-9-CM codes. ICD-9-CM codes were reviewed down to billable code in both diagnosis and procedure categories and culled of codes of minimal relevance to use of a regenerative therapy. Estimates of incidence for the remaining ICD-9-CM codes were made using the Center for Disease Control's National Center for Health Statistics database. Estimates of incidence were captured for first and second diagnoses and all diagnoses. The NHDS is a national probability survey designed to meet the need for information on characteristics of inpatients discharged from nonfederal short-stay hospitals in the United States. From 1988–2007 the NHDS collected data from a sample of ∼270,000 inpatient records acquired from a national sample of about 500 hospitals through 2006. Only hospitals with an average length of stay of fewer than 30 days for all patients, general hospitals, or children's general hospitals are included in the survey.
The design of the NHDS requires that the survey data be inflated or weighted to produce national estimates.15 There are three components to the final weight: inflation by reciprocals of the probabilities of sample selection, adjustment for nonresponse, and population weighting ratio adjustments. A detailed explanation of how NHDS data is gathered and calculated can be found in Dennison et al.16 The size of the treatment opportunity was calculated for years 2004–2006 using the SAS Ver. 9.2 (Cary) statistical programming package. The findings and conclusions derived from this data are those of the authors and do not necessarily represent the views of the Research Data Center, the National Center for Health Statistics, or the Centers for Disease Control and Prevention.
Evaluation of basic research productivity and innovation
Data sets on National Institutes of Health (NIH) research spending were obtained using a beta version of the NIH Research Portfolio Online Reporting Tools (RePORT) and an additional RePORT data summary on NIH Estimate of Funding.17 Data sets were downloaded and analyzed using Microsoft Excel. The correlation of different parameters within the NIH data sets was estimated using the correlation coefficient function in Microsoft Excel. In addition to detailed project information, data on patents citing support from the listed projects and publications citing support from the listed projects were collected from the RePORT query. It was noted that, in the RePORT data, publications citing support from more than one project counted as a publication for each project so the number of total publications did not represent an absolute number of articles but rather a measure of scholarly output from the research. Since publications are the primary measure of scholarly output, we believed that this measure was necessary and despite its limitations, identified overall productivity and trends even though counting some publications more than once.
While the use of patents as a direct measure of innovation can be debated, patents represent inventions that are sufficiently novel or innovative over state-of-the-art. Patent protection is important to commercialization. Patents are a necessary component protecting a company's technology as well as freedom to practice. It is therefore an important component in funding of commercial development, from venture capital and from government programs like the Small Business Innovation and Research Grants as well as interest by larger companies and potential partners. Therefore some measure of patents was important in the evaluation of U.S. productivity and innovation since our focus was on commercial translation. The RePORT patent data for the data sets appeared to contain prior patents related to the listed research or investigator, not exclusively inventions funded during the 2008–2009 period and as such more accurately represented the intellectual property foundation being built upon rather than a specific number of inventions stemming from 2008–2009 funding. The RMSCR data set was created by querying the NIH spending categories Regenerative Medicine and Stem Cell Research for years 2008 and 2009 across all NIH institutions. Since each research grant can fall into multiple categories, Regenerative Medicine and Stem Cell Research categories overlapped but neither was all inclusive. Tissue engineering is not listed as a category, likely usurped by the broader Regenerative Medicine category. Because we wished to collect data specific to tissue engineering, we also queried the database using either the key term “tissue engineering” or, for comparison, “stem cells” in years 2008–2009 across all spending categories.
The subset of most active RMSCR institutions was obtained by selecting for institutions within the RMSCR group with 10 or more distinct projects during 2008–2009 and ≥$10 million dollars in total funding for the 2 years. Cancer research institutions were not included. Universities with more than one site were combined with the exception of the University of California, because of their size, were kept as individual institutions except as noted below. Institutes with a primary focus on cancer research were excluded from the most active list. For comparison, funding data from the “tissue engineering” and “stem cells” data sets were pulled for each institution identified as most active within the RMSCR group.
Pending U.S. patent applications for the field, as well as each of the most active RMSCR institutions was obtained using a U.S. Patent and Trademark Office online patent search and key terms. Pending patent data could not be distinguished for individual sites within a university system and so RePORTer data for the University of California institutions were combined where projected patents were analyzed. RMSCR patent application data from 2001 to September 2010 were obtained from the U.S. Patent and Trademark Office Web site (http://patft.uspto.gov) by performing a search of all patent applications containing in their title or abstract—one or more of the following Boolean search terms: “regeneration,” “stem cell(s),” “tissue engineered,” “tissue repair,” “tissue regeneration,” “progenitor,” “organ repair,” “biological scaffold,” or “tissue scaffold” with and without an institution as the assignee.
Results
Results of the estimate of opportunity analysis
Analysis of the data was performed using a top-down approach to establish and maintain perspective on the relative nature of the target opportunities. Our goal was to objectively determine the relative extent of the opportunities for regenerative therapies in hospitalized patients letting the numbers be our guide. Summing relevant primary and secondary diagnoses and procedures (Fig. 1), there are over 8 million discharged patients per year in the United States who are candidates for a regenerative therapy as defined in this study.
FIG. 1.
Overview of the estimate of opportunity for regenerative therapies. The data estimate the relative opportunity for regenerative therapies in U.S. hospitalized patients across major medical areas for years 2004–2006. Data source: National Hospital Discharge Survey, Centers for Disease Control and Prevention, National Health and Human Services.
Cardiovascular and peripheral vascular targets were combined as a Cardio/Vascular group because many regenerative therapies could be leveraged to meet targets in both. Total incidence within the broad disease categories was sorted into four tiers (Fig. 1A). Incidence ranked Cardio/Vascular as the primary area of opportunity (43%–45%) followed by musculoskeletal diseases (29%), central and peripheral nerve diseases (11%–12%), and genetic and metabolic disorders (9%), represented a second tier. Integument and congenital disorders represented 2%–4%. Opthalmic targets of opportunity that do not currently result in hospitalization would not be captured in this analysis. Clearly, vision restoration is likely to be a significant target for development but its study requires data beyond the scope of this analysis. This is also true for other indications that maybe largely treated in an outpatient setting.
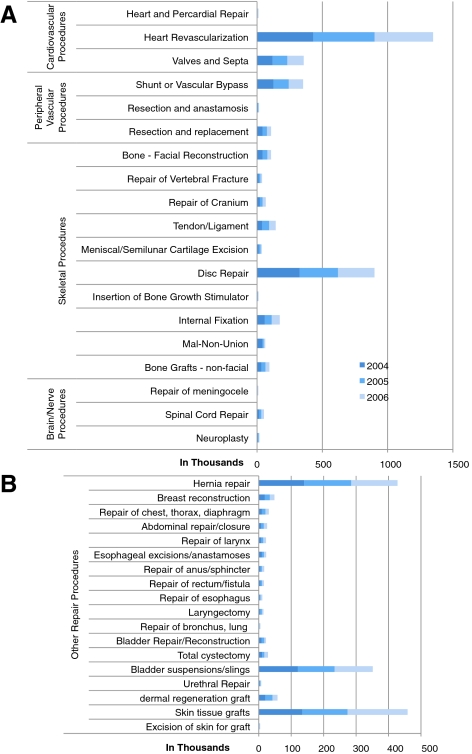
Procedures that could be improved or better enabled by a therapy that stimulates regeneration, or the restoration of functional tissue in the major areas of cardio- and peripheral vascular disease, skeletal, and brain and nerve-related repair numbered over 5 million over the 3-year period between 2004 and 2006 (Fig. 2A) with approximately another 500,000 other assorted soft tissue repair procedures per year (Fig. 2B). The NHDS incidence of liver and pancreas transplantation for genetic and metabolic diseases varied widely year to year, markedly increasing in 2006 to 4,598 liver transplant procedures and 3,660 pancreas transplant procedures (data not shown), perhaps due to new surgical innovations. Pancreatic islet transplants were estimated at 759 in 2006.
FIG. 2.
The incidence of hospital procedures across major medical areas. The graph and table show the relative incidence of U.S. hospital procedures applicable to regenerative therapies across major medical areas for years 2004–2006. (A) Relative incidence of the most prevalent repair procedures. (B) Relative incidence of the less prevalent repair procedures.
The top-down approach identified the areas with the largest concentration of target opportunities and prompted us to begin to examine the opportunities making up the top two tiers in more detail. Several considerations that could impact the clinical adoption of a regenerative therapy came to light.
Cardiovascular and peripheral vascular disease
As might be expected, problems of the heart and vasculature represented the largest target areas for regenerative therapies (Fig. 3A). Cardiovascular targets make up 96% of the opportunity although the incidence of peripheral vascular targets is still significant. Heart failure is the most prominent target within ischemic heart disease (Fig. 3B). Treatment or prevention of heart failure represents major treatment opportunities for stem cell therapy if the therapy would be able to stop progression of cardiac degeneration after acute myocardial infarction (AMI) or would be able to regenerate functional heart muscle in degenerated, scarred, and weakened myocardium, respectively—both different biological targets with different levels of scientific and medical challenge.18–20
FIG. 3.
An estimate of cardiovascular and peripheral vascular treatment opportunities in U.S. hospitalized patients for years 2004–2006. (A) Relative incidence of cardiovascular and peripheral vascular disorders. (B) Breakdown of cardiovascular incidence. (C) Estimate of regenerative drug and device (surgical) opportunity in peripheral vascular disease. AMI, acute myocardial infarction.
For effective translation, consideration of the medical need is as important as overcoming a technological challenge. The EOA data revealed that the current management of acute cardiovascular disease appears to work well. Ninety-seven percent of all cardiac disease patients from 2004 to 2006 were discharged alive, but of those, 11% were discharged to long-term care facilities and 65% of the long-term care patients had a primary or second-listed diagnosis of heart failure representing over 1.1M discharges over the 3-year period. Patients within the long-term care group would likely be in most need of a regenerative therapy capable of restoring functional cardiac muscle. The benefit of early intervention would be reduction of the rate and/or incidence of progression to heart failure. Given what appears to be the successful management of acute cardiac disease, administration of cardiac repair cells at the time of AMI should limit the progression to heart failure to be of ultimate value to the patient and medical system, not simply improve the left ventricular ejection fraction short term. As the EOA data clearly indicate, the greatest medical benefit for a regenerative therapy will not be in the short term for most cardiac disease patients.21 This may pose a challenge for products approved based on near-term endpoints like improvement of ventricular function without a connection to long-term outcomes and may effect reimbursement and the rate of market acceptance until clinical data demonstrating the therapy's ultimate value is either empirically gathered over time, or helped by preclinical models that can establish the connectivity between near- and long-term outcomes. These challenges will not be unique to the cardiovascular space.
Further stratification of the peripheral vascular opportunity into potential drug or cell therapy targets versus potential vascular graft targets was done by combining the incidence of generalized symptoms, pain, and intermittent claudication, or ulceration, gangrene, and bypass grafting, respectively (Fig. 3C). Peripheral vascular disease represents a significant target opportunity for tissue engineering if it is able to deliver a surgically acceptable, small-caliber vascular graft of sufficient length that retains long-term patency. While cell-based therapy to stimulate revascularization and small-caliber grafts may reach a different primary demographic within the target population, they may also be synergistic if the efficacy of the combined treatments justifies the costs. An important return on investment consideration for vascular graft technology is that vascular tissue engineering processes and expertise might also be leveraged to address mid- to large-caliber graft opportunities in vascular access, for example. Indeed, vascular access may be the first medical indication for these grafts and could serve as an important step in gaining regulatory and clinical acceptance of the technology. However as products, biologic therapies targeting vascular access will have to deliver substantially better performance and/or outcome than currently used devices and processed tissues to justify a higher cost in most cases. The EOA data estimate that there are 600,000 or more hemodialysis discharges a year. Drilling further into the EOA data reveals that AV grafts number ∼33,000 per year and that there are 12,000 to 18,000 discharges for revision of arteriovenous grafts per year, which seems an obvious first-target population. However, the greatest medical value may be in providing an easier and faster alternative to the medically preferred arteriovenous fistula and reducing the large number of complication-plagued venous catheters used.22 The opportunities for the application of tissue engineering and stem cells in revascularization, bypass, and vascular access will be covered in greater detail in Part II of this series of reports that compares detailed incidence data with a gap analysis of medical needs.
The most prominent endocardial repair procedure is the replacement of heart valves. An estimated 80,000 valve replacements are performed per year, 70,000 of those are tissue valves. Implanted porcine valves can last up to 30 years,23 creating a significant barrier to entry for any new tissue technology. Where current tissue valves meet less of the medical need is in the pediatric patient where the performance of mechanical valves is poor and tissue autografts (which can grow) or human allografts are used.24 The ideal valve alternative would mitigate the need for extended use of anticoagulants and the need for progressive valve replacements as the child grows. EOA data estimate the total incidence of congenital valve defects for 2004–2006 at between ∼16,000 and just over 22,000 based on primary and second-listed diagnoses, significantly less than the adult incidence. Heart valve replacement in a young child is a high-risk procedure.23 In a study of 146 patients under 18 years of age, freedom from reoperation was 54%±8.1% for homograft and 68%±11.1% for autograft (the Ross procedure using pulmonary valve autograft) at 15 years.24
One difficulty in proving the superior benefit of human valves compared to prosthetics is that in this patient population, experience with prosthetic valves has shown that the patient is a larger determinant of long-term outcome than the device.24 This could be an even greater hurdle for an engineered tissue graft compared against an allograft where differences comparing tissue against tissue may be subtler. This suggests that even a technically perfect tissue engineered valve would meet with several challenges beyond the science and engineering. It may help to explain why the outstanding science and engineering performed to date on valve engineering has not proceeded to clinical implementation.
To summarize, cardiovascular disease represents the most significant area of opportunity for regenerative therapies. Heart failure is the most prominent opportunity within the cardiovascular space. The development of small-caliber vascular grafts, while justified based on incidence alone, may have greater applicability in indications other than coronary bypass grafting and replacement of heart valves will be particularly challenging clinically and commercially.
Musculoskeletal diseases
The musculoskeletal category encompasses bone and cartilage repair as well as tendon and ligament repair, and connective tissue disease. Many of the opportunities within this group could be addressed both by cell-based therapy and acellular biomaterials as well as combination products developed with tissue engineering.
Arthritis was the most prominent area of opportunity within the broad category, followed by the incidence of vertebral disc disorders and osteoporosis. A composite of the subgroups and EOA data for arthritis and joint applications is shown in Figure 4. The arthritis target opportunity was overwhelmingly made up by osteoarthritis. All listed incidence of osteoarthritis averaged 1.8 million discharges per year supporting published reports on prevalence that project an unmet and growing medical need.25 Osteoarthritis also overshadowed the incidence of nonarthritic joint disorders. This suggests that repair of articular cartilage is the most prominent orthopedic opportunity over tendon and ligament repair and well above meniscal disorders and repair.
FIG. 4.
A composite of the treatment opportunities for regenerative therapies to treat musculoskeletal disorders in U.S. hospitalized patients for years 2004–2006. (A) Comparative incidence across major orthopedic areas. (B) The incidence of osteoarthritis in all discharged patients, and those listing osteoarthritis as the first or second disorder upon discharge. (C) Estimates of tendon, ligament, and joint disorders. (D) Estimate of meniscal disorders and procedures. Note that the data do not include procedures done on an outpatient basis.
Examining other orthopedic and skeletal procedures, EOA data clearly identified the repair of the vertebral discs as the most prominent skeletal procedure (Fig. 2A) mirroring the medical need. In contrast, nonunions and malunions, a likely second target population for a regenerative bone product ranged from 10,000 to 40,000 per year (Fig. 2A). Two FDA-approved regenerative bone products containing bone morphogenetic proteins (BMP-2 bone graft [InFUSE®; Medtronic]; a BMP-7 collagen putty [OP-1®; Stryker]) are used as part of several repair procedures, most notably spinal fusion (a treatment for disc disorders). These therapies aid in achieving fusion of the vertebrae to reduce pain but do not repair or replace the vertebral disc. The Charité artificial disc (DePuy Spine) is a medical device to replace the disc and others are in development. Although not perfect solutions, both types of treatment would be medical competitors to a regenerative disc therapy. A more substantive examination of the medical need and opportunity in arthritis and spine applications will be detailed in Part III of this series, which reviews the detailed EOA in orthopedics and spine with a gap analysis of the medical needs as well as the state of the science and current barriers to translation.
In summary, two cartilaginous tissues make up the major opportunity in orthopedics and skeletal procedures: the regeneration of articular cartilage and the repair and regeneration of the intervertebral disc. Limiting and/or repairing the damage caused by osteoarthritis constitutes a major opportunity for regenerative therapies. The medical needs in major joint and intervertebral disc disorders are currently addressed by devices, and in the case of the intervertebral disc, a device/biologic combination is used. A likely question will be whether regenerative therapies can delay the use of these devices or obviate the need for them all together.
Central and peripheral nerve disorders and stroke
Recovery of function following stroke represents the most prominent treatment opportunity in brain and nerve disorders (Fig. 5A). The incidence of late functional deficits, including hemiplegia and hemiparesis (Fig. 5B), is ∼10% of the incidence of cerebral infarction. Regenerative therapies are targeting recovery from motor and mental deficits through either support or stimulation of regeneration or direct contribution of stem cells that can result in growth and functional differentiation of new neurons and supporting glial cells.26
FIG. 5.
A composite of the estimate of opportunity for regenerative therapies to treat central and peripheral nerve disorders in U.S. hospitalized patients for years 2004–2006, including stroke. (A) Comparative incidence across major neurological areas. (B) Breakdown of neuropathies. (C) Breakdown of degenerative disorders.
There has been biotechnology interest in the use of neural stem cell therapy to treat motor disorders (Geron Corp.). The incidence of paraplegia is shown in Figure 5B. Amyotrophic lateral sclerosis (ALS) accounted for 65%–71% of motor neuron disorders. When all diagnoses were counted, the incidence of ALS reached over 8,000 discharges per year.
Degenerative cerebral diseases represent a significant target population where the medical need is still largely unmet and the potential to impact quality of life is high (Fig. 5B). Despite a significant incidence, targeting a degenerative cerebral disease like Alzheimer's using stem cells is predicted to be more difficult due to an active degenerative process and lack of appropriate target cells within the brain.27 In these indications, stem cells might be used indirectly to deliver factors to limit degeneration. This indirect or antagonistic mode of action would naturally make it a more difficult and potentially less-robust medical application and target opportunity for stem cell therapy.28 Also, methods needed to deliver the therapy over time may be particularly difficult compared to a drug approach,27 illuminating the need to consider how a therapy would be used. The methods and results should mesh with acceptable medical practice, risk, and patient needs as well as cost to be an appropriate match to the target opportunity.
Parkinson's disease has long been viewed as a prime target for fetal and stem cell therapy.29 The incidence for Parkinson's disease ranged from 34,000 to 41,000 discharges per year (Fig. 5C). In Parkinson's disease the stem cell therapy is direct. Stable differentiation, integration into the substantia nigra, and persistence of dopaminergic neurons capable of producing sufficient amounts of dopamine with lasting effect will be key to its feasibility and therapeutic value. Of concern in clinical experience to date is the development of dyskinesis30,31 suggesting that knowledge of how to achieve better integration of the cells may be as important to successful translation as delivery and differentiation of the cells. There is a similar hurdle in the use of cardiomyocytes for cardiac repair where the development of arrhythmia will be the concern.32
In diseases with an etiology of autoimmune or inflammatory dysregulation like multiple sclerosis, there is the possibility of using stem cells as drugs to manage the inflammatory response to limit symptoms and possibly progression.33 This will be true for degenerative spinal cord disease as well. But by our more stringent definition, a regenerative therapy for these indications would target stable remyelination, a more complex and difficult objective but also one that is (1) of potentially higher value, (2) less likely to directly compete with more traditional drug approaches, and (3) possibly synergistic with immune therapies. While not all targets in this category will be equally accessible and the scientific match between technology and target equally robust, all represent a significant medical need where a therapy that could improve function and quality of life would be of high value. A more detailed analysis of the medical need, mechanism of action, and regulatory paths as stand-alone or combination therapies will be covered in a future publication.
In summary, the possibility of intervening to limit neural degeneration caused by stroke and ultimately recover lost function through regeneration is attractive and the market sizable. Restoration of spinal cord function will be critical as well. The major issues to overcome in these areas are ones of functional integration within an injured or diseased environment, and from a practical perspective, mode of cell delivery.
Genetic and metabolic disorders
Restoration of liver cell function constitutes the most prominent target opportunity within the genetic metabolic disorders (Fig. 6A). The most prominent liver target is in disorders of lipid metabolism34 although it is reasonable to expect that a single technology able to restore liver cell function could be leveraged to address multiple metabolic targets (Fig. 6B).35 Leveraging of technology would also be likely in bone marrow targets. This is in contrast to the pancreatic islet target for the treatment of diabetes, which is significantly lower than the single lipid metabolism target for a liver technology. Both the liver and pancreas are developmentally related so it is not unreasonable to anticipate that there will be a comparable level of scientific difficulty in generating and validating either liver or islet progenitors36 from either embryonic stem cells or induced pluripotent stem cells. Both have demonstrated some preclinical and early clinical proof of principle.37–39 The differences between liver and islet therapies in development, regulatory, and medical hurdles and how they impact commercial translation will be reviewed in a future report.
FIG. 6.
A composite of the estimate of opportunity for regenerative therapies to treat genetic and metabolic disorders in U.S. hospitalized patients for years 2004–2006. (A) A detailed incidence of liver-, pancreas-, and bone marrow–associated disorders. (B) The total relative treatment opportunity in liver, pancreas, and bone marrow disorders.
The advances for liver and pancreas transplantation have followed parallel paths. However both represent very different kinds of opportunities. A therapy capable of re-establishing functional liver cells within a disease-compromised liver could address disorders in multiple niche and orphan drug markets. Bone marrow stem cells may also address multiple disorders. In contrast, approaches to restore or regenerate pancreatic islets, or more specifically beta cells, will be most applicable to the smaller type 1 diabetic population and then possibly to a niche population within the large type II diabetic population.
Meeting the medical needs identified in the EOA
Even a top-level analysis of the EOA data made it clear that many of the most prominent opportunities for regenerative therapies would not be realized without significant scientific progress and innovation. Without major industry investment and activity, the impetus toward translation must occur in academia and small companies, primarily funded through government grants. Our next goal was to establish objectively a baseline of current research productivity and innovation in U.S.-funded RMSCR and examine how well current activities fit with what was learned from the EOA data.
The NIH recently created a Research, Condition, and Disease Categorization system in response to the NIH Reform Act of 2006. This allowed the collection of data on basic research funding for regenerative medicine and stem cell research categories as well as a break-out of “stem cells” and “tissue engineering” by term using a beta version of the NIH RePORT and an additional RePORT data summary on NIH Estimate of Funding.17 Research output and early translational activity were evaluated by measuring the level of output and innovation attributed to grants awarded by the NIH funded in fiscal years 2008 and 2009. Projects attributed to the categories of regenerative medicine and stem cell research were combined to form the RMSCR data set. Additionally, projects in all categories were searched by key terms to form alternative data groups in stem cells and tissue engineering (not listed by category). Since each project can be assigned multiple categories and key terms, the data sets overlapped but were not equivalent. We examined three contributing factors: (1) U.S. investment in basic and applied research, (2) U.S. scholarly output measured by linked papers, and (3) innovation measured by foundational linked patents and pending patent applications (U.S. Patent data). Naturally, this analysis is dependent on the accuracy of NIH's categorization of funding areas.
U.S. investment in basic and applied research relevant to regenerative therapies
In general, it appeared that both cardiovascular and neurodegenerative research were supported at levels that reflected the medical need (Table 1). The striking exception was arthritis, which despite its prominence as a regenerative medicine target, stood out as being significantly undersupported both as a whole (Table 1) and within the RMSCR group filtered by key terms (Table 2). Yet RMSCR projects filtered using “bone” as a key term identified the highest number of RMSCR projects, even outpacing cancer by a small margin (Table 2). This suggests that while bone-related research is appropriately supported, it includes little focus on arthritis and cartilage regeneration research, indicating a possible disconnect between research focus and the medical need as measured by our EOA.
Table 1.
Relative Ranking of Regenerative Medicine, Stem Cell Research and Tissue Engineering Amongst Major Research Categories
| Ranked by funding | Category or term set | Funding $ (100,000) | No. of projects | No. of publications | No. of patents | % SBIR/STTR funding | % SBIR/STTR projects |
|---|---|---|---|---|---|---|---|
| 1 | Cancer | 111,990 | 32,840 | 58,208 | 1,541 | 2.2 | 2.3 |
| 2 | Bioengineering | 60,080 | 18,843 | 45,410 | 964 | 10.6 | 10.4 |
| 3 | Aging | 49,800 | 17,424 | 38,019 | 659 | 2.4 | 2.1 |
| 4 | Cardiovascular | 40,350 | 11,856 | 27,317 | 477 | 2.5 | 2.1 |
| 5 | Neurodegenerative | 31,740 | 11,030 | 23,913 | 514 | 29.9 | 2.5 |
| 6 | Stem cell (by term) | 30,440 | 8,379 | 20,065 | 647 | 1.6 | 1.8 |
| 7 | Diabetes | 21,100 | 5,737 | 14,349 | 249 | 2.3 | 2.2 |
| 8 | SCR | 19,820 | 8,379 | 20,065 | 647 | 1.6 | 1.3 |
| 9 | RM | 15,220 | 7,754 | 19,230 | 549 | 2.4 | 1.5 |
| 10 | Obesity | 14,090 | 4,685 | 12,118 | 141 | 1.1 | 1.0 |
| 11 | Nanotechnology | 6,470 | 2,191 | 6,358 | 148 | 2.7 | 3.0 |
| 12 | Arthritis | 4,780 | 1,688 | 6,223 | 99 | 3.6 | 3.1 |
| 13 | Gene therapy | 4,700 | 1,542 | 6,103 | 281 | 2.7 | 3.0 |
| 14 | Tissue engineering (by term) | 3,758 | 1,207 | 2,507 | 29 | 5.0 | 4.6 |
SBIR/STTR, small business innovational research and technology transfer grants; SCR, stem cell research; RM, regenerative medicine.
Bold text highlights RMSCR research by category or term.
Table 2.
Regenerative Medicine and Stem Cell Research Activity by Specialty
| Subset within RMSCR group | No. of RMSCR projects identified |
|---|---|
| Aging | 976 |
| Arthritis | 104 |
| Bone | 2,444 |
| Cartilage | 251 |
| Cancer | 2,407 |
| Brain | 1,486 |
| Spinal cord | 464 |
| Cardiovascular | 816 |
| Peripheral vascular | 38 |
| Liver | 567 |
| Pancreas | 239 |
| ALL RMSCR | 7,754 |
RMSCR, Combined regenerative medicine and stem cell research categories.
It was clear that stem cell research dominated RMSCR funding (Table 1). Of the 7,754 projects within the RMSCR group, only 1,417 projects (18%) categorized as regenerative medicine did not also fall under stem cell research, approximating the number of projects (1,207) identified by the term “tissue engineering.” Our EOA analysis clearly indicated that for a clinically effective outcome, many programs would ideally need to combine stem cell research and tissue engineering expertise (among other areas). Although strong support of stem cell research alone will provide an important foundation of basic knowledge, and appears to be doing so (Table 1), it will be important to see the tissue engineering grants and the stem cell research grants begin to merge.
The interest in doing stem cell research was understandable; however, we wanted to understand more about its connection to regenerative medicine and also better understand possible reasons for the lack of tissue engineering support. Table 3 shows the levels of cross-categorization for grants identified by key terms. Although a higher percentage of tissue engineering grants were categorized as regenerative medicine compared with stem cell grants (56% vs. 40%), the results were not dissimilar. However, tissue engineering grants categorized as stem cell research represented 25% of an already small number of grants compared with 56% for the much larger stem cell group. Both groups had a nearly equal number of grants categorized under arthritis, suggesting possible integrated effort although the numbers were very small (Table 3).
Table 3.
Comparative NIH Spending on Stem Cell vs. Tissue Engineering Projects
| NIH spending category(ies)a | Stem cell projects (by term) 2008–2009 $ (millions) | Tissue engineering projects (by terms) 2008–2009 $ (millions) |
|---|---|---|
| All | 3,043 | 376 |
| RM | 1,228 | 211 |
| SCR | 1,726 | 95 |
| Both RM and SCR | 1,136 | 91 |
| RM but not SCR | 92 | 119 |
| SCR but not RM | 590 | 4 |
| Bioengineering | 320 | 250 |
| Biotechnology | 894 | 163 |
| Cardiovascular | 246 | 47 |
| Arthritis | 27 | 24 |
| Uncategorized | 428 | 46 |
Projects can be attributed to more than one category.
Scholarly output and innovation
Publications, patent base, and pending patents were studied to gain a sense of output that could impact translation. Output data reported by the NIH RePORTer system were used to compare RMSCR to other categories of research (Table 1). Publications were those identified as linked to the current research in some way. Since patents take several years to issue once filed, the linked patents in the RePORTer database appeared to past as well as present intellectual property and thus represented a base of technology rather than direct output of the current research. In addition to category and group data, data on institutions with 10 or more unique RMSCR projects in 2008–2009 and ≥$10M in funding were collected to more closely examine the distribution of knowledge generation and innovation, this time adding pending patent information gathered from the United States Patent and Trademark Office (USPTO). One can debate the relative importance of each component, but the translational success of a research program will be related to a combination of its scientific and technical foundation, as well as the current degree of innovation and level of scholarly output. Therefore, a picture of them together gives us some idea of relative performance and potential in the near term.
The relative performance of the Tissue Engineering group in this analysis is not straightforward to understand (Table 1). Gene Therapy and Arthritis, despite a similar level of support, reported significantly higher numbers of linked publications and patents. Bioengineering was well funded. It is possible that researchers are no longer using the term “tissue engineering” to describe their work, but a term search of bioengineering projects using the terms “regenerative medicine” or “tissue engineering” each identified <1,000 projects among the 18,843 projects in the category. Another possibility is that the interest in stem cells has attracted the government and this has influenced investigator interest, resulting in fewer tissue engineering grants being submitted. A third possibility is that while stem cell research is still clearly in a research phase, tissue engineering has been around longer and has had a heavily applied focus. Therefore, tissue engineering projects are less likely to be viewed as new, innovative research. There appeared to be insufficient support for tissue engineering relative to the EOA, but the projects that were funded had a comparatively low level of productivity. This suggests that it is more difficult to produce publishable results in tissue engineering or conversely, it is easier to get stem cell work published. This could be due to the nature and novelty of stem cell work over tissue engineering, the increasing number of journals to publish stem cell work, and general interest in stem cell biology. Indeed, the overall strong showing for RMSCR appeared primarily due to stem cell research. However these data represent a total of all activities and a natural question emerges: are there readily identifiable clusters of excellence, or does the total reflect the overall state of a field like tissue engineering and how is the know-how in stem cell research distributed?
Analysis of the 58 most active RMSCR institutions (excluding institutions with a principal focus on cancer) revealed several surprises. As we might expect, there was some correlation between funding and linked publications (correlation=0.42) and the linked base of patents (correlation=0.68); however, when we broke out groups by major U.S. biotechnology regions for a detailed look, an interesting picture emerged (Table 4). The Boston area (Eastern MA) is home to nine of the top RMSCR institutions and represented a range of funding levels. While attribution of publications and patents is somewhat dependent on investigator reporting and can be subjective, the trend that emerged was one of output variability, particularly in the patent area. This variability was supported by the objective number of pending patent applications. Of note were two institutions that had a strong patent base and future patent potential. Also, a lesser-funded institution with a modest patent base exhibited a strong showing in RMSCR patent applications. Investigators within the institutions appear to value intellectual property differently and do not appear to be positively influenced by proximity to highly innovative institutions or an entrepreneurial environment. The top 1 and 3 ranked institutions were from northern California. Given their level of funding, these institutions had both a limited self-reported intellectual property base and future patent potential. The level of patent activity was not mirrored by linked publications. The data suggest that investigators in these institutions either do not self-report patent activity or are focused on research less likely to lead to patentable innovations. Southern California had a distribution of funding similar to the Boston area with two institutions appearing to be making strides in the generation of new RMSCR intellectual property. Undoubtedly, with 58 institutions conducting 10 or more projects and funded at ≥$10M, investment in the field is spread across many institutions averaging out differences, at least for stem cell research. We also compared funding for projects identified by the terms “tissue engineering” and “stem cells” for the top RMSCR institutions (Fig. 7). It is clear that very few institutions have a major tissue engineering effort and no RMSCR institution received more funding to conduct tissue engineering research than stem cell–related research.
Table 4.
Regional Differences in Funding and Productivity in Major Biotechnology Hubs
| Funding rank among most active RMSCR institutions | Funding | No. of linked publications | No. of linked patients | No. of pending patent applicationsa |
|---|---|---|---|---|
| Region: Eastern MA | ||||
| 7 | $48,158,409 | 283 | 12 | 3 |
| 8 | $41,097,558 | 180 | 4 | 4 |
| 14 | $33,778,188 | 134 | 1 | 0 |
| 26 | $21,944,041 | 286 | 37 | 23 |
| 33 | $19,318,532 | 183 | 19 | 9 |
| 41 | $14,696,506 | 147 | 0 | 2 |
| 47 | $13,458,151 | 102 | 0 | 1 |
| 50 | $12,382,457 | 86 | 0 | 5 |
| 54 | $11,202,036 | 62 | 3 | 10 |
| Total funding | $216,035,878 | |||
| Region: Northern CA | ||||
| 1 | $69,230,915 | 404 | 8 | 8 |
| 3 | $60,042,302 | 316 | 10 | b |
| Total funding | $129,273,217 | |||
| Region: Southern CA | ||||
| 11 | $36,413,010 | 528 | 7 | b |
| 18 | $29,295,268 | 452 | 7 | b |
| 24 | $23,269,184 | 73 | 1 | 7 |
| 29 | $21,080,319 | 59 | 1 | 13 |
| 37 | $16,762,233 | 108 | 5 | b |
| 43 | $14,256,843 | 132 | 8 | 1 |
| 55 | $11,049,652 | 186 | 10 | b |
| Total funding | $152,126,509 | |||
| bUniversity of CA | $164,555,957 | 63 | ||
Represents RMSCR patent applications in the USPTO with the institution as assignee. The current rate of allowance for applications in the United States is 0.47.42
USPTO, United States Patent and Trademark Office.
FIG. 7.
Proportion of tissue engineering funding in the top NIH-funded institutions in regenerative medicine and stem cell research. Data source: NIH RePORTer database.16 Projects were identified by the terms “tissue engineering” or “stem cells.” NIH, National Institutes of Health.
Small business innovation and technology transfer
Small business support through the small business innovational research and technology transfer grants (SBIR/STTR) is an important stepping stone for university technologies. We broke out SBIR/STTR projects to measure the support of small business activity within the major research areas (Table 1). Small business projects and funding of RMSCR were in-line with other categories and accounted for a fairly consistent 1%–3% of the total projects and funding within a research category, regardless of the category's overall level of output and innovation.
Discussion
The estimate of opportunity analysis
While medical device companies are steadily adding biological sophistication to their products and some large pharmaceutical companies are establishing internal expertise in stem cell biology, internal and external investment must be justified with a clear view of how those investments can lead to products that will impact patient care. In addition, the ability to leverage an investment in specialized expertise and processes for a niche market will be key to justify the investment needed to advance many regenerative technologies. This requires a clear picture of the treatment opportunities. Basic research and commercial efforts alike are in need of target-based data that can be matched to and analyzed against a particular technology and medical strategy for reaching it. As we have shown, EOA data can help identify:
Synergies and opportunities for leveraging technology and know-how
Likely reimbursement (and regulatory) issues
The medical value needed for successful adoption and commercialization
Knowledge and technology gaps
Use of target-based EOA data for pipeline analysis may reduce development risk by directing efforts to the most feasible, high-impact applications—even from the earliest stage of innovation. EOA data can also provide clarity with respect to the best path for a technology.
As mentioned in this report, we are preparing more detailed analyses in the key medical areas identified in this report.
Cardiovascular and peripheral vascular disorders
Orthopedic and spine disorders
Central and peripheral nervous system disorders including stroke
These reviews will include EOA detail down to individual disorders, gap analyses of the major medical needs identified by the EOA, and in some cases, additional analysis of scientific barriers to progress.
Despite abundant target opportunities, innovative regenerative medicine, like all therapies, must meet the medical need with therapies capable of delivering value to the patient and the medical system. Without it, reimbursement will continue to be challenged, and business models will remain unconvincing to potential investors. Apart from an initial burst of commercial activity 20 years ago that led to U.S. approvals for regenerative biomaterials (Integra Dermal Regeneration Scaffold; Integra Life Sciences), the first device and biologic cell therapies (Regranex®, Johnson and Johnson; InFuse, Medtronic) and tissue engineered combination products (Apligraf®, Organogenesis Inc.; Dermagraft®, Advanced Tissue Sciences and Smith and Nephew [acquired by Advanced BioHealing]), academia, and industry alike have struggled to expand the list.40
The osteoarthritis target opportunity is one of the most significant treatment opportunities for a regenerative therapy. Effective cartilage repair would reduce the need for knee replacement surgery, and a cartilage repair procedure would likely increase the quality of life for many more patients at a cost savings to the medical system if less than the current cost for knee replacement. The incidence of knee replacement surgeries in the U.S. population doubled for patients aged 45–64 between 2000 and 2006 and in those 65 and older rose from 60.1 to 88.0 per 10,000.15 However, there are regenerative products and procedures currently available that have gained only modest acceptance. How much better does a second-generation cartilage therapy have to be to justify investment in the development of a next generation product? Evaluate Pharma Ltd. reported worldwide sales of Carticel® (Genzyme Biosurgery) of $25M for 2008, an increase of 4% over 2007—after 11 years (Evaluate Pharma Ltd.). This indicates that regenerative therapies for cartilage repair must become significantly better. Venture capital remains unconvinced that a critical mass of science and technology has yet been combined to achieve this (Jeffrey Koran, personal communication) although there is some evidence of a move in that direction with the recent merger of two cartilage repair companies: Histogenics and Prochon. Both large and small companies have struggled with advancing a regenerative cartilage therapy suggesting that financing is not the principal roadblock. It is evident that additional knowledge and innovation is needed and that targeted investment from NIH could have a significant impact. Further investigations focused on better enabling the use of mesenchymal or other stem or progenitor cells in cartilage regeneration would be an effective starting point.41
While we have highlighted the most prominent opportunities in the major disease categories, a small target population is not in itself a limiting factor as is evident from the growing interest in orphan indications and personalized medicine. For example, the low incidence of muscular dystrophy may qualify it for orphan drug and device status, which is based on a U.S. prevalence of <200,000.42 However, an important consideration for the commercialization of orphan regenerative therapies compared to the highly lucrative biologics like antibodies (e.g., Alexion's Soliris®) or enzymes (e.g., Genzyme's Cerezyme®) will be that regenerative therapies beyond molecules will involve more limited or finite dosing and ideally will lead to long lasting results. Depending on the type of therapy, method of delivery, and target, persistent results lasting years may in fact be a prerequisite to approval and acceptance by regulators, patients, and clinicians. A $250,000 yearly cost per patient for an orphan biologic may translate to $250,000 per patient over several years, or the life of the patient, for a regenerative therapy. This model would drive a very high cost per treatment for orphan regenerative (curative) treatments that may not be tolerated by payers without evidence of cost savings over time.
The EOA suggests that not every technology and indication pairing will be equally feasible or valuable—for varying reasons. While top-level analysis of the EOA data provides important perspective and directs us to the major regenerative targets, assessment of a technology's potential to address a specific medical need will require drilling down to the details of the science, technology, medical need, and target patient populations using the EOA data as a road map. Our theory is that an ever clearer understanding of the target opportunities should help academics more readily identify key knowledge gaps and more clearly recognize the opportunities for discovery and innovation in their research—improving the efficiency and effectiveness of translation. We will shortly publish a series of such analyses in each of the main clinical areas.
Analysis of U.S. research output and innovation in RMSCR
The category and institutional analyses leave little doubt that further focusing of investment toward areas of greatest scientific and medical need could improve the current return on investment in tissue engineering. This, and other conclusions of our study, echoes recent conclusions on bioscience progress from the BIGT, which advised that “universities must think and act more strategically” and build a critical mass of knowledge in a particular subject or sector.6
U.S. policy on embryonic stem cell research is often cited as a limiting factor in the pace of discovery in regenerative therapies. Interestingly, most of the opportunities identified in our analysis do not hinge on the use of embryonic stem cells.
Government funding alone, in most cases, will be insufficient to advance a technology through to FDA approval and commercialization. Government funding is not a substitute for private sector investment, though it can be a positive catalyst for this investment. This will require a critical mass of knowledge and innovation to support the connectivity between a technology and treatment target. Of particular interest will be the factors that generate actionable knowledge and patents that enable RMSCR products. Improved patent output should enhance the return on research investment. The need for greater innovation is not specific to RMSCR as U.S. academic patents in biotechnology have declined nearly 50% from highs reached between 1998 and 2001.43 Also, the lack of crossover between the tissue engineering and stem cell research spheres could be a factor in the pace at which RMSCR to advances in key areas of medical need going forward.
The EOA data provide substantial evidence of the clinical and commercial potential of regenerative medicine—as stem cell therapies and tissue engineering, as orphan biologics, and as therapies to fill large unmet medical needs. As in any new field, translating regenerative science is not without its challenges. We have concluded that identifying the challenges and studying them from a scholarly perspective is a critical step in understanding how to overcome them. It is our hope that this and future EOA analyses will help those involved in the field to target scientific, clinical, and commercial pursuits in a way that can sustainably generate high-value regenerative therapies that represent a critical part of the future of medicine.
Acknowledgments
The authors thank Dr. Doug Hardin for his review of the NIH data and manusript, Gregory Bonfiglio for his insights on the current small company and venture capital landscape, and Jeffrey Karan for helpful discussions regarding the perspective of investment banking as well as the pharmaceutical and medical device companies.
Funding: This project was funded by a gift from the Heinz Foundation to the McGowan Institute of Regenerative Medicine.
Disclosure Statement
No competing financial interests exist.
References
- 1.Luce C.B. Giovannetti G. The Ernst & Young Business Risk Report-Life Sciences. New York: EYGM Ltd.; 2009. p. 10. [Google Scholar]
- 2.Frantz S. Pipeline problems are increasing the urge to merge. Nat Rev Drug Discov. 2006;5:977. doi: 10.1038/nrd2206. [DOI] [PubMed] [Google Scholar]
- 3.Mintz C. The potential of personalized medicine: Life Science Leader. Sewickley, PA: VertMarkets; 2010. [Google Scholar]
- 4.Conway B. Big pharma reassesses orphan drug sector. Wall Street BioBeat. 2010;30:5. [Google Scholar]
- 5.Services DoHaH. Regenerative Medicine. Bethesda, MD: US Department of Health and Human Services; 2006. [Sep 25;2010 ]. [Google Scholar]
- 6.Cooksey D. The Review and Refresh of Bioscience 2015. A Report to Government by the Bioscience Innovation and Growth Team London: Office for Life Sciences. 2009. www.berr.gov.uk/files/file49805.pdf. [Sep 3;2010 ]. www.berr.gov.uk/files/file49805.pdf
- 7.Gallico G.G., 3rd O'Connor N.E. Compton C.C. Kehinde O. Green H. Permanent coverage of large burn wounds with autologous cultured human epithelium. N Engl J Med. 1984;311:448. doi: 10.1056/NEJM198408163110706. [DOI] [PubMed] [Google Scholar]
- 8.Parenteau N. Skin: the first tissue-engineered products. Sci Am. 1999;280:83. doi: 10.1038/scientificamerican0499-83. [DOI] [PubMed] [Google Scholar]
- 9.Thomson J.A. Itskovitz-Eldor J. Shapiro S.S. Waknitz M.A. Swiergiel J.J. Marshall V.S., et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 10.Pittenger M.F. Mackay A.M. Beck S.C. Jaiswal R.K. Douglas R. Mosca J.D., et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 11.Takahashi K. Tanabe K. Ohnuki M. Narita M. Ichisaka T. Tomoda K., et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 12.Zhou H. Wu S. Joo J.Y. Zhu S. Han D.W. Lin T., et al. Generation of induced pluripotent stem cells using recombinant proteins. Cell Stem Cell. 2009;4:381. doi: 10.1016/j.stem.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Warren L. Manos P.D. Ahfeldt T. Loh Y.H. Li H. Lau F., et al. Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell. 2010;7:618. doi: 10.1016/j.stem.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson P.C. Bertram T.A. Tawil B. Hellman K.B. Hurdles in tissue engineering/regenerative medicine product commercialization: a survey of North American academia and industry. Tissue Eng Part A. 2011;17:5. doi: 10.1089/ten.TEA.2010.0411. [DOI] [PubMed] [Google Scholar]
- 15.DeFrances C.J. Lucas C.A. Buie V.C. Golosinskiy A. National Hospital Discharge Survey. Natl Health Stat Rep. 2006;2008;1 [PubMed] [Google Scholar]
- 16.Dennison C.F. Pokras R. In: Design and operation of the national hospital discharage survey: 1988 Redesign. Statistics NCfH., editor. Hyattsville, M.D: Center for Disease Control and Prevention; 2000. [PubMed] [Google Scholar]
- 17.Health NIo. Bethesda, MD: National Institutes of Health; 2010. [May 19;2011 ]. Research Portfolio Online Reporting Tools (RePORT) [Google Scholar]
- 18.Kinnaird T. Stabile E. Burnett M.S. Shou M. Lee C.W. Barr S., et al. Local delivery of marrow-derived stromal cells augments collateral perfusion through paracrine mechanisms. Circulation. 2004;109:1543. doi: 10.1161/01.CIR.0000124062.31102.57. [DOI] [PubMed] [Google Scholar]
- 19.Rosenstrauch D. Poglajen G. Zidar N. Gregoric I.D. Stem celltherapy for ischemic heart failure. Tex Heart Inst J. 2005;32:339. [PMC free article] [PubMed] [Google Scholar]
- 20.Menasche P. Alfieri O. Janssens S. McKenna W. Reichenspurner H. Trinquart L., et al. The Myoblast Autologous Grafting in Ischemic Cardiomyopathy (MAGIC) trial: first randomized placebo-controlled study of myoblast transplantation. Circulation. 2008;117:1189. doi: 10.1161/CIRCULATIONAHA.107.734103. [DOI] [PubMed] [Google Scholar]
- 21.Hunt S.A. Baker D.W. Chin M.H. Cinquegrani M.P. Feldman A.M. Francis G.S., et al. ACC/AHA Guidelines for the Evaluation and Management of Chronic Heart Failure in the Adult: Executive Summary A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1995 Guidelines for the Evaluation and Management of Heart Failure): Developed in Collaboration With the International Society for Heart and Lung Transplantation; Endorsed by the Heart Failure Society of America. Circulation. 2001;104:2996. doi: 10.1161/hc4901.102568. [DOI] [PubMed] [Google Scholar]
- 22.DOPPS. Annual Report of the Dialysis Outcomes and Practice Patterns Study. Ann Arbor: Ann Arbor Research Collaborative for Health; 2009. Annual Report of the Dialysis Outcomes and Practice Patterns Study: Hemodialysis Data 1999–2008. [Google Scholar]
- 23.Hickey E. Langley S.M. Allemby-Smith O. Livesey S.A. Monro J.L. Subcoronary allograft aortic valve replacement: parametric risk-hazard outcome analysis to a minimum of 20 years. Ann Thorac Surg. 2007;84:1564. doi: 10.1016/j.athoracsur.2007.02.100. [DOI] [PubMed] [Google Scholar]
- 24.Lupinetti F.M. Duncan B.W. Scifres A.M. Fearneyhough C.T. Kilian K. Rosenthal G.L., et al. Intermediate-term results in pediatric aortic valve replacement. Ann Thorac Surg. 1999;68:521. doi: 10.1016/s0003-4975(99)00642-6. [DOI] [PubMed] [Google Scholar]
- 25.Hootman J.M. Helmick C.G. Projections of US prevalence of arthritis and associated activity limitations. Arthritis Rheum. 2006;54:226. doi: 10.1002/art.21562. [DOI] [PubMed] [Google Scholar]
- 26.Lindvall O. Kokaia Z. Stem cells in human neurodegenerative disorders—time for clinical translation? J Clin Invest. 2010;120:29. doi: 10.1172/JCI40543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mehta T. Feroz A. Thakkar U. Vanikar A. Shah V. Trivedi H. Subarachnoid placement of stem cells in neurological disorders. Transplant Proc. 2008;40:1145. doi: 10.1016/j.transproceed.2008.03.026. [DOI] [PubMed] [Google Scholar]
- 28.Parenteau N.L. Commercial development of cell-based therapeutics: strategic considerations along the drug to tissue spectrum. Regen Med. 2009;4:601. doi: 10.2217/rme.09.29. [DOI] [PubMed] [Google Scholar]
- 29.Brundin P. Barker R.A. Parmar M. Neural grafting in Parkinson's disease problems and possibilities. Prog Brain Res. 2010;184:265. doi: 10.1016/S0079-6123(10)84014-2. [DOI] [PubMed] [Google Scholar]
- 30.Lane E.L. Bjorklund A. Dunnett S.B. Winkler C. Neural grafting in Parkinson's disease unraveling the mechanisms underlying graft-induced dyskinesia. Prog Brain Res. 2010;184:295. doi: 10.1016/S0079-6123(10)84015-4. [DOI] [PubMed] [Google Scholar]
- 31.Politis M. Wu K. Loane C. Quinn N.P. Brooks D.J. Rehncrona S., et al. Serotonergic neurons mediate dyskinesia side effects in Parkinson's patients with neural transplants. Sci Transl Med. 2010;2:38ra46. doi: 10.1126/scitranslmed.3000976. [DOI] [PubMed] [Google Scholar]
- 32.Menasche P. Stem Cell Therapy for Heart Failure: Are Arrrhymias a Real Safety Concern? Circulation. 2009;119:2735. doi: 10.1161/CIRCULATIONAHA.108.812693. [DOI] [PubMed] [Google Scholar]
- 33.Uccelli A. Mancardi G. Stem cell transplantation in multiple sclerosis. Curr Opin Neurol. 2010;23:218. doi: 10.1097/WCO.0b013e328338b7ed. [DOI] [PubMed] [Google Scholar]
- 34.Mitchell C. Mignon A. Guidotti J.E. Besnard S. Fabre M. Duverger N., et al. Therapeutic liver repopulation in a mouse model of hypercholesterolemia. Hum Mol Genet. 2000;9:1597. doi: 10.1093/hmg/9.11.1597. [DOI] [PubMed] [Google Scholar]
- 35.Piscaglia A.C. Campanale M. Gasbarrini A. Gasbarrini G. Stem cell-based therapies for liver diseases: state of the art and new perspectives. Stem Cells Int. 2010;2010:259461. doi: 10.4061/2010/259461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Parenteau N.L. Rosenberg L. Hardin-Young J. The engineering of tissues using progenitor cells. Curr Top Dev Biol. 2004;64:101. doi: 10.1016/S0070-2153(04)64006-3. [DOI] [PubMed] [Google Scholar]
- 37.Quante M. Wang T.C. Stem cells in gastroenterology and hepatology. Nat Rev Gastroenterol Hepatol. 2009;6:724. doi: 10.1038/nrgastro.2009.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Robertson R.P. Islet transplantation a decade later and strategies for filling a half-full glass. Diabetes. 2010;59:1285. doi: 10.2337/db09-1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jun H.S. Cell replacement and regeneration therapy for diabetes. Korean Diabetes J. 2010;34:77. doi: 10.4093/kdj.2010.34.2.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mandel M. The failed promise of innovation in the US: Business Week. New York: Bloomberg; 2010. [Google Scholar]
- 41.Caplan A.I. Review: mesenchymal stem cells: cell-based reconstructive therapy in orthopedics. Tissue Eng. 2005;11:1198. doi: 10.1089/ten.2005.11.1198. [DOI] [PubMed] [Google Scholar]
- 42.US FDA Orphan Drug Act. USA Public Law 97-414. www.fda.gov/RegulatoryInformation/Legislation/FederalFoodDrugandCosmeticActFDCAct/SignificantAmendmentstotheFDCAct/OrphanDrugAct/default.htm. [Jun 27;2011 ]. www.fda.gov/RegulatoryInformation/Legislation/FederalFoodDrugandCosmeticActFDCAct/SignificantAmendmentstotheFDCAct/OrphanDrugAct/default.htm
- 43.National Science Foundation. National Science Board, Science and Engineering Indicators, 2010. 2010. www.nsf.gov/statistics/seind10/ [Jul 26;2010 ]. www.nsf.gov/statistics/seind10/